
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 18 August 2022
Sec. Hematologic Malignancies
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.955080
A correction has been applied to this article in:
Corrigendum: Circulating cell-free DNA and IL-10 from cerebrospinal fluids aid primary vitreoretinal lymphoma diagnosis
Primary vitreoretinal lymphoma (PVRL) is a rare variant of primary central nervous system lymphoma (PCNSL) that presents diagnostic challenges. Here, we focused on circulating cell-free DNA (cfDNA) and interleukin-10 (IL-10) isolated from cerebrospinal fluid. Twenty-three VRL patients (17 PVRL, 2 PCNSL/O, and 4 relapsed VRL, from 10/2018 to 12/2021) and 8 uveitis patients were included in this study. CSF samples from 19 vitreoretinal lymphoma patients had sufficient cfDNA for next-generation sequencing. Of these patients, 73.7% (14/19) had at least one meaningful non-Hodgkin lymphoma-related mutation. The characteristic MYD88L265P mutation was detected in the CSF of 12 VRL patients, with a sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 63.2%, 100%, 100%, and 46.2%, respectively. No meaningful lymphoma related mutations were found in CSF samples from uveitis controls with typical intraocular lesions. Meanwhile, CSF IL-10 levels were elevated in 95.7% of the VRL patients, with a sensitivity, specificity, PPV, and NPV of 95.7%, 100%, 100% and 88.9%, respectively. Key somatic mutations like MYD88L265P and CD79B detected from CSF cfDNA and elevated CSF IL-10 levels can be promising adjuncts for primary vitreoretinal lymphoma diagnosis.
Primary vitreoretinal lymphoma (PVRL) is a rare extranodal non-Hodgkin lymphoma of the retina, vitreous, and optic nerve. Most PVRL patients are of the B-cell lineage; approximately 80% develop intracranial progression eventually, while 15%–20% of patients with primary central nervous system lymphoma (PCNSL) have intraocular involvement at diagnosis (1–4). Thus, PVRL is also considered a subset of PCNSL. Early diagnosis of vitreoretinal lymphoma benefits survival. PVRL often masquerades as chronic posterior uveitis, sometimes as retinitis in clinical manifestations, adding to the difficulties of diagnosis (5–7). Pathological diagnosis is the gold standard. However, the false negative rate of vitreous biopsy cytology or immune cytology is approximately 70% (8, 9). Flow cytometry of vitreous fluid increases the diagnostic sensitivity, up to 82% (10, 11).
In the recent years, numerous studies have focused on exploring potential ancillary techniques and biomarkers for primary vitreoretinal lymphoma (PVRL) diagnosis. The detection of PVRL characteristic gene mutations, including MYD88L265P and CD79B, and immunoglobulin gene rearrangement (4, 12–21) from aqueous humor or vitreous fluid has been considered effective diagnostic approaches. Meanwhile, elevate aqueous humor or vitreous fluid interleukin-10 (IL-10) levels and elevated IL-10/IL-6 ratios have been considered as useful biomarkers (22). Our previous studies also demonstrated the diagnostic and disease monitoring roles of cerebrospinal fluid (CSF) IL-10 levels in PCNSL patients (23, 24). Since PVRL is a special subset of PCNSL, we wonder whether CSF IL-10 levels are also elevated in primary vitreoretinal lymphomas.
Circulating cell-free DNA (cfDNA), double-strand DNA fragments (usually 130–180 base pairs) released from cells into surrounding blood or other body fluids by cellular breakdown or active secretion (25–28), has become a promising biomarker for solid tumors in the past decades (29). Numerous studies have demonstrated the diagnostic, monitoring, and prognostic role of plasma cfDNA in various types of solid tumors (30–38), and the recent use of plasma cfDNA in lymphoma genotyping and prognosis (39–42). For restricted-brain tumors, cerebrospinal fluid (CSF) cfDNA, rather than plasma cfDNA, provides a minimally invasive approach to detect tumor mutations and contribute to diagnosis (43–47).
Vitreous fluid is the key sample to PVRL diagnosis. However, the number of diagnostic tests is limited by the small sample size, while sample dilution adds to false-negative results. Considering the anatomic relationship between vitreoretinal and cerebrospinal fluid, and the role of CSF in PCNSL diagnosis, we conducted this study to evaluate the potential of baseline CSF cfDNA mutation profiles and IL-10 levels for VRL diagnosis and establish the foundation of serial CSF monitoring for early detection of intracranial progression in VRL patients.
Seventeen patients with PVRL, two patients with primary central nervous system lymphoma and intraocular involvement (PCNSL/O), and four patients with relapsed vitreoretinal lymphoma (RVRL) were included in this study as experimental group. All the patients were diagnosed in our center, from 10/2018 to 12/2021 and treated. The PVRL patients were enrolled from our two prospective single-center open-label phase II trials (NCT03746223 and NCT04899453), with the same diagnostic criteria as previously described (48). In brief, pathology is the gold standard for VRL diagnosis. Meanwhile, patients who fulfilled the following criteria of 1 + 2 and two of 3/4/5 were diagnosed with VRL, B-cell type: (1) clinical manifestations including typical vitreous opacities, subretinal lesions, or both; (2) aqueous humor or vitreous fluid IL-10/IL-6 >1; (3) vitreous pathology showing neoplastic lymphoma cells; (4) positive vitreous cell immunoglobulin gene rearrangement (IgH, Igκ, or Igλ); and (5) vitreous flow cytometry positive for lymphoma biomarkers. VRL patients with no evidence of CNS or systemic lymphoma were considered PVRL. Sometimes asymptomatic concurrent intracranial lesions were found in the routine head MRI; these VRL patients were considered PCNSL/O. RVRL patients were those who had previously been treated for systemic lymphoma (n=2) or vitreoretinal lymphoma (n=2), now experienced restricted intraocular relapse. Additionally, eight uveitis patients who presented with typical vitreous opacities and subretinal lesions were enrolled from 11/2019 to 12/2021 as suspected vitreoretinal lymphoma cases. Thorough exams ruled out the possibility of VRL; no malignancy presented on follow-ups (4.5–29 months).
