- 1Department of Medical Oncology and Clinical Pharmacology, The Netherlands Cancer Institute-Antoni van Leeuwenhoek, Amsterdam, Netherlands
- 2Department of Pharmacy and Pharmacology, The Netherlands Cancer Institute-Antoni van Leeuwenhoek, Amsterdam, Netherlands
- 3Department of Pharmacology, Princess Máxima Center for Pediatric Oncology, Utrecht, Netherlands
- 4Department of Clinical Pharmacy, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands
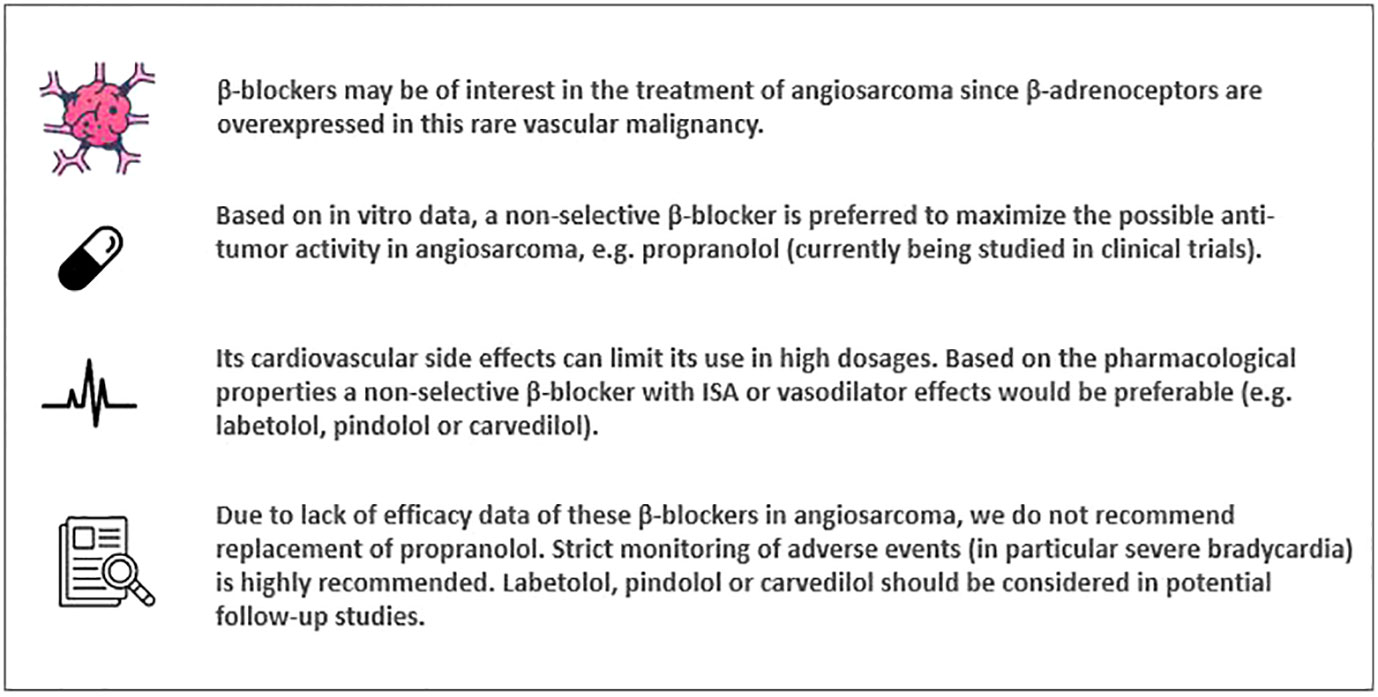
Beta-blockers are currently studied to improve therapeutic options for patients with angiosarcoma. However, most of these patients have no cardiovascular co-morbidity and it is therefore crucial to discuss the most optimal pharmacological properties of beta-blockers for this population. To maximize the possible effectiveness in angiosarcoma, the use of a non-selective beta-blocker is preferred based on in vitro data. To minimize the risk of cardiovascular adverse events a beta-blocker should ideally have intrinsic sympathomimetic activity or vasodilator effects, e.g. labetalol, pindolol or carvedilol. However, except for one case of carvedilol, only efficacy data of propranolol is available. In potential follow-up studies labetalol, pindolol or carvedilol can be considered to reduce the risk of cardiovascular adverse events.
