
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 16 September 2022
Sec. Cancer Epidemiology and Prevention
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.956923
 Oscar Fraile-Martinez1,2
Oscar Fraile-Martinez1,2 Miguel A. Alvarez-Mon1,2,3*
Miguel A. Alvarez-Mon1,2,3* Cielo Garcia-Montero1,2
Cielo Garcia-Montero1,2 Leonel Pekarek1,2,4
Leonel Pekarek1,2,4 Luis G. Guijarro2,5
Luis G. Guijarro2,5 Guillermo Lahera1,2,6
Guillermo Lahera1,2,6 Miguel A. Saez1,2,7
Miguel A. Saez1,2,7 Jorge Monserrat1,2
Jorge Monserrat1,2 Domitila Motogo1
Domitila Motogo1 Javier Quintero3,8
Javier Quintero3,8 Melchor Alvarez-Mon1,2,9†
Melchor Alvarez-Mon1,2,9† Miguel A. Ortega1,2,10†
Miguel A. Ortega1,2,10†In recent years, the incidence of different types of cancer and patient survival have been rising, as well as their prevalence. The increase in survival in recent years exposes the patients to a set of stressful factors such as more rigorous follow-up and more aggressive therapeutic regimens that, added to the diagnosis of the disease itself, cause an increase in the incidence of depressive disorders. These alterations have important consequences for the patients, reducing their average survival and quality of life, and for these reasons, special emphasis has been placed on developing numerous screening tests and early recognition of depressive symptoms. Despite that cancer and major depressive disorder are complex and heterogeneous entities, they also share many critical pathophysiological mechanisms, aiding to explain this complex relationship from a biological perspective. Moreover, a growing body of evidence is supporting the relevant role of lifestyle habits in the prevention and management of both depression and cancer. Therefore, the present study aims to perform a thorough review of the intricate relationship between depression and cancer, with a special focus on its biological links, clinical management, challenges, and the central role of lifestyle medicine as adjunctive and preventive approaches to improve the quality of life of these patients.
Major depressive disorder (MDD) is one of the most important and disabling psychiatric illnesses affecting a large proportion of today’s population. Indeed, it is estimated that approximately more than 260 million people in the world suffer from different degrees of MDD (1). Throughout history, both the diagnosis and treatment of MDD have changed over time. The causes that explain its origin are multifactorial, but a relationship between MDD and cancer has been described for years, both before and after diagnosis, which has led basic and clinical researchers to describe the underlying pathophysiology. In recent years, the incidence of patients affected by some type of cancer has increased gradually, estimating that by 2030, 26 million malignant neoplasms and up to 17 million deaths will be reported worldwide (2). The increase in incidence is in turn accompanied by an increase in prevalence due to both early diagnosis and improved treatment, which have improved the prognosis after diagnosis. The combination of the use of immunotherapy with chemoradiotherapy has made it possible to optimally address malignant tumors that previously had a dire prognosis (3). We cannot forget that both the diagnosis and the disease itself carry a great emotional burden that causes many of the patients to present different psychiatric pathologies such as depression, anxiety, or post-traumatic stress disorder during or after having overcome a malignant neoplasm (4). Numerous studies have shown how depression after cancer diagnosis causes a delay in the start of treatment, a decrease in quality of life, and an increase in the number of suicide attempts (5). It should be noticed that compared to the general population, the overall risk of cancer patients being diagnosed with MDD throughout the course of the disease is two to four times more frequent (6), although the available literature that assesses the prevalence of depression in cancer patients varies from 5% to 60% according to different authors (7).
A growing body of evidence has found common biological mechanisms between cancer and depression. In the same way, an accurate diagnosis and adequate clinical management of oncological patients with MDD are critical, as well as different approaches to prevent their progression. In this sense, lifestyle medicine represents a very important approach with potential benefits in both alleviating and preventing the development of depressive symptoms in cancer patients. Therefore, the purpose of this study is to delve into the different mechanisms that relate depression and cancer, as well as their translation in the clinic, including the challenges to overcome in this field and giving a central role to interventions in the lifestyle to prevent and achieve a better quality of life and clinical management of these patients.
Firstly, the biological basis of both cancer and MDD should be completely unraveled in order to identify and describe their biological links and potential preventive/therapeutic approaches. The etiopathogenesis and pathophysiological mechanisms of cancer and MDD are quite heterogeneous and intricate. Cancer is a complex entity, with unique clinical manifestations characterized by several molecular and cellular alterations brilliantly described by Hanahan and Weinberg (8). Some of the most important features of cancer include sustained proliferation, genome instability, tumor-promoting inflammation, immune evasion, apoptosis avoidance, altered metabolism, and metastasis. Moreover, the proper initiation and progression of tumoral cells entail not only local but also global consequences, affecting the different organs and systems of the organism (9, 10). Following the International Agency for Research on Cancer (IARC), some types of cancer may appear due to some viral, bacterial, parasitic, or fungal infections (11). The heritability of cancer in twins has been estimated at 30%, although the percentage differs according to the type of cancer (12). Other important contributors to the etiology of cancer are lifestyle factors like smoking, alcohol consumption, diet, obesity, and sedentarism, together with hormonal and reproductive factors, pharmaceuticals, or excessive/low sunlight exposure (13).
In the case of MDD, it is a multifactorial disorder frequently manifested as a depressed mood or loss of interest or pleasure (anhedonia) accompanied by a set of somatic and vegetative items like feelings of worthlessness, lack of energy, poor concentration, appetite changes, sleep disturbances, or suicidal thoughts (14). Patients with MDD often exhibit several functional and structural changes in different regions of the brain and neuronal networks, as well as the altered immune system and gut microbiota and multiple systemic alterations (15). MDD onset is associated with many genetic and environmental factors. In a similar manner to cancer, cumulative evidence has estimated that the heritability of MDD is about 30% to 40% (16). Moreover, unhealthy lifestyle factors like low physical activity levels, improper dietary habits, or sleep disturbances are also related to the onset of MDD (17). However, different types of psychological stress, including early life stress (ELS) or chronic stress, are thought to be the major contributors to MDD onset (18). Although there are unique and different pathological signatures in cancer and MDD, being diagnosed with cancer is commonly related to suffering from this psychiatric condition, and in turn, suffering from MDD appears to significantly increase the risk of cancer incidence as well as poorer cancer survival, and higher cancer-specific mortality (19). Moreover, not only psychological stress but also other mechanisms have been described in the connection between cancer and MDD, including circadian disruption, inflammation, gut dysbiosis, and abnormal neurotransmission (20). Indeed, there is an intricate integration of signals between the nervous, immune, and endocrine systems along with the gut microbiota and the psychological functioning of the individual. This is collected under the term psychoneuroimmunoendocrinology (PNIE), resulting in the pivotal etiopathogenic link between complex diseases like cancer and psychiatric disorders like MDD (21). Thus, despite that we will summarize the biological mechanisms in 1) psychological stress and circadian disruption, 2) inflammation and gut dysbiosis, and 3) abnormal neurotransmission, it is important to understand that all these factors are not influencing alone but coordinately in the relationship between depression and cancer.
As above mentioned, being diagnosed with cancer is a strong source of psychological stress for the patients, which can be an important driver of MDD for oncological patients, especially for those with higher stress susceptibility or chronic stress prior to diagnosis. Psychological stress induces the hyperactivation of the hypothalamic–pituitary–adrenal (HPA) axis, leading to an increase of corticotropin-releasing factor (CRF), adrenocorticotropic hormone (ACTH), and cortisol. Importantly, these components are associated with a set of structural and functional changes in the brain and the entire organism (22, 23). Notwithstanding that the IARC does not recognize psychological stress as a cause of any type of cancer, compelling evidence is starting to suggest that, at least, psychological stress and carcinogenesis are tightly associated (24). Nowadays, it is widely accepted that the hyperactivation of the HPA axis and the sympathetic nervous system (SNS) drives decline and dysfunction in the hippocampus and prefrontal cortex along with hyperactivation of the amygdala, which is associated with MDD development (25). Moreover, previous studies have noticed that HPA dysregulation is responsible for the concurrence of several symptoms in patients with advanced stages of lung cancer (26). Li et al. (27) studied a set of genes involved in the alteration of the HPA axis in patients with breast cancer in order to identify women at risk of suffering from psychiatric and depressive symptoms. Interestingly, they found specific polymorphic variants in some critical genes like FKBP5 (rs9394309), NR3C2 (rs5525), and CRHR1 (rs12944712), which can be used to explain the relevance of the HPA axis in women with breast cancer. Systemically, the hyperactivation of the HPA axis is tightly related to inflammation and gut dysbiosis as it will be later discussed. Likewise, an altered HPA axis is frequently related to abnormal biorhythms of cortisol release due to an impaired action of the different circadian clocks (28). Regarding cortisol, the levels of this hormone are high in the morning and diminish in the evening/night. However, chronic stress mismatches the circadian oscillation of cortisol levels, something that could be observed in patients with MDD (20). In turn, the relevance of chronobiological variations in cortisol levels has been demonstrated in different neoplasms and how can they influence the progression of these tumors (29, 30). What is more, patients with cancer exhibit higher plasma cortisol concentrations at 8 AM and 8 PM, and the relative diurnal variation of cortisol was found to be decreased in cancer patients with depression, indicating a disturbed circadian function of the HPA axis, with a sensitivity of 81% and specificity of 88% at a cutoff value of 33.5% (31). Moreover, patients with cancer may present multiple alterations affecting critical genes involved in circadian regulation, especially at advanced stages (32, 33). In this sense, melatonin is a master regulator of circadian rhythms, especially regulating the light–dark cycles in the suprachiasmatic nuclei (SCNs) of the hypothalamus (34). This hormone is crucial for several physiological processes in the brain and the entire organism, and its dysregulation appears to be involved in the onset and development of both MDD and cancer (35, 36). Zaki et al. (37) observed that serum levels of melatonin in 45 women with breast cancer patients correlated significantly with self-reported sleep quality and psychometric profiles of depression. Because of that, there have been some studies showing the promising role of melatonin in reducing the risk of depression in patients with cancer (38). However, to fully understand the effects of chronic stress, HPA dysfunction, and circadian alterations in patients with cancer and MDD, the role of the immune system and gut microbiota as pivotal modulators of several processes needs to be explored.
The immune system and gut microbiota are two major components involved in several physiological processes, being both strongly interconnected (39). Patients with cancer often exhibit an important systemic dysregulation of immune cells and cytokines (40), as well as an abnormal gut microbiota composition (dysbiosis), which in turn has detrimental translational consequences (41). This inflammation can also affect the brain (neuroinflammation), which may represent an adaptive response of the brain to peripheral challenges and tumor growth (42). An exacerbated inflammation is a major feature of MDD, especially in the brain (neuroinflammation), which is associated with different pathophysiological mechanisms implicated in the etiopathogenesis of depression (43). Moreover, patients with MDD present an increase in systemic markers of inflammation, frequently accompanied by gut dysbiosis and an enhanced bacterial translocation (44–46). Likewise, chronic stress has important effects on the immune system and gut microbiota, leading to persistent chronic inflammation and gut dysbiosis, which may be associated with cancer development and progression as well (47).
The gut microbiota affects the brain through several mechanisms through the widely known microbiota–gut–brain (MGB) axis. This axis comprises neurotransmitter/neuropeptide regulation, immunomodulatory effects, and the production of unique microbial metabolites like short-chain fatty acids (SCFAs), thereby regulating the brain in physiological and pathological conditions (48, 49). Interestingly, the immune system, gut microbiota, and their metabolites are essential drivers of tumorigenesis and cancer progression through several mechanisms (41). Gonzalez-Mercado et al. (50) studied the correlation between different bacterial taxa with the onset of depressive symptoms in patients with colorectal cancer after submitting to chemotherapy and radiotherapy. They observed that the relative abundance of Gemella, Bacillales Family XI, Actinomyces, Streptococcus, Lactococcus, Weissella, and Leuconostocaceae were positively correlated with depressive symptoms, whereas Coprobacter, Intestinibacter, Intestinimonas, Lachnospiraceae, Phascolarctobacterium, Ruminiclostridium, Ruminococcaceae, Tyzzerella, and Parasutterella were negatively correlated. In accordance with these results, prior studies have demonstrated that depressed patients with breast cancer exhibit lower gut microbiota diversity, with an augmented relative abundance of Proteobacteria and Escherichia–Shigella than non-depressed patients, with a poor-quality diet essential to understanding these changes (51). Likewise, patients with gastrointestinal cancer and depressive symptoms showed a reduced relative abundance of Gemmiger, Ruminococcus, and Veillonella and a lower alpha diversity in fecal samples (52). Notwithstanding that it is difficult to find out if gut microbiota is a cause or a consequence of the link between cancer and MDD, it seems that oncological patients with MDD may present a distinctive gut microbiota signature in comparison to those without depression. Further studies are needed to unravel biological mechanisms and applications to different types of tumors, as it may represent a promising translational approach for cancer and depressive symptoms (53). In this line, we propose to conduct further studies in the field of microbial metabolites like the abovementioned SCFAs, as these represent a potential opportunity to address not only the MGB axis but also some types of tumors like colorectal cancer (54).
