
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 31 August 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.951292
This article is part of the Research TopicWomen in Gynecological Oncology, volume II, 2022View all 11 articles
Objective: Serous tubal intraepithelial carcinoma (STIC) is a precursor lesion of pelvic high-grade serous carcinoma (HGSC). Information on treatment and outcome of isolated STIC is rare. Therefore, we reviewed systematically the published literature to determine the incidence of subsequent HGSC in the high- and low-risk population and to summarize the current diagnostic and therapeutic options.
Methods: A systematic review of the literature was conducted in MEDLINE-Ovid, Cochrane Library and Web of Science of articles published from February 2006 to July 2021. Patients with an isolated STIC diagnosis and clinical follow-up were included. Study exclusion criteria for review were the presence of synchronous gynaecological cancer and/or concurrent non-gynaecological malignancies.
Results: 3031 abstracts were screened. 112 isolated STIC patients out of 21 publications were included in our analysis with a pooled median follow-up of 36 (interquartile range (IQR): 25.3-84) months. 71.4% of the patients had peritoneal washings (negative: 62.5%, positive: 8%, atypic cells: 0.9%). Surgical staging was performed in 28.6% of all STICs and did not show any malignancies. 14 out of 112 (12.5%) patients received adjuvant chemotherapy with Carboplatin and Paclitaxel. Eight (7.1%) patients developed a recurrence 42.5 (IQR: 33-72) months after isolated STIC diagnosis. Cumulative incidence of HGSC after five (ten) years was 10.5% (21.6%). Recurrence occurred only in BRCA1 carriers (seven out of eight patients, one patient with unknown BRCA status).
Conclusion: The rate of HGSC after an isolated STIC diagnosis was 7.1% with a cumulative incidence of 10.5% (21.6%) after five (ten) years. HGSC was only observed in BRCA1 carriers. The role of adjuvant therapy and routine surveillance remains unclear, however, intense surveillance up to ten years is necessary.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42021278340.
Serous tubal intraepithelial carcinoma (STIC) in the fimbriated end of the fallopian tube is regarded as the precursor lesion of pelvic (i.e. ovarian or peritoneal) high-grade serous cancer (HGSC) (1–3). Women with proven BRCA germline mutations have an increased risk of 10-60% for developing ovarian cancer. For these women, a risk-reducing salpingo-oophorectomy (RRSO) is therefore recommended and presents the most effective method of prevention so far (4, 5). Occult carcinoma and/or STIC is detected in approximately 10-15% of these cases (1), isolated STIC is detected in approximately 2% (6). Metachronous peritoneal carcinomatosis after RRSO in high-risk patients occurs in approximately 4.5% (7) and predominantly in BRCA1 mutation carriers, usually within 5 years (8). Moreover, STIC diagnosis accompanies more than half of the cases with sporadic ovarian, tubal or primary peritoneal cancer (1). The incidence of STIC in patients with a normal risk of ovarian cancer is uncertain; however, a Canadian study reported STIC in eight out of 9392 women (<0.01%) with benign diagnoses (9). Accordingly, a recently published population-based, retrospective cohort study of all individuals in British Columbia, Canada, who underwent opportunistic salpingectomy or a control surgery (hysterectomy alone or tubal ligation), showed that the opportunistic salpingectomy group had significantly fewer serous and epithelial ovarian cancers than the control group (10). In the future, opportunistic salpingectomies will likely increase in routine surgery as a strategy for epithelial ovarian cancer prevention.
The SEE-FIM (Sectioning and Extensively Examining the FIMbria) protocol helps pathologists to detect these STIC lesions and is nowadays established for RRSOs after its first publication in February 2006 (11). Women with a proven isolated STIC lesion are at substantial risk to develop advanced HGSC and the metastatic pattern of a STIC remains unclear (6, 12, 13). Furthermore, consistent information on diagnostic necessities and therapeutical consequences for patients with STIC is lacking so far since most of the literature is focusing on pathological features (7).
The aim of this review was to determine the incidence of HGSC following a proven, isolated STIC diagnosis to discuss the management and follow-up of these women. Additional outcomes comprised the description of therapeutic and diagnostic options for STICs in the clinical routine.
Our systematic review is based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (14). It is registered at PROSPERO (CRD42021278340).
Three electronic bibliographical databases including MEDLINE (via Ovid), the Cochrane Central Register of Controlled Trials (CENTRAL) and Web of Science were searched systematically from February 2006 to July 2021 (15). In February 2006, the SEE-FIM protocol was initially introduced to detect STICs in routine diagnostics regularly (11).
The search strategies for each database were conducted by a librarian from the Johannes Gutenberg- University Mainz according to the PICOS criteria (16). All search strategies included index terms as well as free text related to STIC. The search strategies are provided in the supplementary material (appendix A). The search was performed on 28th July 2021. Furthermore, studies included in related systematic reviews and meta-analyses were screened for eligibility. A de-duplication of database search results in EndNote was performed according to Bramer (17). Grey literature, such as conference abstracts, were not included.
