
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 15 September 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.960204
This article is part of the Research Topic Recurrence of Liver Tumors: The Issue of Iterative Approaches View all 17 articles
Background: Repeat hepatectomy has been proven to be an effective treatment in patients with recurrent hepatocellular carcinoma (RHCC). However, for RHCC, it is still controversial whether laparoscopic hepatectomy is superior to conventional ones. The present meta-analysis was carried out to investigate the safety and overall effect of laparoscopic repeat hepatectomy (LRH) to open repeat hepatectomy (ORH) for patients with RHCC.
Methods: A meta-analysis was registered at PROSPERO, and the registration number is CRD42021257569. PubMed, Web of Science, and EMBASE were searched based on a defined search strategy to identify eligible studies before 25 April 2022. Data on operative times, bleeding volume, overall complications, 90-day mortality, blood transfusion, length of stay, overall survival rate, and long-term recurrence-free survival rate were subjected to meta-analysis.
Results: Overall, we identified nine studies of LRH versus ORH enrolling a total of 945 patients (460 and 485 underwent LRH and ORH, respectively). The present meta-analysis revealed non-significant differences in operative time, blood transfusion, overall complications, 90-day mortality, 3-year overall survival rate, 5-year overall survival rate, and long-term recurrence-free survival rate between the two groups. Alternatively, comparing LRH with ORH, LRH has less bleeding volume (p < 0.001) and a shorter length of stay (p = 0.005).
Conclusion: LRH is a feasible and effective treatment strategy for RHCC.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/#searchadvanced, identifier CRD42021257569.
Liver cancer is the third leading cause of cancer-related death worldwide and ranks sixth in terms of morbidity (1). Hepatocellular carcinoma (HCC) accounts for 75% to 95% of all primary liver cancers (2). Due to its rising incidence and unfavorable prognosis, HCC was considered a major global health problem (3). Hepatectomy has long been the frequent curative treatment for HCC and is especially appropriate for patients at an early stage (4–6). Unfortunately, tumor recurrence occurred in as many as 60%–80% of cases at 5 years, which made the long-term outcomes of HCC to remain unsatisfactory (6–9). No accepted neoadjuvant or adjuvant therapies have been confirmed to reduce the risk of recurrence (6, 7, 10). Hence, an effective therapeutic regimen for recurrence is essential to prolonging survival for HCC patients (11, 12).
Currently, varieties of remedies including repeated hepatectomy, liver transplantation, embolization, ablation, and molecular targeted therapy have been widely used in the clinical treatment of recurrent hepatocellular carcinoma (RHCC) (11, 13, 14). However, guidelines for the management of RHCC remained controversial (11, 12). Multiple studies have endorsed repeat hepatectomy as an effective treatment with favorable long-term surgical outcomes for RHCC in the past few decades (15–17).
Previous operation history had been among the contraindications for laparoscopic surgery (18). Nevertheless, with the improvement of laparoscopic instruments and accumulation of surgical techniques, laparoscopic hepatectomy (LH) has emerged as a viable alternative treatment to open hepatectomy (OH) and has been applied in specific RHCC patients safely (19, 20). Previous literature has confirmed the safety and efficiency of LH, emphasizing that LH was superior to OH due to less bleeding volume, shorter operation time, and faster recovery (21, 22).
However, postoperative adhesions as well as changes in anatomical land marks and liver deformation may cause technical challenges for laparoscopic repeat hepatectomy (LRH). The indication criteria for LRH have yet to be clearly defined (23). Hence, whether LRH or ORH is the preferred treatment for RHCC remains elusive.
To address this issue, we conducted a meta-analysis to compare the clinical efficacy and safety of LRH and ORH for patients with RHCC.
This study was carried out following the PRISMA 2020 guideline (24). The protocol of the present review was registered and allocated the identification number CRD42021257569 in the PROSPERO database.
Published documents before 25 April 2022 were retrieved using the electronic databases PubMed, EMBASE, Web of Science, and Cochrane Central Register, by two independent researchers (FL Hao, HC Li). The following subject terms were employed in the literature search: recurrent liver cancer, recurrent hepatocellular carcinoma, laparoscopic hepatectomy, open hepatectomy, liver resection, and minimally invasive surgery. Supplementary Table S1 shows our search strategy. For gaining additional trials, a manual search of eligible studies in references was complemented.
Two researchers (FL Hao, HC Li) identified and reviewed full-text articles that were regarded as relevant by screening the titles and abstracts. Disagreements were resolved by a team discussion.
Inclusion criteria were as follows (1): participants—patients with RHCC after initial curative liver resection (2); types of interventions—LRH and ORH (3); data available on interesting surgical outcomes.
Exclusion criteria were as follows (1): The publication type was observational clinical studies, case–control studies, abstracts, editorials, case reports, letters, and expert opinion (2); studies without available data, non-English or experimental studies.
Two researchers (FL Hao, HC Li) independently extracted relevant data with a standardized form. The data from studies based on a PSM analysis were extracted from the post-PSM analysis. Any ambiguity was discussed with the third researcher (N Li).
