- 1Medical School of Chinese People’s Liberation Army, Beijing, China
- 2Department of Oncology, The First Medical Centre, Chinese People’s Liberation Army General Hospital, Beijing, China
Background: There are currently no established biomarkers that can predict whether advanced pancreatic carcinoma (PC) patients would benefit from immune checkpoint inhibitors (ICIs). Our study investigated whether the pretreatment composite biomarker of derived neutrophil–lymphocyte ratio (dNLR) and lactate dehydrogenase (LDH) can be used as a reliable prognostic factor for the survival of PC patients receiving PD-1 inhibitor therapy.
Methods: Patients with advanced PC treated with PD-1 inhibitors at a single center from September 2015 to September 2020 were included. The high levels of dNLR (≥3) and LDH (≥250 U/L) were considered to be risk factors. Based on these two risk factors, patients in this study were categorized into two risk groups: the good dNLR-LDH group, without risk factors, and the intermediate/poor dNLR-LDH group, with one to two risk factors. Overall survival (OS) and progression-free survival (PFS) served as this study’s primary and secondary endpoints. Cox regression models were used to identify independent prognostic factors for survival benefit.
Results: There were 98 patients in our study. The good group included 61 (62.2%) patients and the intermediate/poor group included 37 (37.8%). The overall patients with PC who received immunotherapy had a median OS of 12.1 months, and the good dNLR-LDH group had a significantly longer OS compared with the intermediate/poor dNLR-LDH group (44.2 vs. 6.4 months; p < 0.010); median PFS was 3.7 and 2.5 months (p = 0.010). The number of metastatic sites >2 and immunotherapy as third-line or later was associated with worse PFS, and the line of immunotherapy and the dNLR-LDH indicator were independent prognostic factors for OS, according to multivariate analysis.
Conclusion: The pretreatment composite biomarker of dNLR and LDH can be used as a prognostic biomarker in patients with advanced PC treated with PD-1 inhibitors.
Introduction
Pancreatic carcinoma (PC) is one of the deadliest malignant tumors with a low survival rate. Until now, the prognosis remains dismal; the overall 5-year survival rate is less than 5% (1). The main factor for the low survival rate is the late presentation of most patients. Since the disease is relatively asymptomatic at the initial stage, most patients are not examined and treated until the late stage. Not more than 20% of newly diagnosed PC patients have the opportunity to undergo surgery. The tumor is highly malignant and progresses rapidly; chemotherapies and palliative care are still the most essential treatment for PC patients. However, the efficacy of existing drugs is limited (2, 3). The improved chemotherapy regimen FOLFIRINOX significantly increased adverse reactions but only slightly prolonged the median survival time (4).
Although no adequate treatment has been found yet, immunotherapy has gradually become a promising new therapy for PC (5, 6) and other malignancies (7, 8). However, there is still a notable portion of patients with PC that has poor curative effects after receiving this treatment. Therefore, it is critical to identify people who are candidates for immunotherapy and can benefit from it.
Convenient prognostic biomarkers can play a significant role in clinical practice. Previously, Mezquita et al. developed the lung immune prognostic index (LIPI) by combining baseline derived neutrophil–lymphocyte ratio (dNLR) and lactate dehydrogenase (LDH) to classify patients with non-small cell lung cancer treated with programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) inhibitors (9), thus providing a new way to predict prognosis.
The predictive usefulness of dNLR in combination with LDH in a range of solid tumors treated with immunotherapy, including renal cell carcinoma, non-small cell lung cancer, and gastric cancer, has been confirmed in several early studies (10–14). Therefore, we analyzed real-world data to assess the prognostic value of the pretreatment composite biomarker of dNLR and LDH in PC patients treated with PD-1 inhibitors.
Patients and methods
Patients with advanced PC who received PD-1 inhibitors (nivolumab/pembrolizumab/sintilimab) at the Chinese PLA General Hospital (Beijing, China) between September 2015 and September 2020 were included in this study. The inclusion criteria were as follows (1): age > 18 years old (2); Eastern Cooperative Oncology Group (ECOG) performance status of 0–1; (3) diagnosed as locally advanced or advanced PC; and (4) available CT scans and blood test results during immunotherapy.
Demographic, clinical, and pathological data were collected, including age, gender, type of PD-1 inhibitor, smoking history, smoking years, differentiation degree of tumor tissue, number of metastatic sites, prior target therapy, whether PD-1 inhibitor was combined with other drugs, line of immunotherapy, baseline CA19-9 level, white blood cell count, absolute neutrophil count, absolute lymphocyte count, and serum LDH level. This research was authorized by the Ethics Committee of the Chinese PLA General Hospital and performed according to the principles of the Declaration of Helsinki.
The dNLR-LDH indicator was calculated by dNLR (absolute neutrophil count/[white blood cell count − absolute neutrophil count]) and LDH. The cutoff values of dNLR and LDH were 3 and the upper limit of normal value (ULN, 250 U/L), respectively. Patients were divided into the good dNLR-LDH group (dNLR < 3 and LDH normal) and the intermediate/poor dNLR-LDH group (intermediate: dNLR < 3 and LDH ≥ ULN, or dNLR ≥ 3 and LDH < ULN; poor: dNLR ≥ 3 and LDH ≥ ULN).
According to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1), the efficacy of immunotherapy was evaluated. Overall survival (OS) was defined as the time from the first PD-1 inhibitor treatment to death. Progression-free survival (PFS) was defined as the time from the first PD-1 inhibitor treatment to progressive disease (PD) or death.
Statistical analysis
IBM SPSS version 26.0 was used to perform statistical analysis. The Kaplan–Meier method was utilized to analyze OS and PFS, and the differences were evaluated by log-rank test. The clinical characteristics of patients were compared by Chi-square or Fisher’s exact test. The Cox proportional hazard regression model was used for univariate and multivariate analysis. Multivariate analysis was performed on covariates that showed a significant correlation with OS and PFS in univariate analysis. All statistical tests were two-sided, and p < 0.05 was statistically significant.
