- 1Department of biochemistry, Jining Medical University, Jining, China
- 2Collaborative Innovation Center, Jining Medical University, Jining, China
- 3Department of Pathophysiology, Weifang Medical University, Weifang, China
- 4The Affiliated Hospital of Jining Medical University, Jining Medical University, Jining, China
Human cancer statistics report that respiratory related cancers such as lung, laryngeal, oral and nasopharyngeal cancers account for a large proportion of tumors, and tumor metastasis remains the major reason for patient death. The metastasis of tumor cells requires actin cytoskeleton remodeling, in which fascin-1 plays an important role. Fascin-1 can cross-link F-actin microfilaments into bundles and form finger-like cell protrusions. Some studies have shown that fascin-1 is overexpressed in human tumors and is associated with tumor growth, migration and invasion. The role of fascin-1 in respiratory related cancers is not very clear. The main purpose of this study was to provide an updated literature review on the role of fascin-1 in the pathogenesis, diagnosis and management of respiratory related cancers. These studies suggested that fascin-1 can serve as an emerging biomarker and potential therapeutic target, and has attracted widespread attention.
Introduction
Cancer is a major public health issue worldwide (1), and is also the leading cause of death in China and developed countries (2,3). Respiratory related cancers mainly include lung, laryngeal and nasopharyngeal cancers. According to the Annual Cancer Statistics Report 2022, respiratory related cancers account for a large proportion of all cancers (1). Compared with 2021 statistics, the number of lung cancer has no downward trend, and it is still the first cancer in mortality (4). Like most tumors, metastases are also the leading cause of death for patients with respiratory related cancers (5).
In order to achieve metastasis, cancer cells need to spread from the primary tumor to other organs and it will form a secondary tumor (6). At the same time, it is found that the remodeling of cytoskeleton is essential in the migration, invasion, and metastasis spread of cancer cells, in which actin plays a key role (7–10). The major class of actin that regulates these complex processes is fascin, which exists in humans and other vertebrates as fascin-1, fascin-2 and fascin-3, with fascin-1 being the most extensively studied (11). Fascin-1 is hardly expressed in normal human tissues. In contrast, high expression of fascin-1 has been found in a variety of cancers (12). Our previous study and many other studies revealed that fascin-1 can promote tumor cell migration, invasion, and metastasis (13). Our previous study and many other studies revealed that fascin-1 can promote tumor cell migration, invasion, and metastasis (13–16). A growing number of studies have shown that it can be used as a new biomarker and therapeutic target and to assess the prognosis of cancer patients. However, the mechanism of fascin-1 in respiratory related cancers is unclear.
In this review, we illuminate fascin-1 expression in respiratory related cancers and its partial mechanisms by discussing the recent literature dealt with the expression of fascin-1 expression in these cancers. Furthermore, we focus on the correlation between fascin-1 expression and clinicopathological parameters and its relationship with patient prognosis.
Structure and function
Fascin is a globular protein with a size of about 55 KD, which is composed of four tandem fascin protein domains (17, 18) (Figure 1). Fascin-2 is mainly distributed in retinal photoreceptor cells (19), and fascin-3 is distributed in the head of spermatid cells (20). Structural studies have revealed that human fascin-1 protein consists of 493 amino acids, including four β-trefoil domains (21). One actin binding site (ABS) is located on aa33-47 in the β-trefoil 1 of fascin-1, but the second actin binding site has not been fully located (18, 22). At the ser-39 residue of the first binding site, it can be phosphorylated by highly conserved protein kinase C (PKC) (22–24). Ser274 phosphorylation can also regulate the actin binding ability of fascin-1 in human cancer cells (12). In addition, fascin-1 has been shown to interact with many proteins other than F-actin, such as MST2, TGF-β family type I, neurotrophins nerve growth factor (NGF) and neurotrophin-3 (NT-3) (25–29). Functional studies have shown that fascin-1 protein can promote the migration, invasion and metastasis of tumor cells (13, 15, 16). Fascin-1 also plays a role in diseases other than cancer, such as wound healing and neurological diseases. Therefore, it is necessary to further clarify the mechanisms of interaction between the fascin-1 protein and different proteins to understand these novel functions of fascin-1.
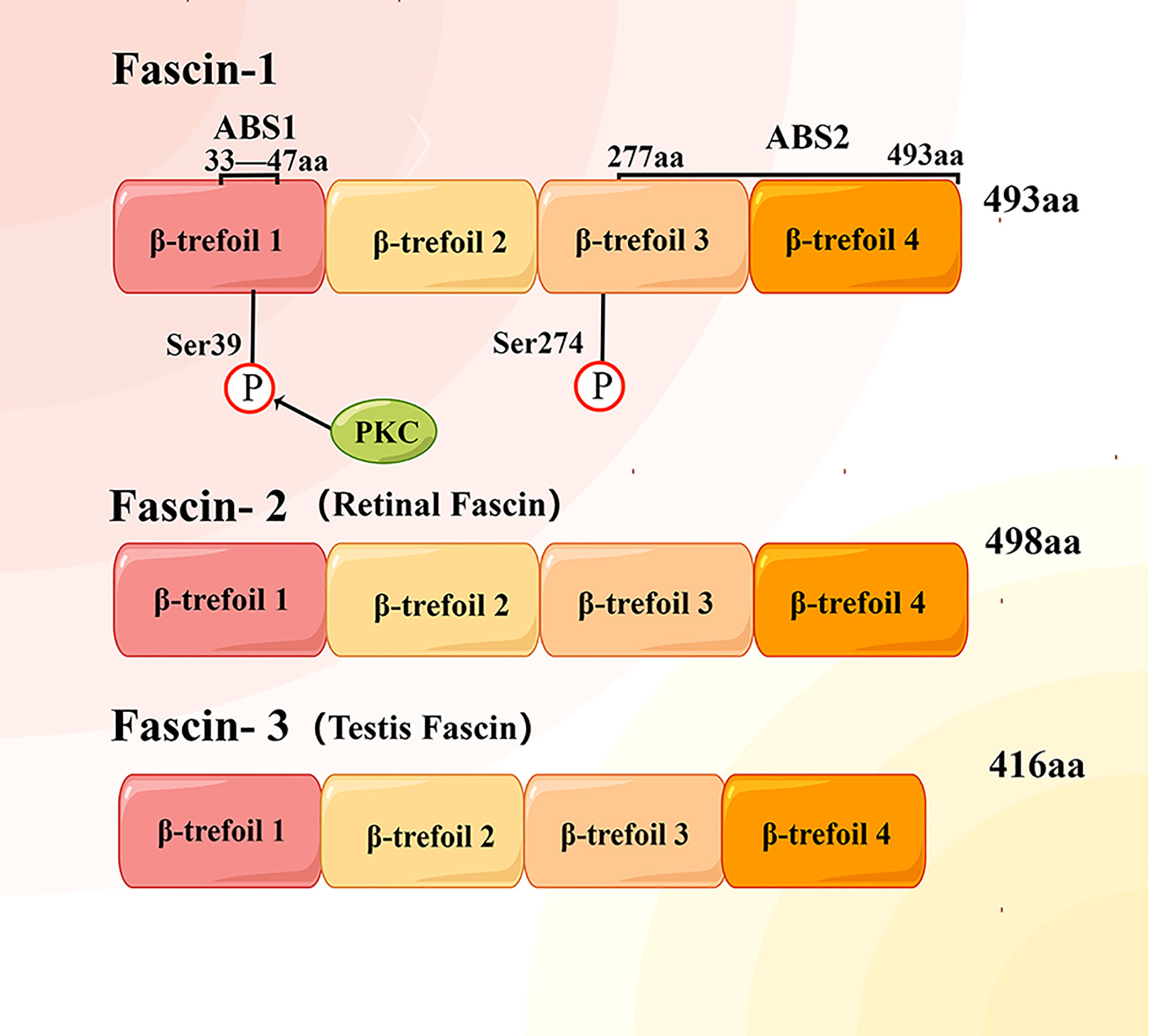
Figure 1 Structural diagram of human fascin protein family. Each fascin protein consists of four β-trefoil domains and with different molecular weight. Actin binding site 1 (ABS1) of fascin-1 protein is located between amino acids (aa) 33 and 47 of the first β-trefoil domain, while the location of ABS2 has not been determined.
