
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 16 August 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.955440
This article is part of the Research Topic Editor's Challenge: Dr. Luciano Mutti - What Is the True Impact of ICIs on Survival in the Treatment of Thoracic Malignancies? View all 6 articles
 Fang Yang1†*
Fang Yang1†* Yucai Wang2†
Yucai Wang2† Lin Tang3†
Lin Tang3† Aaron Scott Mansfield4
Aaron Scott Mansfield4 Alex A. Adjei4
Alex A. Adjei4 Konstantinos Leventakos4
Konstantinos Leventakos4 Narjust Duma5
Narjust Duma5 Jia Wei1
Jia Wei1 Lifeng Wang1
Lifeng Wang1 Baorui Liu1
Baorui Liu1 Julian R. Molina4*
Julian R. Molina4*Background: Immune checkpoint inhibitors (ICIs) have demonstrated remarkable efficacy in non-small cell lung cancer (NSCLC). However, only a minority of NSCLC patients benefit from ICIs, and whether the magnitude of benefit is specific factor-dependent remains unclear. We performed a systematic review to improve our understanding of clinicopathologic and biomolecular features associated with improved survival upon treatment with ICIs for NSCLC.
Methods: We searched PubMed, Web of Science, Embase, and Scopus from database inception to August 31, 2021, for randomized controlled trials (RCTs) comparing overall survival (OS) in NSCLC treated with ICIs vs control therapies. We calculated the pooled OS hazard ratio (HR) and 95% CI in subgroups using a random-effects model, and assessed the heterogeneity between the paired estimates using an interaction test.
Results: A total of 23 RCTs involving 15,829 patients were included. We found that wild-type EGFR, high PD-L1 expression, and high bTMB were associated with a significant OS benefit from ICIs, but not mutant EGFR, low PD-L1 expression, and low bTMB. The differences of OS benefit between wild-type and mutant EGFR (HR=1.53, 95%CI 1.13-2.08), high and low PD-L1 (HR=1.35; 95%CI 1.14-1.61), high and low bTMB (HR=1.71; 95%CI 1.17-2.52) were statistically significant. OS benefit was found in all subgroups regardless of sex, age, ECOG PS, histology, smoking history, baseline brain metastasis, race, and region, and the interaction test demonstrated no significant difference of the OS benefit between these opposed subgroups (e.g. male vs female).
Conclusions: Wild-type EGFR, high PD-L1 expression, and high bTMB are associated with a greater magnitude of efficacy from ICIs vs control therapies in NSCLC. However, the administration of ICIs should not be restricted to other clinicopathological factors (sex, smoking history, race, etc.).
Immune checkpoint inhibitors (ICIs), including inhibitors of programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), or cytotoxic T-lymphocyte protein 4 (CTLA-4), have dramatically changed the NSCLC treatment landscape over the past decade. Based on results from multiple global clinical trials, ICIs have been one of the standard first-line NSCLC treatments, as monotherapy or combined therapy (1–6). However, only a minority of NSCLC patients benefit from ICIs (7), and whether the magnitude of benefit is specific factor-dependent remains unclear. Given the adverse effects (8) and cost of ICIs, and the lack of access to the drug in low-to-middle income countries, it is crucial to identify subpopulations who can derive a larger relative benefit from immunotherapy.
Anti-PD-1/PD-L1 therapy works by blocking the PD-1/PD-L1 pathway, suggesting that high PD-L1 expression could be a reasonable biomarker predicting the efficacy. However, some PD-L1-negative patients also respond to anti-PD-1/PD-L1 therapy (9). Moreover, currently there is no unified standard for defining PD-L1 positivity. Whether there are differences in the relative benefit from ICIs over control therapies remains unclear when using different positive PD-L1 standards.
Sex, age and Eastern Cooperative Oncology Group (ECOG) performance status (PS) might correlate with immune response, thus affecting the efficacy of ICIs. We have explored the association of these three variables with the relative benefit of ICIs in solid tumors. Stratified analysis showed no difference in the survival advantage of immunotherapy among NSCLC patients grouped by sex, age, and ECOG PS (10). However, other clinicopathological and biomolecular characteristics were not analyzed, and several large clinical trials have published updated results in recent years, warranting an updated analysis.
NSCLC harboring epidermal growth factor receptor (EGFR) mutations or low tumor mutation burden (TMB) showed poor clinical outcomes with immunotherapy (11, 12). Histological type, smoking status and race are known to correlate with NSCLC EGFR mutation rate. NSCLC brain metastases showed an increased TMB and genomic instability in comparison with primary NSCLC (13). Therefore, these clinical features mentioned above may also be associated with therapeutic benefit of ICIs.
Given the lack of data on the above issues, we conducted a comprehensive meta-analysis to examine the potential association of the common clinicopathological and biomolecular features with relative advantages of immunotherapy in NSCLC.
The study was registered with PROSPERO (CRD42019123892), an international prospective register of systematic reviews, and performed following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (14). The need for institutional review board approval was waived by Drum Tower Hospital because this meta-analysis utilized publicly available data.
We performed a systematic literature search using PubMed, Web of Science, Embase, and Scopus for phase 2/3 randomized controlled trials (RCTs) from database inception to August 31, 2021. Two investigators (FY and YW) independently searched the databases. Any disagreement was resolved by discussion and consensus. We performed full-text reviews if abstracts were insufficient for determining if the studies met the inclusion criteria. We set the search criteria to include all the PD-1 inhibitors (nivolumab, pembrolizumab, cemiplimab, toripalimab, sintilimab, camrelizumab, tislelizumab, penpulimab, zimberelimab), PD-L1 inhibitors (durvalumab, atezolizumab, avelumab, sugemalimab), and CTLA-4 inhibitors (ipilimumab, tremelimumab) in NSCLC. The references of the included studies were also reviewed for potential additional publications.