Results
Clinical characteristics of patients
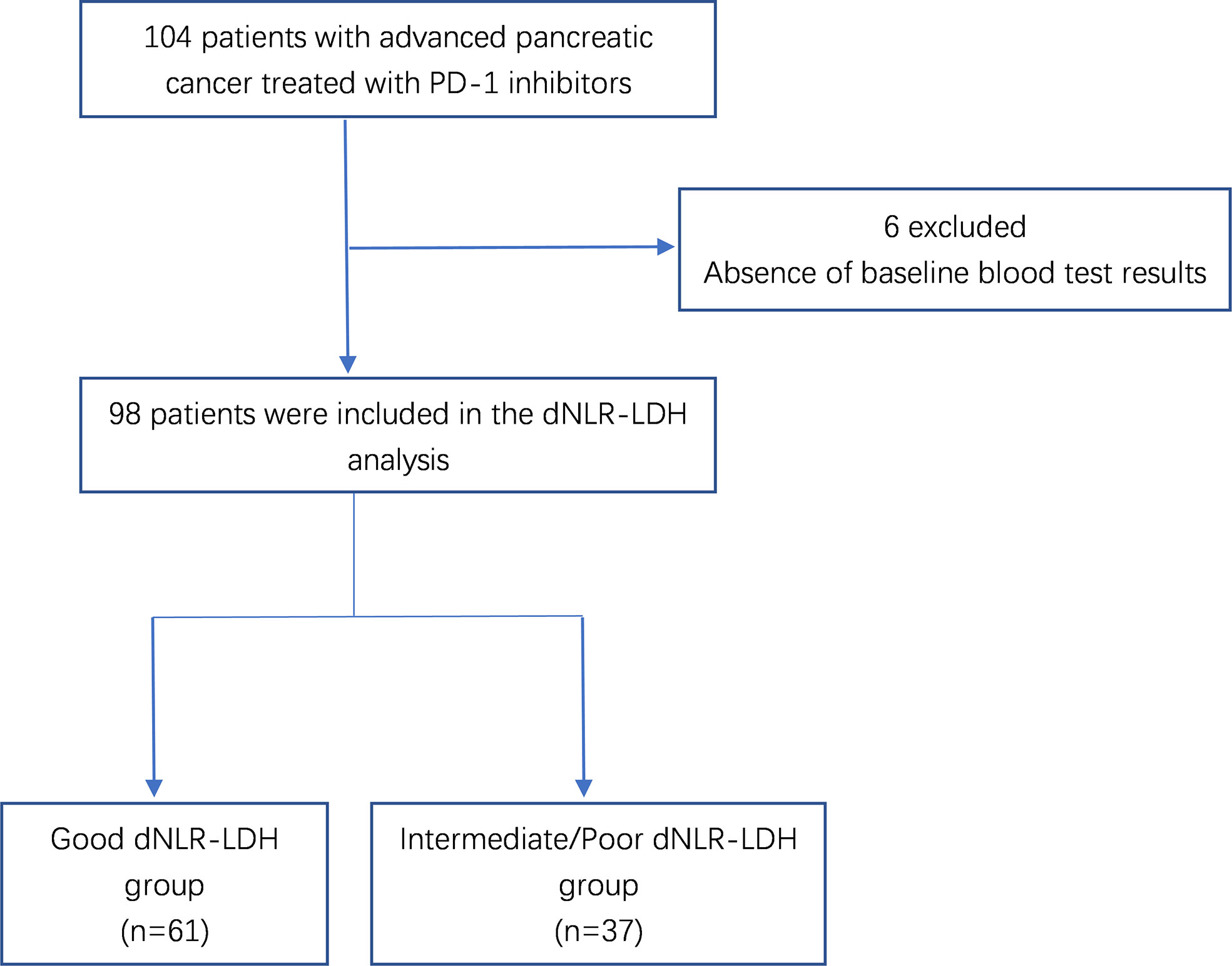
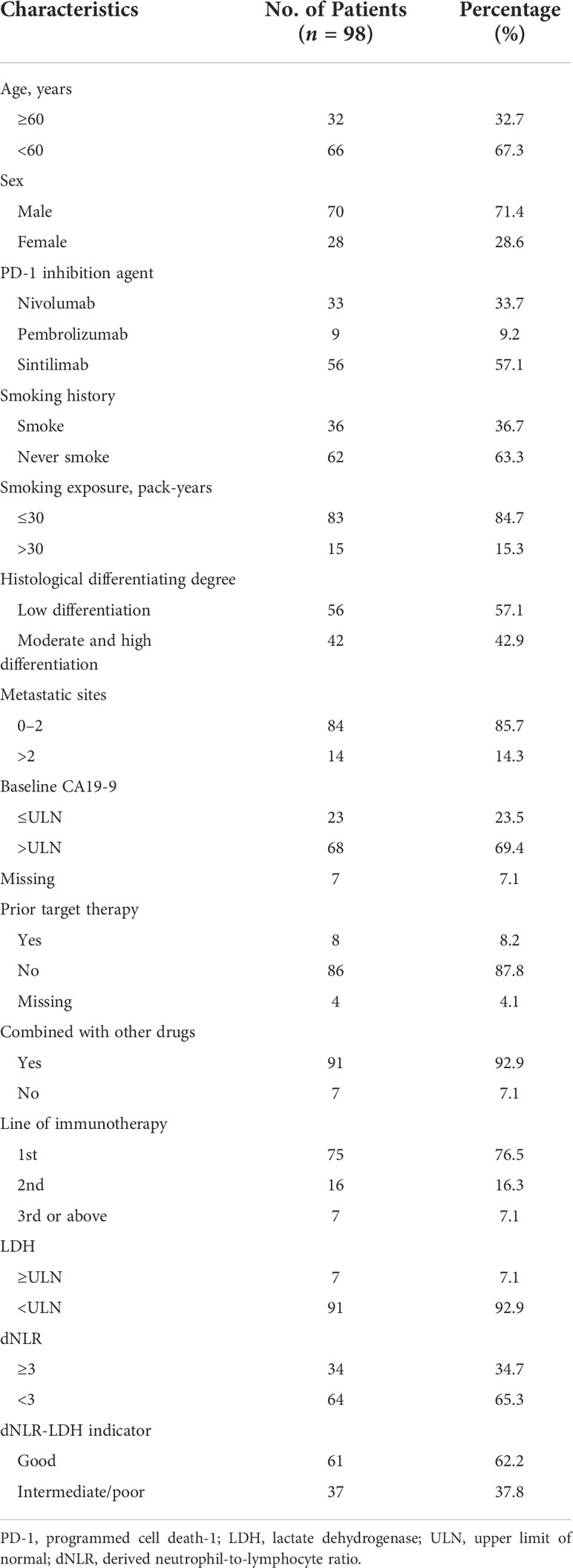
A total of 104 patients with advanced PC were treated with PD-1 inhibitors. After excluding 6 patients without relevant data required for dNLR-LDH grouping, 98 PC patients were included in the study for clinical data analysis (Figure 1). Most patients (92.9%) received PD-1 inhibitors combined with chemotherapy or targeted therapy. The median age was 56 years, and 71.4% were male; 57.1% of patients had low differentiation and 85.7% of patients had ≤2 metastatic sites. Patients with baseline CA19-9 level higher than normal accounted for 69.4%. A total of 61 patients (62.2%) had a good dNLR-LDH indicator. Detailed baseline clinicopathological features of patients are summarized in Tables 1 and 2.