Introduction
Beta (β)-blockers or β-adrenoceptor antagonists were first introduced in the early 1960s for the treatment of angina pectoris (1). The use of β-blockers in ischemic cardiac disease is based on the reduction of the catecholaminergic stimulation of the heart in order to improve the exercise capacity of the cardiac muscle by slowing heart rate and lowering the oxygen consumption. The anti-hypertensive and anti-arrhythmic effects of this drug class have led to its extensive use in the treatment of hypertension and cardiac arrhythmias. Other (off-label) indications for the use of β-blockers are migraine, the treatment of essential tremor, prophylaxis of esophageal variceal hemorrhage caused by portal hypertension, control of anxiety symptoms (exam anxiety and stage fright) and thyrotoxicosis.
The expression of β-adrenergic receptors has been investigated previously in both benign and malignant vascular tumors (2, 3). The registered indication of propranolol, a non-selective β-blocker, has now been extended to the treatment of infantile hemangioma requiring systemic therapy (4, 5). Since β-adrenoceptors are also overexpressed in some cancer types, such as soft tissue sarcomas, propranolol might be a drug of interest in these rare malignancies. Hence, propranolol obtained the orphan designation by the European Commission for the treatment of soft tissue sarcomas (6, 7). Furthermore, the effect of β-receptor blockade with propranolol on angiosarcoma is currently being studied in two clinical trials: The PropAngio trial (NCT04518124, study start date December 2019, status ongoing) and the PROPAN trial (NCT02732678, study start date May 2016, status unknown) (8–10). The aim of the PropAngio trial is to prospectively assess the anti-proliferative effect of neoadjuvant propranolol as monotherapy in primary, recurrent and metastatic angiosarcoma. In the PROPAN trial, propranolol is given in combination with cyclophosphamide in locally advanced or metastatic angiosarcoma (10). The usual daily dose range of propranolol for hypertension is 160 to 320 mg (5). In the PropAngio trial, propranolol is given orally in a dose-escalation schedule (40-80 mg twice daily in the first two weeks, thereafter 80 mg three times a day) until initiation of standard therapy (chemotherapy and/or surgery or radiation). Since the anti-proliferative effect of propranolol on angiosarcoma cells has been shown to be dose dependent, evaluating the effect of dose escalation in this trial is necessary (3). However, these patients have no cardiovascular co-morbidity or other indications for β-receptor blockade, making them more at risk for treatment related cardiovascular adverse events, mainly bradycardia and hypotension. These known side effects of propranolol can limit its use in high dosages in this patient population. Ideally, one should adapt a β-blocker with proven efficacy in angiosarcoma and which is characterized by minimal cardiovascular side effects. Here, we discuss the pharmacological properties of the diverse β-blockers and consider the usage of propranolol in angiosarcoma.
Pharmacological properties
β-blockers can be distinguished based on their pharmacological properties by selectivity, intrinsic sympathomimetic activity (ISA), vasodilator activity and lipophilicity (Table 1). These pharmacological properties can be of use to define treatment and to predict adverse events. Intrinsic sympathomimetic activity or partial agonist activity of β-blockers is caused by inhibition of β-receptors in the presence of agonists, such as catecholamines, and stimulation of β-receptors in rest when these agonists are absent (11). This results in less bradycardia or peripheral vasoconstriction (12). Consequently, β-blockers with ISA can be of use in patients in whom strong decline of heart rate is unwanted. A comparison between pindolol and propranolol confirms this clinical benefit of β-blockers with ISA in patients with angina pectoris (13). Of the non-selective β-blockers with a vasodilator effect, there is some evidence that carvedilol causes less bradycardia compared to other β-blockers (14). Lipophilic β-blockers can more easily cross the blood-brain barrier increasing theoretically the incidence of central nervous system adverse events (12). However, studies were unable to associate lipophilicity to the incidence of adverse events (18).
