
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 14 October 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.956755
This article is part of the Research Topic Optimizing Outcomes and Addressing Adversities of Immunotherapy in Lung Cancer View all 17 articles
Background: Several randomized studies have shown that the combination of programmed cell death 1 (PD-1) inhibitor and chemotherapy is efficacious as a treatment for advanced non-small-cell lung cancer (NSCLC). However, in the neoadjuvant setting, there is scarce evidence of the effectiveness and safety of the combinations in squamous NSCLC. We conducted a retrospective study to evaluate neoadjuvant PD-1 inhibitor plus chemotherapy in resectable squamous NSCLC.
Methods: Patients from Beijing Chest Hospital, Capital Medical University, between October 2019 and October 2021, treated with PD-1 inhibitors and chemotherapy for resectable squamous NSCLC were retrospectively studied. The primary objectives were to assess the pathological tumor response and safety of neoadjuvant PD-1 inhibitors and chemotherapy.
Results: 63 patients with resectable squamous NSCLC stage IIA-IIIB were included. Two to four cycles of PD-1 inhibitors (37 cases with camrelizumab, 11 cases with toripalimab, 8 cases with tislelizumab, and 7 cases with sintilimab) and chemotherapy were administered prior to surgery. 42 patients (66.7%) achieved a major pathologic response (MPR), including 25 (39.7%) with a pathologic complete response (pCR). Twenty-one patients (33.3%) experienced grade 3 neoadjuvant treatment-related adverse events (TRAEs), and no patient had grade 4 or 5 TRAE.
Conclusion: Neoadjuvant PD-1 inhibitors and chemotherapy are feasible therapies for resectable squamous NSCLC. It was associated with a 66.7% MPR rate, 39.7% pCR rate, and tolerable toxicity.
In non-small-cell lung cancer (NSCLC), squamous NSCLC (sqNSCLC) represents approximately 25% to 30% (1), and it is associated with a shorter survival time than nonsquamous NSCLC (2, 3). Squamous NSCLC has historically been treated almost exclusively with cytotoxic chemotherapy due to the lack of targetable aberrations (4).
Patients with resectable NSCLC at high recurrence risk may benefit from neoadjuvant or adjuvant chemotherapy; however, the 5-year overall survival (OS) gain is only 5% (5, 6). Inhibitors of programmed death receptor 1 (PD-1) and its ligand programmed death‐ligand 1 (PD-L1) are effective in the treatment of advanced squamous and nonsquamous NSCLC (7–11). These PD-1/PD-L1 inhibitors are evaluated in multiple clinical trials, rapidly moving from advanced NSCLC to resectable stages and from palliative to curative strategies.
Single-arm phase 2 studies with immunotherapy agents as monotherapy or in combination have recently shown encouraging outcomes (pathologic complete response, event-free survival, and OS) in the neoadjuvant setting (12–16). CheckMate 816 is a randomized, phase 3, open-label study evaluating nivolumab-plus-chemotherapy versus chemotherapy as neoadjuvant treatment for resectable NSCLC, The CheckMate 816 showed statistically significant improvements in the primary endpoints of event-free survival (EFS, median EFS was 31.6 months in the nivolumab-plus-chemotherapy arm and 20.8 months in the chemotherapy-alone arm; hazard ratio, 0.63; 97.38% CI, 0.43 to 0.91), and the pathologic complete response (pCR, pCR rate was 24% in the nivolumab-plus-chemotherapy arm and 2.2% in the chemotherapy-alone arm, odds ratio, 13.94; 99% CI, 3.49 to 55.75) (17). As a result of CheckMate 816, the FDA approved using nivolumab in combination with platinum-doublet chemotherapy for resectable NSCLC patients in the neoadjuvant setting, but in China, this strategy has not yet been approved.
However, in neoadjuvant therapy for NSCLC, few clinical studies on neoadjuvant treatment are designed for squamous cell carcinoma. Therefore, there is scarce evidence of the effectiveness and safety of the neoadjuvant chemoimmunotherapy in squamous NSCLC, especially in several ones approved in China for first-line treatment in advanced sqNSCLC (18–20). In addition, the pathological response of neoadjuvant chemoimmunotherapy in squamous cell carcinoma is not clear. Therefore, we conducted a retrospective study to evaluate pathological response and safety of neoadjuvant PD-1 inhibitor plus chemotherapy in resectable squamous NSCLC.