This study conformed to the Declaration of Helsinki and was approved by the Institutional Ethics Committee of Peking Union Medical College Hospital. Written informed consent was obtained from each participant. Furthermore, patients with VRL received lumber puncture at baseline, before each chemotherapy and every 6 months during maintenance therapy to examine CSF and rule out CNS progression. Ten microliters of cerebrospinal fluid (CSF) and buccal mucosa were obtained from each patient prior to treatment for sequencing. Data on clinical characteristics were collected from electronic health records.
Germline DNA was extracted from buccal mucosa using the DNeasy Tissue Kit (Qiagen, USA) according to the manufacturer’s guidelines. CSF was collected and processed within 4 h. CSF-derived circulating cell-free DNA (cfDNA) was extracted with the QIAamp Circulating Nucleic Acid Kit (Qiagen). Then, the fragment length and quantity of cfDNA were assessed with the Qubit Fluorometer, Qubit dsDNA BR Assay Kit (Invitrogen, USA), and Labchip GX Touch system (PerkinElmer, Shanghai, China). All libraries were hybridized to custom-designed biotinylated oligonucleotide probes (IDT, Coralville, IA, USA) covering 413 genes. DNA sequencing was performed using the GeneSeq-2000 (Geneplus-Suzhou, Suzhou, China) with a read length of PE100 and depth of 500–1,000× (49), and 90 genes related to lymphoma were used for subsequent analysis (Supplementary Table S1).
Terminal adaptor sequences and low-quality reads were removed separately from raw data of paired samples using NCrealSeq (version 1.2.0, Geneplus-Suzhou) and NCfilter (version 2.0.0, Geneplus-Suzhou). The Burrows–Wheeler Aligner (BWA, version 0.7.15-r1140) tool was used to align clean reads to the reference human genome (GRCh37). Duplicate reads of cancer sample derived from PCR amplification were marked using realSeq, which was designed to retain reads containing rare events by treating Unique Molecular Indexes, and the normal sample was marked using Picard tools (version 2.6.0).
Single nucleotide variants (SNVs) and Indels were detected by comparing tumor-normal pairs using TNscope (version 201808) and realDcaller (version 1.7.1, Geneplus-Suzhou), a software developed to review hotspot variants. The results of these analyses were merged using NChot (version 2.7.2, Geneplus-Suzhou) and then annotated to multiple public databases using NCanno (version 1.1.3, Geneplus-Suzhou). For somatic copy-number alteration, CNVKIT (version 0.9.2) (50) was performed, and the matched buccal mucosa samples served as matched controls. Significant copy number variations were calculated as the ratio of adjusted depth between case gDNA and control gDNA. NCSV (0.2.3, Geneplus-Suzhou) was used to identify split-read and discordant read-pair to identify SVs. All candidate variants were manually verified with the integrative genomics viewer browser (51).
One milliliter of fresh CSF samples was used to detect the levels of inflammation factor IL-10 as previously described (23, 24). In brief, CSF samples were centrifuged (10 min at 500×g at 18°C); then, the supernatants were collected, and the IL-10 levels were measured with an electrochemiluminescence immunoassay (ECLIA) analyzer following the manufacturer’s instructions (Siemens Immulite 1000 and IL-10 detection kits). The levels of detected CSF IL-10 range from 5 to 1,000 pg/ml.
RStudio was used to present the results of sequencing. As for validity measurement, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated (52).
We collected CSF samples from 23 patients with B-cell vitreoretinal lymphoma, with or without CNS involvement and lymphoma history (Table 1). Specifically, 17 patients with primary vitreoretinal lymphoma, 2 VRL patients with concomitant CNS lymphoma, 2 patient whose vitreoretinal lymphoma had relapsed, and 2 patients whose previous systemic DLBCL had intraocular relapse were included in this study. Additionally, eight patients who presented with typical vitreous and subretinal lesions but without evidence of malignancy were included as controls, including idiopathic uveitis, cytomegalovirus retinitis, ocular sarcoidosis, and radiation retinopathy. All patients with suspected vitreoretinal lymphoma underwent several diagnostic tests, as shown in the schematic flowchart (Figure 1).
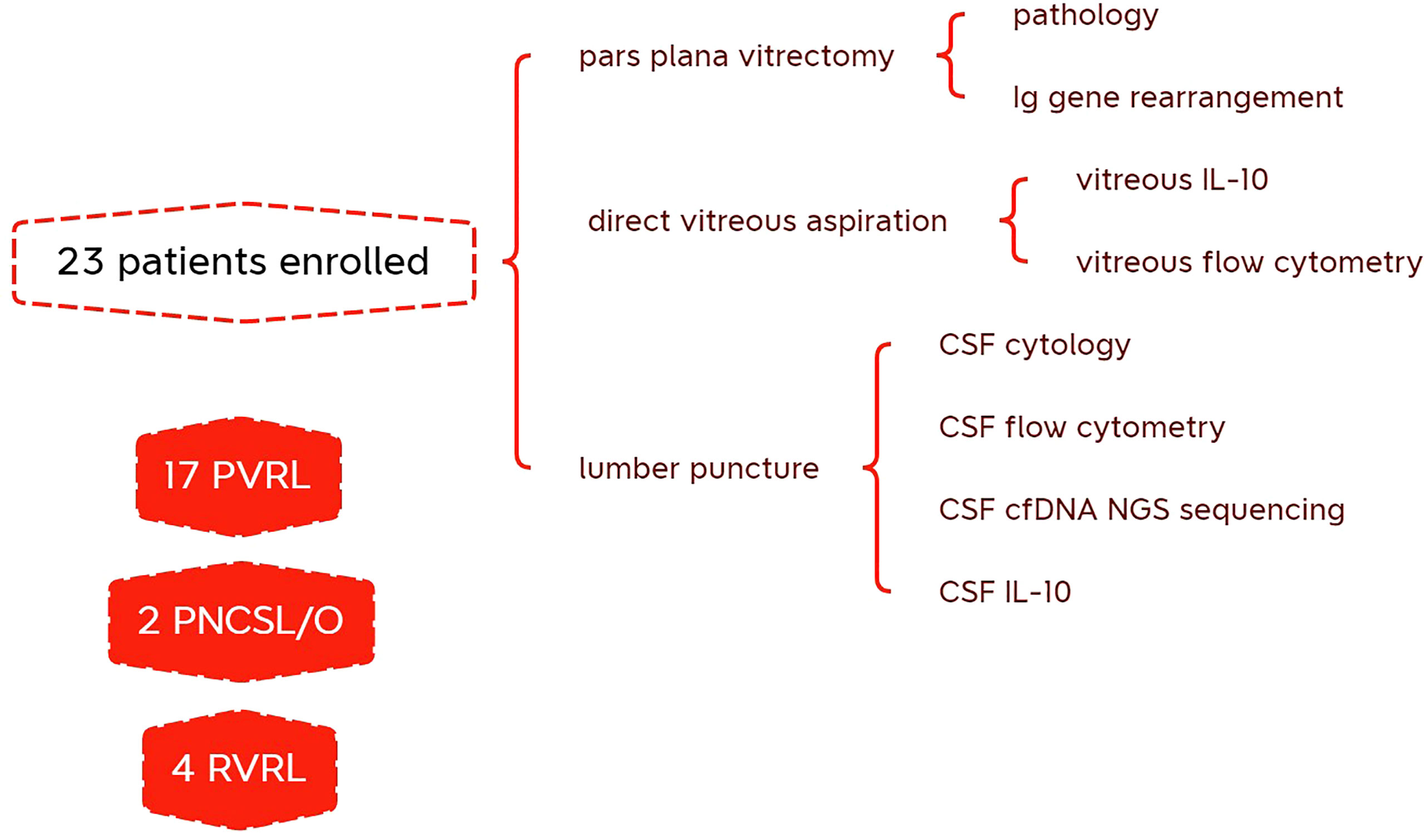
Figure 1 Schematic flowchart of diagnostic test performed on patients with suspected vitreoretinal lymphoma.