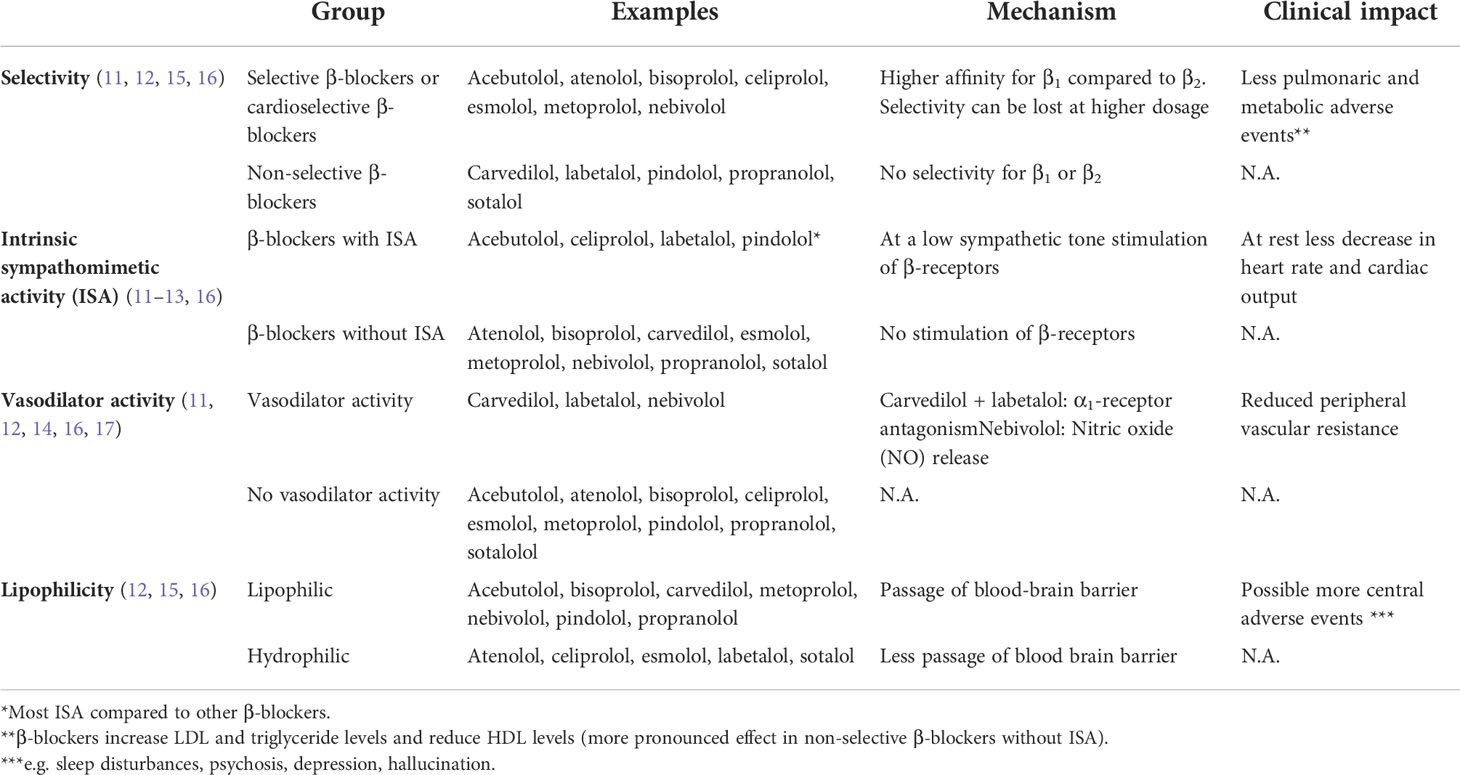
Table 1 Properties of β-blockers divided into selectivity, intrinsic sympathomimetic activity and vasodilator activity (11–17).
β1-receptors are primarily located in the heart and most adverse effects of β-blockers are a result of antagonism of β1-receptors (notably bradycardia), whereas inhibition of β2-receptors, located on vascular and bronchial smooth muscle, can lead to adverse events like constriction of airways or peripheral vasculature (11, 15, 16). Therefore, cardioselective β-blockers are better tolerated and are preferred in patients with respiratory disease (15).
Differences in pharmacological properties can theoretically lead to varying adverse events. However, in head-to-head trials, the incidence of adverse events could not be clearly differentiated among most β-blockers (18, 19). Some studies report a difference in adverse events e.g.; carvedilol showed a higher rate of dizziness compared to metoprolol and propranolol was associated with a higher overall rate of adverse events compared to pindolol (13, 17). However, no β-blocker can be distinguished with highest incidence of adverse events (19).