Based on the predetermined criteria, the following data were extracted: name of the first author, publication year, study design, country, number of patients, mean age, gender, tumor size, tumor number, operative times, bleeding volume, blood transfusion, number of patients converted from laparoscopy to laparotomy, overall complication, hospitalization, 90-day mortality, 1-, 3-, and 5-year survival (OS) rate, and 1-, 3-, and 5-year recurrence-free survival (RFS) rate.
The Newcastle–Ottawa Scale (NOS) developed for evaluating the quality of eligible studies was utilized by two independent reviewers (FL Hao, HC Li) (24). NOS score ≥6 was defined as high-quality. Any disagreements were discussed and resolved through consensus.
Statistical analysis was performed using the Review Manager software (RevMan V.5.3.4). Continuous data were expressed as 95% confidence interval (CI) and mean difference (MD), while dichotomous data used odds ratio (OR). For overall survival data, we used Engauge Digitizer (RevMan V.4.1) to extract OS and RFS data from survival curves (25). Using the method originally described by Hozo et al., medians with ranges were converted into means with standard deviations (26). Publication bias was assessed via Begg’s funnel plot and Egger’s linear regression test. Heterogeneity was examined by the I2 statistic. Statistical heterogeneity is significant when I2 ≥50%, and the random-effect model (REM) is utilized; if not (I2 <50%), the fixed-effect model (FEM) is applied.
The literature search yielded 1,651 relevant English publications which were considered potential studies. Eight hundred twenty-five of them were duplicates. Seven hundred ninety articles were excluded for irrelevance to the objective after screening the abstract and partial full text. Thirty-six full-text articles met the eligibility for assessment. Through reading the full text, 27 studies were excluded due to inappropriate study design or content. Finally, according to the inclusion criteria, nine studies (23, 27–34) of a total of 945 patients (460 and 485 underwent LRH and ORH, respectively) were found to be eligible for the present meta-analysis. Figure 1 shows the procedure of study selection in a flow diagram. Detailed NOS scores are presented in Supplementary Table S2.
