
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 10 August 2022
Sec. Head and Neck Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.945820
Background: Although many studies have shown the predictive value of the high neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) for various cancers, there are conflicting reports regarding their role in laryngeal cancer. This study aimed to evaluate the relationship between high NLR/PLR and laryngeal cancer prognosis with the help of meta-analysis.
Methods: PubMed, Embase and other databases were used to search relevant studies. The pooled hazard ratio (HR) and 95% confidence interval (CI) were calculated using either the random-effect-model or fixed-effect model. Sensitivity analyses and subgroups were used to explore potential sources of heterogeneity. Publication bias was also adopted.
Result: 5716 patients from 20 studies were involved in this meta-analysis. Pooled observed survival (OS) (HR=1.70, 95%CI, 1.41-2.04, p<0.001), progression-free survival (PFS) (HR=1.81, 95%CI, 1.47-2.23, p<0.001), and disease-free survival (DFS) (HR=1.86, 95%CI, 1.45-2.38, p<0.001) showed the prediction of high NLR for poor prognosis. It also suggested that high PLR predicted poor OS (HR=1.89, 95%CI, 1.21-2.94, p<0.001).
Conclusion: This study indicated that high NLR was associated with poor OS, PFS, and DFS in laryngeal cancer patients, and high PLR was related to poor OS. Both could be potential predictors of prognosis.
Laryngeal cancer is one of the most common malignancies, accounting for about 2.4 percent of new malignancies worldwide (1, 2). In 2015, there were 16,300 new cases of laryngeal cancer and 14,500 deaths in China (3). The American Cancer Society also estimates that 12,470 new cases will be diagnosed in the United States in 2022, and 3,820 people will die of laryngeal cancer (4). Despite the continuous progress of radiation therapy, transoral laser microsurgery and other laryngeal cancer treatments (5), no significant improvement in the observed survival (OS) or relative survival rate (RS) has been reported (6). Currently, clinical treatment is selected mainly according to the TNM Classification by the American Joint Commission on Cancer. However, this system focuses only on the anatomical features of tumor size and metastasis, which is not a reliable prognosis for the disease. Prognosis varies among patients with the same stage (7), while immune status (8) and pathological types (9) contribute to prognosis more. The interaction between psychological and functional changes caused by cancer treatment is also important (10). It is essential to find easily accessible new evaluation markers to assess the survival outcome of laryngeal cancer. It has been shown that inflammatory responses can promote cancer development and affect prognosis (11). Tumor-related inflammatory markers have become a focus of many researchers. Previous studies have identified that lymphocyte-to-monocyte ratio, interleukin-12 receptor, immune-inflammatory index and the interleukin-23 receptor can be prognostic indicators for laryngeal cancer (12, 13). High neutrophil/lymphocyte level and low lymphocyte level are closely related to tumor invasion and progression (14–16). As new inflammatory indicators, Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) also have good prognostic value for gastric cancer (17), esophageal cancer (18), bladder cancer (19), breast cancer (20), and ovarian cancer (21). Recent studies showed that high pre-treatment NLR/PLR is a sign of poor prognosis in laryngeal cancer (22–24), but this result has not been confirmed in some other studies (22, 25, 26). Therefore, the role of NLR/PLR in the prognosis of laryngeal cancer remains controversial. Meta-analysis is recognized as a powerful statistical tool that can break down the limitations of different individual studies. It can be used to synthesize different studies and obtain objective and scientific results. In this meta-analysis, we aimed to assess the value of NLR and PLR in the prognosis of laryngeal cancer through meta-analysis.
A systematic search of PubMed, Embase, and other databases was conducted from March 1, 1995, to March 1, 2022. Only English literature could be considered. Both Mesh terms and free-text words were used for article search. The detailed search strategy is as follows: (‘Platelet-to-lymphocyte ratio’ OR ‘platelet-lymphocyte ratio’ OR ‘PLR’ OR ‘platelet to lymphocyte ratio’ OR ‘platelet lymphocyte ratio’ OR ‘neutrophil lymphocyte ratio’ OR ‘neutrophil-to-lymphocyte ratio’ OR ‘neutrophil-lymphocyte ratio’ OR ‘NLR’ OR ‘neutrophil to lymphocyte ratio’ OR ‘neutrophil lymphocyte ratio’) AND (‘Laryngeal Neoplasm’ OR ‘Larynx Neoplasms’ OR ‘Larynx Neoplasm’ OR ‘Cancer of Larynx’ OR ‘Larynx Cancers’ OR ‘Laryngeal Cancer’ OR ‘Laryngeal Cancers’ OR ‘Larynx Cancer’ OR ‘Cancer of the Larynx’ OR ‘Laryngeal Neoplasms’). All reference lists for candidate articles were searched independently by two authors (Xianyang Hu and Tengfei Tian). In case of disagreement, the decision is left to the third author (Qin Sun).
Eligibility criteria are as follows: (1) Studies explored the relationship between pre-treatment NLR or PLR and the prognosis of patients with laryngeal cancer. (2) PLR and NLR were measured by serum-based methods. (3) Studies used COX proportional risk analysis to calculate hazard ratios (HR) and their 95% confidence intervals (95% CI) for OS, progression-free survival (PFS), or disease-free survival (DFS), and provided specific data. (4) Score ≥6 according to the Newcastle-Ottawa Scale (NOS). Two authors independently determined whether the candidate article was selected (Xianyang Hu and Tengfei Tian), and any disagreement was resolved by the third author (Qin Sun).
Exclusion criteria are as follows: (1) Abstracts, case reports, letters, reviews, meta-analysis, system reviews, or non-clinical articles. (2) Articles completed in a language other than English. (3) Studies lacked specific data for HR, 95% CI or p values. (4) Studies data is doubtful (The table shows that high PLR is associated with a good prognosis, which is inconsistent with the description of survival curve in this paper). (5) Duplication of data between studies. Two authors independently determined whether the candidate article was dropped (Xianyang Hu and Tengfei Tian), and any disagreement was resolved by the third author
For all selected articles, we recorded the following information: the author, the year of publication, time of data collection, study region, study design, the number of patients, diagnosis method, the age of patients, tumor type, follow-up time, cut-off value of PLR and NLR, outcomes and HRs with 95%CI.
The quality of the included studies was assessed by NOS, which includes three parts: selection, comparability, and outcome assessment. Each of the three parts has a different score (selection: 0-4, comparability: 0-2, outcome assessment: 0-3). Studies with NOS ≥ 6 were considered high quality. Two authors completed this work (Xianyang Hu and Tengfei Tian).
STATA statistical software (version 12.0) was used for all statistical analyses. For the effect of NLR and PLR on prognosis, we used the pooled HR and 95%CI of OS, PFS, and DFS to assess. When studies had both univariate and multivariate analysis results, we preferred multivariate analysis. Higgins I-squared statistic and Cochran’s Q test were used to assess the heterogeneity. I2>56% or a P heterogeneity<0.1 indicated significant heterogeneity. If considerable heterogeneity existed, the random-effect model would be appropriate. Otherwise, the fixed-effect model would be used. Due to the significant heterogeneity, subgroup analysis, and sensitivity analysis were used to find the sources of heterogeneity. Also, meta-regression analysis was applied to verify the reliability of the results. Publication bias was assessed using Begg’s test and Egger’s tests. For all statistically significant analyses, a two-sided p needed to be less than 0.05.
First, 115 studies were identified through a preliminary data search. Second, by scanning titles and abstracts, sixteen studies were left out as they were non-clinical articles. Forty-five studies were removed because they were not related to the subjects. Seventeen studies were excluded because they were not associated with prognosis. One non-English study was excluded. Afterward, thirty-six articles were eligible for full-text review. Fourteen studies without performing HRs and 95%CI or P values were regarded as lacking data and removed. One article with conflicting data was excluded. Finally, twenty studies with 5716 patients were included. The flow chart shows the detailed selection process (Figure 1).