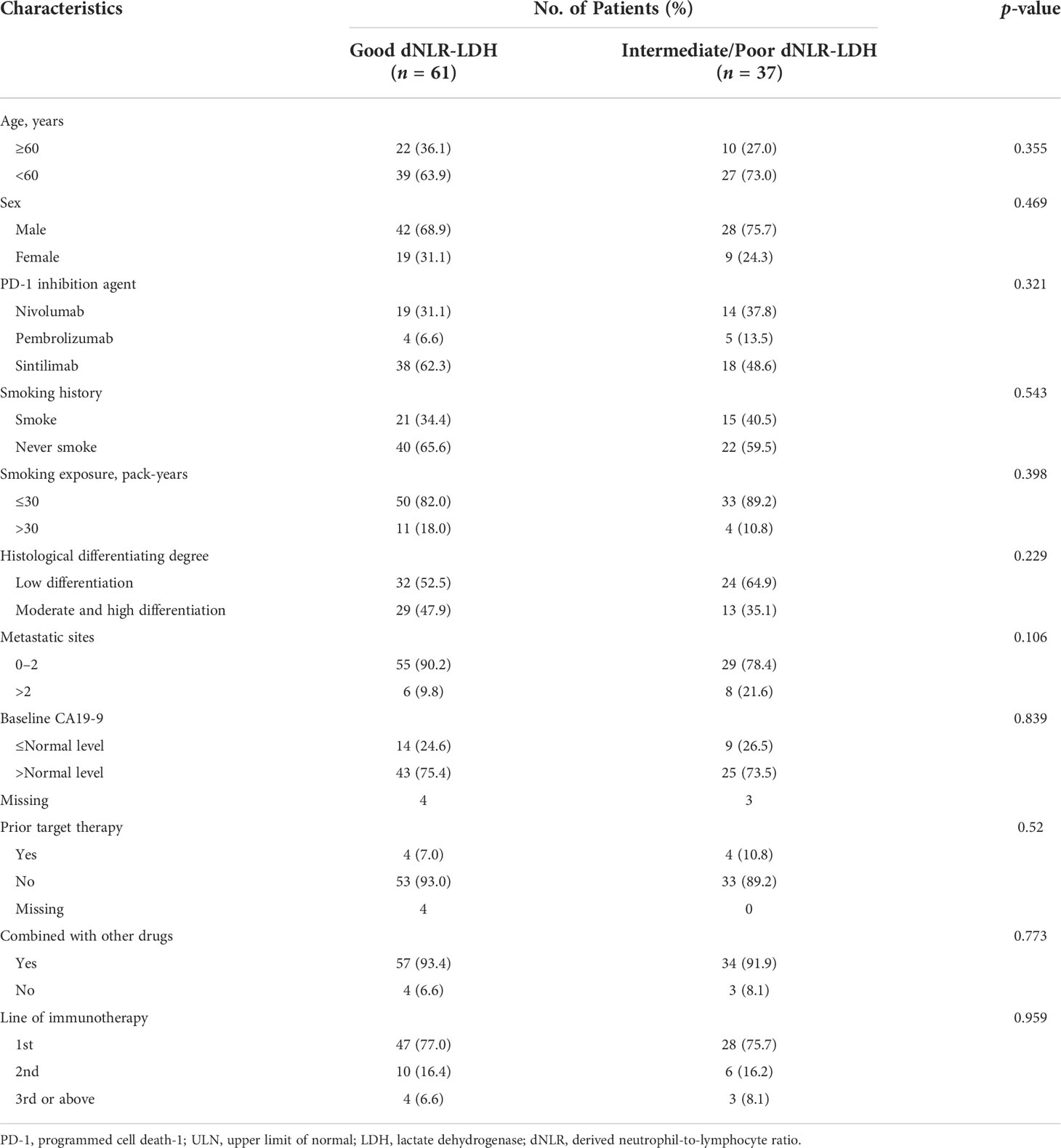
Table 2 Differences of patients’ characteristics between the good dNLR-LDH group and the intermediate/poor dNLR-LDH group.
