
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 24 October 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.961155
This article is part of the Research TopicTranslational Research for Better Diagnosis and Treatment of Endometrial CancerView all 18 articles
Background: A systematic analysis of prognostic factors concerning endometrial clear cell carcinoma (ECCC) is lacking. The current study aimed to construct nomograms predicting the overall survival (OS) of ECCC patients.
Methods: We performed a retrospective study, and predicted nomograms for 3-, 5-, and 10-year OS were established. The nomograms were verified with the consistency index (C-index), calibration curve, and decision curve analysis (DCA).
Results: A total of 1778 ECCC patients, 991 from FIGO stage I/II and 787 from FIGO stage III/IV, were included in this study. The age at diagnosis, marital status, T stage, tumor size, and surgery-independent prognostic factors in FIGO stage I/II, and the age at diagnosis, T stage, lymph node involvement, distant metastasis, tumor size, surgery, radiotherapy, and chemotherapy in FIGO stage III/IV were independent prognostic factors. The C-indexes of the training and validation group were 0.766 and 0.697 for FIGO stage I/II and 0.721 and 0.708 for FIGO stage III/IV, respectively. The calibration curve revealed good agreement between nomogram-predicted and actual observation values. The DCA established that nomograms had better clinical benefits than the traditional FIGO stage.
Conclusions: The predicted nomograms showed good accuracy, excellent discrimination ability, and clinical benefits, depicting their usage in clinical practice.
Endometrial carcinoma (EC) is the sixth most diagnosed cancer among women (1). An estimated 66,570 new cases of uterine corpus cancer and 12,940 deaths were reported in the United States in 2021 (2). EC is usually diagnosed during stage I, and patients have a good prognosis as it induces symptoms from an early stage (3). Postmenopausal vaginal bleeding is the most common symptom of EC (3, 4). Additionally, tumor invasion of the cervix can lead to blood or pus in the uterine cavity, causing abdominal swelling and cramping pain. Patients having advanced disease may suffer pelvic and lumbosacral pain due to the invasion of the tumor within the surrounding tissues or nerves (3, 4). EC is classified as type I and type II based on Bokhman’s dualist model5. Type I EC is estrogen-dependent and accounts for nearly 80% of all EC. Its pathological type is primarily endometrioid carcinoma (3–5). Endometrial clear cell carcinoma (ECCC) is a type II EC accounting for approximately 2–4% of the total EC, and is more common in older women (6). ECCC is an estrogen-independent tumor whose onset has no apparent relationship with estrogen (4, 5, 7). ECCC is more aggressive and prone to early metastasis than endometrioid carcinoma (4, 5, 7). Many studies report a 5-year survival rate of less than 50%, irrespective of the ECCC clinical stage. However, all these studies included small samples having limited persuasion (4). Only a few small retrospective cohort studies and some case reports have explored the prognostic factors in ECCC due to its low incidence. Notably, there are no systematic analyses of ECCC from a large population sample. Therefore, the current study aimed to perform a comprehensive retrospective analysis depending on the Surveillance, Epidemiology, and End Results (SEER) database to evaluate the survival and prognostic risk factors for ECCC. Moreover, it establishes definitive individualized prognostic prediction models to predict the 3-, 5- and 10-year overall survival (OS) in ECCC patients. The findings contribute to developing appropriate treatment and follow-up strategies for ECCC.
The present study recruited ECCC patients from 18 registries of the SEER database between 2000 and 2018 using the SEER* Stat software (version 8.3.9). The National Cancer Institute established the SEER database in 1973, covering approximately 28% of the U.S. population. It includes age, sex, race, and year of diagnosis (8). All the data for this study were retrieved from the SEER database.
The inclusion criteria for this study were: (1) Primary site-labeled: C54.1-Endometrium. (2) ICD-O-3 Hist/behave: 8310/2: Clear cell adenocarcinoma in situ, 8310/3: Clear cell adenocarcinoma, NOS. (3) Year of diagnosis: 2000-2018. (4) Diagnosis confirmed based on histology or cytology. (5) Single primary cancer.
The exclusion criteria were: (1) The survival time was 0 and unknown. (2) The T stage was T0. (3) Unknown race. (4) Unknown AJCC stage.
Each of the following variables was considered for every patient: age at diagnosis, marital status (married, divorced, separated, unmarried, widowed, or unknown), race (white, black, or other), T stage (T1, T2, T3, T4, or TX), lymph node involvement (no, yes, or unknown), distant metastasis (no, yes, or unknown), tumor size (< 4.5 cm, 4.5–6.1 cm, > 6.1 cm or unknown), grade (I: well differentiated, II: moderately differentiated, III: poorly differentiated, IV: undifferentiated, or unknown), the International Federation of Gynecology and Obstetrics (FIGO) stage (I, II, III, or IV), surgery (partial hysterectomy, or total hysterectomy), radiotherapy (no or yes), chemotherapy (no/unknown, or yes), vital status (dead or alive), and time of survival (length in months). The FIGO stage of the patients was obtained based on the TNM staging system since no data on the FIGO stage was available in the SEER database. The endpoint of this study was OS, defined as the time from diagnosis to death or from the last follow-up (patients lost to follow-up).
The Chi-square test and Cox regression analysis were performed with the SPSS software. In contrast, the R software performed the C-index, calibration curve, DCA, and Log-rank tests. The Chi-square test determined the potential statistical differences in the demographic clinicopathological features and treatment patterns among patients with early and advanced ECCC. Then, patients having ECCC in FIGO stages I/II and III/IV were randomly assigned to the training and validation cohort at a 7:3 ratio, respectively. Univariate and multivariate Cox regression analyses were performed in the FIGO I/II and III/IV training cohorts to identify the independent OS risk factors. Predictive nomograms were established depending on the results of the Cox regression analysis for OS to predict the 3-, 5- and 10-year OS. The accuracy of the nomograms was validated with the consistency index (C-index). Moreover, the calibration curves were developed to compare the consistency between the OS predicted by the nomogram at 3, 5, and 10 years and their actual values. The clinical benefit of the nomograms and classical FIGO staging system was compared through a decision curve analysis (DCA). The survival curve of patients from different risk groups was analyzed with the Log-rank tests. The significance threshold had been set at P < 0.05.