Table 1 summarizes the main characteristics of the selected studies. The meta-analysis includes twenty studies published in English from 2015 to 2021. The number of people included in the selected studies ranged from 60 to 1047. All studies were retrospective. These studies were conducted between 1990 and 2018, with sixteen studies from China and four from the United States, United Kingdom, Turkey, and Italy. Eighteen studies focused on Laryngeal Squamous Cell Carcinoma (LSCC), and two studies focused on all types of laryngeal cancers. Fifteen studies used pathology diagnosis, while five used other diagnostic methods or did not mention. All studies recorded pre-treatment NLR or PLR, and some studies reported both pre-and post-treatment data. The post-treatment data were omitted. Fewer than four studies reported the effects of NLR on cancer-specific survival, non-laryngeal cancer survival, recurrence-free survival, and locoregional recurrence-free survival. Therefore, only OS, DFS, and PFS were selected as endpoints for NLR. For the same reason, only OS was selected as the endpoint of PLR. The NOS scores of included studies varied from 6 to 8. Figure 2 shows the details of NOS scoring.
Seventeen studies explored the correlation between NLR and OS, with heterogeneity testing showing high (I2 = 72.2%, p<0.001). The combined HR (HR=1.70, 95%CI, 1.41-2.04, p<0.001) resulting from the random-effect model showed that high NLR correlates closely with poor OS (Figure 3).
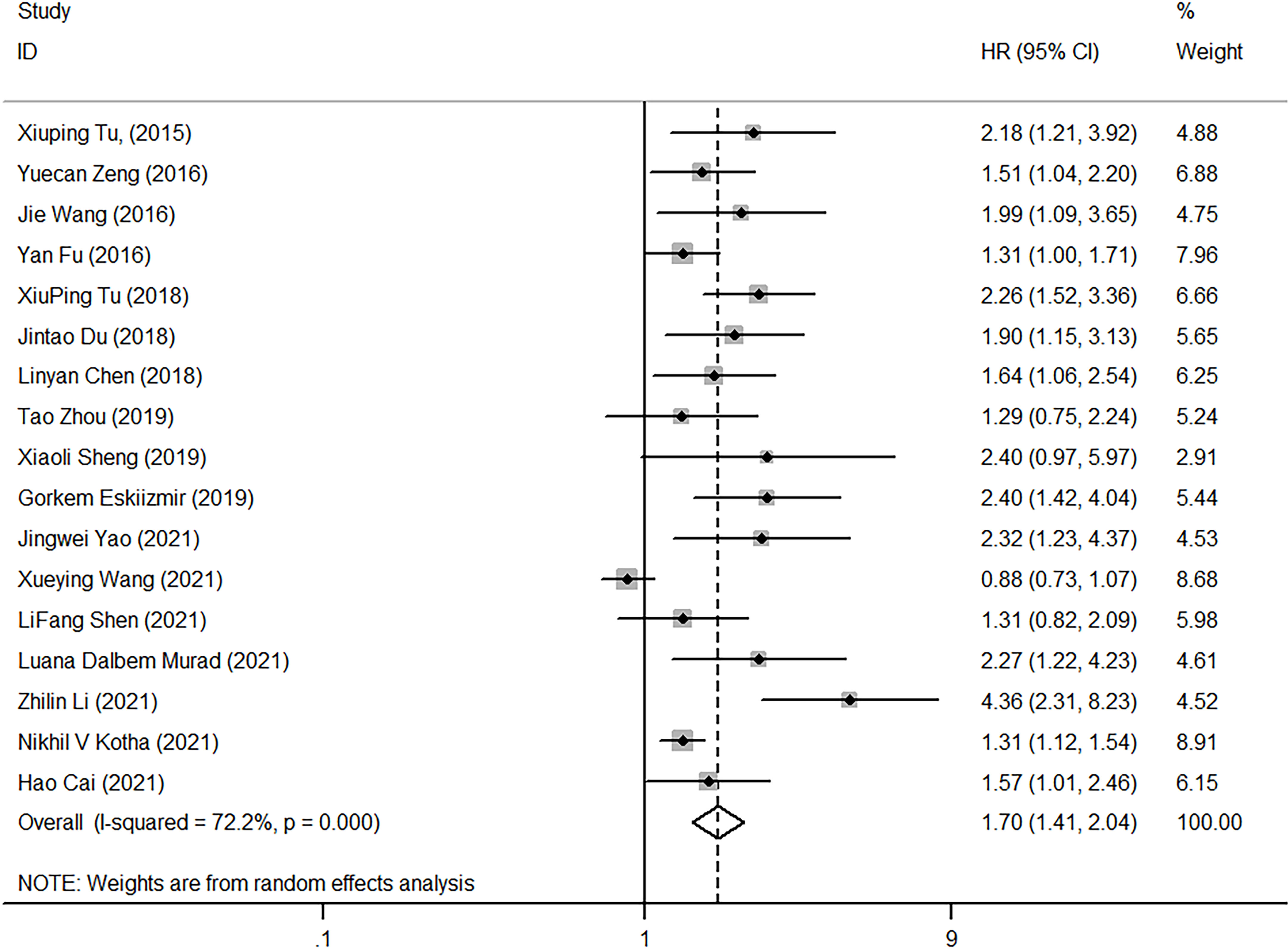
Figure 3 Forest plot of HR for the impact of NLR on OS. NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; HR, hazard ratio; CI, confidence interval.
Factors that might contribute to heterogeneity, such as diagnostic method, follow-up time, tumor type, NLR cutoff, study area, NOS score, and sample size, were selected. Subgroup analysis was then performed based on these, High NLR had predictive value for the prognosis of laryngeal cancer when NLR cut-off<3 mg/L (HR=1.73, 95%CI, 1.51-1.99), and the same when NLR cut-off≥3 mg/L (HR=1.43, 95%CI, 1.25-1.64). The heterogeneity among subgroups was not significant (cut-off<3, I2 = 43.70, p=0.052; cut-off≥3, I2 = 51.90%, p=0.101), suggesting that NLR cut-off was one of the major reasons for the high heterogeneity. Several studies also confirmed that the NLR with a cut-off of 3 is an important prognostic factor (19). As revealed in Table 2, diagnostic method (I2 = 72.20%, p < 0.001), follow-up time (I2 = 82.00%, p<0.001), tumor type (I2 = 72.20%, p<0.001), region of study (I2 = 72.20%, p<0.001), NOS score (I2 = 72.20%, p<0.001), and sample size (I2 = 72.20%, p<0.0010) also worked on the overall heterogeneity.
Regarding the effect of NLR on PFS, heterogeneity was low among the four studies (I2 = 46.9%, p=0.13). Pooled HR (HR=1.81, 95%CI, 1.47-2.23, p<0.001) calculated by the fixed-effect model showed a correlation between high NLR and poor PFS (Figure 4). Five studies reported the impact of NLR on DFS, and their heterogeneity was also mild (I2 = 0, p=0.705), so the fixed-effect model was applied. The result showed that high NLR predicted low DFS in laryngeal cancer (HR=1.86, 95%CI, 1.45-2.38, p<0.001) (Figure 5). Because of limited study information, no subgroup analysis was performed.