In this review, we included nine studies involving 945 patients. The overall characteristics of the included articles are shown in Table 1. The sample sizes varied from 33 to 476, and most study designs were PSM studies.
All of the nine included studies made a comparative evaluation of operative times. Our analysis showed that the operative time in LRH patients was not inferior to those of ORH (MD: 11.63 min; 95% CI: -17.58 to 40.83; p = 0.44). Heterogeneity was high (I2 = 79%) and analyzed in the REM (Figure 2A).
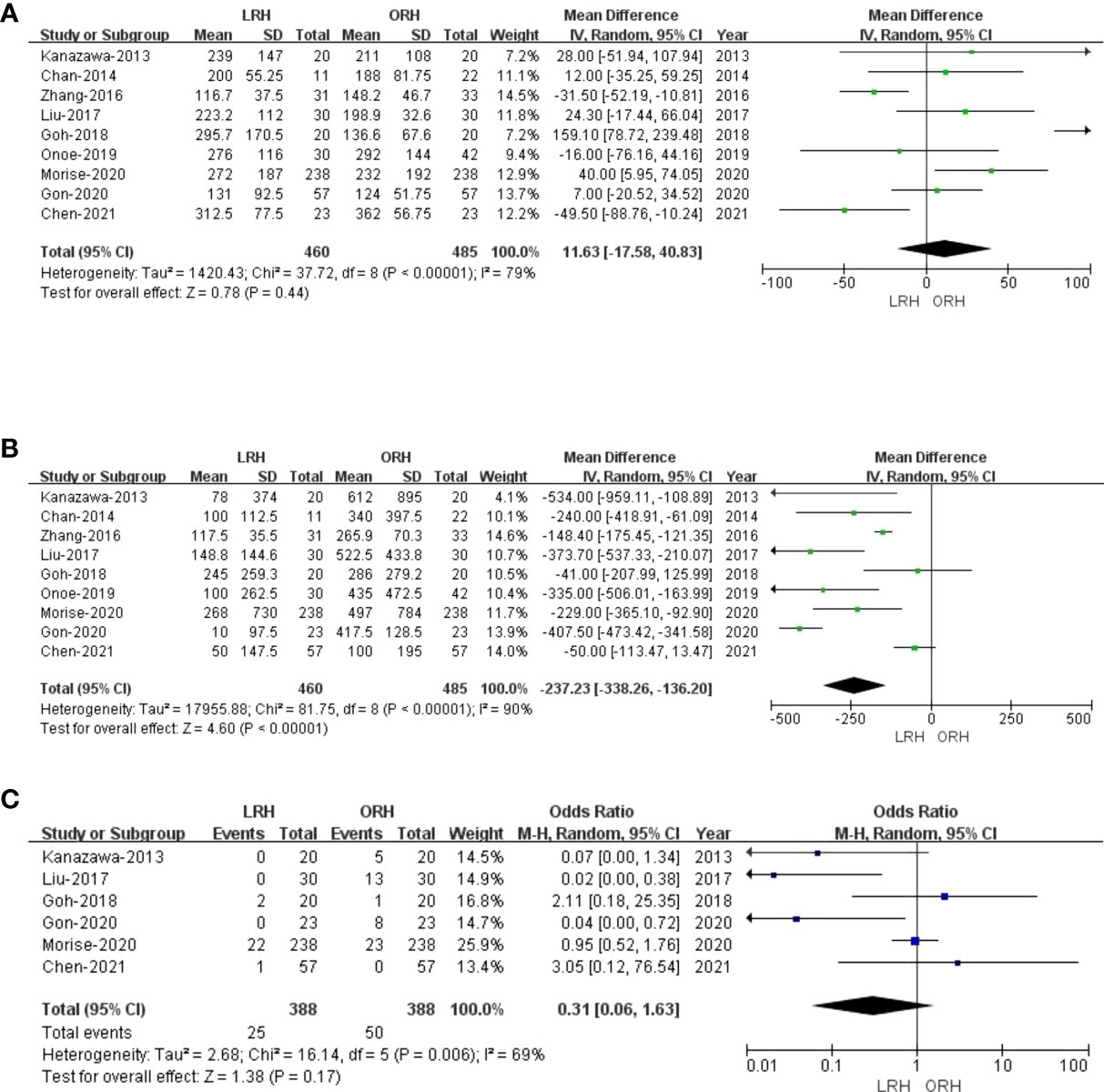
Figure 2 Forest plot of comparison of LRH versus ORH for operative outcomes of survivors.(A), Forest plot for operative time; (B), Forest plot for bleeding volume; (C), Forest plot for blood transfusion.
Nine studies that comprised 945 patients (460 and 485 underwent LRH and ORH, respectively) had reported the bleeding volume. Compared with the ORH group, the bleeding volume was lesser in the LRH group (MD: -237.23 ml; 95% CI: -338.26 to -136.20; p<0.00001). Heterogeneity was high (I2 = 90%) and analyzed in the REM (Figure 2B). A summary of meta-analysis results can be found in Table 2.
Blood transfusion data were available in six studies (23, 27–30, 32). There was no statistical difference in blood transfusion between the two groups (OR: 0.31; 95% CI:0.06 to 1.63; p = 0.17), indicating that LRH and ORH had similar effects on this item. Heterogeneity was high (I2 = 69%) and analyzed in the REM (Figure 2C).
Eight studies (23, 27–33) with a total of 881 patients (429 and 452 underwent LRH and ORH, respectively) mentioned the overall complications, and the result of a comprehensive analysis showed that LRH was associated with a similar overall complication rate for ORH (OR: 0.44; 95% CI: 0.17 to 1.14; p = 0.09). The heterogeneity was high (I2 = 64%) and analyzed in the REM (Figure 3A).
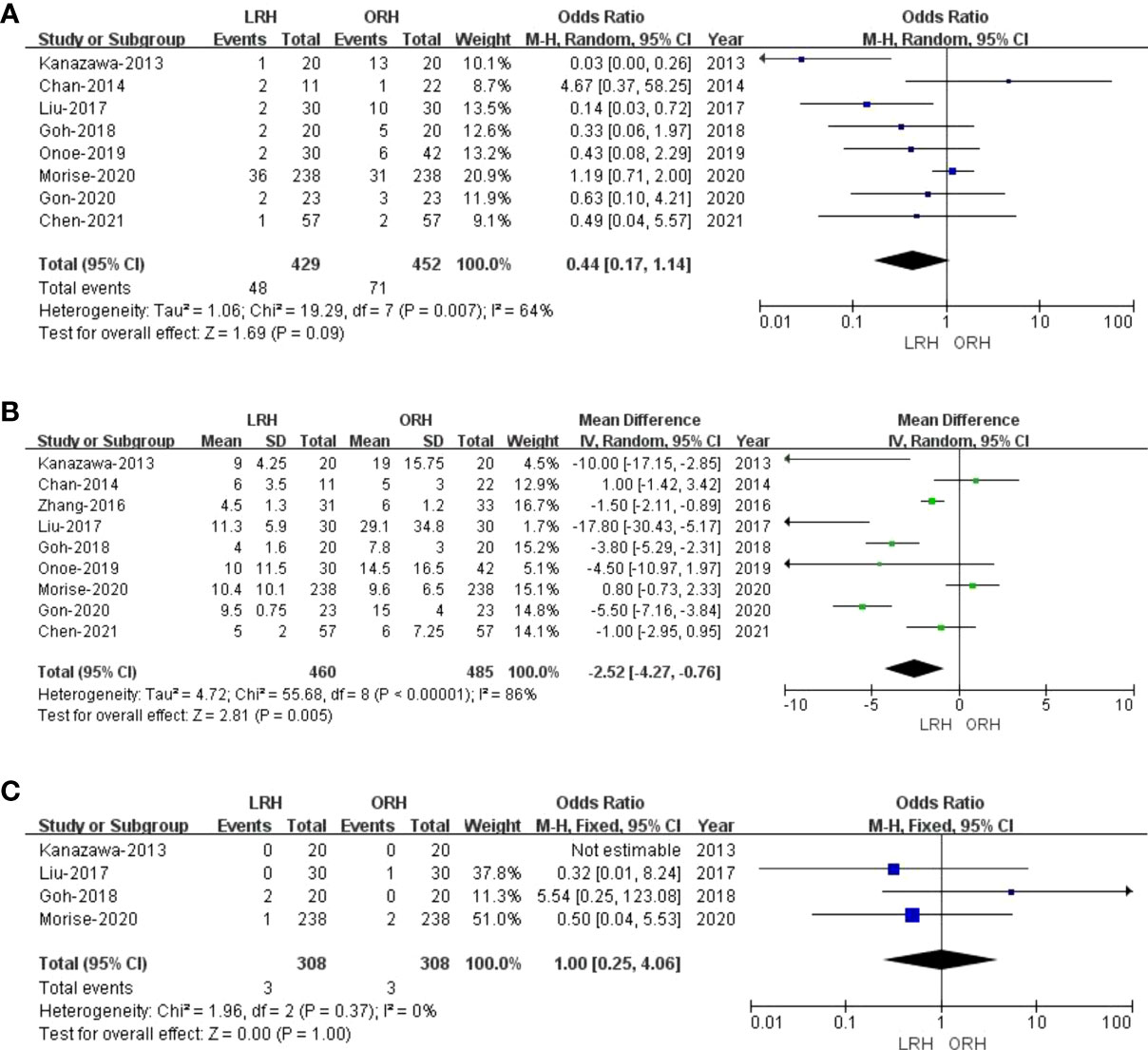
Figure 3 Forest plot of comparison of LRH versus ORH for postoperative outcomes of survivors. (A), Forest plot for overall complication rates; (B), Forest plot for the length of stay; (C), Forest plot for 90-day mortality.
All these nine studies had reported hospitalization time. Noticeably, the meta-analysis certified that RHCC treated with LRH presented shorter hospital stay compared with the ORH group (MD = -2.52; 95% CI: -4.27 to -0.76; p = 0.005), with high heterogeneity (I2 = 86%) in the REM (Figure 3B).