Patients from Beijing Chest Hospital, Capital Medical University, treated with PD-1 inhibitors and chemotherapy for resectable sqNSCLC between October 2019 and October 2021, were retrospectively studied. The inclusion criteria were as follows: age 18 years or older, confirmed histological diagnosis of sqNSCLC, Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 2, clinical stage IIA-IIIB before the treatment, and ≥2 neoadjuvant treatment cycles, adequate organ function and undergone surgical resection. The exclusion criteria were as follows: had previous treatment before diagnosis or lacked completed radiological or pathological data. The resectable criteria were followed by defining the resectability status of the National Comprehensive Cancer Network (NCCN) Guidelines for all stage IIA-IIIA cases. In terms of stage IIIB patients, the cases including tumor T3/T4 with single-station non-bulky N2 disease of mediastinal lymph nodes, excluding tumor T3/T4 with multi-station N2 disease or bulky N2 disease, were judged as potentially resectable or marginal resectable. Therefore, only cases that met the resectable criteria were administrated for neoadjuvant chemoimmunotherapy. Finally, 63 patients were included in the study (Figure 1). The study was carried out following the Declaration of Helsinki (as revised in 2013). It was reviewed and approved by the institutional review board (IRB)/ethics committee of Beijing Chest Hospital, Capital Medical University. In the neoadjuvant chemoimmunotherapy for operable NSCLC of this study, all PD-1 inhibitors were given for off-label use. All patients were fully informed and signed informed consent before starting treatment.
The collected clinicopathologic data of the patients included sex, age, smoking history, ECOG PS, PD-L1 expression (22C3 PD-L1 antibody, Dako, Denmark), clinical TNM (cTNM) stage, neoadjuvant treatment regimen, treatment cycle, surgical treatment, radiological and pathological efficacy evaluation, and treatment-related adverse events (TRAEs). In addition, clinical TNM was determined according to the 8th edition of the lung cancer staging system of the American Joint Committee on Cancer (21).
All of the included patients were scheduled to receive surgery within 4-6 weeks after neoadjuvant chemoimmunotherapy that consisted of 2-4 cycles of a conventional platinum-based doublet chemotherapy regimen with PD-1 inhibitor on day 1 of each 21-day cycle. Patients received one of the following PD-1 inhibitors intravenously as neoadjuvant immunotherapy: camrelizumab (200 mg), toripalimab (240 mg), tislelizumab (200 mg), or sintilimab (200 mg).
As per standard institutional procedures, all surgical resections were performed with thoracotomy or video-assisted thoracoscopic surgery.
The primary objectives were to assess the pathological tumor response of neoadjuvant PD-1 inhibitors and chemotherapy. The pathological tumor response endpoints were MPR, defined as ≤10% residual viable tumor cells in the primary tumor and sampled lymph nodes, and pCR, defined as the complete absence of residual viable tumor cells in the primary tumor and sampled lymph nodes (22).
Secondary endpoints were the imaging response and safety profile of the combination.
Contrast-enhanced CT scans were repeated to assess objective imaging response within seven days before surgery. The imaging responses were evaluated for all patients per the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (23), and the therapeutic response was considered as complete response (CR), partial response (PR), stable disease (SD), or progression disease (PD). The safety endpoints included treatment-related adverse events according to the Common Terminology Criteria for Adverse Events (CTCAE, v.5.0).
All statistical analyses were performed with Stata version 17.0 (StataCorp, TX, USA) or GraphPad Prism version 9.0 (GraphPad Software Inc., CA, USA). Frequency tabulation and summary statistics for the patient’s baseline characteristics, surgical outcomes, and safety evaluation provided data distribution characteristics. Continuous variables were expressed as medians with ranges. Categorical variables were expressed as numbers with percentages. The association of baseline characteristics and pathological response were conducted with the Fisher’s exact test. The association between the clinical response and the pathological response was performed with Pearson correlation coefficient analysis. A two-sided p value less than 0.05 was considered statistically significant.
Sixty-three patients with resectable squamous NSCLC stage IIA-IIIB were included (Table 1). Of these patients, eight were females and 55 males aged from 47 to 75 years old (median age of 63 years old). Most patients (73.0%) had stage IIIA to IIIB disease, according to the IASLC eighth edition of the TNM Classification for Lung Cancer. PD-L1 expression before treatment was detected by the PD-L1 IHC 22C3 pharmDx assay. For the 40 patients with available PD-L1 data, 32 patients (50.8%) had a PD-L1 tumor proportion score of 1% or higher.
Two to four cycles of PD-1 inhibitors (37 cases with camrelizumab, 11 cases with toripalimab, 8 cases with tislelizumab, and 7 cases with sintilimab) and chemotherapy were administered prior to surgery (Table 1). The clinical activity of the chemoimmunotherapy neoadjuvant combination was evaluated according to the RECIST v.1.1 criteria. In particular, 43 out of the 63 cases achieved a partial response (PR, 68.3%), while 20 patients presented a stable disease (SD, 31.7%).
All 63 patients received R0 surgical resection. The results for surgical treatment are shown in Table 2. Surgical methods included video-assisted thoracoscopic surgery (VATS) (n=32) and thoracotomy (n=31), including 47 (74.6%) lobectomy, 9 (14.3%) bilobectomy and 7 (11.1%) pneumonectomy. The median days of hospitalization after surgery operations was 10 (range, 1–68), the median operation time was 154 (range, 85–310) minutes, and the median amount of estimated blood loss was 150 mL (50–1100 mL). One patient died within 48 hours of lobectomy. He had a clinical T3N2 primary tumor. Radiographic SD was observed after two cycles of neoadjuvant chemoimmunotherapy, which resulted in a technically challenging resection. The patient developed severe hypoxemia, required ventilator support, and died 48 hours postoperatively.