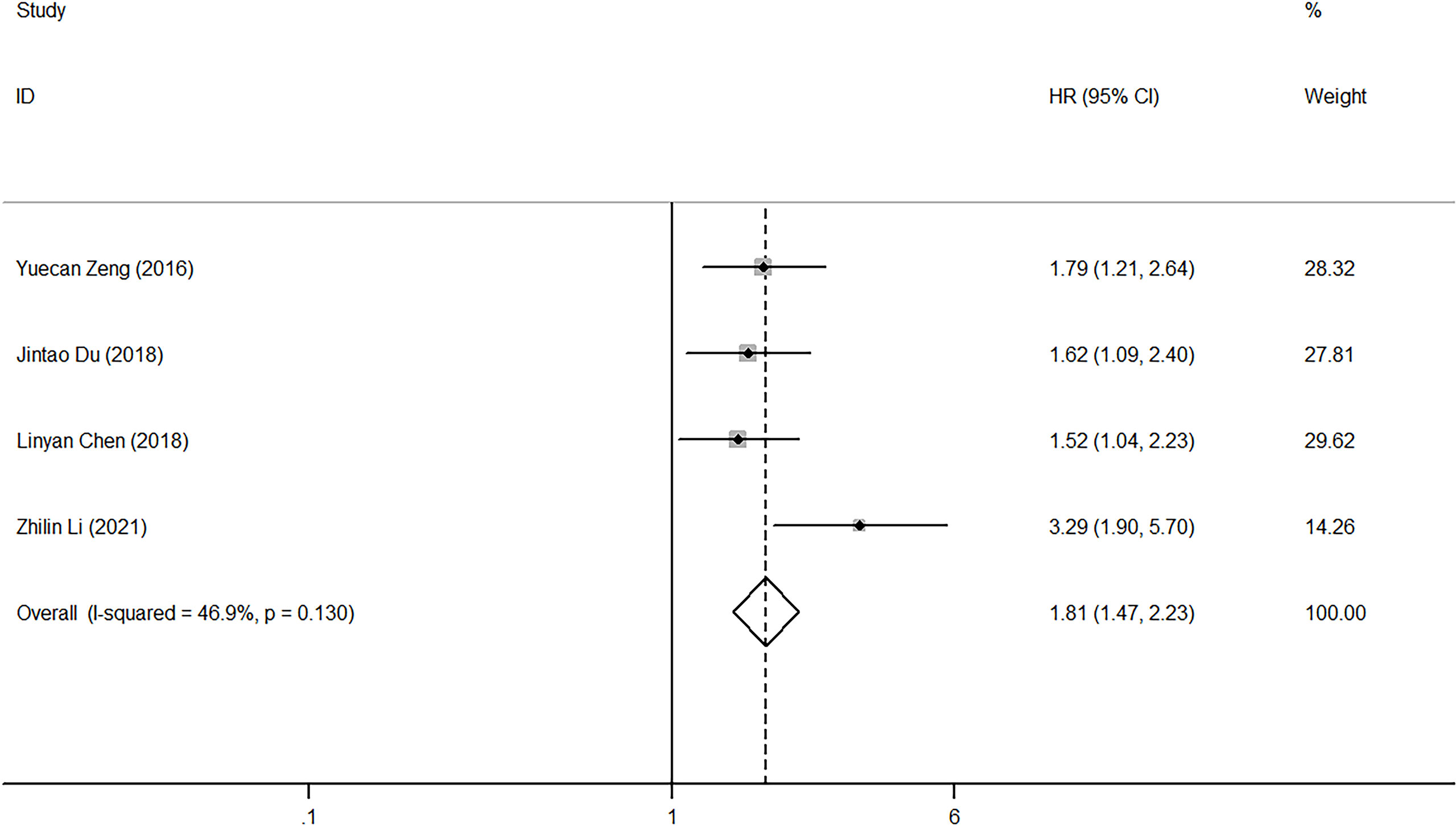
Figure 4 Forest plot of HR for the impact of NLR on PFS. NLR, neutrophil-to-lymphocyte ratio; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval.
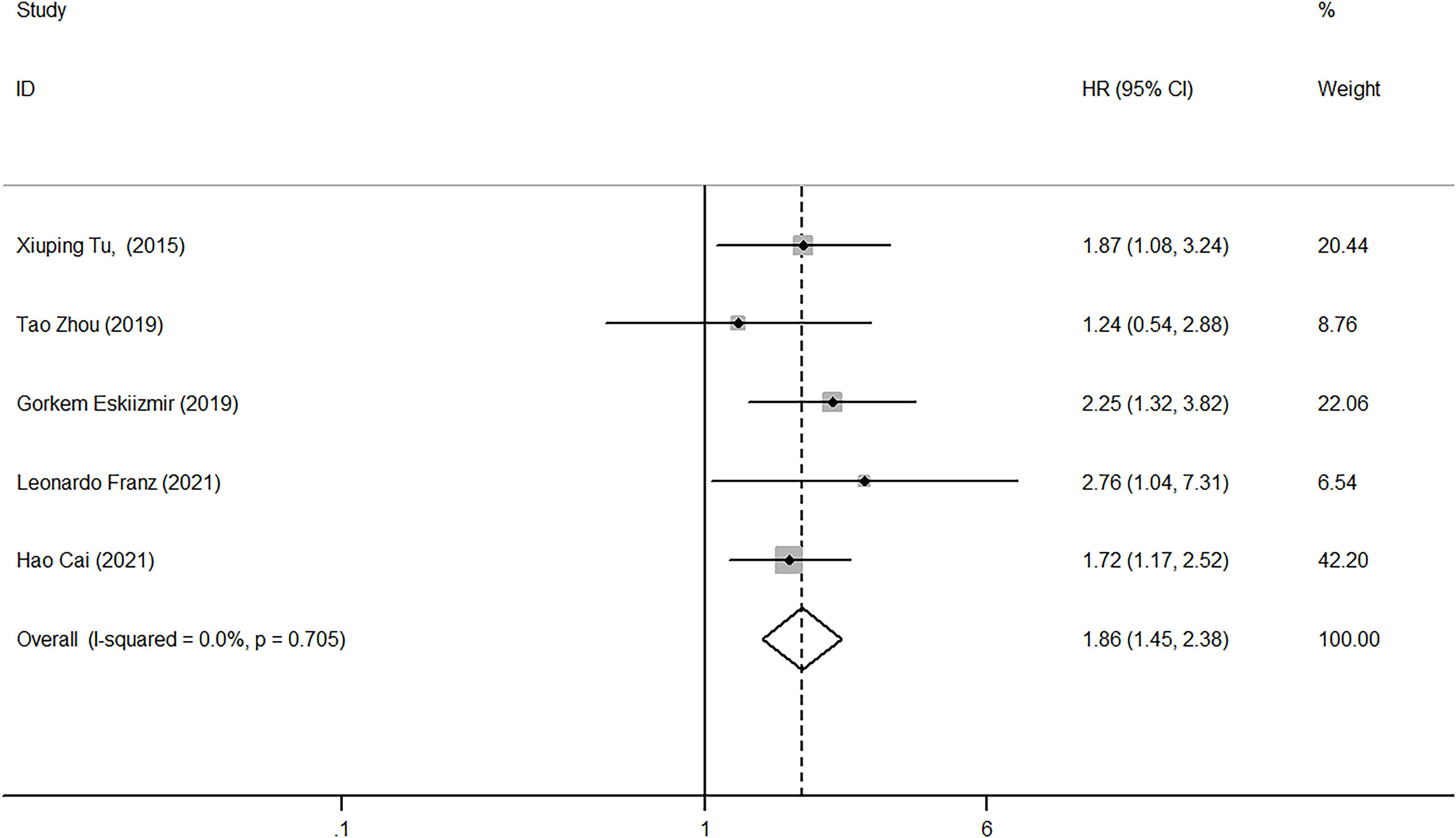
Figure 5 Forest plot of HR for the impact of NLR on DFS. NLR, neutrophil-to-lymphocyte ratio; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval.
Only 8 of the 20 studies explored the relationship between PLR and OS. The random-effect model was used to analyze the effect of PLR on OS due to the presence of high heterogeneity (I2 = 91.6%, p<0.001). The pooled HR equaled 1.89 (95%CI, 1.21-2.94, p<0.001), indicating that high PLR could suggest poor OS (Figure 6).