Literature review
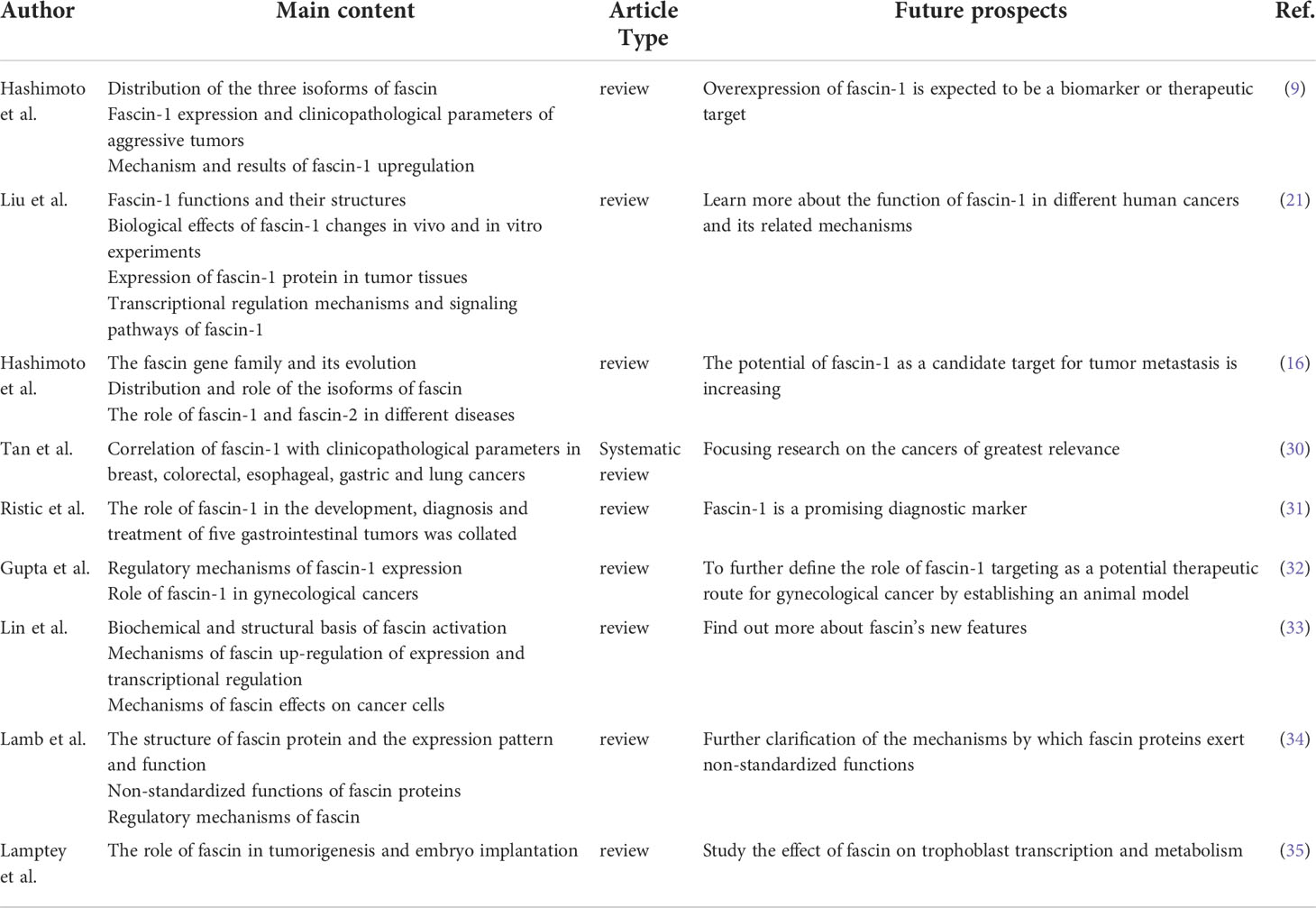
At present, nearly ten reviews on the relationship between fascin-1 and cancer have been published, showing that fascin-1 is overexpressed in a variety of cancers and its pathways of action and regulatory mechanisms have been partially elucidated (Table 1). Meanwhile, fascin-1 also plays a role in a variety of diseases other than cancer and in human embryogenesis.
The up-regulation expression and mechanism of fascin-1 in a variety of cancers were analyzed by Hashimoto et al. (9, 16). They revealed that fascin-1 was expressed in a certain percentage of primary tumors of all tumors (9, 16, 21), most significantly in aggressive pancreatic tumors and non-small cell lung cancer (9). Meanwhile, up regulation of fascin-1 expression could enhance the proliferative activity of cancer cells and promote the formation of cell protrusions, thereby facilitating cell migration (9, 21). Immunohistochemical studies on cancer specimens showed that high expression of fascin-1 was associated with reduced overall survival rate and increased invasiveness, among other parameters (16, 21). The results of a MeTa-analysis by Tan et al. showed that fascin-1 was associated with increased mortality in colorectal, esophageal, and breast cancers and metastasis in gastric and colorectal cancers, but lymph node metastasis of esophageal or lung cancers was not associated with fascin-1 (30). The novel roles of fascin-1 in the pathogenesis, diagnosis, and management of gastrointestinal tumors and gynecological tumors were analyzed by Ristic et al. and Gupta et al. (31, 32). Fascin-1 expression was increased in esophageal squamous cell carcinoma (ESCC), gastric, colorectal, ovarian, uterine, and cervical cancers, and high levels of fascin-1 were related to clinicopathological parameters such as lymph node infiltration, distant metastasis, and reduced survival (31, 32).
The mechanisms of fascin-1 overexpression and promotion of tumor metastasis were elucidated in the article by Lin et al. (33). They concluded that the overexpression of fascin-1 in cancer is unlikely to be due to epigenetic regulation, but rather to activation of NF-κB and JAK-STAT signaling by inflammatory factors in an environment of hypoxia and nutrient deficiency in inflammation (21, 33). For the mechanism by which fascin-1 promotes tumor metastasis, it promotes tumor cell migration by coordinating cell membrane protrusion and cell adhesion on the one hand, and metastatic colonization by promoting capillary extravasation to the mesothelial cell layer on the other (33). Meanwhile, fascin-1 can control metastatic colonization by controlling mitochondrial F-actin and mitochondrial metabolism (33). Fascin-1 participates in the regulation of pivotal oncogenic pathways, such as MAPK, Wnt/β-linked protein, PI3K/AKT, EMT, etc (21).
In addition to the role of fascin-1 in tumors, fascin-1 has been found to be associated with many neurological-related diseases, such as absence seizures, epilepsy, and down syndrome (16, 34). The review by Lamb et al. focused on the various functions of fascin-1 and elucidated that fascin-1, in addition to its bundled actin functions, also had many non-standard functions (34). For example, it interacts with the Linker of the Nucleoskeleton and Cytoskeleton (LINC) Complex, binds to microtubules and regulates actin binding protein activity and mitochondrial function (34). Meanwhile, the review by Lamptey et al. also compiled an analysis of the role of fascin-1 during human embryogenesis (35). It was found to regulate the epithelial-mesenchymal transition in placental formation and early embryogenesis and to achieve homeostasis (35).
Other people still have great expectations for future studies of fascin-1 (9, 16, 21, 30–35). Meanwhile, the results suggest that fascin-1 is a promising marker for tumor diagnosis and prognosis (9, 16, 30–32), but further studies are still needed to test the therapeutic potential of this protein (21, 31).
Fascin-1 and non-small cell lung cancer
Expression of fascin-1 in NSCLC and its potential as a prognostic marker in NSCLC
According to the latest statistical analysis of cancer, lung cancer has the highest mortality of all cancers, of which the five-year survival rate is no more than 22% (1), and the main reason for its low survival rate is the occurrence of distal metastasis (36). Non-small cell lung cancer (NSCLC) accounts for more than 85% of the total number of lung cancer (36). Among the actin-binding protein family, fascin-1 has the greatest impact on distant metastasis in NSCLC (37). Recent studies have found that the expression level of fascin-1 is considerably higher in NSCLC than in normal lung tissue (Table 2). The expression level of fascin-1 is closely related to tumor invasion and metastasis, which affects the survival time of tumor patients (38–40) (Table 2). This is an important reason why fascin-1 is expected to be a prognostic marker for NSCLC.
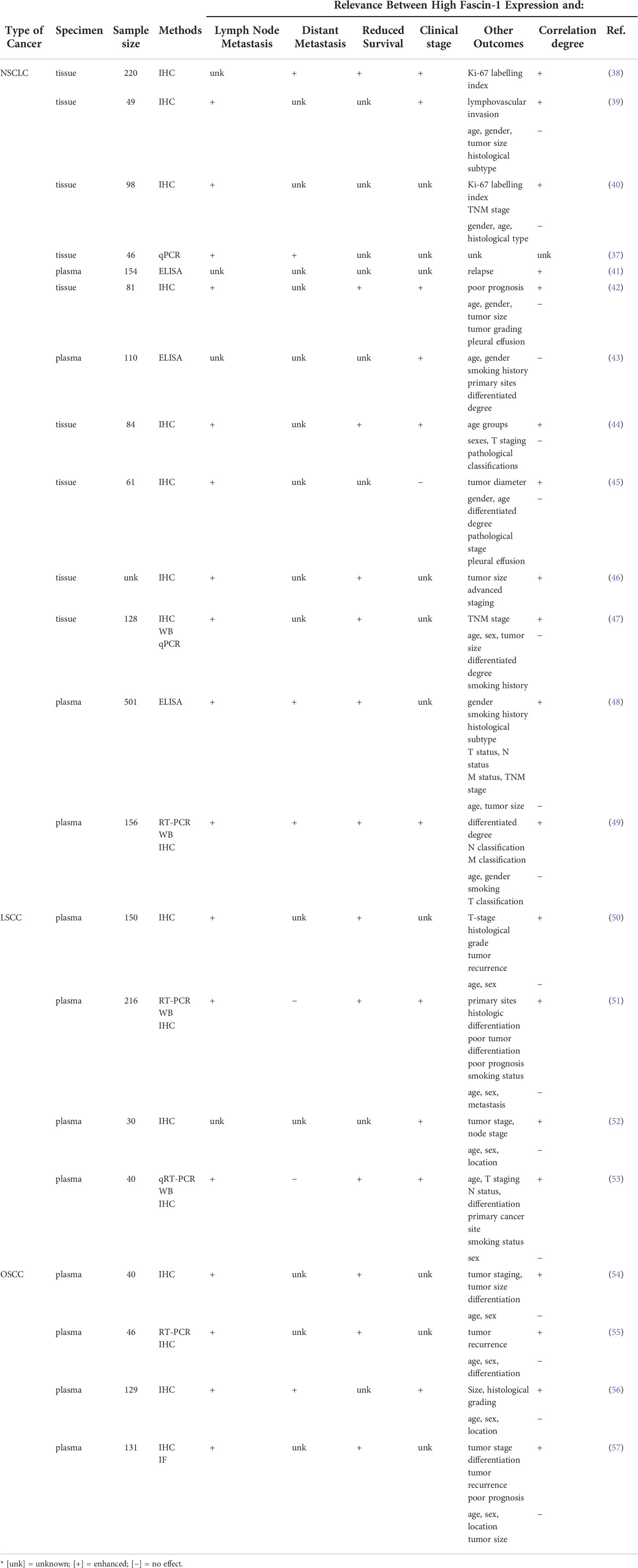
Table 2 Relationship between high expression of fascin-1 and clinical parameters in respiratory related cancers.