A total of 1,778 patients diagnosed with ECCC were enrolled in this study between 2000 and 2018, depending on the inclusion and exclusion criteria. They were divided into 991 (55.7%) patients from FIGO stage I/II and 787 (44.3%) from FIGO stage III/IV. The demographic features of patients with ECCC are listed in Table 1. The median age of the patients was 68 years. Most patients were white (72.5%) and had been subjected to total hysterectomy (87.3%). Almost one-third of the patients (29.3%) had a tumor < 4.5 cm in size, 45.7% were married, 47.6% were in pathological grade III, 42.5% received radiotherapy, and 46.2% received chemotherapy. There were statistical differences between the FIGO stage I/II and FIGO Stage III/IV patients in marital status, race, tumor size, grade, surgery, radiotherapy, and chemotherapy (all P < 0.05). The number of patients with tumor size < 4.5 cm and pathological grade I in FIGO stage I/II was more than that in FIGO stage III/IV (36.2% vs. 20.6% and 1.9% vs. 0.8%, respectively) respectively). In contrast, the number of patients with tumor size > 6.1cm and pathological grade III in FIGO Stage III/IV was more than that in FIGO stage I/II (23.1% vs. 8.2% and 51.1% vs. 44.9%, respectively). Total hysterectomy and radiotherapy rates in the FIGO stage I/II were 92.1% and 46.9%, respectively, higher than the FIGO stage III/IV (81.2% and 36.8%).
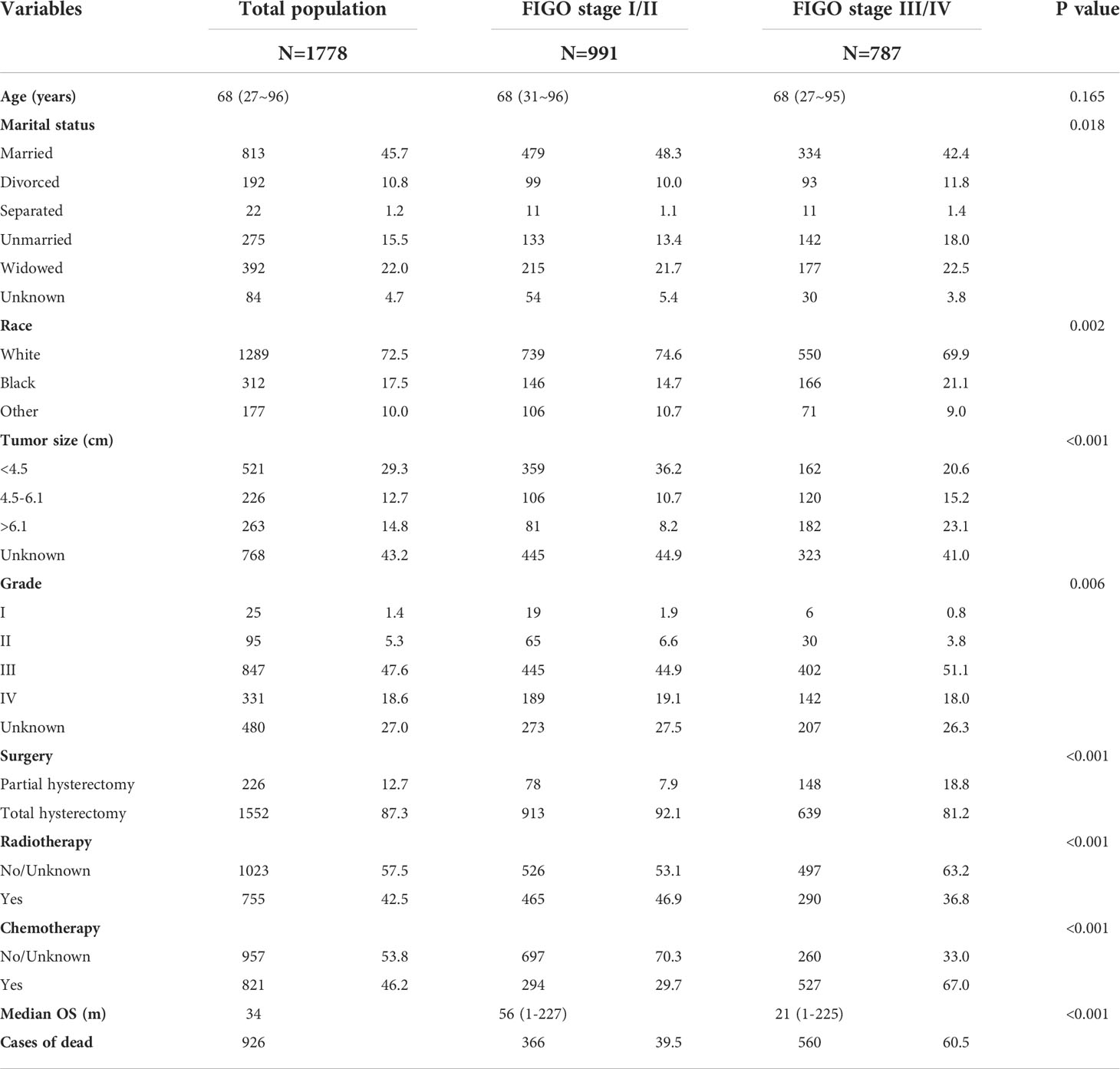
Table 1 Basic characteristics of ECCC patients from the total population, FIGO stage I/II, and FIGO stage III/IV cohorts.
Univariate and multivariate cox analyses indicated that age at diagnosis, marital status, T stage, tumor size, and surgery were independent risk factors for OS among patients with FIGO stage I/II (all P < 0.05) (Table 2). Additionally, age at diagnosis, T stage, lymph node involvement, distant metastasis, tumor size, surgery, radiotherapy, and chemotherapy were independent predictors for OS stage III/IV patients (P < 0.05) (Table 3).