Association between dNLR-LDH and prognosis
Effect of the dNLR-LDH indicator on OS
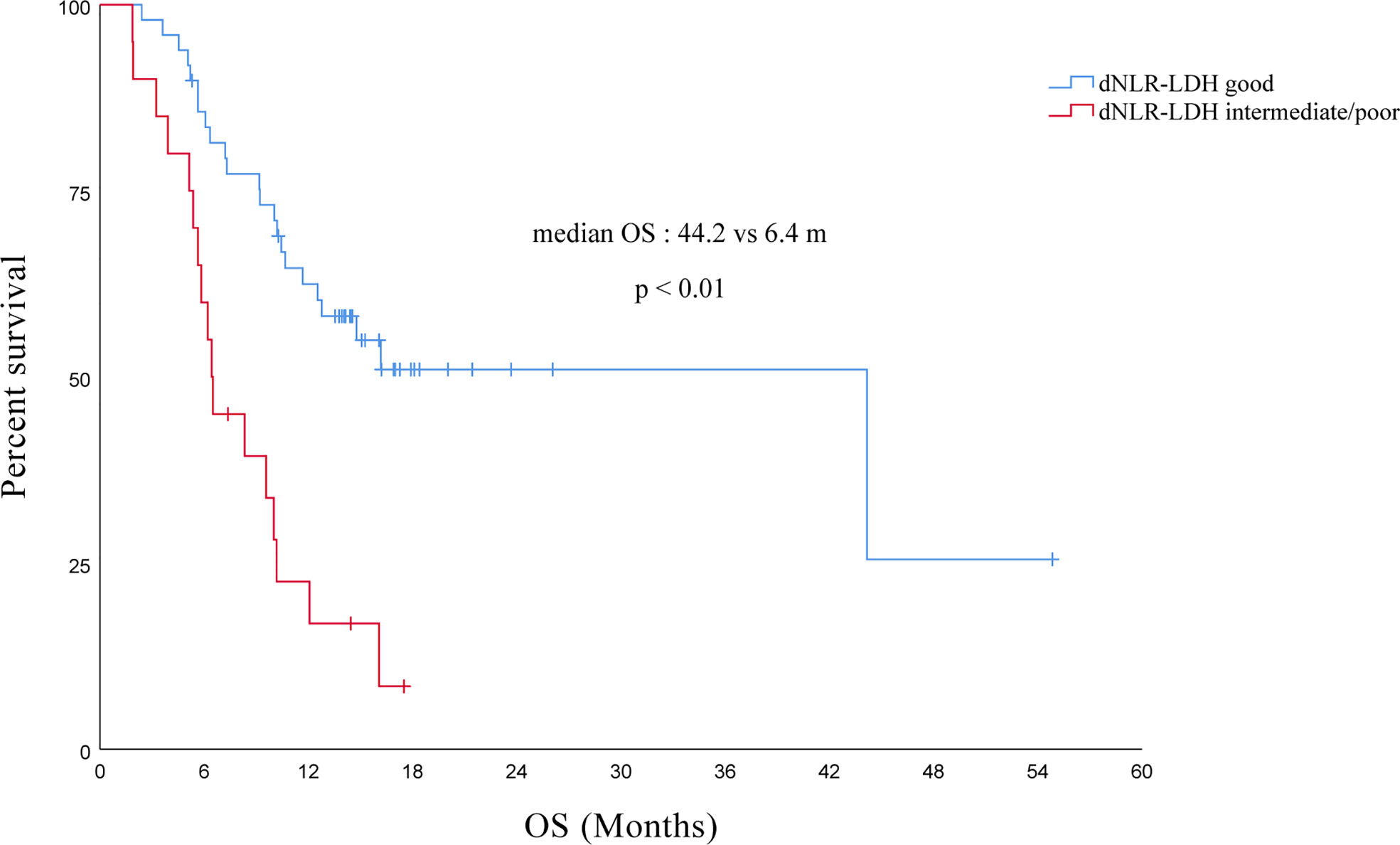
The median OS of all PC patients treated with immunotherapy (single/combined) was 12.1 (95% CI, 7.4–16.8) months. Compared with the intermediate/poor dNLR-LDH group, the OS of the good dNLR-LDH group was significantly prolonged (p < 0.001) (Figure 2). The median OS in the good and intermediate/poor dNLR-LDH groups was 44.2 (95% CI, 9.4–79.0) and 6.4 (95% CI, 5.8–7.1) months, respectively.