The immune system may also play a key role in the link between MDD and cancer. For instance, there are plenty of proinflammatory cytokines produced peripherally by macrophages and lymphocytes, and centrally by astrocytes and microglia, facilitating the onset of depressive symptoms. In this sense, it is known that excessive proinflammatory cytokine production is related to abnormal monoamine neurotransmitter metabolism, limbic system activity, and HPA dysregulation (55). In accordance with this statement, it is known that immune system–tumor interaction causes the release of proinflammatory cytokines such as IL-6 and IL-18, which numerous studies have shown to be related to depressive symptoms (56, 57). For example, authors such as Jehn et al. have described how IL-6 can cause through the activation of the HPA axis (58). In fact, previous studies have found that depressed patients with cancer display enhanced serum levels of IL-6, which is considered a major biomarker of MDD, with a sensitivity of 79% and a specificity of 87% (31). The presence of other cytokines such as IL-1β in the hypothalamus appears to be related to the cachexia–anorexia syndrome that accompanies numerous tumors (59, 60). TNF-α, C-reactive protein (CRP), and serum soluble interleukin-2 receptor (sIL2r) have also been associated with MDD in patients with cancer, directly modulating the HPA axis (61). Likewise, previous studies have described how there are neuroplastic modifications related to the production of different chemotactic cytokines such as CXC or CX3C that cause alterations in neuronal processes, neuronal plasticity, neurogenesis, and synaptic transmission, which can induce the development of depression (62). Moreover, animal models have demonstrated the relevance of social isolation in the sensitization to the detrimental effects of tumor-derived inflammation and proinflammatory cytokines, which may explain the onset of depressive symptoms like anhedonia in oncological patients (63). In turn, the presence of depression and the abovementioned chronic stress is associated with decreased cytotoxic T-cell and natural killer cell activities, thereby affecting immune surveillance of tumors and favoring the development and accumulation of somatic mutations and genomic instability (64). Thus, tumoral cells appear to present a more aggressive phenotype when it co-occurs with MDD and is thus associated with a poorer prognosis (61). Likewise, sustained inflammation, gut dysbiosis, and chronic stress may lead to a wide variety of systemic endocrine changes, including insulin resistance, sex hormone dysregulation, or an aberrant functioning of the renin–angiotensin–aldosterone system (RAAS) (65). Indeed, these changes represent a critical link between cancer and MDD, particularly in the context of an aberrant immune system and gut dysbiosis. Moreover, cancer therapy may be associated with multiple endocrine changes that in turn may lead to the appearance of MDD (66).
Overall, the effects of gut dysbiosis and immune dysfunction in patients with cancer and MDD are numerous, and there is a tight interplay between these components, along with chronic stress.
The third mechanism involved in the relationship between cancer and MDD is an abnormal neurotransmitter and neuropeptide metabolism/action. In this sense, we must highlight the role of monoamines as pivotal neurotransmitters dysregulated in patients with cancer.
Monoamines, represented by dopamine, norepinephrine, and serotonin (5-HT), are central neurotransmitters that play a central role in the pathophysiology of MDD (67). Despite traditionally that it was considered the major cause of depression—mainly because many antidepressants target monoamines such as the widely known serotonin selective reuptake inhibitors (SSRIs)—nowadays, the role of monoamines in the onset of MDD has been taken more cautiously (68). It is well-known that these neurotransmitters mediate many cognitive and emotional domains, including mood, thoughts, attention, behavior, and sensory experiences (69). Thus, their altered levels of MDD are associated with multiple symptoms. For instance, low levels of 5-HT are linked to behavioral changes and somatic dysfunctions (including depressed mood, appetite, sleep, sex, pain response, body temperature, and circadian rhythm); abnormal dopamine action can lead to anhedonia, impaired motivation and concentration, and aggression; and reduced norepinephrine levels can also contribute to changes in sex, appetite, aggression, concentration, interest, and motivation (70). It seems that tumor-derived or tumor-initiated cytokines are able to dysregulate serotonin synthesis through different mechanisms. For instance, there are some tumors that can regulate the metabolism of 5-HT, increasing the local concentration of some metabolites or 5-HT itself (71). This has two main effects that have been postulated. On the one hand, the increase in 5-HT can stimulate the PI3K-Akt-mTOR pathway and therefore promotes tumor metabolism and thus tumor growth (72). On the other hand, this metabolic switch can drive a decrease in serotonin reserves in the brain, which is related to the appearance of depressive symptoms (71). In a similar manner, there are some tumors that may promote the activation of the enzyme indoleamine 2,3-dioxygenase (IDO), which converts tryptophan, a precursor of 5-HT, into kynurenine (KYN) (20). Importantly, these metabolic switches not only deplete serotonin synthesis but also have important neurotoxic effects on the brain (73). KYN is able to pass the blood–brain barrier (BBB) and is metabolized into quinolinic acid (QA), 3-hydroxykynurenine, and 3-hydroxyanthranilic acid by the microglia, exerting neurotoxic effects or into kynurenic acid (KA) in astrocytes, with neuroprotective actions (74). Patients with MDD appear to present reduced serum levels of KA, although there is no evidence of a decreased detection of this component in the brain, and the precise status of the KYN pathway in the brain must be fully studied (75). What seems clearer is that there is an imbalance between excitotoxic and neuro-protective agents in patients with MDD, and this fact can be also related to enhanced susceptibility to stress, inflammation, and gut dysbiosis (76).
However, other studies have also observed other correlations between cancer and monoamines. For instance, it seems that there is a correlation between dysregulation of the Ras oncogene family and impairment in serotonin and dopamine synthesis, resulting in the onset of MDD (77). Moreover, neuroinflammation and proinflammatory cytokines may impair dopamine synthesis, packaging, and release, correlating with anhedonia, fatigue, and psychomotor retardation (78). For instance, studies in non-human primates have demonstrated a pivotal modulatory role of interferon alpha (IFN-α), leading to a decreased expression of dopamine 2 receptors and reduced striatal dopamine release, possibly due to a conversion of the amino acid phenylalanine to tyrosine, resulting in depressive symptoms (5). Regarding norepinephrine, patients with depressive symptoms and feelings of low social support appeared to have higher tumor norepinephrine levels in ovarian cancer, which is directly correlated with tumor growth and stage (79, 80), thereby showing a biopsychosocial relationship between cancer and MDD. Similar to the previous monoamines, the role of some proinflammatory cytokines like TNF-α and IL-1 due to cancer or after cancer therapy appears to increase 5-HT and norepinephrine reuptake transporters, through activation of the p38 mitogen-activated protein kinase (MAPK), driving the onset of depressive symptoms (5).
Glutamate is another neurotransmitter also related to the development of MDD (81). An aberrant glutamate signaling is associated with neuronal excitotoxicity especially under inflammatory and abnormal glial conditions, as is the case of MDD (82). system is an antiporter that is also potentially involved in the release of glutamate to the extracellular space while importing cystine (83). This system is particularly important in cases of oxidative stress, as the entry of cysteine in the cell is critical in synthesizing the antioxidant glutathione (GSH) (20). Oxidative stress frequently co-occurs with neuroinflammation and cell stress, and it is also a major pathophysiological feature of MDD (84). Likewise, tumoral cells can also be a major source of glutamate, taking part in the development of cancer-induced depression (85). Thus, inhibiting the liberation of glutamate and targeting the system have been proposed as a promising anticancer approach in vivo. Other neurotransmitters such as GABA, with opposite effects to glutamate and acetylcholine, along with certain neuropeptides like neuropeptide Y, neurotensin, and oxytocin, can also play a pivotal role in the relationship between cancer and depression, having a tight relationship with chronic stress (86). The last one is oxytocin, which is altered in patients with MDD and is associated with different prodepressant and antidepressant mechanisms (87). Interestingly, this molecule has proven to exert important antitumoral effects in different types of cancer (88). However, further molecular studies are needed to unravel the biological basis of this link.
The multiple biological links explored in this section are shown in Figure 1.
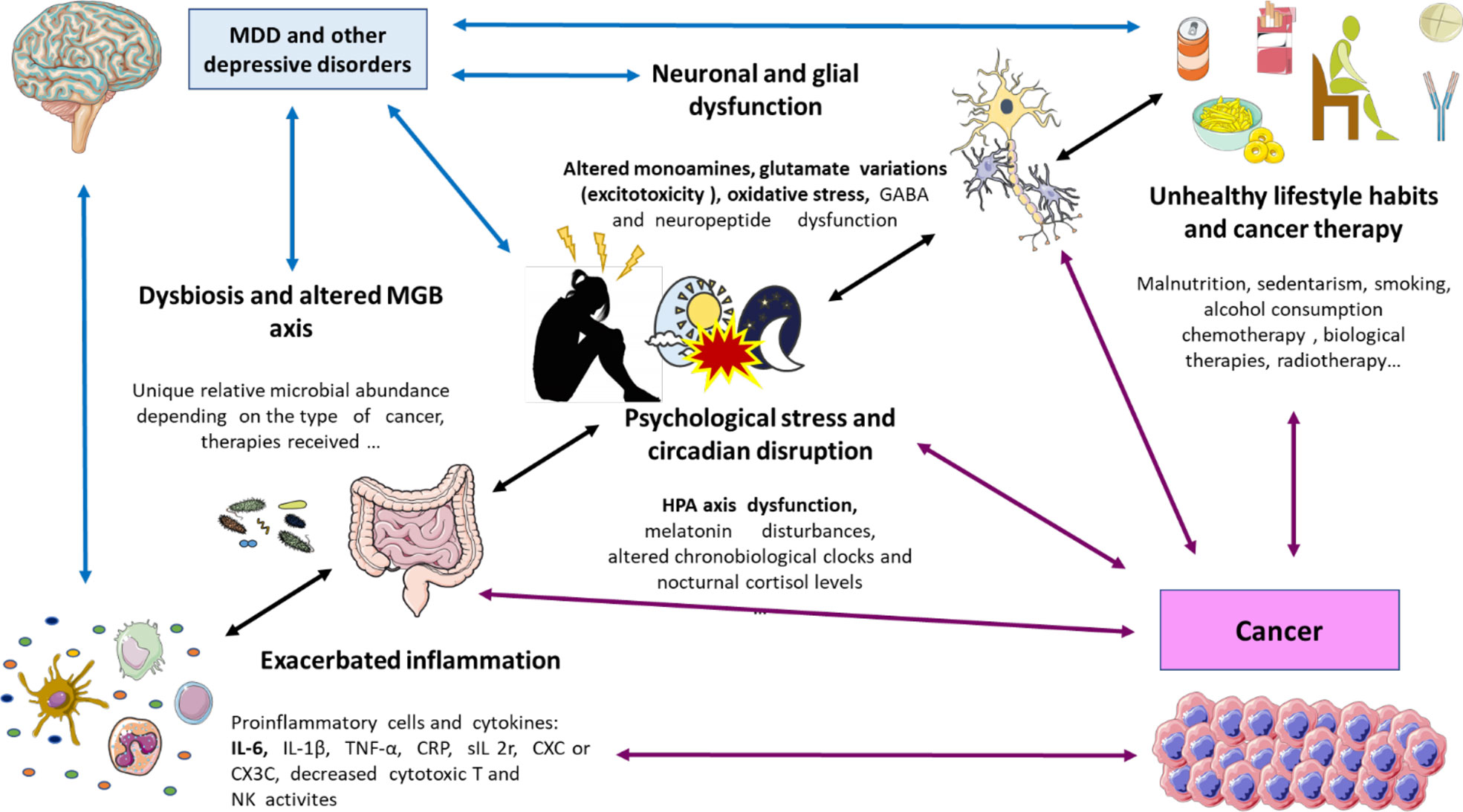
Figure 1 A global perspective on the biological links between cancer and depression. As shown, there are plenty of factors that are interrelated including an exacerbated inflammation (prominently due to augmented proinflammatory cells and cytokines like IL-6); dysbiosis (with different microbial profiles) and disrupted microbiota gut brain (MGB) axis; psychological stress and circadian disruption (prominently through HPA disruption, changes in melatonin, nocturnal cortisol, and circadian clocks); neuronal and glial dysfunction (changes in neuropeptides and neurotransmitters like monoamine metabolism, glutamate, and GABA); unhealthy lifestyle habits like smoking, malnutrition, and sedentarism; and the proper cancer therapy. IL, interleukin; MGB axis, microbiota–gut–brain axis; HPA axis, hypothalamic–pituitary–adrenal axis; GABA, gamma aminobutyric acid.
