- 1Department of Radiation Oncology, Medical College of Wisconsin, Milwaukee, WI, United States
- 2Department of Radiation Oncology, University of California, Los Angeles, Los Angeles, CA, United States
- 3Division of Radiotherapy and Imaging, The Institute of Cancer Research, London, United Kingdom
- 4Depart of Radiation Oncology, Weill Cornell Medicine, Department of Radiation Oncology, New York, NY, United States
- 5Department of Radiation Oncology, Sunnybrook Hospital, University of Toronto, Toronto, ON, Canada
- 6Department of Radiation Oncology, The Netherlands Cancer Institute, Amsterdam, Netherlands
- 7Department of Radiation Oncology, Radboud University Medical Center, Nijmegen, Netherlands
- 8The Royal Marsden NHS Foundation Trust, and the Institute of Cancer Research, Sutton, United Kingdom
Introduction: Prostate cancer is a common malignancy for which radiation therapy (RT) provides an excellent management option with high rates of control and low toxicity. Historically RT has been given with CT based image guidance. Recently, magnetic resonance (MR) imaging capabilities have been successfully integrated with RT delivery platforms, presenting an appealing, yet complex, expensive, and time-consuming method of adapting and guiding RT. The precise benefits of MR guidance for localized prostate cancer are unclear. We sought to summarize optimal strategies to test the benefits of MR guidance specifically in localized prostate cancer.
Methods: A group of radiation oncologists, physicists, and statisticians were identified to collectively address this topic. Participants had a history of treating prostate cancer patients with the two commercially available MRI-guided RT devices. Participants also had a clinical focus on randomized trials in localized prostate cancer. The goal was to review both ongoing trials and present a conceptual focus on MRI-guided RT specifically in the definitive treatment of prostate cancer, along with developing and proposing novel trials for future consideration. Trial hypotheses, endpoints, and areas for improvement in localized prostate cancer that specifically leverage MR guided technology are presented.
Results: Multiple prospective trials were found that explored the potential of adaptive MRI-guided radiotherapy in the definitive treatment of prostate cancer. Different primary areas of improvement that MR guidance may offer in prostate cancer were summarized. Eight clinical trial design strategies are presented that summarize options for clinical trials testing the potential benefits of MRI-guided RT.
Conclusions: The number and scope of trials evaluating MRI-guided RT for localized prostate cancer is limited. Yet multiple promising opportunities to test this technology and potentially improve outcomes for men with prostate cancer undergoing definitive RT exist. Attention, in the form of multi-institutional randomized trials, is needed.
Introduction
Localized prostate cancer is increasing in incidence and represents a major oncologic burden worldwide (1). Fortunately, there are several highly effective therapeutic strategies for men with localized prostate cancer (2, 3). In most cases, localized prostate cancer is highly curable with minimal morbidity. Radiation therapy (RT) represents an effective and curative modality for men with prostate cancer, ranging from low to very high risk, for which the outcomes are excellent. As an example, in men with high risk prostate cancer, rates of five year biochemical control with RT and androgen deprivation therapy exceed 90% in modern trials incorporating advanced imaging (4, 5). This comes at a modest cost, with rates of toxicity that are low, specifically grade 3 or higher rates of less than 5%, and in some cases less than 2%. Historically gastrointestinal (GI) toxicity predominated, however recently urinary and sexual side effects have become the predominate concern for patients (5–7). While RT is already highly effective, and minimally invasive, there are opportunities for improvement. Advances in technology are rapidly enabling this, and it’s imperative that radiation oncologists consider these advances and how they may optimally be applied to improve outcomes for men with localized prostate cancer. Radiation is dramatically and rapidly changing. Radiation oncologists are tasked with understanding novel advances in RT technology, and specifically how these advances could benefit their patients.
The current standard by which the vast majority of prostate cancer patients are treated uses computed tomography (CT) to guide treatment. Often a magnetic resonance image (MRI) is registered to the CT to further delineate treatment volumes. A CT is then acquired on the treatment machine. In other words, an “on board” cone beam CT scan (CBCT) is acquired to align the patient, often daily, and subsequently evaluate for rectal position, bladder size, and location of the prostate gland and target volumes intended for treatment with RT. This represents a current standard of care applied to thousands of men globally with a high degree of success. Outcomes using CBCT based image guidance are truly excellent. Due to significant interfraction motion, as well as intrafraction motion, fiducial markers are often placed to help enhance the accuracy and precision of treatment, allowing smaller volumes of normal tissue to be irradiated. Fiducial placement is an invasive procedure, albeit a minor one, that carries risk of infection and bleeding and requires logistical support. There is also concern with this strategy that the invasive use of fiducials could rarely cause toxicity (8).
