
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 23 September 2022
Sec. Cancer Immunity and Immunotherapy
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.949332
This article is part of the Research Topic Application of Nanotechnology in Diagnosis and/or Therapy of Non-Small Cell Lung Cancer View all 5 articles
Traditional Chinese medicine (TCM), including herbal medicine, acupuncture and meditation, has a wide range of applications in China. In recent years, herbal compounding and active ingredients have been used to control tumor growth, reduce suffering, improve quality of life, and prolong the life span of cancer patients. To reduce side effects, herbal medicine can be used in conjunction with radiotherapy and chemotherapy or can be used as an adjuvant to strengthen the immune effect of anticancer vaccines. In particular, in the immunosuppressed tumor microenvironment, herbal medicine can have antitumor effects by stimulating the immune response. This paper reviews the advances in research on antitumor immunomodulation in Chinese herbal medicine, including the regulation of the innate immune system, which includes macrophages, MDSCs, and natural killer cells, and the adaptive immune system, which includes CD4+ T cells, CD8+ T cells, and regulatory T cells (Tregs), to influence tumor-associated inflammation. In addition, a combination of active ingredients of herbal medicine and modern nanotechnology alter the tumor immune microenvironment. In recent years, immunological antitumor therapy in TCM has been applied on a reasonably large scale both nationally and internationally, and there is potential for further clinical expansion. Investigation of immune modulation mechanisms in Chinese herbal medicine will provide novel perspectives of how herbal medicine controls tumor growth and metastasis, which will contribute to the evolution of tumor research.
Methodology: Experimental research between the years of 2012-2022, meta-analysis and reviews for the period 2002-2022 found on the Databases including PubMed, Embase, and the Cochrane database were used. The inclusion criteria were experimental research literature addressing the anti-tumor immunological effects of active ingredients and nanoparticles in Chinese herbal medicine. Exclusion criteria were articles that addressed Chinese herbal medicine and nano-formulations without discussing anti-tumor immunological effects in innate, adaptive immune cells, MDSCs, and nuclear factors.
Worldwide, tumors have increased in their morbidity and associated mortality, constituting a grave risk to human health and life. According to contemporary research, the development of tumors is correlated with each individual’s immune system. Immunotherapy has been shown to be a powerful approach for treating a broad range of cancers and is widely used in clinical practice.
Given its large population, China accounts for approximately a quarter of the world’s new cancer cases and deaths, which leads to a serious burden of sickness (1). Traditional Chinese Medicine (TCM) is a unique diagnostic and therapeutic method that has been used for thousands of years and is widely used in China. Traditional herbal medicine is mostly used in the form of compound prescriptions in clinical oncology and includes oral herbal medicines, granules or capsules, and injections (2).
Numerous studies indicate that the combination of herbal medicine with antitumor therapy can achieve significant tumor suppression, reduce drug resistance, reduce adverse effects, and improve overall patient health and quality of life (3).
Various studies have demonstrated that herbal medicine can inhibit the proliferation 1 and metastasis of a variety of tumor cells, including lung (4), breast (5), colorectal (6), and other tumor cells, while facilitating their apoptosis. This paper will review the immunomodulatory role of herbal medicine in tumor immunity, including in regulation of the innate immune system, the adaptive immune system, and tumor-associated inflammation, as well as the emerging strengths of integrating herbal medicine with modern nanotechnology (Figure 1).
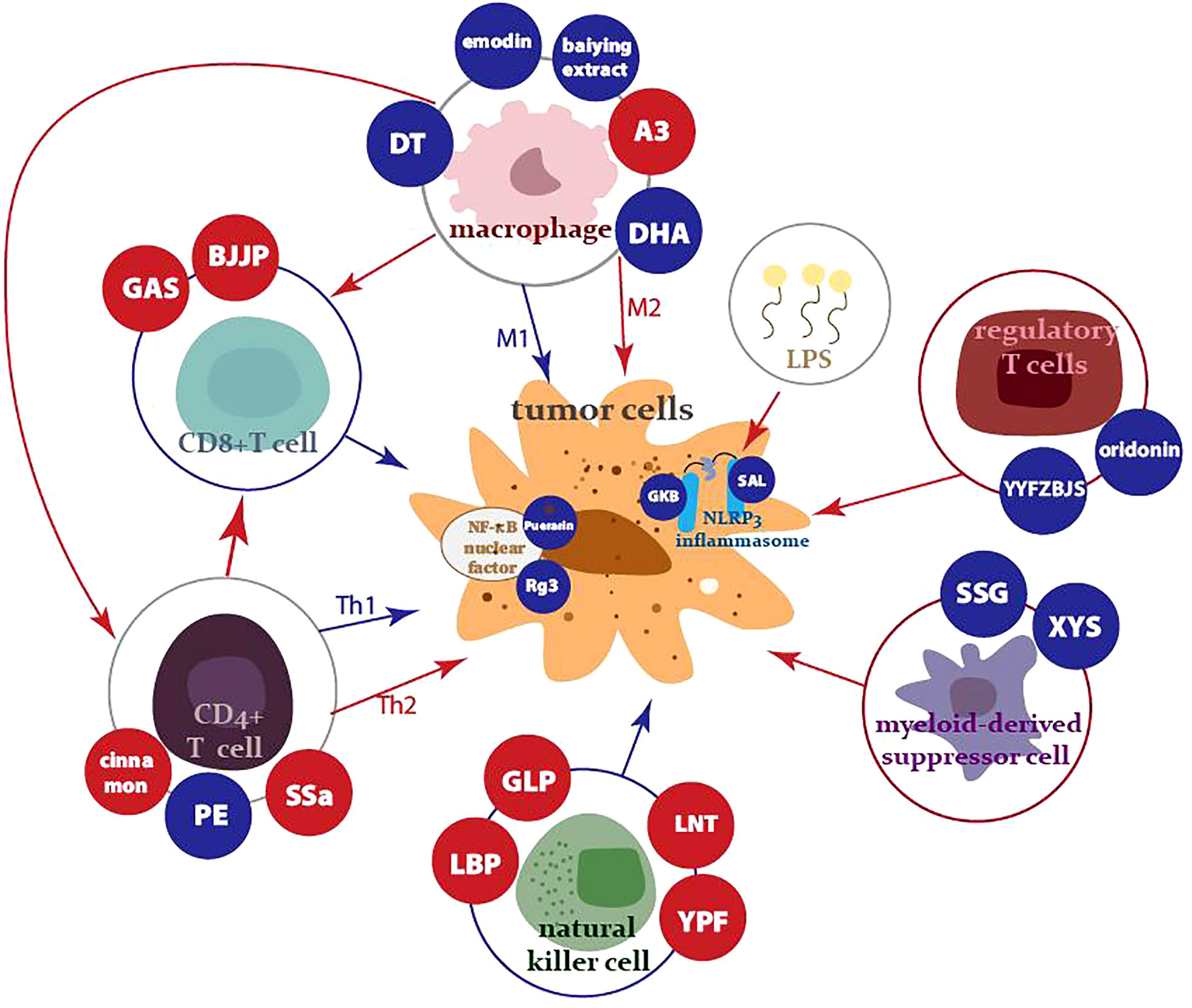
Figure 1 Innate, adaptive immune cells, MDSCs, and nuclear factors controlled by antitumor traditional herbal medicine and its components. The red arrows and dots represent activation, while the blue arrows and dots indicate suppression.
Substantial clinical and experimental evidence indicates that macrophages, which are present abundantly in most tumor types, play a major regulatory role in promoting tumor progression to malignancy (7). The presence of tumor-associated macrophages (TAMs) is generally associated with a poor prognosis in solid tumors.
There are two main categories of activated macrophages: classically activated macrophages (M1; interferon (IFN)-γ/lipopolysaccharide (LPS)-dependent) and alternatively activated macrophages (M2; interleukin (IL)-4)/IL-13/IL-10-dependent) (8).
M1 macrophages have a high capacity for antigen presentation and the ability to secrete a range of cytokines and chemokines that promote inflammatory responses against pathogens and tumor cells as well as mediate the killing of tumor cells. In comparison, M2 macrophages have low antigen-presenting activity and have the functions of suppressing inflammatory responses and enhancing wound healing, angiogenesis, and tissue repair (9).
M1 macrophages support oncogenesis by inducing oxidative stress and an anti-apoptotic response, leading to cell survival and proliferation. During the chronic inflammatory process, M1 macrophages influence the tumor microenvironment and sustain further tumorigenesis. M2 cells also drive tumorigenesis by regulating the formation of extracellular matrix and angiogenesis in the tumor mesenchyme. In contrast, there is some evidence of tumor-suppressive actions of macrophages (10). Yuan R et al. (11) found that an M2 phenotype predominates in TAMs and plays a critical role in promoting tumor growth, invasion, and metastasis.
Evidence indicates that in nascent tumors, TAMs display an M1-like phenotype and can eliminate some immunogenic tumor cells. Subsequently, tumor progression is associated with skewing and subversion of macrophage function by the cues within the tumor microenvironment that could direct an M2-like polarization of TAMs that is pro-tumorigenic (12).
Typically, suppression of M2 polarization may help to stimulate antitumor-specific immune responses and decrease tumor metastasis. Many works have shown that herbal medicine could control the growth and metastasis of tumors by moderating M1/M2 macrophages.
Epithelial-mesenchymal transition (EMT) contributes to cancer progression, particularly metastasis. The reprogramming of gene expression during EMT is triggered and controlled by multiple signaling pathways, in which transforming growth factor-β (TGF-β) signaling plays a primary role (13). M2 macrophages secretes TGF-β1, leading to proliferation, invasion, and migration of tumor cells. Emodin is an anthraquinone compound isolated from Chinese herbs such as Rheum palmatum L., Rheum tanguticum Maxim. ex Bal£. and Rheum officinale Bail1. (The effects and specific information for each herbal medicine are shown in Tables 2, 3).
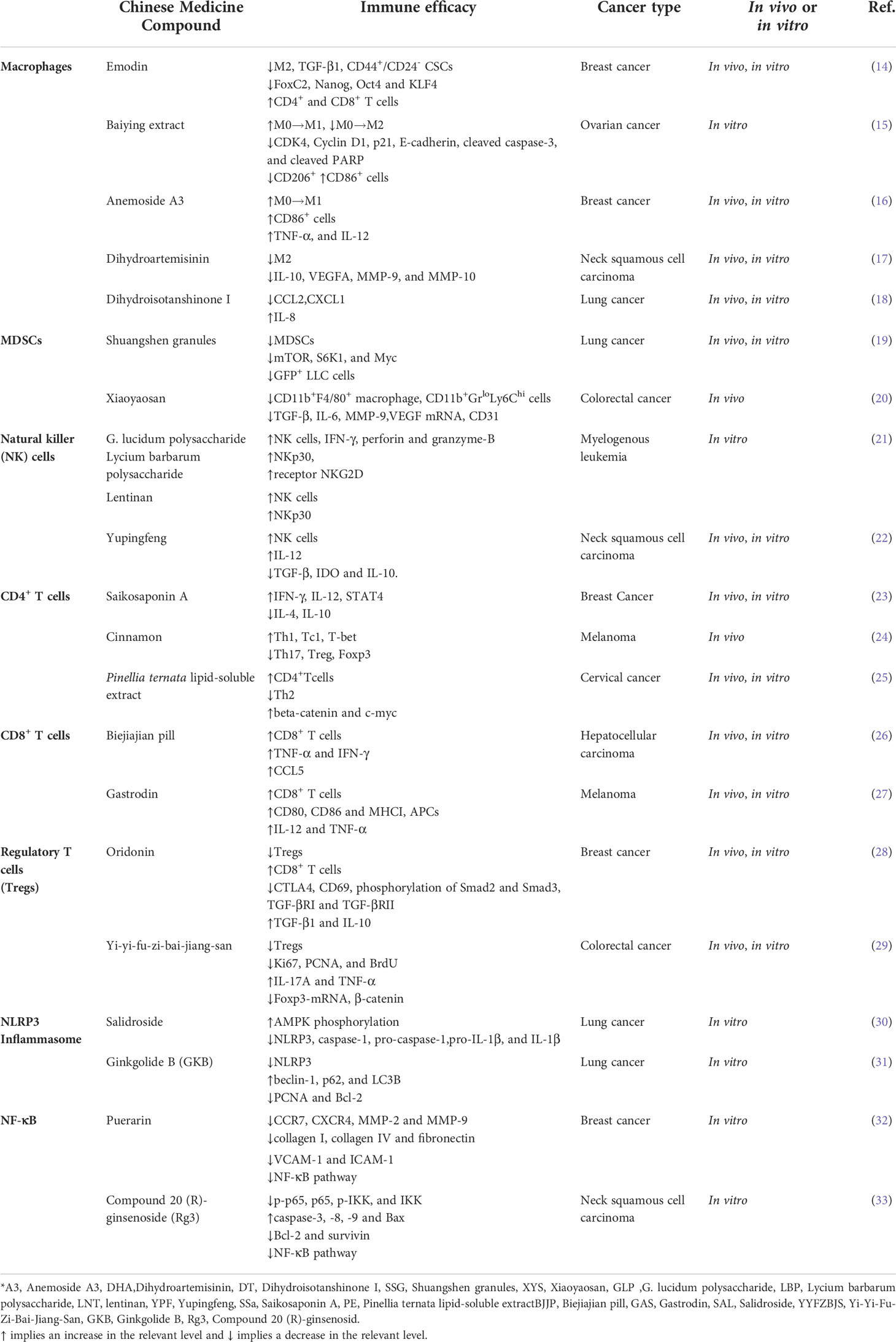
Table 2 Summary of the herbal active ingredients’ immunomodulating effects referred to in this review.