cfDNA was extracted from CSF; then, targeted deep sequencing of NHL-related genes was performed to identify somatic mutations (Figures 2, 3). In four patients with primary vitreoretinal lymphoma, the amounts of extracted cfDNA (ranged from 0.1 to 0.6 ng) were not sufficient for cfDNA library construction, which failed to perform sequencing. Analysis of the cfDNA in the CSF of the remaining 19 vitreoretinal lymphoma patients revealed detectable mutations in 14 patients (Figure 2), at different variant allele frequencies (VAFs), ranging from 1.0% to 96.9%. In PVRL and PCNSL/O, 11/15 of the sequenced patients had MYD88L265P mutation, while 5 were with MYD88L265P and CD79B co-mutation. PIM1 was the most frequently mutated gene. In the meantime, sequencing of non-lymphoma controls’ CSF cfDNA showed no mutation in five, insufficient cfDNA in two, and DNMT3A c.1851+1G>A mutation (VAF 0.9%) in one (CONTROL-5).
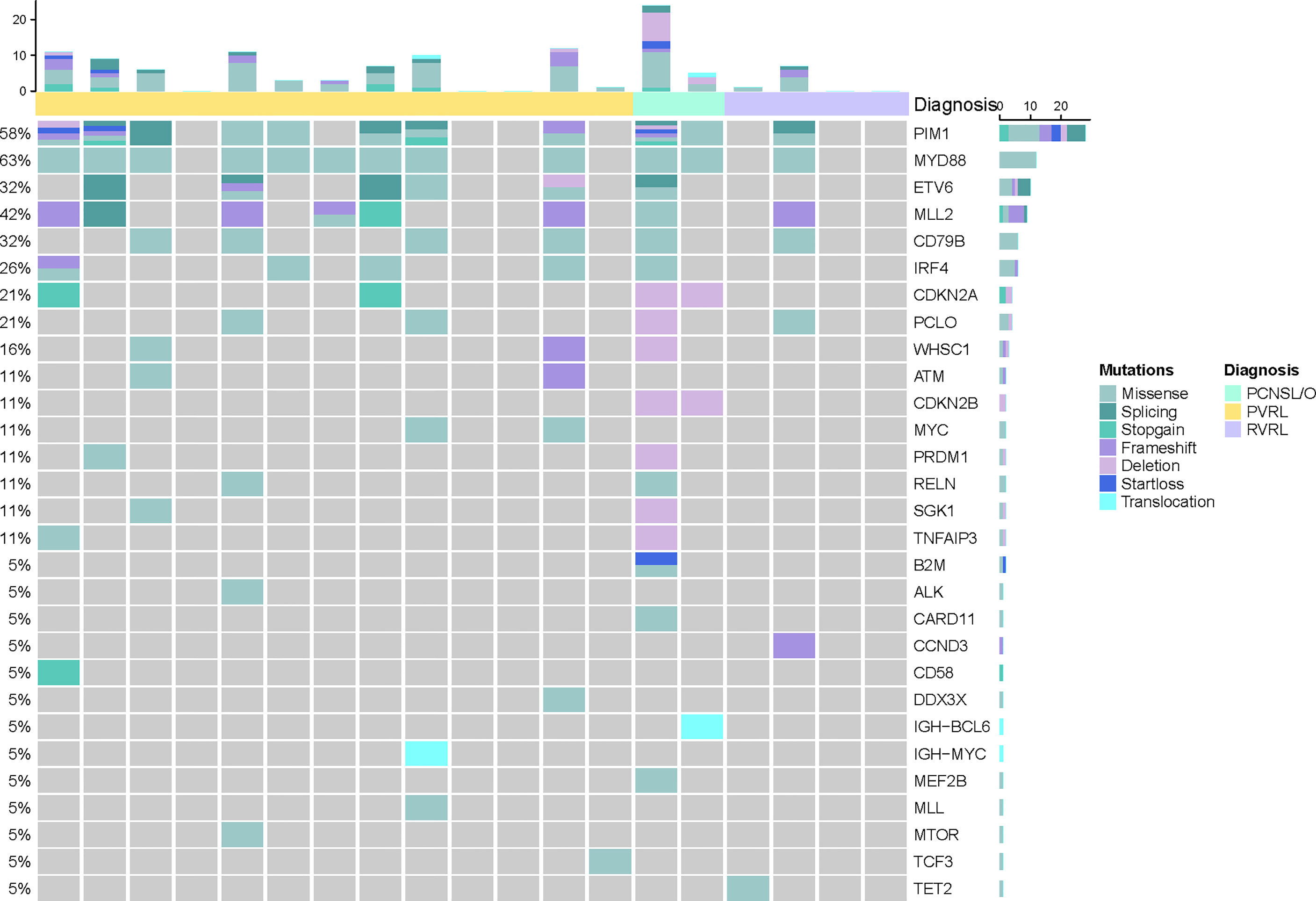
Figure 2 Baseline genomic characteristics of cerebrospinal cfDNA in vitreoretinal lymphoma patients.