Eligible studies met all of the following requirements (1): RCTs assessing PD-1/PD-L1 or CTLA-4 inhibitors for treatment of NSCLC (2); ICIs as monotherapy or part of combination therapy in the intervention arm, and control therapy without ICIs in the control arm (3); data available for hazard ratio (HR) for overall survival (OS) in subgroups defined by clinicopathological and/or biomolecular characteristics; and (4) published in English. If subgroup data of a study were reported in more than one publication, the most updated or comprehensive data were included in this analysis. Outcome data was extracted, including HR and 95% confidence interval (CI) stratified by sex, age, ECOG PS, histology, smoking status, baseline brain metastases, EGFR mutation, PD-L1 expression, TMB, race, and regions.
We calculated the pooled HRs of death in each of the paired subgroups (e.g., male vs female) using the random-effects models to determine whether any subgroup of patients benefited from ICI vs control therapy.
We calculated a study-specific interaction HR (95%CI) in each study based on the reported HRs (95%CIs) in paired subgroups and then combined the study-specific interaction HRs across trials, using a random-effects model, to generate a P value for heterogeneity (Pheterogeneity) as described previously (10, 15, 16). A Pheterogeneity<0.05 indicated that the magnitude of OS benefit from ICI vs control therapy was different between the paired subgroups (e.g., male vs female). The between-study heterogeneity was assessed by the Q test and quantified by I2 values. I2 value <=25% corresponds to a low heterogeneity (17).
All analyses were conducted using Comprehensive Meta-Analysis, version 2. All reported P values are two-sided, and a P<0.05 was considered statistically significant.
The systematic search yielded 6313 results, of which 112 were reviewed in full. Finally, 23 RTCs involving 15829 patients, published from 2015 to 2021, satisfied our inclusion criteria and were included in the meta-analysis (Figure 1) (2, 3, 5, 6, 18–48). Characteristics of the included trials are summarized in Table 1. Most of the trials were phase 3. We found 15 trials conducted for first line, 8 for subsequent lines, 11 trials with anti-PD-1 inhibitors, 10 trials with anti-PD-L1 inhibitors, 1 trial with anti-CTLA-4 inhibitor, and 2 trials with combined ICIs, 14 trials compared immunotherapy alone to control therapy, and 9 trials compared combination of immunotherapy and chemotherapy to control therapy.
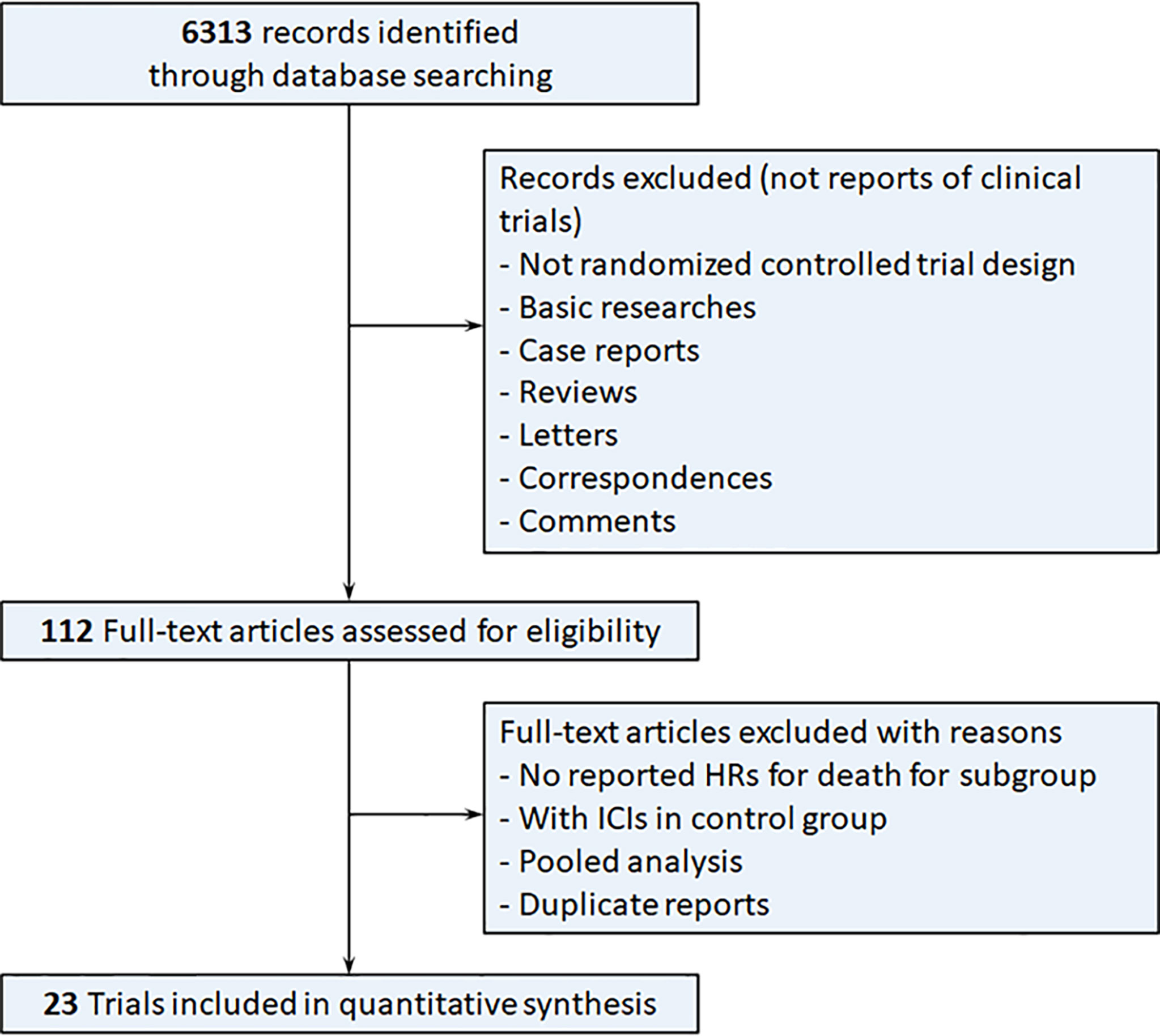
Figure 1 Flow diagram of the study selection process. HRs, hazard ratios; ICIs, immune checkpoint inhibitors.