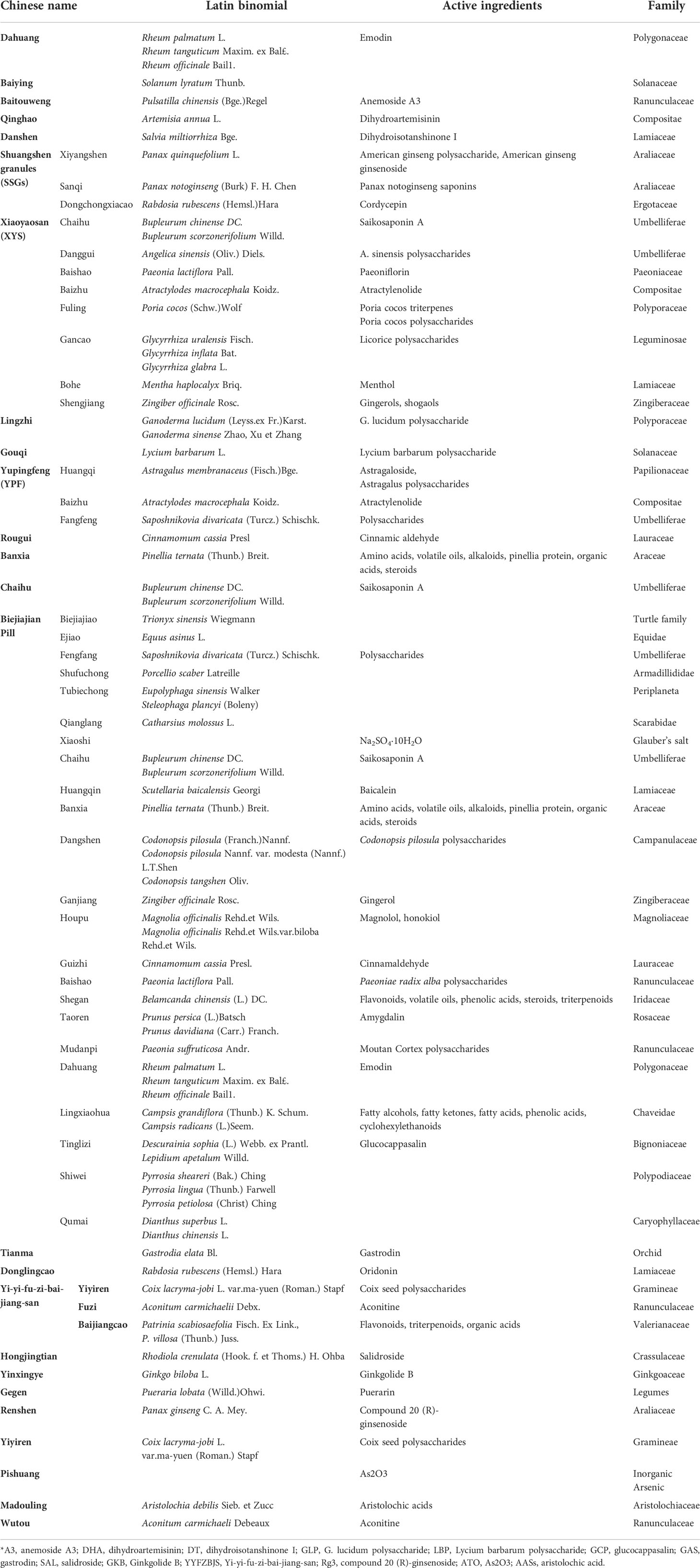
Table 3 List of Chinese herbs, Latin binomials, active ingredients, and species mentioned in the review.
To examine the relationship between macrophage abundance and other properties in breast tumors, Liu Q et al. (14) examined data from 1,105 samples obtained from 1,098 patients with breast cancer from The Cancer Genome Atlas of breast tumors, and 529 samples were selected from these.
Using the expression level of the pan-macrophage marker CD68 as an indicator of macrophage abundance, they sorted CD68lo and CD68hi samples and found that the CD68hi group was associated with pro-tumorigenic markers, including TGF-β1, EMT markers, stemness markers, and related transcription factors. These findings were confirmed by subsequent statistical analysis.
Subsequently, they found that emodin was able to reduce TGF-β1 expression and reverse the increased N-cadherin and reduced E-cadherin protein levels caused by TGF-β1 treatment. They isolated primary macrophages from mice, pretreated them with tumor cell-conditioned medium (TCCM) to generate TAM-like macrophages, and then added them to breast cancer cells. TCCM-pretreated macrophages exhibited production of TGF-β1 and an increased expression of CD206, a marker of M2 macrophages, which was attenuated by emodin. In addition, emodin significantly reduced both baseline peritoneal macrophage-conditioned medium (PMCM) treatment-induced TGF-β1 production in cancer cells and TGF-β1 induced Arg-1 expression, which has been shown to define immunosuppressive subsets of TAMs.
Thus, emodin was found to suppress cancer cell-induced macrophage M2-like polarization, inhibit polarized macrophage-induced TGF-β1 production in breast cancer cells, and block EMT of breast cancer cells induced by TGF-β1 from both macrophages and cancer cells.
As demonstrated by a wound-healing migration assay and Matrigel invasion assay, rhodopsin markedly reduced the migration and invasion of TGF-β1-enhanced breast cancer cells.
Self-renewing cancer stem cells (CSCs) and progenitor cells constitute a minor portion of neoplastic cells within tumors and are collectively defined as tumor-initiating cells (TICs). The TIC population is the key source of metastatic lesions in breast cancer.
Emodin reduced baseline levels of CD44hi/+/CD24lo/-, which are recognized CSC markers, and blocked TGF-β1-induced CSC increasing in 4T1 cells. The TME provides WNT and EGF signals contributed to tumor regeneration and therapy resistance. For primary PyMT tumor cells, emodin suppressed the percentage of progenitor cells and the average CD61 expression levels, a marker of progenitor population in breast cancer. The baseline expression of FoxC2, Nanog, Oct4 and KLF4 was downregulated by emodin in MDA-MB-231 cells, and the TGF-β1-induced expression of Jagged1, KLF4 and FoxC2 was decreased by emodin in 4T1 cells, which are all TIC-related.
Breast cancer TICs can be propagated in vitro as nonadherent spheres; these spherical clusters of self-replicating cells formed in suspension cultures are called mammospheres. Emodin significantly reduced both primary and secondary mammosphere formation in EO771, 4T1, MCF7, and MDA-MB-231 cells. Similarly, C57BL/6 mice transplanted with a prepared emodin EO771 single-cell showed a reduced estimated stem cell frequency, and MDA-MB-231 cells in NOD SCID mice remained effective. Both the in vitro mammosphere formation assays and the in vivo limiting dilution assays demonstrated that emodin reduced breast cancer TICs.
After BALB/C mice were inoculated with 4T1 cells, treatment with emodin notably reduced CD206+ M2-like macrophage infiltration and average cell surface CD206 level of macrophages. In addition, emodin treatment increased both CD4+ and CD8+ T cells in the tumors and the percentage of IFNγ+ cells. Emodin treatment decreased CD44+/CD24- CSCs in the tumors and reduced the expression of EMT markers vimentin and MMP9.
EMT and TICs are exploited as therapeutic targets for metastatic breast cancer. Circulating tumor cells (CTCs) are pioneering tumor cells with stemness properties transendothelially and acquire mesenchymal characteristics following EMT, migrating into the intratumoral microvessels. Thereafter, CTCs can form new tumors and are a sign of breast cancer metastasis. In vivo experiments have confirmed that emodin substantially reduced lung metastasis and increased survival compared with the vehicle-treated control.
Emodin significantly inhibited Smad2/3 phosphorylation and nuclear translocation and suppressed TGF-β1-induced phosphorylation of AKT and the induction of Zeb1 and Twist by TGF-1β by TGF-β1 in both 4T1 cells and MDA-MB-231 cells, suggesting that emodin blocks TGF-β1-mediated crosstalk between TAMs and breast cancer cells.
Furthermore, Pourhajibagher M et al. (34) synthesized a transfersome form of nano-emodin as a novel sono-responsive nanomaterial that could significantly increase expression of caspase-3 and caspase-9, promoting cell apoptosis for head and neck squamous cell carcinoma. Another aloe emodin-encapsulated nanoliposome (35) was used to transfect the r-caspase-3 gene, leading to inhibition of the proliferation rate and an increased apoptotic rate in human gastric cancer cells.
In TCM, Baiying is used to treat malaria, jaundice, edema, gonorrhea, rheumatic joint pain, dampness, and furuncles. Baiying (Solanum lyratum Thunb.) extract (15) (SLT) significantly inhibited the cell viabilities and reduced the protein levels of CDK4 and cyclin D1 in both A2780 and SKOV3 cells. SLT inhibited cell proliferation and EMT and induced cell apoptosis, manifested as increased expression of p21, E-cadherin, cleaved caspase-3, and cleaved PARP and decreased expression of N-cadherin. In addition, SLT can activate ROS/p53 to regulate ovarian cancer progression.
The percentage of CD206+ M2 macrophages was significantly decreased in the A2780/SKOV3+M0 macrophage groups treated with SLT, while the percentage of CD86+ M1 macrophages was significantly increased, indicating that SLT could activate the polarization of M0 macrophages to M1 macrophages but inhibit the polarization of M0 macrophages to M2 macrophages, thus regulating the tumor immune microenvironment and inhibiting ovarian cancer progression.
Anemoside A3 (16) (A3) is an active compound from Pulsatilla saponins (Bge.) Regel, which can active the TLR4/NF-κB/MAPK signaling pathway. In vitro A3 increased the expression of CD86+ M0 macrophages, polarizing M0 macrophages into the M1phenotype and elevated TNF-α and IL-12 expression in a TLR4-dependent manner. Moreover, in a 4T1 murine breast cancer model, A3 induced M1 macrophage polarization and effectively inhibited tumor growth and tumor angiogenesis in vivo.
M2 phagocytosis promotes the progression of neck and head squamous cell carcinoma with tumor cell invasion, migration, and angiogenesis. Dihydroartemisinin (DHA) is a semi-synthetic derivative of Artemisia annua L., which has the function of clearing heat and relieving malaria and itching. In in vitro experiments, DHA (17) led to a significant decrease in the number of M2-like TAMs and reduced IL-10, vascular endothelial growth factor A (VEGFA), MMP-9, and MMP-10 expression. In addition, DHA decreased M2-like TAM-promoted vascular formation by inhibiting phosphorylation of STAT3.
