- 1Department of Hepatobiliary Pancreatic Surgery, Changhai Hospital, Second Military Medical University (Naval Medical University), Shanghai, China
- 2Department of Gastroenterology, The Third People’s Hospital of Chengdu, The Affiliated Hospital of Southwest Jiaotong University, Chengdu, China
- 3Deparment of Neurology, Affiliated Hospital of Jiangsu University, Jiang Su University, Zhenjiang, China
- 4Luodian Clinical Drug Research Center, Shanghai Baoshan Luodian Hospital, Shanghai University, Shanghai, China
Tumor microenvironment (TME), which is characterized by hypoxia, widely exists in solid tumors. As a current research hotspot in the TME, hypoxia is expected to become a key element to break through the bottleneck of tumor treatment. More and more research results show that a variety of biological behaviors of tumor cells are affected by many factors in TME which are closely related to hypoxia. In order to inhibiting the immune response in TME, hypoxia plays an important role in tumor cell metabolism and anti-apoptosis. Therefore, exploring the molecular mechanism of hypoxia mediated malignant tumor behavior and therapeutic targets is expected to provide new ideas for anti-tumor therapy. In this review, we discussed the effects of hypoxia on tumor behavior and its interaction with TME from the perspectives of immune cells, cell metabolism, oxidative stress and hypoxia inducible factor (HIF), and listed the therapeutic targets or signal pathways found so far. Finally, we summarize the current therapies targeting hypoxia, such as glycolysis inhibitors, anti-angiogenesis drugs, HIF inhibitors, hypoxia-activated prodrugs, and hyperbaric medicine.
Introduction
A recent study shows that the global cancer burden is increasing (1). The research on the molecular mechanism and target of the occurrence and development of malignant tumors may be a breakthrough to solve this problem. Recently, it has been proved that TME is a key factor in the occurrence and development of malignant tumors, which is being the well-known hotspot. However, due to the influence of tumor cells and abnormal vascular structure, TME often shows the characteristics of hypoxia, especially in solid tumors.
Under the condition of hypoxia, the expression of HIF increases, and a series of changes have taken place in the metabolic mode and immune function of TME. In order to adapt to the influence of hypoxia, tumor cells have changed their metabolic mode and obtained energy through glycolysis. Meanwhile, immune cells are regulated by hypoxia and have different effects. Among them, the function of immune cells that play an anti-tumor role is inhibited, such as cytotoxic T cells, B cells, and natural killer (NK) cells. However, the expression of immunosuppressive cells such as marrow-derived suppressor cells (MDSC) and regulatory (Treg) T cells is up-regulated. The changes of metabolism and immune effect provide an excellent living environment for tumor cells and hinder the effect of anti-tumor treatment. In addition, while providing survival conditions for tumor cells, TME under hypoxia obstructs the effect of antitumor drugs by hindering drug delivery (2–4). Therefore, traditional chemotherapy and single dose immunotherapy cannot achieve satisfactory results, which makes the treatment of malignant tumors challenging (5, 6).
In conclusion, hypoxia, as an independent prognostic indicator related to poor survival rate of cancer patients, is expected to become an effective target for fighting tumor and alleviating drug resistance (7). After summarizing, we found that people are increasingly interested in the field of hypoxia in TME, and hundreds of relevant academic papers in this field have been published (8). Among the published studies, research targeting metabolic enzymes, HIF, and angiogenesis related factors have made breakthroughs to varying degrees. Currently, what’s exciting is more than 500 clinical trials have been adopt. In this review, we describe the effects of hypoxia on the proliferation, metastasis, and drug resistance of tumor cells in the TME from the perspectives of immunity, metabolism and HIF, and summarize the different treatment strategies targeting hypoxia. Finally, we summarized the current measures to combat drug resistance and the prospects for future research in this field.
Effects of hypoxia on TME
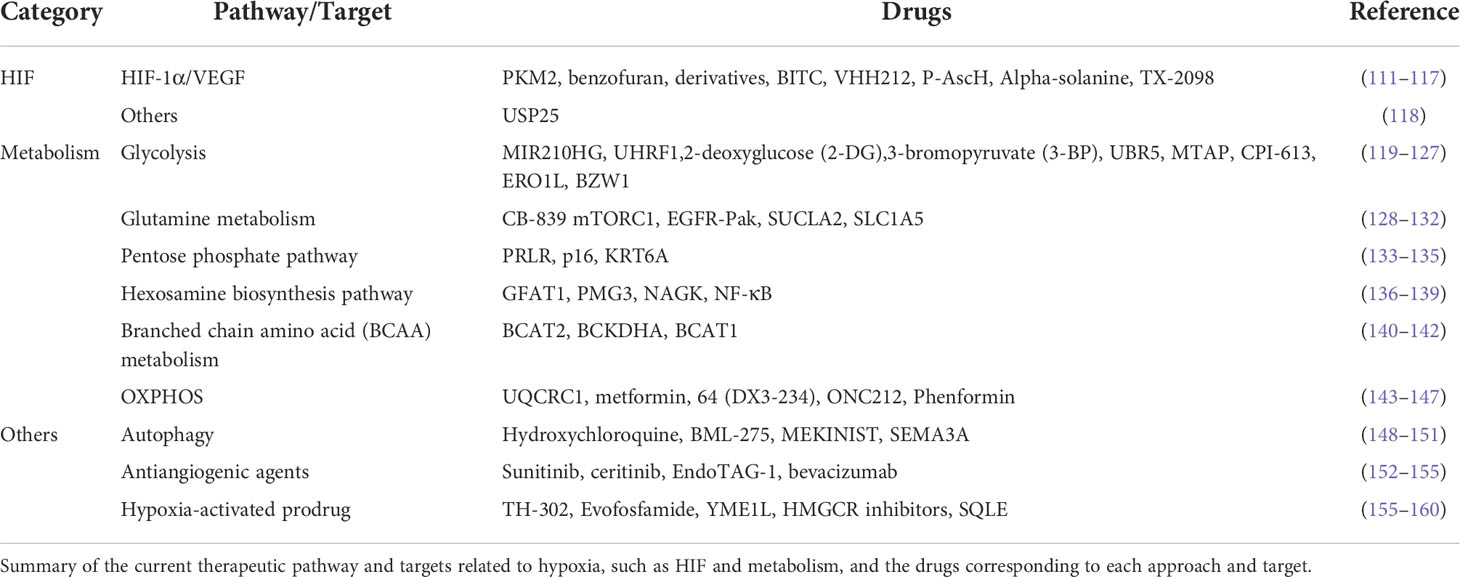
TME is a cellular environment that harbors the tumor, composed of tumor cells, fibroblasts, immune cells (T cells, B cells, natural killer (NK) cells, and tumor-associated macrophages (TAMs)), blood vessels, signaling molecules, and extracellular matrix (9, 10). Hypoxia is ubiquitous property in the TME, especially in solid tumors. Abnormal blood vessels in the tumor tissue cannot meet the excessive oxygen and nutrient demand for its rapid growth, leading to uneven hypoxia. Thus, the area away from the blood vessels was anoxic, while the adjacent tumor tissue was hyper-oxygenated. A recent study suggested that hypoxia affects the immune microenvironment and makes tumor cells escape from immune monitoring and killing (11). As shown in Figure 1, the anoxic area in tumor tissue hinders the infiltration of immune cells and promotes the growth of tumor cells (12).
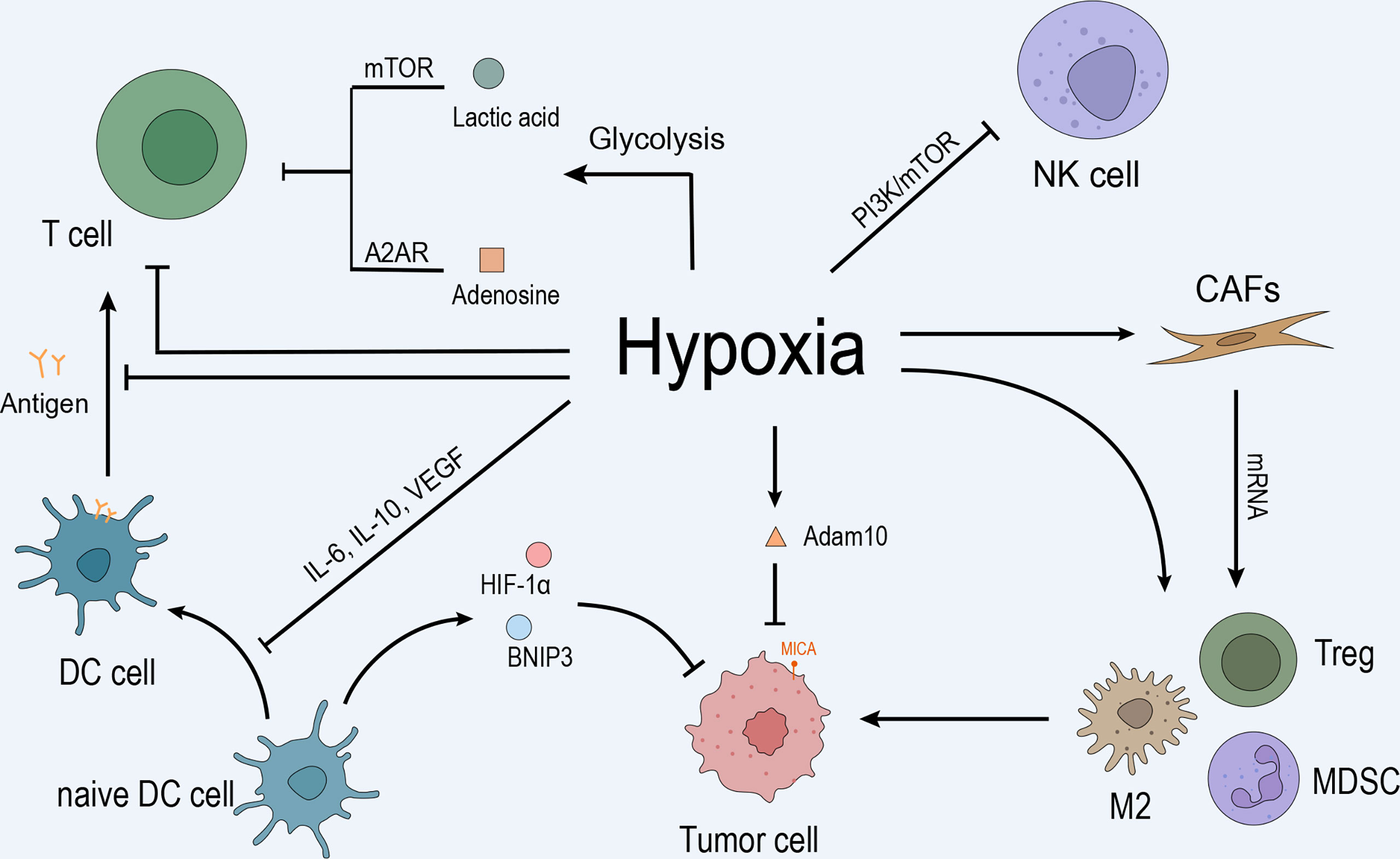
Figure 1 Hypoxia inhibits the immune response by inhibiting immune cells, recruiting immunosuppressive cells, regulating CAFs, promoting tumor cell growth, and mediating immune escape. (A) Anoxic metabolites, lactic acid, and adenosine inhibit T cell effector function and proliferation by blocking the mTOR pathway and interacting with the A2A receptor on the T cell surface. Hypoxia promotes T cell apoptosis and directly inhibits T cell proliferation and differentiation. Hypoxia upregulates IL-10, VEGF, and other cytokines through HIF-1α and inhibits the differentiation and maturation of DCs, leading to the inhibition of T cell function. Moreover, hypoxia-induced high levels of HIF-1α and BNIP3 promote programmed cell death in tumor cells captured by DC. In addition, hypoxia inhibits NK cell function by activating the PI3K/mTOR signaling pathway. (B) Hypoxia induced the mRNA expression of TGF-β, VEGF, IL-6, IL-10, and PD-L1 and promoted CAF participation in the recruitment of MDSCs, Tregs, and type 2 TAMs to maintain the immunosuppressive state of the microenvironment, promoting tumor cells to evade immune surveillance. (C) Hypoxia upregulates the expression of MMP adam10 and induces the immune escape of tumor cells.