Predictive nomograms were developed depending on the independent risk variables to predict the 3-, 5- and 10-year OS (Figure 1). Surgery (31 scores) had the most prognostic impact on FIGO stage I/II among the categorical variables. It was followed by marital status (28 scores), tumor size (24 scores), and T stage (13 scores) (Table 4). Surgery (52 scores) was also the most important factor in the FIGO stage III/IV among the categorical variables, followed by distant metastasis (48 scores), T stage (38 scores), tumor size (25 scores), chemotherapy (23 scores), lymph node involvement (20 scores), and radiotherapy (14 scores) (Table 5). The internal and external validations of the nomograms were performed in the training and validation cohorts, respectively. The C-indexes of the training and validation groups from the FIGO stage I/II and FIGO stage III/IV were 0.766 (95% CI: 0.750-0.782) and 0.697 (95% CI: 0.640–0.754), and 0.721 (95% CI: 0.708–0.734) and 0.708 (95% CI: 0.667–0.749), respectively. These values depicted that the constructed nomograms showed a good predictive performance. In addition, the calibration curves of the two groups had a good agreement between the nomogram-predicted and the real observation values (Figures 2 and 3). The DCA models revealed that the nomograms outperformed the FIGO staging system in clinical benefit (Figures 4 and 5), suggesting that nomograms showed more predictive power than the traditional staging system.
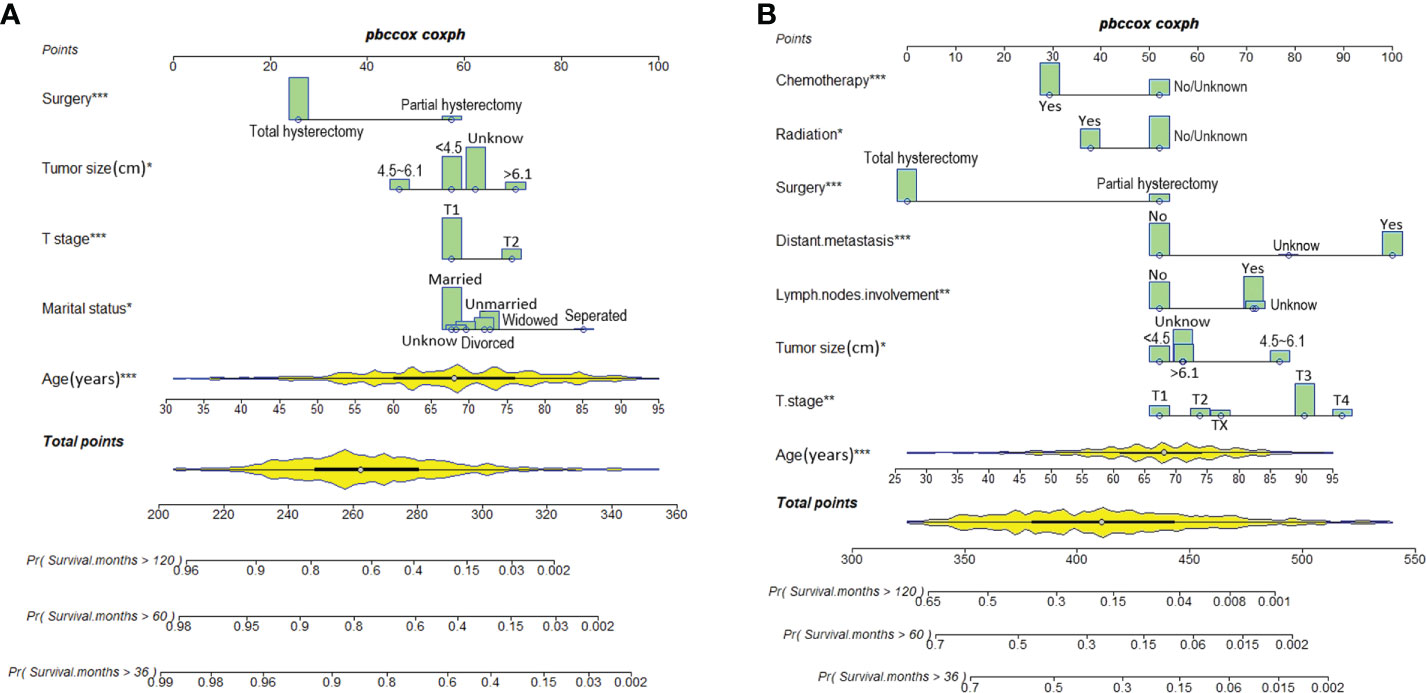
Figure 1 Nomograms for predicting 3-, 5-, and 10-year OS among patients with FIGO stage I/II (A) and FIGO stage III/IV (B) ECCC.

Table 5 Nomogram scores of each independent prognostic factor in the FIGO stage III/IV ECCC patients.
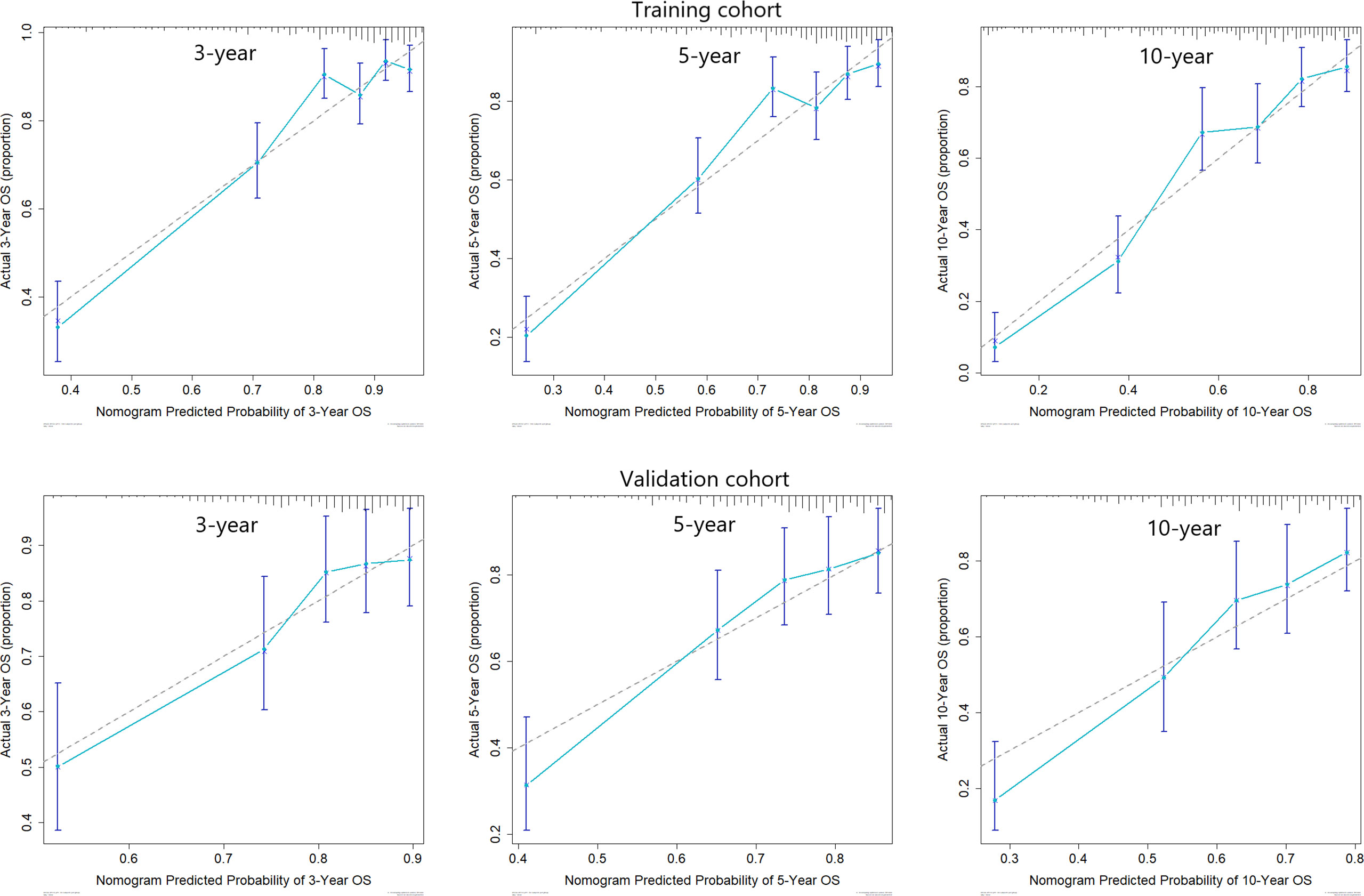
Figure 2 Calibration curves for 3-, 5-, and 10-year OS among patients with FIGO stage I/II ECCC within the training and validation cohorts.
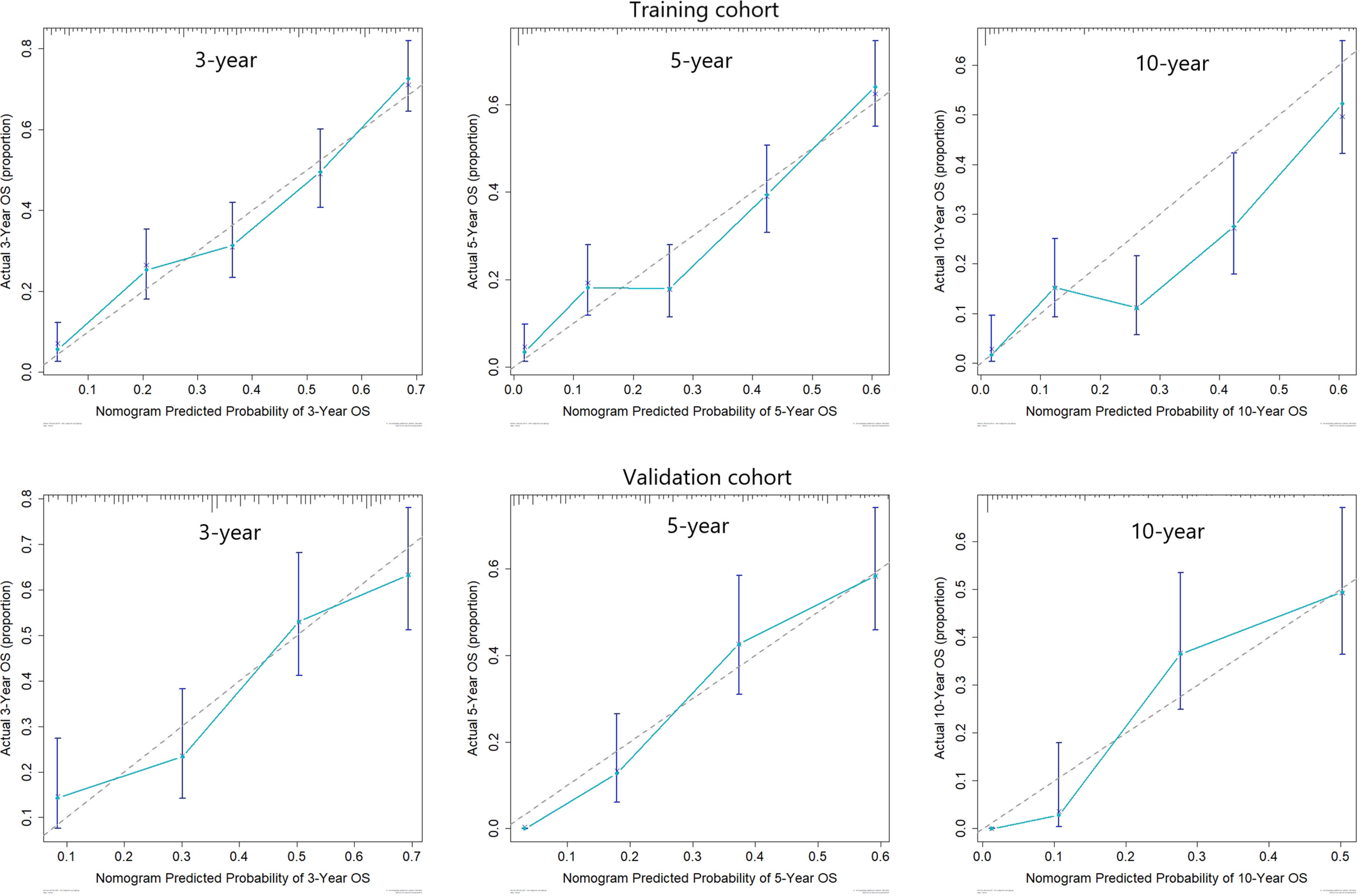
Figure 3 Calibration curves for 3-, 5-, and 10-year OS among patients with FIGO stage III/IV ECCC within the training and validation cohorts.