The Chinese herb danshen (Salvia miltiorrhiza Bge.) is used to treat chest pain, abdominal pain, insomnia, and menstrual disorders. Clinical data analysis has revealed that treatment with salvia is highly correlated with decreased mortality in patients. Dihydroisotanshinone I (DT) is a fat-soluble phenanthrene compound extracted from the Chinese herb Salvia miltiorrhiza Bge. DT (18) treatment resulted in decreased expression of CCL2 and C-X-C motif chemokine ligand 1 (CXCL1) and increased expression of IL-8, blocking the ability of macrophages to promote lung cancer cell migration and the recruitment of macrophages by lung cancer cells. Furthermore, DT treatment significantly inhibited the final tumor volume in a xenograft nude mouse mode in vivo experiment.
Han S et al. (36) used a poly (D,L-lactic-co-glycolic acid) (PLGA)-based nanoparticle (NP) to co-encapsulate dihydrotanshinone I (DIH) and two other drugs, generating chemo-immunotherapeutic effects to reverse an immunosuppressive tumor microenvironment and found significantly increased survival among mice with hepatocellular carcinoma (HCC), without associated toxic signs.
Myeloid-derived suppressor cells (MDSCs) are a population of cells of bone marrow origin with strong immunosuppressive properties that are present in the peripheral blood and tumor tissue of tumor-bearing mice or patients. In normal conditions, this population of cells differentiates into dendritic cells, macrophages, and granulocytes, but in the presence of inflammation, tumors, trauma, and infection, MDSCs can be significantly expanded.
MDSCs can be classified into two subgroups: granulocyte-like MDSCs (G-MDSCs) and monocyte-like MDSCs (M-MDSCs). In most experimental tumor animal models, G-MDSCs have a greater tendency to proliferate than M-MDSCs; hence, G-MDSCs are the predominant type of MDSCs in tumor tissue (37). In clinical practice, the accumulation of MDSCs in the peripheral blood and tumors of patients with liver cancer is strongly associated with HCC staging and poor survival, and the recruitment of MDSCs can cause liver tumor recurrence after transplantation (38). A high level of MDSCs in patients is often a sign of ineffective immunotherapy, and herbal medicine is often used for its antitumor effects to reduce the recruitment of MDSCs.
Immunosuppression is a dominant feature of MDSCs. Although MDSCs are implicated in the suppression of different cells of the immune system, the main target of MDSCs is T cells. MDSCs also impact the tumor microenvironment reformulation and tumor angiogenesis through VEGF expression, thus contributing to tumor development (39). In parallel, MDSCs can trigger the expression of miRNA101 in cancer cells. MiRNA101 subsequently represses CtBP2, which directly targets core stem cell genes, leading to increased stalkiness of cancer cells as well as higher metastatic and tumorigenic potential (40).
Shuangshen granules (SSGs) are composed of Panax quinquefolium L., Panax notoginseng (Burk) F. H. Chen and Rabdosia rubescens (Hemsl.) Hara. In a co-culture model in vitro, SSG treatment (19) exhibited a significant attenuating effect on bone marrow cell differentiation into CD11b+Ly6C+Ly6G+ cells and a partial attenuating effect on differentiation into CD11b+Ly6C+Ly6G– cells in a dose-dependent manner. SSGs suppressed the mTOR, S6K1, and Myc protein levels both in vivo and in vitro. Next, the effect of SSGs on tumor growth and lung metastasis was assessed in vivo, and mice treated with SSGs showed a reduction in tumor weight and GFP+ LLC cells in lung tissue. The results showed that SSGs can attenuate the differentiation of bone marrow by inhibiting the mTOR signal pathway in the bone marrow microenvironment and decrease the systemic levels of MDSCs in the bone marrow, blood, and lungs, suggesting that SSGs not only have an inhibitory effect on tumor cell proliferation but also have a protective effect against lung metastasis.
Chronic restraint stress is one of the most recognized Liver Qi stagnation models as it may mimic symptoms of depression in humans and can promote angiogenesis, resulting in cancer development and impacting the migration of tumor cells (41). Xiaoyaosan (XYS) is a traditional Chinese medical formula consisting of Chaihu (Bupleurum chinense DC., Bupleurum scorzonerifolium Willd.), Danggui (Angelica sinensis (Oliv.) Diels.), Shaoyao (Paeonia lactiflora Pall.), Baizhu (Atractylodes macrocephala Koidz.), Fuling (Poria cocos(Schw.)Wolf.), Gancao (Glycyrrhiza uralensis Fisch., Glycyrrhiza inflata Bat., Glycyrrhiza glabra L.), Bohe (Mentha haplocalyx Briq.), and Shengjiang (Zingiber officinale Rosc.), which has been studied for its effectiveness in treating hepatitis (42).
Zhao L et al. (20) found that in an in vivo experiment, XYS-treated mice had fewer liver metastases and a lower liver weight, and XYS could significantly decrease the CD11b+F4/80+ macrophage population in the primary tumor tissue, accompanied by a significant reduction in CD11b+GrloLy6Chi cells. Treatment of mice undergoing chronic restraint stress with XYS significantly reduced TGF-β, IL-6, MMP-9, and VEGF mRNA and CD31 protein expression compared with mice in the blank-stress (BS) group, which indicated that XYS treatment reduced the effect of chronic restraint stress on the facilitation of cancer cell invasion and metastasis.
Natural killer (NK) cells are intrinsic immune cells that have the rapid capacity to recognize and clear infected cells. A growing number of experimental and clinical studies have shown that NK cell-based antitumor immunotherapy is highly promising (43). NK cells play an essential position in controlling the metastasis and proliferation of cancer cells. NK cells differentiate tumor stem cells or undifferentiated tumors by secreting or membrane-bound IFN-γ and TNF-α and prevent tumor growth by modifying the tumor microenvironment (44). In addition, as documented in multiple experiments, NK cells can distinguish abnormal cells from healthy ones, leading to more specific antitumor cytotoxicity and reducing off-target complications (45).
Mounting evidence in human cancer indicates that NK cell frequency, infiltration, and function improve patient survival (46, 47).
Huyan T et al. (21) researched five polysaccharides derived from plants or fungi including G. frondosa polysaccharide (GFP), lentinan (LNT), G. lucidum polysaccharide (GLP), Lycium barbarum polysaccharide (LBP), and yeast glucan (YG). They found that at a concentration of 100 mg/L, these polysaccharides could have an apparent positive effect on NK cell proliferation, and the three polysaccharides, GLP, LBP and LNT, increased the cytotoxicity of NK cells after 48 h of treatment.
GLP and LBP treatment could significantly upregulate the mRNA expression of IFN-γ, perforin and granzyme-B, while the expression of the surface activating receptor NKp30 was significantly u-regulated after GLP, LBP, and LNT treatment, which can promote NK cell secretion of TNF-α and IFN-γ when stimulated by its ligand, NKp30L. In addition, LBP and GLP treatment enhanced NK cell function under simulated microgravity (SMG) conditions by restoring the expression of the activating receptor NKG2D and reducing early apoptosis and late apoptosis/necrosis.
Another traditional Chinese medicine Danggui (Angelica sinensis (Oliv.) Diels.) can target the liver, and one of its essences lies in the existence of polysaccharide components, A. sinensis polysaccharides (ASP). Liu X et al. (48) synthesized amphiphilic polymer micelles contained ASP, achieving a hypoxic response to drug release, and found enhanced cell uptake and effectively improved proliferation inhibitory activity of HepG2 hepatocellular carcinoma cancer cells.
Yupingfeng (YPF) is a traditional formula in Chinese medicine, consisting of Huangqi (Astragalus membranaceus (Fisch.) Bge.), Baizhu (Atractylodes macrocephala Koidz.), and Fangfeng (Saposhnikovia divaricata (Turcz.) Schischk.), which has Qi nourishing properties. In non-small cell lung cancer (NSCLC) therapy, compared with the untreated control, YPF (22) treatment significantly inhibited the growth of Lewis lung cancer (LLC) cells and prolonged the median survival time for LLC-bearing mice. Furthermore, the results of the flow cytometry analysis revealed that YPF treatment increased NK cell tumor infiltration and the NK cell population in spleen.
By isolating splenic cells and evaluating their cytotoxicity through a calcein-release assay, YPF treatment was demonstrated to enhance NK cell-mediated killing activity. After using anti-NK1.1 antibody PK136, the tumor suppressive effect of YPF was reversed, which implied that the suppression of tumor growth by YPF was NK cell-dependent. YPF probably inhibits cancer development by increasing IL-12 expression to promote NK cell activation while decreasing TGF-β, IDO, and IL-10.
CD4+ T cells contain two diverse subsets of helper T cells, namely, Th1 cells, which produce TNF-α, IFN-γ, IL-2, and IL-12 to mediate antitumor effects, and Th2 cells, which generate IL-4 and IL-10 and foster tumor growth by suppressing the host immune system (49).
According to clinical data, cancer patients suffer from a Th1/Th2 imbalance, with increased cytokine release from Th2 cells. Patients with Th1-led responses have higher survival rates and lower cancer recurrence rates. Consequently, the development of new therapeutic strategies to modify the Th1/Th2 balance may assist in the control of cancer (50).
Saikosaponin A (SSa) is an effective ingredient extracted from the radicals of the root of Bupleurum chinense DC. or Bupleurum scorzonerifolium Willd. Tumor volume was significantly reduced in the tumor-bearing rats treated with SSa (23) compared with those in a control group. After treatment with SSa, there was also weak positive Ki-67 staining, which is a proliferative biomarker of tumor cells and is associated with a poor prognosis.
Detection of signature cytokines revealed that SSa treatment significantly promoted IFN-γ and IL-12 secretion and inhibited IL-4 and IL-10 secretion, indicating that SSa could induce Th1/Th2 shifting into Th1 cell response. Compared with the control group, the SSa group showed significantly increased protein expression levels of IL-12, protein and mRNA expression levels of IL-12R, mRNA expression levels of STAT4, and protein expression levels of pSTAT4, which indicated that SSa could activate the IL-12/STAT4 pathway.
Rougui (Cinnamomum cassia Presl) is a commonly used herb that disperses Cold and relieves pain. It is often used in Chinese medicine to treat impotence, lumbago and menstrual pain.
High-energy ion radiation (IR) has been shown to induce immune damage in clinical applications, investigating the antitumor immune response of single low-dose total body irradiation (SLTBI), Zheng X et al. (24) experimented.
After SLTBI treatment in 57BL/6 mice with melanoma model, both the cell counts and the frequencies of total T cells (CD3+) and CD4+ and CD8+ T cells from the spleen were significantly decreased within 3 to 5 days, and CD3+CD4+T cell restoration started from day 8 and returned to normal levels 10 days after exposure, which showed that the reconstitution of CD4+ T-helper cells was delayed after SLTBI. Although T-cell numbers increased at 10 days after SLTBI, IFN-γ production released by Th1 or by Tc1 was still at a lower level in irradiated mice than in control mice, whereas the proportions of Th2 (CD3+CD4+IL-4+), Th17 (CD3+CD4+IL-17A+), and Treg (CD4+CD25+Foxp3+) cells were significantly increased in the IR group.
Cinnamon-treated up-regulated Th1 and Tc1 cells were significant, whereas Th17 and Treg cells were down-regulated. After administration of cinnamon, T-bet expression increased and expression of Foxp3 decreased, while the transcription levels of RORgt and GATA-3 were not changed significantly. The result showed that cinnamon modulates specific signaling of distinct T-cell subsets, primarily activating Th1 and inhibiting Treg.
Banxia(Pinellia ternata (Thunb.) Breit.), a traditional Chinese herbal medicine, show a function as reducing swelling and drying Dampness. Huang H (25) et al. used a novel Pinellia ternata lipid-soluble extract (PE) to treat HPV(+) tumors in vivo and in vitro.
They co-cultured naïve CD4+ T cells and DCs, and treated with PE solution, founding that PE treatment significantly upregulated beta-catenin and c-myc levels in vitro.
In C57BL/6 mice burdened with the HPV+TC-1 tumor by PE treatment, flow cytometry analysis resulted in the percentage of CD4+ T cells in peripheral blood mononuclear cells (PBMCs) and tumor-infiltrating lymphocyte (TIL) under PE-low treatment and in TIL under PE-high treatment was obviously increased. With PE-low treatment, T-bet expression was expressed more highly in the PBMCs and TIL of mice bearing HPV+TC-1 tumors than in the solvent control group. The IFN-γ production level was also significantly increased in PBMCs compared with TIL, which showed that Th1 cells increased significantly. Additionally, they found that GATA3 and IL-14 had a lower expression in the PBMCs and TIL of mice after PE-low treatment, indicating the downregulation of Th2.