Magnetic Resonance Image (MRI) guidance is an emerging technology that enables routine access to adaptive RT. There are currently two available commercial devices, one by ViewRay (Oakwood Village, Ohio) (9) and a second by Elekta (Elekta AB, Stockholm, Sweden) (10). Key differences have been the subject of prior reivews (11). Broadly speaking across both devices, MRI guidance differs from simply MR registration, which involves aligning an MRI with a CT to facilitate soft tissue delineation, often only at a single time point such as CT simulation. Using MRI guidance involves acquiring an MRI daily before each treatment with RT to precisely guide, or align, the location of high RT dose. The process of MRI guidance also greatly facilitates adaptive RT which involves changing the dose of radiation to account for subtle changes in normal anatomy, often on a daily basis. The concept of adaptive RT has been recently addressed by multiple review articles (11, 12). In brief, this involves daily target volume and surrounding organ at risk contouring and plan recalculation to account for differences in normal organ and tumor position. Routine adaption represents a particularly promising future area for development in RT. This can be done with both CT or MR based solutions, yet MR imaging has superior soft tissue visualization, and CT presents challenges in visualizing local normal structures. However, adaptive radiotherapy is resource intensive with regards to cost and time, and may not be appropriate for all patients. Secondary to these limitations, MRI-guided RT is not universally available. MRI-guided RT remains a technology that is predominantly limited to high volume academic centers, yet is gaining traction within community centers. While these limitations also apply to CT-based methods of adaptation, MR guidance may require additional medical physics support and MR safety precautions. Precisely how this technology can improve outcomes for men with localized prostate cancer beyond highly effective CT based RT treatment strategy remains unclear. We sought to collect a group of radiation oncologists and medical physicists with the objective of organizing consensus around the optimal prospective testing of this technology for patients with localized prostate cancer. This will focus on current definitive prostate treatment trials, areas for improvement not enabled by CT, and future trial design considerations.
Methods
To summarize the current clinical trial landscape, a literature search was performed for trials focused on MR guided RT for localized prostate cancer. Trials that used CT based treatment delivery strategies that incorporated registered diagnostic MRIs or MR simulations only (without MR guided treatment machines) were excluded by review of abstracts. Current data was abstracted from both PubMed, Google Scholar, and Clinicaltrials.gov. In addition, the Medical College of Wisconsin Libraries (MCW) conducted an Ovid Medline search including the following search terms: Magnetic Resonance Imaging, image guided radiation therapy* or image-guided radiation therapy* or image-guided radiotherapy* or radiotherapy target organ alignment were included in the Ovid search criteria. This was further limited to published prospective clinical trials in a sub search.
With the goal of organizing ideas, a central table was collected to formulate where precisely adaptive MR guided RT delivery strategies could improve the treatment of localized prostate cancer. The goal of this effort was to focus specifically on the use of MRI-guided RT, and not MR registration. Collaborative input on potential trial designs, and opportunities for improvement, were collected.
The PRISMA checklist was referenced when conducting the search for this effort, however this was intended to be a narrative review, consequently all criteria specified in PRISMA were not used. A search of clinicaltrials.gov was conducted for ongoing trials. In-general these search terms included (radiotherapy OR radiation therapy OR radiation therapies OR Radiation treatment OR radiation treatments) AND (mri OR magnetic resonance) AND (guided OR guidance) | Prostate Cancer. This search was further restricted to trials that were either recruiting or active and not yet recruiting. Specifically there were several trial concepts that were “in development” however had not yet opened or been made publicly available on clinicaltrials.gov. These were excluded from this summary table.
Results
There were a total of 742 published articles that returned after the initial search query; these were individually reviewed and select articles are summarized in Table 1 with citations. With regard to results from clincaltrials.gov, a total of 123 trials resulted from the search. Again, many of these trials involved registering MRI to a reference planning CT. Selected trials are also summarized in Table 1.

Table 1 Currently published prospective trials to have evaluated mr guided rt in localized definitively treated prostate cancer.