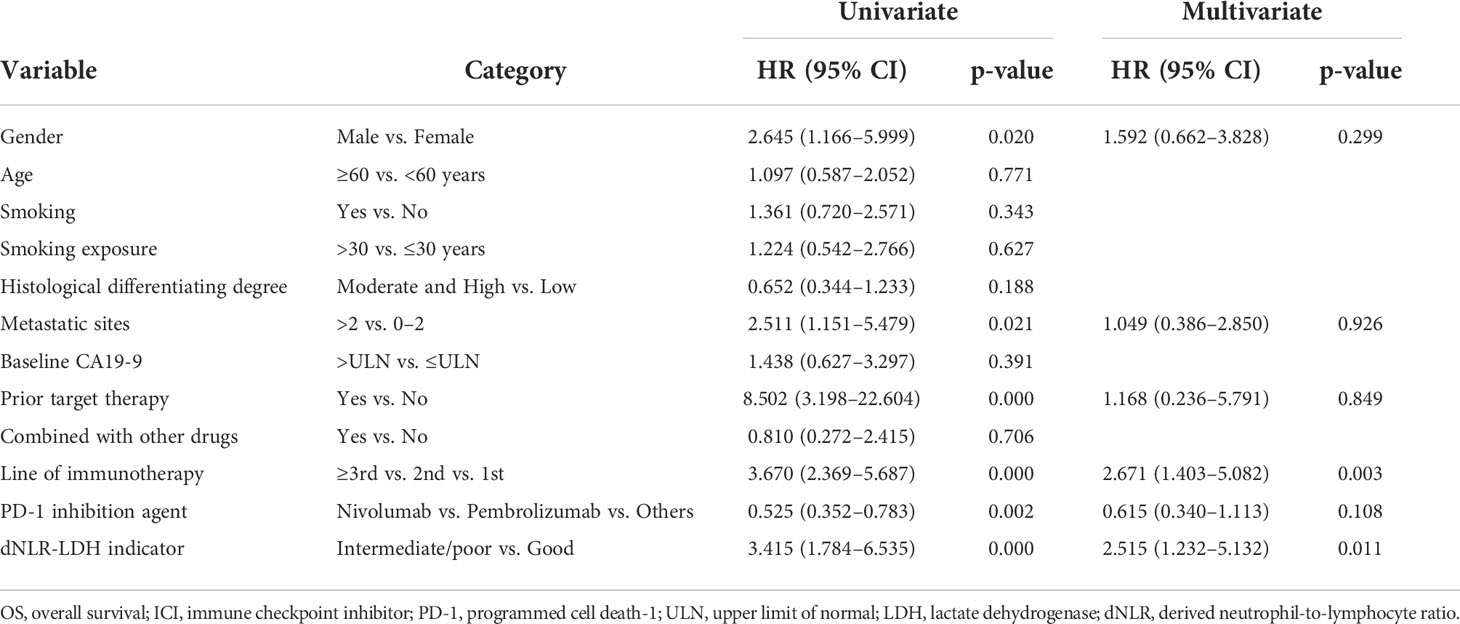
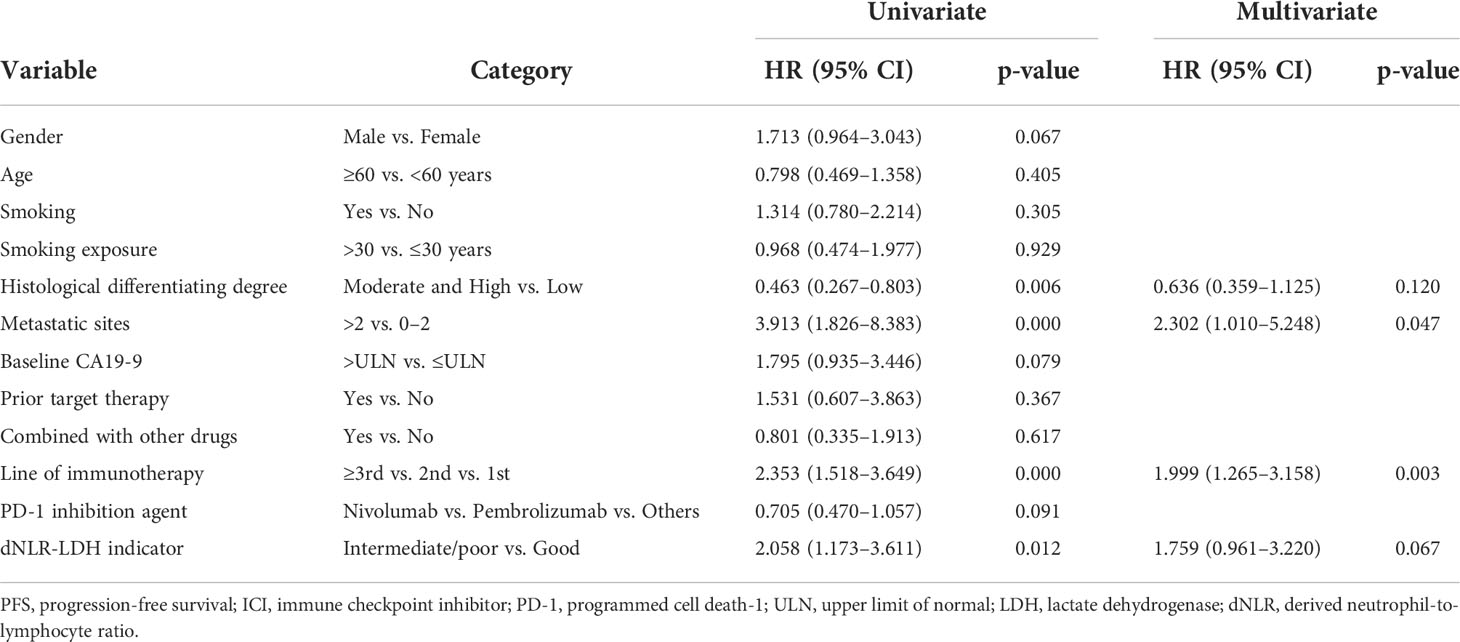
In univariate analysis, the number of metastatic sites, previous targeted therapy, gender, line of immunotherapy, types of PD-1 inhibitors, and the dNLR-LDH indicator were significantly correlated with OS (p < 0.05). Multivariate analysis showed that intermediate/poor dNLR-LDH was associated with a significantly increased risk of death (HR, 2.52; 95% CI, 1.23–5.13; p = 0.011). The line of immunotherapy ≥3 is also an independent poor prognostic factor (Table 3).