The main function of activated CD8+ T cells (cytotoxic T cells) is to secrete perforin, TNF-α, and FASL, specifically killing target cells. Their antitumor function is mainly dependent on two factors: the differentiation of T cells and the infiltration of CD8+ T cells, i.e., the transport of CD8+ T cells into the tumor microenvironment, where they secrete cytotoxic factors and exert tumor lysis (51). Increased levels of CD8+ T cells in the tumor microenvironment have been demonstrated to be correlated with antitumor effects in a wide range of cancer types, demonstrating a correlation between increased levels of CD8+ T cells and cancer prognosis.
Biejiajian pill (BJJP) is a classical formula in the “golden chamber synopsis”, consisting of 23 components. In herbal medicine, it is extensively used in the therapy of liver cancer. Yang X et al. (26) compared the antitumor effects of BJJP in immunodeficient BALB/c-nu/nu and immunocompetent BALB/c mice using the H22 tumor cell model. They found that after treatment with BJJP, the tumor weights and volumes in the immunocompetent BALB/c mice were notably lower than those in the immunodeficient BALB/c-nu/nu mice and that the BJJP-treated immunocompetent BALB/c mice had the highest rate of apoptosis, suggesting BJJP can promote antitumor immunity in HCC. Immunofluorescence or fluorescence-activated cell sorting showed that CD8+ T cell infiltration after treatment with BJJP and BJJP treatment also promoted the effector function of CD8+ T cells by significantly increasing the expression of TNF-α and IFN-γ in tumor-infiltrating CD8+ T cells.
Chemokines and chemokine receptors are necessary for the recruitment of CD8+ T cells into tumors, which were identified to regulate the migration of CD8+ T cells in previous studies. Compared with the control groups, there was a significant increase in the expression of CCL5 in the subcutaneous tumors of mice in the BJJP group. When co-stained CCL5 with CD8+ T cells, fields with higher CCL5 expression showed more CD8+ T cell infiltration, and higher levels of serum CCL5 were observed in the BJJP-treated group.
Spleen cells from BABL/c mice were co-cultured with GFP+H22 cells, and the results revealed a significant increase in the number of migrating CD8+ T cells in the BJJP-treated group, and migration occurred in a dose-dependent manner in vitro. In addition, in the BJJP-treated group, there was a 2- and 3-fold increase in the mRNA and protein expression levels of CCL5.
BJJP is a compounded formula containing many herbs, some of which have been developed at the nanoparticle level.
Zhang M (52) created nanoparticles from ginger and reassembled their lipids into ginger-derived nanovectors (GDNVs), were capable of loading Dox with high efficiency, and showed a better pH-dependent drug-release profile. Furthermore, Dox-GDNVs activated caspase-3/7 and exerted apoptotic effects in Colon-26 and HT-29 cells. In BALB/c nu/nu female mice bearing Colon26 subcutaneous xenografts, treatment with Dox-FA-GDNVs downregulated Ki67 expression, resulting in a significant decrease in the number of cancerous cells compared with saline, free Dox, and FA-GDNVs groups, showing effective in targeting tumors and reducing their volume.
Haggag YA (53) developed a novel PEGylated-PLGA polymeric nanocapsules (NCs) for HK delivery to breast tumor-bearing mice. HK-loaded NCs achieved significant cytotoxic action compared to free HK due to successful HK delivery to the subcellular site of action2 meanwhile preserving its anti-cancer activity after formulation.
HK-loaded NCs showed a significant increase in caspase-3 level and decrease in VEGF-1 level in tumor tissue compared to control animals and the free HK treated group, which confirmed HK-loaded NCs had a potent antiangiogenic and apoptotic activity compared to free drug.
Gastrodin (GAS) is an organic compound extracted from TCM Tianma (Gastrodia elata Bl.), which is used to treat strokes, convulsions, vertigo, headaches, and numbness in the limbs. In vitro, GAS increased CD80, CD86 and MHCI, which are APC activation markers, in DC2.4 cells after treatment compared with the negative control. In vivo, GAS treatment in mice also stimulated the activity of APCs. Intracellular staining of splenocytes and flowcytometric analysis showed that the expression levels of IL-12 and TNF-α were upregulated.
The expression levels of perforin and TNF-α were increased by GAS + cell treatment compared with control group, and cytotoxic lymphocytes were significantly increased in mice immunized with GAS, which suggested that GAS can promote the responses of CD8+ T cells by enhancing T-cell proliferation and inflammation cytokine expression. Liu Z et al. (27)demonstrated the immunogenicity of GAS as an adjuvant in an anti-melanin vaccine when they found that GAS enhanced the activation of antigen-presenting cells (APCs) - both in vivo and in vitro.
Wang J et al. (54) developed a new nano formulation encapsulated with GAS, and GAS was released and delivered into the brain, where its scavenged the oxygen free radicals produced from cavitation, providing an approach to monitor and inhibit cell apoptotic events.
Regulatory T cells (Tregs) are a subset of CD4+ T cells that control autoimmune responses and maintain immune homeostasis. During tumor development, tumor cells and macrophages in the regional tumor microenvironment secrete chemokines that recruit Tregs from peripheral blood into the tumor, allowing Tregs to escape from the host immune system due to the immunosuppressive function (55).
Higher expression of Tregs tends to suggest decreased patient survival. In the field of tumor immunotherapy, there is an acute demand to reduce the number of Tregs to increase antitumor immune response (56).
Oridonin is an active ingredient isolated and extracted from the herb Donglingcao (Rabdosia rubescens (Hemsl.) Hara), which is known for its ability to clear heat, promote blood circulation, and relieve pain. Guo J (28) injected 4T1 cells subcutaneously into WT BALB/c mice to assess the effect of oridonin on the growth of 4T1 murine triple-negative breast cancer (TNBC) in vivo and found that oridonin treatment suppressed tumor progression compared with control mice. TILs from oridonin-treated mice had a significantly reduced number of Tregs, but the proportion of CD8+ T cells was increased. In addition, the spleens of the oridonin-treated mice also demonstrated a decreased percentage of Tregs and an enhanced level of IFN-γ+CD8+ T cells. Similarly, in vitro, oridonin decreased the number of Tregs in a dose-dependent manner and attenuated the induction of Foxp3+CD4+ T cells.
Oridonin-treated Tregs had lower expression of CTLA4 and CD69 and lower protein secretion levels of TGF-β1 and IL-10 than control Tregs. Tregs treated with oridonin displayed a diminished ability to suppress the proliferation of CD8+ T cells, and the phosphorylation of Smad2 and Smad3 was notably decreased in oridonin-treated Tregs. Furthermore, oridonin inhibited the protein levels of TGF-βRI and TGF-RβII, and when utilizing TGF-βreceptor inhibitor SB431542, oridonin could not further suppress Treg differentiation, indicating that oridonin represses Treg polarization by decreasing the TGF-β receptor.
In addition, in treating 4T1 tumors in WT BALB/c mice, therapy with oridonin and anti-PD-1 resulted in superior tumor regression compared with treatment with either oridonin or anti-PD-1 alone. Oridonin+PD-1 treatment resulted in more CD8+ T TILs and increased numbers of IFN-γ+CD8+ T cells and granzyme B+CD8+ T cells, showing that oridonin improves the antitumor activity of anti-PD-1 in breast cancer.
Yi-Yi-Fu-Zi-Bai-Jiang-San (YYFZBJS) is from the Golden Chamber and is commonly used in traditional Chinese medicine to treat gastrointestinal disorders. It is composed of three herbs: Yi-Yi-ren (Coix lacryma-jobi L.var.ma-yuen (Roman.) Stapf), Fuzi (Aconitum carmichaelii Debx.), Baijiangcao (Patrinia scabiosaefolia Fisch. ex Link., P. villosa (Thunb.) Juss.), considered in the theoretical system of Chinese medicine to have the effect of reducing swelling and draining pus, and is used in the treatment of Changyong, known in modern medicine as appendicitis.
Sui H (29) et al. gavaged germ-free ApcMin/+ mice with stool from healthy controls and YYFZBJS volunteers, and discovered that ApcMin/+mice treated with YYFZBJS carried fewer adenomas both in the small and large intestine, and animal weight and hepatorenal toxicity among treatment groups during the experiment, confirming the safety of these herbs. The proliferation markers, nuclear expression levels of Ki67 and PCNA, and BrdU reactivity in intestinal polyp epithelia were reduced after YYFZBJS treatment.
Compared to normal control mice, ApcMin/+ mice secreted higher levels of inflammatory cytokines/chemokines, including IL-17, Eotaxin-2, Leptin and PF4, and YYFZBJS treatment downgraded IL-17, and IL-10 in myeloid precursor differentiation, additionally, IL-6, CCXL13 and IL-10 also had a lower level expression than the untreated group. Further results of ELISA showed that the expression IL-17A and TNF-α were upregulated by YYFZBJS treatment, which suggested that YYFZBJS blocked tumor progression in CRC murine model possibly via inhibiting the accumulation of Treg cells in immune organs, and in tumor microenvironment.
To determine whether the presence of gut commensal bacteria affects regulatory T cells, in vitro experiments showed that YYFZBJS extract treatment could inhibited the increased-Foxp3-mRNA expression when the enterotoxigenic Bacteroides fragilis (ETBF) were co-incubated with CD25+/CD4+ T cells which isolated from the spleens of ApcMin/+ mice. Compared with the ETBF primed Treg group, the proliferation of MC-38 cells were decreased after treated with YYFZBJS extract and ETBF primed Treg. Western blotting results showed that YYFZBJS could reverse the distribution of β-catenin in the nucleus, which is ETBF primed Treg significantly increased.
Inflammation is part of the innate immune response to danger signals, tissue disruption, and/or infection. Properly terminated, inflammation is beneficial; however, chronic inflammation increases cancer risk and has a great effect on the composition of the tumor microenvironment and particularly on the plasticity of both tumor and stromal cells (57).
Extensive epidemiological studies have confirmed that chronic inflammation predisposes people to various cancers. Cancer-related inflammation impacts many aspects of malignancy, including tumor cell proliferation, angiogenesis, metastasis, and tumor response to chemotherapeutic agents and hormones (58, 59).
Tumor-associated inflammation, which entails intricate interactions between epithelial and stromal cells, can in some cases lead to epigenetic alterations that drive malignant progression and even initiate tumorigenesis. Chronic inflammation results in the production of growth factors that support the development of newly emergent tumors (60).
The continuous production of various cytokines, chemokines and growth factors within the tumor microenvironment supports cancer cell proliferation, evolution, and survival as well as tumor vascularization and immune dysregulation, all of which contribute to tumor progression, invasion, metastasis and therapy resistance.
The active components of traditional Chinese herbs can modulate the expression of some cytokines, thus affecting inflammatory inflammasome and nuclear factors to exert a regulatory effect on tumor-associated inflammation.
The NLRP3 inflammasome can be activated by pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), causing caspase-1 cleavage and maturation of IL-1β production. Concurrently, NLRP3 can be activated by LPS in the presence of PAMPs, subsequently exacerbating cancer progression (61).
Studies have shown that LPS-induced pro-inflammatory cytokine expression in NSCLC can be associated with the clinical prognosis of metastatic NSCLC, where Gram-negative bacteria, the primary pathogens, adversely affect NSCLC through the production of LPS (62). Targeting LPS-induced inflammation can effectively ameliorate the proliferation and migration of NSCLC cells in vivo.
Hongjingtian (Rhodiola crenulata (Hook. f. et Thoms) H. Ohba.) is a traditional Chinese medicine with properties of Qi benefit, blood activation, and asthma calming, and salidroside (30) (SAL) is an active extract of Hongjingtian.
Coincubation with SAL for 24 h and 48 h suppressed the LPS-induced increase in A549 cell proliferation in a concentration-dependent manner, and SAL treatment inhibits LPS-induced migration.
