- 1Department of Critical Care Medicine, Tianjin First Central Hospital, Tianjin, China
- 2Department of Radiology, Characteristic Medical Center of Chinese People’s Armed Police Force, Tianjin, China
- 3Department of Pathology, Characteristic Medical Center of Chinese People’s Armed Police Force, Tianjin, China
- 4Department of Pulmonary and Critical Care Medicine, Characteristic Medical Center of Chinese People’s Armed Police Force, Tianjin, China
Background: Extramedullary plasmacytoma (EMP) is an extremely rare kind of soft tissue plasma cell neoplasm without bone marrow involvement or other systemic characteristics of multiple myeloma. Primary pulmonary plasmacytoma (PPP), with no specific clinical manifestations, is an exceedingly rare type of EMP. Because of its complexity, PPP is often difficult to diagnose. Computed tomography-guided percutaneous core needle biopsy (CT-guided PCNB) has been shown to have high sensitivity, specificity and accuracy for characterization of pulmonary lesion, particularly if malignancy is suspected. Here we presented a rare case of PPP diagnosed with CT-guided PCNB.
Case presentation: A 78-year-old female smoker who visited our outpatient clinic for a mass in the left lower lobe of the lung. Pathological based on CT-guided PCNB yielded a PPP with no lymph node or other distant metastasis.
Conclusions: Extramedullary plasmacytoma should be considered in the differential diagnosis of a pulmonary mass.
Background
Extramedullary plasmacytoma (EMP) is a rare monoclonal plasma cell tumor involving tissues outside the bone marrow. The entity comprises approximately 3–5% of all plasma cell neoplasms. More than 80% of EMP cases occur in the head and neck, and most cases involve the upper aerodigestive tract (1). Primary pulmonary plasmacytoma (PPP) is an extremely rare variant of EMP. In a comprehensive literature search reviewing patients with PPP, only 16 reports were found (2–15) summarized in Table 1). Here, we present an extremely unusual presentation as a pulmonary mass and without bone marrow involvement. With respect to the different biologic characteristics and prognoses of PPP, it is essential to consider in differential diagnosis of lung mass. CT-guided needle aspiration biopsy should be considered as first line to gain biopsy sample.
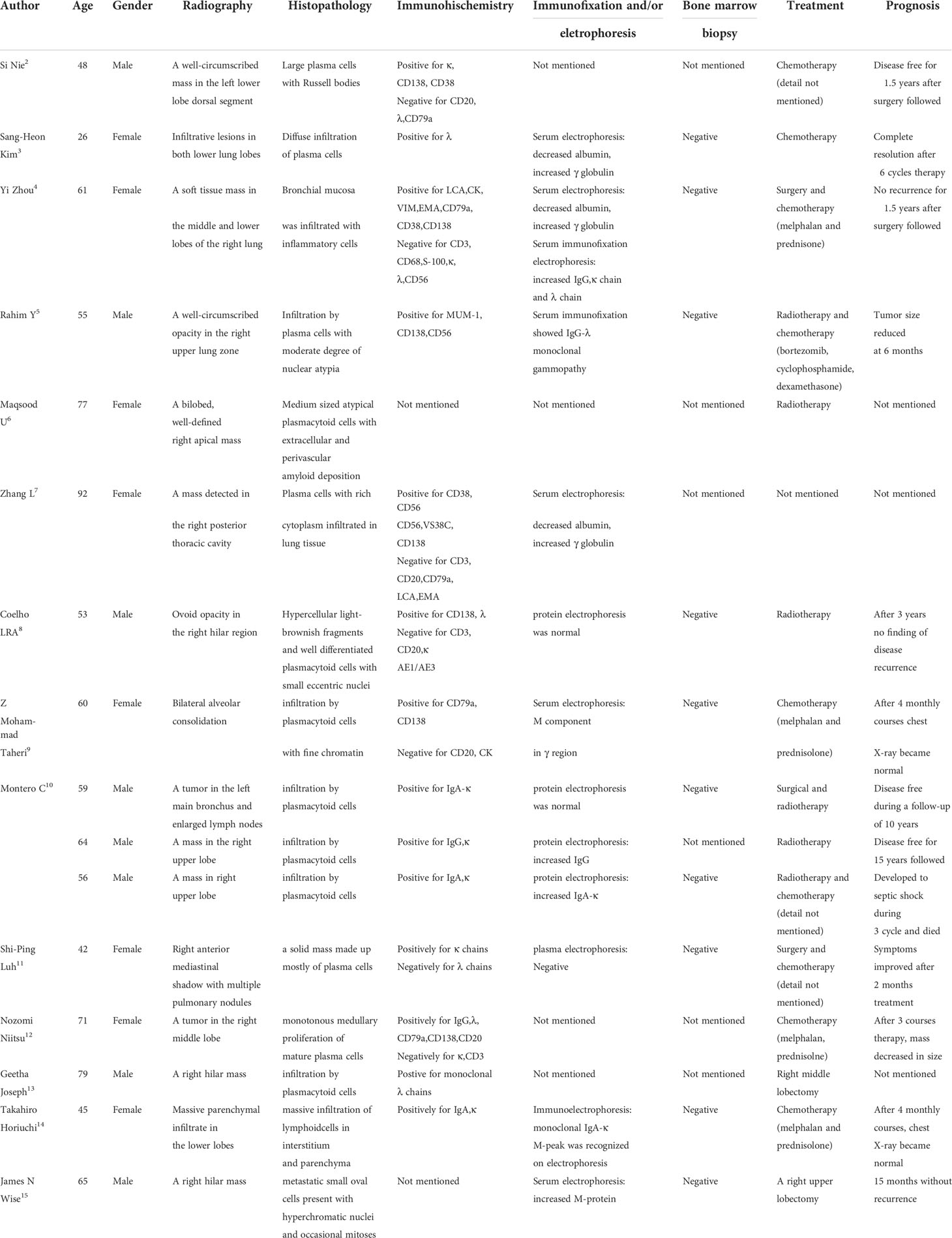
Table 1 Summary of the literature in the clinical treatment and prognosis of primary pulmonary plasmacytoma.