Of the nine studies, four trials (27–29, 32) performed an objective evaluation of the 90-day mortality. The result of the present study considered no difference in 90-day mortality between LRH and ORH groups (OR = 1.00; 95% CI: 0.25 to 4.06; p = 1.00), with low heterogeneity (I2 = 0%) in the FEM (Figure 3C).
Only three studies (27, 29, 31) assessed 1-year overall survival rate, and the result of our meta-analysis demonstrated that the 1-year survival rates for LRH were lower than those for ORH (OR: 0.60; 95% CI: 0.41 to 0.89; p = 0.01), into with moderate heterogeneity (I2 = 20%) in the REM (Figure 4A). Two studies (27, 29) compared the 3-year overall survival rates, and our results revealed no difference in 3-year overall survival rate (OR: 1.06; 95% CI: 0.31 to 3.62; p = 0.93), with high heterogeneity (I2 = 70%) in the REM (Figure 4B). Two studies (27, 29) assessed the 5-year overall survival rate; similarly, LRH had a proximate 5-year overall survival rate compared with the ORH group (OR: 0.76; 95% CI: 0.44 to 1.32; p = 0.33), with moderate heterogeneity (I2 = 31%) in the FEM (Figure 4C).
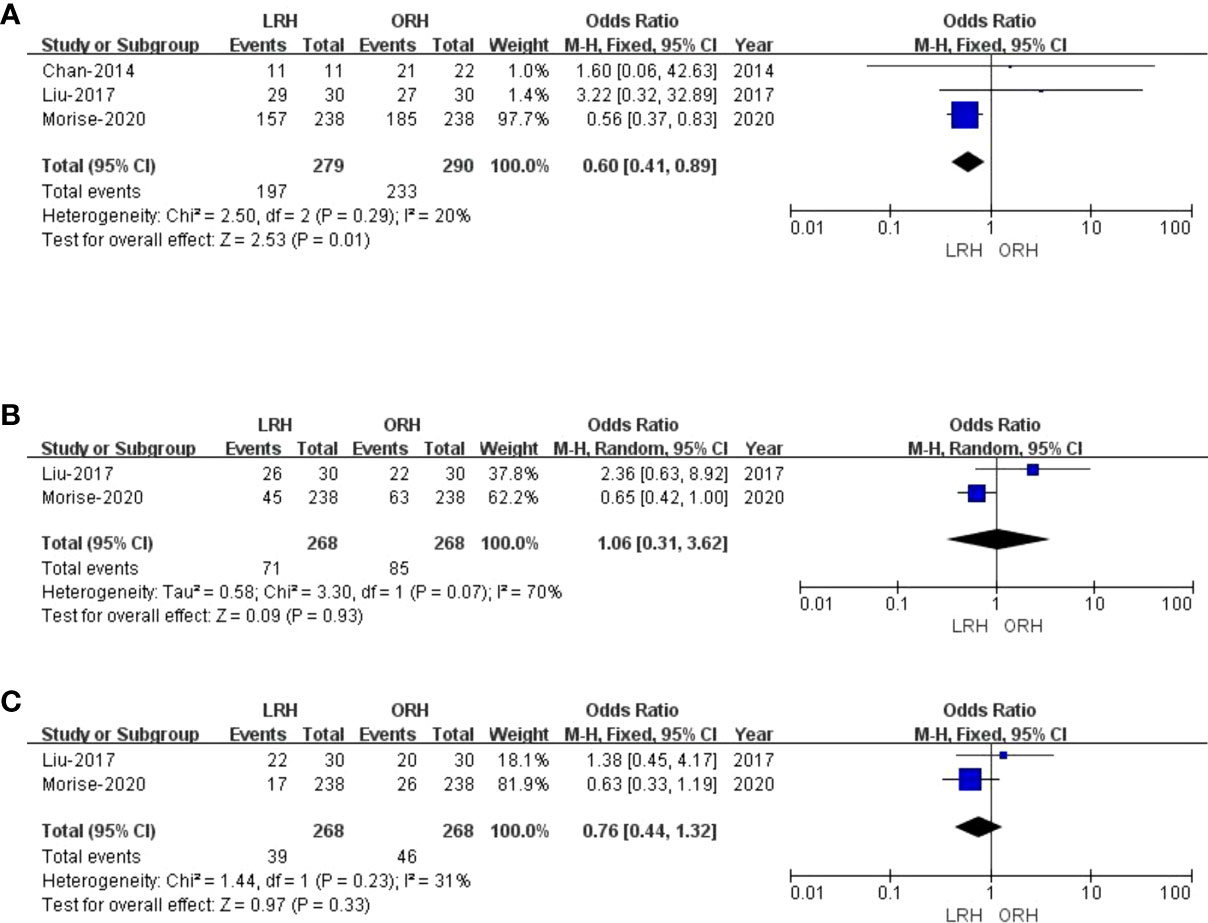
Figure 4 Forest plot of comparison of LRH versus ORH for the overall survival rate of survivors. (A), Forest plot for 1-year overall survival time rate; (B), Forest plot for 3-year survival time rate; (C), Forest plot for 5-year survival time rate.
There were five studies (27–29, 31, 34) that encompassed 673 patients (330 who underwent LRH and 343 who underwent ORH) that evaluated a 1-year recurrence-free survival rate. Overall, the 1-year recurrence-free survival rate did not differ significantly between the two groups (OR: 1.25; 95% CI: 0.53 to 2.92; p = 0.61), with high heterogeneity (I2 = 67%) in the REM (Figure 5A). Three studies (27–29) reported a 3-year recurrence-free survival rate. The result of the comprehensive analysis revealed no difference in the 3-year recurrence-free survival rate between the two regimens (OR: 2.41; 95% CI: 0.62 to 9.30; p = 0.20), with high heterogeneity (I2 = 80%) in the REM (Figure 5B). Additionally, two studies (27, 29) traced a 5-year recurrence-free survival rate, and the pooled data indicated no difference in the 5-year recurrence-free survival rate between LRH and ORH groups (OR: 0.85; 95% CI: 0.16 to 4.46; p = 0.85), with low heterogeneity (I2 = 66%) in the FEM (Figure 5C).