Study inclusion criteria for review were the pathological diagnosis of isolated STIC and clinical follow-up. Patients with a STIC and a positive cytology were also included to maintain consistency with previous publications on this subject (7). Serous intraepithelial neoplasia is also known as STIC and was included (18). Study exclusion criteria for review were missing clinical data (follow-up) and publications restricted to pathological information only. In addition, the presence of synchronous gynaecological cancer and/or concurrent non-gynaecological malignancies were exclusion criteria. Patients with a STIC diagnosis at RRSO and with an upstaging to a HGSC at the following surgical staging were not included, since the HGSC might have been overlooked at the initial surgery. Meta-analyses, systematic reviews, literature review and case reports were not included. Results should be interpreted accordingly. Only the latest published data were reported in case of articles that were an update of previously published patients.
Title and abstract screening, as well as full-text screening, were conducted by two review authors (V.C.L and A.L.) independently. A third independent reviewer (M.J.B) was contacted in case of disagreements between the first two reviewers. Data extraction was performed by V.C.L. and re-checked independently by A.L. using a predefined EXCEL spread sheet. The following information was collected: age, personal history of breast cancer, genetic predispositions, surgical indications, preoperative serum CA-125 levels, preoperative pelvic ultrasound, surgical procedure, peritoneal washings, adjuvant treatment (e.g. completion surgery, chemotherapy), and follow-up.
For each cohort study adequateness was assessed by the following criteria based on a systematic review of Van der Hoeven in 2018 (19): STICs should be diagnosed according to predefined pathological criteria and by an expert pathologist. The reporting bias included the description of the original cohort size, the genetic predisposition, median or mean age at surgery, information about clinical staging and adjuvant treatment for the patients with STIC. The indication was considered adequate if the surgery and the treatment of STIC took place according to a predefined protocol. The reported follow-up was seen as adequate if the follow-up was given in months or years describing the presence or absence of recurrence.
A pooled incidence of subsequent HGSC with a corresponding confidence interval (CI) after an isolated STIC diagnosis was calculated for all patients with an isolated STIC and follow-up. The median is shown with interquartile ranges (IQR) if possible. The Kaplan-Meier estimation was used to calculate the cumulative incidence of HGSC.