Case presentation
A 78-year-old female with a mass in the left lower lobe of the lung was referred to our hospital. The patient was diagnosed with hypertension and coronary heart disease prior. She had an approximately 60-year history of smoking. She has suffered from post-exercising dyspnea for 3 years without any apparent cause. The patient showed up with chest pain intermittent on the left, and the dyspnea was deteriorated a half months ago. No obvious abnormalities were found in physical examinations. A routine laboratory test showed white blood cell count, 4.77 ×109/L; neutrophil percentage, 50.8%; haemoglobin, 122 g/L; blood platelet count, 152×109/L; urine protein +/-. Serum calcium and phosphorus were within normal ranges. The rest of the routine laboratory examinations at the time of admission showed no obvious abnormalities. A chest computed tomography (CT) scan demonstrated a well-circumscribed mass measuring 23.14 × 21.33 × 20.28 mm located in left lower lobe (Figure 1). The mass was homogeneous and without any area of calcification or necrosis on a CT plain scan. It was marginal and spiculated with adjacent pleural retraction without bronchiolar obstruction. No obvious enlarged lymph nodes were found in the mediastinum. CT data resulted in a diagnosis of peripheral lung cancer. All the tumor marker was normal in the blood. Subsequently, the patient received CT-guided needle aspiration biopsy. The histological examination of the specimen revealed multiple lymphocyte and plasma cells were accumulated (Figure 2A). Immunohistochemistry was positive for LCA (CD45) (+++), CD 138 (+++), CD 38 (+++), kappa (+++), CK (-), CD 20(-), INSM1 (-), CD 56(-) (Figures 2B–I). In addition, specific stain of pathology including PAS, GMS were negative (Figures 2J–L). Pathological biopsy indicated extramedullary plasmacytoma. The patient was further evaluated by other diagnostic tests, including urine Bence-Jones protein, serum electrophoresis (Figure 3A), urine electrophoresis (Figure 3B), and bone marrow biopsy. However, all of the above tests had no abnormal change. The PET-CT revealed solid lesions with high grade increase 18F-fluorodeoxyglucose (18F-FDG) uptake in the lower left lobe of lung. There were no signs of abnormal metabolism in other organs and tissues, and no osteolytic lesions (Figure 4). Ultimately, the patient was diagnosed with PPP.