Figure 5 Forest plot of comparison of LRH versus ORH for the recurrence-free survival rate of survivors (A), Forest plot for 1-year recurrence-free survival rate; (b), Forest plot for 3-year recurrence-free survival rate; (C), Forest plot for 5-year recurrence-free survival rate.
Begg’s funnel plot was used to assess potential publication bias. All studies lie inside the 95% CIs in the funnel plot of 90-day mortality which indicated no potential publication bias (Figure 6).
For the past few years, the feasibility and efficacy of LRH for RHCC compared to ORH remained ambiguous. In our latest meta-analysis of nine studies and 945 patients with post-hepatectomy HCC recurrence, we confirmed that patients with LRH had a less bleeding volume and shorter hospital stays. However, the one-year survival rate for LRH was lower than that for ORH No significant intergroup differences were observed in other operative or postoperative outcomes, with similar findings in OS and RFS.
Currently, evidence on the role of LRH in the treatment of RHCC is limited (28). Abdominal adhesions have been reported in 67%–93% of patients following abdominal surgery, particularly in patients with severe portal hypertension (30, 35). Such adhesions restricted liver mobilization and made the recognition of vital blood vessels and specific anatomical structures more difficult, which could lead to accidental vascular or biliary damage (30). Handling the serried or vascularized adhesions, especially those around the hepatic hilum or hepatoduodenal ligaments, presented manipulation challenges for LRH (27, 31). In addition to this, deformation in anatomy, formation of collateral circulation, and impaired liver function due to surgical excision of liver parenchyma may attribute to intractability in re-resection (27). Furthermore, laparoscopic resection may lead to inadequate tumor clearance due to the consideration of surgical margin (36). In particular, tumors located in the caudate lobe or seventh or eighth segment have poor visibility, angular transverse lines, and difficulty in operation limited by costal margin and dynamic diaphragms.