Staging procedures, adjuvant treatment and their outcome were described. Due to the limited number of recurrences, a risk stratification as well as a statistical analysis of the associations between staging, chemotherapy and recurrence was not performed (19).
A statistical analysis was carried out using SPSS version 27 (SPSS, Chicago, IL, USA).
In total, 3031 records were screened and 21 articles met our inclusion criteria as shown in the PRISMA flow chart in Figure 1.
We were able to include 112 patients out of these 21 articles (Table 1 and Table 2 for detailed information; Table 3 for overview). Median age was 52.3 (46.3-60) years. 71 (63.4%) patients were BRCA1 carriers, 18 (16.1%) patients were BRCA2 carriers. Eight (7.1%) patients were either BRCA1 or 2 positive. Four patients (3.6%) had a high risk and four patients a low risk of ovarian cancer. The BRCA status was unknown for five (4.5%) patients. Two (1.8%) were BRCA negative. One patient had a PALB2 mutation (31). RRSO was performed in 100 patients due to BRCA mutations or high-risk personal or family history. An opportunistic salping(o-oophor)ectomy was performed in the remaining twelve patients with an isolated STIC during surgery for benign reasons (ovarian cyst, cholecystectomy) (12, 34–36). In some cases, additional procedures were performed, mostly hysterectomies. All individual procedures are listed for each study in Table 1. Peritoneal washing during RRSO/surgery was reported in 80 (71.4%) cases of which nine (8%) were positive and one (0.9%) showed atypical cells. Six out of these nine patients had immediate reoperation for surgical staging. One patient declined the offer and opted for observation with CA-125 biannually and clinical review yearly for 3.5 years, and afterwards was discharged to the local medical officer (26). All of the surgical stagings showed no pathological findings and no subsequent HGSC was described in the follow-up.
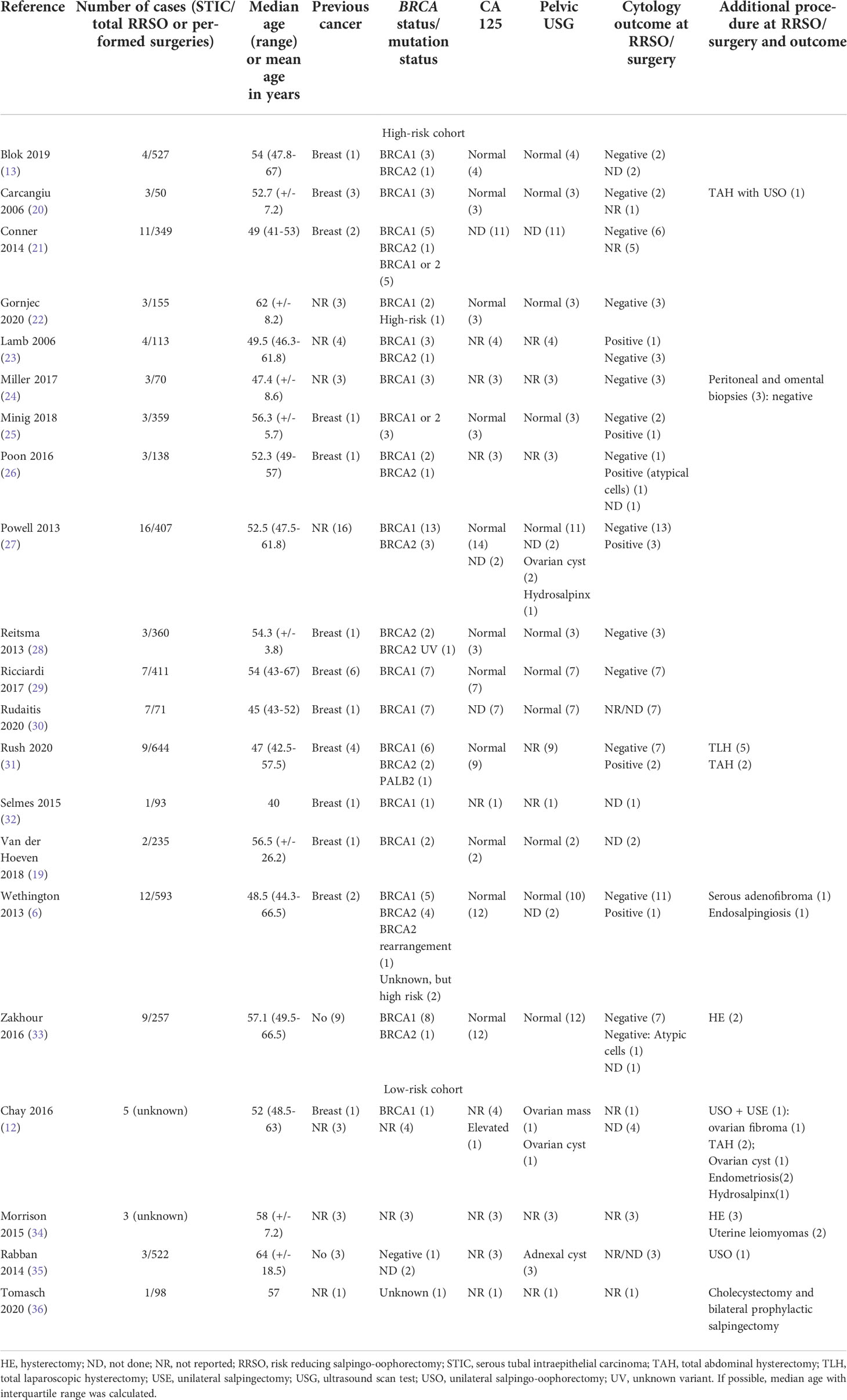
Table 1 Detailed characteristics of all included patients with isolated STIC (white: high-risk cohort; grey: low-risk cohort).
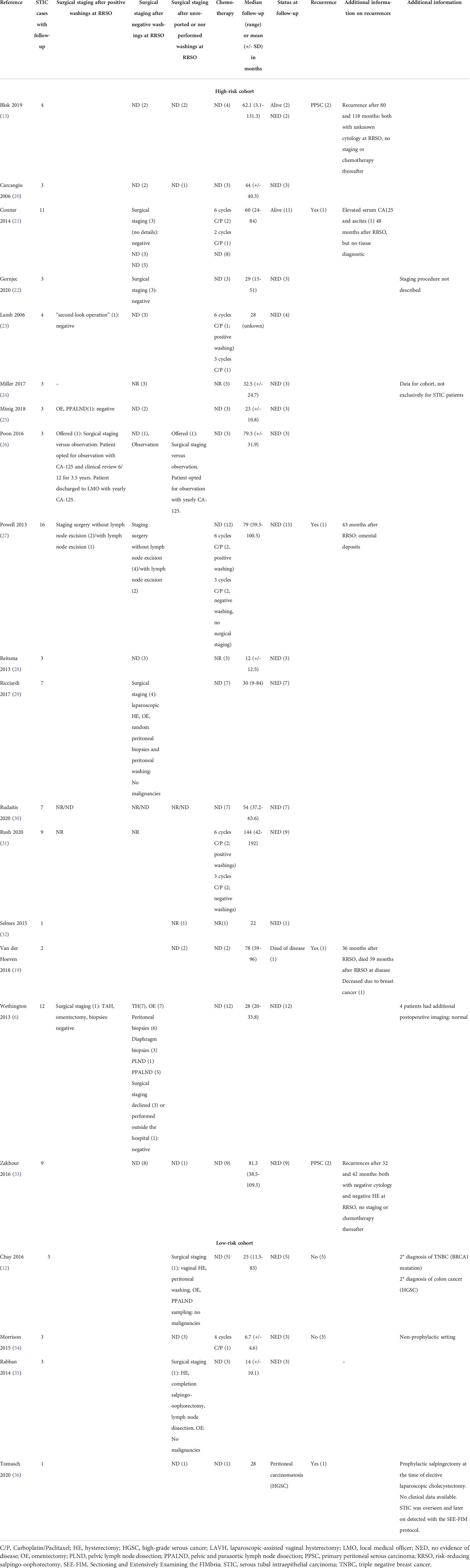
Table 2 Follow-up of patients with STIC included in our systematic review in alphabetical order (white: high-risk cohort; grey: low-risk cohort).
The surgical staging procedures mostly included omentectomy and in some cases a pelvic and paraaortic lymph node dissection (see Table 1).
In the study of Wethington and colleagues, all patients with an isolated STIC were offered a surgical staging, including hysterectomy, omentectomy and in five cases pelvic and paraaortic lymph node dissections. All procedures were without pathological findings. Three patients declined a surgical staging (6). Postoperative imaging as staging was hardly reported. Four out of 12 patients in the cohort of Wethington had an additional postoperative imaging without pathological findings (6).