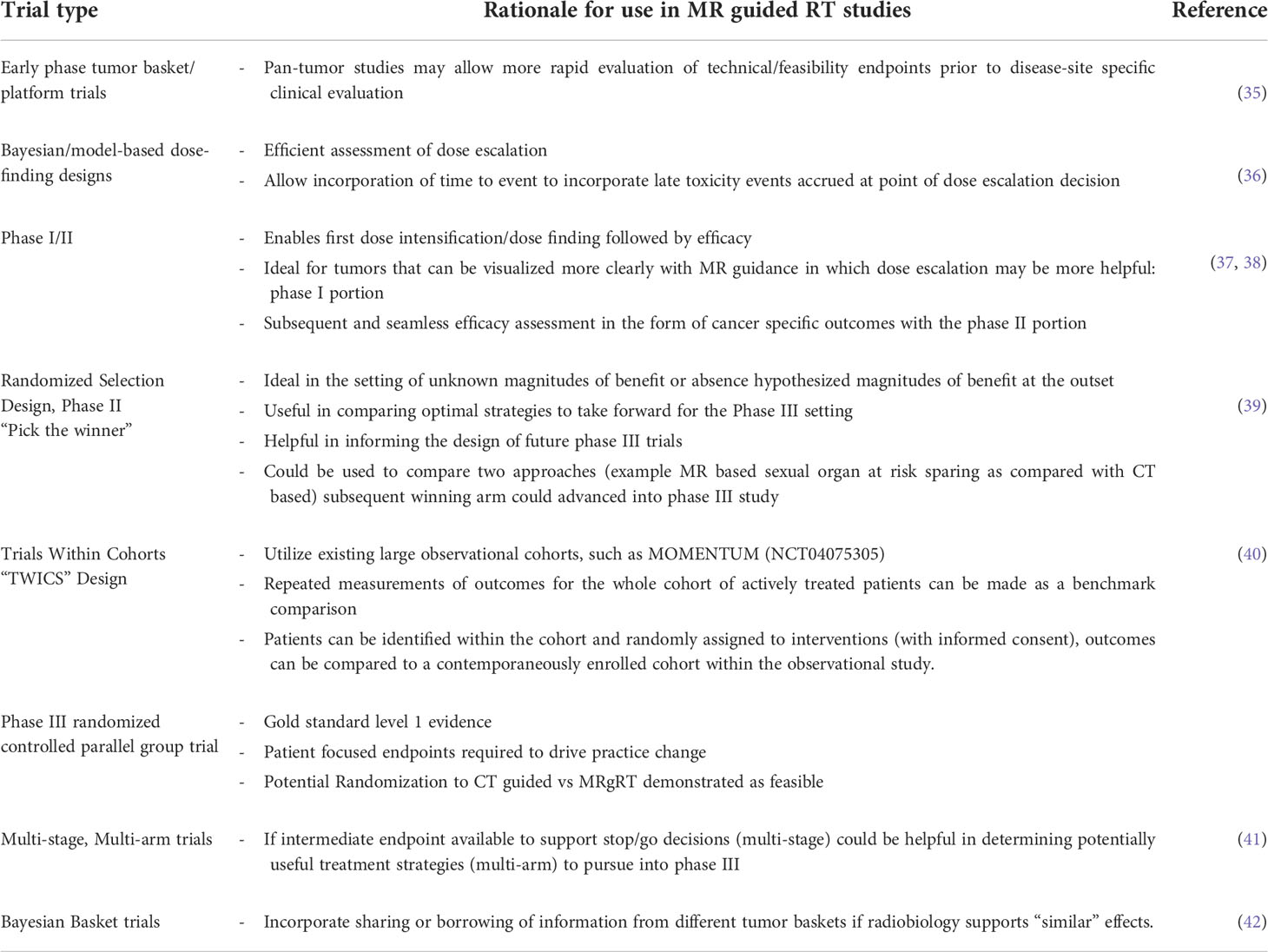
Potential strategies for improvement with MR guidance in localized prostate cancer were reviewed and tabulated accounting for input amongst all authors. These are presented in Table 2 for conceptual consideration and future trial design consideration. Moreover, this table also includes hypotheses that could be addressed with the use of MR guidance specifically over CT based image guidance. Finally, novel trial design strategies, with possible endpoints were suggested in Table 3.