Effect of the dNLR-LDH indicator on PFS
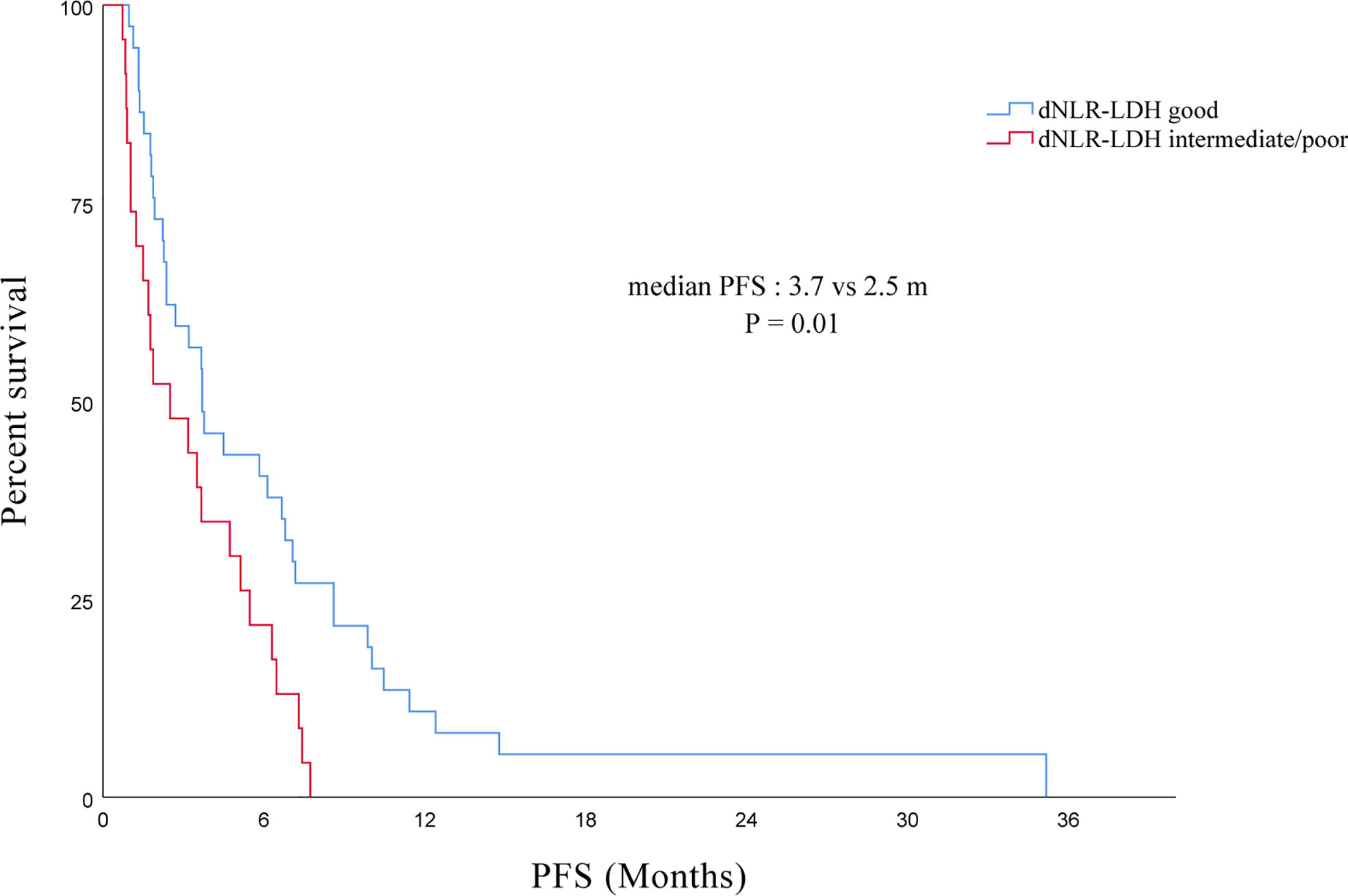
The median PFS of all PC patients treated with immunotherapy (single/combined) was 3.5 (95% CI, 2.4–4.6) months. The PFS of the good dNLR-LDH group was longer than that of the intermediate/poor dNLR-LDH group (p = 0.010) (Figure 3). The median PFS in the good and intermediate/poor dNLR-LDH groups was 3.7 (95% CI, 2.2–5.3) and 2.5 (95% CI, 0.3–4.7) months, respectively.
In univariate analysis, the degree of histological differentiation, the number of metastatic sites, the line of immunotherapy, and the dNLR-LDH indicator were significantly correlated with PFS (p < 0.05). Multivariate analysis showed that the number of metastatic sites and the line of immunotherapy ≥3 were the adverse factors affecting PFS (p < 0.05). The dNLR-LDH indicator was another independent determinant, with a marginal p = 0.067 (HR, 1.76; 95% CI, 0.96–3.22) (Table 4).
Discussion
Immunotherapy is a popular topic in the current medical community and has promising prospects in a variety of cancers. However, not all patients, particularly those with PC, can benefit from PD-1 inhibitor therapy. We need to find out patients who respond to immunotherapy. Therefore, it is crucial to explore biomarkers that can predict treatment prognosis.
Several studies have previously demonstrated the predictive value of the composite biomarker of dNLR and LDH for immunotherapy prognosis in various solid tumors (10–12), which can provide practical guidance for clinical work. The dNLR-LDH indicator, which can be inferred from patient blood test results, has the advantages of being affordable, easily reproducible, and widely available. Monitoring these indicators can early predict the prognosis of some advanced pancreatic cancer patients receiving PD-1 inhibitor therapy, and are able to select and stratify patients who may benefit, facilitating the development of more precise and individualized treatment.
To our knowledge, this is the first study to show that pretreatment dNLR-LDH can serve as a prognostic predictive biomarker in PC patients receiving PD-1 inhibitor therapy. dNLR can reflect the inflammatory state of the whole body. It was calculated as absolute neutrophil count/[white blood cell count − absolute neutrophil count]. It has been implicated in cancer progression through cytokine production, and it can also promote immune evasion (15, 16). It is well known that systemic inflammation is closely associated with the prognosis of immunotherapy. Indicators of inflammation potentially presented in the blood can indicate poor prognosis in patients with malignancies, including elevated levels of neutrophils, platelets, NLR, platelet–lymphocyte ratio (PLR), and LDH (17, 18). dNLR can provide more information than NLR because it includes monocytes and other granulocyte subsets in addition to lymphocytes; it is considered an unfavorable prognostic factor in a wide range of malignancies (19, 20), whereas LDH can reflect the patient’s tumor burden. Several studies have shown the correlation between dNLR and LDH and the prognosis of immunotherapy (18, 21–23), and in previous studies of non-small cell lung cancer, patients with a “good” LIPI score (i.e., dNLR < 3 and normal LDH) had better OS and PFS, regardless of whether they have received immunotherapy, targeted therapy, or chemotherapy (12). To our knowledge, there are no studies that have explored the relationship between the combined indicator of dNLR and LDH and the prognosis of pancreatic cancer treatment. However, there have been some studies showing that NLR can be used as a prognostic indicator in pancreatic cancer patients receiving chemotherapy (24). Furthermore, high LDH levels at baseline may be associated with shorter OS in patients with advanced pancreatic cancer treated with chemotherapy (25). Therefore, we can predict that dNLR-LDH may not only be a prognostic marker for pancreatic cancer patients receiving PD-1 inhibitors, but could also play an important role in the early prediction for the prognosis of other treatment options (e.g., chemotherapy). This speculation remains to be further confirmed by more studies.