In total, 42 patients (66.7%) achieved a major pathologic response (MPR), including 25 (39.7%) with a pathologic complete response (pCR) in the primary tumor and sampled lymph nodes. In two patients, the primary tumor disappeared, but the regional lymph node involvement persisted, achieving an MPR in the final overall evaluation.
The waterfall plot shows pathological regression in the resected primary lung tumor after neoadjuvant administration, according to the subgroup of sex, smoking status, clinical TNM stage, PD-L1 expression, PD-1 inhibitor regimen, and RECIST response (Figure 2). There was correlation between the imaging regression and pathological regression (Spearman correlation coefficient = 0.43; P = 0.0004; Figure 3). The MPR was related to the clinical lymph nodal stage (Fisher’s exact test P = 0.009) and clinical TNM stage (Fisher’s exact test P = 0.027). The pCR was only related to the clinical TNM stage (Fisher’s exact test P = 0.047, Table 3). The Sankey diagram shows the degree of relationship between the pathological response of neoadjuvant therapy in different clinical stages (Figure 4).
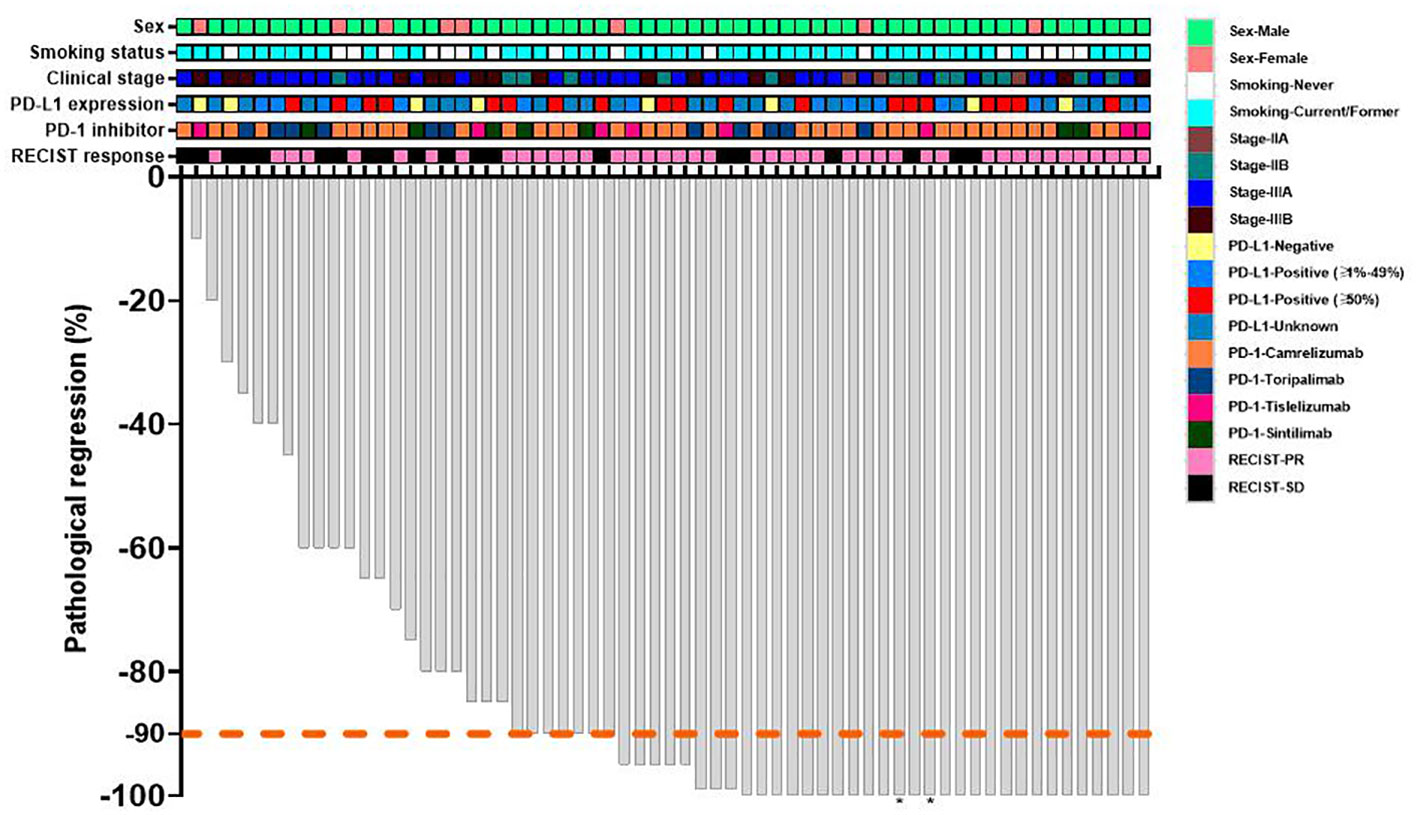
Figure 2 Waterfall plot of pathological response of neoadjuvant therapy. Each bar represents one patient. The upper rows show clinical characteristics and radiological responses. PD-L1, programmed death-ligand 1; PD-1, programmed death 1; RECIST, response evaluation criteria in solid tumors; PR, partial response; SD, stable disease. *The regional lymph node of dissection involvement persisted.