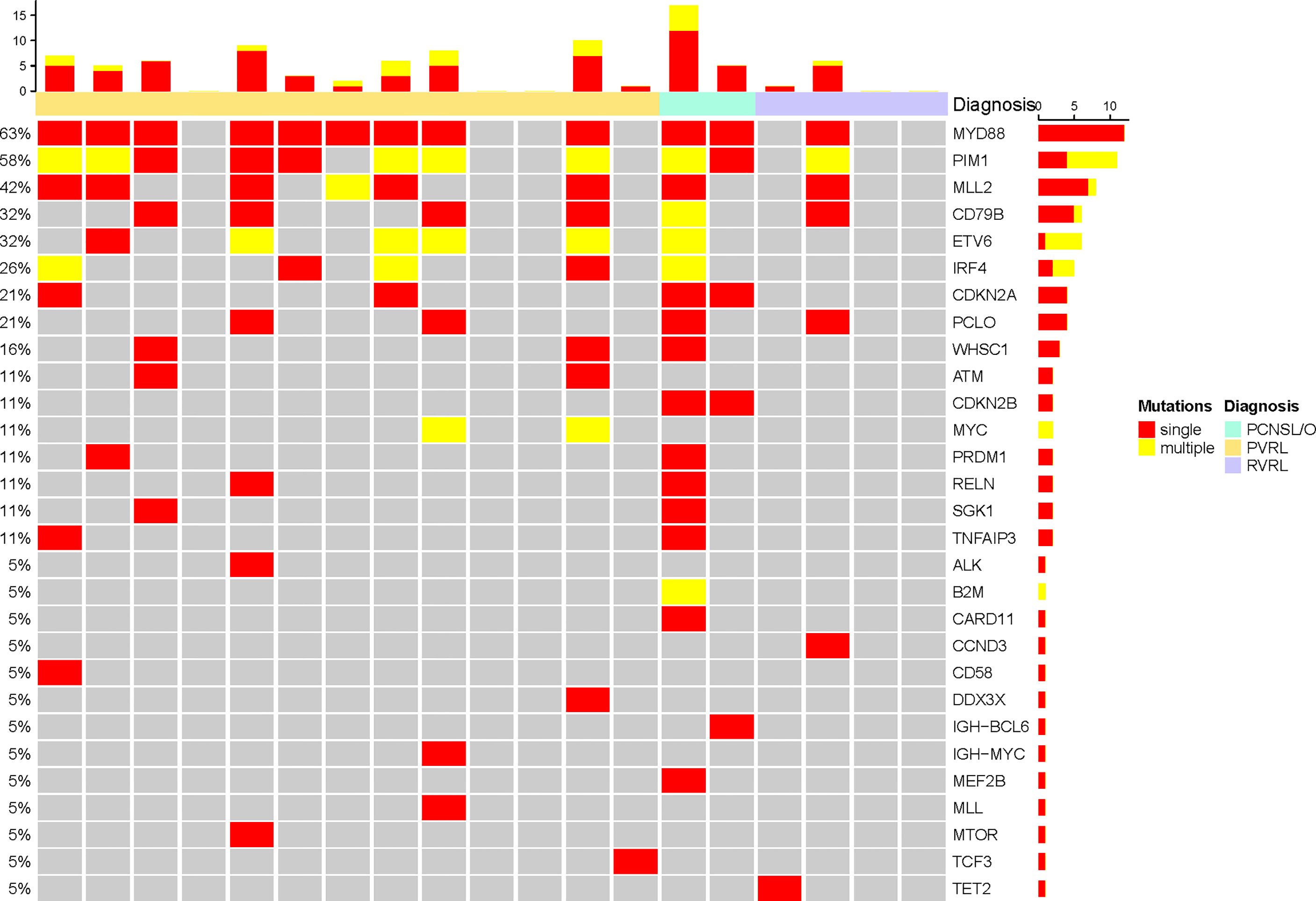
Figure 3 Single and multiple somatic mutations of cerebrospinal cfDNA in vitreoretinal lymphoma patients at baseline.
The detection of cfDNA in CSF was compared with the conventional methods of malignant cells identification at baseline (Table 2) and diagnostic validity was compared (Table 3). Although neoplastic lymphocytes were found in 22 out of the 23 VRL patients, most of the biopsy samples were not enough for immunohistochemical staining. Only four VRL patients were diagnosed with histology. Vitreous flow cytometry detected malignant B cells in 9 out of the 21 VRL patients tested, whereas vitreous cell immunoglobulin gene rearrangement detected 18 out of the 23. In our cohort, CSF cfDNA analysis revealed NHL-related gene mutations in 73.7% of the vitreoretinal lymphoma patients, with higher sensitivity than vitreous histology or flow cytometry (17.4% and 42.9%, respectively), slightly lower sensitivity than vitreous Ig gene rearrangement (78.3%). Notably, in the 14 cases that exhibited cfDNA mutation, 12 were with MYD88L265P mutation (overall sensitivity of 63.2%), and 6 were with MYD88L265P and CD79B co-mutation. Although in some cases lymphoma cells were not detected by cytology (PVRL-02, PVRL-05, PVRL-11, and PCNSL/O-21&22), the characteristic MYD88L265P mutation was detected in the CSF cfDNA (Figure 2). These findings suggest that sequencing CSF cfDNA can act as an adjunct approach to the diagnosis of VRL.
CSF IL-10 was previously demonstrated as a biomarker for the diagnosis and prognosis of primary central nervous system large B-cell lymphoma, with a cutoff value of 8.2 pg/ml (24). Here, we found that the levels of CSF IL-10 were also elevated in 22 out of the 23 vitreoretinal B-cell lymphoma patients, while the CSF IL-10 levels were within normal limits in the control uveitis group. Elevated CSF IL-10 levels had a sensitivity, specificity, PPV, and NPV of 95.7%, 100%, 100%, and 88.9% for the diagnosis of VRL, whereas those were 95.7%, 62.5%, 81.5%, and 75% for vitreous IL-10 levels. Our findings provide evidence that CSF IL-10 could be a good diagnostic marker for primary vitreoretinal lymphoma.