Clinical diagnosis of depression is primarily based on the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-V), which includes, at least during the period of 2 weeks, the presence of either depressed mood or anhedonia (referred to as the main criteria) and a minimum of four additional somatic or non-somatic symptoms like fatigue, suicidal ideation, weight loss or gain, feelings of worthlessness, and loss of attention (89). However, in patients with cancer, it is difficult to identify some symptoms necessary to assess the presence of depression, such as asthenia, weight loss, or insomnia, as these can be confused with symptoms caused by the tumor or by side effects of chemoimmunotherapy treatment (90). However, we cannot forget that the high workload of oncologists, as well as the difficulty in carrying out prospective studies in which adequate follow-up of the symptoms of depression vs. cancer is carried out, makes it even more difficult to establish adequate epidemiology of the current problem. In turn, patients may present MDD throughout their illness, from the initial study to palliative care, so they should undergo periodic reevaluations, something that is sometimes not available in daily clinical practice (91). Similarly, current evidence suggests that the prevalence of depression differs according to the type of tumor, being more representative in digestive, brain, genital tract, or hematological tumors (92). This leads to a generalized situation in which patients are underdiagnosed and are not being adequately treated from a psycho-oncological point of view. In addition, we must add that the spectrum of depressive disorders is very broad, which makes diagnosis even more difficult. For example, the patients may present mixed symptoms of anxiety/depression, minor depression, anxiety, etc. (93). However, to deal with this situation, standardized questionnaires have been implemented in recent years to diagnose depression in cancer patients. Among them, it must be highlighted the PHQ-15 or the GAD-7, although it is the Patient Health Questionnaire-4 (PHQ-4), in which anxiety and depression are evaluated with four short questions, offering the best results in routine clinical practice given the ease of its application (94–96). These questionnaires aid to establish which patients are eligible for further evaluation by a clinical psychologist or psychiatrist. Since these types of screening tools do not have possible adverse effects, they are beginning to be recommended on a day-to-day basis and allow for an adequate psycho-oncological approach to cancer patients (97). Therefore, patients should ideally be screened at the first oncology consultation, as well as at subsequent visits regardless of whether the patients are in remission, recurrence, or progression. This would aid to assess if their clinical manifestations can be treated with psychotropic drugs or different psychological approaches or if, oppositely, the symptoms are a consequence of tumor progression or the adverse effects of some therapeutic line (98). At the same time, we must emphasize that there are symptoms of depression and cancer that overlap, and we should suspect that patients present symptoms of depression when there is a lack of adherence to treatment, hopelessness, anger, rage, or other manifestations that are not consistent with the biopsychosocial situation of the patients (99). Therefore, the prevalence and diagnosis of depression in cancer patients are one of the current challenges in the multidisciplinary management of those affected by any type of malignant neoplasm and whose correct approach allows for improving the quality of life and prognosis of these patients.
In recent years, the advances conducted in the medical care of depression in patients with cancer have been translating into a substantial improvement in the quality of life of these patients and have become a quality standard in the different oncology units (7, 100). As mentioned earlier, the links between depression and cancer are multiple, including numerous metabolic, neuroendocrine, psychological, or immune pathways, without forgetting that depression is an adverse prognostic factor in itself (101, 102). This may be due to a decrease in adherence to chemotherapy treatments or an increase in suicide rates, among other possible causes (5). However, we cannot forget that it is difficult to analyze how median survival is affected by depression since numerous factors have to be taken into account, such as tumor stage, metastatic invasion, or the patients’ social and family support. Even so, the ultimate goal of early diagnosis of major depression in cancer patients is an improvement in the patients’ quality of life.
One of the main tasks of the doctor is to rule out secondary causes that are causing the depressive symptoms, such as hypercalcemia, hypothyroidism, or secondary effects of biological drugs such as interleukins or chemotherapy agents such as vinblastine or vincristine (103). With respect to the possible approach proposed for these patients, current evidence indicates that a combined therapy that integrates psychological interventions with pharmacological treatment is the most effective mechanism to deal with depression in cancer patients. Normally, this type of intervention is carried out by psycho-oncologists together with psychiatrists who carry out periodic evaluations of patients and assess modifications in their treatment. Numerous scales have also been designed, such as those previously mentioned (PHQ-15, GAD-7, or PHQ-4) in addition to the Beck Depression Inventory or the Hospital Anxiety and Depression Scale, which have been proven useful for depression screening and allow clinicians and psychologists to establish which patients are candidates for more intensive evaluations (104, 105). Regarding the use of pharmacotherapy, antidepressants have been shown to be superior to a placebo in MDD or depressive symptoms in cancer patients with few adverse effects (106). The drugs that are best tolerated with the greatest safety indexes are SSRIs. For example, its use has been studied together with the synchronous administration of chemotherapy without causing relevant adverse effects (107). Likewise, there are numerous drugs that can be used to treat these patients depending on the predominant symptomatology, such as atypical antidepressants (trazodone or mirtazapine) in the event that the main symptom is agitation or insomnia; psychostimulants such as methylphenidate improve attention or fatigue and weakness or benzodiazepines in the management of anxiety and insomnia (108–110). There is also a set of antidepressants that have been studied for their analgesic effect acting as adjuvants in pain management, such as duloxetine or desipramine, so they could be considered in cancer patients with chronic pain (111). However, antidepressants are not without risks since they are known to lower the seizure threshold and in the event that the patients present brain metastases, neoplasms of the central nervous system, or metastatic meningitis, neuroleptics (such as aripiprazole or other second-generation neuroleptics) in the management of depressive symptomatology, so antipsychotics are used in the treatment of depression in those fragile people with cancer who are susceptible more to the side effects (112). Despite this, some experts have indicated that a large proportion of cancer patients are not being optimally treated and are receiving insufficient doses, regimens that are not adapted to the patients’ predominant symptoms, or drugs that have fallen into disuse (113).
Another critical aspect in the multidisciplinary management of depression that is interesting in cancer is its prevention. This allows not only to anticipate the disease but also to deal more properly with this underdiagnosed pathology as well as to conduct a more exhaustive control of these patients in order to improve their quality of life. For this reason, in a meta-analysis carried out by Zahid et al., prophylactic doses of different drugs have been evaluated, as well as different psychotherapeutic interventions, obtaining promising results where the incidence of depression in oncological patients was lower compared to that in the control group that did not receive any of the previously described interventions (114). It should be noted that there are some pivotal limitations in the studies included in that meta-analysis, including a wide variety of possible biases observed, like allocation bias, randomization, or double-blinding. However, as we have previously commented, depression in cancer patients is very difficult to evaluate for different reasons, since the progression and therapeutic response to different chemoimmunotherapy lines generate a complex situation to be able to carry out clinical trials without biases, allowing relevant conclusions to be drawn. In this sense and as it will be subsequently discussed, a growing body of evidence is giving the lifestyle factor a pivotal role to prevent and aid in the clinical management of cancer and depression, as these factors appear to modulate a broad spectrum of biological and psychosocial factors implicated in the etiopathogenesis of both conditions.
Some authors have noticed that the augmented incidence and prevalence of depression and cancer are importantly attributed to modernity, which appears as a consequence of a suboptimal lifestyle in which there is low physical activity, inadequate nutrition and rest, low exposure to sunlight, or psychological and social difficulties, among others (115, 116). Lifestyle medicine is a multidisciplinary field of knowledge defined by the American College of Lifestyle Medicine (ACLM) as “the use of evidence-based lifestyle therapeutic approaches, such as a predominately whole food, plant-based diet, physical activity, sleep, stress management, tobacco cessation, and other nondrug modalities, to prevent, treat, and, oftentimes, reverse the lifestyle-related chronic disease that’s all too prevalent” (117). In this sense, there is compelling evidence that lifestyle interventions are critical strategies for the prevention and management of patients with cancer and MDD (118–121).
In this section, we will review some of the most important lifestyle interventions in the field of cancer and MDD, with a focus on a) diet, b) physical activity, c) sleep, and d) psychological and social interventions. Despite not collecting this information in this section, it is of great relevance to focus on certain routines or lifestyle actions to avoid or limit, such as medication/substance abuse (including alcohol and smoking) or the misuse of modern technologies, which are crucial for achieving a healthy lifestyle (122).
Diet could be considered a multitargeting pill, as it affects our organism at many different physiological and homeostatic levels. In the field of cancer and MDD, diet can modulate epigenetics, the HPA axis, inflammation, oxidative stress, metabolism, gut microbiota, neurotransmitter synthesis, and tryptophan-kynurenine metabolism, among other processes (123, 124).
Malnutrition (deficiency, excess, or imbalance of a wide range of nutrients) is one of the major contributors to a wide range of diseases, with detrimental consequences for the patients, affecting the function and recovery of every organ system (125). For instance, overweight and obesity, frequently related to malnutrition, appear to increase the risk to develop MDD, and in turn, this condition frequently leads to malnutrition, overweight, and obesity (126). Similarly, overweight and obesity contribute to increased mortality due to cancer (127), as well as an augmented risk of suffering from cancer, even in metabolically healthy overweight/obese adults (128). Despite nutritional deficiencies being rare in developed countries, suboptimal consumption of different micronutrients (vitamins and minerals) is frequent and also associated with an increased risk of cancer and MDD ( (129, 130). Moreover, there is a close correlation between malnutrition with psychological stress in cancer patients (131). Hospitalized oncological patients appear to be frequently malnourished, and recent studies have found that these patients are 6.29 times more likely to present depressive symptomatology in comparison to those who are well-nourished (132). Worsening of the nutritional status in patients with advanced stages of cancer appears to exacerbate depressive symptoms (133).
In this context, nutritional intervention can be of great aid in the context of MDD and cancer. There are different potential strategies for this goal. There are nutrients with notable relevance to many physiological processes, with a potential preventive and adjunctive therapeutic action. These are the cases of nutraceuticals, which can be defined as food ingredients and dietary supplements, although there is some controversy regarding their nomenclature (134, 135). Moreover, there are a group of foods with interesting nutritional value that may contain multiple nutraceuticals and exert synergic effects. Unlike dietary supplements, foods contain their own matrix, which can be responsible for these combined benefits (136). In the case of oncological patients, nutritional intervention has proven moderate or limited evidence, despite the nutritional status being associated with poorer survival, decreased treatment completion, and higher healthcare consumption (137, 138). These results can be due to the heterogeneity of available studies and the need for personalized nutrition when considering nutritional intervention. However, the evidence seems to indicate that a healthy dietary pattern diminishes the risk of suffering from colorectal and breast cancers, especially in postmenopausal, hormone receptor-negative women (139). Moreover, high adherence to healthy dietary patterns like the Mediterranean diet (MedDiet) appears to be related to lower odds of MDD and lower risk of cancer mortality in the general population, and all-cause mortality among cancer survivors as well as respiratory, colorectal, gastric, liver, bladder, and head and neck cancer risks (140, 141). Regarding the role of diet as adjunctive therapy, few studies have been conducted. An inverse relationship was observed between a healthy diet, coffee, fish, or dietary zinc and the onset of MDD, and probiotics, omega 3 polyunsaturated fatty acids, and acetyl-carnitine supplementations show moderate-quality evidence for depression treatment (142). In the case of cancer patients and cancer survivors, there is little evidence supporting the use of a specific group of foods to improve the clinical outcome and prevention of recurrence, although high adherence to healthy dietary patterns like MedDiet appears to exert potential benefits in this sense (143, 144). There is insufficient evidence to support the efficacy of a low carbohydrate ketogenic diet, whose efficacy can only be extrapolated in some types of cancer in animal models (145, 146). However, the high consumption of vegetables and fruits, due to their high content of polyphenols, can be a potential strategy for further studies in cancer and depression (147, 148).