Furthermore, the results of several studies on tumor tissues showed that elevated fascin-1 RNA and protein levels were significantly relevant to lymph node metastasis and TNM stage, but not with age, gender, tumor size or differentiation (Table 2). The expression level of fascin-1 was significantly inversely proportional to the survival time of NSCLC patients (Table 2). The measurement of fascin-1 concentrations in NSCLC patients’ serum revealed that with the increase of fascin-1 expression in patients’ serum, the tumor is prone to metastasis and indicates a poor prognosis (41, 48). Based on these findings, fascin-1 levels, both in tissues and in serum, are considered promising as new targets and prognostic indicators for assessing the prognosis of NSCLC patients. Although these studies suggest that fascin-1 is a promising prognostic marker in NSCLC, further studies are needed to determine whether it has therapeutic potential.
The role and mechanism of fascin-1 in NSCLC
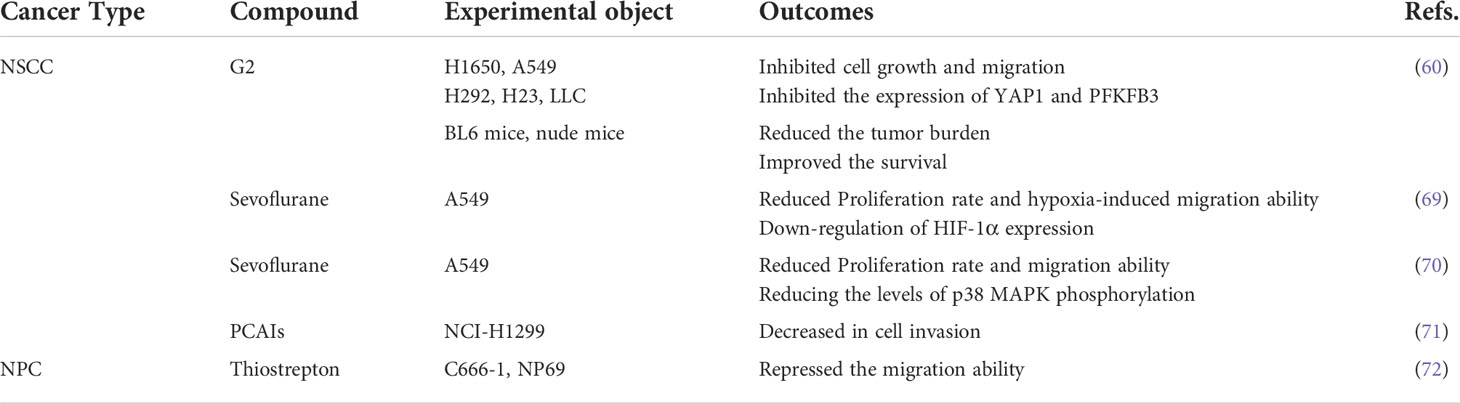
Fascin-1 overexpression promoted the development of NSCLC by boosting cell growth and metastasis (25). Moreover, silencing the expression of fascin-1 in NSCLC cell lines could inhibit the proliferation, invasion and metastasis of NSCLC cells (58). Experiments in mice showed that inhibition of fascin-1 function reduced the migration and metastasis of cancer cells (59). However, an in vitro and in vivo experiment showed that high expression of fascin-1 could improve the migration rate and invasion of tumor cells, but did not promote the growth of tumor nodules (40). One previous study found that fascin-1 promotes lung cancer metastasis and colonization by enhancing resistance to metabolic stress and promoting mitochondrial oxidative phosphorylation (46). Fascin-1 was found to activate PFKFB3 transcription through the YAP1/TEAD binding site in its promoter to further promote glycolysis in NSCLC cells, thereby promoting lung cancer cell metabolism and growth (60) (Figure 2). These experiments suggest that inhibition of fascin-1 may be a potential therapeutic strategy for the treatment of NSCLC, but further studies are still needed.

Figure 2 Molecular regulation mechanism of fascin-1 overexpression in respiratory related cancers. SMAD3/4, CREB, NF-κB, HIF1-α and other transcription factors can be activated by inflammatory microenvironment factors (IL-1β, TGF-β) and hypoxia, so as to up regulate the transcription of fascin-1 protein. Fascin-1 protein can promote tumorigenesis, invasion and metastasis through MAPK, YAP1/TEAD and other signal pathways.
Regulation of fascin-1 expression in non-small cell lung cancer
The mitogen-activated protein kinase (MAPK) pathway has been related to promoting tumor metastasis (Figure 2). The crucial role of the MAPK pathway in the development and progression of NSCLC has been demonstrated in previous studies (48, 61). By further detecting the expression and phosphorylation level of MAPK signaling molecules, it was found that the expression of fascin-1 could be down regulated by regulating MAPK pathway, so as to inhibit the metastasis and invasion of non-small cell lung cancer cells (58). Furthermore, by studying the YAP/TAZ signaling pathway, the core of the Hippo signaling pathway, fascin-1 was found to promote the growth and metastasis of non-small cell lung cancer cells by connecting with kinase MST1 and activating the transcriptional activity of YAP/TEAD complex (25). RSK2 is a Ser/Thr kinase that regulates cell proliferation, cell survival and cycle by phosphorylating cAMP response element binding (CREB) proteins (62–64). As a transcription factor, CREB participates in the signal pathway related to promoting tumor progression, stimulating growth, giving apoptosis resistance and promoting angiogenesis (65, 66). Li et al. found that the RSK2-CREB pathway can up-regulate expression of fascin-1 in lung cancer cell line A549, in clinical samples and in xenograft mouse models, thereby promoting cancer cell filopod formation and thus cancer cell invasion and metastasis (67). They concluded that fascin-1 is expected to become a prognostic marker of metastatic cancer, and RSK2-CREB-fascin-1 signaling pathway is expected to become a therapeutic target of metastatic cancer (67). At the same time, inflammation can promote TGF-β to induce fascin-1 overexpression through direct binding of the SMAD3-SMAD4 complex to the fascin-1 transcriptional start site (68).
A number of compounds were found to exert inhibitory effects on cancer cell invasion and metastasis by blocking the signaling pathway associated with fascin-1 (Table 3). The results in vitro and in vivo showed that G2, a pharmacological inhibitor of fascin-1, could significantly reduce the levels of PFKFB3 and YAP1 in lung cancer cells and nude mice (60). G2 can significantly inhibit the tumor growth on the lung of nude mice, which improves the survival rate of mice (60). And G2 can also significantly reduce the volume of tumor-like culture (60). Meanwhile, it was found that sevoflurane, a commonly used anesthetic, could reduce HIF-α levels by blocking the p38MAPK signaling pathway, further down-regulating fascin-1 expression and thus inhibiting the growth and metastasis of lung cancer cells (69, 70). These studies found the potential role of sevoflurane in lung cancer surgery and gave sevoflurane new clinical significance (69, 70). In another study, it was found that polyisoprene cysteine amide inhibitors (PCAIs) can induce the decrease of fascin-1 protein level in NSCLS cells, so as to inhibit F-actin tissue, filamentous foot and focal adhesions, and further reduce cell invasiveness (71).
Furthermore, microRNAs (miRNAs) regulate gene expression through post transcription, which is another mechanism to affect fascin-1 expression (34). In respiratory related cancers, down-regulation of these miRNAs leads to increase fascin-1 expression, which is associated with increased cell migration and invasion (34) (Figure 3). MiRNAs such as miR-145, miR-200b and miR-326 were labeled as potent repressors of fascin-1 (73–76). Thus, post-transcriptional regulation is one of the key mechanisms regulating fascin-1 expression, and activation of these miRNAs could serve as a potential therapeutic pathway.