Use of β-blockers in angiosarcoma
Overexpression of β1-, β2- and β3-adrenoceptors in angiosarcoma cells has been demonstrated in several preclinical studies (2, 3). This finding suggests that, as in infantile hemangioma, β-blockers may also be of use in the treatment of this aggressive vascular tumor. Several mechanisms behind this anticancer effect have been suggested. Direct effects of β-blockers on tumor cells due to antagonism of β-receptors could lead to less proliferation, migration and differentiation of tumor cells (20). In addition, β-blockers can also influence tumor angiogenesis by downregulation of VEGF (2, 21–23). Furthermore, β-receptors play a role in immunostimulation and β-blockers can have effects on several immune cells (in both the adaptive and innate immune system) and on the tumor microenvironment (20, 22, 23). Several preclinical studies describe the synergy between β-blockers and chemotherapy resulting in less drug-resistance (3, 6, 21–24). Affecting the tumor microenvironment and immune system could also increase sensibility to immune checkpoint inhibitors by increasing T-cell infiltration and decreasing suppression of CD8+ cytotoxic T-cells (20, 25). Additionally the used isomer of β-blockers can be of importance. Studies on the isomers of propranolol suggest that the use of the R-isomer, which has no significant activity against the β-receptor, could lead to less side effects while maintaining efficacy. This supports a mechanism of action independent of β-blockade (24, 26). Using in vitro angiosarcoma models, it was found that the anti-proliferative effect of non-selective β-blockers was superior to cardioselective β-blockers (esmolol and atenolol) (27).
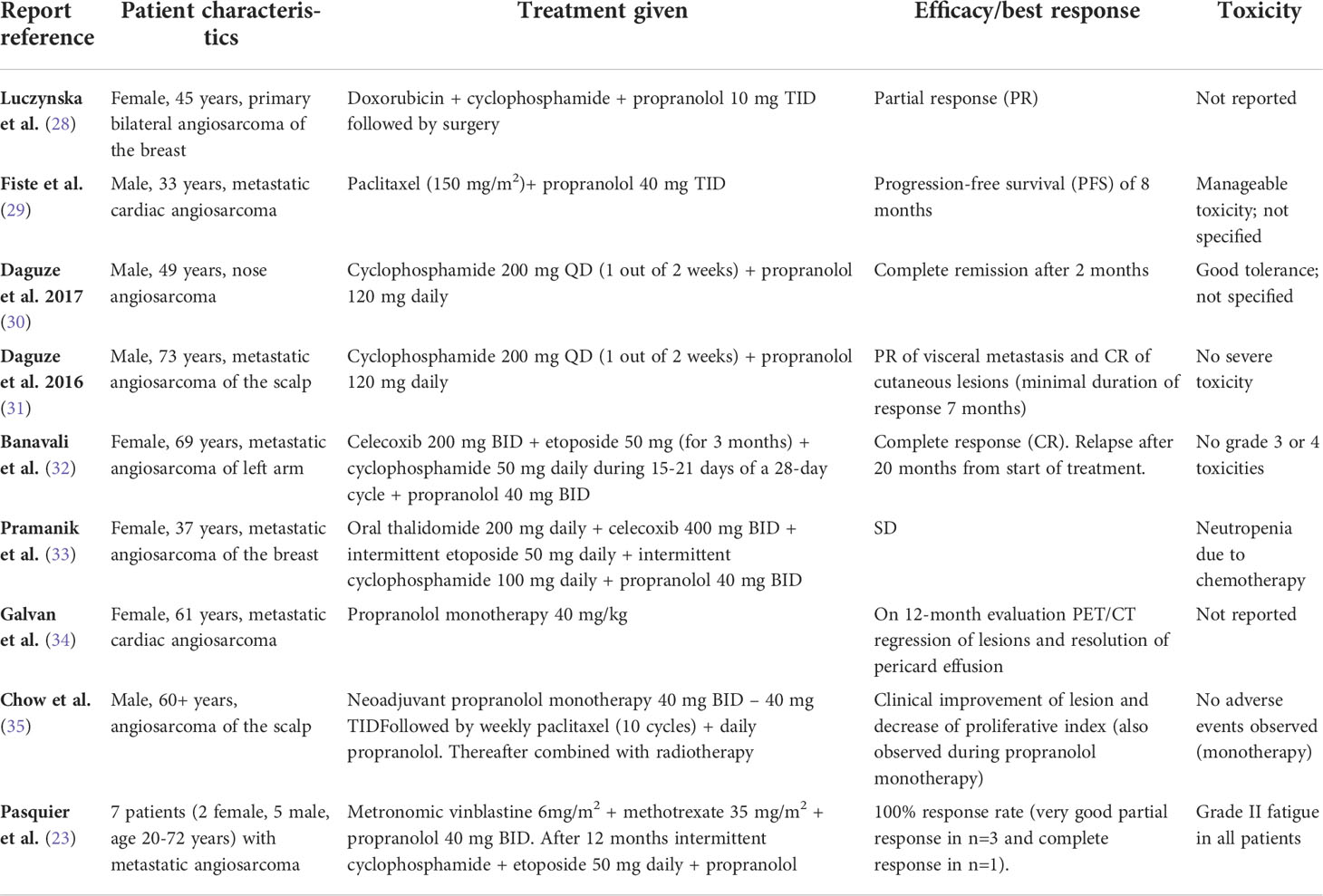
The anti-tumor activity of propranolol (as single agent and in combination with chemotherapy) in different types of angiosarcoma has been described in several case reports and case series (23, 28–35). Table 2 shows the characteristics and results of these reports. Pasquier et al. reported the results of an unpowered pilot study in 7 patients with metastatic angiosarcoma in which propranolol was administered in combination with metronomic vinblastine-based chemotherapy. A response rate of 100% was observed (16). One single arm prospective clinical trial studied the efficacy of beta-blockade with propranolol or carvedilol in metastatic angiosarcoma (27). A total of 9 patients received either propranolol 20-100 mg per day (n=8) or carvedilol 6.25 mg per day (n=1) in combination with standard treatment protocols according to physicians choice. An improvement of the median progression-free survival (PFS) and overall survival (OS) was observed compared with historical controls (PFS 9 months vs. 3-6 months, OS 36 months vs. 12 months) (27).
Discussion