Hypoxia inhibits the function of immune cells
Effector T cells are the main components of immune response in the tumor immune microenvironment, for example, the proliferation and differentiation of T cells determine the strength of the antitumor immune response. Several studies have confirmed that hypoxia is a major regulatory factor that inhibits the function and proliferation of T cells (11, 13). A2A receptor (A2AR) is a kind of G protein coupled receptor with high affinity for adenosine, which is expressed on T cells, NK cells, macrophages, and other immune cells (14). Tumor cells can inhibit the response of immune cells through adenosine-a2ar pathway and promote tumor cells to escape immune surveillance (14). Under hypoxic conditions, tumor cells exploit the glycolytic process to accumulate metabolites, such as lactic acid and adenosine, in the TME. The accumulation of lactic acid and adenosine inhibits T cell effector function and proliferation by blocking the Sirolimus pathway and interacting with the A2AR on the T cell surface (15, 16). On the other hand, hypoxia promotes the apoptosis of T lymphocytes, delays the differentiation of effector cells, and reduces the production of effector T cells and interferon-gamma (IFN-γ) (17). In order to inhibit T cell proliferation, differentiation, and other functional cells, such as dendritic cells (DCs) that present antigens to T cells and activate the Hapten response, which also affected by hypoxia.
B cells, as the main carrier of humoral immunity, play a key role in the production of antibodies. Therefore, the functional defect of B cells will lead to the decline of immune effect. In the hypoxic tumor microenvironment, the transcription and metabolism of B cells are mainly affected by hypoxia inducible factor-alpha (HIF-1α) and myelocytomatosis virus oncogene cellular homolog (MYC) (18, 19). Myc gene specifically regulates the growth and metabolism of these various types of cells and has the potential to cause cancer (19, 20). To meet the energy demand of proliferation, B cells with malignant tendency show high metabolic behavior different from normal cells and show a vicious cycle. However, this differential performance still needs further research, and may become a treatment strategy in the future (19).
DCs are immune cells that capture and present antigens through major histocompatibility complex (MHC) glycoproteins. Many studies have shown that interleukin-6 (IL-6), IL-10, vascular endothelial growth factor (VEGF), and the other cytokines are upregulated by hypoxia, which inhibits the differentiation and maturation of DCs and T cell function (21, 22). BCL2 gene, as a key gene regulating apoptosis, is up-regulated in tumor cells. The BCL2 encoded protein can achieve programmed cell death by regulating proteolytic caspase activation (23, 24). Immature DCs express high levels of HIF-1α and upregulated BCL2 and adenovirus E1B19 kDa interacting protein 3 (BNIP3), inducing programmed cell death in captured cells (25). Yang et al. found that the phagocytic capacity of DCs was lower than normal under hypoxia (26). In addition, hypoxia-stimulated DCs induce the differentiation of naive T cells into the Th2 phenotype, which in turn inhibits T cell proliferation (27). Hypoxia affects the function and differentiation ability of DCs, indicating that the activation ability of DCs to T cells. After that, the effect of T cell immunity on tumor cells is reduced, promoting the immune escape of tumor cells (26, 28).
NK cells constitute a class of naturally occurring cytotoxic lymphocytes. The ability of NK cells to kill tumor cells is inhibited under hypoxia (29) via the activated phosphatidylinositol 3 kinase (PI3K)-mTOR signaling pathway. In addition, hypoxia decreases the expression of the tumor cell surface recognition molecule MICA by upregulating the expression of metalloproteinase 10 (MMP10), thus downregulating the expression of NK and Natural killer group 2 member D (NKG2D) on T cells and inducing the immune escape of tumor cells (30). NKG2D is an activated receptor of immune cells such as T cells and NK cells, which could turn on the immune effect function. The upregulation of its ligand MCIA/MCIB on the surface of tumor cells is conducive to the continuous development of anti-tumor immunity (31). Therefore, the NKG2D ligand (NKG2DL) as a therapeutic target has become a research hotspot in recent years, in which the research progress in the fields of tumor vaccines has made exciting results (32, 33).
Immune checkpoint refers to the ligand-receptor pairs that stimulate or inhibit the immune response, which also affected by hypoxia (34). Hypoxia modulates PD-L1, human leukocyte antigen g (HLA-G), CD47, and the immune checkpoint V-domain IG suppressor of T cell activation (VISTA) to form an inhibitory immune microenvironment, promoting immune escape of tumor cells. PD-1 is widely distributed on the surface of lymphocytes. Under hypoxic conditions, the level of PD-L1 protein on the tumor cell surface is enhanced, and it combines with the PD-1 receptor on the activated T cell surface to produce the immunosuppressive effect (28, 35). Presently, antibodies against PD-1 and PD-L1 have achieved preliminary success in the clinic (36). Human leukocyte antigen G (HLA-G) is another checkpoint molecule involved in tumor immune escape and is strongly associated with increased tumor invasiveness and suppression of immune cell function (37, 38). In addition, the immune checkpoint molecules involved in the inhibition of T cell proliferation and activity under hypoxia conditions include VISTA (11).
Hypoxia modulates immunosuppression
Cancer-associated fibroblasts (CAFs) are similar to inflammation-associated fibroblasts, and participate in tumor cell progression and immune cell regulation during the antitumor immune response (39). Ziani et al. demonstrated that the mRNA expression of CAF-related immunosuppressive modulators, such as tumor growth factor-beta (TGF-β), VEGF, IL6, IL10, and PD-L1 increased significantly under hypoxia (40). CAFs are involved in the collection and differentiation of marrow-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and type 2 TAMs in TME (41, 42). In addition to the recruitment of immunosuppressive cells, CAFs inhibit T cell immune response and enhance the tumor cell immunosuppressive response in the TME, which might be related to the inhibition of CAF, DC, and NK cell functions (43, 44). Under hypoxia, CD4+ T cells differentiate into Tregs by promoting Foxp3 transcription. Tregs are a subset of CD4 T cells and contribute to immunosuppression and tumor tolerance by producing TGF-β and suppressive effector T cells (21). MDSCs are immature myeloid cells that directly inihibit T cells, NK cells, and dendritic cells and promote angiogenesis in tumor tissue (45, 46). Chiu et al. demonstrated that under the influence of hypoxia, the differentiation of MDSCs was inhibited, but its immunosuppressive function was maintained (47). TAM is a major component of the solid TME (48). The two phenotypes of TAMs are M1-like and M2-like phenotypes (48). Type M2 TAMs are detected in anoxic regions and associated with immunosuppression, angiogenesis, tumor cell activation, and metastasis (49). Another study showed that prostaglandin E2, TGF-β, VEGF, IL-4, IL-6, and reactive oxygen species (ROS) were the major factors that induced TAMs to M2-type TAMs (29). In addition, hypoxia-mediated lactic acid accumulation under HIF-1α regulation increases the expression of VEGF and M2-like polarization of TAMs to maintain the immunosuppressive status of the TME (47, 50).
Changes in tumor metabolism caused by hypoxia
Hypoxia affects the TME and alters the tumor and the surrounding tissue metabolism. With the progress of TME studies, metabolic reprogramming has been recognized as a hallmark behavior of malignant tumors. As shown in Figure 2, metabolic reprogramming is the metabolic modification of tumor cells to maintain growth and resist treatment. This reprogramming includes aerobic glycolysis and L-glutamine metabolism et al (51). Thus, it could be deduced that hypoxia inhibits the apoptosis of tumor cells by promoting the metabolic reprogramming of tumor cells.
Figure 2 Hypoxia induces metabolic reprogramming of tumor cells, which provides energy and substrates for tumor cell growth and promotes drug resistance. I. Glucose provides energy to the tumor cells in the form of glycolysis, of which the metabolite Lac is transported to the outside of tumor cells through MCT, effectuating low pH and suppressing the immune effects. The intermediate products in glycolysis contribute to the synthesis of fatty acids and promote the growth and proliferation of tumor cells. II. Gln is broken down into a-KG in tumor cells to provide energy through the TCA cycle or raw materials to synthesize amino acids and nucleic acids in tumor cells. In addition, GLN expresses antioxidant ability by synthesizing GSH, which promotes drug resistance and anti-apoptosis in tumor cells. III. Fatty acids provide materials for the synthesis of biomembranes to meet the growth needs of tumor cells. The synthesis of fatty acids consumes PEP, which relieves the build-up of Lac from glycolysis. The breakdown of fatty acids produces large amounts of ATP, which provides energy for the growth and proliferation of tumor cells. IV. ROS induces drug resistance in tumor cells, associated with the P-gp.
Glucose metabolism
Under aerobic conditions, cells produce pyruvic acid through the glycolytic process, which is oxidized in the mitochondria to produce energy. Under anoxic conditions, the energy of normal cells is mainly provided by glycolysis. However, most tumor cells tend to produce energy by glycolysis even under aerobic conditions. This phenomenon is known as the “Warburg effect” (52, 53). Through this phenomenon, tumor cells use glycolysis for energy and produce lactic acid. The lactic acid accumulates outside the tumor cell via activated monocarboxylic acid transporters, causing a low pH in the extracellular matrix. Some studies have shown that a low pH environment enhances the invasiveness of tumor cells and inhibits the cytotoxicity and proliferation of lymphocytes, which inhibits the functioning of immune effector cells in the TME (54). Nonetheless, as the acidic environment is corrected, the T cell effector function is restored (55). In addition, tumor cells use glycolytic metabolic intermediates to synthesize fats and proteins. The metabolic way of aerobic glycolysis weans the tumor cells off oxygen dependence, which is beneficial to the growth and proliferation of tumor cells in a hypoxic environment (56). Also, the multidrug resistance of tumor cells is closely related to the reprogramming of glucose metabolism. A current study suggested that this process is influenced by a combination of mechanisms, including “ion capture,” decreased apoptotic potential, gene changes (such as p53 mutation), and increased activity of P-gp, a multidrug transporter.