Firstly, we analyzed the associations between clinicopathological characteristics and the relative OS benefit of immunotherapy over control therapy (Table 2). Patients with NSCLC derived an OS benefit from ICIs regardless of sex, age, ECOG PS, histological type, smoking status, and baseline brain metastasis status, and the magnitude of OS benefit was not significantly different between paired subgroups (all Pheterogeneity>0.05) (Table 2). Patients with wild-type EGFR significantly benefited from immunotherapy (HR=0.71, 95%CI: 0.64-0.78), but patients with mutant EGFR did not (HR=1.08, 95%CI: 0.81-1.44) (Table 2). The interaction test confirmed the magnitude of OS benefit was significantly different between the two groups (Pheterogeneity=0.006) (Table 2).
Secondly, we analyzed the associations of OS benefit from ICIs with PD-L1 expression and bTMB. For PD-L1 expression, different cutoffs of tumor proportion score (TPS), tumor cell (TC), or immune cell (IC) were used in various studies. Patients with TPS/TC>=50% significantly benefited from immunotherapy (HR=0.66, 95%CI: 0.59-0.74), but patients with TPS/TC<50% did not (HR=0.88, 95%CI: 0.74-1.06). The interaction test confirmed the magnitude of OS benefit was significantly different between the two groups (Pheterogeneity=0.001) (Table 3). Patients with TPS/TC>=1% or <1% both benefited from immunotherapy, with no statistical difference between the magnitude of OS benefit (Pheterogeneity=0.521) (Table 3). Patients with TC/IC=1/2/3 significantly benefited from immunotherapy (HR=0.73, 95%CI: 0.64-0.82), but patients with TC/IC=0 did not (HR=0.90, 95%CI: 0.79-1.02). The magnitude of OS benefit was significantly different between the two groups (Pheterogeneity=0.020) (Table 3).
We found that patients with higher bTMB significantly benefited from ICI (>=20mut/Mb: HR=0.59, 95%CI: 0.46-0.76; >=16mut/Mb: HR=0.67, 95%CI: 0.54-0.84), but patients with lower bTMB did not (<20mut/Mb; HR=0.98, 95%CI: 0.83-1.16; <16mut/Mb: HR=1.01, 95%CI: 0.84-1.22) (Table 3). The interaction test confirmed the magnitude of OS benefit was significantly different between high and low bTMB groups (cutoff of 20mut/Mb: Pheterogeneity=0.006; cutoff of 16mut/Mb: Pheterogeneity=0.013) (Table 3).
Additionally, we analyzed the associations of patient race and region with OS benefit from ICIs (Table 4). We found that nearly all patients with NSCLC benefited from ICIs over control therapies regardless of race and region. Black/African American patients were under-represented in the studies, with 45 patients included in 4 studies, accounting for only 1.8%-7.0% of the total population size. The OS benefit they derived from ICIs appeared to be statistically insignificant, likely due to a very small sample size. The interaction test demonstrated that there was no significant difference in the magnitude of OS benefit between paired subgroups (all Pheterogeneity>0.05) (Table 4).
We performed subgroup analyses to investigate whether immunotherapy settings affected the relative OS benefit in patients with different were sex, age, ECOG PS, histological type, and smoking status. Specifically, we analyzed the data according to line of therapy (first line or subsequent lines), immunotherapy agent (PD-1 inhibitor or PD-L1 inhibitor), and immunotherapy intervention (immunotherapy alone or chemo-immunotherapy). We found that regardless of the line of treatment, immunotherapy agent, and immunotherapy intervention, the magnitude of OS benefit from ICIs was similar for male vs female, <65 vs >=65 years, ECOG PS=0 vs ECOG PS>=1, squamous vs nonsquamous, and smokers vs non-smokers (Supplementary Tables 1–5).
Between-study heterogeneity was found for female patients (Q=40.72, P=0.004, I²=50.89%), <65 years (Q=38.03, P=0.009, I²=47.42%), ECOG PS>=1 (Q=36.00, P=0.011, I²=47.22%), nonsquamous (Q=23.16, P=0.017, I²=52.50%), never smokers (Q=29.20, P=0.033, I²=41.78%), former or current smokers (Q=29.94, P=0.027, I²=43.22%), PD-L1 TPS/TC<50% (Q=16.75, P=0.005, I²=70.16%), PD-L1 TPS/TC<1% (Q=17.95, P=0.022, I²=55.44%), and PD-L1 TPS/TC>=1% (Q=22.81, P=0.004, I²=64.92%) (Tables 2, 3).
In this meta-analysis, we found that wild-type EGFR, high PD-L1 expression, and high bTMB were associated with a greater OS benefit from ICI therapy vs control therapies in patients with NSCLC. Clinicopathological features such as sex, age, ECOG PS, histological type, smoking history, or baseline brain metastasis status (previously treated) were not associated with OS benefit from ICI therapy. These results highlight the importance of EGFR, PD-L1 expression and bTMB as predictive markers for NSCLC immunotherapy. Moreover, our study suggests that the use of ICIs in NSCLC should not be restricted to certain factors such as sex, smoking history, race, etc.