To determine whether baseline CSF biomarkers have a prognostic potential, we corrected cfDNA levels and their maximal somatic variant allelic frequency (maxVAF) with patients’ clinical outcome–progression-free survival time in the five patients treated with R2 (Rituximab combined with lenalidomide), as shown in Table 4. CSF cfDNA amount and maxVAF did not correlate with VRL PFS (p= 0.89, 0.55, respectively). Neither was the CSF IL-10 level (p=0.47).
The diagnosis of primary vitreoretinal lymphoma is still challenging. PVRLs usually present with bilateral blurry vision and floaters, anterior segment findings of keratic precipitates, and vitreous cellular infiltration of various severities (53). The clinical manifestations of primary vitreoretinal lymphoma are rather masquerading; patients are often misdiagnosed as intraocular inflammation or viral retinitis and wrongly treated (5–7). Cytological and immunohistochemical evidence of malignant lymphoma cells is the gold standard for diagnosis. However, the sensitivity of vitreous biopsy is disappointingly low, due to the lack of lymphoma cells in the vitreous specimen and necrosis during preparation (9). New diagnostic approaches like flow cytometry and molecular analysis of vitreous samples add to the diagnosis. Although Cani et al. (54) proposed with four patients that next-generation sequencing (NGS) test did not compromise the sample volume needed for other diagnostic tests, including cytology and flow cytology. From our experience, after cytology-based tests (cytology, immune cytology, and Ig rearrangement test) and flow cytology, the remaining vitreous samples could not provide enough DNA for NGS. Hence, we wondered whether CSF could be a substitute marker for PVRL diagnosis, since PVRL is a special subset of PCNSL and previous studies have demonstrated the diagnostic role of CSF cfDNA and elevated IL-10 levels (24, 55). Furthermore, serial CSF monitoring might be promising in the early detection of CNS progression in vitreoretinal lymphoma patients. To address the unmet needs of PVRL diagnosis, we conducted this study to analysis the diagnostic roles of CSF biomarkers, circulating cell-free DNA, and IL-10.
MYD88L265P is a unique non-synonymous point mutation in B-cell malignancies (56). Several studies demonstrated the presence of MYD88L265P mutation in the aqueous humor and vitreous fluid of vitreoretinal lymphoma patients. In different PVRL cohorts, the reported sensitivity of MYD88L265P mutation detection ranged from 25% to 88.9%, with direct Sanger sequencing of polymerase chain reaction (PCR), droplet digital PCR, or sequencing (12–19). The vitreous fluid samples showed higher positive rate than paired aqueous humor samples (14). We wondered whether MYD88L265P mutation also presented in the CSF of PVRL patients. In this study, we collected CSF samples from 31 patients with suspected VRL, then performed NGS. The final diagnosis was VRL in 23 patients. Despite the four samples without sufficient cfDNA for NGS, MYD88L265P mutation was confirmed in 12 of the remaining 19 VRL patients. Six patients were with MYD88L265P and CD79B co-mutation. Meanwhile, none of the uveitis controls contained the characteristic lymphoma mutations. The sensitivity, specificity, PPV, and NPV for using CSF MYD88L265P as VRL diagnostic marker were 63.2%, 100%, 100%, and 46.2%, respectively. Our findings suggest that key somatic mutations (i.e., MYD88L265P) detected from CSF samples can be a promising additional approach for the accurate diagnosis of VRL. Notably, mutations without specific clinical meanings might be detected in non-lymphoma patients, like low frequency DNMT3A splicing mutation.
With CSF samples, we were able to overcome the difficulty of insufficient vitreous biopsy samples and picture the genomic features of vitreoretinal lymphomas. This can be a promising adjunct to vitreous fluid samples genomic analyses (19). Although there have been no standard treatment protocols for vitreoretinal lymphomas, the baseline mutation information presents targets for potential precision therapy, which might improve prognosis. Furthermore, we also need disease monitoring biomarkers for PVRL patients. For early detection of disease relapse and CNS progression, biomarkers like MYD88L265P or CD79B variant allele frequencies are promising. This study confirmed the presence of PVRL characteristic mutations in CSF, also established the foundation of assessing CSF samples to monitor disease progression. Furthermore, routine lumbar puncture for cerebrospinal fluid reduces the possible intraocular complications from aqueous puncture or vitreous aspiration.
IL-6 and IL-10 are the most extensively studied cytokines in vitreoretinal lymphomas; studies have demonstrated that elevated aqueous humor or vitreous fluid IL-10 and IL-10/IL-6 ratio can help distinguish vitreoretinal lymphomas from uveitis. However, there are also lymphoma cases with low IL-10 levels or non-lymphoma cases with elevated IL-10 levels (22, 57–59). In this study, we demonstrated that in patients with restricted intraocular lesions, 95.7% had elevated CSF IL-10 levels (ULN, 8.2 pg/ml), while the CSF IL-10 levels were within normal range in the controls. The sensitivity, specificity, PPV, and NPV were 95.7%, 100%, 100%, and 88.9%, respectively. The diagnostic accuracy of CSF IL-10 was slightly higher than vitreous fluid IL-10 (96.8% versus 80.6%). Meanwhile, parallel test of CSF MYD88L265P and CSF IL-10 levels showed a sensitivity of 98.4% and specificity of 100%. Furthermore, we have been detecting the IL-10 levels in serial CSF samples after treatment to assess whether IL-10 can be a potential disease monitoring biomarker.
A significant limit of our study is the small cohort size. Future studies with larger cohorts are needed. Furthermore, cfDNA extraction procedure still needs optimization to eliminate the effect of cfDNA degradation and increase the quantity of extracted cfDNA for sequencing. Nevertheless, we demonstrate that meaningful molecular data can be obtained from CSF cfDNA in PVRL patients. CSF MYD88L265P mutation and CSF IL-10 can be complementary approaches to the current diagnostic standard of PVRL. NGS of the CSF cfDNA also provides targets for precision therapy, including MYD88, CD79B, and CDKN2A. In the meantime, we have been collecting CSF samples of PVRL patients during therapy to investigate whether the mentioned biomarkers can monitor treatment response or indicate disease progression.