Glutamine metabolism
Except for glycolysis, cancer cells under hypoxic conditions tend to choose an alternative substrate for energy metabolism, such as L-glutamine. Some studies have shown the critical role of L-glutamine plays in tumor cell proliferation as an alternative energy source for tumor metabolism (57). L-Glutamine is synthesized as glutamate, which is then converted into α-ketoglutaric acid through transamination and into the tricarboxylic acid cycle for energy metabolism to compensate for the reduced energy production from glycolysis (58, 59). In addition, glutamate provides nitrogen and carbon sources for tumor cells and participates in the synthesis of amino acids and nucleotides, which promotes the development of malignant tumors (60). On the other hand, L-glutamine could be used to synthesize glutathione, a crucial antioxidant that maintains the redox balance and prevents oxidative damage to cells (61, 62). Friesen et al. suggested that glutathione, a metabolite of L-glutamine, is involved in mediating drug resistance and anti-apoptosis in cancer cells which may be related to the antioxidant capacity of glutathione. Strikingly, when glutathione levels are decreased, drug and apoptosis resistance of tumor cells is restored (63).
Fatty acid metabolism
The synthesis of biomembranes and signaling molecules is essential for the rapid proliferation and growth of tumor cells. Fatty acid is a critical raw material. Therefore, tumor cells have high levels of fatty acid synthesis. On the other hand, the synthesis consumes pyruvic acid, which slows the synthesis of lactic acid and prevents the excessive build-up of lactic acid. In addition, the decomposition of fatty acids provides energy for tumor cells and the free fatty acids of metabolic products act as signal molecules that activate various signaling pathways (64, 65). Hypoxia and fatty acid metabolism studies have shown that the occurrence of tumors is closely related to β-oxidation. The enzymes FASN, ACC, and ACLY involved in fatty acid metabolism are upregulated in tumors (66, 67). Another study showed that the efficacy of immunotherapy, T cell longevity, and antitumor effects are also affected by lipid metabolism (68).
The role of HIF
HIF is a major factor that mediates tumor cells to adapt to hypoxia (69). HIF-1α transcription factor directly targets VEGF, TGF-β, IL-10, and PD-L1 genes and regulates the tumor immunosuppressive response to CAFs (70–72). HIF is a heterodimer helical-loop protein consisting of an O2-sensitive α-subunit (including HIF-1α, HIF-2α, and HIF-3α) and a constitutive β-subunit (16). HIF-1α plays a key role in several steps of hypoxia induction (73). In case of hypoxia, HIF-1α breakdown is reduced and transferred to the nucleus when the function of prolyl hydroxylase (PHD-RRB is inhibited. In the cell nucleus, HIF-1α binds to HIF-1β to form heterodimers. HIF-1α/1β heterodimer activates the HIF target gene and promotes HIF expression by combining the HIF-1α/1β heterodimer with p300/CBP and hypoxia response element (HRE), thus regulating various biological processes of tumor cells, including metabolic reprogramming, immunoregulation, angiogenesis, tumor cell invasion, and drug resistance (74, 75).
Among them, HIF could inhibit the immune response by promoting the up regulation of immune checkpoints, apoptosis of cytotoxic T cells and blocking phagocytosis, which is conducive to the occurrence and development of tumor cells. Studies have reported that the up-regulated immune checkpoint PD-L1 in tumor cells presents HIF-1α dependence, promote the apoptosis of cytotoxic T cells, and participate in the immune escape of tumor cells (76, 77). Similarly, the expression of CD47 protein on the surface of tumor cells is also affected by HIF-1α, and hinder the phagocytic ability of phagocytes to tumor cells through phosphorylation of signal regulatory proteins on the surface of macrophages α (SIRP α) (78). In recent years, the research results of CD47 protein showed that the expression of CD47 protein also inhibited the function of cytotoxic T cells and NK cells. Therefore, a series of clinical studies targeting CD47 protein are expected to bring new hope to the field of tumor therapy (79, 80). In addition, vascular endothelial growth factor (VEGF) is up-regulated affected by HIF-1α, which could promote metastasis and immune escape by inducing tumor angiogenesis. It is believed that the disorder of TGF-β is related to the occurrence and development of tumors, and enhances the invasive ability of tumor cells by inducing epithelial to mesenchymal transition (EMT) (81, 82). The research of Huang et al. showed that the HIF-1α regulate the function of TGF-β by forming Smad-HIF-1α complex under hypoxia, and then regulate the progress of tumor cells (83).
In addition to the inhibition of immune effects in TME, HIF-1 activates or inhibits the genes of key proteins in glycolysis pathway to regulate the metabolic process of tumor cells in hypoxic environment. Proteins or genes involved in the regulation of glycolysis pathway and regulated by HIF-1 include those involved in encoding glucose transporters (GLUT1 and GLUT3), hexokinases (HK1 and hK2), lactate dehydrogenase A (LDHA), etc (84–88). At the same time, oxidative phosphorylation related genes and proteins are negatively regulated by HIF-1. The differential regulation of proteins or genes relating glycolysis and oxidative phosphorylation by HIF-1 is conducive to the adaptation of tumor cells to achieve glucose metabolism reorganization. In addition, tumor cells reprogrammed by glucose metabolism have higher “competitiveness” to glucose in the microenvironment, so T cell apoptosis is induced by inhibition of energy metabolism, which aggravates the inhibition of T cell function. Except the glycolysis, HIF, as a major regulator, is involved in regulating glutamine metabolism in tumor cells (89). Under the condition of hypoxia, HIF-2α causes the change in SLC1A5 gene encoding neutral amino acid transporter, which mediates the reprogramming of glutamine metabolism and the resistance to gemcitabine in tumor cells (90). Moreover, HIF inhibit α- Ketoglutarate participates in the tricarboxylic acid cycle by promoting α- Ketoglutarate dehydrogenase (αKGDH) degradation (91). In addition, HIF is also involved in regulating many metabolic pathways, such as fatty acids, pentose phosphate and adenosine, so as to provide a metabolic basis for the progression and metastasis of tumor cells.
In summary, the important role of HIF in tumor progression and its potential mechanism have been widely concerned by researchers. Further research in this field in the future is expected to help us have a deeper understanding of hypoxic TME, and bring new hope to the research of tumor targeted therapy (92).
Effect of hypoxia on tumor oxidative stress
ROS are the main molecules produced by oxidative stress and have been considered major factors in the tumor occurrence, development, and recurrence. The ROS in tumor cells originate from mitochondria (93). Under the influence of hypoxia, the oxygen utilization efficiency of tumor cells is decreased. Therefore, electron transport efficiency through the mitochondrial complexes in the electron transport chain (ETC) is reduced, resulting in abundant ROS in cells (94, 95). Notably, various concentrations of ROS exert different effects on tumor cell production (96). High concentrations of ROS disrupt the proteins and nucleic acids and induce apoptosis of tumor cells through oxidative stress (97). A low concentration of ROS can promote the development and metastasis of tumor cells (98). In addition, the concentration of ROS affects the sensitivity and resistance of tumor cells to chemotherapeutic drugs, which might be related to the level of p-glycoprotein (P-gp) in drug resistance (99) (Figure 2).
Effect of hypoxia on drug resistance of tumor
To date, chemotherapy is the cornerstone of cancer treatment. Anoxic metabolic disorders and changes in the microenvironment severely inhibit the efficacy of drugs such as Bleomycin. This could be because the oxygen-dependent chemotherapy drugs are more active when oxygen is available (100), while hypoxia directly inhibits the antitumor function of oxygen-dependent chemotherapeutic drugs (101). In addition, hypoxia indirectly reduces the efficacy of the drugs by interfering with the cell cycle, promoting DNA repair, and reducing the sensitivity of p53-mediated apoptosis (29, 102). Intriguingly, immunotherapy has developed rapidly in the past decade and has produced significant clinical results. However, the current studies have suggested that hypoxic stimulation significantly limits the effectiveness of immunotherapy (21, 103, 104). About 33% of patients who responded to immunotherapy suffered resistance again (6). Hypoxia effectuates metabolic changes in tumor cells, including low pH, high ROS, abnormal blood vessels, and proliferating fibrous tissue. These changes are beneficial to tumor cell survival, provide anti-apoptosis advantages, inhibit drug penetration, and promote the development of cancer and drug resistance (105).
To confront the challenge of drug resistance, the main methods include early diagnosis, combined multi drug therapy, and adaptive therapy (106). Effective evaluation molecules could provide a good reference for the early diagnosis and progress of malignant tumors. Quantitative monitoring of circulating tumor DNA (ctDNA) is expected to become an important means of early diagnosis and dynamic monitoring of cancer, which will help to improve the overall survival rate and guide individualized treatment (107). Combined with positron emission tomography and computed tomography (PET-CT) imaging results, evaluate the curative effect. And then adjust the drugs, so as to avoid the emergence of drug resistance and achieve better curative effect (108). Meanwhile, precise drug delivery methods such as antibody drug conjugate (ADC) could increase the curative effect by increasing the local drug concentration (109). In addition, multi drug combination therapy is still an effective measure to combat drug resistance at present. The research of Niu et al. shows that the combined application of vibostolimab (anti-TIGIT humanized IgG1 monoclonal antibody) and pembrolizumab can significantly inhibit and improve the drug resistance of non-small cell lung cancer (NSCLC) (110).
Strategies for targeting hypoxic microenvironments
Bibliometric research published on Frontiers in oncology shows that researchers’ interest in the research of tumor microenvironment is continuously rising from 2011 to 2021 (8). Moreover, the current research hotspot in this field focuses on the energy metabolism, oxidative phosphorylation, liposomes and other new drug delivery routes in TME (8). Therefore, in order to facilitate readers to understand the progress in this field, we summarize the treatment strategies that targeted at hypoxia. It is gratifying that several potential anti-tumor targets have been found (Table 1), including targeting hypoxia, glycolytic drugs, abnormal angiogenesis, and HIF drugs.
The energy metabolism of tumor microenvironment is a current research hotspot, and researchers have carried out a series of studies with it. A current study showed that glycolytic inhibitors effectively kill tumor cells that are not sensitive to chemotherapy drugs, even when they were present in multiple drug resistance cells (161, 162). Hexokinase 2 (HK2) plays a critical role in regulating aerobic glycolysis in tumor cells and has become one of the main targets of tumor therapy. A previous study showed that HK2 inhibitor 3-bromopyruvic acid (3-BP) significantly inhibits the progression and proliferation of tumor cells in HK2- expressing colorectal cancer. Moreover, apoptosis of tumor cells was induced by the signaling pathway of mitochondrial apoptosis (163). In recent years, the emergence of chemical dynamic therapy (CDT) has provided a new solution for cancer treatment (164). Interestingly, the amount of glutathione in the tumor cells directly affects the efficacy of chemotherapy. Glycolytic inhibitors could reduce the tumor’s glycolytic process and enhance CDT selectivity to tumor cells, thereby exploiting metabolic differences to achieve the specific treatment for tumor cells (165). In addition, OXPHOS inhibitors, such as metformin, improve hypoxia in the microenvironment by inhibiting the mitochondrial complex I, which reduces oxygen consumption in cells and corrects the hypoxic TME. Besides, OXPHOS also inhibits the upregulation of cancer subtypes, such as ovarian cancer, prostate cancer, and thyroid cancer (166, 167).