A growing body of literature has illustrated sexbased differences in immune responses. Adult females mount stronger innate and adaptive immune responses than males (49), thus could potentially influence the efficacy of immunotherapy. In addition to sex, age and ECOG PS have also been reported to potentially influence the response to ICIs (50, 51). However, we did not find any statistically significant difference in OS benefit from ICIs in patients with different sex, age, or ECOG PS, both in our previous (10) and the current study. Many large-scale clinical trials of immunotherapy were carried out in different races and regions around the world, but there is no research on whether OS benefit from ICI vs control therapy differs by the patient’s race or region. We found that there was no difference in the degree of relative benefit from immunotherapy between races and regions. These findings suggest that ICI immunotherapy is beneficial in NSCLC patients regardless of sex, age, ECOG PS, race and region, and the use of ICIs should not be restricted to any subgroups based on these variables.
Brain metastases are associated with low quality of life and poor survival outcome. The blood-brain barrier extremely limits the therapeutic effectiveness of drugs. A phase 2 trial has demonstrated the positive role of pembrolizumab in treating NSCLC with brain metastases, as the first prospective study of immunotherapy focusing on brain metastasis. The brain metastasis response rate was 29.7% in the PD-L1 TPS≥1% population, with 4 patients achieved complete response and 7 achieved partial response, meeting the prespecified success criteria set for the trial (52). The subgroup analyses from some clinical trials did show that NSCLC patients with brain metastases could benefit from immunotherapies over control therapies (22, 34, 35, 40), whereas some other trials did not (6, 20, 33, 44). The majority of clinical trials with ICIs require that brain metastases be treated by surgical resection or radiation with a subsequent period of stability prior to enrollment. Pooled analyses have suggested that these patients with treated brain metastases still derive significant benefit with ICIs (53, 54). Here, we found NSCLC patients with baseline brain metastases could also benefit from the ICIs, and the magnitude of benefit was similar to that in patients without baseline brain metastases. The results suggest the possibility that ICIs may pass the (likely disrupted) blood-brain barrier and treat brain metastases effectively. However, the number, size, and location of brain metastases may influence the efficacy of ICIs, which needs to be analyzed in future studies.
We demonstrated ICIs resulted in an improved OS compared with controls among EGFR wild-type patients but not among EGFR-mutant patients, consistent with several previous publications (55, 56). Notably, a minority of patients with EGFR-mutant NSCLC could still benefit from ICIs. For example, NSCLC patients with L858 mutations had a similar outcome with ICI treatment compared with those with wild-type EGFR in a large retrospective study (11). Moreover, high PD-L1 expression in patients with EGFR-mutant NSCLC showed a trend toward better outcomes than those with low PD-L1 expression (57). Additionally, add immunotherapy to certain backbone therapies could significantly improve outcomes of NSCLC patients with TKI sensitive EGFR mutations (26). Therefore, NSCLC patients with sensitive EGFR mutations might still benefit from immunotherapy if there is high PD-L1 expression or when used in combination with chemotherapy (58–60). Mutation status in KRAS, TP53, MET, and ROS were associated with PD-L1 expression in patients with lung adenocarcinomas (61, 62). ALK rearrangement status may be associated with response to PD-1/PD-L1 pathway blockade (55). Given the small number of trials, we did not analyze the difference of survival benefit by other genetic mutations.
As the target of anti-PD-L1 antibodies, expression of PD-L1 has become the major initial predictor of benefit from immunotherapy. High tumor PD-L1 expression is correlated with an increased likelihood of response to anti-PD-1/PD-L1 antibodies. In this study, we grouped studies that used the same cutoffs of PD-L1 expression to analyzed the association with OS benefit. Pooled analysis shows that patients with TPS/TC<1% can also benefit from ICIs. The positive result may be partly attributed to some included trials reporting combined ICIs (CTLA4 antibody + PD-1/PD-L1 antibody). A greater OS benefit was observed in those with high PD-L1 levels when dividing subgroups by 50% tumoral PD-L1 expression, but no difference was found when using 1% as the cutoff. This suggested that NSCLC patients with a significantly higher PD-L1 expression may benefit more from ICIs than other groups, but that benefit isn’t restricted to patients with detectable PD-L1 expression. Quantifying PD-L1 expression on immune cells in addition to tumor cells seemed to strengthen the predictive value of PD-L1 for OS benefit, as immune cells expressing PD-L1 also play a key role in regulating antitumor immune response. There are some challenges in establishing PD-L1 expression as a reliable predictive biomarker. First, the attempts to take PD-L1 as a predictor of immunotherapy have yielded variable results using different cutoffs. Second, the antibodies and testing platforms varied, e.g., SP263 (durvamab) and SP142 (atezolizumab) on the Ventana platform, 22C3 (pembrolizumab) and 28-8 (nivolumab) on the DAKO platform, and 73-10 on the Abcam platform (avelumab). It is hard to reach a uniform standard for the detection of PD-L1 expression. The International Association for the Study of Lung Cancer Pathology Committee has made effort to harmonize and standardize testing for PD-L1 by immunohistochemistry (63). Third, PD-L1 expression may have a great temporal and spatial heterogeneity. In one study, the concordance rate for PD-L1 levels was only 67% between paired samples collected more than three months apart (64). In another study, assessment of different fields of view in the same patient sample showed discordant expression at a frequency of 25% (65). We have previously made significant effort to identified the heterogeneity of PDL1 between the tumor microenvironment of paired primary lung cancers and metastatic lesions (66–69). Finally, irresponsiveness and rapid disease progression can be observed in patients with high tumoral PD-L1 expression, while conversely responses can still occur in PD-L1 negative tumors (70). The CheckMate 227 trial showed a similar survival outcome between the high and low PD-L1 expression in NSCLC patient with a high TMB (71). The results indicated that combining biomarkers, such as PD-L1 and TMB, may increase the predictive efficiency.