However, with the improvement of optical technology, the magnified view provided by laparoscopy had greatly enhanced the visual preciseness in identifying vital structures (31). Moreover, modern laparoscope cameras together with the pneumoperitoneum made the adhesion bands tense up, contributing to a more precise dissection (37). On the other hand, the positive pressure of CO2 pneumoperitoneum, intraoperative ultrasound, advanced transection devices, facilitation of liver inflow and outflow control, and proficient laparoscopic skills gradually lessened the uncontrollable bleeding under a laparoscope (38). Consequently, previous abdominal operations were not an absolute contraindication for the LRH (35, 39, 40). Specific selection criteria for patients performing LRH had been documented by Hu et al.: tumor located in segments 2–6, a maximum size of 5 cm, no major vessels invaded by tumors, and well-preserved liver function (41).
During the laparoscopic surgery, open techniques were used to insert the first trocar. Pneumoperitoneum was established at 12–14 mmHg, followed by insertion of remaining four to five additional trocars. Ultrasonic surgical aspiration, an ultrasonic system, and a bipolar clamp coagulation system were utilized during the operation. Resection specimens were stored in plastic bags and removed through a small incision at the umbilical site. A midline and subcostal incision was made when performing ORH procedure. A drainage tube was routinely inserted around the cut surface after operation.
Consistent with the previous meta-analyses by Peng et al. and Cai et al., we reported the advantage of LRH in bleeding volume and hospital stay over ORH. Regrettably, they enrolled only seven and six articles, including 433 and 335 patients, respectively. However, we eliminated studies comprising HCC from colorectal cancer metastasis (42–44) and replenished five pieces of literature that were published after December 2018. Furthermore, shorter hospital stay and less intraoperative blood loss were also demonstrated by Chen et al., which included 12 studies published before 1 October 2020. We included an article that was not detected by Chen. et al, as well as their PSM research. Meanwhile, five studies containing metastatic liver cancer were excluded since they violated our definition of RHCC. Through a rigorous screening and analysis process, we reached conclusions similar to those of other meta-analyses. This may be related to fewer injuries, sooner postoperative activity time, and faster bowel function recovery.
This meta-analysis comprehensively updated the security and effectiveness of LRH and ORH. However, several limitations should also be noted. Firstly, the study design of enrolled original studies was diverse, including retrospective survey, prospective study, case-match analysis, and propensity score matching (PSM). Although the PSM method can minimize selection bias and control unit balance, it will never replace randomized controlled trials on account of inherent flaws in research design. For instance, different studies performed PSM based on different potential influencing factors, and the selected factors might be inconsistent or incomplete. Besides, PSM cannot control for unknown confounders or any covariates that were either not measured or erroneously measured. In addition, retrospective studies might result in significant heterogeneity. Thus, further high-quality research is required to confirm the benefit of LRH. Secondly, the substantial heterogeneity in bleeding volume and postoperative hospital stay indicated that the conclusion should be interpreted with caution. Except for study designs, the baseline characteristics of patients, location and quantity of RHCC, surgical equipment, procedure, etc., could attribute to the heterogeneity. Thirdly, in practice, many patients were considered unsuitable for laparoscopic procedures before surgery but were then used as comparisons between laparotomy and laparoscopic interventions. However, we could not gather data about how many laparoscopic patients were deemed unfeasible. Moreover, included primary studies and our meta-analysis did not evaluate the disease’s overall burden.
There was a higher likelihood that patients undergoing LRH might previously have less complicated HCC/liver disease and resection. This selection bias should be highlighted. Finally, all of the primary research was conducted in Asia, with a particular focus on East Asia. Nevertheless, patient characteristics and diagnostic-therapeutic algorithms frequently differ from those endorsed by Western countries. Thus, we need more research from other regions, to verify the applicability of our study.
Collectively, we found that LRH was likely considered a more favorable approach than ORH in specific RHCC cases for the similar risk of oncological outcomes and a quicker recovery from the procedure. However, accurate indications of LRH should be identified, and more studies are needed to reach an evidence-based conclusion.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
HW conceived and designed the study. FH, HL, NL, and JL, participated in the literature search and data collection. FH, HL, and NL analyzed the data and wrote the paper. HW reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from Sichuan University from 0 to 1 project (2022SCUH0017) and Sichuan Science and Technology Plan Project “International cooperation in science and technology innovation/technological innovation cooperation in Hong Kong, Macao and Taiwan” (2021YFH0095).