The aim of this perspective was to give an overview of the efficacy and safety of different β-blockers in angiosarcoma in order to recommend the use of the most appropriate β-blocker in this patient population. In accordance with the summarized preclinical and clinical data, we hypothesize that use of a non-selective β-blocker is preferred to maximize the possible effectiveness in angiosarcoma. Propranolol has been reported to be effective as monotherapy and in combination with chemotherapy in diverse cases of angiosarcoma. This is possibly the result of a synergistic effect of this non-selective β-blocker with chemotherapy by decreasing drug-resistance of tumor cells. β-blockade is also expected to enhance the immunomodulatory effects combinations of checkpoint inhibitors due to their positive effects on T-cells. However, propranolol is known to cause (cardiovascular) side effects by β-receptor antagonism, repurposing the R-isomer of propranolol could be a strategy to decrease side effects.
Given the abovementioned pharmacological features of β-blockers, one should ideally adopt a non-selective β-blocker with ISA to lower the risk of potential severe cardiovascular events such as bradycardia in patients with angiosarcoma. β-blockers that fulfill these criteria are labetalol and pindolol. As mentioned earlier, carvedilol has no ISA, but is known to cause less bradycardia because of its mainly vasodilator effects and may, therefore, be a suitable drug. To our knowledge, these agents have never been studied in angiosarcoma (except of carvedilol which is described in one case). Therefore, we do not recommend replacement of propranolol with another β-adrenoceptor antagonist in angiosarcoma due to lack of efficacy data. Hence, strict monitoring of adverse events and in particular (severe) bradycardia is highly recommended. The use of non-selective β-blockers with ISA should be considered in potential follow-up studies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
Conception and design: AE, LM, AH, and NS; Literature search and collection of data: AE and LM; Data interpretation: AE and LM; Manuscript writing: All authors. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Baker JG, Hill SJ, Summers RJ. Evolution of β-blockers: from anti-anginal drugs to ligand-directed signalling. Trends Pharmacol Sci (2011) 32(4):227–34. doi: 10.1016/j.tips.2011.02.010
2. Chisholm KM, Chang KW, Truong MT, Kwok S, West RB, Heerema-Mckenney AE. β-adrenergic receptor expression in vascular tumors. Modern Pathol (2012) 25(11):1446–51. doi: 10.1038/modpathol.2012.108
3. Stiles JM, Amaya C, Rains S, Diaz D, Pham R, Battiste J, et al. Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PloS One (2013) 8(3):e60021. doi: 10.1371/journal.pone.0060021
4. Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med (2015) 372(8):735–46. doi: 10.1056/NEJMoa1404710
5. Propranolol 10mg tablets BP - summary of product characteristics. Available at: https://www.medicines.org.uk/emc/product/5888/smpc#MACHINEOPS.