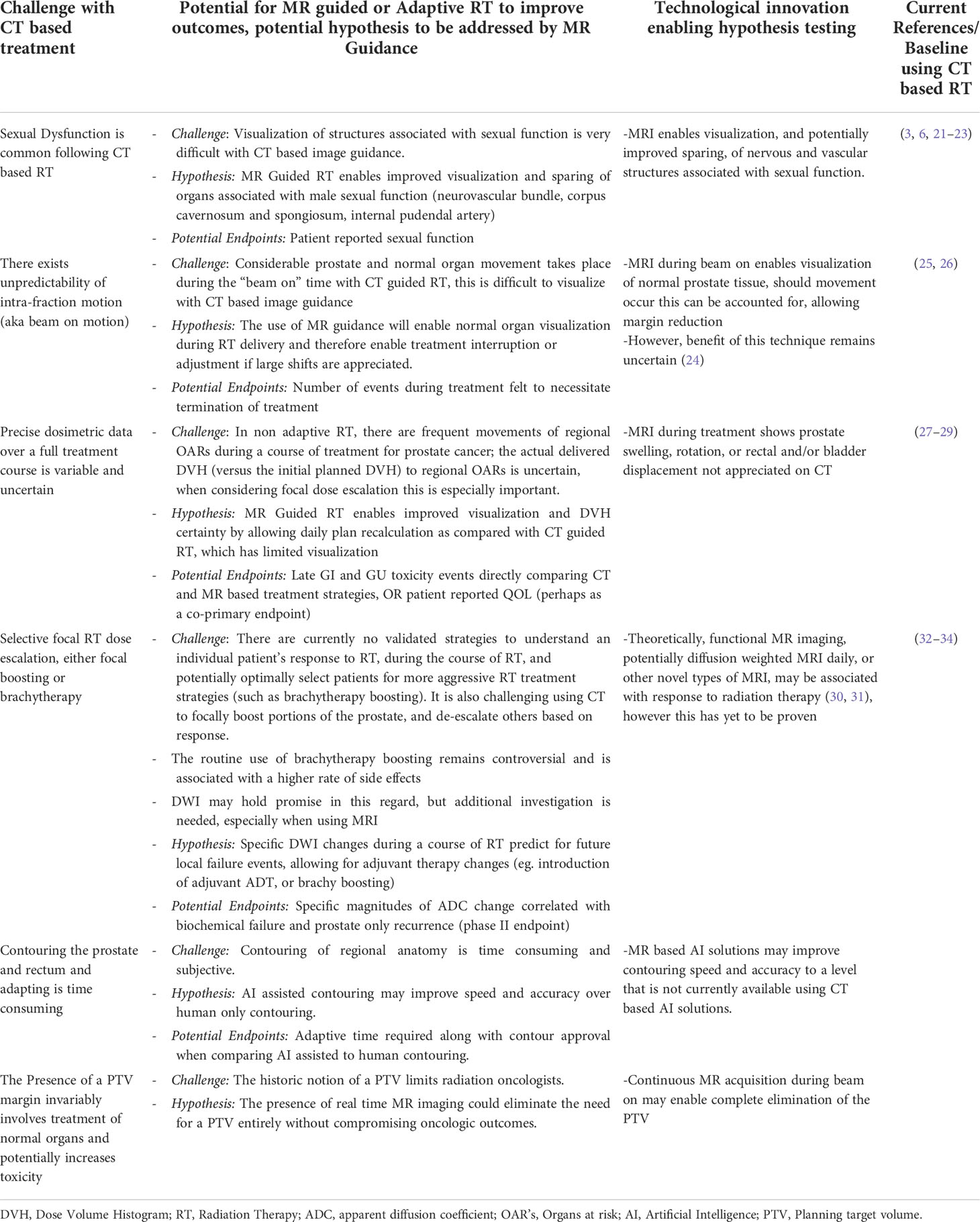
Table 2 a: Opportunities for Improvement in localized prostate cancer when considering adaptive MR guided RT trial designs.
Discussion
The ability to use MRI guidance has considerable promise for patients with localized prostate cancer (12, 43). At the present time these advantages are mostly theoretical, however data is emerging that is showing exciting improvements associated with the use of MRI-guided RT (13). A concerted focus on how to optimally demonstrate the advantages of MR guidance in localized prostate cancer is needed. There is an imperative to test this technology against current established standards. This imperative is critical for two overarching reasons. First, the current standard of care when using CT based RT is resulting in truly excellent outcomes with relatively low rates of toxicity. One such example is the recently published Prostate-Only Versus Whole-Pelvic Radiation Therapy in High-Risk and Very High-Risk Prostate Cancer (POP-RT) trial (4). In this study, the five year biochemical failure free survival was an impressive 95% when using PSMA PET combined with prostate and whole pelvic RT. This impressive rate of control was achieved with very acceptable toxicity rates (4). Carefully iterating on currently established standards of care, with a focus of how RT can be refined further, is essential. A second important imperative to consider is that the use of MR guidance is more logistically complex and potentially time consuming than CT based treatment. However, this could be considerably impacted by artificial intelligence (AI) based contouring solutions and the potential use of ultra-hypofractionation schedules. Interestingly, despite a commonly exchanged narrative, the use of MRI-guided RT technology does not appear to add considerable cost when accounting for potential differences in toxicity and fractionation schedules (44). Despite this, adopting this technology does not come without the very real potential for costs to both patients and healthcare systems. For both of these reasons, the magnitudes of improvement with MR based RT and adaptive therapy in localized prostate cancer are essential to study and quantify.