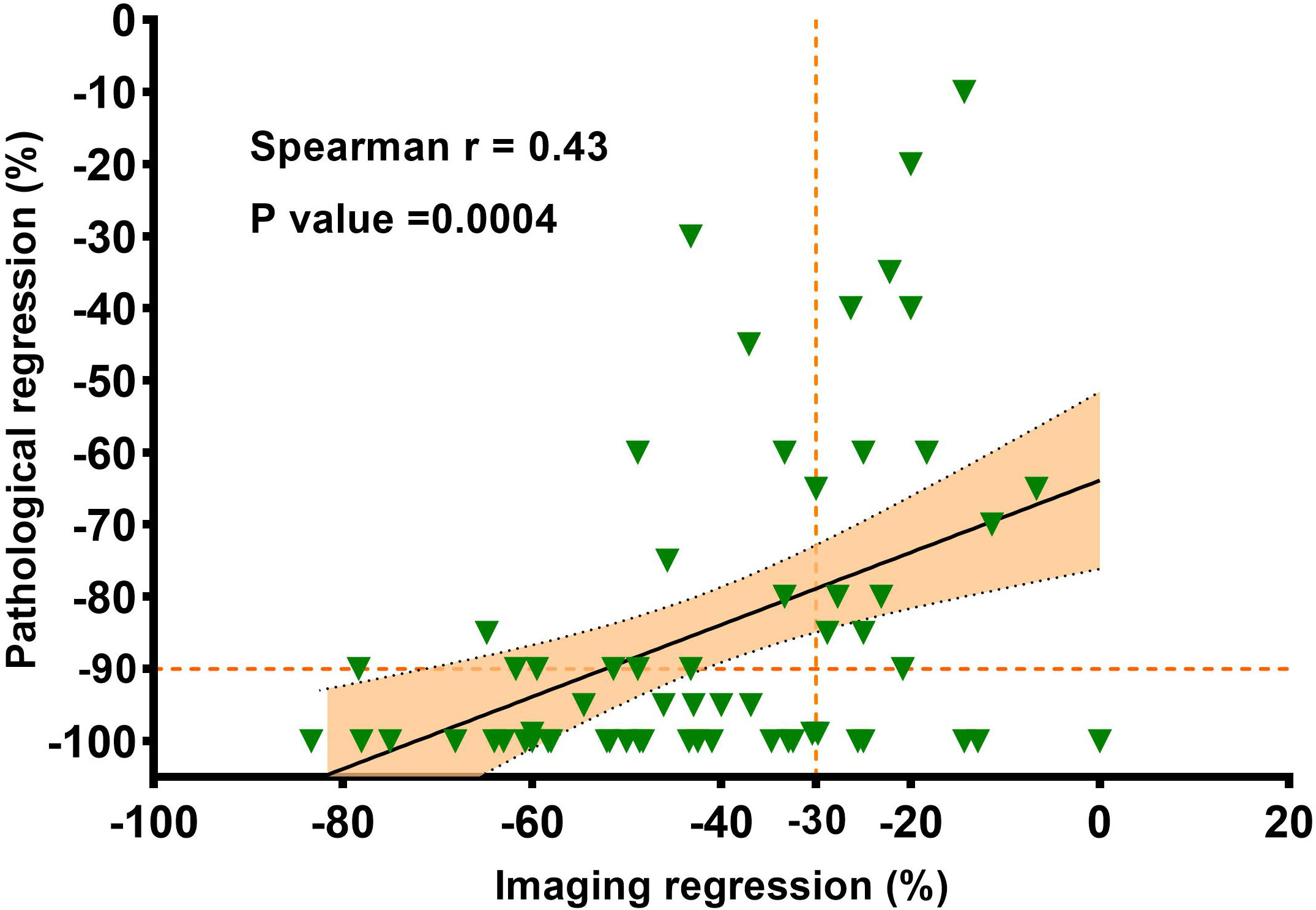
Figure 3 The correlation between the imaging regression and the pathologic regression. Pearson correlation coefficient and two-sided P value are shown.