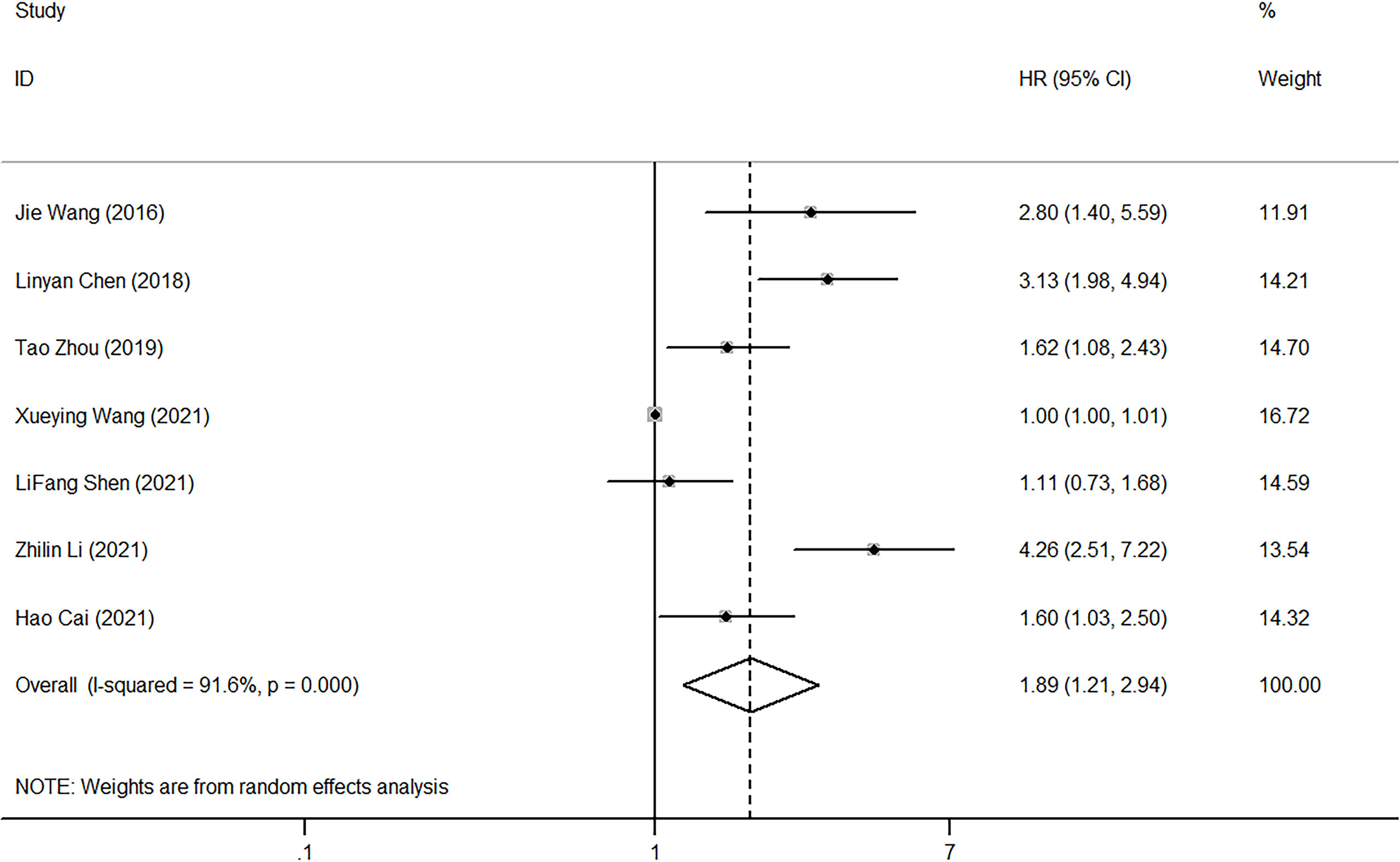
Figure 6 Forest plot of HR for the impact of PLR on OS. PLR, platelet-to-lymphocyte ratio; OS, overall survival; HR, hazard ratio; CI, confidence interval.
A subgroup analysis was performed according to the diagnostic method, NOS score, sample size, and statistical model (univariate or multivariate). As shown in Table 3, the above diagnostic methods (I2 = 91.60%, p<0.001), NOS scores (I2 = 91.60%, p<0.001), sample size (I2 = 91.60%, p<0.001), and statistical model (univariate or multivariate) (I2 = 91.60%, p<0.001) all contributed to the overall heterogeneity. Subgroup analysis by NOS score suggested that high PLR predicted poor OS when NOS score was 7 (HR=1.62, 95%CI, 1.08-2.43) or 8 (HR=2.177, 95%CI, 1.198-3.955), but not when NOS score was 6 (HR=1.59, 95%CI, 0.5-4.29). Given that there were only two studies with a NOS value of 6, this finding needs further validation. Moreover, high PLR was related to poor OS for sample sizes less than 200 (HR=2.289, 95%CI, 1.428-3.667), while this result could not be obtained when sample sizes were more than 200 (HR=1.472, 95%CI, 0.790-2.741) (Table 3).
Publication bias was assessed using Egger’s test and Begg’s test (Figure 7). When analyzing the impact of NLR on OS, publication bias was found in both testing methods (Egger’s test, p<0.001<0.05; Begg’s test, p<0.0016<0.05) (Figure 7A). Analyzing the impact of high NLR on OS revealed no publication bias by Begg’s test (p=0.072>0.05), but showed publication bias through Egger’s test (p=0.01<0.05). Due to limitations in the number of studies, NLR publication bias for PFS and DFS was not performed (Figure 7B).
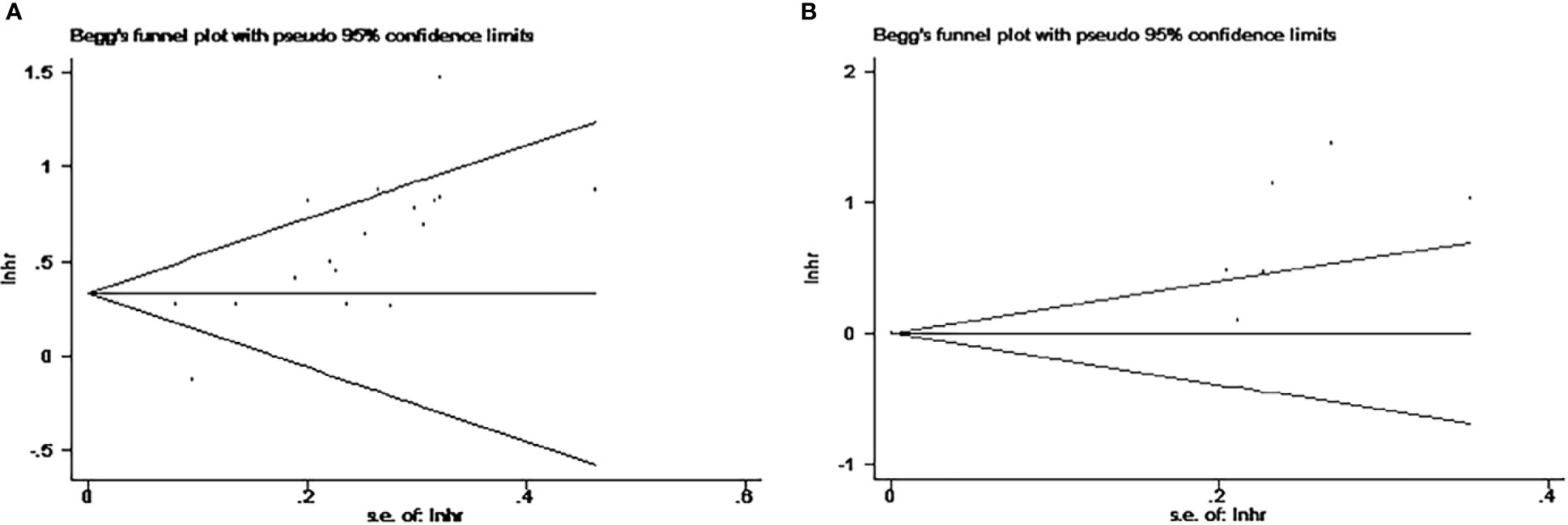
Figure 7 Begg funnel plots for publication bias. (A) Studies for the impact of NLR on OS. (B) Studies for the impact of PLR on OS. NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; OS, overall survival.