As described previously, TMB could be a potential biomarker predicting better response to ICIs. Tumors with high TMB are more likely to generate neoantigens for immune recognition and tumor cell killing, thus resulting in stronger antitumor immune responses to PD-1/PD-L1 or CTLA-4 blockade. NCCN guidelines endorsed TMB as a predictor of immunotherapy based on the positive findings in advanced NSCLC from CheckMate 227 (4). CheckMate 227 also assessed the benefit of ICIs for patients with TMBhighPD-L1low, and found that these patients could significantly benefit from ICIs (HR 0.51, 95%CI 0.30–0.87) (4). The findings suggested that combining TMB with PD-L1 can identify more patients who can potentially benefit from ICIs. This need to be verified in a larger sample size. The result in our study was consistent with previous publications, but our analysis is limited by the few trials reporting this analysis. A larger effort is needed before taking TMB as a convenient and affordable tool in clinical practice. The first challenge is the use of variable cutoffs for high TMB among different studies and between blood or tumor tissues (72–75).
Our study has several strengths. First, we performed an exceedingly comprehensive literature search and included most updated data. Second, we included multiple clinicopathological and biomolecular characteristics in the analysis, some of which such as race and region were analyzed for the first time. Third, we performed analyses with various cutoffs for PD-L1 and bTMB as well as different categorization of race and region. Additionally, we performed subgroups analyses to explore whether immunotherapy settings affected the association of ICI OS benefit with clinical variable such as sex, age and ECOG PS.
Our study also has limitations. First, we conducted the analysis based on published study-level data but not on individual patient-level data. Second, statistically significant heterogeneity was found among the studies for some subgroups. Third, the number of studies that were included in the subgroups analyses and in TMB analysis was small. Fourth, clinical trial enrollment criteria limit the range of many variables that are available for our analysis. For example, patients with an ECOG PS of 2 or lower typically are not included in trials, and patients with brain metastasis typically have undergone resection or received radiation. Finally, our results may not be generalizable to all patients and clinician experiences, because we did not include non-English studies and real-world studies.
This meta-analysis, which included an interaction test, demonstrated that the OS benefit from ICIs did not appear to differ on the basis of patients’ sex, age, ECOG PS, histology, smoking history, baseline brain metastasis, race, and region. These data suggest that the use of ICIs in NSCLC patients should not be restricted by those variables. However, EGFR status, PD-L1 expression, and TMB are important biomarkers that could potentially predict the efficacy of ICIs. The value of using multiple factors in predicting the efficacy of ICIs might be an important future research topic.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conception and design: FY, YW, and JM. Collection and assembly of data: FY, YW, and LT. Assessed the eligibilities of feasible studies: FY, YW, and LT. Statistical analysis: FY, YW, and LT. Wrote the first draft of the manuscript: FY and YW. Revised and edited the manuscript: all authors. Final approval of manuscript: all authors.
This research was funded by National Natural Science Foundation of China (No. 82002783), Nanjing Outstanding Youth Fund (No. JQX21001), The Double Innovation Talent Program of Jiangsu Province (2019).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.955440/full#supplementary-material
1. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-Small-Cell lung cancer: A randomised, phase 2 cohort of the open-label keynote-021 study. Lancet Oncol (2016) 17(11):1497–508. doi: 10.1016/S1470-2045(16)30498-3
2. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, pd-L1-Expressing, locally advanced or metastatic non-Small-Cell lung cancer (Keynote-042): A randomised, open-label, controlled, phase 3 trial. Lancet (2019) 393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7
3. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-Small-Cell lung cancer (Impower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(7):924–37. doi: 10.1016/S1470-2045(19)30167-6
4. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non-Small-Cell lung cancer. New Engl J Med (2019) 381(21):2020–31. doi: 10.1056/NEJMoa1910231
5. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of pd-L1-Selected patients with nsclc. New Engl J Med (2020) 383(14):1328–39. doi: 10.1056/NEJMoa1917346
6. Sezer A, Kilickap S, Gumus M, Bondarenko I, Ozguroglu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-Small-Cell lung cancer with pd-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet (2021) 397(10274):592–604. doi: 10.1016/S0140-6736(21)00228-2
7. Liu Y, Chen P, Wang H, Wu S, Zhao S, He Y, et al. The landscape of immune checkpoints expression in non-small cell lung cancer: A narrative review. Transl Lung Cancer Res (2021) 10(2):1029–38. doi: 10.21037/tlcr-20-1019
8. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-related adverse events of pd-1 and pd-L1 inhibitors in clinical trials: A systematic review and meta-analysis. JAMA Oncol (2019) 5(7):1008–19. doi: 10.1001/jamaoncol.2019.0393