A549 cells exposed to LPS for 4–24 h showed lower levels of AMPK phosphorylation and higher levels of NLRP3, caspase-1, and IL-1β than untreated cells, and LPS-treated cells also exhibited impaired AMPK activity and enhanced NLRP3 inflammasome activation. In contrast, cells treated with SAL following LPS-induction showed higher levels of phosphorylated AMPK.
In addition, when AMPK was inhibited by compound C, LPS and SAL cotreated cells showed increased levels of NLRP3, pro-caspase-1, caspase-1, pro-IL-1β, and IL-1β, which suggests that SAL suppresses LPS-induced activation of the ROS/NLRP3 inflammasome axis in A549 cells through its effects on AMPK. The efficacy of SAL in NSCLC has been demonstrated, particularly in cases of concomitant bacterial infection.
Ginkgolide B (GKB) is the main active ingredient of Yinxingye (Ginkgo biloba L.) extract, which activates blood circulation, resolves blood stasis, relieves pain, and calms asthma.
Wang X et al. (31) treated H1975 and A549 cells with GKB, and GKB time-dependently inhibited tumor cell proliferation. The expression of beclin-1, p62, and LC3B proteins was strongly induced in response to GKB treatment in lung tumor cells in a time-dependent manner by western blot analysis. Furthermore, the expression of PCNA and Bcl-2 decreased in a time-dependent manner in GKB-treated A549 and H1975 cells. After using the selective autophagy inhibitor 3-MA to block autophagy, GKB decreased the number of invasive cells in vitro, and the treatment interfered with the GKB-induced decreased number of cell colonies.
Treatment of H1975 cells with GKB for 24 h significantly reduced NLRP3 expression, leading to pro-IL-1β and pro-IL-18 being cleaved to create the mature bioactive form. Furthermore, after using autophagy inhibitor 3-MA, the GKB-induced inhibition of IL-1β and IL-18 expression were reversed. These results showed that GKB-induced inhibition of the NLRP3 inflammasome can be reversed by beclin-1 knockdown in H1975 cells.
The nuclear factor kappa B (NF-kB) family consists of transcription factors that act as an integral part of a network that determines its pattern of expression on other genes, regulating a variety of functions, including inflammation, immune responses, cell proliferation, and survival (63). The NF-kB pathway contributes to the progression and metastasis of several cancer types, including breast cancer (64), prostate cancer (65), and nasopharyngeal carcinoma.
Chemokines and their receptors (CXCR4 and CCR7) are critical in determining tumor cell metastasis. The expression of CCR7 and CXCR4 mRNA in LPS-induced MCF-7 and MDAMB-231 breast cancer cells was significantly reduced by treatment with puerarin, a flavonoid isolated from Gegen (Pueraria lobata (Willd.) Ohwi.), thereby significantly dampening the adhesion of cells to extracellular matrix components. Liu X et al. (32) found that puerarin inhibited LPS-stimulated migration of MCF-7 and MDA-MB-231 cells and dose-dependently reduced the mRNA expression and protein levels of CCR7 and CXCR4 in LPS-induced MCF-7 and MDA-MB-231 cells.
Co-treatment with puerarin inhibited LPS-induced cell invasion in breast cancer, and the mRNA and protein expression levels of MMP-2 and MMP-9 were reversed by puerarin treatment. In addition, compared with the LPS group, puerarin significantly reduced cell attachment to collagen I, collagen IV, and fibronectin. Furthermore, the mRNA and protein expression levels of the adhesive molecules VCAM-1 and ICAM-1 were obviously reduced in the puerarin group.
Prestimulation with LPS triggered phosphorylation of p65, and this effect was reversed by co-treatment with puerarin in MCF-7 and MDA-MB-231 cells. Puerarin also suppressed the phosphorylation of IκBα and Erk1/2 in breast cancer cells, which indicated that puerarin disrupted NF-κB signaling via suppression of p65 nuclear translocation and IκBα phosphorylation.
Xu H et al. (66) developed a novel puerarin nanoemulsion (nanoPue) that facilitated the chemotherapy effects of nano-paclitaxel in a desmoplastic TNBC model and increased intra-tumoral infiltration of cytotoxic T cells to synergize PD-L1 blockade therapy in this TNBC model.
Cisplatin chemotherapy is a standard clinical option for the treatment of NSCLC (67). Hypoxia is prevalent in multiple solid tumors and is closely related to tumor proliferation, metastasis, clinical stage, and treatment outcome as well as patient prognosis. Under hypoxic conditions, the NF-κB pathway is activated, tumor proliferation and metastasis are enhanced, human NSCLC cells are less sensitive to cisplatin, and the efficacy of cisplatin chemotherapy is compromised.
Wang J et al. (68) found that CoCl2-induced hypoxia decreased the sensitivity of NSCLC cells to cisplatin and significantly reduced the colony formation rate and total apoptosis rate of cisplatin-treated cells compared with cells in normoxia. They demonstrated that expression of the pro-apoptotic proteins (caspase-3, -8, and -9 and Bax) and apoptosis markers (PARP) were lower in hypoxia than in normoxia with cisplatin; furthermore, anti-apoptotic proteins (Bcl-2 and survivin) were significantly increased. In addition, hypoxia significantly activated the expression of HIF-1α, Snail, N-cadherin, and vimentin, while it decreased the expression of E-cadherin. The expression of SOX2, NANOG, OCT4, and CD44 were upregulated in hypoxia, which indicated that NSCLC cells undergo EMT and obtain stemness in hypoxia.
Under hypoxic conditions, there was increased expression of p-p65, p65, p-IKK, and IKK and downregulated expression of EMT-related molecules including Snail, N-cadherin, and vimentin, while E-cadherin protein expression was upregulated. In addition, the stemness-related molecules SOX2, NANOG, OCT4, and CD44 were downregulated. The results suggested that inhibition of the NF-κB pathway could reverse hypoxia-mediated EMT and stemness.
Compound 20 (R)-ginsenoside (Rg3) is an active monomer extracted from the TCM herb Renshen (Panax ginseng C. A. Mey.), which is considered to be a tonic for Qi and nourishes Yin in traditional Chinese medicine and has been shown to inhibit cancer cells. Some studies indicate that Rg3 can suppress EMT via suppressing Notch-Hes1-EMT signaling (33).
Rg3 treatment of hypoxic NSCLC cells decreased the expression of p-p65 and p65 in a concentration- and time-dependent manner, and NF-κB DNA binding in the Rg3 + cisplatin group was less than that of the two drugs used individually in hypoxic cells. The expression levels of p-p65 and p65 in nuclear protein extracts were the lowest in the Rg3 + cisplatin-treated group in hypoxia, and the expression levels of p-p65, p65, p-IKK, and IKK in total protein extracts were lowest in the cells treated with Rg3 + cisplatin. Thus, Rg3 inhibited the activation of the NF-κB signaling pathway under hypoxic conditions, and Rg3 + cisplatin increased this inhibitory effect.
They found that hypoxic NSCLC cells treated with Rg3 + cisplatin had significantly decreased cell viability compared with treatment with Rg3 or cisplatin individually, and the Rg3 + cisplatin-treated cells had a significantly lower colony formation rate than cells treated with cisplatin or Rg3 alone in hypoxia. Furthermore, the flow cytometry results showed that Rg3 + cisplatin treatment led to an increase in the total apoptosis rate compared with treatment with cisplatin or Rg3 alone in hypoxic cells. The Rg3 + cisplatin group showed higher expression of pro-apoptotic proteins (caspase-3, -8, -9 and Bax) and apoptosis markers (PARP) than the individually treated groups, and the expression of anti-apoptotic proteins (Bcl-2 and survivin) showed the opposite trend.
Cao M (69) isolated and characterized from Panax ginseng C. A. Mey., and developed a novel EVs-liked ginseng-derived nanoparticles (GDNPs) with superb stability and biocompatibility. After treatment with GDNPs, the expression of CD80, CD86, MHC-II, TLR2/4, IL-6 and TNF-α was up-regulated, which revealed that GDNP exposure significantly induced M1-related markers. Moreover, GDNPs treatment increased the production of M1-related cytokines and chemokines, such as CCL5, IL-6, MCP-1, TNF-α IL-1α and IL-12, altering the M2-like polarization of macrophages in vitro. Treatment with GDNP-stimulated macrophages significantly increased apoptosis of B16F10 melanoma cells and caspase- 3/7 expression compared to treatment with a control medium.
In recent years, nanotechnology has been applied in various fields of biomedicine, with the emergence of new drug delivery systems based on nano-formulations that are expected to overcome the current limitations of tumor therapy, offering the advantages of increased efficacy, improved absorption, and reduced adverse effects (70). This paper focuses on the antitumor effects of the active ingredients of TCM in combination with liposomes, carbon nanomaterials, and metal nanoparticles.
Zhu J et al. (71) developed a novel nano-lipid carrier formulation, NCNLC, using coix seed oil (CSO) extracted from the TCM Yiyiren (Coix lacryma-jobi L.var.ma-yuen (Roman.) Stapf) as a functional liquid lipid, loaded with naringin (NG), a model anti-HCC drug, by ultrasonic melt emulsification. Drug release was promoted when CSO acted as a liquid lipid. However, when comparing nanostructured lipid carrier (NLC) prepared from free NG, neodecanoic triglyceride (NDNLC), and oleic acid (NONLC), NCNLC was more effective in antitumor proliferation and promoting apoptosis among tumor cells. In particular, NG + CSO upregulated the expression of IL-6 and IL-10 in the serum of tumor-bearing mice and significantly increased the spleen index, suggesting that the combination of the two via nanotechnology could enhance the antitumor immune effect.
Doxorubicin (DOX) is one of the most widespread antitumor drugs used in prostate cancer (pCa), but its side effects are unavoidable. Tanshinone is an active extract of the Chinese herb Danshen (Salvia miltiorrhiza Bge.), which has now been shown to possess wide-ranging anticancer effects.
Prostate-specific membrane antigen (PSMA) is a cell surface protein that is a recognized tumor marker, including for pCa, and is an established target for targeted drug delivery. Sun G et al. (72) combined pH-sensitive nanoparticles and prepared lipid nanoparticles loaded with DOX and TAN by emulsification and solvent diffusion to form a P-N-DOX/TAN nanodelivery system. This system can be adapted to the acidic environment of tumor tissue (pH 6.5) and can be released more entirely at the tumor area. Due to the modification of the PSMA-targeting ligands, the LNCaP tumor cells showed a high degree of absorption of P-N-DOX/TAN, which facilitated the accumulation of the drug in vivo. The drug-loaded nanoparticles were more stable than those in the drug-free group and had a superior effect on apoptosis, showing a potent inhibitory effect on cancer cells.
Pishuang is a natural product of TCM, and As2O3 (ATO), the main compound of arsenic, can be used to remedy a variety of cancers such as breast cancer (73) and pCa (74). Several limitations exist in its clinical application, such as poor pharmacokinetics and unacceptable systemic toxicity. There are also severe limitations to the use of ATO in the treatment of gliomas, as its poor ability to penetrate the blood-brain barrier makes it less effective.
RGDArg-Gly-Asp (RGDyC) acts as a ligand that preferentially binds to receptors on the surface of leukocytes, bringing NPs into the brain for targeted delivery to tumor tissue. Simultaneously, polyamidoamines (PAMAMs) may be useful carriers that can add to the biodistribution of the drug and potentially augment the targeting effect. Lu Y et al. (75) proposed a targeted drug delivery system based on RGDyC and PEG co-modified PAMAM (RGDyC-mpeg-PAMAM/ATO). RGDyC-mPEG-PAMAM/ATO treatment significantly inhibited the growth of glioma in Wistar rats by repressing cell proliferation and inducing apoptosis while reducing the toxicity of ATO, prolonging the survival time of rats and enhancing the antitumor effects of ATO.
With their excellent biocompatibility and autologous liver targeting, iron-based NPs show significant advantages as multifunctional carriers for chemotherapeutic drug delivery. The ginsenoside Rg3 is a potent extract of the TCM ginseng, which has been demonstrated to induce cellular autophagy and sensitize liver cancer cells to adriamycin (76).
Ren Z et al. (77) developed a novel nanomedicine (NpRg3) by synthesizing and coupling Fe@Fe3O4 NPs with the ginsenoside Rg3. NpRg3 treatment significantly prolonged the survival time of tumor-bearing mice, improved biochemical parameters such as ALT and AST, as well as reduced tumor proliferation and aggressiveness.