All authors have completed the ICMJE uniform disclosure form.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.960204/full#supplementary-material
1. Li H, Lan T, Liu H, Liu C, Dai J, Xu L, et al. IL-6-induced cGGNBP2 encodes a protein to promote cell growth and metastasis in intrahepatic cholangiocarcinoma. Hepatology (2022) 75(6):1402–19. doi: 10.1002/hep.32232
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int J Mol Sci (2020) 21(21). doi: 10.3390/ijms21218165
4. Peter R, Alejandro F, Joseph F L, Vincenzo M, Fabio P, Jean-Luc R. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
5. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology (2018) 68(2):723–50. doi: 10.1002/hep.29913
6. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet (2018) 391(10127):1301–14. doi: 10.1016/S0140-6736(18)30010-2
7. Villanueva A. Hepatocellular carcinoma. N Engl J Med (2019) 380(15):1450–62. doi: 10.1056/NEJMra1713263
8. Chan DL, Morris DL, Chua TC. Clinical efficacy and predictors of outcomes of repeat hepatectomy for recurrent hepatocellular carcinoma - a systematic review. Surg Oncol (2013) 22(2):e23–30. doi: 10.1016/j.suronc.2013.02.009
9. Roayaie S, Obeidat K, Sposito C, Mariani L, Bhoori S, Pellegrinelli A, et al. Resection of hepatocellular cancer ≤2 cm: Results from two Western centers. Hepatology (2013) 57(4):1426–35. doi: 10.1002/hep.25832
10. Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol (2015) 16(13):1344–54. doi: 10.1016/S1470-2045(15)00198-9
11. Yang Y, Yu H, Tan X, You Y, Liu F, Zhao T, et al. Liver resection versus radiofrequency ablation for recurrent hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hyperthermia (2021) 38(1):875–86. doi: 10.1080/02656736.2021.1933218
12. Zhou Y, Sui C, Li B, Yin Z, Tan Y, Yang J, et al. Repeat hepatectomy for recurrent hepatocellular carcinoma: A local experience and a systematic review. World J Surg Oncol (2010) 8:55. doi: 10.1186/1477-7819-8-55
13. Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: Patterns, treatments, and prognosis. Ann Surg (2015) 261(5):947–55. doi: 10.1097/SLA.0000000000000710
14. Lu LH, Mei J, Kan A, Ling YH, Li SH, Wei W, et al. Treatment optimization for recurrent hepatocellular carcinoma: Repeat hepatic resection versus radiofrequency ablation. Cancer Med (2020) 9(9):2997–3005. doi: 10.1002/cam4.2951
15. Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg (2000) 232(1):10–24. doi: 10.1097/00000658-200007000-00003
16. Sugimachi K, Maehara S, Tanaka S, Shimada M, Sugimachi K. Repeat hepatectomy is the most useful treatment for recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Surg (2001) 8(5):410–6. doi: 10.1007/s005340100002
17. Wu CC, Cheng SB, Yeh DC, Wang J, P'Eng FK. Second and third hepatectomies for recurrent hepatocellular carcinoma are justified. Br J Surg (2009) 96(9):1049–57. doi: 10.1002/bjs.6690
18. Morise Z. Status and perspective of laparoscopic repeat liver resection. World J Hepatol (2018) 10(7):479–84. doi: 10.4254/wjh.v10.i7.479
19. Moris D, Vernadakis S. Laparoscopic hepatectomy for hepatocellular carcinoma: The opportunities, the challenges, and the limitations. Ann Surg (2018) 268(1):e16. doi: 10.1097/SLA.0000000000002458
20. Yoon YI, Kim KH, Kang SH, Kim WJ, Shin MH, Lee SK, et al. Pure laparoscopic versus open right hepatectomy for hepatocellular carcinoma in patients with cirrhosis: A propensity score matched analysis. Ann Surg (2017) 265(5):856–63. doi: 10.1097/SLA.0000000000002072
21. Komatsu S, Brustia R, Goumard C, Perdigao F, Soubrane O, Scatton O. Laparoscopic versus open major hepatectomy for hepatocellular carcinoma: A matched pair analysis. Surg Endosc (2016) 30(5):1965–74. doi: 10.1007/s00464-015-4422-4
22. Singhirunnusorn J, Niyomsri S, Dilokthornsakul P. The cost-effectiveness analysis of laparoscopic hepatectomy compared with open liver resection in the early stage of hepatocellular carcinoma: A decision-analysis model in Thailand. HPB (Oxford) (2022) 24(2):183–91. doi: 10.1016/j.hpb.2021.06.005
23. Gon H, Kido M, Tanaka M, Kuramitsu K, Komatsu S, Awazu M, et al. Laparoscopic repeat hepatectomy is a more favorable treatment than open repeat hepatectomy for contralateral recurrent hepatocellular carcinoma cases. Surg Endosc (2021) 35(6):2896–906. doi: 10.1007/s00464-020-07728-9
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst Rev (2021) 10(1):89. doi: 10.1186/s13643-021-01626-4
25. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: Comparing reviewers' to authors' assessments. BMC Med Res Methodol (2014) 14:45. doi: 10.1186/1471-2288-14-45
26. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol (2005) 5:13. doi: 10.1186/1471-2288-5-13