In a similar manner to diet, physical activity (PA) also has multiple targets, influencing the proper tumor biology and the brain through the modulation of oxidative stress, inflammation, overweight and obesity, neurotransmission, neurogenesis, cognitive processes, and memory due to the secretion of certain products like endorphins, myokines, and enhancing thermogenesis (149, 150). Conversely, sedentarism is one of the greatest contributors to the development of multiple chronic maladies, including MDD and cancer (151). Indeed, some authors have found a direct correlation between sedentary time with higher scores of depressive symptoms (152). Because of that, the prescription of PA is actually considered a potential preventive and adjunctive support for the management of MDD in patients with cancer.
Different studies have shown how physical activity decreases sarcopenia by improving muscle strength, quality of life, and chronic fatigue in cancer patients (153). Current expert recommendations such as those reported by the American College of Sports Medicine (ACSM) claimed that aerobic training performed three times per week and for at least 12 weeks or twice weekly with combined aerobic plus resistance training can significantly reduce depressive symptoms in cancer survivors during and after treatment, although there are some controversial data regarding the most adequate dose of exercise for depressive patients with cancer (154). A meta-analysis conducted by Brown et al. analyzed the benefits of up to 40 different types of physical exercises (aerobic, relaxation, and muscle hypertrophy) in approximately 3,000 patients with hematological neoplasms, colon, breast, or prostate cancer and from 37 different studies. Interestingly, they obtained a slight improvement in depressive symptomatology in patients with different malignant neoplasms, with a dose-response increase fashion following aerobic exercise. Resistance training alone did not seem to present benefits for these patients, such as those that stimulate the body’s physical endurance capacity in the face of sustained effort, through both aerobic or anaerobic efforts, as well as local (focused) or general (whole body) efforts. Moreover, the maximum benefits were obtained in breast cancer survivors aged between 47 and 62 years and when exercise was supervised by a professional (155). Consistently with these data, Craft et al. (156) found modest positive outcomes from PA to improve depressive symptoms in oncological patients, with the largest benefits when the programs were supervised or partially supervised, not conducted at home, and at least 30 min in duration per session. Another meta-analysis conducted by Patsou et al. (157) concluded that when progressive exercise programs are adapted by cancer survivors according to their individual needs, capabilities, and preferences, they offer a valid alternative to depression mood management. Cancer treatment, especially chemotherapy, appears to have negative effects at various cognitive levels in cancer patients, leading to the onset of depressive symptoms that could be ameliorated by moderate and vigorous levels of PA (158). Conversely, for breast cancer survivors, light-to-moderate but not vigorous PA levels exert the maximum antidepressant benefits (159).
The earlier PA is adopted after a cancer diagnosis, the more benefits it will bring. In this sense, Salam et al. (160) have noticed that statistically significant improvements in levels of depression were identified following the exercise intervention, supporting that post-diagnosis physical activity leads to a decrease in depression scores. In more detail, post-diagnosis exercise as a part of the daily routine led to a 37% reduction in the rate of breast cancer-specific mortality and a decrease of 39% in the all-cause mortality rate. However, it is important to underline that when depressive symptoms present, PA can be experienced as more difficult and demanding, eventually leading to patients engaging in less exercise (161). Thus, other strategies should be considered in conjunction with PA. For instance, group training can be an interesting approach for some patients with cancer, as they may achieve the benefits not only from physical activity but also from social interactions and support (150).
Overall, despite a growing body of evidence supporting the imperative need for PA as an adjunctive and potential lifestyle intervention in patients with cancer and depression, some questions remain to be answered yet. Further studies providing more high-quality evidence for the efficacy of PA in depressed patients with cancer are needed, as well as additional efforts examining the better dose of exercise in this vulnerable population (154).
As explained before, altered circadian rhythms are a major feature of patients with MDD and cancer. In turn, sleep disturbances are importantly related to depression persistence and cancer progression in these patients, thereby representing an important pathophysiological link between both conditions (162). Thus, circadian and sleep-based interventions have offered promising results regarding the prevention and management of both cancer and depression.
In this context, different strategies can be considered here, including pharmacological and non-pharmacological approaches. Melatonin supplementation is the pharmacological intervention most widely studied. Despite some studies that have obtained some positive results from its prophylactic use in the field of MDD and alleviation of depressive symptoms, the effectiveness of this substance appears to be limited (163, 164). In patients with cancer, an intervention with 6 mg of oral melatonin or placebo for 3 months in women with breast cancer after surgery showed that melatonin significantly reduced the risk of depressive symptoms (38). Similarly, 20 mg of melatonin before and during the first cycle of adjuvant chemotherapy for breast cancer exerted neuroprotective actions for these women, improving sleep quality, depressive symptoms, and cognitive functions (165).
However, non-pharmacological interventions are perhaps more adequate strategies to influence sleep and circadian rhythms. A meta-analysis conducted by Gee et al. (166), with a sample of 5,908 patients and 49 clinical trials, concluded that non-pharmacological sleep interventions are effective in reducing the severity of depression, particularly in clinical populations. Different types of non-pharmacological sleep interventions have been studied, including a) relaxation training, b) sleep restriction, c) stimulus control therapy, d) cognitive behavioral therapy (CBT), and e) psychoeducation/sleep hygiene rules (167, 168). Of them, there have been some studies demonstrating the benefits of CBT in the amelioration of depressive symptoms in cancer patients, also improving their overall quality of life (169). In the case of breast cancer patients, the maximum benefits of CBT were observed in younger patients and those suffering from more severe insomnia (170). Similar results were observed in cancer survivors, and importantly, the benefits of CBT were also found even 3 months after the completion of this therapeutic approach (171). Improving sleep hygiene can also have the potential to reduce depressive symptoms in cancer patients, as there is a direct correlation between poor sleep hygiene with fatigue, sleepiness, anxiety, depressive symptoms, and worse insomnia in men with prostate cancer (172). In this sense, light exposure and, especially, sunlight exposure, are a prominent and potential approach for improving sleep quality and ameliorating depression in cancer patients (173, 174). These benefits can be maximized if combined with PA outdoors, including different intensities like light, moderate, and vigorous exercises.
As previously discussed, psychological stress, feelings of loneliness, and social isolation are critical drivers of MDD in patients with cancer, entailing multiple biological mechanisms especially mediated by an altered HPA axis. Because of that, psychosocial interventions are great approaches to preventing and aiding in the clinical management of oncological patients with depression (175). A meta-analysis including 78 studies reported that psychological intervention is of great aid to improve the overall quality of life in patients with cancer, especially when combined with the management of depressive symptoms (176). Patients with mild to moderately severe depression may benefit the most from the combination of antidepressants and psychotherapy, although for patients with severe depression, psychotherapy appears to move into the background (177). In the case of patients with advanced cancer, psychotherapy can be especially useful for patients with depressive symptoms, but not with MDD (178). However, psychological intervention with different approaches such as CBT, mindfulness, and group support therapy has proven to be useful in causing an improvement in depressive symptoms in cancer patients and cancer survivors (179–181). It is also true that there are numerous biases in this type of intervention since it is difficult to analyze the type of intervention that is best suited to each patient or the improvement in quality of life and depressive symptoms in patients whose staging, prognosis, or oncological treatment varies among them (182). In this sense, some studies have reported better results of this therapy when performed individually instead of in groups (179). The integration of pharmacotherapeutic treatment with the psycho-oncological approach has been shown in numerous clinical trials to cause an improvement in the quality of life of these patients. However, there are numerous difficulties in the development of clinical intervention trials that evaluate the effect of the use of different antidepressants and psychotherapy. For example, difficulties have been reported in recruiting patients with cancer and depression in these studies, as well as the refusal by patients or physicians to use a placebo in trials or to establish appropriate control groups in the context of psychotherapy, leading to strong side effects. Placebo in the same (183). In the same way, the approach of the patients’ relatives and caregivers should take on important importance in order to analyze a possible intrafamily burnout syndrome and prevent the appearance of anxiety, depression, or anticipated pathological mourning. Family overload has been studied, and an increased incidence of depression has been observed in male family members of women who have breast cancer (184). The entire psycho-oncological approach not only improves the quality of life of cancer patients but also improves adherence to treatment, improves follow-up after active treatment, and ultimately improves the survival of cancer patients.
However, authors such as Komatsu et al. have described the benefits of implementing self-help recommendations in patients with breast cancer in order to prevent depression and anxiety and improve quality of life, although there are no recommendations in the form of books or pamphlets that have managed to reduce depression in patients undergoing chemotherapy (185). We must emphasize that the preventive management of depression is complex despite the numerous psychopharmacological approaches that have been studied. We have previously indicated how the multifactorial etiopathogenesis of depression makes not only the diagnosis and treatment of this entity difficult, but also prevention remains complex, and numerous studies must be carried out in order to analyze different preventive interventions in cancer patients to improve the quality of life and reduce the incidence of depression and anxiety associated with this entity. In this sense, numerous studies have shown how psychopharmacological drugs in cancer can have effects on cancer patients at different biological levels and can be another tool for the comprehensive management of these patients (186–188).
In this work, we intended to explore the complex and multiple links between two single and quite heterogeneous entities like cancer and MDD. Despite their singularities, they share many pathophysiological mechanisms like an altered HPA axis, circadian disruption, inflammation, gut dysbiosis, and changes in the endocrine and nervous systems, which should be understood as interconnected factors encompassed in the PNIE. Patients with MDD have an increased risk of suffering from cancer, and patients with cancer frequently exhibit depressive symptoms due to the emotional impact of the disease. Moreover, the co-occurrence of cancer and MDD is associated with a worse prognosis for the patients, accelerated cancer development, reduced quality of life, and survival. Thus, the diagnosis, prevention, and therapy of depression in patients with cancer are essential for improving the clinical management of these patients, although it is an issue undoubtedly complex. Notwithstanding some improvements have been done in this area, further translational approaches are required in this population. Lifestyle medicine and implementing healthy lifestyle habits are critical strategies to prevent or diminish the risk of suffering from both MDD and cancer. Moreover, they represent imperative adjunctive support to limit the impact of cancer and depression, boosting the therapy received and limiting its side effects, therefore improving the quality of life of these patients. The most relevant evidence collected about this topic is summarized in Figure 2. Overall, despite there being multiple difficulties in this field and no single formula, it must be individualized and adapted to the patients; the cumulative evidence encourages the numerous benefits of implementing this kind of approach in healthcare systems for depression in patients with cancer.