An important consideration in this discussion is that evaluating novel radiation technological innovations is difficult. Road maps and methodologies for introducing new RT techniques have been proposed (37). But conducting randomized trials comparing novel RT based technologies to historically established standards remains relatively rare. There are a few reasons for this. One is an absence of clearly tangible incentives for device manufactures to sponsor and conduct this type of research. There is minimal (to no) regulatory imperative from the Food and Drug Administration (FDA) for companies to conduct randomized trials robustly proving the added value of novel technologies. In addition, there are substantial costs associated with conducting a randomized trial, which often serves as a disincentive for device manufacturers to sponsor. The other challenge is that advantages may seem overwhelmingly apparent to some radiation oncologists who evaluate this novel technology. In fact, one may easily ponder the question: “if a device enables more clear visualization of a target, does such a device really require comparative randomized data before it can be adopted, particularly if it can be purchased and used without randomized evidence?” This is difficult to address in the real world setting of RT delivery. It is also important to consider that there is a common perception amongst radiation oncologists that many of these technologies are, at worst, equivalent to current treatment strategies using CT. Therefore, taking time to prove the magnitude of this advantage, as opposed to just adopting the technology (that has seemingly apparent advantages) presents a distinct obstacle that our field faces with exceeding frequency. Despite these barriers, there are some examples of radiation device manufacturers funding (but not sponsoring) novel interventional studies testing radiation treatment strategies in prostate cancer (45). As radiation oncologists we are often reminded of the age old adage that just because we can, does not mean we should. Its something that should be very carefully reflected on by all radiation oncologists.
The concept of when a randomized trial is needed has been recently debated (46). Indeed, precisely when a randomized trial is necessary is not entirely clear. There does not seem to be robust consensus on this issue. One proposal has been that a trial may be needed when an intervention is thought to be beneficial by physicians, but offers only modest benefit with the potential for harm (46). Another indication would be when a standard of care is debated, or poorly understood, early or mid-phase randomized trials provide an important strategy to identify an optimal approach. MR guided radiation certainly may fall into this category, and this could potentially be the case in localized prostate cancer where current CT based RT interventions offer remarkable success. We have proposed several conceptual areas in which CT based RT for localized prostate cancer could be uniquely improved by MR guidance. These range from sexual function improvements, enabled by better visualization of the structures enabling male sexual function to biologically adapted therapy based on changes in DWI. Each of these conceptual areas could uniquely leverage MR guided technology.
The current landscape of randomized trials in prostate cancer testing the benefits of MR guidance is modest. Extremely important trials, such as the MIRAGE trial (NCT04384770), have been pioneered recently and may set a standard moving forward making future randomization to CT based treatment difficult in some centers. More questions using MR guidance could be asked, perhaps with larger multi-center trials. MIRAGE has clearly and critically demonstrated that randomization of this population is feasible, but its results may make future randomization between MR and CT challenging (13). Moreover, such a trial provides strong data that acute toxicity can indeed be improved with the use of MR guidance over CT based image guidance. This is an important step toward future MR guided trials that could be larger, multi-center, randomized trial designs testing cost effectiveness and even further improve patient benefit. The US National Cancer Institute (NCI), Elekta MR Linac Consoritum, and ViewRay Consortium cooperative groups could play a central role in expanding and refining this portfolio. Industry sponsored consortium registries (such as MOMENTUM; NCT04075305) offer another opportunity for potentially conducting nested trials examining novel approaches within the spectrum of MR guidance. Trials could potentially test the ability of MR guidance to further improve endpoints including: late toxicity, sexual toxicity, or even further reduce the existing small magnitudes of acute toxicity. Such a trial should ideally be device agnostic and include enough patients to enable robust estimates of the magnitude of effect sizes. Such a proposal is in development and planned for submission in the coming year. Finally, there is an important need to both test and standardize dose schedules along with constraints of applied RT doses when possible.