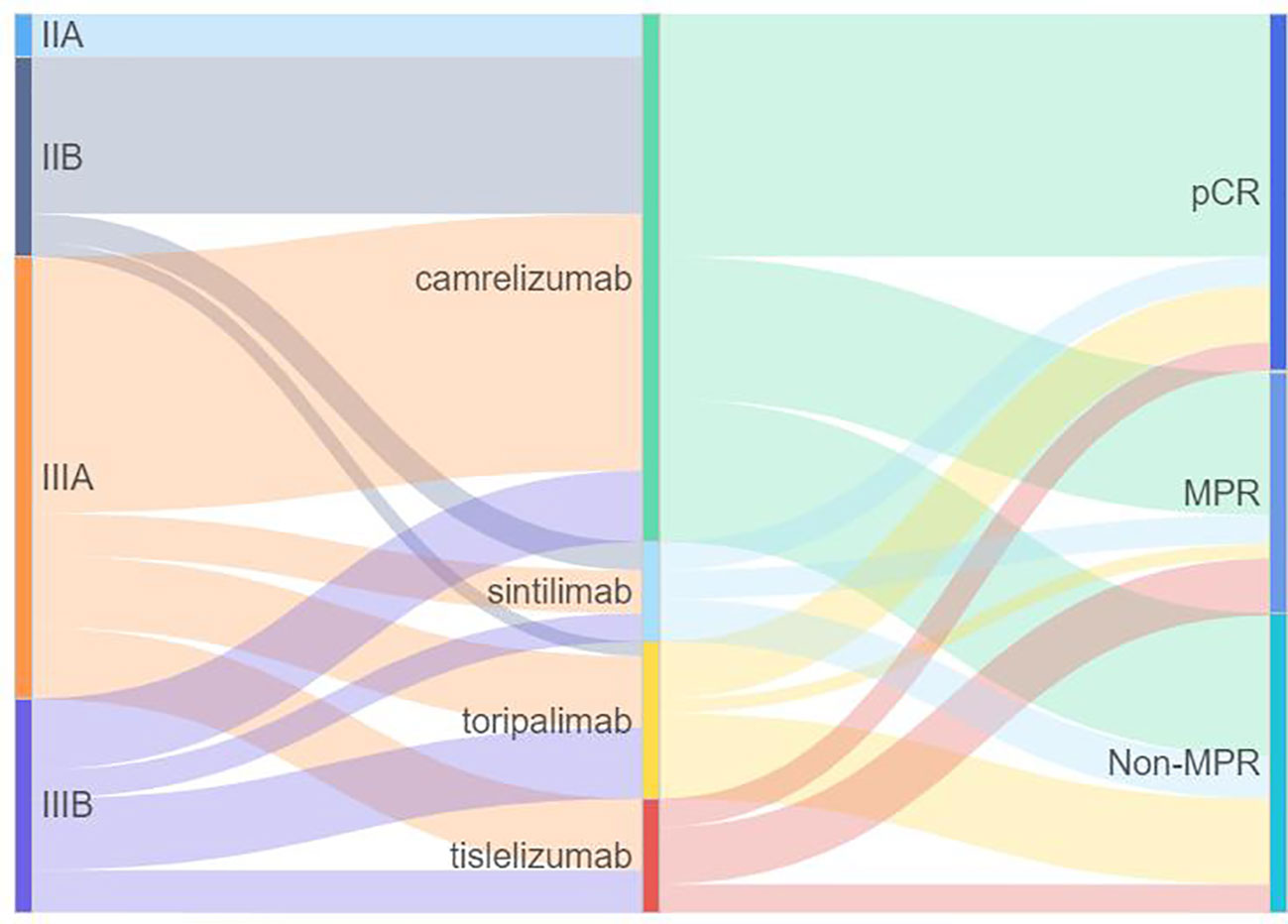
Figure 4 Sankey diagram of the relationship between pathological response of neoadjuvant therapy in different clinical stages. PD-1, programmed death 1; MPR, major pathologic response; pCR, pathologic complete response.
Treatment-related adverse events were reported for 62 (98.4%) patients treated with neoadjuvant PD-1 inhibitors plus chemotherapy. Most of the adverse events were in grades 1-2. Grade 3 treatment-related adverse events occurred in 21 (33.3%) patients, including decreased neutrophil count 11 (17.5%) patients, pneumonia 7 (11.1%), and decreased white blood cell count 5 (7.9%), (Table 4). No grade 4-5 toxicities occurred during the neoadjuvant treatment phase.
Resectable sqNSCLC is usually treated with a combination of surgery, radiation, and systemic chemotherapy. However, it has been proven that immunotherapy is a very effective front-line treatment for advanced sqNSCLC (8, 11, 18–20). Additionally, perioperative immunotherapy has been proven successful in NSCLC (13, 17, 24), but the effect of chemoimmunotherapy in resectable sqNSCLC has rarely been reported. In this study, we retrospectively analyzed 63 squamous NSCLC with stage II-IIIB treated with neoadjuvant chemoimmunotherapy. Our study revealed that PD-1 inhibitors plus chemotherapy were prescribed preoperatively, thus resulting in 66.7% (42/63) of patients achieving an MPR and 39.7% (25/63) cases achieving a pCR. Meanwhile, no unexpected adverse reactions were observed.
NSCLC is classified into squamous cell carcinomas and non-squamous cell carcinomas based on their unique biological behavior, clinical molecular characteristics, and therapeutic responses (25). The study found that compared with adenocarcinoma, the expression of PD-L1 in squamous cell carcinoma is more common, and the infiltration of macrophages and other immune cells is more prominent, which brings an opportunity for the treatment of patients with advanced squamous cell carcinoma, and also leads to the different response of squamous cell carcinoma and nonsquamous cell carcinoma to immunotherapy (26).