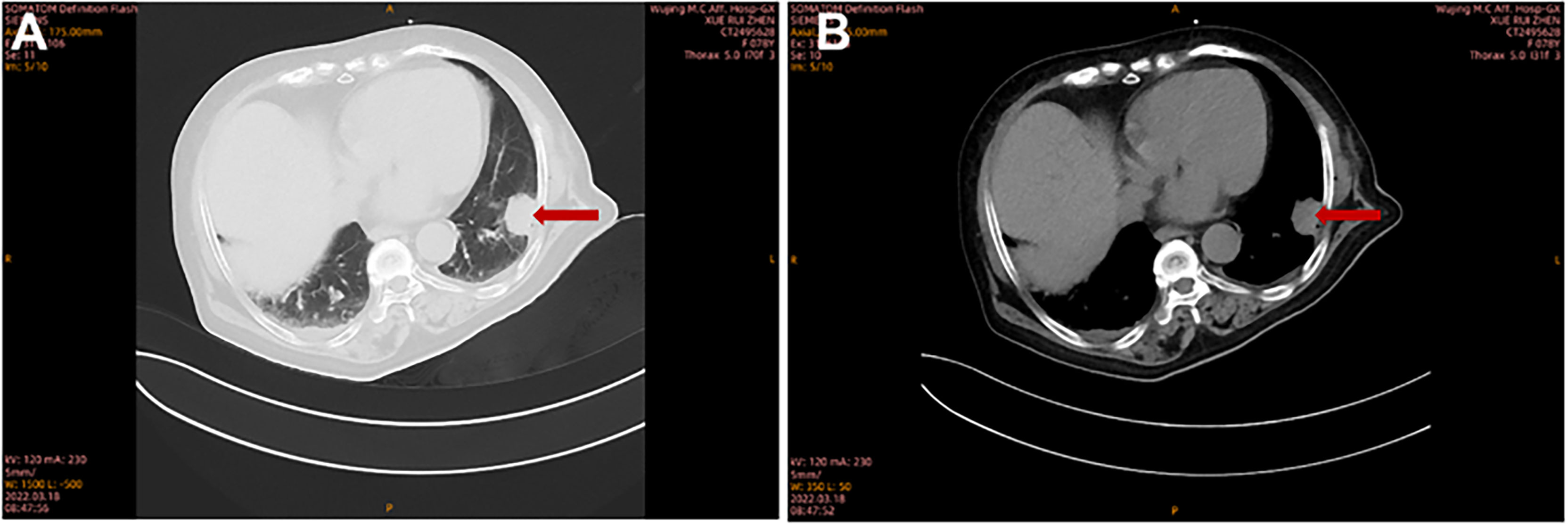
Figure 1 The imaging characteristics of PPP on pulmonary CT scan. Chest CT showed a solid lesion in the left lower lobe at lung window (A) and at mediastinal window (B).
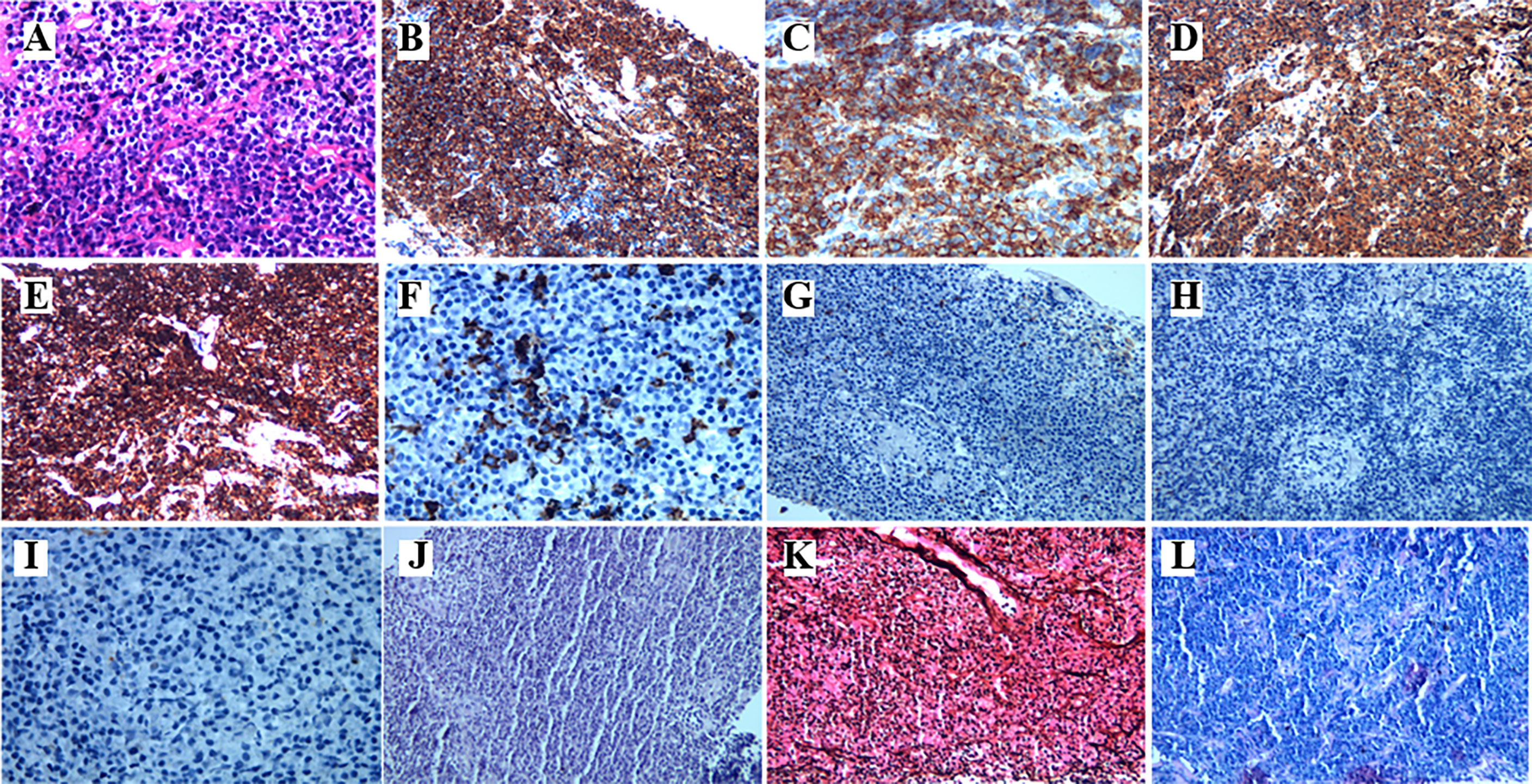
Figure 2 The histopathological characteristics of the tumor in the lung demonstrated by H&E and immunohistochemical staining. (A) Hematoxylin and eosin staining. The higher power view shows uniform small