Sensitivity analysis assessed the impact of individual studies on the overall results. As shown in Figure 8, the omission of one study did not affect the outcomes, indicating the stability and reliability of our results.
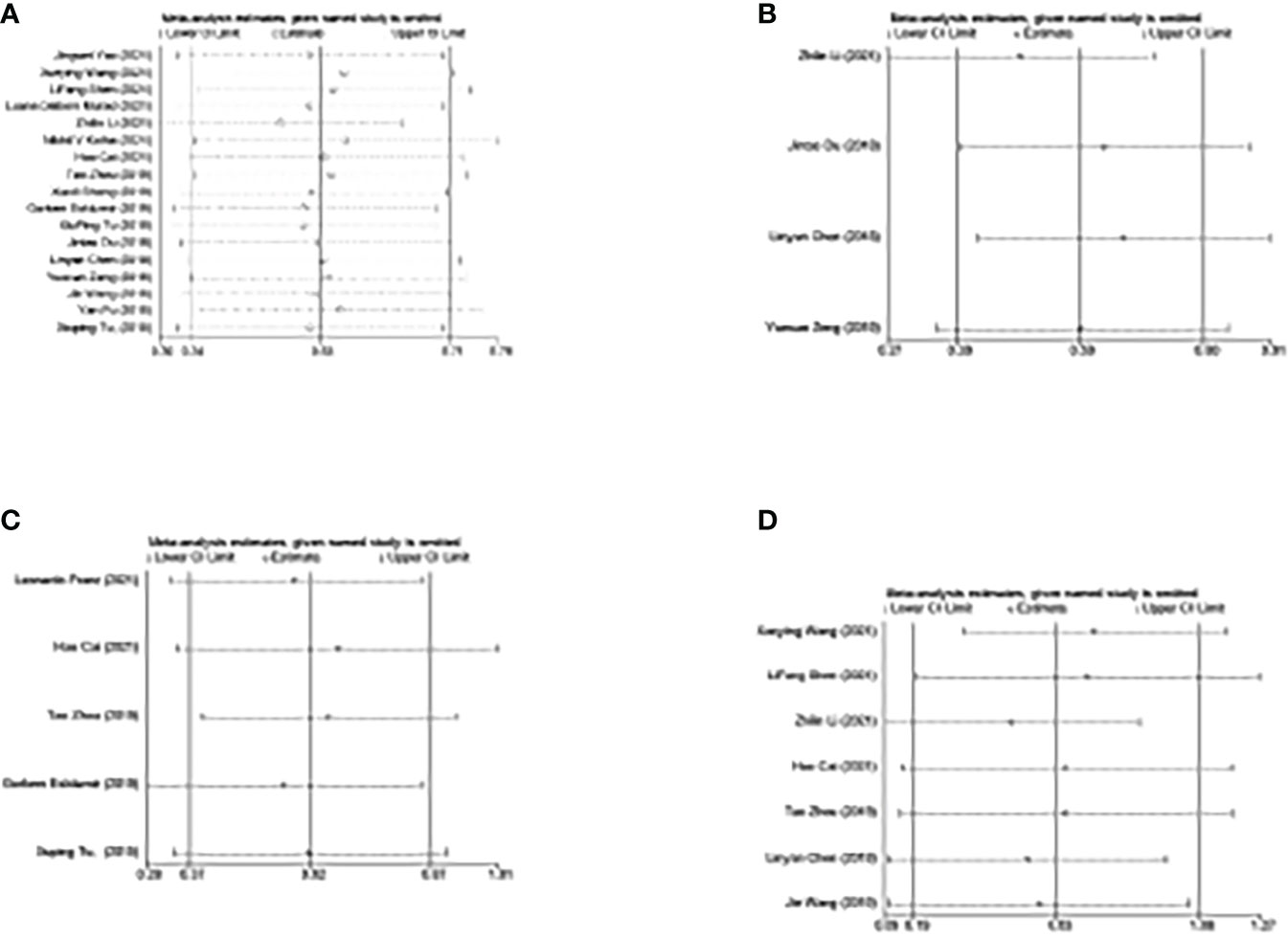
Figure 8 Sensitivity analysis of the corresponding included studies in each study. (A) Study for the impact of NLR on OS. (B) Study for the impact of NLR on PFS. (C) Study for the impact of NLR on DFS. (D) Study for the impact of PLR on OS. NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; OS, overall survival; PFS, progression-free survival; DFS, disease-free survival.
Biomarkers play an important role in the diagnosis of many diseases (43, 44), and urine biomarker monitoring has become an effective approach for molecular diagnosis and individualized drug use of bladder (45). At the same time, inflammatory response plays a vital role in the development, progression, and metastasis of cancer. And it is closely associated with death and recurrence in patients with malignancy after surgery (11, 46). Previous studies have shown that various validated indicators such as C-reactive protein (47) and interleukin-6 (48) are closely associated with the prognosis of cancers. Na Liu et al. concluded that both high NLR and PLR predicted poor prognosis in non-small cell lung cancer (49). A study by Song et al. on osteosarcoma also revealed that high NLR/PLR was associated with poor prognosis (50). And Yan Jiang et al. found the same predictive effect of NLR/PLR for esophageal cancer (51).
This study is the most comprehensive and complete meta-analysis of the predictive value of NLR and PLR in laryngeal cancer as is known to us. We included 5716 patients from 20 studies and confirmed that high NLR and PLR predicted poor OS. We also concluded that high NLR was associated with poor PFS and DFS. A recent meta-analysis published by Lin Yang et al. about the role of NLR in predicting the prognosis of head and neck squamous cell carcinoma is available (7). Compared with them, we narrowed the disease to laryngeal cancer, which is more in line with precision medicine. We also added PLR as a predictive index, making the assessment more comprehensive. In addition, Fangyu Yang et al. also conducted a meta-analysis of the relationship between NLR and laryngeal cancer (52), but we included more studies (20vs12 studies in Fangyu Yang’s) and more patients (5716vs3746 patients in Fangyu Yang’s). More studies and patients make our research more accurate and scientific. And we comprehensively analyze the impact of NLR and PLR on OS, DFS, PFS, which makes our conclusion of more clinical value. Notably, the similar results of the above two meta-analyses further support the results obtained from our study, giving more conviction to this study.
Numerous studies reported the relationship between NLR/PLR and the prognosis of laryngeal cancer, while their results are contradictory (22–26). Some studies confirm that high NLR/PLR predicts poor prognosis (22–24), but others fail to do so (22, 25, 26). Therefore, in this study, we performed a meta-analysis to accurately predict the predictive value of pre-treatment NLR/PLR in laryngeal cancer.
Our results show that high pre-treatment NLR predicted poor OS (HR=1.70, 95%CI, 1.41-2.04, p<0.001), PFS (HR=1.81, 95%CI, 1.47-2.23, p<0.001) and DFS (HR=1.86, 95%CI, 1.45-2.38, p<0.001). Similarly, high PLR was associated with poor OS (HR=1.89, 95%CI, 1.21-2.94, p<0.001). Subgroup analysis revealed that various factors such as diagnostic method, follow-up time, tumor type, study area, NOS score, cut-off values, and sample size were sources of heterogeneity. More studies and analyses are needed to explore the effects of subgroups. The reliability of our findings was verified by sensitivity analysis, but the tests showed a significant publication bias. Results of studies with significance are more likely to be reported and published than those with insignificance and invalidity (53). Based on this result, we judged that pre-treatment NLR and PLR might be vital indicators to predict the prognosis of laryngeal cancer. NLR and PLR are inexpensive and readily available blood-borne parameters, so we suggested hospitals paying attention to NLR and PLR values and providing appropriate treatment based on them.