Notably, our study only included patients with squamous cell carcinoma. After neoadjuvant immunotherapy combined with chemotherapy, we achieved an excellent pathological response from a numerical point of view. Two-thirds of the patients obtained MPR, and nearly 40% of the cases achieved pCR. Our findings further confirmed the findings of previous small samples of neoadjuvant immunotherapy for lung squamous carcinoma (27, 28).
A major pathological response is more likely to be observed in patients with squamous cell carcinoma (26%) than in those with adenocarcinoma (12%) following neoadjuvant chemotherapy studies, possibly because of greater baseline tumor necrosis in squamous cell carcinomas (29). However, the pCR rates of squamous and nonsquamous NSCLC to neoadjuvant nivolumab plus chemotherapy were similar in the CheckMate 816 study, with 25.3% in squamous and 22.8% in nonsquamous. Therefore, more studies are needed to investigate whether the efficacy of neoadjuvant immunotherapy varies against squamous and nonsquamous NSCLC.
In terms of treatment course before surgery, most previous studies choose 2 to 4 cycles. The neoadjuvant single-agent immunotherapy in CheckMate159 and LCMC3, was performed for two cycles (12, 30). Neoadjuvant immunotherapy combined with chemotherapy in NADIM and CheckMate 816, or a combination of two checkpoint inhibitors in NEOSTAR, was performed for three to four cycles (13, 17, 31). In our study, 41 (65.1%) patients received two cycles of preoperative treatment, 18 (28.6%) patients received three cycles, and only four patients received four cycles of treatment (Table 1). In terms of efficacy (Table 3), further comparing the difference between 2 cycles treatment and 3-4 cycles treatment, we found no statistical correlation (data not shown). In determining the best neoadjuvant treatment course, various factors are taken into account, including efficacy, timing of surgery, and patient compliance. In order to determine the optimal course of treatment, there is a need for more clinical evidence.
Of the 63 patients included in our study, 43 achieved radiological PR, of which 35 (81.4%) achieved pathological MPR or pCR, we found that there was a positive correlation between the imaging regression and pathological regression (Spearman correlation coefficient = 0.43; P = 0.0004; Figure 3). However, 20 patients were evaluated as radiological SD, with seven (35.0%) achieving pathological MPR or pCR. The primary role of immunotherapy promotes the immune cells to infiltrate the tumor and then kill the tumor cells. Patients may benefit from neoadjuvant immunotherapy without initial tumor shrinkage, which is likely to contribute to immune cells infiltrating the tumor (32).
A long-standing method of evaluating neoadjuvant therapy is to examine the pathological changes after surgery. Major pathological response to neoadjuvant treatment is a potential surrogate endpoint for survival (33). Several studies in NSCLC suggest an association between pCR and survival (HR, 0.49; 95% CI, 0.42-0.57) (34). Of note, resectable NSCLC treated with neoadjuvant chemotherapy shows low rates of pCR (median, 4%; range, 0-16%) (33). In CheckMate 816 of neoadjuvant chemoimmunotherapy, the pCR rate was 24% in the nivolumab-plus-chemotherapy arm and 2.2% in the control arm (odds ratio, 13.94; 99% CI, 3.49 to 55.75), the event-free survival appeared to be longer in patients who had a pCR than those who did not (17). Our study found that neoadjuvant PD-1 inhibitors and chemotherapy resulted in a 66.7% MPR rate and a 39.7% pCR rate. Patients who achieved either an MPR or a pCR might benefit long-term survival. In the future follow-up period, this point will be clarified further. For the 40 patients with available PD-L1 data in our study, There was no correlation between the PD-L1 expression of the primary baseline tumor and pathological regression (Spearman correlation coefficient = -0.131; P = 0.42; Supplementary Figure 1).
In advanced NSCLC, PD-L1 expression is a critical marker to guide treatment selection. Among patients with PD-L1 expression ≥ 50%, PD-1 or PD-L1 inhibitor monotherapy can be selected for first-line treatment (9, 35, 36), and patients with high PD-L1 expression may benefit more from the combined immunotherapy (18, 37). However, in a chemoimmunotherapy neoadjuvant setting, PD-L1 expression is not an ideal therapeutic or prognostic marker, and the results differ in different studies. A benefit with nivolumab plus chemotherapy was seen across PD-L1 subgroups in CheckMate 816 study, with a greater event-free survival benefit in patients with a tumor PD-L1 expression level of 1% or more than in those with a level of less than 1% (18, 37). There was a significant difference in PD-L1 tumor proportion score between patients who had a complete pathological response and those who had an incomplete pathological response in the NADIM study (p=0.042) (13), but PD-L1 staining was not predictive of survival (38).
The association of PD-L1 expression in tumor tissues with the efficacy and prognosis of neoadjuvant immunotherapy is unclear and requires continued studies with a larger sample size. Neoadjuvant immunotherapy for NSCLC requires biomarkers that accurately predict efficacy to select people who benefit (13, 38). A single biomarker may be challenging to meet the clinical needs of the published clinical studies. Combining multiple biomarkers is the future trend, and the best biomarkers to predict the efficacy also need to be explored.