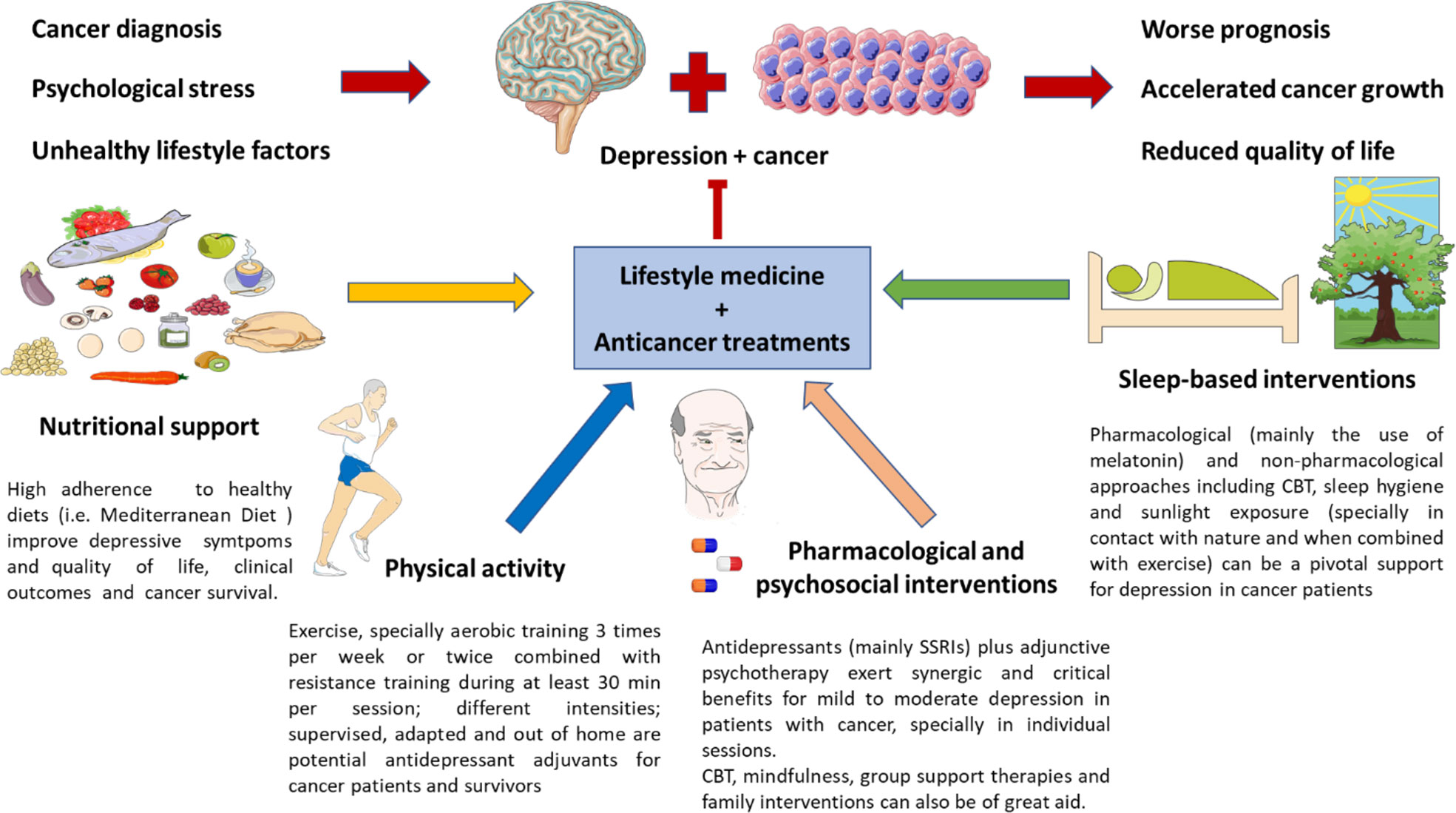
Figure 2 Lifestyle medicine in the clinical management of depression in patients with cancer. As previously mentioned, due to the proper diagnosis of cancer and its emotional impact, psychological stress, and unhealthy habits, patients with cancer can suffer from depressive disorders, which are related to a poorer prognosis, accelerated cancer growth, and a reduced quality of life. Nutritional support, together with encouraging physical activity, psychosocial plus pharmacological adjuvants and sleep-based interventions are critical for preventing and ameliorating depressive symptoms in cancer patients. However, it cannot be denied that some of these measures are not easy to implement in this population. Thus, the main evidence collected up to date in each field is briefly exposed in this picture.
All authors contributed to the article and approved the submitted version.
The study was supported by the Comunidad de Madrid (B2017/BMD-3804 MITIC-CM) and HALEKULANI, S.L. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Friedrich MJ. Depression is the leading cause of disability around the world. JAMA (2017) 317:1517–7. doi: 10.1001/JAMA.2017.3826
2. Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: Priorities for prevention. Carcinogenesis (2010) 31:100–10. doi: 10.1093/CARCIN/BGP263
3. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res (2014) 74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155
4. Yang X, Wu X, Gao M, Wang W, Quan L, Zhou X. Heterogeneous patterns of posttraumatic stress symptoms and depression in cancer patients. J Affect Disord (2020) 273:203–9. doi: 10.1016/J.JAD.2020.04.033
5. Smith HR. Depression in cancer patients: Pathogenesis, implications and treatment (Review). Oncol Lett (2015) 9:1509. doi: 10.3892/OL.2015.2944
6. Hartung TJ, Brähler E, Faller H, Härter M, Hinz A, Johansen C, et al. The risk of being depressed is significantly higher in cancer patients than in the general population: Prevalence and severity of depressive symptoms across major cancer types. Eur J Cancer (2017) 72:46–53. doi: 10.1016/J.EJCA.2016.11.017
7. Walker J, Holm Hansen C, Martin P, Sawhney A, Thekkumpurath P, Beale C, et al. Prevalence of depression in adults with cancer: A systematic review. Ann Oncol (2013) 24:895–900. doi: 10.1093/ANNONC/MDS575
8. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell (2011) 144:646–74. doi: 10.1016/J.CELL.2011.02.013
9. Holly JMP, Biernacka K, Perks CM. Systemic metabolism, its regulators, and cancer: Past mistakes and future potential. Front Endocrinol (2019) 10:65/BIBTEX. doi: 10.3389/FENDO.2019.00065/BIBTEX
10. Alečković M, McAllister SS, Polyak K. Metastasis as a systemic disease: Molecular insights and clinical implications. Biochim Biophys Acta Rev Cancer (2019) 1872:89–102. doi: 10.1016/J.BBCAN.2019.06.002
11. Hatta MNA, Mohamad Hanif EA, Chin SF, Neoh HM. Pathogens and carcinogenesis: A review. Biol (Basel) (2021) 10:533. doi: 10.3390/BIOLOGY10060533
12. Mucci LA, Hjelmborg JB, Harris JR, Czene K, Havelick DJ, Scheike T, et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA (2016) 315:68–76. doi: 10.1001/JAMA.2015.17703
13. Blackadar CB. Historical review of the causes of cancer. World J Clin Oncol (2016) 7:54–86. doi: 10.5306/WJCO.V7.I1.54
14. Bains N, Abdijadid S. Major depressive disorder. Major Depressive Disord (2021), 1–189. doi: 10.1016/C2017-0-01421-0
15. Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Primers (2016) 2:16065. doi: 10.1038/nrdp.2016.65
16. Mullins N, Lewis CM. Genetics of depression: Progress at last. Curr Psychiatry Rep (2017) 19:43. doi: 10.1007/s11920-017-0803-9
17. Furihata R, Konno C, Suzuki M, Takahashi S, Kaneita Y, Ohida T, et al. Unhealthy lifestyle factors and depressive symptoms: A Japanese general adult population survey. J Affect Disord (2018) 234:156–61. doi: 10.1016/J.JAD.2018.02.093
18. Yang L, Zhao Y, Wang Y, Liu L, Zhang X, Li B, et al. The effects of psychological stress on depression. Curr Neuropharmacol (2015) 13:494–504. doi: 10.2174/1570159X1304150831150507
19. Wang YH, Li JQ, Shi JF, Que JY, Liu JJ, Lappin JM, et al. Depression and anxiety in relation to cancer incidence and mortality: A systematic review and meta-analysis of cohort studies. Mol Psychiatry (2020) 25:1487–99. doi: 10.1038/S41380-019-0595-X
20. Young K, Singh G. Biological mechanisms of cancer-induced depression. Front Psychiatry (2018) 9:299. doi: 10.3389/FPSYT.2018.00299
21. González-Díaz SN, Arias-Cruz A, Elizondo-Villarreal B, Monge-Ortega OP. Psychoneuroimmunoendocrinology: Clinical implications. World Allergy Organ J (2017) 10:973. doi: 10.3390/biology11070973
22. Tafet GE, Nemeroff CB. The links between stress and depression: Psychoneuroendocrinological, genetic, and environmental interactions. J Neuropsychiatry Clin Neurosci (2016) 28:77–88. doi: 10.1176/APPI.NEUROPSYCH.15030053
23. Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-Pituitary-Adrenocortical stress response. Compr Physiol (2016) 6:603. doi: 10.1002/CPHY.C150015
24. Abate M, Citro M, Caputo M, Pisanti S, Martinelli R. Psychological stress and cancer: New evidence of an increasingly strong link. Trans Med @ UniSa (2020) 23:53. doi: 10.37825/2239-9747.1010
25. Lupien SJ, Juster RP, Raymond C, Marin MF. The effects of chronic stress on the human brain: From neurotoxicity, to vulnerability, to opportunity. Front Neuroendocrinol (2018) 49:91–105. doi: 10.1016/J.YFRNE.2018.02.001
26. Oh IJ, Kim KS, Kim YC, Park JY, Yoo KY, Do SH, et al. Altered hypothalamus-Pituitary-Adrenal axis function: A potential underlying biological pathway for multiple concurrent symptoms in patients with advanced lung cancer. Psychosom Med (2019) 81:41–50. doi: 10.1097/PSY.0000000000000648
27. Li H, Marsland AL, Conley YP, Sereika SM, Bender CM. Genes involved in the HPA axis and the symptom cluster of fatigue, depressive symptoms, and anxiety in women with breast cancer during 18 months of adjuvant therapy. Biol Res Nurs (2020) 22:277–86. doi: 10.1177/1099800419899727
28. Nader N, Chrousos GP, Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol Metab (2010) 21:277. doi: 10.1016/J.TEM.2009.12.011
29. Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun (2013) 30 Suppl:S163–70. doi: 10.1016/J.BBI.2012.07.019
30. Zeitzer JM, Nouriani B, Rissling MB, Sledge GW, Kaplan KA, Aasly L, et al. Aberrant nocturnal cortisol and disease progression in women with breast cancer. Breast Cancer Res Treat (2016) 158:43. doi: 10.1007/S10549-016-3864-2
31. Jehn CF, Kuehnhardt D, Bartholomae A, Pfeiffer S, Krebs M, Regierer AC, et al. Biomarkers of depression in cancer patients. Cancer (2006) 107:2723–9. doi: 10.1002/CNCR.22294
32. Lin H-H, Taylor SR, Farkas ME. Circadian alterations increase with progression in a patient-derived cell culture model of breast cancer. Clocks Sleep (2021) 3:598–608. doi: 10.3390/CLOCKSSLEEP3040042
33. Morgan MN, Dvuchbabny S, Martinez C-A, Kerr B, Cistulli PA, Cook KM. The cancer clock is (Not) ticking: Links between circadian rhythms and cancer. Clocks Sleep (2019) 1:435–58. doi: 10.3390/CLOCKSSLEEP1040034
34. Zisapel N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br J Pharmacol (2018) 175:3190. doi: 10.1111/BPH.14116
35. Schernhammer ES, Schulmeister K. Melatonin and cancer risk: Does light at night compromise physiologic cancer protection by lowering serum melatonin levels? Br J Cancer (2004) 90:941. doi: 10.1038/SJ.BJC.6601626
36. Tonon AC, Pilz LK, Markus RP, Hidalgo MP, Elisabetsky E. Melatonin and depression: A translational perspective from animal models to clinical studies. Front Psychiatry (2021) 12:638981. doi: 10.3389/FPSYT.2021.638981
37. Zaki NFW, Sabri YM, Farouk O, Abdelfatah A, Spence DW, Bahammam AS, et al. Depressive symptoms, sleep profiles and serum melatonin levels in a sample of breast cancer patients. Nat Sci Sleep (2020) 12:135–49. doi: 10.2147/NSS.S206768
38. Hansen MV, Andersen LT, Madsen MT, Hageman I, Rasmussen LS, Bokmand S, et al. Effect of melatonin on depressive symptoms and anxiety in patients undergoing breast cancer surgery: A randomized, double-blind, placebo-controlled trial. Breast Cancer Res Treat (2014) 145:683–95. doi: 10.1007/S10549-014-2962-2
39. García-Montero C, Fraile-Martínez O, Gómez-Lahoz AM, Pekarek L, Castellanos AJ, Noguerales-Fraguas F, et al. Nutritional components in Western diet versus Mediterranean diet at the gut microbiota-immune system interplay. implications for health and disease. Nutrients (2021) 13:1–53. doi: 10.3390/nu13020699
40. Roxburgh CSD, McMillan DC. Cancer and systemic inflammation: Treat the tumour and treat the host. Br J Cancer (2014) 110:1409. doi: 10.1038/BJC.2014.90
41. Vimal J, Himal I, Kannan S. Role of microbial dysbiosis in carcinogenesis & cancer therapies. Indian J Med Res (2020) 152:553. doi: 10.4103/IJMR.IJMR_1026_18
42. Molfino A, Gioia G, Fanelli FR, Laviano A. Contribution of neuroinflammation to the pathogenesis of cancer cachexia. Mediators Inflammation (2015) 2015:801685. doi: 10.1155/2015/801685
43. Miller AH, Raison CL. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat Rev Immunol (2016) 16:22. doi: 10.1038/NRI.2015.5
44. Alvarez-Mon MA, Gomez-Lahoz AM, Orozco A, Lahera G, Sosa-Reina MD, Diaz D, et al. Blunted expansion of regulatory T lymphocytes is associated with increased bacterial translocation in patients with major depressive disorder. Front Psychiatry (2021) 11. doi: 10.3389/FPSYT.2020.591962