Abnormal tumor blood vessels are major factors in the continuous hypoxia of TME that hinder drug delivery (168). In the hypoxic TME, angiogenesis-promoting cytokines, such as VEGF and TGF-β, impede the differentiation and maturation of endothelial cells in neovascularization. As a result, malformed and poorly permeable new blood vessels aggravate the anoxic state of the tumor, making it difficult to deliver drugs effectively to the tumor (169). Antiangiogenic drugs, such as anti-VEGF antibodies, correct the abnormal blood vessels and promote the normalization of tumor blood vessels, which in turn alleviates hypoxia and improves the efficacy of conventional antitumor drugs (170, 171). However, angiogenesis inhibitors alone do not receive ideal therapeutic results, which might be related to the complex mechanisms of angiogenesis compensation (172). Therefore, VEGF inhibitors need to be used in combination with chemotherapy or immunotherapy, which has achieved satisfactory results in solid tumors, such as ovarian and breast cancer (173, 174).
HIF activity is mainly dependent on HIF-1α and plays a critical role in the regulation of hypoxic TME. Presently, studies on targeting HIF-1α are being widely carried out. Targeting the HIF-1α signaling pathway is effective in the treatment of solid tumors, such as pancreatic cancer (175, 176). Ubiquitin carboxy-terminal hydrolase L 1(UCHL1) is a ubiquitin-free enzyme that stabilizes its α-subunit (HIF-1α). UCHL1 inhibitors promote the degradation of HIF-1α and inhibit the activity of its downstream genes. Li et al. showed that the inhibition of the UCHL1-HIF-1 pathway decreases the expression of malignant tumor-related factors and eliminates UCHL1-mediated tumor cell proliferation and metastasis (177). In addition, Nelson et al. found that in the hypoxic microenvironment of pancreatic cancer, downregulation of USP25 reduced the transcriptional activity of HIF-1α, leading to cell death in the hypoxic core of the tumor without normal tissue affected (118). In another mouse model of pancreatic cancer, Xu et al. demonstrated that the Benzofuran derivative inhibited tumor growth by acting on the HIF-1α/VEGF pathway under hypoxia (111). In addition, the combination of the HIF-1α inhibitor px-478 and the immune-checkpoint inhibitor enhances the cytotoxicity of T cells against tumor cells, which might be related to the blocking of the HIF-1α/LOXL2 signaling pathway (178). Currently, clinical trials of combined therapy with HIF-1α inhibitors are underway. Thus, HIF-1α inhibitors seem to be a promising cancer therapy in the future.
Of note, the selective is not negligible for drug development, anoxic prodrug is an inactive compound that could be activated automatically in a specific anoxic region. This exploits the selective metabolism of precursor drugs in an anoxic environment, diffuses the killing compounds to the whole TME, and realizes the selective killing of tumor cells (179). A randomized controlled trial for the treatment of advanced pancreatic cancer showed that hypoxia-activated prodrug TH-302 combined with GissiTabine drug yields promising results; the combination group achieved more median progression-free survival than the single Gissi treatment group (180). In addition, TH-302 combined immune checkpoint blocking therapy cured >80% of the tumors in a mouse model of prostate cancer, which prolongs the suppression of bone MDSCs and relieves the inhibition of T cell proliferation (181). Another study showed that CP-506, an anoxic prodrug of nitrogen mustard, also yielded satisfactory effects in tumor tissue (182).
Hyperbaric medicine improves hypoxia in the TME by increasing the amount of dissolved oxygen in the blood (183). In previous studies, hyperbaric medicine has shown a satisfactory excellent curative effect in some cancers (breast and ovarian cancer) (184, 185). Hyperbaric oxygen (HBO) can be used as an adjuvant therapy to inhibit tumors by improving the hypoxic microenvironment (186). A recent study showed that hyperbaric medicine in mice with lung cancer improves the anoxic state of tumors, promotes tumor cell apoptosis, and inhibits tumor growth (187).
Conclusion
Hypoxia is the key factor regulating TME, which mediates the occurrence, development, and drug resistance of tumor cells. Under the condition of hypoxia, TME show immunosuppression and metabolic reprogramming. Therefore, the proliferation and differentiation of immune cells were inhibited. Immunosuppressive cells such as MDSCs, TAM and Tregs are recruited to the hypoxic zone to promote the escape of tumor cells. The metabolic reprogramming of tumor cells is conducive to obtaining energy in a hypoxic environment while maintaining an acidic microenvironment. In addition, glutamine metabolism and fatty acid metabolism have made great contributions to the balanced redox, anti-apoptosis, growth promotion and drug resistance of tumor cells. More importantly, while the hypoxia inhibits the function of PHD, HIF-1α will be activated and promotes the expression of downstream target genes, which further promotes the formation of hypoxic microenvironment and the progress of tumor cells. Therefore, anti-tumor therapy targeting hypoxia and related factors has attracted many researchers’ exploration. Finally, drug resistance induced by hypoxia still plays an important role in the process of anti-tumor treatment, which significantly affects the outcome of treatment. In this review, we summarize the treatment schemes for hypoxia, such as glycolysis inhibitors, anti-angiogenesis drugs, HIF inhibitors, hypoxia-activated prodrugs, and hyperbaric medicine, and finally the found targets and signal pathways in the form of table.
In conclusion, hypoxia is still the key to fight against malignant tumors. It is very necessary to clarify the molecular mechanism of hypoxia on the formation of tumor microenvironment and drug resistance, which will contribute to the breakthrough of tumor targeted therapy in the following work.
Author contributions
(I) Conception and design: TH, DX. (II) Searching literature and led the writing of the review: DX, GC. (III) Making figures and table: GC, KW, HL. (IV) Final approval of manuscript: All authors.
Funding
This work was supported in part by the Shanghai “Rising Stars of Medical Talent” Youth Development Program, Outstanding Youth Medical Talents.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
TME, tumor microenvironment; NK cells, natural killer cells; TAMs, tumor-associated macrophages; IFN-γ, interferon-gamma; DCs, dendritic cells; MHC, Major histocompatibility complex; IL-6, interleukin-6; IL-10, interleukin-10; VEGF, vascular endothelial growth factor; HIF-1α, hypoxia-inducible factor-alpha; BNIP3, BCL2 and adenovirus E1B19 kDa interacting protein 3; PI3K, phosphatidylinositol 3 kinase; MMP10, metalloproteinase 10; VISTA, V-domain Ig suppressor of T cell activation; HLA-G, Human leukocyte antigen G; CAFs, Cancer-associated fibroblasts; TGF-β, tumor growth factor-beta; MDSCs, marrow-derived suppressor cells; Tregs, regulatory T cells; Foxp3, forkhead box P3; ROS, reactive oxygen species; HIFs, Hypoxia-Inducible Factors; PHD, prolyl hydroxylase; HRE, hypoxia response element; P-gp, p-glycoprotein; ETC, electron transport chain; HBO, Hyperbaric oxygen; HK2, Hexokinase 2; 3-BP, 3-bromopyruvic acid; CDT, chemical dynamic therapy; LDHA, lactate dehydrogenase A; αKGDH, α- Ketoglutarate dehydrogenase; ctDNA, circulating tumor DNA; PET-CT, positron emission tomography and computed tomography; ADC, antibody drug conjugate; NSCLC, non-small cell lung cancer.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Castells M, Thibault B, Delord J-P, Couderc B. Implication of tumor microenvironment in chemoresistance: Tumor-associated stromal cells protect tumor cells from cell death. Int J Mol Sci (2012) 13(8):9545–71. doi: 10.3390/ijms13089545
3. Khalaf K, Hana D, Chou JT-T, Singh C, Mackiewicz A, Kaczmarek M. Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Front Immunol (2021) 12:656364. doi: 10.3389/fimmu.2021.656364
4. Seebacher NA, Krchniakova M, Stacy AE, Skoda J, Jansson PJ. Tumour microenvironment stress promotes the development of drug resistance. Antioxidants (Basel) (2021) 10(11):1801. doi: 10.3390/antiox10111801
5. Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: From enhancement to normalization. Cell (2018) 175(2):313–26. doi: 10.1016/j.cell.2018.09.035
6. Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity (2020) 52(1):17–35. doi: 10.1016/j.immuni.2019.12.011
7. McDonald P, Chafe S, Dedhar S. Overcoming hypoxia-mediated tumor progression: Combinatorial approaches targeting ph regulation, angiogenesis and immune dysfunction. Front Cell Dev Biol (2016) 4:27. doi: 10.3389/fcell.2016.00027
8. Wu K, Liu Y, Liu L, Peng Y, Pang H, Sun X, et al. Emerging trends and research foci in tumor microenvironment of pancreatic cancer: A bibliometric and visualized study. Front In Oncol (2022) 12:810774. doi: 10.3389/fonc.2022.810774
9. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med (2013) 19(11):1423–37. doi: 10.1038/nm.3394
10. Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature (2013) 501(7467):346–54. doi: 10.1038/nature12626
11. Noman MZ, Hasmim M, Lequeux A, Xiao M, Duhem C, Chouaib S, et al. Improving cancer immunotherapy by targeting the hypoxic tumor microenvironment: New opportunities and challenges. Cells (2019) 8(9):1083. doi: 10.3390/cells8091083
12. Wan M, Ding Y, Li Z, Wang X, Xu M. Metabolic manipulation of the tumour immune microenvironment. Immunology (2022) 165(3):290–300. doi: 10.1111/imm.13444
13. Vuillefroy de Silly R, Dietrich P-Y, Walker PR. Hypoxia and antitumor Cd8 T cells: An incompatible alliance? Oncoimmunology (2016) 5(12):e1232236. doi: 10.1080/2162402X.2016.1232236