The limitations of our study include, but are not limited to, the bias of a retrospective single cohort study, the small number of patients who were included, and the lack of survival follow-up. Therefore, larger randomized control studies are needed to reduce bias and determine the most effective PD-1 blockades of neoadjuvant therapy. Furthermore, long-term follow-up of these studies will be necessary to define the role of neoadjuvant PD-1 blockade in reducing recurrences and curing resectable cancers. In addition, PD-L1 was detected in some but not all patients. At the same time, the ctDNA and tumor mutational burden were not recorded in our study, and adequate biomarker studies are needed to identify the best predictive biomarkers of response and to correlate the pathologic response of neoadjuvant chemoimmunotherapy.
Neoadjuvant PD-1 inhibitors and chemotherapy are feasible therapies for resectable squamous NSCLC. It was associated with a 66.7% MPR rate, 39.7% pCR rate, and tolerable toxicity.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the institutional review board (IRB)/ethics committee of Beijing Chest Hospital, Capital Medical University. All patients were fully informed and signed informed consent before starting treatment.
ZL and LS conceived the study. LS, QM, LT, HL, YD, and CS collected the data. LS, HL, and ZL analyzed the data. LS, QM, LT, HL, YD, CS, and ZL interpreted the data. LS and ZL wrote the first draft of the manuscript. All authors read and contributed to the final version of the manuscript and approved its submission for publication.
This study was supported by Beijing Tongzhou District High Level Talent Project (Grant number: YH201910 to ZL and YH201916 to LS).
We thank patients and their families for their support and participation in this study. Part of this work was presented as a poster at 2021 World Conference on Lung Cancer, worldwide virtual event, September 8-14 2021.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JN declared a shared parent affiliation with the authors to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.956755/full#supplementary-material
1. Wahbah M, Boroumand N, Castro C, El-Zeky F, Eltorky M. Changing trends in the distribution of the histologic types of lung cancer: a review of 4,439 cases. Ann Diagn Pathol (2007) 11(2):89–96. doi: 10.1016/j.anndiagpath.2006.04.006
2. Soldera SV, Leighl NB. Update on the treatment of metastatic squamous non-small cell lung cancer in new era of personalized medicine. Front Oncol (2017) 7:50. doi: 10.3389/fonc.2017.00050
3. Socinski MA, Obasaju C, Gandara D, Hirsch FR, Bonomi P, Bunn PJ, et al. Current and emergent therapy options for advanced squamous cell lung cancer. J Thorac Oncol (2018) 13(2):165–83. doi: 10.1016/j.jtho.2017.11.111
4. Gandara DR, Hammerman PS, Sos ML, Lara PJ, Hirsch FR. Squamous cell lung cancer: from tumor genomics to cancer therapeutics. Clin Cancer Res (2015) 21(10):2236–43. doi: 10.1158/1078-0432.CCR-14-3039
5. Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le Chevalier T, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet (2010) 375(9722):1267–77. doi: 10.1016/S0140-6736(10)60059-1
6. Group NMC. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet (2014) 383(9928):1561–71. doi: 10.1016/S0140-6736(13)62159-5
7. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-Small-Cell lung cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
8. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus chemotherapy for squamous non-Small-Cell lung cancer. N Engl J Med (2018) 379(21):2040–51. doi: 10.1056/NEJMoa1810865
9. Mok T, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (2019) 393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7
10. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(7):924–37. doi: 10.1016/S1470-2045(19)30167-6
11. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodriguez-Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): Results from a randomized phase III trial. J Thorac Oncol (2020) 15(8):1351–60. doi: 10.1016/j.jtho.2020.03.028
12. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med (2018) 378(21):1976–86. doi: 10.1056/NEJMoa1716078
13. Provencio M, Nadal E, Insa A, Garcia-Campelo MR, Casal-Rubio J, Domine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol (2020) 21(11):1413–22. doi: 10.1016/S1470-2045(20)30453-8
14. Gao S, Li N, Gao S, Xue Q, Ying J, Wang S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol (2020) 15(5):816–26. doi: 10.1016/j.jtho.2020.01.017
15. Shu CA, Gainor JF, Awad MM, Chiuzan C, Grigg CM, Pabani A, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol (2020) 21(6):786–95. doi: 10.1016/S1470-2045(20)30140-6
16. Rothschild SI, Zippelius A, Eboulet EI, Savic PS, Betticher D, Bettini A, et al. SAKK 16/14: Durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-Small-Cell lung cancer-a multicenter single-arm phase II trial. J Clin Oncol (2021) 39(26):2872–80. doi: 10.1200/JCO.21.00276
17. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med (2022) 386(21):1973–85. doi: 10.1056/NEJMoa2202170
18. Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-Small-Cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol (2021) 7(5):709–17. doi: 10.1001/jamaoncol.2021.0366
19. Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen G, et al. Sintilimab plus platinum and gemcitabine as first-line treatment for advanced or metastatic squamous NSCLC: Results from a randomized, double-blind, phase 3 trial (ORIENT-12). J Thorac Oncol (2021) 16(9):1501–11. doi: 10.1016/j.jtho.2021.04.011
20. Ren S, Chen J, Xu X, Jiang T, Cheng Y, Chen G, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-sq): A phase 3 trial. J Thorac Oncol (2021) 17(4):544–57. doi: 10.1016/j.jtho.2021.11.018
21. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest (2017) 151(1):193–203. doi: 10.1016/j.chest.2016.10.010
22. Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol (2018) 29(8):1853–60. doi: 10.1093/annonc/mdy218
23. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
24. Felip E, Altorki N, Zhou C, Csoszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet (2021) 398(10308):1344–57. doi: 10.1016/S0140-6736(21)02098-5
25. Thomas A, Liu SV, Subramaniam DS, Giaccone G. Refining the treatment of NSCLC according to histological and molecular subtypes. Nat Rev Clin Oncol (2015) 12(9):511–26. doi: 10.1038/nrclinonc.2015.90
26. Chen Q, Fu YY, Yue QN, Wu Q, Tang Y, Wang WY, et al. Distribution of PD-L1 expression and its relationship with clinicopathological variables: an audit from 1071 cases of surgically resected non-small cell lung cancer. Int J Clin Exp Pathol (2019) 12(3):774–86.