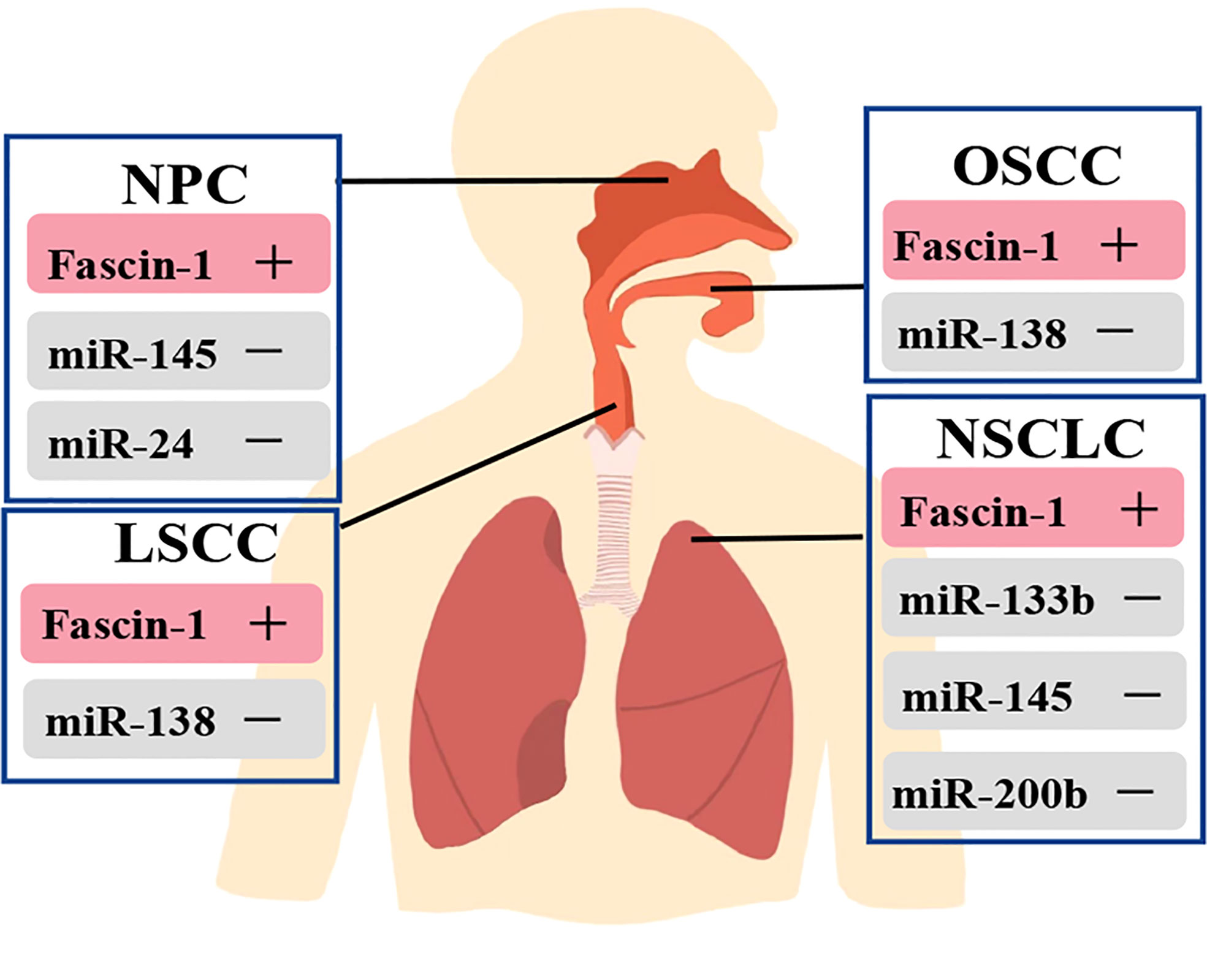
Figure 3 Relationship between miRNAs and regulation of fascin-1 expression in respiratory related tumors. Fascin-1 is highly expressed in respiratory related tumors, and activation of these miRNAs can inhibit the expression of fascin-1 in cancers.
Fascin-1 and laryngeal squamous cell carcinoma
Fascin-1 as a prognostic marker for LSCC
Apart from lung cancer, laryngeal squamous cell carcinoma (LSCC) of the larynx is the most usual cancer of the respiratory system (77). Immunostaining showed that fascin-1 was mainly distributed in the cytoplasm of tumor cells and the immune response to fascin-1 was homogeneous in the center of the tumor but enhanced at the tumor margin (50, 51). The expression of fascin-1 is significantly higher in LSCC than in adjacent normal margin tissue (51, 52, 78). Several studies have shown that fascin-1 expression in LSCC tissues is associated with T-stage, cervical lymphatic tract metastasis and clinical stage (Table 2). Also, high expression of fascin-1 was found to be associated with poor prognosis (51, 53, 79). To estimate the validity of fascin-1 expression as a prognostic marker in LSCC, it was found by using logistic regression models that fascin-1 could be an important independent predictor of LSCC recurrence (50). However, the results of another study showed that fascin-1 expression was not related to prognosis and that immunohistochemical studies were not helpful in predicting prognosis, and more emphasis should be placed on morphological findings (80). Based on these findings, most people believe that fascin-1 can be used as a potential molecular marker to judge the prognosis of LSCC (50, 51, 53, 79). Compared with other tumors, there are fewer studies on fascin-1 and LSCC, so further studies can be conducted to determine the potential of fascin-1 as a prognostic indicator for laryngeal cancer.
The role of high expression of fascin-1 in LSCC
Based on the relationship between fascin-1 expression and clinical pathological prognostic parameters of LSCC, LSCC with high fascin-1 expression may be more aggressive than laryngeal carcinoma with low expression (52). Meanwhile, with the increase of fascin-1 level in LSCC patients, the tumor recurrence rate increased significantly, while the 3-year disease-free survival rate decreased significantly (50). In LSCC cells, by inhibiting the expression of fascin-1, it was found that the integrity of cytoskeleton structure was destroyed and the ability of cell migration decreased (81). Also, through in-depth bioinformatics analysis, fascin-1 may play a more important role in cancer progression than before (26).
Regulation of fascin-1 expression in LSCC
In LSCC, aminoacyl tRNA synthetase complex interacting multifunctional protein 1 (AIMP1) and leukotriene A4 hydrolase (LTA4H) bind to and co-localize with fascin-1 (82). AIMP1 exerts its role in regulating cellular structure by binding to fascin-1 (82). Fascin-1 may be a substrate for LTA4H, and LTA4H may act by binding to fascin-1 binding to regulate the activity of fascin-1 to act (82). Meanwhile, knockdown of AIMP1 and LTA4H inhibits proliferation, migration, and invasion of LSCC cells (82). The results of another study showed that miR145-5p plays a key role in inhibiting LSCC progression through inhibition of fascin-1 (53). Luciferase analysis and xenograft model assay showed that miR-145-5p could inhibit the migration, invasion and growth of LSCC by downregulated the expression of fascin-1 gene (53). Therefore, they concluded that both miR-145-5p and fascin-1 are significant latent prognostic markers and therapeutic targets for LSCC (53).
Fascin-1 and oral squamous cell carcinoma
Results of fascin-1 overexpression in oral squamous cell carcinoma
It was observed that overexpression of fascin-1 can lead to the increase of F-actin structure such as filopodia and lamellipodia (57). According to the results of several studies, fascin-1 was found to be more expressed in human oral squamous carcinoma cells than in normal cells (54–56, 83, 84). Also, they found that high levels of fascin-1 expression had to do with lymph node metastasis and reduced disease-free survival (55, 57) (Table 2). Meanwhile, other studies found that the decreased expression of fascin-1 can reduce the migration and invasion of oral squamous cell carcinoma (OSCC) cells and increase cell adhesion (83–85). Overexpression of fascin-1 could promote cell proliferation (57). Also, univariate and multivariate survival analyses showed that fascin-1 expression levels could be an independent predictor of poor prognosis in OSCC (83).
Combined with the above findings, most believe that fascin-1 is linked to the poor prognosis of oral squamous carcinoma (Table 2), however, the results of one study revealed that although high expression of fascin-1 was present in most samples, it did not correlate significantly with patient prognosis (86).
Mechanism of fascin-1 in OSCC
A known hallmark of aggressive tumor is the loss of E-cadherin expression, which results in reduced cell contact (87). Therefore, to assess the correlation between fascin-1 expression and E-cadherin, a negative correlation was found between fascin-1 and E-cadherin expression by detecting E-cadherin expression in OSCC specimens (55). Overexpression of fascin-1 is thought to enhance OSCC invasiveness by reducing the expression of E-cadherin (55). Furthermore, the mRNA expression levels of cathepsin B, cathepsin D, MMP-9 and MMP-10 were reduced, while the mRNA expression level of kinin release enzyme 5 (KLK5) was increased (88). This suggests that fascin-1 affects cancer invasion and progression by influencing the activity of matrix-degrading proteases (88). Furthermore, high expression of fascin-1 in OSCC derived cells leads to increased cell membrane protrusions, disruption of cell contacts and alterations in the actin cytoskeleton (57). Therefore, these data demonstrated that fascin-1 could be involved in OSCC invasion and progression through multiple pathways.
Regulation of fascin-1 expression in OSCC
In the process of tumor invasion and progression, microenvironment plays a key role in the regulation of cancer cells (84). The study results showed that IL-1β is a key inducer of fascin-1 expression and can increase the invasiveness of oral cancer (84). Meanwhile, IL-1β by using ERK1/2 and JNK as intermediate signal molecules, NF-κB and CREB act as the signal pathway composed of transcription factors to induce the expression of fascin-1, which increases the invasiveness of cancer cells (84). Furthermore, Keratins 8 (K8) was found to be aberrantly expressed in squamous cell carcinoma (SCC) in previous studies, and its expression correlated with invasion and poor prognosis (89–92). Also, in the OSCC-derived cell line AW13516, knockout of the K8 gene leads to reduced fascin levels, resulting in altered actin organization and reduced cell migration (93). The results showed that there was a significant negative correlation between miR-138 and fascin-1 but not in miR-145. Meanwhile, miR-138 has the ability to target and regulate fascin-1 expression in OSCC, thus affecting the migration rate of cells (83) (Figure 3). Moreover, the results of study showed that fascin-1 activates AKT and MAPK pathways in OSCC-derived cells, thus promoting tumor progression (57). Therefore, fascin-1 is likely to be a new therapeutic target for human oral cancer (57).
Fascin-1 and nasopharyngeal carcinoma
Expression and role of fascin-1 in nasopharyngeal carcinoma
Nasopharyngeal carcinoma (NPC) is relatively rare compared to other cancers, but its global geographical distribution is highly uneven, with > 70% of new cases occurring in East and Southeast Asia (94). It is an Epstein-Barr virus-associated (EBA) malignancy and is the most usual head and neck cancer in China, mainly concentrated in the southern region (95). The role of fascin-1 in several human cancers has been confirmed through studies, but the number of studies on the presence and role of fascin-1 in NPC is relatively limited. In this context, immunohistochemical staining showed that the positive signal of fascin-1 was mainly concentrated in the cytoplasm (96). Fascin-1 was found to be overexpressed in NPC (78, 97, 98), and can participate in the progress of NPC by enhancing cell migration and adhesion (98).