Rg3 acts as a cancer suppressor by regulating VEGF-dependent tumor angiogenesis, while the long-term circulation of iron-based NpRg3 could have a therapeutic effect on circulating tumor cells by activating immune cells in the blood. In comparison to the other groups, no lung metastases from HCC were observed in the NpRg3-treated group, and lung metastases from HCC were significantly inhibited, demonstrating its superior antitumor metastatic effect.
Chinese herbal medicine has long been used traditionally to treat patients with cancer. In particular, combined TCM and Western medicine are usually used for cancer patients who have undergone surgical resection in conjunction with radiotherapy and chemotherapy as a maintenance or alternative treatment. Nonetheless, as cases of adverse drug reactions (ADRs) increase with the popularity of Chinese herbal medicine, attention should also be drawn to the adverse effects caused by herbal medicine.
Madouling (Aristolochia debilis Sieb. et Zucc) is an herb that is believed in TCM theory to be effective in clearing heat and relieving coughs and asthma. Aristolochic acids (AASs) are potent carcinogens and are extremely nephrotoxic (78). Excessive intake of AASs is a major cause of aristolochic acid nephropathy (AAN).
The herb Wutou (Aconitum carmichaeli Debeaux) is used to treat impotence, abdominal pain, edema and joint pain, and it is also known as a poison because of the presence of aconitine, a highly toxic alkaloid. Fresh Wutou must be fried, steamed, or fumigated to reduce its toxicity for oral administration. Aconitine’s toxic effects inhibit the myocardial tricarboxylic acid cycle and oxidative respiratory chain phosphorylation, resulting in impaired myocardial aerobic metabolism and cardiac dysfunction, causing severe cardiotoxicity (79).
Generally speaking, except for drugs that have been demonstrated to be poisonous, the side effects of oral Chinese medicine are not obvious; the main side effects exist in Chinese medicine injections.
ADRs primarily involve cutaneous and appendage injuries, general body disturbances, and autonomic nervous system disturbances, including rash, pruritus, anaphylactic reactions, and palpitations. Hypersensitivity shock and anaphylactic-like reactions are among the rarest serious ADRs associated with herbal injections, posing an increased threat to patient safety and even death (80).
The active ingredients in TCM are very complex and contain a variety of different types of compounds. In the theory of TCM, polysaccharides and saponins, such as G. lucidum polysaccharides and ginsenosides, are usually found in medicines that can help make Qi and blood flow better.
Modern research has also shown that ginsenosides can help the immune system switch from a Th1 to a Th2 phenotype, release interferons, facilitate dendritic cell growth, and promote immunomodulatory effects in different types of cancer (81) (82).
Flavonoids can be found in the human diet in fruits, vegetables, legumes, tea, dark chocolate, etc. Some specific parts of these foods are richer in flavonoids than others, such as the peels of certain fruits (83). Flavonoids are generally considered to be associated with intestinal metabolism (84), and the herbs that are considered to promote digestion in TCM theory also basically contain flavonoids, such as Shanzha (Crataegus pinnatifida Bge. var. major N. E. Br., Crataegus pinnatifida Bge.). Flavonoids are generally considered to have anti-inflammatory properties; for example, one flavonoid, quercetin, is able to achieve anti-inflammatory effects by inhibiting the NF-κB pathway (85).
Alkaloids are the main active ingredients of another group of herbs, generally contained in medicines that are considered to clear Heat in TCM theory. For instance, berberine has been reported to possess anticancer activities. Among the various cellular targets of berberine is AMPK, which regulates tumor progression and metastasis. Berberine activated AMPK in human colon cancer cell lines. Notably, berberine-induced activation of AMPK, reduced integrin β1 protein levels, and decreased the phosphorylation of integrin β1 signaling targets (81).
In addition to the above, there are many unexplored and under-explained active ingredients in Chinese herbal medicine that remain to be discovered through further experiments. (The effects of some of the actively ingredients commonly used in herbal medicine are listed in Table 4).
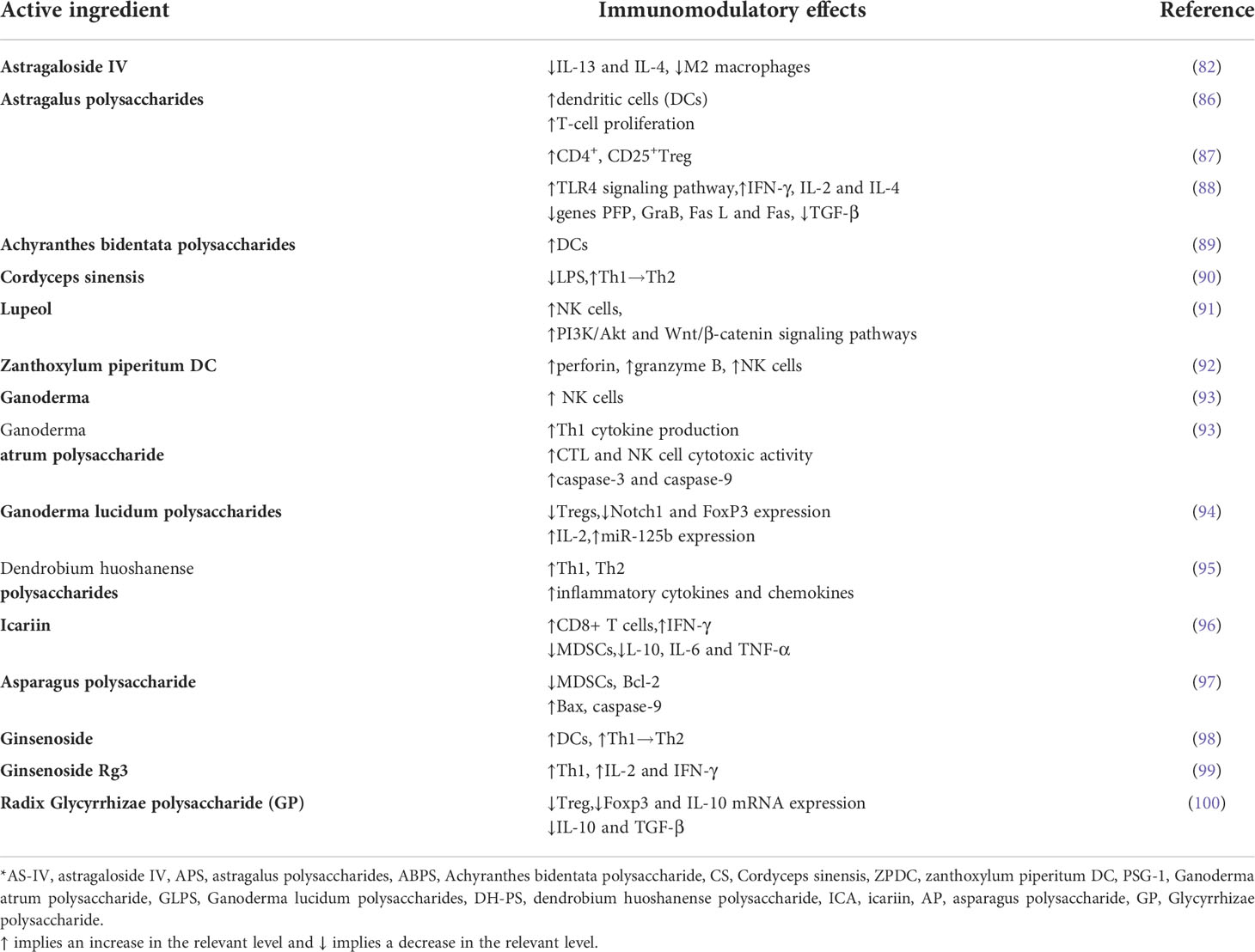
Table 4 A list of active ingredients of Chinese herbal medicines proven to have anti-cancer immune effects.
The effect of Chinese medicine on the immune system can be sophisticated. Chinese herbal medicines are rich in a variety of chemical components, including polysaccharides, adenosine, and alkaloids. Different herbs used individually or different combinations of different herbs and various active ingredients in multiple preparations can also yield distinct immunomodulatory outcomes.
TCM, particularly herbal medicine, enjoys a distinguished history of clinical application in China and has enormous potential for development. Thousands of years of experience in clinical practice have provided the diagnostic basis for the medical treatment of diseases, but the chemical composition of some traditional antitumor Chinese medicines in clinical use has not yet been entirely identified. Therefore, further in-depth research is warranted. Despite the fact that research on the mechanisms of antitumor action of Chinese medicine is still in an early phase, it is still a worthwhile consideration to use Chinese medicine as a promising direction for antitumor immunotherapy.
There are limitations in the application of TCM in antitumor therapy; however, further research is required to identify the valid components of TCM with antitumor effects and elaborate their mechanisms of action. Moreover, some Chinese medicines have a high degree of toxicity, and their safety in clinical applications has been called into question, thus limiting the promotion of clinical applications of these Chinese medicines in the international community.
Although a large proportion of Chinese medicines and compound prescriptions still have unclear antitumor mechanisms or toxic reactions (101), most of the Chinese medicines currently used clinically are those with proven safety. Nevertheless, if further developments are to be pursued, the definitive toxicity of herbal medicines has yet to be thoroughly researched (102).
More fundamental studies are necessary to confirm the pharmacological mechanisms of action of Chinese medicines and compounded formulations to achieve wider clinical application and a definite improvement in the quality of life of patients.
Chinese herbal components have the disadvantages of poor absorption, high toxicity, and lack of targeting, which may be complemented by nanomaterials in various aspects to develop safer, more effective, and stable clinical applications. The combination of Chinese medicine and materials science, especially for the integration of the active components of Chinese medicine and nanoparticles, will perhaps serve as a novel breakthrough.