Conclusions
MRI guidance is rapidly gaining popularity, with over 150 devices installed from various vendors. The technology holds considerable promise for continuing to refine and improve outcomes in patients with localized prostate cancer. The specialty, and academic radiation oncologists, must remain vigilantly focused on proving this technologies value and throughput safely. While radiation oncologists may be enthusiastic to start using this technology on patients, we must simultaneously test novel hypothesis as to how this technological advance can improve their outcomes. The future is promising for the continued intersection of technological imaging advances and precision RT delivery.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
WH, EP, and AT receives research and travel support from Elekta AB, Stockholm Sweden. HN: ViewRay Ad board research funds BSci ad board and research funds Lantheus research funds Veracyte research funds. AK: ViewRay Research funding, honoraria, consulting Varian Honoraria Boston Scientific Advisory Board Janssen Research FundingPointBiopharma Research funding. EH: Prof. Hall reports grants from Accuray Inc., grants from Varian Medical Systems Inc., outside the submitted work; EH acknowledges support from a Cancer Research UK Network Accelerator Award Grant (A21993) to the ART-NET consortium; and from the NIHR Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and ICR (London, UK). DV reports speaker fees from Elekta. AT acknowledges support from Cancer Research UK (C33589/A28284 and C7224/A28724) and the National Institute for Health Research (NIHR) Cancer Research Network. This project represents independent research supported by the National Institute for Health research (NIHR) Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med (2016) 375(15):1415–24. doi: 10.1056/NEJMoa1606220
3. Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med (2016) 375(15):1425–37. doi: 10.1056/NEJMoa1606221
4. Murthy V, Maitre P, Kannan S, Panigrahi G, Krishnatry R, Bakshi G, et al. Prostate-only versus whole-pelvic radiation therapy in high-risk and very high-risk prostate cancer (POP-RT): Outcomes from phase III randomized controlled trial. J Clin Oncol (2021) 39(11):1234–42. doi: 10.1200/jco.20.03282
5. Kerkmeijer LGW, Groen VH, Pos FJ, Haustermans K, Monninkhof EM, Smeenk RJ, et al. Focal boost to the intraprostatic tumor in external beam radiotherapy for patients with localized prostate cancer: Results from the FLAME randomized phase III trial. J Clin Oncol (2021) 39(7):787–96. doi: 10.1200/jco.20.02873
6. Hall WA, Deshmukh S, Bruner DW, Michalski JM, Purdy JA, Bosch W, et al. Quality of life implications of dose-escalated external beam radiation for localized prostate cancer: Results of a prospective randomized phase 3 clinical trial, NRG/RTOG 0126. Int J Radiat Oncol Biol Phys Jan 1 (2022) 112(1):83–92. doi: 10.1016/j.ijrobp.2021.07.004
7. Michalski JM, Moughan J, Purdy J, Bosch W, Bruner DW, Bahary JP, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: The NRG oncology RTOG 0126 randomized clinical trial. JAMA Oncol Jun 14 (2018) 4(6):e180039. doi: 10.1001/jamaoncol.2018.0039
8. Gill S, Li J, Thomas J, Bressel M, Thursky K, Styles C, et al. Patient-reported complications from fiducial marker implantation for prostate image-guided radiotherapy. Br J Radiol (1015) 2012:85. doi: 10.1259/bjr/68127917
9. Mutic S, Dempsey JF. The ViewRay system: magnetic resonance–guided and controlled radiotherapy. Elsevier (2014) p. 196–9.
10. Lagendijk JJ, Raaymakers BW, Van Vulpen M. The magnetic resonance imaging–linac system. Elsevier (2014) p. 207–9.
11. Hall WA, Paulson ES, van der Heide UA, Fuller CD, Raaymakers BW, Lagendijk JJW, et al. The transformation of radiation oncology using real-time magnetic resonance guidance: A review. Eur J Cancer (2019) 122:42–52. doi: 10.1016/j.ejca.2019.07.021
12. Hall WA, Paulson E, Li XA, Erickson B, Schultz C, Tree A, et al. Magnetic resonance linear accelerator technology and adaptive radiation therapy: An overview for clinicians. CA Cancer J Clin (2022) 72(1):34–56. doi: 10.3322/caac.21707