45. Alvarez-Mon MA, Gomez-Lahoz AM, Orozco A, Lahera G, Diaz D, Ortega MA, et al. Expansion of CD4 T lymphocytes expressing interleukin 17 and tumor necrosis factor in patients with major depressive disorder. J Pers Med (2021) 11:220. doi: 10.3390/JPM11030220
46. Alvarez-Mon MA, Gómez AM, Orozco A, Lahera G, Sosa MD, Diaz D, et al. Abnormal distribution and function of circulating monocytes and enhanced bacterial translocation in major depressive disorder. Front Psychiatry (2019) 10:812. doi: 10.3389/FPSYT.2019.00812
47. Dai S, Mo Y, Wang Y, Xiang B, Liao Q, Zhou M, et al. Chronic stress promotes cancer development. Front Oncol (2020) 10:1492. doi: 10.3389/FONC.2020.01492
48. Westfall S, Caracci F, Estill M, Frolinger T, Shen L, Pasinetti GM. Chronic stress-induced depression and anxiety priming modulated by gut-Brain-Axis immunity. Front Immunol (2021) 12:670500/FULL. doi: 10.3389/FIMMU.2021.670500/FULL
49. Ortega MA, Alvarez-Mon MA, García-Montero C, Fraile-Martinez O, Guijarro LG, Lahera G, et al. Gut microbiota metabolites in major depressive disorder-deep insights into their pathophysiological role and potential translational applications. Metabolites (2022) 12:50. doi: 10.3390/METABO12010050
50. Gonzalez-Mercado VJ, Lim J, Saligan LN, Perez N, Rodriguez C, Bernabe R, et al. Gut microbiota and depressive symptoms at the end of CRT for rectal cancer: A cross-sectional pilot study. Depress Res Treat (2021) 2021:7967552. doi: 10.1155/2021/7967552
51. Maitiniyazi G, Cao X, Chen Y, Zhang R, Liu Y, Li Z, et al. Impact of gut microbiota on the association between diet and depressive symptoms in breast cancer. Nutrients (2022) 14:1186. doi: 10.3390/NU14061186
52. Zhu J, Li M, Shao D, Ma S, Wei W. Altered fecal microbiota signatures in patients with anxiety and depression in the gastrointestinal cancer screening: A case-control study. Front Psychiatry (2021) 12:757139. doi: 10.3389/FPSYT.2021.757139
53. Dehhaghi M, Kazemi Shariat Panahi H, Heng B, Guillemin GJ. The gut microbiota, kynurenine pathway, and immune system interaction in the development of brain cancer. Front Cell Dev Biol (2020) 8:562812. doi: 10.3389/FCELL.2020.562812
54. Hou H, Chen D, Zhang K, Zhang W, Liu T, Wang S, et al. Gut microbiota-derived short-chain fatty acids and colorectal cancer: Ready for clinical translation? Cancer Lett (2022) 526:225–35. doi: 10.1016/J.CANLET.2021.11.027
55. Currier MB, Nemeroff CB. Depression as a risk factor for cancer: From pathophysiological advances to treatment implications. Annu Rev Med (2014) 65:203–21. doi: 10.1146/ANNUREV-MED-061212-171507
56. Botwinick IC, Pursell L, Yu G, Cooper T, Mann JJ, Chabot JAA. Biological basis for depression in pancreatic cancer. HPB (Oxford) (2014) 16:740–3. doi: 10.1111/HPB.12201
57. Jara MDJ, Gautam AS, Peesapati VSR, Sadik M, Khan S. The role of interleukin-6 and inflammatory cytokines in pancreatic cancer-associated depression. Cureus (2020) 12:e9969. doi: 10.7759/CUREUS.9969
58. Jehn CF, Kühnhardt D, Bartholomae A, Pfeiffer S, Schmid P, Possinger K, et al. Association of IL-6, hypothalamus-Pituitary-Adrenal axis function, and depression in patients with cancer. Integr Cancer Therapies (2010) 9:270–5. doi: 10.1177/1534735410370036
59. Bennani-Baiti N, Davis MP. Review article: Cytokines and cancer anorexia cachexia syndrome. Am J Hospice Palliative Med (2008) 25:407–11. doi: 10.1177/1049909108315518
60. van Norren K, Dwarkasing JT, Witkamp RF. The role of hypothalamic inflammation, the hypothalamic-Pituitary-Adrenal axis and serotonin in the cancer anorexia-cachexia syndrome. Curr Opin Clin Nutr Metab Care (2017) 20:396–401. doi: 10.1097/MCO.0000000000000401
61. Aldea M, Craciun L, Tomuleasa C, Crivii C, Crivii C. The role of depression and neuroimmune axis in the prognosis of cancer patients. 19:5–14.
62. Oliveira Miranda D, Anatriello E, Ribeiro Azevedo L, Santos JC, Cordeiro JFC, Peria FM, et al. Fractalkine (C-X3-C motif chemokine ligand 1) as a potential biomarker for depression and anxiety in colorectal cancer patients. BioMed Rep (2017) 7:188–92. doi: 10.3892/BR.2017.937
63. Lamkin DM, Lutgendorf SK, Lubaroff D, Sood AK, Beltz TG, Johnson AK. Cancer induces inflammation and depressive-like behavior in the mouse: Modulation by social housing. Brain Behav Immun (2011) 25:555–64. doi: 10.1016/J.BBI.2010.12.010
64. Reiche EMV, Nunes SOV, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol (2004) 5:617–25. doi: 10.1016/S1470-2045(04)01597-9
65. Straub RH. Interaction of the endocrine system with inflammation: A function of energy and volume regulation. Arthritis Res Ther (2014) 16:203. doi: 10.1186/AR4484
66. Gebauer J, Higham C, Langer T, Denzer C, Brabant G. Long-term endocrine and metabolic consequences of cancer treatment: A systematic review. Endocrine Rev (2019) 40:711–67. doi: 10.1210/ER.2018-00092
67. Hindmarch I. Beyond the monoamine hypothesis: Mechanisms, molecules and methods. Eur Psychiatry (2002) 17 Suppl 3:294–9. doi: 10.1016/S0924-9338(02)00653-3
68. Hirschfeld RMA. History and evolution of the monoamine hypothesis of depression Vol. 61. Physicians Postgraduate Press, Inc. (2000) 61(Suppl 6):4–6.
69. Azizi SA. Monoamines: Dopamine, norepinephrine, and serotonin, beyond modulation, “Switches” that alter the state of target networks. Neuroscientist (2022) 28:121–43. doi: 10.1177/1073858420974336
70. Jesulola E, Micalos P, Baguley IJ. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model - are we there yet? Behav Brain Res (2018) 341:79–90. doi: 10.1016/j.bbr.2017.12.025
71. Jiang SH, Li J, Dong FY, Yang JY, Liu DJ, Yang XM, et al. Increased serotonin signaling contributes to the warburg effect in pancreatic tumor cells under metabolic stress and promotes growth of pancreatic tumors in mice. Gastroenterology (2017) 153:277–291.e19. doi: 10.1053/J.GASTRO.2017.03.008
72. Liang Y, Li H, Gan Y, Tu H. Shedding light on the role of neurotransmitters in the microenvironment of pancreatic cancer. Front Cell Dev Biol (2021) 9:688953. doi: 10.3389/FCELL.2021.688953
73. Savitz J. Role of kynurenine metabolism pathway activation in major depressive disorders. Curr Top Behav Neurosci (2017) 31:249–68. doi: 10.1007/7854_2016_12
74. Schwarcz R, Stone TW. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology (2017) 112:237–47. doi: 10.1016/J.NEUROPHARM.2016.08.003
75. Brown SJ, Huang XF, Newell KA. The kynurenine pathway in major depression: What we know and where to next. Neurosci Biobehav Rev (2021) 127:917–27. doi: 10.1016/J.NEUBIOREV.2021.05.018
76. Barone P. The “Yin” and the “Yang” of the kynurenine pathway: Excitotoxicity and neuroprotection imbalance in stress-induced disorders. Behav Pharmacol (2019) 30:163–86. doi: 10.1097/FBP.0000000000000477
77. Brewer JK. Behavioral genetics of the Depression/Cancer correlation: A look at the ras oncogene family and the “Cerebral diabetes paradigm”. J Mol Neurosci (2008) 35:307–22. doi: 10.1007/S12031-008-9078-2
78. Felger JC. The role of dopamine in inflammation-associated depression: Mechanisms and therapeutic implications. Curr Top Behav Neurosci (2017) 31:199–220. doi: 10.1007/7854_2016_13
79. Lutgendorf SK, DeGeest K, Dahmoush L, Farley D, Penedo F, Bender D, et al. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun (2011) 25:250–5. doi: 10.1016/J.BBI.2010.10.012
80. Lutgendorf SK, DeGeest K, Sung CY, Arevalo JM, Penedo F, Lucci J, et al. Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain Behav Immun (2009) 23:176–83. doi: 10.1016/J.BBI.2008.04.155
81. Moriguchi S, Takamiya A, Noda Y, Horita N, Wada M, Tsugawa S, et al. Glutamatergic neurometabolite levels in major depressive disorder: A systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry (2019) 24:952–64. doi: 10.1038/S41380-018-0252-9
82. Haroon E, Miller AH, Sanacora G. Inflammation, glutamate, and glia: A trio of trouble in mood disorders. Neuropsychopharmacology (2017) 42:193. doi: 10.1038/NPP.2016.199
83. Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, et al. The Cystine/Glutamate antiporter system xc– in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signaling (2013) 18:522. doi: 10.1089/ARS.2011.4391
84. Bhatt S, Nagappa AN, Patil CR. Role of oxidative stress in depression. Drug Discovery Today (2020) 25:1270–6. doi: 10.1016/J.DRUDIS.2020.05.001
85. Nashed MG, Ungard RG, Young K, Zacal NJ, Seidlitz EP, Fazzari J, et al. Behavioural effects of using sulfasalazine to inhibit glutamate released by cancer cells: A novel target for cancer-induced depression. Sci Rep (2017) 7:41382. doi: 10.1038/SREP41382
86. Liu Hm, Ma L, Li C, Cao B, Jiang Y, Han L, et al. The molecular mechanism of chronic stress affecting the occurrence and development of breast cancer and potential drug therapy. Trans Oncol (2022) 15:101281. doi: 10.1016/J.TRANON.2021.101281
87. McQuaid RJ, McInnis OA, Abizaid A, Anisman H. Making room for oxytocin in understanding depression. Neurosci Biobehav Rev (2014) 45:305–22. doi: 10.1016/J.NEUBIOREV.2014.07.005
88. Mankarious A, Dave F, Pados G, Tsolakidis D, Gidron Y, Pang Y, et al. The pro-social neurohormone oxytocin reverses the actions of the stress hormone cortisol in human ovarian carcinoma cells in vitro. Int J Oncol (2016) 48:1805–14. doi: 10.3892/IJO.2016.3410
89. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Arlington, TX, USA: American Psychiatric Association (2013).