In univariate analysis, our findings imply that the pretreatment dNLR-LDH indicator was associated with PFS and OS in ICI-treated advanced PC patients. The multivariate analysis showed that the pretreatment dNLR-LDH indicator is an independent prognostic factor for OS (p < 0.05). However, in the correlation analysis of PFS, marginal p = 0.067. We must take into account the limits of this retrospective study: We cannot arbitrarily define the results as not statistically significant. Since PFS is affected by several factors, such as the time and frequency of tumor evaluation, radiological assessments were performed within a time range of ±2 weeks. From mPFS, we can also see that patients with a good dNLR-LDH indicator had a better prognosis, with an mPFS of 3.7 (95% CI, 2.2–5.3) and 2.5 (95% CI, 0.3–4.7) months in the good and intermediate/poor dNLR-LDH groups, respectively. In addition, although many factors were considered in the multivariate analysis, we failed to incorporate some factors into the study that may also affect the final results because some tests in patients with PC were not routinely performed in clinical work, resulting in missing test reports, such as PD-L1 and tumor mutation burden (TMB) (26). However, recent studies have shown that the predictive value of the composite biomarker of dNLR and LDH is independent of PD-L1 expression and TMB (27, 28). Furthermore, in multivariate analysis, our study also found that the line of immunotherapy and the number of metastatic sites were associated with the PFS of PC patients treated with immunotherapy, and the line of immunotherapy is also related to the patient’s OS. The number of metastasis sites reflected the tumor burden of patients; in other words, the heavier the tumor load, the higher the possibility of short-term progress after receiving immunotherapy. If patients who had already undergone a lengthy course of treatment before received third-line immunotherapy or later, their overall condition at this point may be worse than those who received first-line immunotherapy. This may be one of the reasons why the use of immunotherapy as a third-line or later treatment is associated with worse PFS and OS.
There are some shortcomings within our investigation. First, this is a single-center retrospective study with a limited sample size, which could not avoid possible confounding factors and selective bias. Due to the small sample size, we divided the cohort into two groups (good dNLR-LDH and intermediate/poor dNLR-LDH) instead of three (good, intermediate, and poor dNLR-LDH) for analysis. Nonetheless, our study provides a simple and non-invasive method to help identify patients with advanced PC who might benefit from PD-1 inhibitor therapy in clinical practice.
Conclusion
Our finding confirms the utility of the dNLR-LDH indicator in the prognostic prediction of advanced PC patients treated with PD-1 inhibitors, and their potential to help with patient stratification and clinical decision. Further prospective studies of the dNLR-LDH indicator in anti-PD-1-based randomized clinical trials are warranted in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was reviewed and approved by The Ethics Committee of Chinese PLA General Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SC was in charge of writing and analysis. GD and ZT provided the guide and idea. SG, MG, YP, MF, and NZ contributed to analysis. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
dNLR, derived neutrophil–lymphocyte ratio; ECOG, Eastern Cooperative Oncology Group; ICIs, immune checkpoint inhibitors; LDH, lactate dehydrogenase; LIPI, lung immune prognostic index; OS, overall survival; PC, pancreatic carcinoma; PD-L1, programmed death ligand-1; PD-1, programmed death-1; PFS, progression-free survival; PD, progressive disease; TMB, tumor mutation burden; ULN, upper limit of normal.
References
1. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA: Cancer J Clin (2019) 69(5):363–85. doi: 10.3322/caac.21565
2. Löhr M. Is it possible to survive pancreatic cancer? Nat Clin Pract Gastroenterol Hepatol (2006) 3(5):236–7. doi: 10.1038/ncpgasthep0469
3. Niederhuber JE, Brennan MF, Menck HR. The national cancer data base report on pancreatic cancer. Cancer (1995) 76(9):1671–7. doi: 10.1002/1097-0142(19951101)76:9<1671
4. Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. Folfirinox or gemcitabine as adjuvant therapy for pancreatic cancer. New Engl J Med (2018) 379(25):2395–406. doi: 10.1056/NEJMoa1809775
5. Wu AA, Jaffee E, Lee V. Current status of immunotherapies for treating pancreatic cancer. Curr Oncol Rep (2019) 21(7):60. doi: 10.1007/s11912-019-0811-5
6. Guddati AK, Komiya T, Patel SJ, Sharma N, Powell E. Impact of immunotherapy use in patients with stage iv pancreatic carcinoma. J gastrointestinal Oncol (2020) 11(4):654–62. doi: 10.21037/jgo-20-191
7. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (Checkmate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (London England) (2017) 389(10088):2492–502. doi: 10.1016/s0140-6736(17)31046-2
8. Brower V. Checkpoint blockade immunotherapy for cancer comes of age. J Natl Cancer Institute (2015) 107(3):djv069. doi: 10.1093/jnci/djv069
9. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol (2018) 4(3):351–7. doi: 10.1001/jamaoncol.2017.4771
10. Meyers DE, Stukalin I, Vallerand IA, Lewinson RT, Suo A, Dean M, et al. The lung immune prognostic index discriminates survival outcomes in patients with solid tumors treated with immune checkpoint inhibitors. Cancers (2019) 11(11):1713. doi: 10.3390/cancers11111713