6. Porcelli L, Garofoli M, di Fonte R, Fucci L, Volpicella M, Strippoli S, et al. The β-adrenergic receptor antagonist propranolol offsets resistance mechanisms to chemotherapeutics in diverse sarcoma subtypes: a pilot study. Sci Rep (2020) 10(1):10465. doi: 10.1038/s41598-020-67342-6
7. Medicines Agency EPublic summary of opinion on orphan designation propranolol for the treatment of soft tissue sarcoma. Available at: www.ema.europa.eu/contact.
8. Heinhuis KM, Ijzerman NS, Koenen AM, van der Graaf WTA, Haas RL, Beijnen JH, et al. PropAngio study protocol: a neoadjuvant trial on the efficacy of propranolol monotherapy in cutaneous angiosarcoma-a proof of principle study. BMJ Open (2020) 10(9). doi: 10.1136/bmjopen-2020-039449
9. Propranolol in angiosarcoma. Available at: https://clinicaltrials.gov/ct2/show/NCT04518124?term=propranolol&cond=Angiosarcoma&draw=2&rank=1.
10. Dose-finding of propranolol in combination with metronomic fixed oral cyclophosphamide based on bivariate efficacy-tolerability outcome in patients with locally advanced or metastatic angiosarcoma: A collaborative and innovative phase I-II sequential trial by the French sarcoma group (GSF/GETO). Available at: https://clinicaltrials.gov/ct2/show/study/NCT02732678.
11. Oliver E, Mayor F Jr, D’Ocon P. Beta-blockers: Historical perspective and mechanisms of action. Rev espanola cardiol (English ed) (2019) 72(10):853–62. doi: 10.1016/j.recesp.2019.02.023
12. Poirier L, Tobe SW. Contemporary use of β-blockers: clinical relevance of subclassification. Can J Cardiol (2014) 30(5 Suppl):S9–15. doi: 10.1016/j.cjca.2013.12.001
13. Frishman W, Kostis J, Strom J, Hossler M, Elkayam U, Goldner S, et al. Clinical pharmacology of the new beta-adrenergic blocking drugs. part 6. a comparison of pindolol and propranolol in treatment of patients with angina pectoris. the role of intrinsic sympathomimetic activity. Am Heart J (1979) 98(4):526–35. doi: 10.1016/0002-8703(79)90261-8
14. Pedersen ME, Cockcroft JR. The vasodilatory beta-blockers. Curr Hypertens Rep (2007) 9(4):269–77. doi: 10.1007/s11906-007-0050-2
15. Farzam K, Jan A. Beta Blockers. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2022).
16. Borchard U. Pharmacological properties of β-adrenoceptor blocking drugs. J Clin Bas Cardiol (1998) 1(1):5–9.
17. Metra M, Giubbini R, Nodari S, Boldi E, Modena MG, Cas LD. Differential effects of beta-blockers in patients with heart failure: A prospective, randomized, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation (2000) 102(5):546–51. doi: 10.1161/01.CIR.102.5.546
18. Ko DT, Hebert PR, Coffey CS, Sedrakyan A, Curtis JP, Krumholz HM. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA (2002) 288(3):351–7. doi: 10.1001/jama.288.3.351
19. Helfand M, Peterson K, Christensen V, Dana T, Thakurata S. Drug class review: Beta adrenergic blockers. Oregon Health and Science University (2009) p. 1–616.