9. Shen X, Zhao B. Efficacy of pd-1 or pd-L1 inhibitors and pd-L1 expression status in cancer: Meta-analysis. Bmj (2018) 362:k3529. doi: 10.1136/bmj.k3529
10. Yang F, Markovic SN, Molina JR, Halfdanarson TR, Pagliaro LC, Chintakuntlawar AV, et al. Association of sex, age, and Eastern cooperative oncology group performance status with survival benefit of cancer immunotherapy in randomized clinical trials: A systematic review and meta-analysis. JAMA Netw Open (2020) 3(8):e2012534. doi: 10.1001/jamanetworkopen.2020.12534
11. Hastings K, Yu HA, Wei W, Sanchez-Vega F, DeVeaux M, Choi J, et al. Egfr mutation subtypes and response to immune checkpoint blockade treatment in non-Small-Cell lung cancer. Ann Oncol (2019) 30(8):1311–20. doi: 10.1093/annonc/mdz141
12. Xu Y, Li H, Huang Z, Chen K, Yu X, Sheng J, et al. Predictive values of genomic variation, tumor mutational burden, and pd-L1 expression in advanced lung squamous cell carcinoma treated with immunotherapy. Transl Lung Cancer Res (2020) 9(6):2367–79. doi: 10.21037/tlcr-20-1130
13. Wang H, Ou Q, Li D, Qin T, Bao H, Hou X, et al. Genes associated with increased brain metastasis risk in non-small cell lung cancer: Comprehensive genomic profiling of 61 resected brain metastases versus primary non-small cell lung cancer (Guangdong association study of thoracic oncology 1036). Cancer (2019) 125(20):3535–44. doi: 10.1002/cncr.32372
14. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. J Clin Epidemiol (2009) 62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005
15. Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, et al. Cancer immunotherapy efficacy and patients' sex: A systematic review and meta-analysis. Lancet Oncol (2018) 19(6):737–46. doi: 10.1016/S1470-2045(18)30261-4
16. Wallis CJD, Butaney M, Satkunasivam R, Freedland SJ, Patel SP, Hamid O, et al. Association of patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: A systematic review and meta-analysis. JAMA Oncol (2019) 5(4):529–36. doi: 10.1001/jamaoncol.2018.5904
17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
18. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-Small-Cell lung cancer. New Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
19. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage iv or recurrent non-Small-Cell lung cancer. New Engl J Med (2017) 376(25):2415–26. doi: 10.1056/NEJMoa1613493
20. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-Small-Cell lung cancer. New Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
21. Lu S, Wang J, Cheng Y, Mok T, Chang J, Zhang L, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non-small cell lung cancer: 2-year follow-up from a randomized, open-label, phase 3 study (Checkmate 078). Lung Cancer (2021) 152:7–14. doi: 10.1016/j.lungcan.2020.11.013
22. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-Small-Cell lung cancer (Checkmate 9la): An international, randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0
23. Jassem J, de Marinis F, Giaccone G, Vergnenegre A, Barrios CH, Morise M, et al. Updated overall survival analysis from Impower110: Atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1-selected nsclc. J Thorac Oncol (2021) 16(11):1872–82. doi: 10.1016/j.jtho.2021.06.019
24. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodriguez-Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous nsclc (Impower131): Results from a randomized phase iii trial. J Thorac Oncol (2020) 15(8):1351–60. doi: 10.1016/j.jtho.2020.03.028
25. Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous nsclc: Results from the randomized phase 3 Impower132 trial. J Thorac Oncol (2021) 16(4):653–64. doi: 10.1016/j.jtho.2020.11.025
26. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-Small-Cell lung cancer (Impower150): Key subgroup analyses of patients with egfr mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med (2019) 7(5):387–401. doi: 10.1016/S2213-2600(19)30084-0
27. Socinski MA, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, et al. Impower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous nsclc. J Thorac Oncol (2021) 16(11):1909–24. doi: 10.1016/j.jtho.2021.07.009
28. Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-Small-Cell lung cancer (Javelin lung 200): An open-label, randomised, phase 3 study. Lancet Oncol (2018) 19(11):1468–79. doi: 10.1016/S1470-2045(18)30673-9
29. Park K, Ozguroglu M, Vansteenkiste J, Spigel D, Yang JCH, Ishii H, et al. Avelumab versus docetaxel in patients with platinum-treated advanced nsclc: 2-year follow-up from the javelin lung 200 phase 3 trial. J Thorac Oncol (2021) 16(8):1369–78. doi: 10.1016/j.jtho.2021.03.009
30. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, pd-L1-Positive, advanced non-Small-Cell lung cancer (Keynote-010): A randomised controlled trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
31. Herbst RS, Garon EB, Kim DW, Cho BC, Perez-Gracia JL, Han JY, et al. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1positive, advanced nonsmall-cell lung cancer in the keynote-010 study. J Clin Oncol (2020) 38(14):1580–90. doi: 10.1200/JCO.19.02446
32. Herbst RS, Garon EB, Kim DW, Cho BC, Gervais R, Perez-Gracia JL, et al. Five year survival update from keynote-010: Pembrolizumab versus docetaxel for previously treated, programmed death-ligand 1-positive advanced nsclc. J Thorac Oncol (2021) 16(10):1718–32. doi: 10.1016/j.jtho.2021.05.001
33. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Updated analysis of keynote-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-Small-Cell lung cancer with pd-L1 tumor proportion score of 50% or greater. J Clin Oncol (2019) 37(7):537–46. doi: 10.1200/JCO.18.00149
34. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-Small-Cell lung cancer. New Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
35. Rodriguez-Abreu D, Powell SF, Hochmair MJ, Gadgeel S, Esteban E, Felip E, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous nsclc: Protocol-specified final analysis from keynote-189. Ann Oncol (2021) 32(7):881–95. doi: 10.1016/j.annonc.2021.04.008
36. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus chemotherapy for squamous non-Small-Cell lung cancer. New Engl J Med (2018) 379(21):2040–51. doi: 10.1056/NEJMoa1810865
37. Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazieres J, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous nsclc: Protocol-specified final analysis of keynote-407. J Thorac Oncol (2020) 15(10):1657–69. doi: 10.1016/j.jtho.2020.06.015
38. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: The mystic phase 3 randomized clinical trial. JAMA Oncol (2020) 6(5):661–74. doi: 10.1001/jamaoncol.2020.0237
39. Govindan R, Szczesna A, Ahn MJ, Schneider CP, Gonzalez Mella PF, Barlesi F, et al. Phase iii trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-Small-Cell lung cancer. J Clin Oncol (2017) 35(30):3449–57. doi: 10.1200/JCO.2016.71.7629
40. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-Small-Cell lung cancer (Oak): A phase 3, open-label, multicentre randomised controlled trial. Lancet (2017) 389(10066):255–65. doi: 10.1016/S0140-6736(16)32517-X
41. Fehrenbacher L, von Pawel J, Park K, Rittmeyer A, Gandara DR, Ponce Aix S, et al. Updated efficacy analysis including secondary population results for oak: A randomized phase iii study of atezolizumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer. J Thorac Oncol (2018) 13(8):1156–70. doi: 10.1016/j.jtho.2018.04.039
42. Hida T, Kaji R, Satouchi M, Ikeda N, Horiike A, Nokihara H, et al. Atezolizumab in Japanese patients with previously treated advanced non-Small-Cell lung cancer: A subgroup analysis of the phase 3 oak study. Clin Lung Cancer (2018) 19(4):e405–e15. doi: 10.1016/j.cllc.2018.01.004
43. Gadgeel SM, Lukas RV, Goldschmidt J, Conkling P, Park K, Cortinovis D, et al. Atezolizumab in patients with advanced non-small cell lung cancer and history of asymptomatic, treated brain metastases: Exploratory analyses of the phase iii oak study. Lung Cancer (2019) 128:105–12. doi: 10.1016/j.lungcan.2018.12.017
44. Yang Y, Sun J, Wang Z, Fang J, Yu Q, Han B, et al. Updated overall survival data and predictive biomarkers of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous nsclc in the phase 3 orient-11 study. J Thorac Oncol (2021) 16(12):2109–20. doi: 10.1016/j.jtho.2021.07.015
45. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage iii nsclc. New Engl J Med (2018) 379(24):2342–50. doi: 10.1056/NEJMoa1809697
46. Paz-Ares L, Spira A, Raben D, Planchard D, Cho BC, Ozguroglu M, et al. Outcomes with durvalumab by tumour pd-L1 expression in unresectable, stage iii non-Small-Cell lung cancer in the pacific trial. Ann Oncol (2020) 31(6):798–806. doi: 10.1016/j.annonc.2020.03.287
47. Faivre-Finn C, Vicente D, Kurata T, Planchard D, Paz-Ares L, Vansteenkiste JF, et al. Four-year survival with durvalumab after chemoradiotherapy in stage iii nsclc-an update from the pacific trial. J Thorac Oncol (2021) 16(5):860–7. doi: 10.1016/j.jtho.2020.12.015
48. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-Small-Cell lung cancer (Poplar): A multicentre, open-label, phase 2 randomised controlled trial. Lancet (2016) 387(10030):1837–46. doi: 10.1016/S0140-6736(16)00587-0
49. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol (2016) 16(10):626–38. doi: 10.1038/nri.2016.90
50. Kugel CH 3rd, Douglass SM, Webster MR, Kaur A, Liu Q, Yin X, et al. Age correlates with response to anti-Pd1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin Cancer Res (2018) 24(21):5347–56. doi: 10.1158/1078-0432.CCR-18-1116
51. Pluvy J, Brosseau S, Naltet C, Opsomer MA, Cazes A, Danel C, et al. Lazarus Syndrome in nonsmall cell lung cancer patients with poor performance status and major leukocytosis following nivolumab treatment. Eur Respir J (2017) 50(1):1700310. doi: 10.1183/13993003.00310-2017
52. Goldberg SB, Schalper KA, Gettinger SN, Mahajan A, Herbst RS, Chiang AC, et al. Pembrolizumab for management of patients with nsclc and brain metastases: Long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol (2020) 21(5):655–63. doi: 10.1016/S1470-2045(20)30111-X
53. Mansfield AS, Herbst RS, de Castro G Jr., Hui R, Peled N, Kim DW, et al. Outcomes with pembrolizumab monotherapy in patients with programmed death-ligand 1-positive nsclc with brain metastases: Pooled analysis of keynote-001, 010, 024, and 042. JTO Clin Res Rep (2021) 2(8):100205. doi: 10.1016/j.jtocrr.2021.100205
54. Powell SF, Rodriguez-Abreu D, Langer CJ, Tafreshi A, Paz-Ares L, Kopp HG, et al. Outcomes with pembrolizumab plus platinum-based chemotherapy for patients with nsclc and stable brain metastases: Pooled analysis of keynote-021, -189, and -407. J Thorac Oncol (2021) 16(11):1883–92. doi: 10.1016/j.jtho.2021.06.020
55. Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, et al. Egfr mutations and alk rearrangements are associated with low response rates to pd-1 pathway blockade in non-small cell lung cancer: A retrospective analysis. Clin Cancer Res (2016) 22(18):4585–93. doi: 10.1158/1078-0432.CCR-15-3101
56. Li J, Gu J. Pd-L1 expression and egfr status in advanced non-Small-Cell lung cancer patients receiving pd-1/Pd-L1 inhibitors: A meta-analysis. Future Oncol (2019) 15(14):1667–78. doi: 10.2217/fon-2018-0639
57. Garassino MC, Cho BC, Kim JH, Mazieres J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-Small-Cell lung cancer (Atlantic): An open-label, single-arm, phase 2 study. Lancet Oncol (2018) 19(4):521–36. doi: 10.1016/S1470-2045(18)30144-X
58. Masuda K, Horinouchi H, Tanaka M, Higashiyama R, Shinno Y, Sato J, et al. Efficacy of anti-Pd-1 antibodies in nsclc patients with an egfr mutation and high pd-L1 expression. J Cancer Res Clin Oncol (2021) 147(1):245–51. doi: 10.1007/s00432-020-03329-0
59. Lin C, Chen X, Li M, Liu J, Qi X, Yang W, et al. Programmed death-ligand 1 expression predicts tyrosine kinase inhibitor response and better prognosis in a cohort of patients with epidermal growth factor receptor mutation-positive lung adenocarcinoma. Clin Lung Cancer (2015) 16(5):e25–35. doi: 10.1016/j.cllc.2015.02.002
60. Tozuka T, Seike M, Minegishi Y, Kitagawa S, Kato T, Takano N, et al. Pembrolizumab and salvage chemotherapy in egfr T790m-positive non-Small-Cell lung cancer with high pd-L1 expression. OncoTargets Ther (2018) 11:5601–5. doi: 10.2147/OTT.S168598
61. Schoenfeld AJ, Rizvi H, Bandlamudi C, Sauter JL, Travis WD, Rekhtman N, et al. Clinical and molecular correlates of pd-L1 expression in patients with lung adenocarcinomas. Ann Oncol (2020) 31(5):599–608. doi: 10.1016/j.annonc.2020.01.065
62. Petrelli F, Maltese M, Tomasello G, Conti B, Borgonovo K, Cabiddu M, et al. Clinical and molecular predictors of pd-L1 expression in non-Small-Cell lung cancer: Systematic review and meta-analysis. Clin Lung Cancer (2018) 19(4):315–22. doi: 10.1016/j.cllc.2018.02.006
63. Tsao MS, Kerr KM, Dacic S, Yatabe Y, Hirsch F. Iaslc atlas of pd-L1 immunohistochemistry testing in lung cancer. In: Aurora, CO: International association for the study of lung cancer. Aurora, CO: International Association for the Study of Lung Cancer (IASLC) (2017).
64. Cho JH, Sorensen SF, Choi YL, Feng Y, Kim TE, Choi H, et al. Programmed death ligand 1 expression in paired non-small cell lung cancer tumor samples. Clin Lung Cancer (2017) 18(6):e473–e9. doi: 10.1016/j.cllc.2017.04.008
65. McLaughlin J, Han G, Schalper KA, Carvajal-Hausdorf D, Pelekanou V, Rehman J, et al. Quantitative assessment of the heterogeneity of pd-L1 expression in non-Small-Cell lung cancer. JAMA Oncol (2016) 2(1):46–54. doi: 10.1001/jamaoncol.2015.3638
66. Mansfield AS, Murphy SJ, Peikert T, Yi ES, Vasmatzis G, Wigle DA, et al. Heterogeneity of programmed cell death ligand 1 expression in multifocal lung cancer. Clin Cancer Res (2016) 22(9):2177–82. doi: 10.1158/1078-0432.CCR-15-2246
67. Mansfield AS, Aubry MC, Moser JC, Harrington SM, Dronca RS, Park SS, et al. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol (2016) 27(10):1953–8. doi: 10.1093/annonc/mdw289
68. Mansfield AS, Ren H, Sutor S, Sarangi V, Nair A, Davila J, et al. Contraction of T cell richness in lung cancer brain metastases. Sci Rep (2018) 8(1):2171. doi: 10.1038/s41598-018-20622-8
69. Hazim A, Majithia N, Murphy SJ, Wigle D, Aubry MC, Mansfield AS. Heterogeneity of pd-L1 expression between invasive and lepidic components of lung adenocarcinomas. Cancer Immunol Immunother (2021) 70(9):2651–6. doi: 10.1007/s00262-021-02883-x
70. Camidge DR, Doebele RC, Kerr KM. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of nsclc. Nat Rev Clin Oncol (2019) 16(6):341–55. doi: 10.1038/s41571-019-0173-9
71. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. New Engl J Med (2018) 378(22):2093–104. doi: 10.1056/NEJMoa1801946
72. Karn T, Denkert C, Weber KE, Holtrich U, Hanusch C, Sinn BV, et al. Tumor mutational burden and immune infiltration as independent predictors of response to neoadjuvant immune checkpoint inhibition in early tnbc in geparnuevo. Ann Oncol (2020) 31(9):1216–22. doi: 10.1016/j.annonc.2020.05.015
73. McGrail DJ, Pilie PG, Rashid NU, Voorwerk L, Slagter M, Kok M, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol (2021) 32(5):661–72. doi: 10.1016/j.annonc.2021.02.006
74. Valero C, Lee M, Hoen D, Wang J, Nadeem Z, Patel N, et al. The association between tumor mutational burden and prognosis is dependent on treatment context. Nat Genet (2021) 53(1):11–5. doi: 10.1038/s41588-020-00752-4
Keywords: non-small cell lung cancer, immune checkpoint inhibitor, efficacy, survival, meta-analysis
Citation: Yang F, Wang Y, Tang L, Mansfield AS, Adjei AA, Leventakos K, Duma N, Wei J, Wang L, Liu B and Molina JR (2022) Efficacy of immune checkpoint inhibitors in non-small cell lung cancer: A systematic review and meta-analysis. Front. Oncol. 12:955440. doi: 10.3389/fonc.2022.955440
Received: 17 June 2022; Accepted: 28 July 2022;
Published: 16 August 2022.
Edited by:
Santiago Viteri, Clínica Mi Tres Torres, SpainReviewed by:
Paul Zarogoulidis, G. Papanikolaou General Hospital, GreeceCopyright © 2022 Yang, Wang, Tang, Mansfield, Adjei, Leventakos, Duma, Wei, Wang, Liu and Molina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Yang, eWFuZ2ZhbmduanVAaG90bWFpbC5jb20=; Julian R. Molina, bW9saW5hLmp1bGlhbkBtYXlvLmVkdQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.