11. Benitez JC, Recondo G, Rassy E, Mezquita L. The lipi score and inflammatory biomarkers for selection of patients with solid tumors treated with checkpoint inhibitors. Q J Nucl Med Mol Imaging Off Publ Ital Assoc Nucl Med (AIMN) [and] Int Assoc Radiopharmacol (IAR) [and] Section So (2020) 64(2):162–74. doi: 10.23736/s1824-4785.20.03250-1
12. Kazandjian D, Gong Y, Keegan P, Pazdur R, Blumenthal GM. Prognostic value of the lung immune prognostic index for patients treated for metastatic non-small cell lung cancer. JAMA Oncol (2019) 5(10):1481–5. doi: 10.1001/jamaoncol.2019.1747
13. Pan Y, Si H, Deng G, Chen S, Zhang N, Zhou Q, et al. A composite biomarker of derived neutrophil-lymphocyte ratio and platelet-lymphocyte ratio correlates with outcomes in advanced gastric cancer patients treated with anti-Pd-1 antibodies. Front Oncol (2021) 11:798415. doi: 10.3389/fonc.2021.798415
14. Li L, Pi C, Yan X, Lu J, Yang X, Wang C, et al. Prognostic value of the pretreatment lung immune prognostic index in advanced small cell lung cancer patients treated with first-line pd-1/Pd-L1 inhibitors plus chemotherapy. Front Oncol (2021) 11:697865. doi: 10.3389/fonc.2021.697865
15. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol (2011) 11(8):519–31. doi: 10.1038/nri3024
16. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol (2014) 15(11):e493–503. doi: 10.1016/s1470-2045(14)70263-3
17. Russo A, Franchina T, Ricciardi GRR, Battaglia A, Scimone A, Berenato R, et al. Baseline neutrophilia, derived neutrophil-to-Lymphocyte ratio (Dnlr), platelet-to-Lymphocyte ratio (Plr), and outcome in non small cell lung cancer (Nsclc) treated with nivolumab or docetaxel. J Cell Physiol (2018) 233(10):6337–43. doi: 10.1002/jcp.26609
18. Diem S, Kasenda B, Spain L, Martin-Liberal J, Marconcini R, Gore M, et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-Pd-1 therapy in metastatic melanoma. Br J Cancer (2016) 114(3):256–61. doi: 10.1038/bjc.2015.467
19. Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, et al. Baseline neutrophils and derived neutrophil-to-Lymphocyte ratio: Prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol Off J Eur Soc Med Oncol (2016) 27(4):732–8. doi: 10.1093/annonc/mdw016
20. Dalpiaz O, Luef T, Seles M, Stotz M, Stojakovic T, Pummer K, et al. Critical evaluation of the potential prognostic value of the pretreatment-derived neutrophil-lymphocyte ratio under consideration of c-reactive protein levels in clear cell renal cell carcinoma. Br J Cancer (2017) 116(1):85–90. doi: 10.1038/bjc.2016.393
21. Muhammed A, Fulgenzi CAM, Dharmapuri S, Pinter M, Balcar L, Scheiner B, et al. The systemic inflammatory response identifies patients with adverse clinical outcome from immunotherapy in hepatocellular carcinoma. Cancers (2021) 14(1):186. doi: 10.3390/cancers14010186
22. Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, et al. Pretreatment neutrophil-to-Lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-Small-Cell lung cancer. Lung Cancer (Amsterdam Netherlands) (2017) 106:1–7. doi: 10.1016/j.lungcan.2017.01.013
23. Hsieh AH, Tahkar H, Koczwara B, Kichenadasse G, Beckmann K, Karapetis C, et al. Pre-treatment serum lactate dehydrogenase as a biomarker in small cell lung cancer. Asia-Pacific J Clin Oncol (2018) 14(2):e64–70. doi: 10.1111/ajco.12674
24. Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic cancer: Long-term survival from a phase iii trial. J Natl Cancer Institute (2015) 107(2):dju413. doi: 10.1093/jnci/dju413
25. Xiao Y, Chen W, Xie Z, Shao Z, Xie H, Qin G, et al. Prognostic relevance of lactate dehydrogenase in advanced pancreatic ductal adenocarcinoma patients. BMC Cancer (2017) 17(1):25. doi: 10.1186/s12885-016-3012-8
26. Cortellini A, Di Maio M, Nigro O, Leonetti A, Cortinovis DL, Aerts JG, et al. Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non-small cell lung cancer treated with first-line pembrolizumab versus cytotoxic chemotherapy. J immunother Cancer (2021) 9(4):e002421. doi: 10.1136/jitc-2021-002421
27. Ali WAS, Hui P, Ma Y, Wu Y, Zhang Y, Chen Y, et al. Determinants of survival in advanced non-small cell lung cancer patients treated with anti-Pd-1/Pd-L1 therapy. Ann Trans Med (2021) 9(22):1639. doi: 10.21037/atm-21-1702
Keywords: pancreatic cancer, immune checkpoint inhibitors, overall survival, progression-free survival, derived neutrophil–lymphocyte ratio, lactate dehydrogenase
Citation: Chen S, Guo S, Gou M, Pan Y, Fan M, Zhang N, Tan Z and Dai G (2022) A composite indicator of derived neutrophil–lymphocyte ratio and lactate dehydrogenase correlates with outcomes in pancreatic carcinoma patients treated with PD-1 inhibitors. Front. Oncol. 12:951985. doi: 10.3389/fonc.2022.951985
Received: 24 May 2022; Accepted: 10 October 2022;
Published: 25 October 2022.
Edited by:
Anupam Kotwal, University of Nebraska Medical Center, United StatesReviewed by:
Yunfeng Cheng, Fudan University, ChinaJingping Zhang, Memorial Sloan Kettering Cancer Center, United States
Copyright © 2022 Chen, Guo, Gou, Pan, Fan, Zhang, Tan and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanghai Dai, ZGFpZ2gzMDFAdmlwLnNpbmEuY29t; Zhaoli Tan, emhhb2xpdGFuQHllYWgubmV0
 Shiyun Chen
Shiyun Chen Shiyuan Guo
Shiyuan Guo Miaomiao Gou
Miaomiao Gou Yuting Pan
Yuting Pan Mengjiao Fan
Mengjiao Fan Nan Zhang
Nan Zhang Zhaoli Tan
Zhaoli Tan Guanghai Dai
Guanghai Dai