KS searched for the literature and wrote the manuscript; LW and SW searched for the literature, WD extensively edited the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Health System Innovation Project of Shanghai Putuo Science and Technology Commission (No.ptkwws202002), Traditional Chinese Medicine Clinical Key Specialty Construction Project of Shanghai Putuo District (No.ptzyzk2101), Clinical Specialized Discipline of Health System of Putuo District in Shanghai (No. 2021tszk01) and Construction of Colorectal Cancer Special Disease Alliance with Integrated Traditional Chinese and Western Medicine (No. ZY(2021-2023)-0302).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wu C, Li M, Meng H, Liu Y, Niu W, Zhou Y, et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci (2019) 62(5):640–7. doi: 10.1007/s11427-018-9461-5
2. Liu J, Mao JJ, Wang XS, Lin H. Evaluation of traditional Chinese medicine herbs in oncology clinical trials. Cancer J (2019) 25(5):367–71. doi: 10.1097/PPO.0000000000000404
3. Huang S, Peng W, Mao D, Zhang S, Xu P, Yi P, et al. Kangai injection, a traditional Chinese medicine, improves efficacy and reduces toxicity of chemotherapy in advanced colorectal cancer patients: A systematic review and meta-analysis. Evid Based Complement Alternat Med (2019):8423037. doi: 10.1155/2019/8423037
4. Su XL, Wang JW, Che H, Wang CF, Jiang H, Lei X, et al. Clinical application and mechanism of traditional Chinese medicine in treatment of lung cancer. Chin Med J (Engl) (2020) 133(24):2987–97. doi: 10.1097/CM9.0000000000001141
5. Lu Y, Ding Y, Wei J, He S, Liu X, Pan H, et al. Anticancer effects of traditional Chinese medicine on epithelial-mesenchymal transition (EMT) in breast cancer: Cellular and molecular targets. Eur J Pharmacol (2021) 907:174275. doi: 10.1016/j.ejphar.2021.174275
6. Sun Q, He M, Zhang M, Zeng S, Chen L, Zhao H, et al. Traditional Chinese medicine and colorectal cancer: Implications for drug discovery. Front Pharmacol (2021) 12:685002. doi: 10.3389/fphar.2021.685002
7. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity (2014) 41(1):49–61. doi: 10.1016/j.immuni.2014.06.010
8. Sica A, Allavena P, Mantovani A. Cancer related inflammation: The macrophage connection. Cancer Lett (2008) 267(2):204–15. doi: 10.1016/j.canlet.2008.03.028
9. Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J Pathol (2002) 196(3):254–65. doi: 10.1002/path.1027
10. Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol (2020) 353:104119. doi: 10.1016/j.cellimm.2020.104119
11. Yuan R, Li S, Geng H, Wang X, Guan Q, Li X, et al. Reversing the polarization of tumor-associated macrophages inhibits tumor metastasis. Int Immunopharmacol (2017) 49:30–7. doi: 10.1016/j.intimp.2017.05.014
12. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol (2017) 14(7):399–416. doi: 10.1038/nrclinonc.2016.217
13. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol (2014) 15(3):178–96. doi: 10.1038/nrm3758
14. Liu Q, Hodge J, Wang J, Wang Y, Wang L, Singh U, et al. Emodin reduces breast cancer lung metastasis by suppressing macrophage-induced breast cancer cell epithelial-mesenchymal transition and cancer stem cell formation. Theranostics (2020) 10(18):8365–81. doi: 10.7150/thno.45395
15. Zhang C, Li Z, Wang J, Jiang X, Xia M, Wang J, et al. Ethanol extracts of solanum lyratum thunb regulate ovarian cancer cell proliferation, apoptosis, and epithelial-to-Mesenchymal transition (EMT) via the ROS-mediated p53 pathway. J Immunol Res (2021) 2021:5569354. doi: 10.1155/2021/5569354
16. Yin L, Fan Z, Liu P, Chen L, Guan Z, Liu Y, et al. Anemoside A3 activates TLR4-dependent M1-phenotype macrophage polarization to represses breast tumor growth and angiogenesis. Toxicol Appl Pharmacol (2021) 432:115755. doi: 10.1016/j.taap.2021.115755
17. Chen R, Lu X, Li Z, Sun Y, He Z, Li X. Dihydroartemisinin prevents progression and metastasis of head and neck squamous cell carcinoma by inhibiting polarization of macrophages in tumor microenvironment. Onco Targets Ther (2020) 13:3375–87. doi: 10.2147/OTT.S249046
18. Wu CY, Cherng JY, Yang YH, Lin CL, Kuan FC, Lin YY, et al. Danshen improves survival of patients with advanced lung cancer and targeting the relationship between macrophages and lung cancer cells. Oncotarget (2017) 8(53):90925–47. doi: 10.18632/oncotarget.18767
19. Wei H, Guo C, Zhu R, Zhang C, Han N, Liu R, et al. Shuangshen granules attenuate lung metastasis by modulating bone marrow differentiation through mTOR signalling inhibition. J Ethnopharmacol (2021) 281:113305. doi: 10.1016/j.jep.2020.113305
20. Zhao L, Zhu X, Ni Y, You J, Li A. Xiaoyaosan, a traditional Chinese medicine, inhibits the chronic restraint stress-induced liver metastasis of colon cancer in vivo. Pharm Biol (2020) 58(1):1085–91. doi: 10.1080/13880209.2020.1839513
21. Huyan T, Li Q, Yang H, Jin ML, Zhang MJ, Ye LJ, et al. Protective effect of polysaccharides on simulated microgravity-induced functional inhibition of human NK cells. Carbohydr Polym (2014) 101:819–27. doi: 10.1016/j.carbpol.2013.10.021
22. Luo Y, Wu J, Zhu X, Gong C, Yao C, Ni Z, et al. NK cell-dependent growth inhibition of Lewis lung cancer by yu-Ping-Feng, an ancient Chinese herbal formula. Mediators Inflammation (2016) 2016:3541283. doi: 10.1155/2016/3541283
23. Zhao X, Liu J, Ge S, Chen C, Li S, Wu X, et al. Saikosaponin a inhibits breast cancer by regulating Th1/Th2 balance. Front Pharmacol (2019) 10:624. doi: 10.3389/fphar.2019.00624
24. Zheng X, Guo Y, Wang L, Zhang H, Wang S, Wang L, et al. Recovery profiles of T-cell subsets following low-dose total body irradiation and improvement with cinnamon. Int J Radiat Oncol Biol Phys (2015) 93(5):1118–26. doi: 10.1016/j.ijrobp.2015.08.034
25. Huang H, Zhang M, Yao S, Zhang M, Peng J, with the Pinellia pedatisecta (PE) advisory group. Immune modulation of a lipid-soluble extract of pinellia pedatisecta schott in the tumor microenvironment of an HPV+ tumor-burdened mouse model. J Ethnopharmacol (2018) 225:103–15. doi: 10.1016/j.jep.2018.04.037
26. Yang X, Sun J, Wen B, Wang Y, Zhang M, Chen W, et al. Biejiajian pill promotes the infiltration of CD8+ T cells in hepatocellular carcinoma by regulating the expression of CCL5. Front Pharmacol (2021) 12:771046. doi: 10.3389/fphar.2021.771046
27. Liu Z, Wang S, Zhang J, Wang Y, Wang Y, Zhang L, et al. Gastrodin, a traditional Chinese medicine monomer compound, can be used as adjuvant to enhance the immunogenicity of melanoma vaccines. Int Immunopharmacol (2019) 74:105699. doi: 10.1016/j.intimp.2019.105699
28. Guo J, Chen T, Ma Z, Qiao C, Yuan F, Guo X, et al. Oridonin inhibits 4T1 tumor growth by suppressing treg differentiation via TGF-β receptor. Int Immunopharmacol (2020) 88:106831. doi: 10.1016/j.intimp.2020.106831
29. Sui H, Zhang L, Gu K, Chai N, Ji Q, Zhou L, et al. YYFZBJS ameliorates colorectal cancer progression in ApcMin/+ mice by remodeling gut microbiota and inhibiting regulatory T-cell generation. Cell Commun Signal (2020) 18(1):113. doi: 10.1186/s12964-020-00596-9
30. Ma W, Wang Z, Zhao Y, Wang Q, Zhang Y, Lei P, et al. Salidroside suppresses the proliferation and migration of human lung cancer cells through AMPK-dependent NLRP3 inflammasome regulation. Oxid Med Cell Longev (2021) 2021:6614574. doi: 10.1155/2021/6614574
31. Wang X, Shao QH, Zhou H, Wu JL, Quan WQ, Ji P, et al. Ginkgolide b inhibits lung cancer cells promotion via beclin-1-dependent autophagy. BMC Complement Med Ther (2020) 20(1):194. doi: 10.1186/s12906-020-02980-x
32. Liu X, Zhao W, Wang W, Lin S, Yang L. Puerarin suppresses LPS-induced breast cancer cell migration, invasion and adhesion by blockage NF-κB and erk pathway. BioMed Pharmacother (2017) 92:429–36. doi: 10.1016/j.biopha.2017.05.102
33. Li X, Liu W, Geng C, Li T, Li Y, Guo Y, et al. Ginsenoside Rg3 suppresses epithelial-mesenchymal transition via downregulating notch-Hes1 signaling in colon cancer cells. Am J Chin Med (2021) 49(1):217–35. doi: 10.1142/S0192415X21500129
34. Pourhajibagher M, Etemad-Moghadam S, Alaeddini M, Bahador A. Modulation of the triggered apoptosis by nano emodin transfersome-mediated sonodynamic therapy on head and neck squamous cell carcinoma cell lines. Photodiagnosis Photodyn Ther (2021) 34:102253. doi: 10.1016/j.pdpdt.2021.102253
35. Li KT, Duan QQ, Chen Q, He JW, Tian S, Lin HD, et al. The effect of aloe emodin-encapsulated nanoliposome-mediated r-caspase-3 gene transfection and photodynamic therapy on human gastric cancer cells. Cancer Med (2016) 5(2):361–9. doi: 10.1002/cam4.584
36. Han S, Bi S, Guo T, Sun D, Zou Y, Wang L, et al. Nano co-delivery of plumbagin and dihydrotanshinone I reverses immunosuppressive TME of liver cancer. J Control Release (2022) 348:250–63. doi: 10.1016/j.jconrel.2022.05.057
37. Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity (2010) 32(6):790–802. doi: 10.1016/j.immuni.2010.05.010
38. Liu H, Ling CC, Yeung WHO, Pang L, Liu J, Zhou J, et al. Monocytic MDSC mobilization promotes tumor recurrence after liver transplantation via CXCL10/TLR4/MMP14 signaling. Cell Death Dis (2021) 12(5):489. doi: 10.1038/s41419-021-03788-4
39. Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, et al. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci U.S.A. (2009) 106(16):6742–7. doi: 10.1073/pnas.0902280106
40. Cui TX, Kryczek I, Zhao L, Zhao E, Kuick R, Roh MH, et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity (2013) 39(3):611–21. doi: 10.1016/j.immuni.2013.08.025
41. Le CP, Nowell CJ, Kim-Fuchs C, Botteri E, Hiller JG, Ismail H, et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun (2016) 7:10634. doi: 10.1038/ncomms10634
42. Qi FH, Wang ZX, Cai PP, Zhao L, Gao JJ, Kokudo N, et al. Traditional Chinese medicine and related active compounds: a review of their role on hepatitis b virus infection. Drug Discovery Ther (2013) 7(6):212–24. doi: 10.5582/ddt.2013.v7.6.212
43. López-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of metastasis by NK cells. Cancer Cell (2017) 32(2):135–54. doi: 10.1016/j.ccell.2017.06.009
44. Jewett A, Kos J, Fong Y, Ko MW, Safaei T, Perišić Nanut M, et al. NK cells shape pancreatic and oral tumor microenvironments; role in inhibition of tumor growth and metastasis. Semin Cancer Biol (2018) 53:178–88. doi: 10.1016/j.semcancer.2018.08.001
45. Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol (2016) 17(9):1025–36. doi: 10.1038/ni.3518
46. Li B, Jiang Y, Li G, Fisher GA Jr, Li R. Natural killer cell and stroma abundance are independently prognostic and predict gastric cancer chemotherapy benefit. JCI Insight (2020) 5(9):e136570. doi: 10.1172/jci.insight.136570
47. Gil M, Kim KE. Interleukin-18 is a prognostic biomarker correlated with CD8+ T cell and natural killer cell infiltration in skin cutaneous melanoma. J Clin Med (2019) 8(11):1993. doi: 10.3390/jcm8111993
48. Liu X, Wu Z, Guo C, Guo H, Su Y, Chen Q, et al. Hypoxia responsive nano-drug delivery system based on angelica polysaccharide for liver cancer therapy. Drug Delivery (2022) 1):138–48. doi: 10.1080/10717544.2021.2021324
49. Ostroumov D, Fekete-Drimusz N, Saborowski M, Kühnel F, Woller N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci (2018) 75(4):689–713. doi: 10.1007/s00018-017-2686-7
50. Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res (2011) 71(4):1263–71. doi: 10.1158/0008-5472.CAN-10-2907