round blue cells with scant cytoplasm. (B–I) Immunohistochemical staining (400×magnification) was positive for CD38, CD138, Kappa, and LCA(CD45), CD20, but negative for CD56, CK, INSM1, respectively. (J–L) Specific stain of pathology of PAS, GMS, and acid fast stain, respectively.
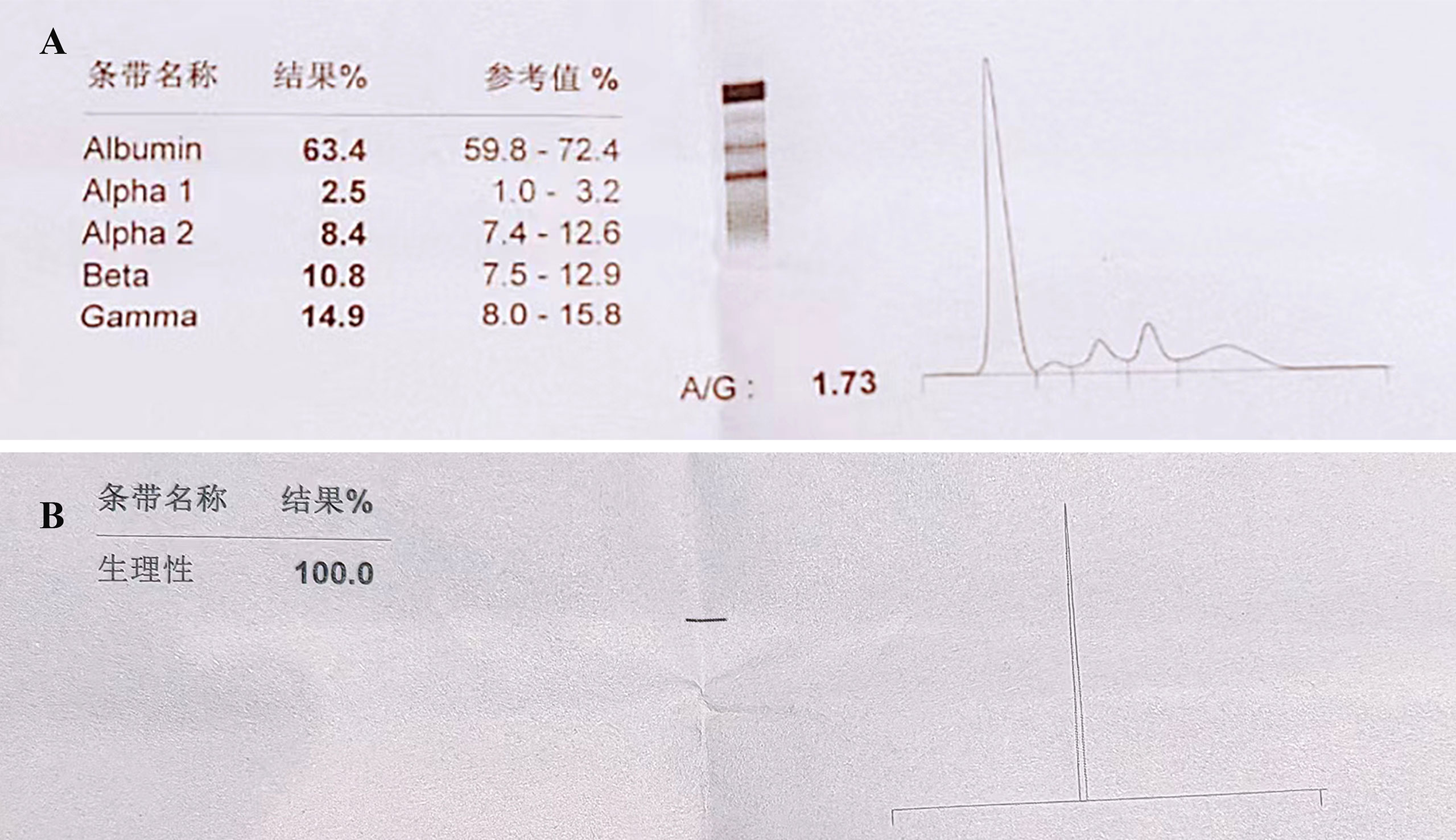
Figure 3 Serum and urine electrophoresis. No obvious abnormalities are detected in (A) Serum electrophoreis and (B) urine electrophoresis.