14 out of 112 (12.5%) patients received adjuvant chemotherapy consisting of a combination of Carboplatin and Paclitaxel. Five out of nine patients with a positive washing received chemotherapy as well as seven patients with a negative cytology and one patient with a non-reported cytology. Follow-up mostly included clinical observation with CA-125 yearly. Pooled median follow up was 36 months (IQR: 25.3-84).
Eight out of 112 patients developed a subsequent HGSC (7.1%, 95% CI 2.3-12%), listed in Table 4. Pooled median time to recurrence were 42.5 (IQR: 33-72) months. The five (ten)- year- HGSC rate was 10.5% (21.6%), determined by the Kaplan-Meier estimation (Figure 2). The latest HGSC recurred 118 months after the diagnosis of STIC at RRSO/surgery. Seven out of eight patients were BRCA1 carriers and one patient had an unknown BRCA status since STIC was detected after revaluation of a salpingectomy during cholecystectomy (36). No BRCA2 carrier presented a recurrence in the selected studies. A recurrence occurred in four patients with a negative peritoneal washing, in three patients in which no pelvic washing was done and in one patient without a reported peritoneal cytology at the time of the first surgery.
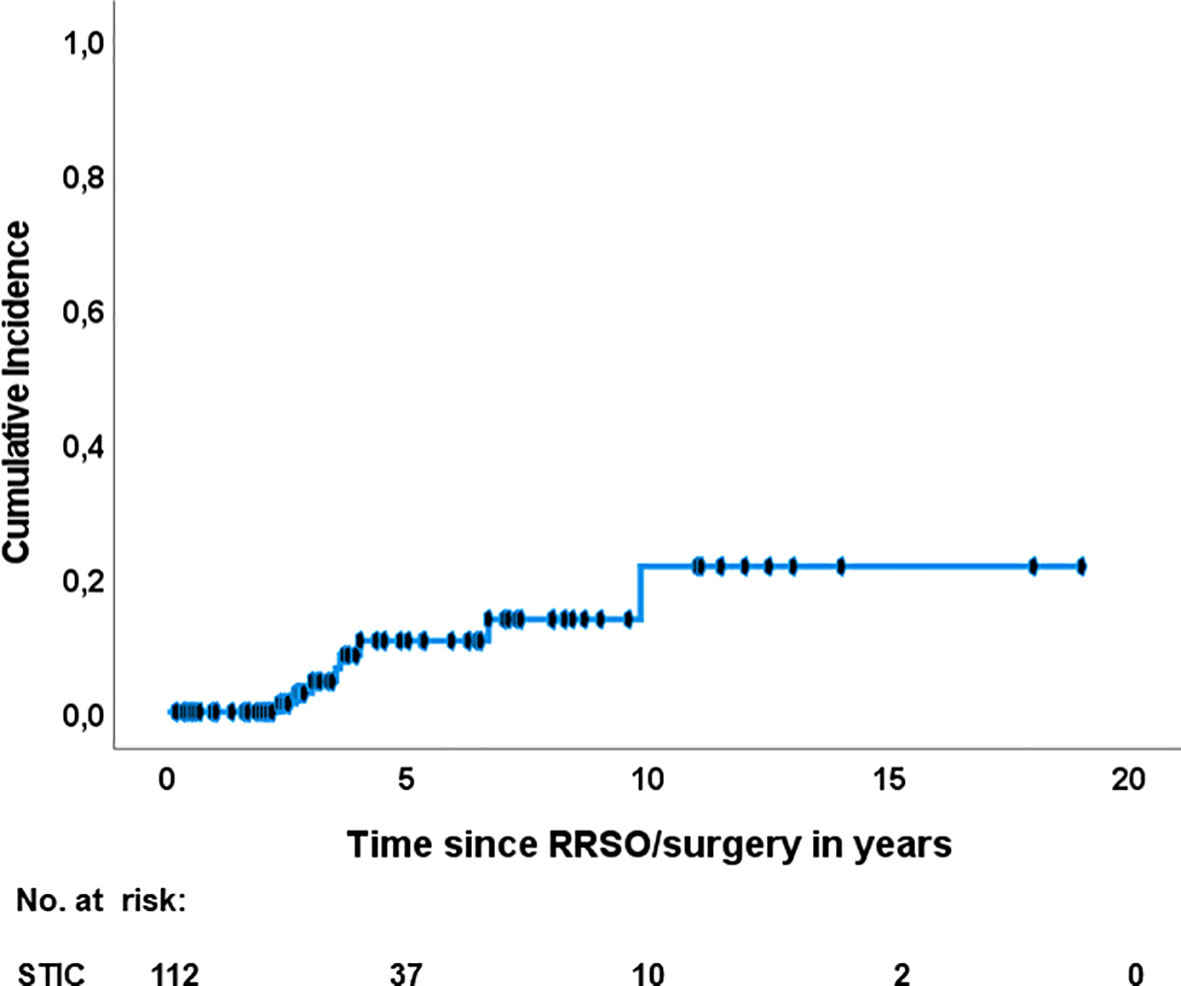
Figure 2 Recurrence of HGSC after isolated STIC diagnosis. RRSO risk reducing salpingo-oophorectomy; STIC, serous tubal intraepithelial carcinoma.