14. Leone RD, Emens LA. Targeting adenosine for cancer immunotherapy. J For Immunotherapy Cancer (2018) 6(1):57. doi: 10.1186/s40425-018-0360-8
15. Payen VL, Porporato PE, Baselet B, Sonveaux P. Metabolic changes associated with tumor metastasis, part 1: Tumor ph, glycolysis and the pentose phosphate pathway. Cell Mol Life Sci CMLS (2016) 73(7):1333–48. doi: 10.1007/s00018-015-2098-5
16. Vito A, El-Sayes N, Mossman K. Hypoxia-driven immune escape in the tumor microenvironment. Cells (2020) 9(4):992. doi: 10.3390/cells9040992
17. Mpekris F, Voutouri C, Baish JW, Duda DG, Munn LL, Stylianopoulos T, et al. Combining microenvironment normalization strategies to improve cancer immunotherapy. Proc Natl Acad Sci United States America (2020) 117(7):3728–37. doi: 10.1073/pnas.1919764117
18. Burrows N, Maxwell PH. Hypoxia and b cells. Exp Cell Res (2017) 356(2):197–203. doi: 10.1016/j.yexcr.2017.03.019
19. Franchina DG, Grusdat M, Brenner D. B-cell metabolic remodeling and cancer. Trends Cancer (2018) 4(2):138–50. doi: 10.1016/j.trecan.2017.12.006
20. Sander S, Calado DP, Srinivasan L, Köchert K, Zhang B, Rosolowski M, et al. Synergy between Pi3k signaling and myc in burkitt lymphomagenesis. Cancer Cell (2012) 22(2):167–79. doi: 10.1016/j.ccr.2012.06.012
21. Noman MZ, Hasmim M, Messai Y, Terry S, Kieda C, Janji B, et al. Hypoxia: A key player in antitumor immune response. a review in the theme: Cellular responses to hypoxia. Am J Physiol Cell Physiol (2015) 309(9):C569–C79. doi: 10.1152/ajpcell.00207.2015
22. Chang WH, Lai AG. The hypoxic tumour microenvironment: A safe haven for immunosuppressive cells and a therapeutic barrier to overcome. Cancer Lett (2020) 487:34–44. doi: 10.1016/j.canlet.2020.05.011
23. Edlich F. Bcl-2 proteins and apoptosis: Recent insights and unknowns. Biochem Biophys Res Commun (2018) 500(1):26–34. doi: 10.1016/j.bbrc.2017.06.190
24. Radha G, Raghavan SC. Bcl2: A promising cancer therapeutic target. Biochim Biophys Acta Rev Cancer (2017) 1868(1):309–14. doi: 10.1016/j.bbcan.2017.06.004
25. Daniel SK, Sullivan KM, Labadie KP, Pillarisetty VG. Hypoxia as a barrier to immunotherapy in pancreatic adenocarcinoma. Clin Transl Med (2019) 8(1):10. doi: 10.1186/s40169-019-0226-9
26. Yang M, Ma C, Liu S, Sun J, Shao Q, Gao W, et al. Hypoxia skews dendritic cells to a T helper type 2-stimulating phenotype and promotes tumour cell migration by dendritic cell-derived osteopontin. Immunology (2009) 128(1 Suppl):e237–e49. doi: 10.1111/j.1365-2567.2008.02954.x
27. Weigert A, Weichand B, Sekar D, Sha W, Hahn C, Mora J, et al. Hif-1α is a negative regulator of plasmacytoid dc development in vitro and in vivo. Blood (2012) 120(15):3001–6. doi: 10.1182/blood-2012-03-417022
28. Labiano S, Palazon A, Melero I. Immune response regulation in the tumor microenvironment by hypoxia. Semin Oncol (2015) 42(3):378–86. doi: 10.1053/j.seminoncol.2015.02.009
29. Chouaib S, Noman MZ, Kosmatopoulos K, Curran MA. Hypoxic stress: Obstacles and opportunities for innovative immunotherapy of cancer. Oncogene (2017) 36(4):439–45. doi: 10.1038/onc.2016.225
30. Torres N, Regge MV, Secchiari F, Friedrich AD, Spallanzani RG, Raffo Iraolagoitia XL, et al. Restoration of antitumor immunity through anti-mica antibodies elicited with a chimeric protein. J immunotherapy Cancer (2020) 8(1):e000233. doi: 10.1136/jitc-2019-000233
31. Fuertes M, Domaica C, Zwirner N. Leveraging Nkg2d ligands in immuno-oncology. Front Immunol (2021) 12:713158. doi: 10.3389/fimmu.2021.713158
32. Badrinath S, Dellacherie M, Li A, Zheng S, Zhang X, Sobral M, et al. A vaccine targeting resistant tumours by dual T cell plus nk cell attack. Nature (2022) 606(7916):992–8. doi: 10.1038/s41586-022-04772-4
33. Ferrari de Andrade L, Tay R, Pan D, Luoma A, Ito Y, Badrinath S, et al. Antibody-mediated inhibition of mica and micb shedding promotes nk cell-driven tumor immunity. Sci (New York NY) (2018) 359(6383):1537–42. doi: 10.1126/science.aao0505
34. Zhang Y, Zheng J. Functions of immune checkpoint molecules beyond immune evasion. Adv Exp Med Biol (2020) 1248:201–26. doi: 10.1007/978-981-15-3266-5_9
35. Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. Pd-L1 is a novel direct target of hif-1α, and its blockade under hypoxia enhanced mdsc-mediated T cell activation. J Exp Med (2014) 211(5):781–90. doi: 10.1084/jem.20131916
36. Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol (2020) 20(1):25–39. doi: 10.1038/s41577-019-0218-4
37. Curigliano G, Criscitiello C, Gelao L, Goldhirsch A. Molecular pathways (Hla-G). Clin Cancer Res an Off J Am Assoc Cancer Res (2013) 19(20):5564–71. doi: 10.1158/1078-0432.CCR-12-3697
38. Garziera M, Scarabel L, Toffoli G. Hypoxic modulation of hla-G expression through the metabolic sensor hif-1 in human cancer cells. J Immunol Res (2017) 2017:4587520. doi: 10.1155/2017/4587520
39. Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer (2020) 20(3):174–86. doi: 10.1038/s41568-019-0238-1
40. Ziani L, Buart S, Chouaib S, Thiery J. Hypoxia increases melanoma-associated fibroblasts immunosuppressive potential and inhibitory effect on T cell-mediated cytotoxicity. Oncoimmunology (2021) 10(1):1950953. doi: 10.1080/2162402X.2021.1950953
41. Kuen J, Darowski D, Kluge T, Majety M. Pancreatic cancer Cell/Fibroblast Co-culture induces M2 like macrophages that influence therapeutic response in a 3d model. PloS One (2017) 12(7):e0182039. doi: 10.1371/journal.pone.0182039
42. Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, et al. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene (2014) 33(19):2423–31. doi: 10.1038/onc.2013.191
43. Freeman P, Mielgo A. Cancer-associated fibroblast mediated inhibition of Cd8+ cytotoxic T cell accumulation in tumours: Mechanisms and therapeutic opportunities. Cancers (2020) 12(9):2687. doi: 10.3390/cancers12092687
44. Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, et al. Hepatocellular carcinoma-associated fibroblasts trigger nk cell dysfunction Via Pge2 and ido. Cancer Lett (2012) 318(2):154–61. doi: 10.1016/j.canlet.2011.12.020
45. Dysthe M, Parihar R. Myeloid-derived suppressor cells in the tumor microenvironment. Tumor Microenviron (2020) 1224:117–40.
46. Hou A, Hou K, Huang Q, Lei Y, Chen W. Targeting myeloid-derived suppressor cell, a promising strategy to overcome resistance to immune checkpoint inhibitors. Front Immunol (2020) 11:783. doi: 10.3389/fimmu.2020.00783
47. Chiu DK-C, Tse AP-W, Xu IM-J, Di Cui J, Lai RK-H, Li LL, et al. Hypoxia inducible factor hif-1 promotes myeloid-derived suppressor cells accumulation through Entpd2/Cd39l1 in hepatocellular carcinoma. Nat Commun (2017) 8(1):517. doi: 10.1038/s41467-017-00530-7
48. Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab (2019) 30(1):36–50. doi: 10.1016/j.cmet.2019.06.001
49. Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv Drug Delivery Rev (2016) 99(Pt B):180–5. doi: 10.1016/j.addr.2015.11.009
50. Colegio OR, Chu N-Q, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature (2014) 513(7519):559–63. doi: 10.1038/nature13490
51. Schiliro C, Firestein BL. Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells (2021) 10(5):1056. doi: 10.3390/cells10051056
52. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the warburg effect: The metabolic requirements of cell proliferation. Sci (New York NY) (2009) 324(5930):1029–33. doi: 10.1126/science.1160809
53. Urbano AM. Otto Warburg: The journey towards the seminal discovery of tumor cell bioenergetic reprogramming. Biochim Biophys Acta Mol Basis Dis (2021) 1867(1):165965. doi: 10.1016/j.bbadis.2020.165965
54. Kobliakov AV. Hypoxia and glycolysis as factors determining the malignant phenotype. Tsitologiia (2018) 58(7):499–506.
55. Sgarbi G, Gorini G, Liuzzi F, Solaini G, Baracca A. Hypoxia and If1 expression promote ros decrease in cancer cells. Cells (2018) 7(7):64. doi: 10.3390/cells7070064
56. Rob A, Cairns I, , Harris S. Regulation of cancer cell metabolism. Nat Rev Cancer (2012) 11(2):85–95.
57. Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces myc-dependent apoptosis in human cells. J Cell Biol (2007) 178(1):93–105
58. Zhang J, Pavlova NN, Thompson CB. Cancer cell metabolism: The essential role of the nonessential amino acid, glutamine. EMBO J (2017) 36(10):1302–15. doi: 10.15252/embj.201696151
59. Zhdanov AV, Waters AHC, Golubeva AV, Dmitriev RI, Papkovsky DB. Availability of the key metabolic substrates dictates the respiratory response of cancer cells to the mitochondrial uncoupling. Biochim Biophys Acta (2014) 1837(1):51–62. doi: 10.1016/j.bbabio.2013.07.008
60. Kodama M, Oshikawa K, Shimizu H, Yoshioka S, Takahashi M, Izumi Y, et al. A shift in glutamine nitrogen metabolism contributes to the malignant progression of cancer. Nat Commun (2020) 11(1):1320. doi: 10.1038/s41467-020-15136-9
61. Matés JM, Campos-Sandoval JA, JdL Santos-Jiménez, Márquez J. Dysregulation of glutaminase and glutamine synthetase in cancer. Cancer Lett (2019) 467:29–39. doi: 10.1016/j.canlet.2019.09.011
62. Matés JM, Segura JA, Alonso FJ, Márquez J. Oxidative stress in apoptosis and cancer: An update. Arch Toxicol (2012) 86(11):1649–65. doi: 10.1007/s00204-012-0906-3
63. Friesen C, Kiess Y, Debatin KM. A critical role of glutathione in determining apoptosis sensitivity and resistance in leukemia cells. Cell Death Differ (2004) 11 Suppl 1:S73–85.
64. Domblides C, Lartigue L, Faustin B. Metabolic stress in the immune function of T cells, macrophages and dendritic cells. Cells (2018) 7(7):68. doi: 10.3390/cells7070068
65. Cheng C, Geng F, Cheng X, Guo D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun (Lond) (2018) 38(1):27. doi: 10.1186/s40880-018-0301-4
66. Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer (2007) 7(10):763–77.