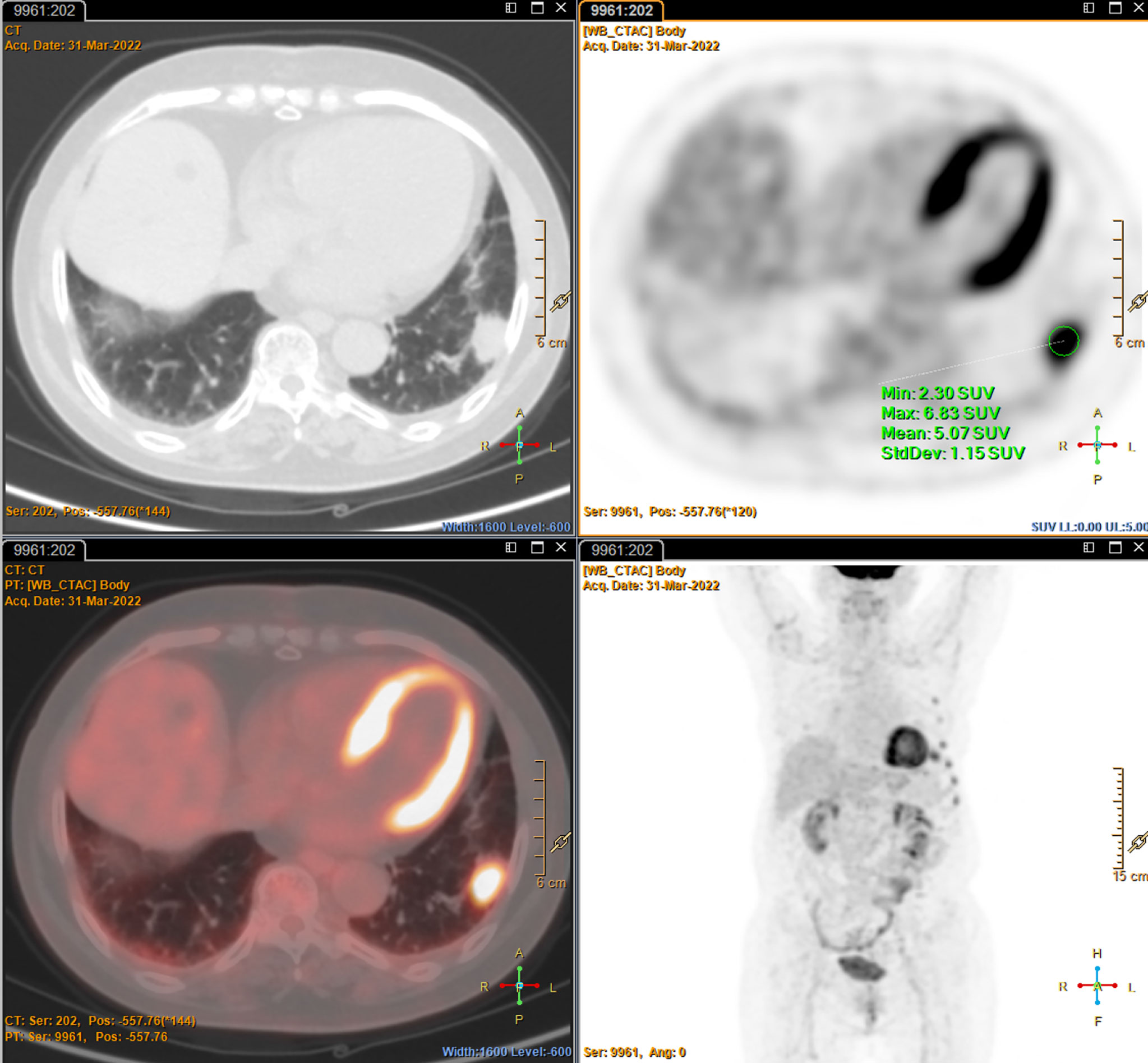
Figure 4 The Positron emission tomography-CT imaging characteristics shows a soft tissue density with hypermetabolism in the left lower lobe, but no abnormal metabolism in other organs and tissues, and no osteolytic lesions.