The risk of bias assessment is shown in appendix B. In 10/21 (48%) studies, STIC was diagnosed according to predefined pathological criteria. 17/21 (81%) studies reported the mutation status for the cohort. 11/21 (52%) studies operated according to a predefined protocol and only one study had a predefined treatment protocol for STIC. In general, adjuvant treatment was adequately described in 13/21 (61%) studies. Two studies had a predefined protocol for the follow-up of patients with STIC. Finally, 18/21 (86%) studies reported an adequate follow-up for patients with STIC.
In our review, the rate for subsequent HGSC after an isolated STIC diagnosis was 7.1%. In literature, recurrence rates in patients with isolated STIC ranged from 0-22% (21, 26, 28, 33), mostly due to small numbers of patients per study. One systematic review reported a rate of 4.5% in 2015 (7) and a more recent one in 2018 a rate of 11% (19). Our rate of recurrence may be more accurate since all patients with isolated STIC and available follow-up of the current literature were included. It is important to note that our rate might be probably increased with a longer follow-up of patients after an isolated STIC diagnosis, because the pooled median follow-up was 36 months and the pooled recurrence was detected more than half a year later after 42.5 months. A long follow-up is necessary to be able to determine the real incidence of HGSC. Our study determined a high and clinically relevant cancer risk for HGSC after STIC diagnosis of 10.5% (21.6%) after five (ten) years according to the Kaplan-Meier estimation. This again underlines the importance of a long follow-up, especially if we consider that the latest recurrence occurred almost 10 years after initial surgery. During the preparation of the manuscript, Steenbeek and colleagues published a systematic review about the risk of peritoneal carcinomatosis after RRSO with similar results in February 2022. They report a five- and ten- year- risk of developing peritoneal carcinomatosis of 10.5% and 27.5% after RRSO, respectively (37). Due to the prior closure of our data collection, we could not include their newly published STIC cases.
Interestingly, only BRCA1 carriers developed a subsequent HGSC. In general, BRCA1 carriers have the highest risk of occult neoplasia at RRSO (31). For all BRCA mutation carriers, a 3.5% cumulative risk for peritoneal cancer after prophylactic oophorectomy was reported after 20 years of follow-up (38). One STIC patient had a PALB2 gene mutation which is also involved in hereditary breast and ovarian cancer but insufficiently determines the ovarian cancer risk (39, 40).
We present a comprehensive review on published clinical outcomes and treatment modalities of patients with isolated STIC. Our strength is that our study contains the largest patient collective with isolated STIC and follow-up in the high-risk and especially the low-risk population so far. An increase in the number of STIC patients in the low-risk population is expected because opportunistic salpingectomies are recommended during routine surgery to prevent epithelial ovarian cancer (10).
However, our study reanalysed published data. The quality of collected data was low and with significant risk of bias (see appendix B). The latter included incomplete clinical data, heterogeneous follow-up data, e.g. only the mean data was given, the lack of data regarding a standard diagnostic, staging, treatment and surveillance. An important confounder in many studies was the short follow-up period after the STIC diagnosis, which might disguise the real rate of subsequent HGSC after RRSO/salpingectomy.
The impact of STIC in a low risk population is difficult to assess due to the lack of information. A Canadian study reported STIC in eight out of 9392 women (<0.01%) with benign diagnoses who had a normal risk of ovarian cancer using the SEE-FIM protocol (9). However, the SEE-FIM protocol was not routinely applied in non-RRSO surgery in the past and therefore published data on the incidence of STIC low-risk populations should be interpreted with caution. In general, diagnosing STIC is challenging with only moderate reproducibility. A recently published systematic review suggests not only the use of the SEE-FIM protocol, but also evaluation by a subspecialized pathologist, rational use of immunohistochemical staining, and obtaining a second opinion from a colleague to secure the diagnosis (41). Furthermore, there can also be a HGSC unrelated to a STIC diagnosis. Another bias is that STIC patients with positive washings were included to maintain the comparability to previous studies (7). However, not every patient received a subsequent surgical staging to eliminate the risk of a HGSC. The impact of positive peritoneal washings remains unclear as well. The routine use of peritoneal biopsies during RRSO does not seem to improve the detection of occult malignancies (42). In our review, nine patients had a positive washing and six underwent surgical staging without pathological findings. No patient with a positive washing developed a recurrence. According to the study of Wethington 15% of the peritoneal washings were positive at the time of RRSO and therefore recommended as a component of RRSO (6).
Clinical management of STIC is still a matter of debate. It is important that patients are informed about their potential risk of developing pelvic HGSC after a STIC diagnosis. A surgical staging should be considered (43), especially in cases of a positive peritoneal washing at initial RRSO/surgery to reduce the risk of synchronous HGSC. A surgical staging mostly included hysterectomy, omentectomy, pelvic and paraaortic lymph node dissection and peritoneal washing in the published studies. In case of a positive peritoneal washing at initial surgery, which implies circulating malignant cells in the peritoneal cavity, some institutions offered adjuvant chemotherapy. The latter usually comprised six cycles of Carboplatin and Paclitaxel. However, if the surgical staging is without evidence of disease, observation remains a reasonable option and avoids possible chemotherapy-induced adverse events (6). Adjuvant chemotherapy for intraepithelial neoplasia is not recommended any longer (31, 43). A radiological staging was rarely reported in our study.