51. Nolz JC. Molecular mechanisms of CD8(+) T cell trafficking and localization. Cell Mol Life Sci (2015) 72(13):2461–73. doi: 10.1007/s00018-015-1835-0
52. Zhang M, Xiao B, Wang H, Han MK, Zhang Z, Viennois E, et al. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol Ther (2016) 24(10):1783–96. doi: 10.1038/mt.2016.159
53. Haggag YA, Ibrahim RR, Hafiz AA. Design, formulation and in vivo evaluation of novel honokiol-loaded PEGylated PLGA nanocapsules for treatment of breast cancer. Int J Nanomed (2020) 15:1625–42. doi: 10.2147/IJN.S241428
54. Wang J, Xie L, Shi Y, Ao L, Cai F, Yan F. Early detection and reversal of cell apoptosis induced by focused ultrasound-mediated blood-brain barrier opening. ACS Nano (2021) 15(9):14509–21. doi: 10.1021/acsnano.1c04029
55. Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res (2017) 27(1):109–18. doi: 10.1038/cr.2016.151
56. Ohue Y, Nishikawa H, Regulatory T. (Treg) cells in cancer: Can treg cells be a new therapeutic target? Cancer Sci (2019) 110(7):2080–9. doi: 10.1111/cas.14069
57. Greten FR, Grivennikov SI. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity (2019) 51(1):27–41. doi: 10.1016/j.immuni.2019.06.025
58. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205
59. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol (2014) 15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3
60. Hou J, Karin M, Sun B. Targeting cancer-promoting inflammation - have anti-inflammatory therapies come of age? Nat Rev Clin Oncol (2021) 18(5):261–79. doi: 10.1038/s41571-020-00459-9
61. Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol (2019) 19(8):477–89. doi: 10.1038/s41577-019-0165-0
62. Sun M, Bai Y, Zhao S, Liu X, Gao Y, Wang L, et al. Gram-negative bacteria facilitate tumor progression through TLR4/IL-33 pathway in patients with non-small-cell lung cancer. Oncotarget (2018) 9(17):13462–73. doi: 10.18632/oncotarget.24008
63. Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer (2013) 12:86. doi: 10.1186/1476-4598-12-86
64. Torrealba N, Vera R, Fraile B, Martínez-Onsurbe P, Paniagua R, Royuela M. TGF-β/PI3K/AKT/mTOR/NF-kB pathway. clinicopathological features in prostate cancer. Aging Male (2020) 23(5):801–11. doi: 10.1080/13685538.2019.1597840
65. Deng M, Dai W, Yu VZ, Tao L, Lung ML. Cylindromatosis lysine 63 deubiquitinase (CYLD) regulates NF-kB signaling pathway and modulates fibroblast and endothelial cells recruitment in nasopharyngeal carcinoma. Cancers (Basel) (2020) 12(7):1924. doi: 10.3390/cancers12071924
66. Xu H, Hu M, Liu M, An S, Guan K, Wang M, et al. Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model. Biomaterials (2020) 235:119769. doi: 10.1016/j.biomaterials.2020.119769
67. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non-small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther (2016) 16(6):653–60. doi: 10.1586/14737140.2016.1170596
68. Wang J, Tian L, Khan MN, Zhang L, Chen Q, Zhao Y, et al. Ginsenoside Rg3 sensitizes hypoxic lung cancer cells to cisplatin via blocking of NF-κB mediated epithelial-mesenchymal transition and stemness. Cancer Lett (2018) 415:73–85. doi: 10.1016/j.canlet.2017.11.037
69. Cao M, Yan H, Han X, Weng L, Wei Q, Sun X, et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunother Cancer (2019) 7(1):326. doi: 10.1186/s40425-019-0817-4
70. Ma Z, Fan Y, Wu Y, Kebebe D, Zhang B, Lu P, et al. Traditional Chinese medicine-combination therapies utilizing nanotechnology-based targeted delivery systems: a new strategy for antitumor treatment. Int J Nanomed (2019) 14:2029–53. doi: 10.2147/IJN.S197889
71. Zhu J, Huang Y, Zhang J, Feng Y, Shen L. Formulation, preparation and evaluation of nanostructured lipid carrier containing naringin and coix seed oil for anti-tumor application based on “Unification of medicines and excipients”. Drug Des Devel Ther (2020) 14:1481–91. doi: 10.2147/DDDT.S236997
72. Sun G, Sun K, Sun J. Combination prostate cancer therapy: Prostate-specific membranes antigen targeted, pH-sensitive nanoparticles loaded with doxorubicin and tanshinone. Drug Delivery (2021) 28(1):1132–40. doi: 10.1080/10717544.2021.1931559
73. Zhang L, Zhou Y, Kong J, Zhang L, Yuan M, Xian S, et al. Effect of arsenic trioxide on cervical cancer and its mechanisms. Exp Ther Med (2020) 20(6):169. doi: 10.3892/etm.2020.9299
74. Mirzaei A, Akbari MR, Tamehri Zadeh SS, Khatami F, Mashhadi R, Aghamir SMK. Novel combination therapy of prostate cancer cells with arsenic trioxide and flutamide: An in-vitro study. Tissue Cell (2022) 74:101684. doi: 10.1016/j.tice.2021.101684
75. Lu Y, Han S, Zheng H, Ma R, Ping Y, Zou J, et al. A novel RGDyC/PEG co-modified PAMAM dendrimer-loaded arsenic trioxide of glioma targeting delivery system. Int J Nanomed (2018) 13:5937–52. doi: 10.2147/IJN.S175418
76. Kim DG, Jung KH, Lee DG, Yoon JH, Choi KS, Kwon SW, et al. 20(S)-ginsenoside Rg3 is a novel inhibitor of autophagy and sensitizes hepatocellular carcinoma to doxorubicin. Oncotarget (2014) 5(12):4438–51. doi: 10.18632/oncotarget.2034
77. Ren Z, Chen X, Hong L, Zhao X, Cui G, Li A, et al. Nanoparticle conjugation of ginsenoside Rg3 inhibits hepatocellular carcinoma development and metastasis. Small (2020) 16(2):e1905233. doi: 10.1002/smll.201905233
78. Debelle FD, Nortier JL, De Prez EG, Garbar CH, Vienne AR, Salmon IJ, et al. Aristolochic acids induce chronic renal failure with interstitial fibrosis in salt-depleted rats. J Am Soc Nephrol (2002) 13(2):431–6. doi: 10.1681/ASN.V132431
79. Zhou W, Liu H, Qiu LZ, Yue LX, Zhang GJ, Deng HF, et al. Cardiac efficacy and toxicity of aconitine: A new frontier for the ancient poison. Med Res Rev (2021) 41(3):1798–811. doi: 10.1002/med.21777
80. Li H, Deng J, Deng L, Ren X, Xia J. Safety profile of traditional Chinese herbal injection: An analysis of a spontaneous reporting system in China. Pharmacoepidemiol Drug Saf (2019) 28(7):1002–13. doi: 10.1002/pds.4805
81. Park JJ, Seo SM, Kim EJ, Lee YJ, Ko YG, Ha J, et al. Berberine inhibits human colon cancer cell migration via AMP-activated protein kinase-mediated downregulation of integrin β1 signaling. Biochem Biophys Res Commun (2012) 426(4):461–7. doi: 10.1016/j.bbrc.2012.08.091
82. Xu F, Cui WQ, Wei Y, Cui J, Qiu J, Hu LL, et al. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J Exp Clin Cancer Res (2018) 37(1):207. doi: 10.1186/s13046-018-0878-0
83. Li X, Wang T, Zhou B, Gao W, Cao J, Huang L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem (2014) 152:531–8. doi: 10.1016/j.foodchem.2013.12.010
84. Thilakarathna SH, Rupasinghe HP. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients (2013) 5(9):3367–87. doi: 10.3390/nu5093367
85. Rogerio AP, Dora CL, Andrade EL, Chaves JS, Silva LF, Lemos-Senna E, et al. Anti-inflammatory effect of quercetin-loaded microemulsion in the airways allergic inflammatory model in mice. Pharmacol Res (2010) 61(4):288–97. doi: 10.1016/j.phrs.2009.10.005
86. Liu QY, Yao YM, Zhang SW, Sheng ZY. Astragalus polysaccharides regulate T cell-mediated immunity via CD11c(high)CD45RB(low) DCs in vitro. J Ethnopharmacol (2011) 136(3):457–64. doi: 10.1016/j.jep.2010.06.041
87. Li Q, Bao JM, Li XL, Zhang T, Shen XH. Inhibiting effect of astragalus polysaccharides on the functions of CD4+CD25 highTreg cells in the tumor microenvironment of human hepatocellular carcinoma. Chin Med J (Engl) (2012) 125(5):786–93. doi: 10.3760/cma.j.issn.0366-6999.2012.05.012
88. Du X, Chen X, Zhao B, Lv Y, Zhang H, Liu H, et al. Astragalus polysaccharides enhance the humoral and cellular immune responses of hepatitis b surface antigen vaccination through inhibiting the expression of transforming growth factor β and the frequency of regulatory T cells. FEMS Immunol Med Microbiol (2011) 63(2):228–35. doi: 10.1111/j.1574-695X.2011.00845.x
89. Zhou ZD, Xia DJ. [Effect of achyranthes bidentata polysaccharides stimulated dendritic cells co-cultured with cytokine induced killer cells against SW480 cells]. Zhongguo Zhong Yao Za Zhi (2013) 38(7):1056–60. doi: 10.4268/cjcmm20130726
90. Li CY, Chiang CS, Tsai ML, Hseu RS, Shu WY, Chuang CY, et al. Two-sided effect of cordyceps sinensis on dendritic cells in different physiological stages. J Leukoc Biol (2009) 85(6):987–95. doi: 10.1189/jlb.0908573
91. Wu XT, Liu JQ, Lu XT, Chen FX, Zhou ZH, Wang T, et al. The enhanced effect of lupeol on the destruction of gastric cancer cells by NK cells. Int Immunopharmacol (2013) 16(2):332–40. doi: 10.1016/j.intimp.2013.04.017
92. Lee J, Lee SJ, Lim KT. ZPDC glycoprotein (24 kDa) induces apoptosis and enhances activity of NK cells in n-nitrosodiethylamine-injected balb/c. Cell Immunol (2014) 289(1-2):1–6. doi: 10.1016/j.cellimm.2014.03.002
93. Yu Q, Nie SP, Wang JQ, Huang DF, Li WJ, Xie MY. Toll-like receptor 4 mediates the antitumor host response induced by ganoderma atrum polysaccharide. J Agric Food Chem (2015) 63(2):517–25. doi: 10.1021/jf5041096
94. Li A, Shuai X, Jia Z, Li H, Liang X, Su D, et al. Ganoderma lucidum polysaccharide extract inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation and function by inducing microRNA-125b. J Transl Med (2015) 13:100. doi: 10.1186/s12967-015-0465-5
95. Lin J, Chang YJ, Yang WB, Yu AL, Wong CH. The multifaceted effects of polysaccharides isolated from dendrobium huoshanense on immune functions with the induction of interleukin-1 receptor antagonist (IL-1ra) in monocytes. PloS One (2014) 9(4):e94040. doi: 10.1371/journal.pone.0094040
96. Zhou J, Wu J, Chen X, Fortenbery N, Eksioglu E, Kodumudi KN, et al. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int Immunopharmacol (2011) 11(7):890–8. doi: 10.1016/j.intimp.2011.01.007
97. Zhang W, He W, Shi X, Li X, Wang Y, Hu M, et al. An asparagus polysaccharide fraction inhibits MDSCs by inducing apoptosis through toll-like receptor 4. Phytother Res (2018) 32(7):1297–303. doi: 10.1002/ptr.6058
98. Takei M, Tachikawa E, Hasegawa H, Lee JJ. Dendritic cells maturation promoted by M1 and M4, end products of steroidal ginseng saponins metabolized in digestive tracts, drive a potent Th1 polarization. Biochem Pharmacol (2004) 68(3):441–52. doi: 10.1016/j.bcp.2004.04.015
99. Wu R, Ru Q, Chen L, Ma B, Li C. Stereospecificity of ginsenoside Rg3 in the promotion of cellular immunity in hepatoma H22-bearing mice. J Food Sci (2014) 79(7):H1430–5. doi: 10.1111/1750-3841.12518
100. He X, Li X, Liu B, Xu L, Zhao H, Lu A. Down-regulation of treg cells and up-regulation of TH1/TH2 cytokine ratio were induced by polysaccharide from radix glycyrrhizae in H22 hepatocarcinoma bearing mice. Molecules (2011) 16(10):8343–52. doi: 10.3390/molecules16108343
101. Liu R, Li X, Huang N, Fan M, Sun R. Toxicity of traditional Chinese medicine herbal and mineral products. Adv Pharmacol (2020) 87:301–46. doi: 10.1016/bs.apha.2019.08.001
Keywords: oncology, traditional Chinese medicine, TCM, tumor immune microenvironment, nano-formulation
Citation: Sun K, Wu L, Wang S and Deng W (2022) Antitumor effects of Chinese herbal medicine compounds and their nano-formulations on regulating the immune system microenvironment. Front. Oncol. 12:949332. doi: 10.3389/fonc.2022.949332
Received: 20 May 2022; Accepted: 25 August 2022;
Published: 23 September 2022.
Edited by:
Franz Rödel, University Hospital Frankfurt, GermanyReviewed by:
Robert David Hoffman, Zhejiang Chinese Medical University, ChinaCopyright © 2022 Sun, Wu, Wang and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wanli Deng, tcmdwl@163.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.