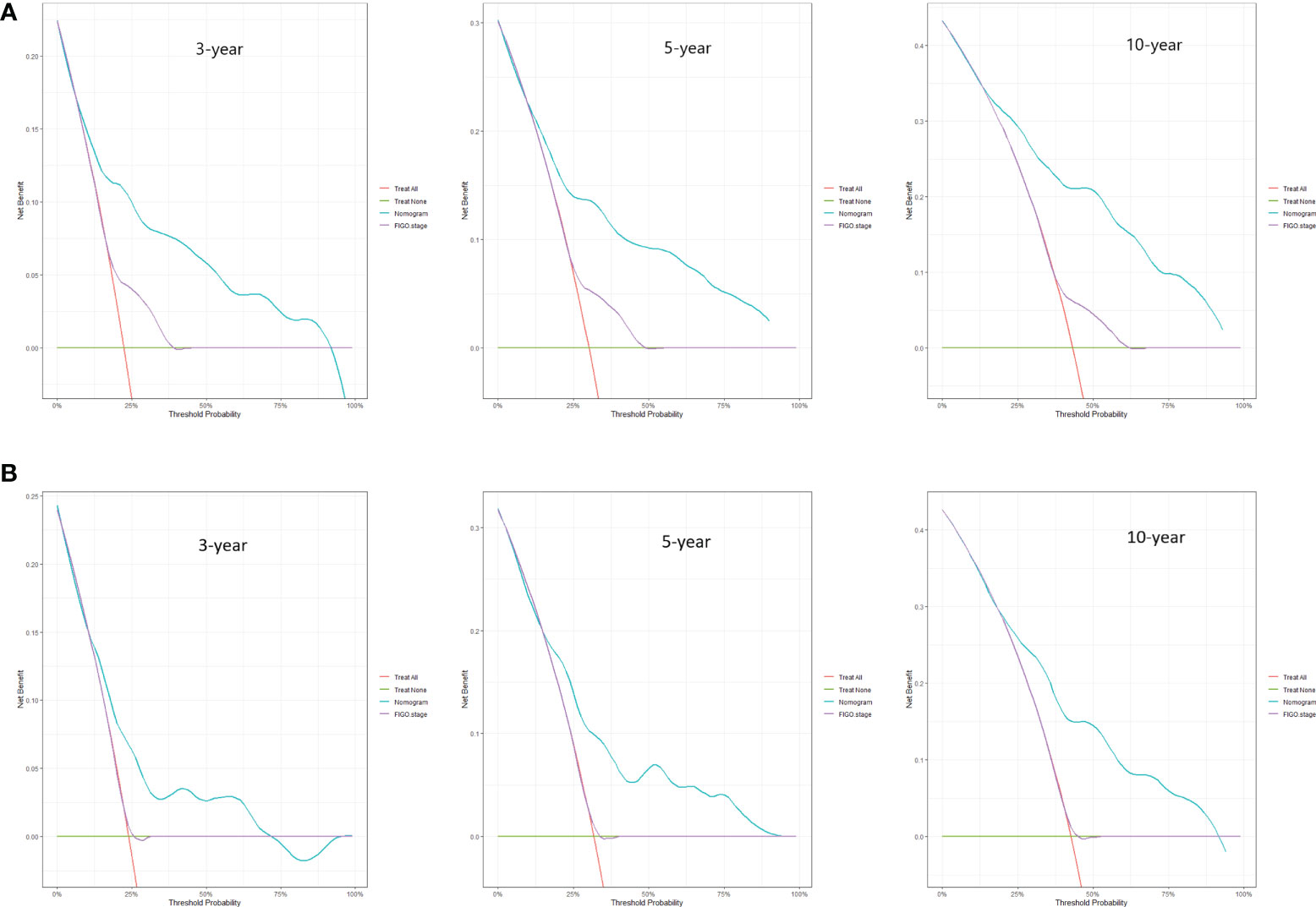
Figure 4 DCA curves for 3-, 5-, and 10-year OS among ECCC patients with FIGO stage I/II within the training cohort (A) and validation cohort (B).
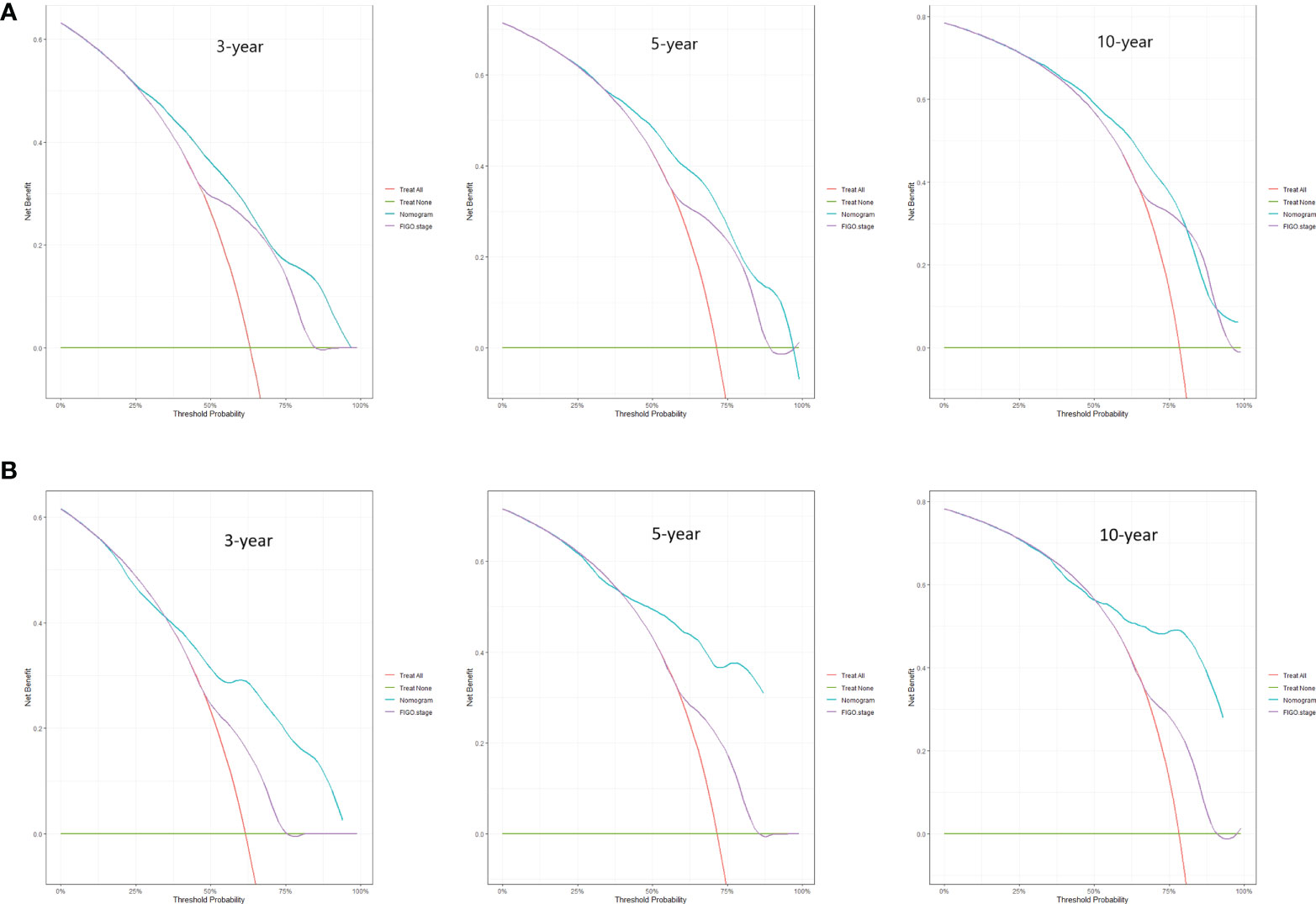
Figure 5 DCA curves for 3-, 5-, and 10-year OS among ECCC patients with FIGO stage III/IV within the training cohort (A) and validation cohort (B).