Although many studies have reported the predictive role of PLR/NLR on cancer prognosis, the mechanisms have not been fully clarified (16). The increase of NLR and PLR levels suggests a relative decrease of lymphocytes and a relative increase of neutrophils and platelets. Two reasons for high NLR predicting poor cancer prognosis are available. One explanation is that, as a typical inflammatory cell, neutrophils represent the inflammatory state of the tumor (54–56). Neutrophils can produce vascular endothelial growth factors, hepatocyte growth factors, chemokines, intercellular adhesion molecules-1, IL-6, IL-8, transforming growth factor-β, and matrix metalloproteinases. All these secretions have been shown to promote angiogenesis, and cell growth, thus facilitating tumorigenesis and development (57–63). Evidence suggested that neutrophils could promote tumor metastasis and invasion (14, 64–66). Meanwhile, neutrophils can secrete reactive oxygen species, nitric oxide, and arginase to suppress lymphocytes and natural killer cells (67, 68). They can also inhibit T-cell proliferation through hydrogen peroxide and integrin Mac-1 (69). Therefore, high neutrophils indicate a poor prognosis of cancer. Another explanation is that lymphocytes, a fundamental component of the immune system, can reflect anti-tumor immunity (29, 70). Enough studies showed that lymphocytes could suppress the proliferation and metastasis of tumor cells and avoid the rise and spread (15, 55, 56). Therefore, a decrease in lymphocytes often indicates a poor prognosis for cancer. For PLR, the same two factors come into play. Besides the lymphocytes mentioned above, elevated platelets are also essential. Tumor cells can secrete inflammatory mediators (71), thrombopoietin, and leukemia inhibitory factor (72), which promote the differentiation of megakaryocytes to platelets (73). These secretions can also facilitate the activation of platelets (74, 75). The elevated platelets in turn release inflammatory mediators and vascular endothelial growth factor to promote tumor angiogenesis (16). Various studies reported that platelets could promote tumor growth and metastasis and fight immune responses (71, 76–78). Myriam Labelle’s study indicated that platelets induce tumor cells epithelial-mesenchymal transition through activation of TGF-β/Smad and NF-κB pathways, thereby promoting their metastasis (79). Thus, increased platelets are closely associated with tumor progression.
In short, NLR and PLR reflect the balance of tumor inflammatory response and anti-tumor immune response. Their increase indicates an inadequate immune response to tumors and the formation of an immune microenvironment conducive to tumor growth, thus enabling to prediction of the prognosis. In addition, various studies have shown that NLR is more effective in predicting cancer prognosis than PLR (39, 80). All of these coincide with the results of our study.
However, besides publication bias, our meta-analysis also had some limitations. First, significant heterogeneity existed in the HR for OS (NLR, I2 = 72.2%, p<0.001; PLR, I2 = 91.6%, p<0.001). Despite using sensitivity and subgroup analyses, it is not easy to accurately trace the source of heterogeneity. Second, all included studies were retrospective. Therefore, some prospective randomized studies were necessary to confirm our results. Third, when analyzing the impact of NLR on PFS/DFS, there are fewer eligible papers. The same applied when analyzing the relationship between PLR and OS. And we limited the language of publication to English so that we might have missed a lot of high-quality studies.
Our results indicated that high NLR and PLR before treatment suggested poor prognosis in patients with laryngeal cancer. As inexpensive and easily accessible markers, NLR and PLR have the potential to become indicators to guide clinical treatment. In the future, further confirmation of our findings through numerous prospective studies is needed.
XH wrote the manuscript with contributions from all authors. TT collected data. QS performed the analysis. WJ conceived and designed the study. All authors critically revised and approved the manuscript.
This work was supported by National Natural Science Foundation of China Grant 81725004.
We thank all the authors of the included studies.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lin HW, Bhattacharyya N. Staging and survival analysis for nonsquamous cell carcinomas of the larynx. Laryngoscope. (2008) 118(6):1003–13. doi: 10.1097/MLG.0b013e3181671b3d
2. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin (2005) 55(2):74–108. doi: 10.3322/canjclin.55.2.74
3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
4. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
5. Baird BJ, Sung CK, Beadle BM, Divi V. Treatment of early-stage laryngeal cancer: A comparison of treatment options. Oral Oncol (2018) 87:8–16. doi: 10.1016/j.oraloncology.2018.09.012
6. Li MM, Zhao S, Eskander A, Rygalski C, Brock G, Parikh AS, et al. Stage migration and survival trends in laryngeal cancer. Ann Surg Oncol (2021) 28(12):7300–9. doi: 10.1245/s10434-021-10318-1
7. Yang L, Huang Y, Zhou L, Dai Y, Hu G. High pretreatment neutrophil-to-lymphocyte ratio as a predictor of poor survival prognosis in head and neck squamous cell carcinoma: Systematic review and meta-analysis. Head Neck (2019) 41(5):1525–35. doi: 10.1002/hed.25583
8. Wang X, Cao K, Guo E, Mao X, An C, Guo L, et al. Assessment of immune status of laryngeal squamous cell carcinoma can predict prognosis and guide treatment. Cancer Immunol Immunother (2022) 71(5):1199–220. doi: 10.1007/s00262-021-03071-7
9. Tsinias G, Nikou S, Mastronikolis N, Bravou V, Papadaki H. Expression and prognostic significance of YAP, TAZ, TEAD4 and p73 in human laryngeal cancer. Histol Histopathol (2020) 35(9):983–95. doi: 10.14670/HH-18-228
10. Maggi M, Gentilucci A, Salciccia S, Gatto A, Gentile V, Colarieti A, et al. Psychological impact of different primary treatments for prostate cancer: A critical analysis. Andrologia (2019) 51(1):e13157. doi: 10.1111/and.13157
11. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol (2014) 15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3
12. Woodley N, Rogers ADG, Turnbull K, Slim MAM, Ton T, Montgomery J, et al. Prognostic scores in laryngeal cancer. Eur Arch Otorhinolaryngol 279(7):3705–15 (2022). doi: 10.1007/s00405-021-07233-2
13. Tao Y, Tao T, Gross N, Peng X, Li Y, Huang Z, et al. Combined effect of il-12rβ2 and il-23r expression on prognosis of patients with laryngeal cancer. Cell Physiol Biochem (2018) 50(3):1041–54. doi: 10.1159/000494515
14. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer (2016) 16(7):431–46. doi: 10.1038/nrc.2016.52
15. Bastid J, Bonnefoy N, Eliaou JF, Bensussan A. Lymphocyte-derived interleukin-17A adds another brick in the wall of inflammation-induced breast carcinogenesis. Oncoimmunology (2014) 3:e28273. doi: 10.4161/onci.28273
16. Wang J, Wang S, Song X, Zeng W, Wang S, Chen F, et al. The prognostic value of systemic and local inflammation in patients with laryngeal squamous cell carcinoma. Onco Targets Ther (2016) 9:7177–85. doi: 10.2147/OTT.S113307
17. Peng X, Zeng W, Tang B, He A, Zhang M, Luo R. Utility of pretreatment blood platelet-to-lymphocyte ratio in prediction of clinical outcomes and chemosensitivity in patients with advanced gastric cancer: a meta-analysis. Med Sci Monit (2022) 28:e933449. doi: 10.12659/MSM.933449
18. Li B, Xiong F, Yi S, Wang S. Prognostic and clinicopathologic significance of neutrophil-to-lymphocyte ratio in esophageal cancer: an update meta-analysis. Technol Cancer Res Treat (2022) 21:15330338211070140. doi: 10.1177/15330338211070140
19. Ferro M, Babă DF, de Cobelli O, Musi G, Lucarelli G, Terracciano D, et al. Neutrophil percentage-to-albumin ratio predicts mortality in bladder cancer patients treated with neoadjuvant chemotherapy followed by radical cystectomy. Future Sci OA (2021) 7(7):Fso709. doi: 10.2144/fsoa-2021-0008
20. Jia W, Wu J, Jia H, Yang Y, Zhang X, Chen K, et al. The peripheral blood neutrophil-to-lymphocyte ratio is superior to the lymphocyte-to-monocyte ratio for predicting the long-term survival of triple-negative breast cancer patients. PLoS One (2015) 10(11):e0143061. doi: 10.1371/journal.pone.0143061
21. Xun L, Zhai L, Xu H. Comparison of conventional, doppler and contrast-enhanced ultrasonography in differential diagnosis of ovarian masses: a systematic review and meta-analysis. BMJ Open (2021) 11(12):e052830. doi: 10.1136/bmjopen-2021-052830
22. Cai H, Zhang ZH, Zhou YJ, Liu J, Chen HQ, Lin RY. The prognostic value of preoperative plasma fibrinogen and neutrophil-to-lymphocyte ratio in patients with laryngeal squamous cell carcinoma. Ear Nose Throat J (2021) 100(10):731–6. doi: 10.1177/0145561320920746
23. Zhou T, Yu ST, Chen WZ, Xie R, Yu JC. Pretreatment albumin globulin ratio has a superior prognostic value in laryngeal squamous cell carcinoma patients: a comparison study. J Cancer (2019) 10(3):594–601. doi: 10.7150/jca.28817
24. Tu XP, Tian T, Chen LS, Luo XN, Lu ZM, Zhang SY, et al. Prognostic values of preoperative NLR and PLR in patients with laryngeal squamous cell carcinoma. Trans Cancer Res (2018) 7(2):301–9. doi: 10.21037/tcr.2018.03.03
25. Rachidi S, Wallace K, Wrangle JM, Day TA, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. (2016) 38 Suppl 1(Suppl 1):E1068–74. doi: 10.1002/hed.24159
26. Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm (2016) 2016:6058147. doi: 10.1155/2016/6058147
27. Yao J, Wen M, Deng K, Xiong Q, Zeng R, Pan M, et al. The role of neutrophil-lymphocyte ratio (NLR) in predicting tumor prognosis and enlightenment for future treatment of tumor. Int J Clin Exp Med (2021) 14(4):1666–80.