Discussion and conclusions
In the present study, we encountered an extremely rare case of primary lung plasmacytomas without involving bone marrow. According to the classification of the Wilshaw method (4): Stage I, the tumour is confined to the primary site; stage II, the tumour has invaded local lymph nodes; and stage III, there are obvious widespread metastases. Therefore, in this case, the tumor should be classified as stage I.
Solitary plasmacytomas (SP) are rare neoplasms, involving solitary plasmacytoma of the bone (SPB), solitary extramedullary (extra-ossesous) plasmacytoma (SEP) or multiple solitary plasmacytomas (MSP) (16). Generally SP do not involve systemic manifestation or bone marrow. However, the entity has a propensity to eventually progress to MM (16). SEP is encountered more frequently in sites having a rich lymphatic drainage such as nasal cavity, nasopharynx, and upper respiratory tract (16).
In the present study, we encountered an unusual site of SEP. The average age of PPP was 60 years old, one fourth of patients were under 50 years old. No gender difference was showed. The symptoms of PPP were nonspecific and depended on the location of the neoplasms and their tumor classification. Most PPP presented solitary pulmonary masses, multiple shadowed masses was rare on CT image. Therefore, PPP is often misdiagnosed as tuberculosis or lung cancer. The imaging findings of PPP reported in the literature are mostly solitary pulmonary nodules or masses, mostly located around the hilar, with round or quasi-circular shape, relatively uniform density and clear boundary (2). A few patients also show multiple nodules, masses or diffuse lesions in the lung (3). Consistently with previous reports (2), the tumor in this case on CT image was rounded masses with well-defined margins that was initially misdiagnosed as peripheral lung cancer. There are some subtle differences between PPP and peripheral lung cancer, tuberculoma, but there is no characteristic difference. In general, peripheral lung cancer is deeply lobulated with short hard burrs. The radiography of tuberculoma shows no lobule with calcified foci, and satellite lesions around. Peripheral lung cancer shows obvious enhancement, but tuberculoma shows no or light strengthening on enhanced CT scan. PPP presents with moderate uniform reinforcement on enhanced CT scan (2). The tumor markers of pulmonary carcinoma can be elevated, while tumor marker was negative in tuberculoma and PPP. Consistent with the CT presentation of previous cases, the mass was homogeneous and necrosis and calcification are rarely seen in this case (17). Few patients showed diffuse consolidation in bilateral lung (3, 9). Castleman disease is also a lymphoproliferative disease with hyperplastic germinal center of the lymphatic node. According to the distribution of lymph nodes, it was divided into Unicentric Castlman disease and Multicentric Castlman disease. According to pathology, There are three pathologic subtype, including hyaline vascular type, plasma cell type and intermediate type (18). Unicentric Castleman’s disease, which mimic PPP, usually shows well-circumscribed and homogenous masses in mediatum with intense reinforcement on enhanced CT scan. Nevertheless, Unicentric Castleman’s disease predominantly consists of the hyaline vessel variant (90%) (19). Histopathology is the only method to make a definitive diagnosis. The feature of Castleman disease is multiple concentric rings of mantle zone lymphocytes encircling atretic follicles. However, CD 38 and CD 138 are indicative of the diagnosis of plasmacytoma, especially CD138 (2, 4). In most circumstances, features of PPP tumor cells are positive for CD138, CD38, CD45, PC, EMA, and CD20, while negative for CD15. In a few cases, CK and EMA are positive, but negative for CD45 (4).
The use of PET/CT in evaluation of plasma cell malignancy has opportunities of detecting MSP or additional bone lesions when biochemical and laboratory investigations are within normal limits (20). Eletrophoresis of most cases showed decreased albumin and increased γ globulin (3–5, 7) or M-peak (9, 14, 15). Consistent with few cases serum eletrophoresis showed normal (8, 10, 11). In this case, immunohistochemistry findings showed the infiltration of numerous lymphocyte and plasma cells positive for CD138 and CD38. PET-CT showed no evidence of osteolytic lesions and distant metastasis. Thus, a diagnosis of PPP was made.