13. Kishan AU, Lamb J, Casado M, Wang X, Ma TM, Low D, et al. Magnetic resonance imaging-guided versus computed tomography-guided stereotactic body radiotherapy for prostate cancer (MIRAGE): Interim analysis of a phase III randomized trial. J Clin Oncol (2022) 40(6_suppl):255–5. doi: 10.1200/JCO.2022.40.6_suppl.255
14. Ma TM, Lamb JM, Casado M, Wang X, Basehart TV, Yang Y, et al. Magnetic resonance imaging-guided stereotactic body radiotherapy for prostate cancer (mirage): a phase iii randomized trial. BMC Cancer (2021) 21(1):538. doi: 10.1186/s12885-021-08281-x
15. Tetar SU, Bruynzeel AME, Oei SS, Senan S, Fraikin T, Slotman BJ, et al. Magnetic resonance-guided stereotactic radiotherapy for localized prostate cancer: Final results on patient-reported outcomes of a prospective phase 2 study. Eur Urol Oncol (2021) 4(4):628–34. doi: 10.1016/j.euo.2020.05.007
16. Leeman JE, Cagney DN, Mak RH, Huynh MA, Tanguturi SK, Singer L, et al. Magnetic resonance–guided prostate stereotactic body radiation therapy with daily online plan adaptation: Results of a prospective phase 1 trial and supplemental cohort. Adv Radiat Oncol (2022) 7(5). doi: 10.1016/j.adro.2022.100934
17. Pathmanathan A, Bower L, Creasey H, Dunlop A, Hall E, Hanson I, et al. The PRISM trial-first UK experience of MRI-guided adaptive radiotherapy. Int J Radiat Oncol Biol Phys (2019) 105(1):E301. doi: 10.1016/j.ijrobp.2019.06.1856
18. Westley R, Hall E, Tree A. HERMES: Delivery of a speedy prostate cancer treatment. Clin Oncol (R Coll Radiol) (2022). doi: 10.1016/j.clon.2022.01.003
19. de Mol van Otterloo SR, Christodouleas JP, Blezer ELA, Akhiat H, Brown K, Choudhury A, et al. Patterns of care, tolerability, and safety of the first cohort of patients treated on a novel high-field MR-linac within the MOMENTUM study: Initial results from a prospective multi-institutional registry. Int J Radiat Oncol Biol Phys (2021) 111(4):867–75. doi: 10.1016/j.ijrobp.2021.07.003
20. de Mol van Otterloo SR, Christodouleas JP, Blezer ELA, Akhiat H, Brown K, Choudhury A, et al. The MOMENTUM study: An international registry for the evidence-based introduction of MR-guided adaptive therapy. Front Oncol (2020) 10:1328. doi: 10.3389/fonc.2020.01328
21. Spratt DE, Lee JY, Dess RT, Narayana V, Evans C, Liss A, et al. Vessel-sparing radiotherapy for localized prostate cancer to preserve erectile function: A single-arm phase 2 trial. Eur Urol (2017) 72(4):617–24. doi: 10.1016/j.eururo.2017.02.007
22. Murray J, Gulliford S, Griffin C, Wilkins A, Syndikus I, Staffurth J, et al. Evaluation of erectile potency and radiation dose to the penile bulb using image guided radiotherapy in the CHHiP trial. Clin Trans Radiat Oncol (2020) 21:77–84. doi: 10.1016/j.ctro.2019.12.006
23. Teunissen FR, Wortel RC, Hes J, Willigenburg T, Breugel Groot-van EN, Boer JC, et al. Adaptive magnetic resonance-guided neurovascular-sparing radiotherapy for preservation of erectile function in prostate cancer patients. Phys Imaging Radiat Oncol (2021) 20:5–10. doi: 10.1016/j.phro.2021.09.002
24. Wahlstedt I, Andratschke N, Behrens CP, Ehrbar S, Gabryś HS, Schüler HG, et al. Gating has a negligible impact on dose delivered in MRI-guided online adaptive radiotherapy of prostate cancer. Radiother Oncol (2022). doi: 10.1016/j.radonc.2022.03.013
25. Crook JM, Raymond Y, Salhani D, Yang H, Esche B. Prostate motion during standard radiotherapy as assessed by fiducial markers. Radiother Oncol (1995) 37(1):35–42. doi: 10.1016/0167-8140(95)01613-l
26. Wahl M, Descovich M, Shugard E, Pinnaduwage D, Sudhyadhom A, Chang A, et al. Interfraction anatomical variability can lead to significantly increased rectal dose for patients undergoing stereotactic body radiotherapy for prostate cancer. Technol Cancer Res Treat (2017) 16(2):178–87. doi: 10.1177/1533034616649495
27. Liu F, Ahunbay E, Lawton C, Li XA. Assessment and management of interfractional variations in daily diagnostic-quality-CT guided prostate-bed irradiation after prostatectomy. Med Phys (2014) 41(3):031710. doi: 10.1118/1.4866222
28. Eckl M, Sarria GR, Springer S, Willam M, Ruder AM, Steil V, et al. Dosimetric benefits of daily treatment plan adaptation for prostate cancer stereotactic body radiotherapy. Radiat Oncol (2021) 16(1):145. doi: 10.1186/s13014-021-01872-9
29. Li W, Lu L, Stephans KL, Sharma N, Vassil A, Shen ZL, et al. Volumetric-based image guidance is superior to marker-based alignments for stereotactic body radiotherapy of prostate cancer. J Appl Clin Med Phys (2018) 19(2):198–203. doi: 10.1002/acm2.12280
30. Kooreman ES, van Houdt PJ, Keesman R, Pelt van VWJ, Nowee ME, Pos F, et al. Daily intravoxel incoherent motion (IVIM) in prostate cancer patients during MR-guided radiotherapy-a multicenter study. Front Oncol (2021) 11:705964. doi: 10.3389/fonc.2021.705964