Routine surveillance is recommended for the next years of follow-up, because the time from STIC to invasive cancer has been suggested to be approximately seven years and has guided the recommendation for RRSO in BRCA1 patients at the age of 35–40 years (44). This is coherent with the findings of Stanciu and colleagues who published seven cases with isolated STICs. Two of these patients (28%) developed peritoneal HGSC within 53 and 75 months after RRSO. The publication was not included in our study, because the follow-up of the five other patients with isolated STIC was missing (45).
To date, no effective screening tool exists to monitor STIC patients (26). Most of the published studies included annual clinical check-ups with pelvic ultrasound and in some cases routine evaluation of serum CA-125. BRCA status should be checked in cases of isolated STIC as well. No routine screening for ovarian HGSC should be offered to women of the general population. Two prospective randomized trials could not reduce ovarian cancer mortality with simultaneous screening of CA-125 and transvaginal ultrasound compared with usual care in the normal population (46, 47).
To summarize, several questions concerning STIC remain unclear and the therapy may require an individualized treatment plan. We are urgently in need of registries for longer follow-up data of STIC patients to assess the real incidence of HGSC after a STIC diagnosis. Future multicentre and international efforts are needed to generate a large cohort of patients with STIC to allow further subgroup analyses, e.g. regarding histopathological characteristics. In the meantime, systematic reviews will help to gather information and to define and update guidelines for the management of STIC.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conceptualization: VL, AL, AH and MB. Design: VL. and AL. Data acquisition: VL, AL and MB. Analysis and interpretation: VL, AL and MB. Writing- original draft of the manuscript: VL. Writing - review and editing: VL, AL, JV, AH and MB. All authors have read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
We would like to thank Lorena Cascant Ortolano from the library of the Johannes Gutenberg- University Mainz for her support in conducting the literature search.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.951292/full#supplementary-material
1. Li HX, Lu ZH, Shen K, Cheng WJ, Malpica A, Zhang J, et al. Advances in serous tubal intraepithelial carcinoma: correlation with high grade serous carcinoma and ovarian carcinogenesis. Int J Clin Exp Pathol (2014) 7(3):848–57.
2. Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol (2010) 34(3):433–43. doi: 10.1097/PAS.0b013e3181cf3d79
3. Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, et al. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathol (2001) 195(4):451–6. doi: 10.1002/path.1000
4. Eleje GU, Eke AC, Ezebialu IU, Ikechebelu JI, Ugwu EO, Okonkwo OO. Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst Rev (2018) 8:CD012464. doi: 10.1002/14651858.CD012464.pub2
5. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst (2009) 101(2):80–7. doi: 10.1093/jnci/djn442
6. Wethington SL, Park KJ, Soslow RA, Kauff ND, Brown CL, Dao F, et al. Clinical outcome of isolated serous tubal intraepithelial carcinomas (STIC). Int J Gynecol Cancer (2013) 23(9):1603–11. doi: 10.1097/IGC.0b013e3182a80ac8
7. Patrono MG, Iniesta MD, Malpica A, Lu KH, Fernandez RO, Salvo G, et al. Clinical outcomes in patients with isolated serous tubal intraepithelial carcinoma (STIC): A comprehensive review. Gynecologic Oncol (2015) 139(3):568–72. doi: 10.1016/j.ygyno.2015.09.018
8. Harmsen MG, Piek JMJ, Bulten J, Casey MJ, Rebbeck TR, Mourits MJ, et al. Peritoneal carcinomatosis after risk-reducing surgery in BRCA1/2 mutation carriers. Cancer (2018) 124(5):952–9. doi: 10.1002/cncr.31211
9. Samimi G, Trabert B, Geczik AM, Duggan MA, Sherman ME. Population frequency of serous tubal intraepithelial carcinoma (STIC) in clinical practice using SEE-fim protocol. JNCI Cancer Spectr (2018) 2(4):pky061. doi: 10.1093/jncics/pky061
10. Hanley GE, Pearce CL, Talhouk A, Kwon JS, Finlayson SJ, McAlpine JN, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Netw Open (2022) 5(2):e2147343. doi: 10.1001/jamanetworkopen.2021.47343
11. Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol (2006) 30(2):230–6. doi: 10.1097/01.pas.0000180854.28831.77
12. Chay WY, McCluggage WG, Lee CH, Kobel M, Irving J, Millar J, et al. Outcomes of incidental fallopian tube high-grade serous carcinoma and serous tubal intraepithelial carcinoma in women at low risk of hereditary breast and ovarian cancer. Int J Gynecol Cancer (2016) 26(3):431–6. doi: 10.1097/IGC.0000000000000639