67. Pike LS, Smift AL, Croteau NJ, Ferrick DA, Wu M. Inhibition of fatty acid oxidation by etomoxir impairs nadph production and increases reactive oxygen species resulting in atp depletion and cell death in human glioblastoma cells. Biochim Biophys Acta (2011) 1807(6):726–34.
68. Xiao L, Ma X, Ye L, Su P, Xiong W, Bi E, et al. Il-9/Stat3/Fatty acid oxidation-mediated lipid peroxidation contributes to Tc9 cell longevity and enhanced antitumor activity. J Clin Invest (2022) 132(7):e153247. doi: 10.1172/JCI153247
69. Semenza GL. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci (2012) 33(4):207–14. doi: 10.1016/j.tips.2012.01.005
70. Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. modulation of transcriptional activity by oxygen tension. J Biol Chem (1997) 272(31):19253–60.
71. Deng B, Zhu J-M, Wang Y, Liu T-T, Ding Y-B, Xiao W-M, et al. Intratumor hypoxia promotes immune tolerance by inducing regulatory T cells Via tgf-β1 in gastric cancer. PloS One (2013) 8(5):e63777. doi: 10.1371/journal.pone.0063777
72. Meng X, Grötsch B, Luo Y, Knaup KX, Wiesener MS, Chen X-X, et al. Hypoxia-inducible factor-1α is a critical transcription factor for il-10-Producing b cells in autoimmune disease. Nat Commun (2018) 9(1):251. doi: 10.1038/s41467-017-02683-x
73. Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol (2014) 9:47–71. doi: 10.1146/annurev-pathol-012513-104720
74. Bosco MC, D'Orazi G, Del Bufalo D. Targeting hypoxia in tumor: A new promising therapeutic strategy. J Exp Clin Cancer Res CR (2020) 39(1):8. doi: 10.1186/s13046-019-1517-0
75. Qiu G-Z, Jin M-Z, Dai J-X, Sun W, Feng J-H, Jin W-L. Reprogramming of the tumor in the hypoxic niche: The emerging concept and associated therapeutic strategies. Trends Pharmacol Sci (2017) 38(8):669–86. doi: 10.1016/j.tips.2017.05.002
76. Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res (2014) 74(3):665–74. doi: 10.1158/0008-5472.CAN-13-0992
77. Shen D-D, Bi Y-P, Pang J-R, Zhao L-J, Zhao L-F, Gao Y, et al. Generation, secretion and degradation of cancer immunotherapy target pd-L1. Cell Mol Life Sci CMLS (2022) 79(8):413. doi: 10.1007/s00018-022-04431-x
78. Logtenberg MEW, Scheeren FA, Schumacher TN. The Cd47-sirpα immune checkpoint. Immunity (2020) 52(5):742–52. doi: 10.1016/j.immuni.2020.04.011
79. Samanta D, Park Y, Ni X, Li H, Zahnow CA, Gabrielson E, et al. Chemotherapy induces enrichment of Cd47/Cd73/Pdl1 immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci United States America (2018) 115(6):E1239–E48. doi: 10.1073/pnas.1718197115
80. Jia X, Yan B, Tian X, Liu Q, Jin J, Shi J, et al. Cd47/Sirpα pathway mediates cancer immune escape and immunotherapy. Int J Biol Sci (2021) 17(13):3281–7. doi: 10.7150/ijbs.60782
81. Bachman KE, Park BH. Duel nature of tgf-beta signaling: Tumor suppressor vs. tumor promoter. Curr Opin Oncol (2005) 17(1):49–54.
83. Huang Y, Chen Z, Lu T, Bi G, Li M, Liang J, et al. Hif-1α switches the functionality of tgf-β signaling Via changing the partners of smads to drive glucose metabolic reprogramming in non-small cell lung cancer. J Exp Clin Cancer Res CR (2021) 40(1):398. doi: 10.1186/s13046-021-02188-y
84. Massari F, Ciccarese C, Santoni M, Iacovelli R, Mazzucchelli R, Piva F, et al. Metabolic phenotype of bladder cancer. Cancer Treat Rev (2016) 45:46–57. doi: 10.1016/j.ctrv.2016.03.005
85. Sampedro-Núñez M, Bouthelier A, Serrano-Somavilla A, Martínez-Hernández R, Adrados M, Martín-Pérez E, et al. Lat-1 and glut-1 carrier expression and its prognostic value in gastroenteropancreatic neuroendocrine tumors. Cancers (2020) 12(10):2968. doi: 10.3390/cancers12102968
86. Zhang J, Zhang Y, Mo F, Patel G, Butterworth K, Shao C, et al. The roles of hif-1α in radiosensitivity and radiation-induced bystander effects under hypoxia. Front Cell Dev Biol (2021) 9:637454. doi: 10.3389/fcell.2021.637454
87. Scarini J, Rosa L, Souza R, Egal E, Tincani A, Martins A, et al. Gene and immunohistochemical expression of hif-1α, glut-1, fasn, and adipophilin in carcinoma ex pleomorphic adenoma development. Oral Dis (2020) 00:1–10. doi: 10.1111/odi.13332
88. Wu Z, Wu J, Zhao Q, Fu S, Jin J. Emerging roles of aerobic glycolysis in breast cancer. Clin Trans Oncol Off Publ Fed Spanish Oncol Societies Natl Cancer Institute Mexico (2020) 22(5):631–46. doi: 10.1007/s12094-019-02187-8
89. Samanta D, Semenza G. Metabolic adaptation of cancer and immune cells mediated by hypoxia-inducible factors. Biochim Biophys Acta Rev Cancer (2018) 1870(1):15–22. doi: 10.1016/j.bbcan.2018.07.002
90. Yoo H, Park S, Nam M, Kang J, Kim K, Yeo J, et al. A variant of Slc1a5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab (2020) 31(2):267–83.e12. doi: 10.1016/j.cmet.2019.11.020
91. Sun R, Denko N. Hypoxic regulation of glutamine metabolism through Hif1 and Siah2 supports lipid synthesis that is necessary for tumor growth. Cell Metab (2014) 19(2):285–92. doi: 10.1016/j.cmet.2013.11.022
92. Infantino V, Santarsiero A, Convertini P, Todisco S, Iacobazzi V. Cancer cell metabolism in hypoxia: Role of hif-1 as key regulator and therapeutic target. Int J Mol Sci (2021) 22(11):5703. doi: 10.3390/ijms22115703
93. Weinberg F, Ramnath N, Nagrath D. Reactive oxygen species in the tumor microenvironment: An overview. Cancers (2019) 11(8):1191. doi: 10.3390/cancers11081191
94. Zhu X, Zuo L. Characterization of oxygen radical formation mechanism at early cardiac ischemia. Cell Death Dis (2013) 4:e787. doi: 10.1038/cddis.2013.313
95. Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, et al. Mitochondrial complex iii is required for hypoxia-induced ros production and cellular oxygen sensing. Cell Metab (2005) 1(6):401–8.
97. Bridge G, Rashid S, Martin SA. DNA Mismatch repair and oxidative DNA damage: Implications for cancer biology and treatment. Cancers (2014) 6(3):1597–614. doi: 10.3390/cancers6031597
98. Liao Z, Chua D, Tan NS. Reactive oxygen species: A volatile driver of field cancerization and metastasis. Mol Cancer (2019) 18(1):65. doi: 10.1186/s12943-019-0961-y
99. Galadari S, Rahman A, Pallichankandy S, Thayyullathil F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic Biol Med (2017) 104:144–64. doi: 10.1016/j.freeradbiomed.2017.01.004
101. Minassian LM, Cotechini T, Huitema E, Graham CH. Hypoxia-induced resistance to chemotherapy in cancer. Adv Exp Med Biol (2019) 1136:123–39.
102. Phung CD, Tran TH, Pham LM, Nguyen HT, Jeong J-H, Yong CS, et al. Current developments in nanotechnology for improved cancer treatment, focusing on tumor hypoxia. J Control Release (2020) 324:413–29. doi: 10.1016/j.jconrel.2020.05.029
103. Chouaib S, Janji B, Tittarelli A, Eggermont A, Thiery JP. Tumor plasticity interferes with anti-tumor immunity. Crit Rev Immunol (2014) 34(2):91–102.