There are no established treatments for patients with PPP. Anatomic pulmonary resection with or without radiotherapy are the most therapeutic approach for PPP (6). Adjuvant chemotherapy is usually conservative treatment in case of diffuse infiltration, aggressive lesion on histopathology, or poor local control after local surgery or radiotherapy (21, 22). Melphalan and prednisone are commonly used chemotherapy scheme (21, 22). Chemotherapy is considered in patients with tumors larger than 5cm (4). Taking into account, the size of the primary lesion without local infiltration or distant metastasis. We planned complete radiation therapy for the elderly patients with multiple comorbidities. Long-term survival in PPP remains unclear, due to limited follow-up data on too few patients. PPP has a relative favourable prognosis, as evidence by previous findings that these patients survived 10-20 years (10, 21). 20%-40% rate of progress to MM was noted (23). After 4 courses of radiotherapy, the chest X-ray became normal. Further follow-up is needed to monitor the progression of this case, and determine optimal treatment strategies for similar case.
In conclusion, we report an extremely rare presentation of SEP. This case highlights that attention should be paid to the differential diagnosis of pulmonary mass. Precise biopsy and optimal pathological evaluation will confirm the diagnosis. Doctors should be mindful of PPP as a differential diagnosis.
Ethics statement
This case report was approved by the Medical Ethics Committee of Characteristic Medical Center of Chinese People’s Armed Police Force. The patient and her family consented to participate the study.
Author contributions
The authors’ contributions are described as followed. JW and BL collected the data of medical history. JW wrote the manuscript. XY and XL collected the imaging data. TH collected the pathological data. LY and JL did the CT-guided needle aspiration biopsy. JL and FY revised the manuscript. JL and FY were the guarantors of this work and take responsibility for the contents of the article. All authors have contributed significantly and are in agreement with the content of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank the patient for her participation and her agreement to the publication of the report. We thank Tianjin Key Medical Discipline (Specialty) Construction Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Nie S, Peng DC, Gong HH, Ye CL, Nie X, Li HJ. Primary pulmonary plasmacytoma: a case report introduction. World J Surg Oncol (2016) 14(1):205. doi: 10.1186/s12957-016-0948-8
3. Kim S-H, Kim TH, Sohn JW, Yoon HJ, Shin DH, Kim IS, et al. Primary pulmonary plasmacytoma presenting as multiple lung nodules. Korean J Intern Med (2012) 27(1):111–3. doi: 10.3904/kjim.2012.27.1.111
4. Zhou Y, Wang XH, Meng SS, Wang HC, Li YX, Xu R, et al. Primary pulmonar y plasmacytoma accompan ied by overlap syndrome: A case report and review of the literature. World J Clin cases (2020) 8(20):4999–5006. doi: 10.12998/wjcc.v8.i20
5. Rahim Y, Tareen FZ, Ahmed R, Khan JA. Primary pulmonary plasmacytom a presenting with rare IgG lambda monoclonal gammopathy. BMJ Case Rep (2019) 12(3):e227514. doi: 10.1136/bcr-2018-227514
6. Maqsood U, Jones H, Gey van Pittius D, Haris M. Primary pulmonary plasmacyt oma mimicking lung cancer diagnosed on endobronchia l ultrasound (EBUS) –guided biopsy. BMJ Case Rep (2016) 2016:bcr2016215785. doi: 10.1136/bcr-2016-215785
7. Zhang L, Zhang X, Zhang R, Fan W. 18F-FDG PET/CT metabolic activity in a patient with solitary extramedullary plasmacytoma of the lung. Clin Nucl Med (2016) 41(3):232–4. doi: 10.1097/RLU.0000000000001030
8. Coelho LRA, Coelho GP, Queiroz RM, Valentin MVN. Extramedullary plasmacytoma in the right pulmonary hilum. Radiol Bras (2015) 48(6):401–2. doi: 10.1590/0100-3984.2015.0074