13. Blok F, Dasgupta S, Dinjens WNM, Roes EM, van Beekhuizen HJ, Ewing-Graham PC. Retrospective study of a 16year cohort of BRCA1 and BRCA2 carriers presenting for RRSO: Prevalence of invasive and in-situ carcinoma, with follow-up. Gynecol Oncol (2019) 153(2):326–34. doi: 10.1016/j.ygyno.2019.03.003
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
15. Goossen K, Tenckhoff S, Probst P, Grummich K, Mihaljevic AL, Buchler MW, et al. Optimal literature search for systematic reviews in surgery. Langenbecks Arch Surg (2018) 403(1):119–29. doi: 10.1007/s00423-017-1646-x
16. da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. The PICO strategy for the research question construction and evidence search. Rev Lat Am Enfermagem (2007) 15(3):508–11. doi: 10.1590/S0104-11692007000300023
17. Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc (2016) 104(3):240–3. doi: 10.3163/1536-5050.104.3.014
18. Perrone ME, Reder NP, Agoff SN, Garcia RL, Agnew KJ, Norquist BM, et al. An alternate diagnostic algorithm for the diagnosis of intraepithelial fallopian tube lesions. Int J Gynecol Pathol (2020) 39(3):261–9. doi: 10.1097/PGP.0000000000000604
19. Van der Hoeven NMA, Van Wijk K, Bonfrer SE, Beltman JJ, Louwe LA, De Kroon CD, et al. Outcome and prognostic impact of surgical staging in serous tubal intraepithelial carcinoma: A cohort study and systematic review. Clin Oncol (Royal Coll Radiologists) (2018) 30(8):463–71. doi: 10.1016/j.clon.2018.03.036
20. Carcangiu ML, Peissel B, Pasini B, Spatti G, Radice P, Manoukian S. Incidental carcinomas in prophylactic specimens in BRCA1 and BRCA2 germ-line mutation carriers, with emphasis on fallopian tube lesions: Report of 6 cases and review of the literature. Am J Surg Pathol (2006) 30(10):1222–30. doi: 10.1097/01.pas.0000202161.80739.ac
21. Conner JR, Meserve E, Pizer E, Garber J, Roh M, Urban N, et al. Outcome of unexpected adnexal neoplasia discovered during risk reduction salpingo-oophorectomy in women with germ-line BRCA1 or BRCA2 mutations. Gynecologic Oncol (2014) 132(2):280–6. doi: 10.1016/j.ygyno.2013.12.009
22. Gornjec A, Merlo S, Novakovic S, Stegel V, Gazic B, Perhavec A, et al. The prevalence of occult ovarian cancer in the series of 155 consequently operated high risk asymptomatic patients - Slovenian population based study. Radiol Oncol (2020) 54(2):180–6. doi: 10.2478/raon-2020-0020
23. Lamb JD, Garcia RL, Goff BA, Paley PJ, Swisher EM. Predictors of occult neoplasia in women undergoing risk-reducing salpingo-oophorectomy. Am J Obstet Gynecol (2006) 194(6):1702–9. doi: 10.1016/j.ajog.2006.03.006
24. Miller H, Pipkin LS, Tung C, Hall TR, Masand RP, Anderson ML. The role of routine peritoneal and omental biopsies at risk-reducing salpingo-oophorectomy. J Minimally Invasive Gynecol (2017) 24(5):772–6. doi: 10.1016/j.jmig.2017.03.001
25. Minig L, Cabrera S, Oliver R, Couso A, Rubio MJ, Iacoponi S, et al. Pathology findings and clinical outcomes after risk reduction salpingo-oophorectomy in BRCA mutation carriers: a multicenter Spanish study. Clin Trans Oncol: Off Publ Fed Spanish Oncol Societes Natl Cancer Institute Mexico (2018) 20(10):1337–44. doi: 10.1007/s12094-018-1865-9
26. Poon C, Hyde S, Grant P, Newman M, Ireland Jenkin K. Incidence and characteristics of unsuspected neoplasia discovered in high-risk women undergoing risk reductive bilateral salpingooophorectomy. Int J Gynecol Cancer (2016) 26(8):1415–20. doi: 10.1097/IGC.0000000000000791
27. Powell CB, Swisher EM, Cass I, McLennan J, Norquist B, Garcia RL, et al. Long term follow up of BRCA1 and BRCA2 mutation carriers with unsuspected neoplasia identified at risk reducing salpingo-oophorectomy. Gynecologic Oncol (2013) 129(2):364–71. doi: 10.1016/j.ygyno.2013.01.029
28. Reitsma W, de Bock GH, Oosterwijk JC, Bart J, Hollema H, Mourits MJ. Support of the ‘fallopian tube hypothesis’ in a prospective series of risk-reducing salpingo-oophorectomy specimens. Eur J Cancer (2013) 49(1):132–41. doi: 10.1016/j.ejca.2012.07.021
29. Ricciardi E, Tomao F, Aletti G, Bazzurini L, Bocciolone L, Boveri S, et al. Risk-reducing salpingo-oophorectomy in women at higher risk of ovarian and breast cancer: A single institution prospective series. Anticancer Res (2017) 37(9):5241–8. doi: 10.21873/anticanres.11948
30. Rudaitis V, Mikliusas V, Januska G, Jukna P, Mickys U, Janavicius R. The incidence of occult ovarian neoplasia and cancer in BRCA1/2 mutation carriers after the bilateral prophylactic salpingo-oophorectomy (PBSO): A single-center prospective study. Eur J Obstetrics Gynecol Reprod Biol (2020) 247:26–31. doi: 10.1016/j.ejogrb.2020.01.040
31. Rush SK, Swisher EM, Garcia RL, Pennington KP, Agnew KJ, Kilgore MR, et al. Pathologic findings and clinical outcomes in women undergoing risk-reducing surgery to prevent ovarian and fallopian tube carcinoma: A large prospective single institution experience. Gynecologic Oncol (2020) 157(2):514–20. doi: 10.1016/j.ygyno.2020.02.006