104. Hasmim M, Messai Y, Ziani L, Thiery J, Bouhris J-H, Noman MZ, et al. Critical role of tumor microenvironment in shaping nk cell functions: Implication of hypoxic stress. Front Immunol (2015) 6:482. doi: 10.3389/fimmu.2015.00482
105. Jain RK. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell (2014) 26(5):605–22. doi: 10.1016/j.ccell.2014.10.006
106. Sharma M, Bakshi AK, Mittapelly N, Gautam S, Marwaha D, Rai N, et al. Recent updates on innovative approaches to overcome drug resistance for better outcomes in cancer. J Control Release (2022) 346:43–70. doi: 10.1016/j.jconrel.2022.04.007
107. Baylin SB, Jones PA. Epigenetic determinants of cancer. Cold Spring Harb Perspect Biol (2016) 8(9):a019505. doi: 10.1101/cshperspect.a019505
108. Ansell SM, Armitage JO. Positron emission tomographic scans in lymphoma: Convention and controversy. Mayo Clin Proc (2012) 87(6):571–80. doi: 10.1016/j.mayocp.2012.03.006
109. Baah S, Laws M, Rahman KM. Antibody-drug conjugates-a tutorial review. Molecules (2021) 26(10):2943. doi: 10.3390/molecules26102943
110. Attili I, Passaro A, de Marinis F. Anti-tigit to overcome resistance to immune checkpoint inhibitors in lung cancer: Limits and potentials. Ann Oncol (2022) 33(2):119–22. doi: 10.1016/j.annonc.2021.11.008
111. Xu X-L, Yang Y-R, Mo X-F, Wei J-L, Zhang X-J, You Q-D. Design, synthesis, and evaluation of benzofuran derivatives as novel anti-pancreatic carcinoma agents Via interfering the hypoxia environment by targeting hif-1α pathway. Eur J Med Chem (2017) 137:45–62. doi: 10.1016/j.ejmech.2017.05.042
112. Azoitei N, Becher A, Steinestel K, Rouhi A, Diepold K, Genze F, et al. Pkm2 promotes tumor angiogenesis by regulating hif-1α through nf-κb activation. Mol Cancer (2016) 15:3. doi: 10.1186/s12943-015-0490-2
113. Hu X, Xiao Y, Sun J, Ji B, Luo S, Wu B, et al. New possible silver lining for pancreatic cancer therapy: Hydrogen sulfide and its donors. Acta Pharm Sin B (2021) 11(5):1148–57. doi: 10.1016/j.apsb.2020.10.019
114. Kang G, Hu M, Ren H, Wang J, Cheng X, Li R, et al. Vhh212 nanobody targeting the hypoxia-inducible factor 1α suppresses angiogenesis and potentiates gemcitabine therapy in pancreatic cancer. Cancer Biol Med (2021) 18(3):772–87. doi: 10.20892/j.issn.2095-3941.2020.0568
115. Cho I-R, Kaowinn S, Moon J, Soh J, Kang HY, Jung C-R, et al. Oncotropic h-1 parvovirus infection degrades hif-1α protein in human pancreatic cancer cells independently of vhl and Rack1. Int J Oncol (2015) 46(5):2076–82. doi: 10.3892/ijo.2015.2922
116. Wen Z, Huang C, Xu Y, Xiao Y, Tang L, Dai J, et al. α-solanine inhibits vascular endothelial growth factor expression by down-regulating the Erk1/2-Hif-1α and Stat3 signaling pathways. Eur J Pharmacol (2016) 771:93–8. doi: 10.1016/j.ejphar.2015.12.020
117. Miyake K, Nishioka M, Imura S, Batmunkh E, Uto Y, Nagasawa H, et al. The novel hypoxic cytotoxin, tx-2098 has antitumor effect in pancreatic cancer; possible mechanism through inhibiting vegf and hypoxia inducible factor-1α targeted gene expression. Exp Cell Res (2012) 318(13):1554–63. doi: 10.1016/j.yexcr.2012.03.013
118. Nelson JK, Thin MZ, Evan T, Howell S, Wu M, Almeida B, et al. Usp25 promotes pathological hif-1-Driven metabolic reprogramming and is a potential therapeutic target in pancreatic cancer. Nat Commun (2022) 13(1):2070. doi: 10.1038/s41467-022-29684-9
119. Yu T, Li G, Wang C, Gong G, Wang L, Li C, et al. Mir210hg regulates glycolysis, cell proliferation, and metastasis of pancreatic cancer cells through mir-125b-5p/Hk2/Pkm2 axis. RNA Biol (2021) 18(12):2513–30. doi: 10.1080/15476286.2021.1930755
120. Hu Q, Qin Y, Ji S, Xu W, Liu W, Sun Q, et al. Uhrf1 promotes aerobic glycolysis and proliferation Via suppression of Sirt4 in pancreatic cancer. Cancer Lett (2019) 452:226–36. doi: 10.1016/j.canlet.2019.03.024
121. Halbrook CJ, Lyssiotis CA. Employing metabolism to improve the diagnosis and treatment of pancreatic cancer. Cancer Cell (2017) 31(1):5–19. doi: 10.1016/j.ccell.2016.12.006
122. Chapiro J, Sur S, Savic LJ, Ganapathy-Kanniappan S, Reyes J, Duran R, et al. Systemic delivery of microencapsulated 3-bromopyruvate for the therapy of pancreatic cancer. Clin Cancer Res an Off J Am Assoc Cancer Res (2014) 20(24):6406–17. doi: 10.1158/1078-0432.CCR-14-1271
123. Chen L, Yuan R, Wen C, Liu T, Feng Q, Deng X, et al. E3 ubiquitin ligase Ubr5 promotes pancreatic cancer growth and aerobic glycolysis by downregulating Fbp1 Via destabilization of C/Ebpα. Oncogene (2021) 40(2):262–76. doi: 10.1038/s41388-020-01527-1
124. Hu Q, Qin Y, Ji S, Shi X, Dai W, Fan G, et al. Mtap deficiency-induced metabolic reprogramming creates a vulnerability to cotargeting purine synthesis and glycolysis in pancreatic cancer. Cancer Res (2021) 81(19):4964–80. doi: 10.1158/0008-5472.CAN-20-0414
125. Vasan K, Werner M, Chandel NS. Mitochondrial metabolism as a target for cancer therapy. Cell Metab (2020) 32(3):341–52.
126. Zhang J, Yang J, Lin C, Liu W, Huo Y, Yang M, et al. Endoplasmic reticulum stress-dependent expression of Ero1l promotes aerobic glycolysis in pancreatic cancer. Theranostics (2020) 10(18):8400–14. doi: 10.7150/thno.45124
127. Li Z, Ge Y, Dong J, Wang H, Zhao T, Wang X, et al. Bzw1 facilitates glycolysis and promotes tumor growth in pancreatic ductal adenocarcinoma through potentiating Eif2α phosphorylation. Gastroenterology (2022) 162(4):1256–1271.e14. doi: 10.1053/j.gastro.2021.12.249
128. Hamada S, Matsumoto R, Tanaka Y, Taguchi K, Yamamoto M, Masamune A. Nrf2 activation sensitizes K-ras mutant pancreatic cancer cells to glutaminase inhibition. Int J Mol Sci (2021) 22(4):1870. doi: 10.3390/ijms22041870
129. Tsai P-Y, Lee M-S, Jadhav U, Naqvi I, Madha S, Adler A, et al. Adaptation of pancreatic cancer cells to nutrient deprivation is reversible and requires glutamine synthetase stabilization by Mtorc1. Proc Natl Acad Sci United States America (2021) 118(10):e2003014118. doi: 10.1073/pnas.2003014118
130. Lee S-W, Zhang Y, Jung M, Cruz N, Alas B, Commisso C. Egfr-pak signaling selectively regulates glutamine deprivation-induced macropinocytosis. Dev Cell (2019) 50(3):381–392.e5. doi: 10.1016/j.devcel.2019.05.043
131. Tong Y, Guo D, Lin S-H, Liang J, Yang D, Ma C, et al. Sucla2-coupled regulation of gls succinylation and activity counteracts oxidative stress in tumor cells. Mol Cell (2021) 81(11):2303–2316.e8. doi: 10.1016/j.molcel.2021.04.002
132. Yoo HC, Park SJ, Nam M, Kang J, Kim K, Yeo JH, et al. A variant of Slc1a5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab (2020) 31(2):267–283.e12. doi: 10.1016/j.cmet.2019.11.020
133. Nie H, Huang P-Q, Jiang S-H, Yang Q, Hu L-P, Yang X-M, et al. The short isoform of prlr suppresses the pentose phosphate pathway and nucleotide synthesis through the Nek9-hippo axis in pancreatic cancer. Theranostics (2021) 11(8):3898–915. doi: 10.7150/thno.51712
134. Buj R, Chen C-W, Dahl ES, Leon KE, Kuskovsky R, Maglakelidze N, et al. Suppression of P16 induces Mtorc1-mediated nucleotide metabolic reprogramming. Cell Rep (2019) 28(8):1971–1980.e8. doi: 10.1016/j.celrep.2019.07.084
135. Che D, Wang M, Sun J, Li B, Xu T, Lu Y, et al. Krt6a promotes lung cancer cell growth and invasion through myc-regulated pentose phosphate pathway. Front Cell Dev Biol (2021) 9:694071. doi: 10.3389/fcell.2021.694071
136. Sharma NS, Gupta VK, Garrido VT, Hadad R, Durden BC, Kesh K, et al. Targeting tumor-intrinsic hexosamine biosynthesis sensitizes pancreatic cancer to anti-Pd1 therapy. J Clin Invest (2020) 130(1):451–65. doi: 10.1172/JCI127515
137. Ricciardiello F, Gang Y, Palorini R, Li Q, Giampà M, Zhao F, et al. Hexosamine pathway inhibition overcomes pancreatic cancer resistance to gemcitabine through unfolded protein response and egfr-akt pathway modulation. Oncogene (2020) 39(20):4103–17. doi: 10.1038/s41388-020-1260-1
138. Campbell S, Mesaros C, Izzo L, Affronti H, Noji M, Schaffer BE, et al. Glutamine deprivation triggers nagk-dependent hexosamine salvage. Elife (2021) 10:e62644. doi: 10.7554/eLife.62644
139. Ma Z, Vocadlo DJ, Vosseller K. Hyper-O-Glcnacylation is anti-apoptotic and maintains constitutive nf-κb activity in pancreatic cancer cells. J Biol Chem (2013) 288(21):15121–30. doi: 10.1074/jbc.M113.470047
140. Li J-T, Yin M, Wang D, Wang J, Lei M-Z, Zhang Y, et al. Bcat2-mediated bcaa catabolism is critical for development of pancreatic ductal adenocarcinoma. Nat Cell Biol (2020) 22(2):167–74. doi: 10.1038/s41556-019-0455-6
141. Lee JH, Cho Y-R, Kim JH, Kim J, Nam HY, Kim SW, et al. Branched-chain amino acids sustain pancreatic cancer growth by regulating lipid metabolism. Exp Mol Med (2019) 51(11):1–11. doi: 10.1038/s12276-019-0350-z
142. Zhu Z, Achreja A, Meurs N, Animasahun O, Owen S, Mittal A, et al. Tumour-reprogrammed stromal Bcat1 fuels branched-chain ketoacid dependency in stromal-rich pdac tumours. Nat Metab (2020) 2(8):775–92. doi: 10.1038/s42255-020-0226-5
143. Wang Q, Li M, Gan Y, Jiang S, Qiao J, Zhang W, et al. Mitochondrial protein Uqcrc1 is oncogenic and a potential therapeutic target for pancreatic cancer. Theranostics (2020) 10(5):2141–57. doi: 10.7150/thno.38704
144. Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS. Oxidative phosphorylation as an emerging target in cancer therapy. Clin Cancer Res an Off J Am Assoc Cancer Res (2018) 24(11):2482–90. doi: 10.1158/1078-0432.CCR-17-3070
145. Xue D, Xu Y, Kyani A, Roy J, Dai L, Sun D, et al. Discovery and lead optimization of benzene-1,4-Disulfonamides as oxidative phosphorylation inhibitors. J Med Chem (2022) 65(1):343–68. doi: 10.1021/acs.jmedchem.1c01509
146. Ferrarini I, Louie A, Zhou L, El-Deiry WS. Onc212 is a novel mitocan acting synergistically with glycolysis inhibition in pancreatic cancer. Mol Cancer Ther (2021) 20(9):1572–83. doi: 10.1158/1535-7163.MCT-20-0962