Regulation of fascin-1 expression in NPC
FoxM1 belongs to the fox transcription factor family FoxM1, which is a key factor involved in the regulation of the cell cycle from G1 to S phase, G2 to M phase and the transition to mitosis (99–101). FOXM1 has now been shown to be up-regulated in many human malignancies and to be a promising therapeutic target (44, 102–106). Thiostrepton, an inhibitor of FOXM1 (107), was found to significantly reduce the level of fascin-1 in C666-1 cells, thereby inhibiting the migration of NPC cells. However, because fascin-1 has not been reported as a direct or indirect target gene of FOXM1, the thiostrepton-FOXM1 pathway has not been established (72) (Table 3). Moreover, studies in NPC have identified miR-145 and miR-24 as inhibitors of fascin-1 expression, which were negatively correlated with fascin-1 expression (108) (Figure 3). MiR-145 and miR-24 were found to be significantly down-regulated in NPC cell lines and tissue samples, and that their ectopic expression could inhibit the growth and invasion of NPC cells by targeting fascin-1 (108, 109). Therefore, the miR-145-fascin-1 pathway and the miR-24-fascin-1 pathway may be potential new therapeutic targets for NPC patients (108, 109).
Treatment potential and future directions
The treatment targets of fascin-1 in cancers mainly focused on small molecule inhibitors, miRNAs and inhibitory nanobodies.
Small-molecule inhibitors of fascin-1 reduce tumor cell migration and invasion (13, 59, 110–112). G2, a pharmacological inhibitor of fascin-1, significantly inhibited the growth of tumors and improved the survival rate of mice (60). In first-in-human clinical trial of ovarian cancer patients, NP-G2-044 (derivatives of G2) was safe and well tolerated (113). Also, compounds such as sevoflurane and polyisoprene cysteine amide inhibitors (PCAIs) can reduce the expression of fascin-1 (69–71). In non-respiratory related cancers, leucine aminopeptidase 3 (LAP3) inhibitors, migraine inhibitors could block fascin-1 activity (13, 110, 114).
Similarly, many miRNAs are tagged as potent post-transcriptional repressors of fascin-1 (Figure 3) by inhibiting cell proliferation, migration, and invasion (108, 115–117). Although it is possible to develop relevant therapeutic drugs based on miRNAs, there are still some limitations to using them in the clinical setting. To date, only 10 miRNA-based drugs have entered clinical trials, and none have reached Phase III (118). During clinical trials, the emergence of multiple immune-related side effects and severe hyperbilirubinemia forced the suspension of the trials (119–121). Targeting effects, routes of delivery, dosing issues, and drug delivery systems are the main challenges that must be overcome to develop miRNA-based cancer therapies (118).
In vitro experiments using inhibitory nanobody against fascin-1 protein in breast and prostate cancer cells have shown that they can inhibit invasion base formation and cell invasion (122). However, there are relatively few studies on inhibitory nanobody and no data on studies in respiratory related cancers. Therefore, there is a need to expand the research surface to determine the adaptability of inhibitory nanobodies in different tumors and also to determine whether they can be applied in clinical settings.
Conclusions
Fascin-1 is overexpressed in a variety of human cancers, including respiratory related cancers, and is associated with several tumor clinicopathological parameters, such as increased tumor invasiveness, promotion of regional and distant metastasis, and reduced patient survival time. Meanwhile, fascin-1 is transcriptionally regulated by a variety of transcription factors (SMAD3/4, CREB, NF-κB, HIF1-α) and participates in a variety of cancer promoting signaling pathways, such as MAPK, YAP/TAZ, AKT, RSK2, etc. Therefore, fascin-1 is considered as a promising diagnostic marker and prognostic marker. Both in vitro and in vivo experiments have yielded good results on the therapeutic effects of fascin-1 as an anti-cancer target. Small molecular inhibitors, inhibitory nanobody and miRNAs have been found to potential therapeutic measure. Most studies focused on small molecule inhibitors and achieved good results. However, the development of anticancer drugs based on miRNAs still faces such major problems as targeting effect, drug delivery route and drug delivery system. The emerging inhibition nanobody is also a potential treatment method with good future prospects. At present, the study of the molecular regulatory mechanism of fascin-1 and its role with other proteins is still in its early stage, and we still have great expectations for the future study of fascin-1. Although most studies have shown a strong relationship between fascin-1 and the aggressive clinical course of multiple human cancers, most studies remain at the in vitro stage, lacking enough in vivo experiments to further prove the potential of fascin-1 for clinical application. A host of studies are still needed to determine whether fascin-1 can be used as a new biomarker and whether it exceeds the biomarkers currently in clinical.
Author contributions
HY and NZ contributed to conception and design of the study. NZ wrote the first draft of the manuscript. YG organized the database. YG, QB, QW, YS and ZZ wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Shandong Provincial Natural Science Foundation (grant numbers: ZR2020MH078 and ZR2020MH070), Shandong Province Medicine and Health Science and Technology Development Plan Project (grant number: 2019WS368), and College students' Innovative Entrepreneurial Training Plan Program of Jining Medical University (grant number: cx2021094).
Acknowledgments
We made Figures 1 and 2 by figdraw plotform. Thanks for the support of figdraw platform.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ELISA, enzyme-linked immunosorbentassay; ESCC, esophageal squamous cell carcinoma; IF, immunofluorescence; IHC, immunohistochemistry; LSCC, laryngeal squamous cell carcinoma; NPC, nasopharyngeal carcinoma; NSCLC, non-small cell lung cancer; OSCC, oral squamous cell carcinoma; PCAIs, polyisoprenylated cysteinyl amide inhibitors; WB, western blot.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer-Am Cancer Soc (2021) 127(16):3029–30. doi: 10.1002/cncr.33587
4. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA: Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin (2015) 65(1):5–29. doi: 10.3322/caac.21254
6. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. (2011) 331(6024):1559–64. doi: 10.1126/science.1203543
7. Saad A, Bijian K, Qiu D, Da SS, Marques M, Chang CH, et al. Insights into a novel nuclear function for fascin in the regulation of the amino-acid transporter SLC3A2. Sci Rep (2016) 6:36699. doi: 10.1038/srep36699
8. Machesky LM, Li A. Fascin: Invasive filopodia promoting metastasis. Commun Integr Biol (2010) 3(3):263–70. doi: 10.4161/cib.3.3.11556
9. Hashimoto Y, Skacel M, Adams JC. Roles of fascin in human carcinoma motility and signaling: prospects for a novel biomarker? Int J Biochem Cell Biol (2005) 37(9):1787–804. doi: 10.1016/j.biocel.2005.05.004
10. Adams JC. Roles of fascin in cell adhesion and motility. Curr Opin Cell Biol (2004) 16(5):590–6. doi: 10.1016/j.ceb.2004.07.009
11. De Arcangelis A, Georges-Labouesse E, Adams JC. Expression of fascin-1, the gene encoding the actin-bundling protein fascin-1, during mouse embryogenesis. Gene Expr Patterns. (2004) 4(6):637–43. doi: 10.1016/j.modgep.2004.04.012
12. Zhang FR, Tao LH, Shen ZY, Lv Z, Xu LY, Li EM. Fascin expression in human embryonic, fetal, and normal adult tissue. J Histochem Cytochem (2008) 56(2):193–9. doi: 10.1369/jhc.7A7353.2007
13. Huang F, Han S, Xing B, Huang J, Liu B, Bordeleau F, et al. Targeted inhibition of fascin function blocks tumour invasion and metastatic colonization. Nat Commun (2015) 6(1):7465. doi: 10.1038/ncomms8465
14. Zhao Z, Wang Y, Zhang JJ, Huang XY. Fascin inhibitors decrease cell migration and adhesion while increase overall survival of mice bearing bladder cancers. Cancers (Basel). (2021) 13(11):2698. doi: 10.3390/cancers13112698
15. Darnel AD, Behmoaram E, Vollmer RT, Corcos J, Bijian K, Sircar K, et al. Fascin regulates prostate cancer cell invasion and is associated with metastasis and biochemical failure in prostate cancer. Clin Cancer Res (2009) 15(4):1376–83. doi: 10.1158/1078-0432.CCR-08-1789
16. Hashimoto Y, Kim DJ, Adams JC. The roles of fascins in health and disease. J Pathology. (2011) 224(3):289–300. doi: 10.1002/path.2894
17. Ponting CP, Russell RB. Identification of distant homologues of fibroblast growth factors suggests a common ancestor for all β-trefoil proteins 1 1Edited by J. Thornton. J Mol Biol (2000) 302(5):1041–7. doi: 10.1006/jmbi.2000.4087
18. Sedeh RS, Fedorov AA, Fedorov EV, Ono S, Matsumura F, Almo SC, et al. Structure, evolutionary conservation, and conformational dynamics of homo sapiens fascin-1, an f-actin crosslinking protein. J Mol Biol (2010) 400(3):589–604. doi: 10.1016/j.jmb.2010.04.043
19. Saishin Y, Ishikawa R, Ugawa S, Guo W, Ueda T, Morimura H, et al. Retinal fascin: Functional nature, subcellular distribution, and chromosomal localization. Invest Ophth Vis Sci (2000) 41(8):2087–95.