28. Wang X, Cao K, Guo E, Mao X, An C, Guo L, et al. Assessment of immune status of laryngeal squamous cell carcinoma can predict prognosis and guide treatment. Cancer Immunol Immunother (2022) 71(5):1199–220. doi: 10.21203/rs.3.rs-673283/v1
29. Shen LF, Wang QY, Yu Q. The systemic immune-inflammation index and albumin as prognostic predictors in laryngeal carcinoma. Nutr Cancer (2021) 73(10):1916–23. doi: 10.1080/01635581.2020.1812677
30. Murad LD, Silva TQ, Schilithz AOC, Monteiro MC, Murad LB, Fialho E. Body mass index alters the predictive value of the neutrophil-to-Lymphocyte ratio and systemic inflammation response index in laryngeal squamous cell carcinoma patients. Nutr Cancer (2022) 74(4):1261–9. doi: 10.1080/01635581.2021.1952447
31. Li Z, Qu Y, Yang Y, An W, Li S, Wang B, et al. Prognostic value of the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index in patients with laryngeal squamous cell carcinoma. Clin Otolaryngol (2021) 46(2):395–405. doi: 10.1111/coa.13689
32. Kotha NV, Voora RS, Qian AS, Kumar A, Qiao EM, Stewart TF, et al. Prognostic utility of pretreatment neutrophil-lymphocyte ratio in advanced larynx cancer. Biomark Insights (2021) 16:11772719211049848. doi: 10.1177/11772719211049848
33. Franz L, Alessandrini L, Fasanaro E, Gaudioso P, Carli A, Nicolai P, et al. Prognostic impact of neutrophils-to-lymphocytes ratio (NLR), PD-L1 expression, and tumor immune microenvironment in laryngeal cancer. Ann Diagn Pathol (2021) 50:151657. doi: 10.1016/j.anndiagpath.2020.151657
34. Chen H, Song S, Zhang L, Dong W, Chen X, Zhou H. Preoperative platelet-lymphocyte ratio predicts recurrence of laryngeal squamous cell carcinoma. Future Oncol (2020) 16(6):209–17. doi: 10.2217/fon-2019-0527
35. Song S, Chen H, Dong W, Zhou H. The prognostic value of preoperative derived neutrophil-to-lymphocyte ratio in patients undergoing total laryngectomy with laryngeal carcinoma. Acta Otolaryngol (2019) 139(3):294–8. doi: 10.1080/00016489.2019.1566780
36. Sheng X, Zhang H, Ge P, Chen L, Zhang S. A retrospective study of the prognostic significance of preoperative plasma fibrinogen, mean platelet volume, and the neutrophil-to-Lymphocyte ratio in patients with laryngeal squamous cell carcinoma. Med Sci Monit (2019) 25:4527–34. doi: 10.12659/MSM.914426
37. Eskiizmir G, Uz U, Onur E, Ozyurt B, Karaca Cikrikci G, Sahin N, et al. The evaluation of pretreatment neutrophil–lymphocyte ratio and derived neutrophil–lymphocyte ratio in patients with laryngeal neoplasms. Braz J Otorhinolaryngol (2019) 85(5):578–87. doi: 10.1016/j.bjorl.2018.04.013
38. Du J, Liu J, Zhang X, Chen X, Yu R, Gu D, et al. Pre-treatment neutrophil-to-lymphocyte ratio predicts survival in patients with laryngeal cancer. Oncol Lett (2018) 15(2):1664–72. doi: 10.3892/ol.2017.7501
39. Chen L, Zeng H, Yang J, Lu Y, Zhang D, Wang J, et al. Survival and prognostic analysis of preoperative inflammatory markers in patients undergoing surgical resection for laryngeal squamous cell carcinoma. BMC Cancer (2018) 18(1):816. doi: 10.1186/s12885-018-4730-x
40. Zeng YC, Chi F, Xing R, Xue M, Wu LN, Tang MY, et al. Pre-treatment neutrophil-to-lymphocyte ratio predicts prognosis in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy. Jpn J Clin Oncol (2016) 46(2):126–31. doi: 10.1093/jjco/hyv175
41. Fu Y, Liu W, OuYang D, Yang A, Zhang Q. Preoperative neutrophil-to-lymphocyte ratio predicts long-term survival in patients undergoing total laryngectomy with advanced laryngeal squamous cell carcinoma: a single-center retrospective study. Med (Baltimore) (2016) 95(6):e2689. doi: 10.1097/MD.0000000000002689
42. Tu XP, Qiu QH, Chen LS, Luo XN, Lu ZM, Zhang SY, et al. Preoperative neutrophil-to-lymphocyte ratio is an independent prognostic marker in patients with laryngeal squamous cell carcinoma. BMC Cancer (2015) 15:743. doi: 10.1186/s12885-015-1727-6
43. Nicolazzo C, Busetto GM, Del Giudice F, Sperduti I, Giannarelli D, Gradilone A, et al. The long-term prognostic value of survivin expressing circulating tumor cells in patients with high-risk non-muscle invasive bladder cancer (NMIBC). J Cancer Res Clin Oncol (2017) 143(10):1971–6. doi: 10.1007/s00432-017-2449-8
44. Giovannone R, Busetto GM, Antonini G, De Cobelli O, Ferro M, Tricarico S, et al. Hyperhomocysteinemia as an early predictor of erectile dysfunction: international index of erectile function (iief) and penile doppler ultrasound correlation with plasma levels of homocysteine. Med (Baltimore) (2015) 94(39):e1556. doi: 10.1097/MD.0000000000001556
45. Ferro M, La Civita E, Liotti A, Cennamo M, Tortora F, Buonerba C, et al. Liquid biopsy biomarkers in urine: A route towards molecular diagnosis and personalized medicine of bladder cancer. J Pers Med (2021) 11(3):237. doi: 10.3390/jpm11030237
46. Balkwill F, Mantovani A. 2001 Inflammation and cancer: back to virchow? Lancet 357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0
47. Chen Y, Cong R, Ji C, Ruan W. The prognostic role of c-reactive protein in patients with head and neck squamous cell carcinoma: A meta-analysis. Cancer Med (2020) 9(24):9541–53. doi: 10.1002/cam4.3520
48. Rezaei F, Mohammadi H, Heydari M, Sadeghi M, Mozaffari HR, Khavid A, et al. Association between IL-8 (-251T/A) and IL-6 (-174G/C) polymorphisms and oral cancer susceptibility: A systematic review and meta-analysis. Medicina (Kaunas) (2021) 57(5):405. doi: 10.3390/medicina57050405
49. Liu N, Mao J, Tao P, Chi H, Jia W, Dong C. The relationship between NLR/PLR/LMR levels and survival prognosis in patients with non-small cell lung carcinoma treated with immune checkpoint inhibitors. Med (Baltimore) (2022) 101(3):e28617. doi: 10.1097/MD.0000000000028617
50. Song X, Zhang H, Yin F, Guo P, Yang X, Liu J, et al. Systemic inflammatory markers for predicting overall survival in patients with osteosarcoma: a systematic review and meta-analysis. Mediators Inflamm (2021) 2021:3456629. doi: 10.1155/2021/3456629