27. Shen D, Wang J, Wu J, Chen S, Li J, Liu J, et al. Neoadjuvant pembrolizumab with chemotherapy for the treatment of stage IIB-IIIB resectable lung squamous cell carcinoma. J Thorac Dis (2021) 13(3):1760–8. doi: 10.21037/jtd-21-103
28. Ling Y, Li N, Li L, Guo C, Wei J, Yuan P, et al. Different pathologic responses to neoadjuvant anti-PD-1 in primary squamous lung cancer and regional lymph nodes. NPJ Precis Oncol (2020) 4(1):32. doi: 10.1038/s41698-020-00135-2
29. Qu Y, Emoto K, Eguchi T, Aly RG, Zheng H, Chaft JE, et al. Pathologic assessment after neoadjuvant chemotherapy for NSCLC: Importance and implications of distinguishing adenocarcinoma from squamous cell carcinoma. J Thorac Oncol (2019) 14(3):482–93. doi: 10.1016/j.jtho.2018.11.017
30. Rusch V, Chaft J, Johnson B, Wistuba I, Kris M, Lee J, et al. MA04.09 neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): Updated results from a multicenter study (LCMC3). J Thorac Oncol (2018) 13(10, Supplement):S369. doi: 10.1016/j.jtho.2018.08.346
31. Cascone T, William WJ, Weissferdt A, Leung CH, Lin HY, Pataer A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med (2021) 27(3):504–14. doi: 10.1038/s41591-020-01224-2
32. Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol (2016) 34(13):1510–7. doi: 10.1200/JCO.2015.64.0391
33. Hellmann MD, Chaft JE, William WJ, Rusch V, Pisters KM, Kalhor N, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol (2014) 15(1):e42–50. doi: 10.1016/S1470-2045(13)70334-6
34. Waser NA, Adam A, Schweikert B, Vo L, McKenna M, Breckenridge M, et al. Pathologic response as early endpoint for survival following neoadjuvant therapy (NEO-AT) in resectable non-small cell lung cancer (rNSCLC): Systematic literature review and meta-analysis. Ann Oncol (2020) 31:S806. doi: 10.1016/j.annonc.2020.08.116
35. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1-Selected patients with NSCLC. N Engl J Med (2020) 383(14):1328–39. doi: 10.1056/NEJMoa1917346
36. Sezer A, Kilickap S, Gumus M, Bondarenko I, Ozguroglu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet (2021) 397(10274):592–604. doi: 10.1016/S0140-6736(21)00228-2
37. Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R, et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol (2022) 23(2):220–33. doi: 10.1016/S1470-2045(21)00650-1
38. Provencio M, Serna-Blasco R, Nadal E, Insa A, Garcia-Campelo MR, Casal RJ, et al. Overall survival and biomarker analysis of neoadjuvant nivolumab plus chemotherapy in operable stage IIIA non-Small-Cell lung cancer (NADIM phase II trial). J Clin Oncol (2022) 40(25):2924–33. doi: 10.1200/JCO.21.02660
Keywords: resectable non-small-cell lung cancer, squamous cell carcinoma, neoadjuvant chemoimmunotherapy, programmed death-1 inhibitors, pathologic response
Citation: Shi L, Meng Q, Tong L, Li H, Dong Y, Su C and Liu Z (2022) Pathologic response and safety to neoadjuvant PD-1 inhibitors and chemotherapy in resectable squamous non-small-cell Lung cancer. Front. Oncol. 12:956755. doi: 10.3389/fonc.2022.956755
Received: 30 May 2022; Accepted: 03 October 2022;
Published: 14 October 2022.
Edited by:
Jun Zhang, University of Kansas Medical Center, United StatesReviewed by:
Jingying Nong, Xuanwu Hospital, Capital Medical University, ChinaCopyright © 2022 Shi, Meng, Tong, Li, Dong, Su and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhe Liu, bHphQHZpcC4xNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.