Our study provides mutation landscape of vitreoretinal lymphomas with next-generation sequencing. MYD88L265P or CD79B mutation detected from CSF circulating cell-free DNA aids in primary vitreoretinal lymphoma diagnosis. Patient-specific genomic alterations are also pictured, which provide therapeutic targets for personalized medicine. Furthermore, IL-10 levels are also elevated in the CSF of VRL patients, with higher specificity than vitreous fluid IL-10.
The original contributions presented in the study are publicly available. This data can be found here: Link - https://ngdc.cncb.ac.cn/gsa-human/browse; Accession - HRA002732/HRA002732.
The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study.
ZZ, YZ, XZ, MFZ, and WZ designed the experiment. YZ, XZ, MFZ, and WZ enrolled participants and treated the enrolled patients. ZZ and DMZ collected patient samples and data. ZZ, DMZ, and LZ conducted the experiments. CWJ double checked all the histology samples. ZZ wrote the first draft manuscript. All authors edited and approved the manuscript.
This study was funded by the CAMS Innovation Fund of Medical Sciences (CIFMS) 2019-I2M-2-009, the CAMS Innovation Fund for Medical Sciences (CIFMS) [2021-I2M-C&T-B-005], and Natural Science Foundation of Beijing Municipality 7202154.
We thank all the patients for their consent in participating in the study and sharing their medical records. We thank the medical staff and physicians who participated in this study. We are also grateful to Dr. Nanzhao for her help in statistical analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.955080/full#supplementary-material
1. Chan CC, Sen HN. Current concepts in diagnosing and managing primary vitreoretinal (intraocular) lymphoma. Discov Med (2013) 15(81):93–100.
2. Sobolewska B, Chee SP, Zaguia F, Goldstein DA, Smith JR, Fend F, et al. Vitreoretinal Lymphoma Cancers (2021) 13(16):3921. doi: 10.3390/cancers13163921
3. Chan CC, Rubenstein JL, Coupland SE, Davis JL, Harbour JW, Johnston PB, et al. Primary vitreoretinal lymphoma: a report from an international primary central nervous system lymphoma collaborative group symposium. Oncologist (2011) 16(11):1589–99. doi: 10.1634/theoncologist.2011-0210
4. Kimura K, Usui Y, Goto H, The Japanese Intraocular Lymphoma Study G. Clinical features and diagnostic significance of the intraocular fluid of 217 patients with intraocular lymphoma. Japanese J Ophthalmol (2012) 56(4):383–9. doi: 10.1007/s10384-012-0150-7
5. Zloto O, Elkader AE, Fabian ID, Vishnevskia-Dai V. Primary vitreoretinal lymphoma masquerading as refractory retinitis. Case Rep Ophthalmol (2015) 6(3):345–50. doi: 10.1159/000440762
6. Almas T, Stephenson KA, Ehtesham M, Murphy CC. Beware the quiet eye: primary vitreoretinal lymphoma masquerading as posterior uveitis. BMJ Case Rep (2020) 13(12):e240802. doi: 10.1136/bcr-2020-240802
7. Hormigo A, Abrey L, Heinemann MH, DeAngelis LM. Ocular presentation of primary central nervous system lymphoma: diagnosis and treatment. Br J Haematol (2004) 126(2):202–8. doi: 10.1111/j.1365-2141.2004.05028.x
8. Venkatesh R, Bavaharan B, Mahendradas P, Yadav NK. Primary vitreoretinal lymphoma: Prevalence, impact, and management challenges. Clin Ophthalmol (Auckland NZ) (2019) 13:353–64. doi: 10.2147/OPTH.S159014
9. Gonzales JA, Chan CC. Biopsy techniques and yields in diagnosing primary intraocular lymphoma. Int Ophthalmol (2007) 27(4):241–50. doi: 10.1007/s10792-007-9065-6
10. Tan WJ, Wang MM, Ricciardi-Castagnoli P, Chan ASY, Lim TS. Cytologic and molecular diagnostics for vitreoretinal lymphoma: Current approaches and emerging single-cell analyses. Front Mol Biosci (2020) 7:611017. doi: 10.3389/fmolb.2020.611017
11. Missotten T, Tielemans D, Bromberg JE, van Hagen PM, van Lochem EG, van Dongen JJ, et al. Multicolor flowcytometric immunophenotyping is a valuable tool for detection of intraocular lymphoma. Ophthalmology (2013) 120(5):991–6. doi: 10.1016/j.ophtha.2012.11.007
12. Miserocchi E, Ferreri AJM, Giuffrè C, Cangi MG, Francaviglia I, Calimeri T, et al. MYD88 L265P MUTATION DETECTION IN THE AQUEOUS HUMOR OF PATIENTS WITH VITREORETINAL LYMPHOMA. Retina (Philadelphia Pa) (2019) 39(4):679–84. doi: 10.1097/IAE.0000000000002319
13. Carreno E, Clench T, Steeples LR, Salvatore S, Lee RWJ, Dick AD, et al. Clinical spectrum of vitreoretinal lymphoma and its association with MyD88 L265P mutation. Acta Ophthalmol (2019) 97(1):e138–e9. doi: 10.1111/aos.13808
14. Hiemcke-Jiwa LS, Ten Dam-van Loon NH, Leguit RJ, Nierkens S, Ossewaarde-van Norel J, de Boer JH, et al. Potential diagnosis of vitreoretinal lymphoma by detection of MYD88 mutation in aqueous humor with ultrasensitive droplet digital polymerase chain reaction. JAMA Ophthalmol (2018) 136(10):1098–104. doi: 10.1001/jamaophthalmol.2018.2887
15. Bonzheim I, Giese S, Deuter C, Süsskind D, Zierhut M, Waizel M, et al. High frequency of MYD88 mutations in vitreoretinal b-cell lymphoma: A valuable tool to improve diagnostic yield of vitreous aspirates. Blood (2015) 126(1):76–9. doi: 10.1182/blood-2015-01-620518
16. Wang CZ, Lin J, Qian J, Shao R, Xue D, Qian W, et al. Development of high-resolution melting analysis for the detection of the MYD88 L265P mutation. Clin Biochem (2013) 46(4-5):385–7. doi: 10.1016/j.clinbiochem.2012.11.007