27. Liu K, Chen Y, Wu X, Huang Z, Lin Z, Jiang J, et al. Laparoscopic liver re-resection is feasible for patients with posthepatectomy hepatocellular carcinoma recurrence: A propensity score matching study. Surg Endosc (2017) 31(11):4790–8. doi: 10.1007/s00464-017-5556-3
28. Goh BKP, Syn N, Teo JY, Guo YX, Lee SY, Cheow PC, et al. Perioperative outcomes of laparoscopic repeat liver resection for recurrent HCC: Comparison with open repeat liver resection for recurrent HCC and laparoscopic resection for primary HCC. World J Surg (2019) 43(3):878–85. doi: 10.1007/s00268-018-4828-y
29. Morise Z, Aldrighetti L, Belli G, Ratti F, Belli A, Cherqui D, et al. Laparoscopic repeat liver resection for hepatocellular carcinoma: A multicentre propensity score-based study. Br J Surg (2020) 107(7):889–95. doi: 10.1002/bjs.11436
30. Chen JF, Fu XT, Gao Z, Shi YH, Tang Z, Liu WR, et al. Laparoscopic vs. open repeat hepatectomy for recurrent liver tumors: A propensity score-matched study and meta-analysis. Front Oncol (2021) 11:646737. doi: 10.3389/fonc.2021.646737
31. Chan AC, Poon RT, Chok KS, Cheung TT, Chan SC, Lo CM. Feasibility of laparoscopic re-resection for patients with recurrent hepatocellular carcinoma. World J Surg (2014) 38(5):1141–6. doi: 10.1007/s00268-013-2380-3
32. Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamamoto S, Yamazoe S, et al. Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Sci (2013) 20(5):512–7. doi: 10.1007/s00534-012-0592-9
33. Onoe T, Yamaguchi M, Irei T, Ishiyama K, Sudo T, Hadano N, et al. Feasibility and efficacy of repeat laparoscopic liver resection for recurrent hepatocellular carcinoma. Surg Endosc (2020) 34(10):4574–81. doi: 10.1007/s00464-019-07246-3
34. Zhang J, Zhou ZG, Huang ZX, Yang KL, Chen JC, Chen JB, et al. Prospective, single-center cohort study analyzing the efficacy of complete laparoscopic resection on recurrent hepatocellular carcinoma. Chin J Cancer (2016) 35:25. doi: 10.1186/s40880-016-0088-0
35. Szomstein S, Lo Menzo E, Simpfendorfer C, Zundel N, Rosenthal RJ. Laparoscopic lysis of adhesions. World J Surg (2006) 30(4):535–40. doi: 10.1007/s00268-005-7778-0
36. Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, Chan SC, et al. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg (2013) 257(3):506–11. doi: 10.1097/SLA.0b013e31827b947a
37. Goh BK, Teo JY, Chan CY, Lee SY, Cheow PC, Chung AY. Review of 103 cases of laparoscopic repeat liver resection for recurrent hepatocellular carcinoma. J Laparoendosc Adv Surg Tech A (2016) 26(11):876–81. doi: 10.1089/lap.2016.0281
38. Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in morioka. Ann Surg (2015) 261(4):619–29. doi: 10.1097/SLA.0000000000001184
39. Law WL, Lee YM, Chu KW. Previous abdominal operations do not affect the outcomes of laparoscopic colorectal surgery. Surg Endosc (2005) 19(3):326–30. doi: 10.1007/s00464-004-8114-8
40. Nunobe S, Hiki N, Fukunaga T, Tokunaga M, Ohyama S, Seto Y, et al. Previous laparotomy is not a contraindication to laparoscopy-assisted gastrectomy for early gastric cancer. World J Surg (2008) 32(7):1466–72. doi: 10.1007/s00268-008-9542-8
41. Hu M, Zhao G, Xu D, Liu R. Laparoscopic repeat resection of recurrent hepatocellular carcinoma. World J Surg (2011) 35(3):648–55. doi: 10.1007/s00268-010-0919-0
42. Hallet J, Sa Cunha A, Cherqui D, Gayet B, Goéré D, Bachellier P, et al. Laparoscopic compared to open repeat hepatectomy for colorectal liver metastases: A multi-institutional propensity-matched analysis of short- and long-term outcomes. World J Surg (2017) 41(12):3189–98. doi: 10.1007/s00268-017-4119-z
43. Noda T, Eguchi H, Wada H, Iwagami Y, Yamada D, Asaoka T, et al. Short-term surgical outcomes of minimally invasive repeat hepatectomy for recurrent liver cancer. Surg Endosc (2018) 32(1):46–52. doi: 10.1007/s00464-017-5632-8
Keywords: recurrence, hepatocellular carcinoma, laparoscopic repeat hepatectomy, open repeat hepatectomy, meta-analysis
Citation: Hao F, Li H, Li N, Li J and Wu H (2022) Laparoscopic repeat hepatectomy versus conventional open repeat hepatectomy for recurrent hepatocellular carcinoma: A systematic review and meta-analysis. Front. Oncol. 12:960204. doi: 10.3389/fonc.2022.960204
Received: 02 June 2022; Accepted: 15 August 2022;
Published: 15 September 2022.
Edited by:
Alessandro Vitale, University Hospital of Padua, ItalyReviewed by:
Francesca Marcon, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, ItalyCopyright © 2022 Hao, Li, Li, Li and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Wu, NDA3NzIzMDgwQHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.