32. Selmes G, Ferron G, Filleron T, Querleu D, Mery E. Early epithelial lesions in prophylactic annexectomies in patients at high risk of ovarian cancer: Report of a series of 93 cases. Gynecologie Obstetrique Fertilite (2015) 43(10):659–64. doi: 10.1016/j.gyobfe.2015.07.006
33. Zakhour M, Danovitch Y, Lester J, Rimel BJ, Walsh CS, Li AJ, et al. Occult and subsequent cancer incidence following risk-reducing surgery in BRCA mutation carriers. Gynecologic Oncol (2016) 143(2):231–5. doi: 10.1016/j.ygyno.2016.08.336
34. Morrison JC, Blanco LZ Jr., Vang R, Ronnett BM. Incidental serous tubal intraepithelial carcinoma and early invasive serous carcinoma in the nonprophylactic setting: analysis of a case series. Am J Surg Pathol (2015) 39(4):442–53. doi: 10.1097/PAS.0000000000000352
35. Rabban JT, Garg K, Crawford B, Chen LM, Zaloudek CJ. Early detection of high-grade tubal serous carcinoma in women at low risk for hereditary breast and ovarian cancer syndrome by systematic examination of fallopian tubes incidentally removed during benign surgery. Am J Surg Pathol (2014) 38(6):729–42. doi: 10.1097/PAS.0000000000000199
36. Tomasch G, Lemmerer M, Oswald S, Uranitsch S, Schauer C, Schutz AM, et al. Prophylactic salpingectomy for prevention of ovarian cancer at the time of elective laparoscopic cholecystectomy. Br J Surg (2020) 107(5):519–24. doi: 10.1002/bjs.11419
37. Steenbeek MP, van Bommel MHD, Bulten J, Hulsmann JA, Bogaerts J, Garcia C, et al. Risk of peritoneal carcinomatosis after risk-reducing salpingo-oophorectomy: A systematic review and individual patient data meta-analysis. J Clin Oncol (2022) 40(17):JCO2102016. doi: 10.1200/JCO.21.02016
38. Casey MJ, Synder C, Bewtra C, Narod SA, Watson P, Lynch HT. Intra-abdominal carcinomatosis after prophylactic oophorectomy in women of hereditary breast ovarian cancer syndrome kindreds associated with BRCA1 and BRCA2 mutations. Gynecol Oncol (2005) 97(2):457–67. doi: 10.1016/j.ygyno.2005.01.039
39. Bono M, Fanale D, Incorvaia L, Cancelliere D, Fiorino A, Calo V, et al. Impact of deleterious variants in other genes beyond BRCA1/2 detected in breast/ovarian and pancreatic cancer patients by NGS-based multi-gene panel testing: looking over the hedge. ESMO Open (2021) 6(4):100235. doi: 10.1016/j.esmoop.2021.100235
40. Samuel D, Diaz-Barbe A, Pinto A, Schlumbrecht M, George S. Hereditary ovarian carcinoma: Cancer pathogenesis looking beyond BRCA1 and BRCA2. Cells (2022) 11(3). doi: 10.3390/cells11030539
41. Bogaerts JMA, Steenbeek MP, van Bommel MHD, Bulten J, van der Laak J, de Hullu JA, et al. Recommendations for diagnosing STIC: A systematic review and meta-analysis. Virchows Arch (2022) 480(4):725–37. doi: 10.1007/s00428-021-03244-w
42. Marchetti C, Arcieri M, Vertechy L, Ergasti R, Russo G, Zannoni GF, et al. Risk reducing surgery with peritoneal staging in BRCA1-2 mutation carriers. a prospective study. Eur J Surg Oncol (2022). doi: 10.1016/j.ejso.2022.07.007
43. Colombo N, Sessa C, du Bois A, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger. Ann Oncol (2019) 30(5):672–705. doi: 10.1093/annonc/mdz062
44. Labidi-Galy SI, Papp E, Hallberg D, Niknafs N, Adleff V, Noe M, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun (2017) 8(1):1093. doi: 10.1038/s41467-017-00962-1
45. Stanciu PI, Ind TEJ, Barton DPJ, Butler JB, Vroobel KM, Attygalle AD, et al. Development of peritoneal carcinoma in women diagnosed with serous tubal intraepithelial carcinoma (stic) following risk-reducing salpingo-oophorectomy (rrso). J Ovarian Res (2019) 12(1):50. doi: 10.1186/s13048-019-0525-1
46. Menon U, Gentry-Maharaj A, Burnell M, Singh N, Ryan A, Karpinskyj C, et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK collaborative trial of ovarian cancer screening (UKCTOCS): A randomised controlled trial. Lancet (2021) 397(10290):2182–93. doi: 10.1016/S0140-6736(21)00731-5
Keywords: serous tubal intraepithelial carcinoma (STIC), high-grade serous carcinoma (HGSC), precursor, peritoneal carcinomatosis, incidence, treatment, outcome
Citation: Linz VC, Löwe A, van der Ven J, Hasenburg A and Battista MJ (2022) Incidence of pelvic high-grade serous carcinoma after isolated STIC diagnosis: A systematic review of the literature. Front. Oncol. 12:951292. doi: 10.3389/fonc.2022.951292
Received: 23 May 2022; Accepted: 09 August 2022;
Published: 31 August 2022.
Edited by:
Sophia George, University of Miami, United StatesReviewed by:
Federica Perelli, Santa Maria Annunziata Hospital, ItalyCopyright © 2022 Linz, Löwe, van der Ven, Hasenburg and Battista. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valerie Catherine Linz, dmFsZXJpZS5saW56QHVuaW1lZGl6aW4tbWFpbnouZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.