20. Tubb B, Mulholland DJ, Vogl W, Lan Z, Niederberger C, Cooney A, et al. Testis fascin (FSCN3): A novel paralog of the actin-bundling protein fascin expressed specifically in the elongate spermatid head. Exp Cell Res (2002) 275(1):92–109. doi: 10.1006/excr.2002.5486
21. Liu H, Zhang Y, Li L, Cao J, Guo Y, Wu Y, et al. Fascin actin-bundling protein 1 in human cancer: Promising biomarker or therapeutic target? Mol Ther - Oncolytics. (2021) 20:240–64. doi: 10.1016/j.omto.2020.12.014
22. Anilkumar N, Parsons M, Monk R, Ng T, Adams JC. Interaction of fascin and protein kinase calpha: a novel intersection in cell adhesion and motility. EMBO J (2003) 22(20):5390–402. doi: 10.1093/emboj/cdg521
23. Ono S, Yamakita Y, Yamashiro S, Matsudaira PT, Gnarra JR, Obinata T, et al. Identification of an actin binding region and a protein kinase c phosphorylation site on human fascin. J Biol Chem (1997) 272(4):2527–33. doi: 10.1074/jbc.272.4.2527
24. Parsons M, Adams JC. Rac regulates the interaction of fascin with protein kinase c in cell migration. J Cell Sci (2008) 121(Pt 17):2805–13. doi: 10.1242/jcs.022509
25. Liang Z, Wang Y, Shen Z, Teng X, Li X, Li C, et al. Fascin 1 promoted the growth and migration of non-small cell lung cancer cells by activating YAP/TEAD signaling. Tumor Biol (2016) 37(8):10909–15. doi: 10.1007/s13277-016-4934-0
26. Liu H, Cui J, Zhang Y, Niu M, Xue X, Yin H, et al. Mass spectrometry-based proteomic analysis of FSCN1-interacting proteins in laryngeal squamous cell carcinoma cells. IUBMB Life (2019) 71(11):1771–84. doi: 10.1002/iub.2121
27. Kang J, Wang J, Yao Z, Hu Y, Ma S, Fan Q, et al. Fascin induces melanoma tumorigenesis and stemness through regulating the hippo pathway. Cell Commun Signal (2018) 16(1):37. doi: 10.1186/s12964-018-0250-1
28. Liu Z, Ning G, Xu R, Cao Y, Meng A, Wang Q. Fscn1 is required for the trafficking of TGF-β family type I receptors during endoderm formation. Nat Commun (2016) 7(1):12603. doi: 10.1038/ncomms12603
29. Shonukan O, Bagayogo I, McCrea P, Chao M, Hempstead B. Neurotrophin-induced melanoma cell migration is mediated through the actin-bundling protein fascin. Oncogene. (2003) 22(23):3616–23. doi: 10.1038/sj.onc.1206561
30. Tan VY, Lewis SJ, Adams JC, Martin RM. Association of fascin-1 with mortality, disease progression and metastasis in carcinomas: a systematic review and meta-analysis. BMC Med (2013) 11:52. doi: 10.1186/1741-7015-11-52
31. Ristic B, Kopel J, Sherazi SAA, Gupta S, Sachdeva S, Bansal P, et al. Emerging role of fascin-1 in the pathogenesis, diagnosis, and treatment of the gastrointestinal cancers. Cancers. (2021) 13(11):2536. doi: 10.3390/cancers13112536
32. Gupta I, Vranic S, Al-Thawadi H, Al MA. Fascin in gynecological cancers: An update of the literature. Cancers (Basel). (2021) 13(22):5760. doi: 10.3390/cancers13225760
33. Lin S, Taylor MD, Singh PK, Yang S. How does fascin promote cancer metastasis? FEBS J (2021) 288(5):1434–46. doi: 10.1111/febs.15484
34. Lamb MC, Tootle TL. Fascin in cell migration: More than an actin bundling protein. Biology. (2020) 9(11):403. doi: 10.3390/biology9110403
35. Lamptey J, Czika A, Aremu JO, Pervaz S, Adu-Gyamfi EA, Otoo A, et al. The role of fascin in carcinogenesis and embryo implantation. Exp Cell Res (2021) 409(1):112885. doi: 10.1016/j.yexcr.2021.112885
36. Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc (2019) 94(8):1623–40. doi: 10.1016/j.mayocp.2019.01.013
37. Kolegova ES, Kakurina GV, Kostromitskiy DN, Dobrodeev AY, Kondakova IV. [Increases in mRNA and protein levels of the genes for the actin-binding proteins profilin, fascin, and ezrin promote metastasis in non-small cell lung cancer]. Mol Biol (Mosk). (2020) 54(2):285–92. doi: 10.1134/S0026893320020065
38. Pelosi G, Pastorino U, Pasini F, Maissoneuve P, Fraggetta F, Iannucci A, et al. Independent prognostic value of fascin immunoreactivity in stage I nonsmall cell lung cancer. Br J Cancer. (2003) 88(4):537–47. doi: 10.1038/sj.bjc.6600731
39. Choi P, Yang D, Son C, Lee K, Lee J, Roh M. Fascin immunoreactivity for preoperatively predicting lymph node metastases in peripheral adenocarcinoma of the lung 3cm or less in diameter. Eur J Cardio-Thorac. (2006) 30(3):538–42. doi: 10.1016/j.ejcts.2006.06.029
40. Zhao J, Zhou Y, Zhang Z, Tian F, Ma N, Liu T, et al. Upregulated fascin1 in non-small cell lung cancer promotes the migration and invasiveness, but not proliferation. Cancer Lett (2010) 290(2):238–47. doi: 10.1016/j.canlet.2009.09.013
41. Yang L, Teng Y, Han TP, Li FG, Yue WT, Wang ZT. Clinical significance of fascin-1 and laminin-5 in non-small cell lung cancer. Genet Mol Res (2017) 16(2). doi: 10.4238/gmr16029617
42. Zhang Y, Liang B, Dong H. Expression of fascin_1 protein in cancer tissues of patients with nonsmall cell lung cancer and its relevance to patients' clinicopathologic features and prognosis. J Cancer Res Ther (2018) 14(4):856–9. doi: 10.4103/jcrt.JCRT_732_17
43. Liu YG, Gao XD, Yue WT. [Expression and diagnosis value of fascin in non-small cell lung cancer patients]. Zhonghua Yi Xue Za Zhi. (2013) 93(31):2505–7. doi: 10.3760/cma.j.issn.0376-2491.2013.31.019
44. Yang DK, Son CH, Lee SK, Choi PJ, Lee KE, Roh MS. Forkhead box M1 expression in pulmonary squamous cell carcinoma: correlation with clinicopathologic features and its prognostic significance. Hum Pathol (2009) 40(4):464–70. doi: 10.1016/j.humpath.2008.10.001
45. Zhang J, Wang X, Zhang Y, Wu J, Zhou N. Leucine-rich repeats and immunoglobulin-like domains protein 1 and fascin actin-bundling protein 1 expression in nonsmall cell lung cancer. J Cancer Res Ther (2016) 12(Supplement):C248–51. doi: 10.4103/0973-1482.200749
46. Lin S, Huang C, Gunda V, Sun J, Chellappan SP, Li Z, et al. Fascin controls metastatic colonization and mitochondrial oxidative phosphorylation by remodeling mitochondrial actin filaments. Cell Rep (2019) 28(11):2824–36. doi: 10.1016/j.celrep.2019.08.011
47. Ling XL, Zhang T, Hou XM, Zhao D. Clinicopathological significance of fascin-1 expression in patients with non-small cell lung cancer. Onco Targets Ther (2015) 8:1589–95.