90. Pasquini M, Biondi M. Depression in cancer patients: A critical review. Clin Pract Epidemiol Ment Health : CP EMH (2007) 3:2. doi: 10.1186/1745-0179-3-2
91. Khalil A, Faheem M, Fahim A, Innocent H, Mansoor Z, Rizvi S, et al. Prevalence of depression and anxiety amongst cancer patients in a hospital setting: A cross-sectional study. Psychiatry J (2016) 2016:1–6. doi: 10.1155/2016/3964806
92. Walker J, Hansen CH, Martin P, Symeonides S, Ramessur R, Murray G, et al. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: A cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry (2014) 1:343–50. doi: 10.1016/S2215-0366(14)70313-X
93. Zhu L, Ranchor AV, van der Lee M, Garssen B, Sanderman R, Schroevers MJ. Subtypes of depression in cancer patients: An empirically driven approach. Support Care Cancer (2016) 24:1387–96. doi: 10.1007/S00520-015-2919-Y
94. Muñoz-Navarro R, Cano-Vindel A, Ruiz-Rodríguez P, Medrano LA, González-Blanch C, Moriana JA, et al. Modelo jerárquico de diagnóstico y derivación de Los trastornos mentales comunes en centros de atención primaria. una propuesta a partir del ensayo clínico PsicAP. Ansiedad y Estrés (2017) 23:124–9. doi: 10.1016/J.ANYES.2017.10.002
95. Esser P, Hartung TJ, Friedrich M, Johansen C, Wittchen HU, Faller H, et al. The generalized anxiety disorder screener (GAD-7) and the anxiety module of the hospital and depression scale (HADS-a) as screening tools for generalized anxiety disorder among cancer patients. Psychooncology (2018) 27:1509–16. doi: 10.1002/PON.4681
96. Kroenke K, Spitzer RL, Williams JBW. The PHQ-15: Validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med (2002) 64:258–66. doi: 10.1097/00006842-200203000-00008
97. Meijer A, Roseman M, Milette K, Coyne JC, Stefanek ME, Ziegelstein RC, et al. Depression screening and patient outcomes in cancer: A systematic review. PloS One (2011) 6. doi: 10.1371/JOURNAL.PONE.0027181
98. Thekkumpurath P, Walker J, Butcher I, Hodges L, Kleiboer A, O’Connor M, et al. Screening for major depression in cancer outpatients: The diagnostic accuracy of the 9-item patient health questionnaire. Cancer (2011) 117:218–27. doi: 10.1002/CNCR.25514
99. Yusof S, Zakaria FN, Hashim NK, Dasiman R. Depressive symptoms among cancer patients undergoing chemotherapy. Proc - Soc Behav Sci (2016) 234:185–92. doi: 10.1016/J.SBSPRO.2016.10.233
100. Oberst S, van Harten W, Sæter G, de Paoli P, Nagy P, Burrion JB, et al. 100 European core quality standards for cancer care and research centres. Lancet Oncol (2020) 21:1009–11. doi: 10.1016/S1470-2045(20)30318-1/ATTACHMENT/BE7A74EC-8506-4AEB-AEBC-1BC334156850/MMC1.PDF
101. Wang X, Wang N, Zhong L, Wang S, Zheng Y, Yang B, et al. Prognostic value of depression and anxiety on breast cancer recurrence and mortality: A systematic review and meta-analysis of 282,203 patients. Mol Psychiatry (2020) 25:3186–97. doi: 10.1038/S41380-020-00865-6
102. McFarland DC, Saracino RM, Miller AH, Breitbart W, Rosenfeld B, Nelson C. Prognostic implications of depression and inflammation in patients with metastatic lung cancer. Future Oncol (2020) 17:183–96. doi: 10.2217/FON-2020-0632
103. Jia Y, Li F, Liu YF, Zhao JP, Leng MM, Chen L. Depression and cancer risk: A systematic review and meta-analysis. Public Health (2017) 149:138–48. doi: 10.1016/J.PUHE.2017.04.026
104. Wedding U, Koch A, Röhrig B, Pientka L, Sauer H, Höffken K, et al. Requestioning depression in patients with cancer: Contribution of somatic and affective symptoms to beck’s depression inventory. Ann Oncol (2007) 18:1875–81. doi: 10.1093/ANNONC/MDM353
105. Shreders A, Niazi S, Hodge D, Chimato N, Agarwal A, Gustetic E, et al. Universal screening for depression in cancer patients and its impact on management patterns. J Clin Oncol (2016) 34:232–2. doi: 10.1200/JCO.2016.34.26_SUPPL.232
106. Caruso R, GiuliaNanni M, Riba MB, Sabato S, Grassi L. Depressive spectrum disorders in cancer: Diagnostic issues and intervention. a critical review. Curr Psychiatry Rep (2017) 19:33. doi: 10.1007/S11920-017-0785-7
107. Grassi L, Nanni MG, Rodin G, Li M, Caruso R. The use of antidepressants in oncology: A review and practical tips for oncologists. Ann Oncol (2018) 29:101–11. doi: 10.1093/ANNONC/MDX526
108. Howard P, Twycross R, Shuster J, Mihalyo M, Wilcock A. Benzodiazepines. J Pain Symptom Manage (2014) 47:955–64. doi: 10.1016/J.JPAINSYMMAN.2014.03.001
109. Theobald DE, Kirsh KL, Holtsclaw E, Donaghy K, Passik SD. An open-label, crossover trial of mirtazapine (15 and 30 mg) in cancer patients with pain and other distressing symptoms. J Pain Symptom Manage (2002) 23:442–7. doi: 10.1016/S0885-3924(02)00381-0
110. Gong S, Sheng P, Jin H, He H, Qi E, Chen W, et al. Effect of methylphenidate in patients with cancer-related fatigue: A systematic review and meta-analysis. PloS One (2014) 9:e84391. doi: 10.1371/JOURNAL.PONE.0084391
111. Matsuoka H, Iwase S, Miyaji T, Kawaguchi T, Ariyoshi K, Oyamada S, et al. Additive duloxetine for cancer-related neuropathic pain nonresponsive or intolerant to opioid-pregabalin therapy: A randomized controlled trial (JORTC-PAL08). J Pain Symptom Manage (2019) 58:645–53. doi: 10.1016/J.JPAINSYMMAN.2019.06.020
112. Davis MP, Khawam E, Pozuelo L, Lagman R. Management of symptoms associated with advanced cancer: Olanzapine and mirtazapine. a world health organization project. Expert Rev Anticancer Ther (2002) 2:365–76. doi: 10.1586/14737140.2.4.365
113. Pilevarzadeh M, Amirshahi M, Afsargharehbagh R, Rafiemanesh H, Hashemi SM, Balouchi A. Global prevalence of depression among breast cancer patients: A systematic review and meta-analysis. Breast Cancer Res Treat (2019) 176:519–33. doi: 10.1007/S10549-019-05271-3
114. Zahid JA, Grummedal O, Madsen MT, Gögenur I. Prevention of depression in patients with cancer: A systematic review and meta-analysis of randomized controlled trials. J Psychiatr Res (2020) 120:113–23. doi: 10.1016/J.JPSYCHIRES.2019.10.009
115. Hidaka BH. Depression as a disease of modernity: Explanations for increasing prevalence. J Affect Disord (2012) 140:205–14. doi: 10.1016/J.JAD.2011.12.036
116. Corbett S, Courtiol A, Lummaa V, Moorad J, Stearns S. The transition to modernity and chronic disease: Mismatch and natural selection. Nat Rev Genet (2018) 19:419–30. doi: 10.1038/S41576-018-0012-3
117. Guthrie GE. What is lifestyle medicine? Am J Lifestyle Med (2018) 12:363. doi: 10.1177/1559827618759992
118. Kelly DL, Yang GS, Starkweather AR, Siangphoe U, Alexander-Delpech P, Lyon DE. Relationships among fatigue, anxiety, depression, and pain and health-promoting lifestyle behaviors in women with early-stage breast cancer. Cancer Nurs (2020) 43:134–46. doi: 10.1097/NCC.0000000000000676
119. Rippe JM. Lifestyle medicine: The health promoting power of daily habits and practices. Am J Lifestyle Med (2018) 12:499–512. doi: 10.1177/1559827618785554
120. Rivers D. Lifestyle interventions for cancer survivors. Nat Rev Cancer (2021) 22:130–0. doi: 10.1038/s41568-021-00434-1
121. Hwang ES, Nho J-H. Lifestyle intervention for breast cancer women. J Lifestyle Med (2019) 9:12. doi: 10.15280/JLM.2019.9.1.12
123. Sapienza C, Issa JP. Diet, nutrition, and cancer epigenetics. Annu Rev Nutr (2016) 36:665–81. doi: 10.1146/ANNUREV-NUTR-121415-112634
124. Marx W, Lane M, Hockey M, Aslam H, Berk M, Walder K, et al. Diet and depression: Exploring the biological mechanisms of action. Mol Psychiatry (2020) 26:134–50. doi: 10.1038/s41380-020-00925-x
125. Saunders J, Smith T. Malnutrition: Causes and consequences. Clin Med (2010) 10:624. doi: 10.7861/CLINMEDICINE.10-6-624
126. Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, et al. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry (2010) 67:220–9. doi: 10.1001/ARCHGENPSYCHIATRY.2010.2
127. Krupa-Kotara K, Dakowska D. Impact of obesity on risk of cancer. Cent Eur J Public Health (2021) 29:38–44. doi: 10.21101/CEJPH.A5913
128. Lin CJ, Chang YC, Hsu HY, Tsai MC, Hsu LY, Hwang LC, et al. Metabolically healthy Overweight/Obesity and cancer risk: A representative cohort study in Taiwan. Obes Res Clin Pract (2021) 15:564–9. doi: 10.1016/J.ORCP.2021.10.004
129. Alvarez-Mon MA, Ortega MA, García-Montero C, Fraile-Martinez O, Monserrat J, Lahera G, et al. Exploring the role of nutraceuticals in major depressive disorder (MDD): Rationale, state of the art and future prospects. Pharm (Basel) (2021) 14:821. doi: 10.3390/PH14080821
130. Ames BN, Wakimoto P. Are vitamin and mineral deficiencies a major cancer risk? Nat Rev Cancer (2002) 2:694–704. doi: 10.1038/nrc886
131. Zhu C, Wang B, Gao Y, Ma X. Prevalence and relationship of malnutrition and distress in patients with cancer using questionnaires. BMC Cancer (2018) 18:1272. doi: 10.1186/S12885-018-5176-X
132. Sánchez-Torralvo FJ, Contreras-Bolívar V, Ruiz-Vico M, Abuín-Fernández J, González-Almendros I, Barrios M, et al. Relationship between malnutrition and the presence of symptoms of anxiety and depression in hospitalized cancer patients. Support Care Cancer (2022) 30:1607–13. doi: 10.1007/S00520-021-06532-Y
133. Yildirim D. Relationship between the depression levels and nutritional statuses of advanced stage cancer patients. Palliat Support Care (2021). doi: 10.1017/S1478951521001516
134. Nasri H, Baradaran A, Shirzad H, Kopaei MR. New concepts in nutraceuticals as alternative for pharmaceuticals. Int J Prev Med (2014) 5:1487.
135. Aronson JK. Defining ‘Nutraceuticals’: Neither nutritious nor pharmaceutical. Br J Clin Pharmacol (2017) 83:8. doi: 10.1111/BCP.12935
136. Aguilera JM. The food matrix: Implications in processing, nutrition and health. Crit Rev Food Sci Nutr (2019) 59:3612–29. doi: 10.1080/10408398.2018.1502743
137. Hamaker ME, Oosterlaan F, van Huis LH, Thielen N, Vondeling A, van den Bos F. Nutritional status and interventions for patients with cancer – a systematic review. J Geriatric Oncol (2021) 12:6–21. doi: 10.1016/J.JGO.2020.06.020
138. Blackwood HA, Hall CC, Balstad TR, Solheim TS, Fallon M, Haraldsdottir E, et al. Systematic review examining nutrition support interventions in patients with incurable cancer. Support Care Cancer (2020) 28:1877–89. doi: 10.1007/S00520-019-04999-4/FIGURES/1
139. Grosso G, Bella F, Godos J, Sciacca S, del Rio D, Ray S, et al. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr Rev (2017) 75:405–19. doi: 10.1093/NUTRIT/NUX012
140. Shafiei F, Salari-Moghaddam A, Larijani B, Esmaillzadeh A. Adherence to the Mediterranean diet and risk of depression: A systematic review and updated meta-analysis of observational studies. Nutr Rev (2019) 77:230–9. doi: 10.1093/NUTRIT/NUY070
141. Morze J, Danielewicz A, Przybyłowicz K, Zeng H, Hoffmann G, Schwingshackl L. An updated systematic review and meta-analysis on adherence to Mediterranean diet and risk of cancer. Eur J Nutr (2021) 60:1561–86. doi: 10.1007/S00394-020-02346-6
142. Xu Y, Zeng L, Zou K, Shan S, Wang X, Xiong J, et al. Role of dietary factors in the prevention and treatment for depression: An umbrella review of meta-analyses of prospective studies. Trans Psychiatry (2021) 11:1–13. doi: 10.1038/s41398-021-01590-6
143. Zaragoza-Martí A, García EC. [Influence of food or food groups intake on the occurrence and/or protection of different types of cancer: Systematic review]. Nutricion hospitalaria (2020) 37:169–92. doi: 10.20960/NH.02588
144. Jochems SHJ, van Osch FHM, Bryan RT, Wesselius A, van Schooten FJ, Cheng KK, et al. Impact of dietary patterns and the main food groups on mortality and recurrence in cancer survivors: A systematic review of current epidemiological literature. BMJ Open (2018) 8:e014530. doi: 10.1136/BMJOPEN-2016-014530
145. Yang YF, Mattamel PB, Joseph T, Huang J, Chen Q, Akinwunmi BO, et al. Efficacy of low-carbohydrate ketogenic diet as an adjuvant cancer therapy: A systematic review and meta-analysis of randomized controlled trials. Nutrients (2021) 13:1388. doi: 10.3390/NU13051388
146. Li J, Zhang H, Dai Z. Cancer treatment with the ketogenic diet: A systematic review and meta-analysis of animal studies. Front Nutr (2021) 8:594408. doi: 10.3389/FNUT.2021.594408
147. Sharma A, Kaur M, Katnoria JK, Nagpal AK. Polyphenols in food: Cancer prevention and apoptosis induction. Curr Med Chem (2018) 25:4740–57. doi: 10.2174/0929867324666171006144208
148. Bayes J, Schloss J, Sibbritt D. Effects of polyphenols in a Mediterranean diet on symptoms of depression: A systematic literature review. Adv Nutr (2020) 11:602. doi: 10.1093/ADVANCES/NMZ117
149. Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Primary Care Companion to J Clin Psychiatry (2004) 6:104. doi: 10.4088/PCC.V06N0301
150. Ortega MA, Fraile-Martínez O, García-Montero C, Pekarek L, Guijarro LG, Castellanos AJ, et al. Physical activity as an imperative support in breast cancer management. Cancers (Basel) (2021) 13:1–30.