9. Mohammad Taheri Z, Mohammadi F, Karbasi M, Seyfollahi L, Kahkoei S, Ghadiany M, et al. Primary pulmonary plasmacytoma with diffuse alveolar consolidation: a case report. Pathol Res Int (2010) 2010:463465. doi: 10.4061/2010/463465
10. Montero C, Souto A, Vidal I, Fernandez M, Blanco M, Verea H. Three cases of primary pulmonary plasmacytoma. Arch Bronconeumol (2009) 45:564–6. doi: 10.1016/j.arbres.2009.04.009
11. Luh S-P, Lai Y-S, Tsai C-H, Tsao TC-Y. Extramedullary plasmacytoma (EMP): report of a case manifested as a mediastinal mass and multiple pulmonary nodules and review of literature. World J Surg (2007) 5:123. doi: 10.1186/1477-7819-5-123
12. Niitsu N, Kohri M, Hayama M, Nakamine H, Nakamura N, Bessho M, et al. Primary pulmonary plasmacytoma involving bilateral lungs and marked hypergammaglobulinemia: differentiation from extranodal marginal zone b-cell lymphoma of mucosa-associated lymphoid tissue. Leuk Res (2005) 29(11):1361–4. doi: 10.1016/j.leukres.2005.04.009
13. Joseph G, Pundit M. Primary pulmonary plasmacytoma. Cancer (1993) 3(27):721–4. doi: 10.1002/1097-0142(19930201)71:3<721::aid-cncr2820710311>3.0.co;2-u
14. Horiuchi T, Hirokawa M, Oyama Y, Kitabayashi A, Satoh K, Shindoh T, et al. Diffuse pulmonary infiltrates as a roentgenographic manifestation of primary pulmonary plasmacytoma. Am J Med (1998) 105(1):72–4. doi: 10.1016/S0002-9343(98)00131-4
15. Wise JN, Schaefer RF. Primary pulmonary plasmacytoma. Chest (2001) 120:1405–7. doi: 10.1378/chest.120.4.1405
16. Kilciksiz S, Karakoyun-Celik O, Agaoglu FY, Haydaroglu A. A review for solitary plasmacytoma of bone and extramedullary plasmacytoma. Sci World J (2012) 2012:895765. doi: 10.1100/2012/895765
17. Kaneko Y, Satoh H, Haraguchi N, Imagawa S, Sekizawa K. Radiologic findings in primary pulmonary plasmacytoma. J Thorac Imaging (2005) 20(1):53–4. doi: 10.1097/01.rti.0000139389.88019.63
18. Kligerman SJ, Auerbach A, Franks TJ, Galvin JR. Castleman disease of the thorax: Clinical, radiologic, and pathologic correlation: From the radiologic pathology archives. Radiographics (2016) 36(5):1309–32. doi: 10.1148/rg.2016160076
19. Pribyl K, Vakayil V, Farooqi N, Arora N, Kreitz B, Ikramuddin S, et al. Castleman disease: A single-center case series. Int J Surg Case Rep (2021) 80:105650. doi: 10.1016/j.ijscr.2021.105650
20. Basavaiah SH, Lobo FD, Philipose CS, Suresh PK, Sreeram S, Kini H, et al. Clinicopathological spectrum of solitary plasmacytoma: a single center experience from coastal India. BMC Cancer (2019) 19(1):801. doi: 10.1186/s12885-019-5976-7
21. Etienne G, Grenouillet M, Ghiringhelli C, Vatan R, Lazaro E, Germain P, et al. Pulmonary plasmacytoma: about two new cases and review of the literature. Rev Med Interne (2004) 25:591–5. doi: 10.1016/j.revmed.2004.04.024
22. Lacaze O, Khaddage O, Court-Fortune I, Tiffet O, Vergnon JM. Isolated intrapulmonary plasmacytoma; diagnostic and therapeutic difficulties. Rev Mal Respir (2002) 19:648–50. doi: MDOI-RMR-10-2002-19-5-0761-8425-101019-ART18
Keywords: primary pulmonary plasmacytoma, extramedullary plasmacytoma, CT scan, pathology, prognosis
Citation: Wang J, Yang X, Liu X, He T, Liu B, Yang L, Yuan F and Li J (2022) Solitary extramedullary plasmacytoma in the lung misdiagnosed as lung cancer: A case report and literature review. Front. Oncol. 12:950383. doi: 10.3389/fonc.2022.950383
Received: 22 May 2022; Accepted: 08 August 2022;
Published: 30 August 2022.
Edited by:
Helmut H. Popper, Medical University of Graz, AustriaReviewed by:
Thomas Thomopoulos, Attikon University Hospital, GreeceMainak Dutta, Medical College and Hospital, Kolkata, India
Copyright © 2022 Wang, Yang, Liu, He, Liu, Yang, Yuan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Li, bGlqaW5nMTk4NTAxMjVAaG90bWFpbC5jb20=; Fei Yuan, ZmVpeXVhbjIwMjEyMDIyQDE2My5jb20=
 Jingjing Wang
Jingjing Wang Xiaoyun Yang2
Xiaoyun Yang2