48. Teng Y, Xu S, Yue W, Ma L, Zhang L, Zhao X, et al. Serological investigation of the clinical significance of fascin in non-small-cell lung cancer. Lung Cancer. (2013) 82(2):346–52. doi: 10.1016/j.lungcan.2013.08.017
49. Luo A, Yin Y, Li X, Xu H, Mei Q, Feng D. The clinical significance of FSCN1 in non-small cell lung cancer. BioMed Pharmacother. (2015) 73:75–9. doi: 10.1016/j.biopha.2015.05.014
50. Zou J, Yang H, Chen F, Zhao H, Lin P, Zhang J, et al. Prognostic significance of fascin-1 and e-cadherin expression in laryngeal squamous cell carcinoma. Eur J Cancer Prev (2010) 19(1):11–7. doi: 10.1097/CEJ.0b013e32832f9aa6
51. Gao W, Zhang C, Feng Y, Chen G, Wen S, Huangfu H, et al. Fascin-1, ezrin and paxillin contribute to the malignant progression and are predictors of clinical prognosis in laryngeal squamous cell carcinoma. PloS One (2012) 7(11):e50710. doi: 10.1371/journal.pone.0050710
52. Durmaz A, Kurt B, Ongoru O, Karahatay S, Gerek M, Yalcın S. Significance of fascin expression in laryngeal squamous cell carcinoma. J Laryngol Otology. (2010) 124(2):194–8. doi: 10.1017/S0022215109991630
53. Gao W, Zhang C, Li W, Li H, Sang J, Zhao Q, et al. Promoter methylation-regulated miR-145-5p inhibits laryngeal squamous cell carcinoma progression by targeting FSCN1. Mol Ther (2019) 27(2):365–79. doi: 10.1016/j.ymthe.2018.09.018
54. Routray S, Kheur S, Chougule HM, Mohanty N, Dash R. Establishing fascin over-expression as a strategic regulator of neoplastic aggression and lymph node metastasis in oral squamous cell carcinoma tumor microenvironment. Ann Diagn Pathol (2017) 30:36–41. doi: 10.1016/j.anndiagpath.2017.05.013
55. Lee TK, Poon RTP, Man K, Guan X, Ma S, Liu XB, et al. Fascin over-expression is associated with aggressiveness of oral squamous cell carcinoma. Cancer Lett (2007) 254(2):308–15. doi: 10.1016/j.canlet.2007.03.017
56. Chen SF, Yang SF, Li JW, Nieh PC, Lin SY, Fu E, et al. Expression of fascin in oral and oropharyngeal squamous cell carcinomas has prognostic significance - a tissue microarray study of 129 cases. Histopathology. (2007) 51(2):173–83. doi: 10.1111/j.1365-2559.2007.02755.x
57. Alam H, Bhate AV, Gangadaran P, Sawant SS, Salot S, Sehgal L, et al. Fascin overexpression promotes neoplastic progression in oral squamous cell carcinoma. BMC Cancer. (2012) 12(1):32. doi: 10.1186/1471-2407-12-32
58. Zhao D, Zhang T, Hou XM, Ling XL. Knockdown of fascin-1 expression suppresses cell migration and invasion of non-small cell lung cancer by regulating the MAPK pathway. Biochem Biophys Res Commun (2018) 497(2):694–9. doi: 10.1016/j.bbrc.2018.02.134
59. Han S, Huang J, Liu B, Xing B, Bordeleau F, Reinhart-King CA, et al. Improving fascin inhibitors to block tumor cell migration and metastasis. Mol Oncol (2016) 10(7):966–80. doi: 10.1016/j.molonc.2016.03.006
60. Lin S, Li Y, Wang D, Huang C, Marino D, Bollt O, et al. Fascin promotes lung cancer growth and metastasis by enhancing glycolysis and PFKFB3 expression. Cancer Lett (2021) 518:230–42. doi: 10.1016/j.canlet.2021.07.025
61. Ciuffreda L, Incani UC, Steelman LS, Abrams SL, Falcone I, Curatolo AD, et al. Signaling intermediates (MAPK and PI3K) as therapeutic targets in NSCLC. Curr Pharm Des (2014) 20(24):3944–57. doi: 10.2174/13816128113196660763
62. Buck M, Poli V, Hunter T, Chojkier M. C/EBPbeta phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol Cell (2001) 8(4):807–16. doi: 10.1016/S1097-2765(01)00374-4
63. Kang S, Chen J. Targeting RSK2 in human malignancies. Expert Opin Ther Targets. (2011) 15(1):11–20. doi: 10.1517/14728222.2010.531013
64. Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol (2008) 9(10):747–58. doi: 10.1038/nrm2509
65. Abramovitch R, Tavor E, Jacob-Hirsch J, Zeira E, Amariglio N, Pappo O, et al. A pivotal role of cyclic AMP-responsive element binding protein in tumor progression. Cancer Res (Chicago Ill.). (2004) 64(4):1338–46. doi: 10.1158/0008-5472.CAN-03-2089
66. Jiang H, Chen SS, Yang J, Chen J, He B, Zhu LH, et al. CREB-binding protein silencing inhibits thrombin-induced endothelial progenitor cells angiogenesis. Mol Biol Rep (2012) 39(3):2773–9. doi: 10.1007/s11033-011-1035-4
67. Li D, Jin L, Alesi GN, Kim YM, Fan J, Seo JH, et al. The prometastatic ribosomal S6 kinase 2-cAMP response element-binding protein (RSK2-CREB) signaling pathway up-regulates the actin-binding protein fascin-1 to promote tumor metastasis. J Biol Chem (2013) 288(45):32528–38. doi: 10.1074/jbc.M113.500561
68. Sun J, He H, Xiong Y, Lu S, Shen J, Cheng A, et al. Fascin protein is critical for transforming growth factor β protein-induced invasion and filopodia formation in spindle-shaped tumor cells. J Biol Chem (2011) 286(45):38865–75. doi: 10.1074/jbc.M111.270413
69. Liang H, Gu M, Yang C, Wang H, Wen X, Zhou Q. Sevoflurane inhibits invasion and migration of lung cancer cells by inactivating the p38 MAPK signaling pathway. J Anesth (2012) 26(3):381–92. doi: 10.1007/s00540-011-1317-y
70. Liang H, Yang CX, Zhang B, Wang HB, Liu HZ, Lai XH, et al. Sevoflurane suppresses hypoxia-induced growth and metastasis of lung cancer cells via inhibiting hypoxia-inducible factor-1α. J Anesth (2015) 29(6):821–30. doi: 10.1007/s00540-015-2035-7
71. Ntantie E, Allen MJ, Fletcher J, Nkembo AT, Lamango NS, Ikpatt OF. Suppression of focal adhesion formation may account for the suppression of cell migration, invasion and growth of non-small cell lung cancer cells following treatment with polyisoprenylated cysteinyl amide inhibitors. Oncotarget. (2018) 9(40):25781–95. doi: 10.18632/oncotarget.25372
72. Jiang L, Wang P, Chen H. Overexpression of FOXM1 is associated with metastases of nasopharyngeal carcinoma. Upsala J Med Sci (2014) 119(4):324–32. doi: 10.3109/03009734.2014.960053
73. Zhang N, Nan A, Chen L, Li X, Jia Y, Qiu M, et al. Circular RNA circSATB2 promotes progression of non-small cell lung cancer cells. Mol Cancer. (2020) 19(1). doi: 10.1186/s12943-020-01221-6
74. Zhang Y, Lin Q. MicroRNA-145 inhibits migration and invasion by down-regulating FSCN1 in lung cancer. Int J Clin Exp Med (2015) 8(6):8794–802. doi: 10.1002/jbt.21771
75. Zhang Y, Yang X, Wu H, Zhou W, Liu Z. MicroRNA-145 inhibits migration and invasion via inhibition of fascin 1 protein expression in non-small-cell lung cancer cells. Mol Med Rep (2015) 12(4):6193–8. doi: 10.3892/mmr.2015.4163
76. Xiao P, Liu W, Zhou H. miR-200b inhibits migration and invasion in non-small cell lung cancer cells via targeting FSCN1. Mol Med Rep (2016) 14(2):1835–40. doi: 10.3892/mmr.2016.5421
77. Cattaruzza MS, Maisonneuve P, Boyle P. Epidemiology of laryngeal cancer. Eur J Cancer Part B: Oral Oncol (1996) 32(5):293–305. doi: 10.1016/0964-1955(96)00002-4
78. Gao X, Wu DH. [Fascin expression in human epithelial tumors and its clinical significance]. Nan Fang Yi Ke Da Xue Xue Bao. (2008) 28(6):953–5.
79. Gao W, Wang BQ, Zhang CM, Wen SX, Chen GG, Huangfu H, et al. Expressions of key molecules affiliated cytoskeleton in laryngeal squamous cell carcinoma and their implications for prognosis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2012) 47(10):841–7.
80. Öztürk Ç, Paşaoğlu HE, Emre F, Tetikkurt ÜS, Şentürk Ege T. Do immunohistochemical studies have a role in predicting prognosis of laryngeal squamous cell carcinomas? CD44 and fascin experience. Acta bio-medica Atenei Parmensis. (2022) 92(6):e2021309. doi: 10.23750/abm.v92i6.10432
81. Zon J, Chen F, Wu J, Li W, Lou L, Liu S, et al. [The cytoskeleton and immigration of laryngeal squamous carcinoma cell affected by fascin-1 expression]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. (2010) 27(5):1034–8. dio: 10.3788/gzxb20103905.0855
82. Gao W, An C, Xue X, Zheng X, Niu M, Zhang Y, et al. Mass spectrometric analysis identifies AIMP1 and LTA4H as FSCN1-binding proteins in laryngeal squamous cell carcinoma. Proteomics (2019) 19:e1900059. doi: 10.1002/pmic.201900059
83. Rodrigues PC, Sawazaki-Calone I, Ervolino DOC, Soares MC, Dourado MR, Cervigne NK, et al. Fascin promotes migration and invasion and is a prognostic marker for oral squamous cell carcinoma. Oncotarget. (2017) 8(43):74736–54. doi: 10.18632/oncotarget.20360
84. Lee MK, Park JH, Gi SH, Hwang YS. IL-1beta induces fascin expression and increases cancer invasion. Anticancer Res (2018) 38(11):6127–32. doi: 10.21873/anticanres.12964
85. Chen SF, Lin CY, Chang YC, Li JW, Fu E, Chang FN, et al. Effects of small interfering RNAs targeting fascin on gene expression in oral cancer cells. J Oral Pathol Med (2009) 38(9):722–30. doi: 10.1111/j.1600-0714.2009.00769.x
86. Domingueti CB, Castilho D, de Oliveira CE, Janini J, González-Arriagada WA, Salo T, et al. Eukaryotic translation elongation factor 1δ, n-terminal propeptide of type I collagen and cancer-associated fibroblasts are prognostic markers of oral squamous cell carcinoma patients. Oral Surg Oral Med Oral Pathol Oral Radiol (2020) 130(6):700–7. doi: 10.1016/j.oooo.2020.09.003