17. Narasimhan S, Joshi M, Parameswaran S, Rishi P, Khetan V, Ganesan S, et al. MYD88 L265P mutation in intraocular lymphoma: A potential diagnostic marker. Indian J Ophthalmol (2020) 68(10):2160–5. doi: 10.4103/ijo.IJO_1712_19
18. Raja H, Salomão DR, Viswanatha DS, Pulido JS. PREVALENCE OF MYD88 L265P MUTATION IN HISTOLOGICALLY PROVEN, DIFFUSE LARGE b-CELL VITREORETINAL LYMPHOMA. Retina (Philadelphia Pa) (2016) 36(3):624–8. doi: 10.1097/IAE.0000000000000996
19. Tan WJ, Wang MM, Castagnoli PR, Tang T, Chan ASY, Lim TS. Single b-cell genomic analyses differentiate vitreoretinal lymphoma from chronic inflammation. Ophthalmology (2021) 128(7):1079–90. doi: 10.1016/j.ophtha.2020.11.018
20. Lee J, Kim B, Lee H, Park H, Ho Byeon S, Choi JR, et al. Whole exome sequencing identifies mutational signatures of vitreoretinal lymphoma. Haematologica (2020) 105(9):e458–60. doi: 10.3324/haematol.2019.233783
21. Wang X, Su W, Gao Y, Feng Y, Wang X, Chen X, et al. A pilot study of the use of dynamic cfDNA from aqueous humor and vitreous fluid for the diagnosis and treatment monitoring of vitreoretinal lymphomas. Haematologica (2022). doi: 10.3324/haematol.2021.279908
22. Kuo DE, Wei MM, Knickelbein JE, Armbrust KR, Yeung IYL, Lee AY, et al. Logistic regression classification of primary vitreoretinal lymphoma versus uveitis by interleukin 6 and interleukin 10 levels. Ophthalmology (2020) 127(7):956–62. doi: 10.1016/j.ophtha.2020.01.042
23. Zhang Y, Zou D, Yin J, Zhang L, Zhang X, Wang W, et al. Changes in cerebrospinal fluid interleukin-10 levels display better performance in predicting disease relapse than conventional magnetic resonance imaging in primary central nervous system lymphoma. BMC Cancer (2021) 21(1):183. doi: 10.1186/s12885-020-07774-5
24. Song Y, Zhang W, Zhang L, Wu W, Zhang Y, Han X, et al. Cerebrospinal fluid IL-10 and IL-10/IL-6 as accurate diagnostic biomarkers for primary central nervous system Large b-cell lymphoma. Sci Rep (2016) 6:38671. doi: 10.1038/srep38671
25. Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev (2016) 35(3):347–76. doi: 10.1007/s10555-016-9629-x
26. Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, et al. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat Rev Cancer. (2017) 17(4):223–38. doi: 10.1038/nrc.2017.7
27. Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, et al. DNA Fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res (2001) 61(4):1659–65.
28. Stroun M, Lyautey J, Lederrey C, Olson-Sand A, Anker P. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clinica Chimica acta; Int J Clin Chem (2001) 313(1-2):139–42. doi: 10.1016/S0009-8981(01)00665-9
29. Dudley JC, Diehn M. Detection and diagnostic utilization of cellular and cell-free tumor DNA. Annu Rev Pathol (2021) 16:199–222. doi: 10.1146/annurev-pathmechdis-012419-032604
30. Aldea M, Hendriks L, Mezquita L, Jovelet C, Planchard D, Auclin E, et al. Circulating tumor DNA analysis for patients with oncogene-addicted NSCLC with isolated central nervous system progression. J Thorac Oncol (2020) 15(3):383–91. doi: 10.1016/j.jtho.2019.11.024
31. Azad TD, Jin MC, Bernhardt LJ, Bettegowda C. Liquid biopsy for pediatric diffuse midline glioma: A review of circulating tumor DNA and cerebrospinal fluid tumor DNA. Neurosurg focus (2020) 48(1):E9. doi: 10.3171/2019.9.FOCUS19699
32. Kimura H, Kasahara K, Kawaishi M, Kunitoh H, Tamura T, Holloway B, et al. Detection of epidermal growth factor receptor mutations in serum as a predictor of the response to gefitinib in patients with non-small-cell lung cancer. Clin Cancer Res (2006) 12(13):3915–21. doi: 10.1158/1078-0432.CCR-05-2324
33. Gray ES, Rizos H, Reid AL, Boyd SC, Pereira MR, Lo J, et al. Circulating tumor DNA to monitor treatment response and detect acquired resistance in patients with metastatic melanoma. Oncotarget (2015) 6(39):42008–18. doi: 10.18632/oncotarget.5788
34. Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med (2008) 14(9):985–90. doi: 10.1038/nm.1789
35. Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. New Engl J Med (2013) 368(13):1199–209. doi: 10.1056/NEJMoa1213261
36. Lecomte T, Berger A, Zinzindohoué F, Micard S, Landi B, Blons H, et al. Detection of free-circulating tumor-associated DNA in plasma of colorectal cancer patients and its association with prognosis. Int J Cancer (2002) 100(5):542–8. doi: 10.1002/ijc.10526
37. Hamfjord J, Guren TK, Dajani O, Johansen JS, Glimelius B, Sorbye H, et al. Total circulating cell-free DNA as a prognostic biomarker in metastatic colorectal cancer before first-line oxaliplatin-based chemotherapy. Ann Oncol (2019) 30(7):1088–95. doi: 10.1093/annonc/mdz139
38. Valpione S, Gremel G, Mundra P, Middlehurst P, Galvani E, Girotti MR, et al. Plasma total cell-free DNA (cfDNA) is a surrogate biomarker for tumour burden and a prognostic biomarker for survival in metastatic melanoma patients. Eur J Cancer (Oxford England: 1990) (2018) 88:1–9. doi: 10.1016/j.ejca.2017.10.029
39. Rossi D, Diop F, Spaccarotella E, Monti S, Zanni M, Rasi S, et al. Diffuse large b-cell lymphoma genotyping on the liquid biopsy. Blood (2017) 129(14):1947–57. doi: 10.1182/blood-2016-05-719641