The ECCC patients were divided into low-risk, medium-risk, and high-risk groups based on the total scores from each variable. The median survival time in the high, medium, and low-risk groups for the FIGO stage I/II were 14.5, 40, and 69 months (Figure 6A), respectively, and 7, 18, and 33 months, respectively (Figure 6B) for the FIGO stage III/IV. The log-rank tests revealed that the survival times for the three risk groups differed significantly (both P < 0.001).
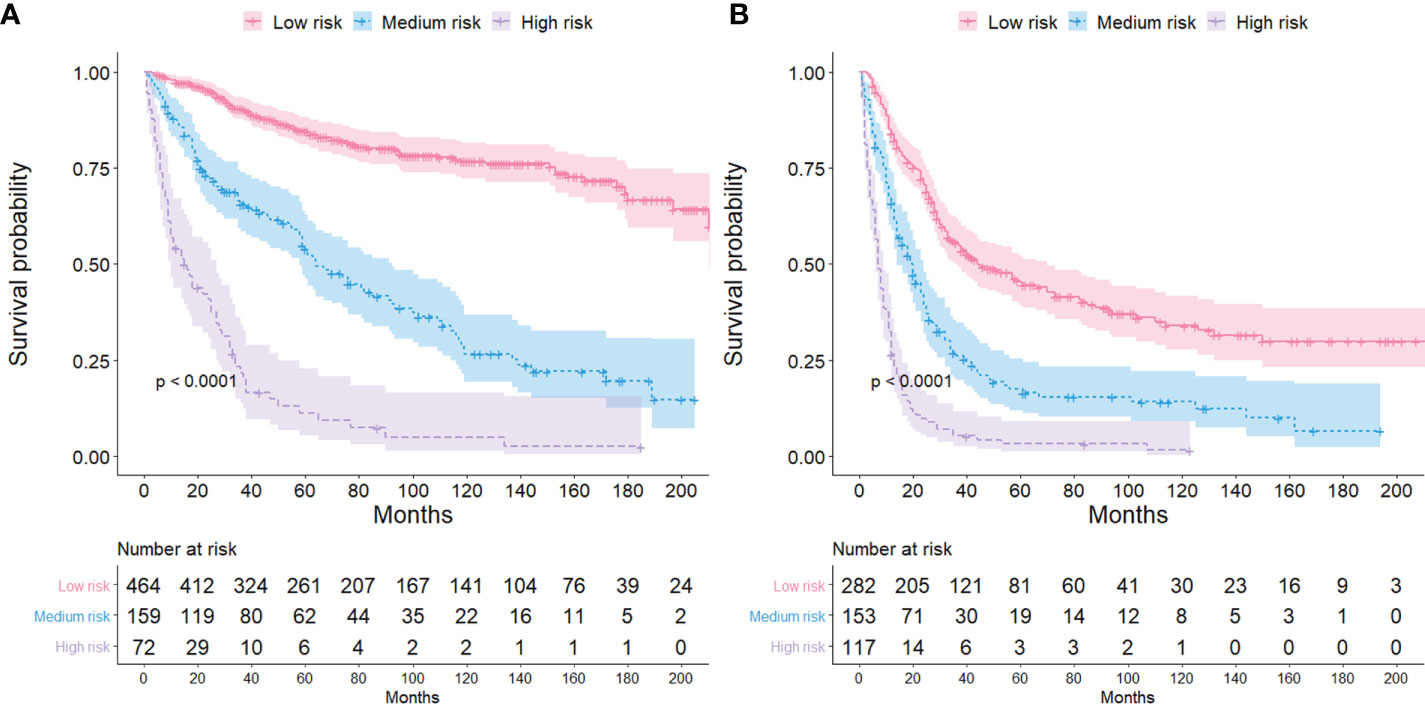
Figure 6 Kaplan-Meier survival curves for different risk groups among ECCC patients with FIGO stage I/II (A) and FIGO stage III/IV (B).
Unlike EC, most ECCC patients have a tumor negative for the estrogen and progesterone receptors. However, positive for hepatocyte nuclear factor 1β and Napsin A. Notably, TP53 is the most commonly mutated gene in ECCC (4, 9–11). The abnormal p53 expression is considered a poor prognostic factor for EC (11). Previous studies observed that the mutation rate of the TP53 gene in POLE wild-type ECCC is 46%, while that of non-POLE endometrioid carcinoma is only 11% (11). ECCC patients are accompanied by high-risk factors for poor prognoses, including advanced clinical stage, deep muscular infiltration, lymphovascular space involvement, and distant metastasis, with a high recurrence rate, high mortality, and poor prognosis than in type I EC (3, 4, 7). Currently, The Cancer Genome Atlas (TCGA) classification is the most authoritative classification system of EC. However, it does not include ECCC patients. Therefore, it is essential to analyze the demographic and clinicopathological characteristics of ECCC patients independently. Moreover, we must comprehensively evaluate their prognosis to develop an adequate treatment guide for ECCC patients.