31. van Schie MA, van Houdt PJ, Ghobadi G, Pos FJ, Walraven I, Boer HCJ, et al. Quantitative MRI changes during weekly ultra-hypofractionated prostate cancer radiotherapy with integrated boost. Front Oncol (2019) 9:1264. doi: 10.3389/fonc.2019.01264
32. Pasquier D, Hadj Henni A, Escande A, Tresch E, Reynaert N, Colot O, et al. Diffusion weighted MRI as an early predictor of tumor response to hypofractionated stereotactic boost for prostate cancer. Sci Rep (2018) 8(1):10407. doi: 10.1038/s41598-018-28817-9
33. Wolf MB, Edler C, Tichy D, Röthke MC, Schlemmer HP, Herfarth K, et al. Diffusion-weighted MRI treatment monitoring of primary hypofractionated proton and carbon ion prostate cancer irradiation using raster scan technique. J Magn Reson Imaging (2017) 46(3):850–60. doi: 10.1002/jmri.25635
34. Park SY, Kim CK, Park BK, Park W, Park HC, Han DH, et al. Early changes in apparent diffusion coefficient from diffusion-weighted MR imaging during radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys (2012) 83(2):749–55. doi: 10.1016/j.ijrobp.2011.06.2009
35. Polley M-YC, Cheung YK. Early-phase platform trials: A new paradigm for dose finding and treatment screening in the era of precision oncology. JCO Precis Oncol (2019) 3):1–8. doi: 10.1200/po.19.00057
36. Giovagnoli A. The Bayesian design of adaptive clinical trials. Int J Environ Res Public Health (2021) 18(2). doi: 10.3390/ijerph18020530
37. Verkooijen HM, Kerkmeijer LGW, Fuller CD, Huddart R, Faivre-Finn C, Verheij M, et al. R-IDEAL: A framework for systematic clinical evaluation of technical innovations in radiation oncology. Front Oncol (2017) 7:59. doi: 10.3389/fonc.2017.00059
38. Yan F, Thall PF, Lu KH, Gilbert MR, Yuan Y. Phase I-II clinical trial design: A state-of-the-art paradigm for dose finding. Ann Oncol (2018) 29(3):694–9. doi: 10.1093/annonc/mdx795
39. Lee JJ, Feng L. Randomized phase II designs in cancer clinical trials: current status and future directions. J Clin Oncol (2005) 23(19):4450–7. doi: 10.1200/JCO.2005.03.197
40. Relton C, Torgerson D, O’Cathain A, Nicholl J. Rethinking pragmatic randomised controlled trials: introducing the “cohort multiple randomised controlled trial” design. BMJ (2010) 340:c1066. doi: 10.1136/bmj.c1066
41. Millen GC, Yap C. Adaptive trial designs: what are multiarm, multistage trials? Arch Dis Childhood - Educ Pract Edition (2020) 105(6):376–8. doi: 10.1136/archdischild-2019-317826
42. Asano J, Hirakawa A. A Bayesian basket trial design accounting for uncertainties of homogeneity and heterogeneity of treatment effect among subpopulations. Pharm Stat (2020) 19(6):975–1000. doi: 10.1002/pst.2049
43. Pathmanathan AU, van As NJ, Kerkmeijer LGW, Christodouleas J, Lawton CAF, Vesprini D, et al. Magnetic resonance imaging-guided adaptive radiation therapy: A "Game changer" for prostate treatment? Int J Radiat Oncol Biol Phys (2018) 100(2):361–73. doi: 10.1016/j.ijrobp.2017.10.020
44. Hehakaya C, van der Voort van Zyp JRN, Vanneste BGL, Grutters JPC, Grobbee DE, Verkooijen HM, et al. Early health economic analysis of 1.5 T MRI-guided radiotherapy for localized prostate cancer: Decision analytic modelling. Radiother Oncol (2021) 161:74–82. doi: 10.1016/j.radonc.2021.05.022
45. Brand DH, Tree AC, Ostler P, Voet der van H, Loblaw A, Chu W, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-b): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol (2019) 20(11):1531–43. doi: 10.1016/s1470-2045(19)30569-8
Keywords: MR guided radiation therapy, prostate cancer, adaptive radiation therapy prostate cancer, adaptive radiation therapy, FLAME prostate, MR guided radiation prostate cancer, MIRAGE trial
Citation: Hall WA, Kishan AU, Hall E, Nagar H, Vesprini D, Paulson E, Van der Heide UA, Lawton CAF, Kerkmeijer LGW and Tree AC (2022) Adaptive magnetic resonance image guided radiation for intact localized prostate cancer how to optimally test a rapidly emerging technology. Front. Oncol. 12:962897. doi: 10.3389/fonc.2022.962897
Received: 06 June 2022; Accepted: 04 July 2022;
Published: 05 September 2022.
Edited by:
Neil B. Desai, University of Texas Southwestern Medical Center, United StatesReviewed by:
Aurelie Garant, University of Texas Southwestern Medical Center, United StatesChad Tang, University of Texas MD Anderson Cancer Center, United States
Copyright © 2022 Hall, Kishan, Hall, Nagar, Vesprini, Paulson, Van der Heide, Lawton, Kerkmeijer and Tree. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: William A. Hall, d2hhbGxAbWN3LmVkdQ==
 William A. Hall
William A. Hall Amar U. Kishan
Amar U. Kishan Emma Hall
Emma Hall Himanshu Nagar
Himanshu Nagar Danny Vesprini5
Danny Vesprini5 Eric Paulson
Eric Paulson Uulke A. Van der Heide
Uulke A. Van der Heide Linda G. W. Kerkmeijer
Linda G. W. Kerkmeijer Alison C. Tree
Alison C. Tree