20. Wagner MJ, Cranmer LD, Loggers ET, Pollack SM. Propranolol for the treatment of vascular sarcomas 2018. J Exp Pharmacol (2018) 10:51. doi: 10.2147/JEP.S146211
21. Pasquier E, Ciccolini J, Carre M, Giacometti S, Fanciullino R, Pouchy C, et al. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: implication in breast cancer treatment. Oncotarget (2011) 2(10):797–809. doi: 10.18632/oncotarget.343
22. Pantziarka P, Bouche G, Sukhatme V, Meheus L, Rooman I, Sukhatme VP. Repurposing drugs in oncology (ReDO) - propranolol as an anti-cancer agent. ecancermedicalscience (2016) 12:10. doi: 10.3332/ecancer.2016.680
23. Pasquier E, André N, Street J, Chougule A, Rekhi B, Ghosh J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: A bench to bedside study. EBioMedicine (2016) 6:87–95. doi: 10.1016/j.ebiom.2016.02.026
24. Saha J, Kim JH, Amaya CN, Witcher C, Khammanivong A, Korpela DM, et al. Propranolol sensitizes vascular sarcoma cells to doxorubicin by altering lysosomal drug sequestration and drug efflux. Front Oncol (2021) 1:10 doi: 10.3389/fonc.2020.614288
25. Fjæstad KY, Rømer AMA, Goitea V, Johansen AZ, Thorseth ML, Carretta M, et al. Blockade of beta-adrenergic receptors reduces cancer growth and enhances the response to anti-CTLA4 therapy by modulating the tumor microenvironment. Oncogene (2022) 41(9):1364–75. doi: 10.1038/s41388-021-02170-0
26. Sasaki M, North PE, Elsey J, Bubley J, Rao S, Jung Y, et al. Propranolol exhibits activity against hemangiomas independent of beta blockade. NPJ Precis Oncol (2019) 3(1):27. doi: 10.1038/s41698-019-0099-9
27. Amaya CN, Perkins M, Belmont A, Herrera C, Nasrazadani A, Vargas A, et al. Non-selective beta blockers inhibit angiosarcoma cell viability and increase progression free- and overall-survival in patients diagnosed with metastatic angiosarcoma. Oncoscience (2018) 5(3–4):109–19. doi: 10.18632/oncoscience.413
28. Luczynska E, Rudnicki W, Kargol J, Szpor J, Hodorowicz-Zaniewska D, Wysocki PJ, et al. Primary bilateral angiosarcoma of the breast treated with neoadjuvant chemotherapy combined with propranolol. Breast J (2021) 27(10):781–6. doi: 10.1111/tbj.14272
29. Fiste O, Dimos A, Kardara VE, Ballasis K, Karampeazis A. Propranolol and weekly paclitaxel in the treatment of metastatic heart angiosarcoma. Cureus (2020) 12(12). doi: 10.7759/cureus.12262
30. Daguzé J, Saint-Jean M, Dréno B. Large Nose angiosarcoma treated effectively with oral cyclophosphamide combined with propranolol. J Eur Acad Dermatol Venereol (2018) 32(2):e52–4. doi: 10.1111/jdv.14528
31. Daguzé J, Saint-Jean M, Peuvrel L, Cassagnau E, Quéreux G, Khammari A, et al. Visceral metastatic angiosarcoma treated effectively with oral cyclophosphamide combined with propranolol. JAAD Case Rep (2016) 2(6):497–9. doi: 10.1016/j.jdcr.2016.10.005
32. Banavali S, Pasquier E, Andre N. Targeted therapy with propranolol and metronomic chemotherapy combination: sustained complete response of a relapsing metastatic angiosarcoma. Ecancermedicalscience (2015) 8:9. doi: 10.3332/ecancer.2015.499
33. Pramanik R, Gogia A, Malik PS, Gogi R. Metastatic primary angiosarcoma of the breast: Can we tame it the metronomic way. Indian J Med Paediatr Oncol (2017) 38(2):228–31. doi: 10.4103/ijmpo.ijmpo_156_16
34. Galván DC, Ayyappan AP, Bryan BA. Regression of primary cardiac angiosarcoma and metastatic nodules following propranolol as a single agent treatment. Oncoscience (2018) 5(9–10):264–8. doi: 10.18632/oncoscience.472
Keywords: beta-blockade, angiosarcoma, drug repurposing, pharmacological characteristics, propranolol
Citation: Embaby A, van Merendonk L, Steeghs N, Beijnen J and Huitema A (2022) Beta-adrenergic receptor blockade in angiosarcoma: Which beta-blocker to choose? Front. Oncol. 12:940582. doi: 10.3389/fonc.2022.940582
Received: 10 May 2022; Accepted: 30 August 2022;
Published: 15 September 2022.
Edited by:
Sandeep Singh, Central University of Punjab, IndiaReviewed by:
Nicolas Andre, Aix Marseille Université, FranceRajkumar S. Kalra, Okinawa Institute of Science and Technology Graduate University, Japan
Copyright © 2022 Embaby, van Merendonk, Steeghs, Beijnen and Huitema. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alaa Embaby, YS5lbWJhYnlAbmtpLm5s
†These authors have contributed equally to this work and share first authorship
 Alaa Embaby
Alaa Embaby Lisanne van Merendonk
Lisanne van Merendonk Neeltje Steeghs
Neeltje Steeghs Jos Beijnen2
Jos Beijnen2 Alwin Huitema
Alwin Huitema