Our study identified that age was an essential prognostic factor among ECCC patients, positively correlating with the risk of death. The findings of this study concerning the relationship between age and prognosis in EC patients were consistent with previous studies. A retrospective study found that patients aged ≤ 40 include more favorable prognostic factors, such as a higher proportion of non-invasive carcinoma, a lower proportion in the uterine segment involvement, and less invasion of the lymphatic vascular space than in EC patients aged 40-60 years (12). Furthermore, EC patients aged ≤ 40 years had a lower probability of mismatch repair defects due to MLH1 methylation, a mutation associated with poor prognosis, than patients aged 41-60 years (12). Another study also found that ECCC patients aged ≥ 70 had worse progression-free survival time and OS independent of the treatment modality they were subjected to (13). An investigation on the influence of marital status on the diagnosis and prognosis of EC revealed that marriage was a protective prognostic factor for OS and cancer-specific survival among EC patients. Unmarried, divorced/separated, and widowed patients showed a higher risk of death than married patients (14). This phenomenon was because married patients were more likely to be diagnosed early, possibly due to the stability of the endogenous hormone levels in women associated with emotional benefits (14). In this study, separated and widowed patients having early ECCC had a higher risk of death than married patients at the same stage. However, marital status had no significant effect on the prognosis of patients with advanced ECCC. Previous studies have evaluated the relationship between tumor diameter, myometrium invasion depth, and prognosis of EC patients. Nilufer et al. observed that more than half of the ECCC patients with a tumor diameter > 2 cm were prone to myometrial invasion (15). Kohei et al. identified that large tumor size is associated with deeper myometrial infiltration and lymph node metastasis among EC patients (16). In this study, ECCC patients with large tumor sizes and late T stages significantly enhanced the risk of death. These findings were consistent with a retrospective study that inferred that large tumor size and deep muscle invasion could be associated with poor prognosis among ECCC patients (17). Lymphatic metastasis is the main route of EC metastasis. The survival time of patients is significantly shortened once they develop lymph node metastasis, indicating disease progression (18). This study also depicted that OS is significantly decreased in ECCC patients with lymph node involvement.
A study revealed that black patients with EC were more likely to develop invasive and non-endometrioid cancer than white EC patients from America (19). However, this study did not observe a correlation between race and prognosis in ECCC patients. The degree of differentiation was not an independent prognostic factor for ECCC. Therefore, we hypothesized that the prognosis was poor irrespective of the degree of differentiation due to the high invasiveness of ECCC and thus had no significant effect on the prognosis of ECCC patients.
The preferred treatment method to cure ECCC is extensive staging surgery, including total uterine and bilateral adnexectomy, pelvic and para-aortic lymph node dissection, more significant omentum biopsy, and examination of the peritoneal washing fluid (20–22). The advantage of surgery is that the tumor stage is more accurately identified, facilitating the subsequent selection of the appropriate adjuvant treatment. Our results revealed that total hysterectomy was a favorable factor for a good prognosis. The risk of death after total hysterectomy was lower than after partial hysterectomy in both early and advanced stages. Therefore, active surgical treatment was recommended for ECCC patients. Patients who cannot undergo radical surgery should also be treated with tumor-reducing surgery, depending on their physical condition. Adjuvant radiotherapy and chemotherapy are fundamental approaches in treating ECCC. Numerous studies underline that the choice of adjuvant radiotherapy and chemotherapy is associated with the stage of ECCC (23). The adjuvant therapy in patients with early ECCC should be chosen based on prognosis-related factors, such as age and the depth of myometrial invasion. Although our results depicted that radiotherapy and chemotherapy had no role in improving the prognosis of patients with early ECCC, this factor did not hinder patients with early ECCC from benefiting from radiotherapy and chemotherapy. Our results were attributed to the SEER database limitations, which did not allow us to know the adjuvant treatment regimen and course, thus preventing the specific stratification study of the enrolled patients.
The FIGO stage of EC represents the pathological surgical stage, which includes factors related to patient prognoses, such as depth of muscular invasion, nodal metastasis, and distant metastasis. It is the primary tool clinicians use to evaluate the prognosis of EC patients. However, the FIGO stage does not include other factors associated with the survival of patients, such as age, marital status, and treatment methods. At the same time, the nomograms contain the demographic, clinical characteristics, and therapeutic approaches of the ECCC patients. Additionally, the DCA curves applied to ECCC patients established that nomograms had better clinical benefits than traditional FIGO stages in stages I/II and III/IV. Therefore, the nomograms had a significant practical value due to their good accuracy, excellent discrimination ability, and clinical benefits.
Compared to the existing prognostic classification, our predictive model demonstrated several strengths. All the clinical variables included in the survival prediction model were easily accessible. This study enrolled ECCC patients; thus, our nomogram was more applicable to ECCC patients than other classification systems. Moreover, the nomogram intuitively and clearly showed 3-, 5- and 8-year survival rates, which is convenient for clinicians. However, this study had several limitations. Firstly, all the variables selected in our study were clinical characteristics. Several genetic and epigenetic features, including Non-Coding RNAs, identified as predictors of EC patients in previous studies (24, 25), were not included in this study due to the limitations of the SEER database. The absence of these new molecular characteristics deteriorated the practicability of the nomogram model. Secondly, this was a retrospective study; thus, the bias significantly affected the results because the information about the patients was partially missing. For instance, the tumor size of 43.2% of the patients was unknown, which significantly reduced the accuracy of the prediction model. Finally, it was unclear whether the patients received neoadjuvant therapy, and the specific information based on surgery, radiotherapy, chemotherapy, and a potential targeted therapy was unknown.
Nomograms for predicting 3-, 5-, and 10-year OS in ECCC patients were successfully constructed. Moreover, new risk stratification systems were further built to stratify patients into different risk groups. These predictive models may be valuable tools to aid ECCC management and treatment in clinical practice.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
ZL, XC, and PC conceived and designed the study. YZ and HZ helped in data collection. PC and YZ performed the analysis and wrote the manuscript. All the authors have reviewed the final draft of the manuscript and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
3. Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet (2016) 387(10023):1094–108. doi: 10.1016/S0140-6736(15)00130-0
4. Kurman RJ, Carcangiu ML, Herrington CS, Young RH eds. World health organization classification of tumours of female reproductive organs. Lyon: IARC Press (2014).
5. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol (1983) 15(1):10–7. doi: 10.1016/0090-8258(83)90111-7
6. Bogani G, Ray-Coquard I, Concin N, Ngoi NYL, Morice P, Enomoto T, et al. Uterine serous carcinoma. Gynecol Oncol (2021) 162(1):226–34. doi: 10.1016/j.ygyno.2021.04.029
7. Nieto K, Adams W, Pham N, Block AM, Grover S, Small W Jr, et al. Adjuvant therapy in patients with clear cell endometrial carcinoma: An analysis of the national cancer database. Gynecol Oncol (2018) 148(1):147–53. doi: 10.1016/j.ygyno.2017.11.010
8. Wang S, Chen L, Feng Y, Swinnen JV, Jonscher C, Van Ongeval C, et al. Heterogeneity of synchronous lung metastasis calls for risk stratification and prognostic classification: Evidence from a population-based database. Cancers (Basel) (2022) 14(7):1608. doi: 10.3390/cancers14071608
9. Bogani G, Ray-Coquard I, Concin N, Ngoi NYL, Morice P, Enomoto T, et al. Clear cell carcinoma of the endometrium. Gynecol Oncol (2022) 164(3):658–66. doi: 10.1016/j.ygyno.2022.01.012
10. Kim SR, Cloutier BT, Leung S, Cochrane D, Britton H, Pina A, et al. Molecular subtypes of clear cell carcinoma of the endometrium: Opportunities for prognostic and predictive stratification. Gynecol Oncol (2020) 158(1):3–11. doi: 10.1016/j.ygyno.2020.04.043
11. DeLair DF, Burke KA, Selenica P, Lim RS, Scott SN, Middha S, et al. The genetic landscape of endometrial clear cell carcinomas. J Pathol (2017) 243(2):230–41. doi: 10.1002/path.4947
12. Son J, Carr C, Yao M, Radeva M, Priyadarshini A, Marquard J, et al. Endometrial cancer in young women: prognostic factors and treatment outcomes in women aged ≤40 years. Int J Gynecol Cancer (2020) 30(5):631–9. doi: 10.1136/ijgc-2019-001105
13. Armbruster SD, Previs R, Soliman PT, Westin SN, Fellman B, Jhingran A, et al. Clinicopathologic features and treatment in patients with early stage uterine clear cell carcinoma: A 16-year experience. Gynecol Oncol (2019) 154(2):328–32. doi: 10.1016/j.ygyno.2019.06.001
14. Dong J, Dai Q, Zhang F. The effect of marital status on endometrial cancer-related diagnosis and prognosis: a surveillance epidemiology and end results database analysis. Future Oncol (2019) 15(34):3963–76. doi: 10.2217/fon-2019-0241
15. Cetinkaya N, Selcuk İ, Ozdal B, Meydanli MM, Gungor T. Prognostic factors in endometrial clear cell carcinoma. Arch Gynecol Obstet (2017) 295(1):189–95. doi: 10.1007/s00404-016-4183-x
16. Nakamura K, Nakayama K, Ishikawa N, Minamoto T, Ishibashi T, Ohnishi K, et al. Preoperative tumor size is associated with deep myometrial invasion and lymph node metastases and is a negative prognostic indicator for patients with endometrial carcinoma. Oncotarget (2018) 9(33):23164–72. doi: 10.18632/oncotarget.25248
17. Zhang Z, Gao P, Bao Z, Zeng L, Yao J, Chai D, et al. Clear cell carcinoma of the endometrium: Evaluation of prognostic parameters in 27 cases. Front Oncol (2021) 11:732782. doi: 10.3389/fonc.2021.732782
18. AlHilli MM, Mariani A. The role of para-aortic lymphadenectomy in endometrial cancer. Int J Clin Oncol (2013) 18(2):193–9. doi: 10.1007/s10147-013-0528-7
19. Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet (2022) 399(10333):1412–28. doi: 10.1016/S0140-6736(22)00323-3
20. Sarı ME, Meydanlı MM, Türkmen O, Cömert GK, Turan AT, Karalök A, et al. Prognostic factors and treatment outcomes in surgically-staged non-invasive uterine clear cell carcinoma: a Turkish gynecologic oncology group study. J Gynecol Oncol (2017) 28(4):e49. doi: 10.3802/jgo.2017.28.e49
21. Mahdi H, Lockhart D, Moselmi-Kebria M. Prognostic impact of lymphadenectomy in uterine clear cell carcinoma. J Gynecol Oncol (2015) 26(2):134–40. doi: 10.3802/jgo.2015.26.2.134
22. Sugiyama T, Takeuchi S, Itamochi H. Surgical management of non-invasive uterine clear cell carcinoma. J Gynecol Oncol (2017) 28(4):e55. doi: 10.3802/jgo.2017.28.e55
23. Olawaiye AB, Leath CA 3rd. Contemporary management of uterine clear cell carcinoma: A society of gynecologic oncology (SGO) review and recommendation. Gynecol Oncol (2019) 155(2):365–73. doi: 10.1016/j.ygyno.2019.08.031
24. Cavaliere AF, Perelli F, Zaami S, Piergentili R, Mattei A, Vizzielli G, et al. Towards personalized medicine: Non-coding RNAs and endometrial cancer. Healthcare (Basel) (2021) 9(8):965. doi: 10.3390/healthcare9080965
Keywords: endometrial clear cell carcinoma, prognosis, nomogram, FIGO stage, risk classification system
Citation: Cui P, Cong X, Zhang Y, Zhang H and Liu Z (2022) Endometrial clear cell carcinoma: A population-based study. Front. Oncol. 12:961155. doi: 10.3389/fonc.2022.961155
Received: 04 June 2022; Accepted: 11 October 2022;
Published: 24 October 2022.
Edited by:
Andrzej Semczuk, Medical University of Lublin, PolandReviewed by:
Olugbenga Akindele Silas, University of Jos, NigeriaCopyright © 2022 Cui, Cong, Zhang, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ziling Liu, emlsaW5nQGpsdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.