40. Rivas-Delgado A, Nadeu F, Enjuanes A, Casanueva-Eliceiry S, Mozas P, Magnano L, et al. Mutational landscape and tumor burden assessed by cell-free DNA in diffuse Large b-cell lymphoma in a population-based study. Clin Cancer Res (2021) 27(2):513–21. doi: 10.1158/1078-0432.CCR-20-2558
41. Hur JY, Kim YJ, Yoon SE, Son DS, Park WY, Kim SJ, et al. Plasma cell-free DNA is a prognostic biomarker for survival in patients with aggressive non-Hodgkin lymphomas. Ann Hematol (2020) 99(6):1293–302. doi: 10.1007/s00277-020-04008-3
42. Zhang W, Wang W, Han X, Gan Y, Qian L, Zhang Y, et al. Circulating tumor DNA by high-throughput sequencing of T cell receptor monitored treatment response and predicted treatment failure in T cell lymphomas. Int J Lab Hematol (2021) 43(5):1041–9. doi: 10.1111/ijlh.13498
43. Wang Y, Springer S, Zhang M, McMahon KW, Kinde I, Dobbyn L, et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci USA (2015) 112(31):9704–9. doi: 10.1073/pnas.1511694112
44. Bobillo S, Crespo M, Escudero L, Mayor R, Raheja P, Carpio C, et al. Cell free circulating tumor DNA in cerebrospinal fluid detects and monitors central nervous system involvement of b-cell lymphomas. Haematologica (2021) 106(2):513–21. doi: 10.3324/haematol.2019.241208
45. Zorofchian S, Lu G, Zhu JJ, Duose DY, Windham J, Esquenazi Y, et al. Detection of the MYD88 p.L265P mutation in the CSF of a patient with secondary central nervous system lymphoma. Front Oncol (2018) 8:382. doi: 10.3389/fonc.2018.00382
46. Rimelen V, Ahle G, Pencreach E, Zinniger N, Debliquis A, Zalmaï L, et al. Tumor cell-free DNA detection in CSF for primary CNS lymphoma diagnosis. Acta Neuropathol Commun (2019) 7(1):43. doi: 10.1186/s40478-019-0692-8
47. De Mattos-Arruda L, Mayor R, Ng CKY, Weigelt B, Martínez-Ricarte F, Torrejon D, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun (2015) 6:8839. doi: 10.1038/ncomms9839
48. Zhang Y, Zhang X, Zou D, Yin J, Zhang L, Wang X, et al. Lenalidomide and rituximab regimen combined with intravitreal methotrexate followed by lenalidomide maintenance for primary vitreoretinal lymphoma: A prospective phase II study. Front Oncol (2021) 11:701507. doi: 10.3389/fonc.2021.701507
49. Lv X, Zhao M, Yi Y, Zhang L, Guan Y, Liu T, et al. Detection of rare mutations in CtDNA using next generation sequencing. J Visualized Experiments: JoVE (2017) 126:56342. doi: 10.3791/56342
50. Talevich E, Shain AH, Botton T, Bastian BC. CNVkit: Genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput Biol (2016) 12(4):e1004873. doi: 10.1371/journal.pcbi.1004873
51. Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative genomics viewer (IGV): high-performance genomics data visualization and exploration. Briefings Bioinf (2013) 14(2):178–92. doi: 10.1093/bib/bbs017
52. Parikh R, Mathai A, Parikh S, Chandra Sekhar G, Thomas R. Understanding and using sensitivity, specificity and predictive values. Indian J Ophthalmol (2008) 56(1):45–50. doi: 10.4103/0301-4738.37595
53. Carbonell D, Mahajan S, Chee SP, Sobolewska B, Agrawal R, Bülow T, et al. Consensus recommendations for the diagnosis of vitreoretinal lymphoma. Ocular Immunol Inflamm (2021) 29(3):507–20. doi: 10.1080/09273948.2021.1878233
54. Cani AK, Hovelson DH, Demirci H, Johnson MW, Tomlins SA, Rao RC. Next generation sequencing of vitreoretinal lymphomas from small-volume intraocular liquid biopsies: new routes to targeted therapies. Oncotarget (2017) 8(5):7989–98. doi: 10.18632/oncotarget.14008
55. Ferreri AJM, Calimeri T, Lopedote P, Francaviglia I, Daverio R, Iacona C, et al. MYD88 L265P mutation and interleukin-10 detection in cerebrospinal fluid are highly specific discriminating markers in patients with primary central nervous system lymphoma: results from a prospective study. Br J Haematol (2021) 193(3):497–505. doi: 10.1111/bjh.17357
56. Yu X, Li W, Deng Q, Li L, Hsi ED, Young KH, et al. MYD88 L265P mutation in lymphoid malignancies. Cancer Res (2018) 78(10):2457–62. doi: 10.1158/0008-5472.CAN-18-0215
57. Akpek EK, Maca SM, Christen WG, Foster CS. Elevated vitreous interleukin-10 level is not diagnostic of intraocular-central nervous system lymphoma. Ophthalmology (1999) 106(12):2291–5. doi: 10.1016/S0161-6420(99)90528-6
58. Sugita S, Takase H, Sugamoto Y, Arai A, Miura O, Mochizuki M. Diagnosis of intraocular lymphoma by polymerase chain reaction analysis and cytokine profiling of the vitreous fluid. Japanese J Ophthalmol (2009) 53(3):209–14. doi: 10.1007/s10384-009-0662-y
Keywords: cerebrospinal fluid, circulating cell-free DNA, IL-10, MYD88, vitreoretinal lymphoma
Citation: Zhuang Z, Zhang Y, Zhang X, Zhang M, Zou D, Zhang L, Jia C and Zhang W (2022) Circulating cell-free DNA and IL-10 from cerebrospinal fluids aid primary vitreoretinal lymphoma diagnosis. Front. Oncol. 12:955080. doi: 10.3389/fonc.2022.955080
Received: 28 May 2022; Accepted: 29 June 2022;
Published: 18 August 2022.
Edited by:
Sylvain Choquet, Hôpitaux Universitaires Pitié Salpêtrière, FranceReviewed by:
Vincent Camus, Centre Henri Becquerel Rouen, FranceCopyright © 2022 Zhuang, Zhang, Zhang, Zhang, Zou, Zhang, Jia and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhang, dnYxMjIzQHZpcC5zaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.