151. González K, Fuentes J, Márquez JL. Physical inactivity, sedentary behavior and chronic diseases. Korean J Family Med (2017) 38:111. doi: 10.4082/KJFM.2017.38.3.111
152. Doré I, Plante A, Peck SS, Bedrossian N, Sabiston CM. Physical activity and sedentary time: Associations with fatigue, pain, and depressive symptoms over 4 years post-treatment among breast cancer survivors. Support Care Cancer (2022) 30:785–92. doi: 10.1007/S00520-021-06469-2
153. Rajarajeswaran P, Vishnupriya R. Exercise in cancer. Indian J Med Paediatr Oncol (2009) 30:61–70. doi: 10.4103/0971-5851.60050
154. Marconcin P, Marques A, Ferrari G, Gouveia ÉR, Peralta M, Ihle A. Impact of exercise training on depressive symptoms in cancer patients: A critical analysis. Biol (Basel) (2022) 11:614. doi: 10.3390/BIOLOGY11040614
155. Brown JC, Huedo-Medina TB, Pescatello LS, Ryan SM, Pescatello SM, Moker E, et al. The efficacy of exercise in reducing depressive symptoms among cancer survivors: A meta-analysis. PloS One (2012) 7:e30955. doi: 10.1371/JOURNAL.PONE.0030955
156. Craft LL, VanIterson EH, Helenowski IB, Rademaker AW, Courneya KS. Exercise effects on depressive symptoms in cancer survivors: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev (2012) 21:3–19. doi: 10.1158/1055-9965.EPI-11-0634
157. Patsou ED, Alexias GD, Anagnostopoulos FG, Karamouzis M. V. effects of physical activity on depressive symptoms during breast cancer survivorship: A meta-analysis of randomised control trials. ESMO Open (2017) 2:e000271. doi: 10.1136/ESMOOPEN-2017-000271
158. Bedillion MF, Ansell EB, Thomas GA. Cancer treatment effects on cognition and depression: The moderating role of physical activity. Breast (2019) 44:73–80. doi: 10.1016/J.BREAST.2019.01.004
159. Sylvester BD, Ahmed R, Amireault S, Sabiston CM. Changes in light-, moderate-, and vigorous-intensity physical activity and changes in depressive symptoms in breast cancer survivors: A prospective observational study. Support Care Cancer (2017) 25:3305–12. doi: 10.1007/s00520-017-3745-1
160. Salam A, Woodman A, Chu A, Al-Jamea LH, Islam M, Sagher M, et al. Effect of post-diagnosis exercise on depression symptoms, physical functioning and mortality in breast cancer survivors: A systematic review and meta-analysis of randomized control trials. Cancer Epidemiol (2022) 77:102111. doi: 10.1016/J.CANEP.2022.102111
161. Padin AC, Wilson SJ, Bailey BE, Malarkey WB, Lustberg MB, Farrar WB, et al. Physical activity after breast cancer surgery: Does depression make exercise feel more effortful than it actually is? Int J Behav Med (2019) 26:237–46. doi: 10.1007/S12529-019-09778-3
162. Irwin MR. Depression and insomnia in cancer: Prevalence, risk factors, and effects on cancer outcomes. Curr Psychiatry Rep (2013) 15:614. doi: 10.1007/S11920-013-0404-1
163. Hansen MVbu/JTForAbbrev.xml, Danielsen AK, Hageman I, Rosenberg J, Gögenur I. The therapeutic or prophylactic effect of exogenous melatonin against depression and depressive symptoms: A systematic review and meta-analysis. Eur Neuropsychopharmacol (2014) 24:1719–28. doi: 10.1016/J.EURONEURO.2014.08.008
164. Li C, Ma D, Li M, Wei T, Zhao X, Heng Y, et al. The therapeutic effect of exogenous melatonin on depressive symptoms: A systematic review and meta-analysis. Front Psychiatry (2022) 13:737972/FULL. doi: 10.3389/FPSYT.2022.737972/FULL
165. Palmer ACS, Zortea M, Souza A, Santos V, Biazús JV, Torres ILS, et al. Clinical impact of melatonin on breast cancer patients undergoing chemotherapy; effects on cognition, sleep and depressive symptoms: A randomized, double-blind, placebo-controlled trial. PloS One (2020) 15:e0231379. doi: 10.1371/JOURNAL.PONE.0231379
166. Gee B, Orchard F, Clarke E, Joy A, Clarke T, Reynolds S. The effect of non-pharmacological sleep interventions on depression symptoms: A meta-analysis of randomised controlled trials. Sleep Med Rev (2019) 43:118–28. doi: 10.1016/J.SMRV.2018.09.004
167. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M, Schutte-Rodin SL. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med (2008) 4:487–504. doi: 10.5664/jcsm.27286
168. Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, et al. European Guideline for the diagnosis and treatment of insomnia. J Sleep Res (2017) 26:675–700. doi: 10.1111/JSR.12594
169. Garland SN, Johnson JA, Savard J, Gehrman P, Perlis M, Carlson L, et al. Sleeping well with cancer: A systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat (2014) 10:1113. doi: 10.2147/NDT.S47790
170. Dozeman E, Verdonck-de Leeuw IM, Savard J, van Straten A. Guided web-based intervention for insomnia targeting breast cancer patients: Feasibility and effect. Internet Interv (2017) 9:1–6. doi: 10.1016/J.INVENT.2017.03.005
171. Peoples AR, Garland SN, Pigeon WR, Perlis ML, Wolf JR, Heffner KL, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med (2019) 15:129–37. doi: 10.5664/JCSM.7586
172. Galvin KT, Garland SN, Wibowo E. The relationship between sleep hygiene, mood, and insomnia symptoms in men with prostate cancer. Support Care Cancer (2022) 30:4055–64. doi: 10.1007/S00520-021-06680-1
173. Cui Y, Gong Q, Huang C, Guo F, Li W, Wang Y, et al. The relationship between sunlight exposure duration and depressive symptoms: A cross-sectional study on elderly Chinese women. PloS One (2021) 16:e0254856. doi: 10.1371/JOURNAL.PONE.0254856
174. Sun JL, Wu SC, Chang LI, Chiou JF, Chou PL, Lin CC. The relationship between light exposure and sleep, fatigue, and depression in cancer outpatients: Test of the mediating effect. Cancer Nurs (2014) 37:382–90. doi: 10.1097/NCC.0000000000000106
175. Die Trill M. Psychological aspects of depression in cancer patients: An update. Ann Oncol (2012) 23:x302–5. doi: 10.1093/ANNONC/MDS350
176. de la Torre-Luque A, Gambara H, López E, Cruzado JA. Psychological treatments to improve quality of life in cancer contexts: A meta-analysis. Int J Clin Health Psychol (2016) 16:211–9. doi: 10.1016/J.IJCHP.2015.07.005
177. Naser AY, Hameed AN, Mustafa N, Alwafi H, Dahmash EZ, Alyami HS, et al. Depression and anxiety in patients with cancer: A cross-sectional study. Front Psychol (2021) 12:585534/BIBTEX. doi: 10.3389/FPSYG.2021.585534/BIBTEX
178. Akechi T, Okuyama T, Onishi J, Morita T, Furukawa TA. Psychotherapy for depression among incurable cancer patients. Cochrane Database Syst Rev (2008) 2008. doi: 10.1002/14651858.CD005537.PUB2
179. Osborn RL, Demoncada AC, Feuerstein M. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: Meta-analyses. Int J Psychiatry Med (2006) 36:13–34. doi: 10.2190/EUFN-RV1K-Y3TR-FK0L
180. Yang YL, Sui GY, Liu GC, Huang DS, Wang SM, Wang L. The effects of psychological interventions on depression and anxiety among Chinese adults with cancer: A meta-analysis of randomized controlled studies. BMC Cancer (2014) 14. doi: 10.1186/1471-2407-14-956
181. Piet J, Würtzen H, Zachariae R. The effect of mindfulness-based therapy on symptoms of anxiety and depression in adult cancer patients and survivors: A systematic review and meta-analysis. J Consult Clin Psychol (2012) 80:1007–20. doi: 10.1037/A0028329
182. Zhang X, Liu J, Zhu H, Zhang X, Jiang Y, Zhang J. Effect of psychological intervention on quality of life and psychological outcomes of colorectal cancer patients. Psychiatry (2020) 83:58–69. doi: 10.1080/00332747.2019.1672440
183. Li M, Kennedy EB, Byrne N, Gérin-Lajoie C, Katz MR, Keshavarz H, et al. Management of depression in patients with cancer: A clinical practice guideline. J Oncol Pract (2016) 12:747–56. doi: 10.1200/JOP.2016.011072
184. Clark MM, Atherton PJ, Lapid MI, Rausch SM, Frost MH, Cheville AL, et al. Caregivers of patients with cancer fatigue: A high level of symptom burden. Am J Hosp Palliat Care (2014) 31:121–5. doi: 10.1177/1049909113479153
185. Komatsu H, Hayashi N, Suzuki K, Yagasaki K, Iioka Y, Neumann J, et al. Guided self-help for prevention of depression and anxiety in women with breast cancer. ISRN Nurs (2012) 2012:1–8. doi: 10.5402/2012/716367
186. Busby J, Mills K, Zhang SD, Liberante FG, Cardwell CR. Selective serotonin reuptake inhibitor use and breast cancer survival: a population-based cohort study. Breast Cancer Res (2018) 20(1):4. doi: 10.1186/s13058-017-0928-0
187. Stapel B, Melzer C, von der Ohe J, Hillemanns P, Bleich S, Kahl KG, et al. Effect of SSRI exposure on the proliferation rate and glucose uptake in breast and ovary cancer cell lines. Sci Rep (2021) 11(1):1250. doi: 10.1038/s41598-020-80850-9
Keywords: cancer, depression, lifestyle medicine, multidisciplinary approaches, clinical challenges, Translational medicine
Citation: Fraile-Martinez O, Alvarez-Mon MA, Garcia-Montero C, Pekarek L, Guijarro LG, Lahera G, Saez MA, Monserrat J, Motogo D, Quintero J, Alvarez-Mon M and Ortega MA (2022) Understanding the basis of major depressive disorder in oncological patients: Biological links, clinical management, challenges, and lifestyle medicine. Front. Oncol. 12:956923. doi: 10.3389/fonc.2022.956923
Received: 30 May 2022; Accepted: 23 August 2022;
Published: 16 September 2022.
Edited by:
Huaidong Cheng, The Second Affiliated Hospital of Anhui Medical University, ChinaCopyright © 2022 Fraile-Martinez, Alvarez-Mon, Garcia-Montero, Pekarek, Guijarro, Lahera, Saez, Monserrat, Motogo, Quintero, Alvarez-Mon and Ortega. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miguel A. Alvarez-Mon, bWFhbHZhcmV6ZGVtb25AaWNsb3VkLmNvbQ==; bWlndWVsLmFuZ2VsLm9ydGVnYTkyQGdtYWlsLmNvbQ==
†These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.