51. Jiang Y, Xu D, Song H, Qiu B, Tian D, Li Z, et al. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: a systematic review and meta-analysis. BMJ Open (2021) 11(9):e048324. doi: 10.1136/bmjopen-2020-048324
52. Yang F, Huang Q, Guan Z, Diao Q. Prognostic significance of pretreatment neutrophil-to-lymphocyte ratio in patients with laryngeal cancer: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol (2021) 278(2):417–25. doi: 10.1007/s00405-020-06337-5
53. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. (2018) 74(3):785–94. doi: 10.1111/biom.12817
54. Brigati C, Noonan DM, Albini A, Benelli R. Tumors and inflammatory infiltrates: friends or foes? Clin Exp Metastasis (2002) 19(3):247–58. doi: 10.1023/A:1015587423262
55. Rakoff-Nahoum S. Why cancer and inflammation? Yale J Biol Med (2006) 79(3-4):123–30. doi: 10.1097/00054725-200604002-00010
56. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer (2011) 105(1):93–103. doi: 10.1038/bjc.2011.189
57. McCourt M, Wang JH, Sookhai S, Redmond HP. Proinflammatory mediators stimulate neutrophil-directed angiogenesis. Arch Surg (1999) 134(12):1325–31. doi: 10.1001/archsurg.134.12.1325
58. McCourt M, Wang JH, Sookhai S, Redmond HP. Activated human neutrophils release hepatocyte growth factor/scatter factor. Eur J Surg Oncol (2001) 27(4):396–403. doi: 10.1053/ejso.2001.1133
59. Jabłońska E, Kiluk M, Markiewicz W, Piotrowski L, Grabowska Z, Jabłoński J. TNF-alpha, IL-6 and their soluble receptor serum levels and secretion by neutrophils in cancer patients. Arch Immunol Ther Exp (Warsz) (2001) 49(1):63–9.
60. Schaider H, Oka M, Bogenrieder T, Nesbit M, Satyamoorthy K, Berking C, et al. Differential response of primary and metastatic melanomas to neutrophils attracted by IL-8. Int J Cancer (2003) 103(3):335–43. doi: 10.1002/ijc.10775
61. Shamamian P, Schwartz JD, Pocock BJ, Monea S, Whiting D, Marcus SG, et al. Activation of progelatinase a (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol (2001) 189(2):197–206. doi: 10.1002/jcp.10014
62. Orellana R, Kato S, Erices R, Bravo ML, Gonzalez P, Oliva B, et al. Platelets enhance tissue factor protein and metastasis initiating cell markers, and act as chemoattractants increasing the migration of ovarian cancer cells. BMC Cancer (2015) 15:290. doi: 10.1186/s12885-015-1304-z
63. Liu S, Li N, Yu X, Xiao X, Cheng K, Hu J, et al. Expression of intercellular adhesion molecule 1 by hepatocellular carcinoma stem cells and circulating tumor cells. Gastroenterology (2013) 144(5):1031–41.e10. doi: 10.1053/j.gastro.2013.01.046
64. Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. (2003) 6(4):283–7. doi: 10.1023/B:AGEN.0000029415.62384.ba
65. Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, Komuta K, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan multinational trial organisation LC00-03. Eur J Cancer (2009) 45(11):1950–8. doi: 10.1016/j.ejca.2009.01.023
66. Lança T, Silva-Santos B. The split nature of tumor-infiltrating leukocytes: Implications for cancer surveillance and immunotherapy. Oncoimmunology. (2012) 1(5):717–25. doi: 10.4161/onci.20068
67. Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol (1985) 134(1):230–4.
68. el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. dependence on the myeloperoxidase system. J Immunol (1987) 139(7):2406–13.
69. Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through mac-1. J Clin Invest (2012) 122(1):327–36. doi: 10.1172/JCI57990
70. Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. (2001) 411(6835):380–4. doi: 10.1038/35077246
71. Alexandrakis MG, Passam FH, Moschandrea IA, Christophoridou AV, Pappa CA, Coulocheri SA, et al. Levels of serum cytokines and acute phase proteins in patients with essential and cancer-related thrombocytosis. Am J Clin Oncol (2003) 26(2):135–40. doi: 10.1097/01.COC.0000017093.79897.DE
72. Ciurea SO, Hoffman R. Cytokines for the treatment of thrombocytopenia. Semin Hematol (2007) 44(3):166–82. doi: 10.1053/j.seminhematol.2007.04.005
73. Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell (2018) 33(6):965–83. doi: 10.1016/j.ccell.2018.03.002
74. Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol (2010) 30(12):2362–7. doi: 10.1161/ATVBAHA.110.207514
75. Suzuki K, Aiura K, Ueda M, Kitajima M. The influence of platelets on the promotion of invasion by tumor cells and inhibition by antiplatelet agents. Pancreas. (2004) 29(2):132–40. doi: 10.1097/00006676-200408000-00008
76. Sharma D, Brummel-Ziedins KE, Bouchard BA, Holmes CE. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol (2014) 229(8):1005–15. doi: 10.1002/jcp.24539
77. Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell (2006) 10(5):355–62. doi: 10.1016/j.ccr.2006.10.002
78. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost (2011) 9(2):237–49. doi: 10.1111/j.1538-7836.2010.04131.x
79. Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell (2011) 20(5):576–90. doi: 10.1016/j.ccr.2011.09.009
80. Choi WJ, Cleghorn MC, Jiang H, Jackson TD, Okrainec A, Quereshy FA. Preoperative neutrophil-to-lymphocyte ratio is a better prognostic serum biomarker than platelet-to-lymphocyte ratio in patients undergoing resection for nonmetastatic colorectal cancer. Ann Surg Oncol (2015) 22(Suppl 3):S603–13. doi: 10.1245/s10434-015-4571-7
Keywords: prognosis, meta-analysis, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, laryngeal cancer
Citation: Hu X, Tian T, Sun Q and Jiang W (2022) Prognostic value of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in laryngeal cancer: What should we expect from a meta-analysis? Front. Oncol. 12:945820. doi: 10.3389/fonc.2022.945820
Received: 17 May 2022; Accepted: 08 July 2022;
Published: 10 August 2022.
Edited by:
Matteo Ferro, European Institute of Oncology (IEO), ItalyReviewed by:
Vincenzo Asero, Consultant, Rome, ItalyCopyright © 2022 Hu, Tian, Sun and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxiu Jiang, and4ZW50QGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.