87. Chang HW, Chow V, Lam KY, Wei WI, Yuen A. Loss of e-cadherin expression resulting from promoter hypermethylation in oral tongue carcinoma and its prognostic significance. Cancer-Am Cancer Soc (2002) 94(2):386–92. doi: 10.1002/cncr.10211
88. Lee MK, Park JH, Gi SH, Hwang YS. Proteases are modulated by fascin in oral cancer invasion. J Cancer Prev (2018) 23(3):141–6. doi: 10.15430/JCP.2018.23.3.141
89. Fillies T, Werkmeister R, Packeisen J, Brandt B, Morin P, Weingart D, et al. Cytokeratin 8/18 expression indicates a poor prognosis in squamous cell carcinomas of the oral cavity. BMC Cancer. (2006) 6:10. doi: 10.1186/1471-2407-6-10
90. Vaidya MM, Borges AM, Pradhan SA, Bhisey AN. Cytokeratin expression in squamous cell carcinomas of the tongue and alveolar mucosa. European journal of cancer. Part B Oral Oncol (1996) 32(5):333–6. doi: 10.1016/0964-1955(96)00012-7
91. Schaafsma HE, van der Velden LA, Manni JJ, Peters H, Link M, Rutter DJ, et al. Increased expression of cytokeratins 8, 18 and vimentin in the invasion front of mucosal squamous cell carcinoma. J Pathol (1993) 170(1):77–86. doi: 10.1002/path.1711700113
92. Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol (2008) 129(6):705–33. doi: 10.1007/s00418-008-0435-6
93. Alam H, Kundu ST, Dalal SN, Vaidya MM. Loss of keratins 8 and 18 leads to alterations in 6 4-integrin-mediated signalling and decreased neoplastic progression in an oral-tumour-derived cell line. J Cell Sci (2011) 124(12):2096–106. doi: 10.1242/jcs.073585
94. Chen YP, Chan A, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. (2019) 394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0
95. Tang F, Zou F, Peng Z, Huang D, Wu Y, Chen Y, et al. N,N'-dinitrosopiperazine-mediated ezrin protein phosphorylation via activation of rho kinase and protein kinase c is involved in metastasis of nasopharyngeal carcinoma 6-10B cells. J Biol Chem (2011) 286(42):36956–67. doi: 10.1074/jbc.M111.259234
96. Liu Q, Han A, You S, Yang Q, Liang Y, Dong Y. The association of genomic variation of Epstein-Barr virus BamHI f fragment with the proliferation of nasopharyngeal carcinoma. APMIS. (2010) 118(9):657–64. doi: 10.1111/j.1600-0463.2010.02642.x
97. Krikelis D, Bobos M, Karayannopoulou G, Resiga L, Chrysafi S, Samantas E, et al. Expression profiling of 21 biomolecules in locally advanced nasopharyngeal carcinomas of Caucasian patients. BMC Clin Pathol (2013) 13:1. doi: 10.1186/1472-6890-13-1
98. Liu QY, Han AJ, You SY, Dong Y, Yang QX, Wu JH, et al. [Correlation of Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) to fascin and phosphorylated Stat3 in nasopharyngeal carcinoma]. Ai Zheng. (2008) 27(10):1070–6. doi: 10.1016/j.coi.2007.11.005
99. Wang IC, Chen YJ, Hughes D, Petrovic V, Major ML, Park HJ, et al. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol (2005) 25(24):10875–94. doi: 10.1128/MCB.25.24.10875-10894.2005
100. Leung TW, Lin SS, Tsang AC, Tong CS, Ching JC, Leung WY, et al. Over-expression of FoxM1 stimulates cyclin B1 expression. FEBS Lett (2001) 507(1):59–66. doi: 10.1016/S0014-5793(01)02915-5
101. Wang X, Kiyokawa H, Dennewitz MB, Costa RH. The forkhead box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc Natl Acad Sci USA. (2002) 99(26):16881–6. doi: 10.1073/pnas.252570299
102. Li Q, Zhang N, Jia Z, Le X, Dai B, Wei D, et al. Critical role and regulation of transcription factor FoxM1 in human gastric cancer angiogenesis and progression. Cancer Res (2009) 69(8):3501–9. doi: 10.1158/0008-5472.CAN-08-3045
103. Zeng J, Wang L, Li Q, Li W, Björkholm M, Jia J, et al. FoxM1 is up-regulated in gastric cancer and its inhibition leads to cellular senescence, partially dependent on p27kip1. J pathol (2009) 218(4):419–27. doi: 10.1002/path.2530
104. Chan DW, Yu SY, Chiu PM, Yao KM, Liu VW, Cheung AN, et al. Over-expression of FOXM1 transcription factor is associated with cervical cancer progression and pathogenesis. J Pathol (2008) 215(3):245–52. doi: 10.1002/path.2355
105. Gialmanidis IP, Bravou V, Amanetopoulou SG, Varakis J, Kourea H, Papadaki H. Overexpression of hedgehog pathway molecules and FOXM1 in non-small cell lung carcinomas. Lung Cancer. (2009) 66(1):64–74. doi: 10.1016/j.lungcan.2009.01.007
106. Bektas N, Haaf A, Veeck J, Wild PJ, Luscher-Firzlaff J, Hartmann A, et al. Tight correlation between expression of the forkhead transcription factor FOXM1 and HER2 in human breast cancer. BMC Cancer. (2008) 8:42. doi: 10.1186/1471-2407-8-42
107. Hegde NS, Sanders DA, Rodriguez R, Balasubramanian S. The transcription factor FOXM1 is a cellular target of the natural product thiostrepton. Nat Chem (2011) 3(9):725–31. doi: 10.1038/nchem.1114
108. Li YQ, Lu JH, Bao XM, Wang XF, Wu JH, Hong WQ. MiR-24 functions as a tumor suppressor in nasopharyngeal carcinoma through targeting FSCN1. J Exp Clin Cancer Res (2015) 34:130. doi: 10.1186/s13046-015-0242-6
109. Li YQ, He QM, Ren XY, Tang XR, Xu YF, Wen X, et al. MiR-145 inhibits metastasis by targeting fascin actin-bundling protein 1 in nasopharyngeal carcinoma. PloS One (2015) 10(3):e122228. doi: 10.1371/journal.pone.0122228
110. Chen L, Yang S, Jakoncic J, Zhang JJ, Huang X. Migrastatin analogues target fascin to block tumour metastasis. Nature. (2010) 464(7291):1062–6. doi: 10.1038/nature08978
111. Alburquerque-González B, Bernabé-García M, Montoro-García S, Bernabé-García Á, Rodrigues PC, Ruiz Sanz J, et al. New role of the antidepressant imipramine as a Fascin1 inhibitor in colorectal cancer cells. Exp Mol Med (2020) 52(2):281–92. doi: 10.1038/s12276-020-0389-x
112. Montoro-García S, Alburquerque-González B, Bernabé-García Á, Bernabé-García M, Rodrigues PC, Den-Haan H, et al. Novel anti-invasive properties of a Fascin1 inhibitor on colorectal cancer cells. J Mol Med (2020) 98(3):383–94. doi: 10.1007/s00109-020-01877-z
113. Chung V, Jhaveri KL, Von Hoff DD, Huang X, Garmey EG, Zhang J, et al. Phase 1A clinical trial of the first-in-class fascin inhibitor NP-G2-044 evaluating safety and anti-tumor activity in patients with advanced and metastatic solid tumors. J Clin Oncol (2021) 39(15_suppl):2548. doi: 10.1200/JCO.2021.39.15_suppl.2548
114. Wang X, Shi L, Deng Y, Qu M, Mao S, Xu L, et al. Inhibition of leucine aminopeptidase 3 suppresses invasion of ovarian cancer cells through down-regulation of fascin and MMP-2/9. Eur J Pharmacol (2015) 768:116–22. doi: 10.1016/j.ejphar.2015.10.039
115. Feng Y, Zhu J, Ou C, Deng Z, Chen M, Huang W, et al. MicroRNA-145 inhibits tumour growth and metastasis in colorectal cancer by targeting fascin-1. Brit J Cancer. (2014) 110(9):2300–9. doi: 10.1038/bjc.2014.122
116. Zhao H, Kang X, Xia X, Wo L, Gu X, Hu Y, et al. miR-145 suppresses breast cancer cell migration by targeting FSCN-1 and inhibiting epithelial-mesenchymal transition. Am J Transl Res (2016) 8(7):3106–14.
117. Ma L, Li L. miR-145 contributes to the progression of cervical carcinoma by directly regulating FSCN1. Cell Transplant. (2019) 28(9-10):1299–305. doi: 10.1177/0963689719861063
118. Reda El Sayed S, Cristante J, Guyon L, Denis J, Chabre O, Cherradi N. MicroRNA therapeutics in cancer: Current advances and challenges. Cancers. (2021) 13(11):2680. doi: 10.3390/cancers13112680
119. Lindow M, Kauppinen S. Discovering the first microRNA-targeted drug. J Cell Biol (2012) 199(3):407–12. doi: 10.1083/jcb.201208082
120. van der Ree MH, van der Meer AJ, van Nuenen AC, de Bruijne J, Ottosen S, Janssen HL, et al. Miravirsen dosing in chronic hepatitis c patients results in decreased microRNA-122 levels without affecting other microRNAs in plasma. Aliment Pharm Ther (2016) 43(1):102–13. doi: 10.1111/apt.13432
121. Beg MS, Brenner AJ, Sachdev J, Borad M, Kang Y, Stoudemire J, et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drug (2017) 35(2):180–8. doi: 10.1007/s10637-016-0407-y
Keywords: fascin-1, respiratory related cancers, pathogenesis, diagnosis, biomarker, treatment
Citation: Zhang N, Gao Y, Bian Q, Wang Q, Shi Y, Zhao Z and Yu H (2022) The role of fascin-1 in the pathogenesis, diagnosis and management of respiratory related cancers. Front. Oncol. 12:948110. doi: 10.3389/fonc.2022.948110
Received: 19 May 2022; Accepted: 25 July 2022;
Published: 11 August 2022.
Edited by:
Giuseppe Giaccone, Cornell University, United StatesReviewed by:
Chunxia Su, Shanghai Pulmonary Hospital, ChinaVaishali Aggarwal, School of Medicine, University of Pittsburgh, United States
Copyright © 2022 Zhang, Gao, Bian, Wang, Shi, Zhao and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Honglian Yu, yuhonglian@mail.jnmc.edu.cn
 Naibin Zhang
Naibin Zhang Yankun Gao1
Yankun Gao1 Honglian Yu
Honglian Yu