147. Masoud R, Reyes-Castellanos G, Lac S, Garcia J, Dou S, Shintu L, et al. Targeting mitochondrial complex I overcomes chemoresistance in high oxphos pancreatic cancer. Cell Rep Med (2020) 1(8):100143. doi: 10.1016/j.xcrm.2020.100143
148. Bigelsen S. Evidence-based complementary treatment of pancreatic cancer: A review of adjunct therapies including paricalcitol, hydroxychloroquine, intravenous vitamin c, statins, metformin, curcumin, and aspirin. Cancer Manag Res (2018) 10:2003–18. doi: 10.2147/CMAR.S161824
149. Duong H-Q, Hwang JS, Kim HJ, Seong Y-S, Bae I. Bml-275, an ampk inhibitor, induces DNA damage, G2/M arrest and apoptosis in human pancreatic cancer cells. Int J Oncol (2012) 41(6):2227–36. doi: 10.3892/ijo.2012.1672
150. Zhu X, Cao Y, Liu W, Ju X, Zhao X, Jiang L, et al. Stereotactic body radiotherapy plus pembrolizumab and trametinib versus stereotactic body radiotherapy plus gemcitabine for locally recurrent pancreatic cancer after surgical resection: An open-label, randomised, controlled, phase 2 trial. Lancet Oncol (2021) 22(8):1093–102. doi: 10.1016/S1470-2045(21)00286-2
151. Gioelli N, Maione F, Camillo C, Ghitti M, Valdembri D, Morello N, et al. A rationally designed Nrp1-independent superagonist Sema3a mutant is an effective anticancer agent. Sci Transl Med (2018) 10(442):eaah4807. doi: 10.1126/scitranslmed.aah4807
152. Martínez-Bosch N, Guerrero PE, Moreno M, José A, Iglesias M, Munné-Collado J, et al. The pancreatic niche inhibits the effectiveness of sunitinib treatment of pancreatic cancer. Oncotarget (2016) 7(30):48265–79. doi: 10.18632/oncotarget.10199
153. Jamshed MB, Munir F, Shahid N, Sadiq U, Muhammad SA, Ghanem NB, et al. Antitumor activity and combined inhibitory effect of ceritinib with gemcitabine in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol (2020) 318(1):G109–G19. doi: 10.1152/ajpgi.00130.2019
154. Zhang Z, Song J, Xie C, Pan J, Lu W, Liu M. Pancreatic cancer: Recent progress of drugs in clinical trials. AAPS J (2021) 23(2):29. doi: 10.1208/s12248-021-00556-2
155. Miller AL, Garcia PL, Yoon KJ. Developing effective combination therapy for pancreatic cancer: An overview. Pharmacol Res (2020) 155:104740. doi: 10.1016/j.phrs.2020.104740
156. Sun JD, Liu Q, Ahluwalia D, Li W, Meng F, Wang Y, et al. Efficacy and safety of the hypoxia-activated prodrug Th-302 in combination with gemcitabine and nab-paclitaxel in human tumor xenograft models of pancreatic cancer. Cancer Biol Ther (2015) 16(3):438–49. doi: 10.1080/15384047.2014.1003005
157. Kishimoto S, Brender JR, Chandramouli GVR, Saida Y, Yamamoto K, Mitchell JB, et al. Hypoxia-activated prodrug evofosfamide treatment in pancreatic ductal adenocarcinoma xenografts alters the tumor redox status to potentiate radiotherapy. Antioxid Redox Signal (2021) 35(11):904–15. doi: 10.1089/ars.2020.8131
158. MacVicar T, Ohba Y, Nolte H, Mayer FC, Tatsuta T, Sprenger H-G, et al. Lipid signalling drives proteolytic rewiring of mitochondria by Yme1l. Nature (2019) 575(7782):361–5. doi: 10.1038/s41586-019-1738-6
159. Zhou L, Wang Q, Zhang H, Li Y, Xie S, Xu M. Yap inhibition by nuciferine Via ampk-mediated downregulation of hmgcr sensitizes pancreatic cancer cells to gemcitabine. Biomolecules (2019) 9(10):620. doi: 10.3390/biom9100620
160. Bian X, Liu R, Meng Y, Xing D, Xu D, Lu Z. Lipid metabolism and cancer. J Exp Med (2021) 218(1):e20201606. doi: 10.1084/jem.20201606
161. Reyes R, Wani NA, Ghoshal K, Jacob ST, Motiwala T. Sorafenib and 2-deoxyglucose synergistically inhibit proliferation of both sorafenib-sensitive and -resistant hcc cells by inhibiting atp production. Gene Expr (2017) 17(2):129–40. doi: 10.3727/105221616X693855
162. Yoo J-J, Yu SJ, Na J, Kim K, Cho YY, Lee YB, et al. Hexokinase-ii inhibition synergistically augments the anti-tumor efficacy of sorafenib in hepatocellular carcinoma. Int J Mol Sci (2019) 20(6):1292. doi: 10.3390/ijms20061292
163. Zhong J, Lu S, Jia X, Li Q, Liu L, Xie P, et al. Role of endoplasmic reticulum stress in apoptosis induced by Hk2 inhibitor and its potential as a new drug combination strategy. Cell Stress Chaperones (2022) 27(3):273–83. doi: 10.1007/s12192-022-01267-z
164. Zhou Y, Fan S, Feng L, Huang X, Chen X. Manipulating intratumoral fenton chemistry for enhanced chemodynamic and chemodynamic-synergized multimodal therapy. Adv Mater (2021) 33(48):e2104223. doi: 10.1002/adma.202104223
165. Huang Y, Wu S, Zhang L, Deng Q, Ren J, Qu X. A metabolic multistage glutathione depletion used for tumor-specific chemodynamic therapy. ACS Nano (2022) 16(3):4228–38. doi: 10.1021/acsnano.1c10231
166. Reznik E, Miller ML, Şenbabaoğlu Y, Riaz N, Sarungbam J, Tickoo SK, et al. Mitochondrial DNA copy number variation across human cancers. Elife (2016) 5:e10769. doi: 10.7554/eLife.10769
167. Zannella VE, Dal Pra A, Muaddi H, McKee TD, Stapleton S, Sykes J, et al. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clin Cancer Res an Off J Am Assoc Cancer Res (2013) 19(24):6741–50. doi: 10.1158/1078-0432.CCR-13-1787
168. Choueiri TK, Kaelin WG. Targeting the Hif2-vegf axis in renal cell carcinoma. Nat Med (2020) 26(10):1519–30. doi: 10.1038/s41591-020-1093-z
169. Akman M, Belisario DC, Salaroglio IC, Kopecka J, Donadelli M, De Smaele E, et al. Hypoxia, endoplasmic reticulum stress and chemoresistance: Dangerous liaisons. J Exp Clin Cancer Res CR (2021) 40(1):28. doi: 10.1186/s13046-020-01824-3
170. Pietrobon V, Marincola FM. Hypoxia and the phenomenon of immune exclusion. J Transl Med (2021) 19(1):9. doi: 10.1186/s12967-020-02667-4
171. Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Sci (New York NY) (2005) 307(5706):58–62.
172. Augustin HG, Koh GY. Antiangiogenesis: Vessel regression, vessel normalization, or both? Cancer Res (2022) 82(1):15–7. doi: 10.1158/0008-5472.CAN-21-3515
173. Li Q, Wang Y, Jia W, Deng H, Li G, Deng W, et al. Low-dose anti-angiogenic therapy sensitizes breast cancer to pd-1 blockade. Clin Cancer Res an Off J Am Assoc Cancer Res (2020) 26(7):1712–24. doi: 10.1158/1078-0432.CCR-19-2179
174. Klemba A, Bodnar L, Was H, Brodaczewska KK, Wcislo G, Szczylik CA, et al. Hypoxia-mediated decrease of ovarian cancer cells reaction to treatment: Significance for chemo- and immunotherapies. Int J Mol Sci (2020) 21(24):9492. doi: 10.3390/ijms21249492
175. Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: Master regulators of metastasis. Clin Cancer Res an Off J Am Assoc Cancer Res (2010) 16(24):5928–35. doi: 10.1158/1078-0432.CCR-10-1360
176. Tao J, Yang G, Zhou W, Qiu J, Chen G, Luo W, et al. Targeting hypoxic tumor microenvironment in pancreatic cancer. J Hematol Oncol (2021) 14(1):14. doi: 10.1186/s13045-020-01030-w
177. Li X, Hattori A, Takahashi S, Goto Y, Harada H, Kakeya H. Ubiquitin carboxyl-terminal hydrolase L1 promotes hypoxia-inducible factor 1-dependent tumor cell malignancy in spheroid models. Cancer Sci (2020) 111(1):239–52. doi: 10.1111/cas.14236
178. Luo F, Lu F-T, Cao J-X, Ma W-J, Xia Z-F, Zhan J-H, et al. Hif-1α inhibition promotes the efficacy of immune checkpoint blockade in the treatment of non-small cell lung cancer. Cancer Lett (2022) 531:39–56. doi: 10.1016/j.canlet.2022.01.027
179. Denny WA. Hypoxia-activated prodrugs in cancer therapy: Progress to the clinic. Future Oncol (2010) 6(3):419–28. doi: 10.2217/fon.10.1
180. Borad MJ, Reddy SG, Bahary N, Uronis HE, Sigal D, Cohn AL, et al. Randomized phase ii trial of gemcitabine plus Th-302 versus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol (2015) 33(13):1475–81. doi: 10.1200/JCO.2014.55.7504
181. Jayaprakash P, Ai M, Liu A, Budhani P, Bartkowiak T, Sheng J, et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J Clin Invest (2018) 128(11):5137–49. doi: 10.1172/JCI96268
182. Jackson-Patel V, Liu E, Bull MR, Ashoorzadeh A, Bogle G, Wolfram A, et al. Tissue pharmacokinetic properties and bystander potential of hypoxia-activated prodrug cp-506 by agent-based modelling. Front Pharmacol (2022) 13:803602. doi: 10.3389/fphar.2022.803602
183. Moen I, Stuhr LEB. Hyperbaric oxygen therapy and cancer–a review. Target Oncol (2012) 7(4):233–42. doi: 10.1007/s11523-012-0233-x
184. Selvendiran K, Kuppusamy ML, Ahmed S, Bratasz A, Meenakshisundaram G, Rivera BK, et al. Oxygenation inhibits ovarian tumor growth by downregulating Stat3 and cyclin-D1 expressions. Cancer Biol Ther (2010) 10(4):386–90.
185. Batenburg MCT, Maarse W, van der Leij F, Baas IO, Boonstra O, Lansdorp N, et al. The impact of hyperbaric oxygen therapy on late radiation toxicity and quality of life in breast cancer patients. Breast Cancer Res Treat (2021) 189(2):425–33. doi: 10.1007/s10549-021-06332-2
186. Li YC, Chen CH, Chang CL, Chiang JY, Chu CH, Chen HH, et al. Melatonin and hyperbaric oxygen therapies suppress colorectal carcinogenesis through pleiotropic effects and multifaceted mechanisms. Int J Biol Sci (2021) 17(14):3728–44. doi: 10.7150/ijbs.62280
Keywords: Tumor microenvironment, immunity, metabolism, hypoxia, targeted therapy
Citation: Chen G, Wu K, Li H, Xia D and He T (2022) Role of hypoxia in the tumor microenvironment and targeted therapy. Front. Oncol. 12:961637. doi: 10.3389/fonc.2022.961637
Received: 05 June 2022; Accepted: 01 September 2022;
Published: 23 September 2022.
Edited by:
Shaoquan Zheng, The First Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Ouyang Chen, Duke University, United StatesJing Mu, Shenzhen Hospital, Peking University, China
Copyright © 2022 Chen, Wu, Li, Xia and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Demeng Xia, ZGVtZW5neGlhQDE2My5jb20=; Tianlin He, c2t5cmFpbmhlQDE2My5jb20=
†These authors have contributed equally to this work
 Gaoqi Chen1†
Gaoqi Chen1† Kaiwen Wu
Kaiwen Wu Demeng Xia
Demeng Xia