- 1Department of Breast Oncology, Henan Provincial People’s Hospital; Zhengzhou University People’s Hospital, Zhengzhou, China
- 2Department of Breast Oncology, Henan Provincial People’s Hospital; Henan University People’s Hospital, Zhengzhou, China
- 3Department of Medical Oncology, Henan Provincial People’s Hospital; Zhengzhou University People’s Hospital, Zhengzhou, China
- 4Department of Medical Oncology, Henan Provincial People’s Hospital; Henan University People’s Hospital, Zhengzhou, China
Background: Antiangiogenic agents provides an optional treatment strategy for patients with metastatic breast cancer. The present study was conducted to evaluate the efficacy and safety of anlotinib as third-line or above therapy for patients with HER-2 negative metastatic breast cancer.
Methods: Patients with HER-2 negative metastatic breast cancer who have failed from prior therapy and treated with anlotinib monotherapy or combined with chemotherapy or immunotherapy from June 2018 to December 2020 were retrospectively analyzed based on real-world clinical practice. The primary end point was progression free survival (PFS). Secondary end points included objective response rate (ORR), disease control rate (DCR), overall survival (OS) and safety.
Results: 47 patients with HER-2 negative metastatic breast cancer received anlotinib monotherapy or combination therapy as third-line or above therapy. In the general population, 10 patients achieved PR, 25 patients had SD and 12 patients had PD. The overall ORR and DCR were 21.3% and 74.5%, respectively. Subgroup analysis suggested that there were no statistically significant differences in ORR and DCR with respect to HR status (positive vs. negative), treatment programs (monotherapy vs. combination) and treatment type in combination group (chemotherapy vs. immunotherapy). The patients who did not received previously anti-angiogenesis therapy had superior DCR (84.8% vs. 50.0%, P=0.012). Median PFS and OS were 5.0 months (95% CI=4.3-5.7) and 21.0 (95% CI=14.9-27.1) months, respectively. The PFS (6.5m vs. 3.5m, P=0.042)and OS (28.2m vs. 12.6m, P=0.040) were better in HR positive patients than HR negative patients. And simultaneously, patients who received anlotinib combination therapy obtained better PFS (5.5m vs. 3.0m, P=0.045). The incidence of Grade 3-4 adverse events(AEs) was 31.9%.
Conclusions: Anlotinib monotherapy or combination therapy provide a viable third-line or above therapeutic strategy in patients with HER-2 negative metastatic breast cancer, a median PFS of 5.0 months was obtained with well tolerated toxicity.
Introduction
According to the global cancer registration data in 2020, breast cancer has become the most common malignant tumor in the world, accounting for 11.7% of all cancers (1). Through the development of medicine in the past decade, the treatment level of breast cancer has undergone tremendous changes. The improvement of the overall treatment level of breast cancer benefits from the deepening understanding of the biological behavior of breast cancer and the progress of comprehensive treatment methods, including chemotherapy, targeted therapy, endocrine therapy and immunotherapy (2–5). Even after comprehensive treatment, a considerable proportion of patients will progress to metastatic breast cancer, which still lacking effective treatment manner.
Angiogenesis is one of the key factors of tumorigenesis and progression, and it is also a significant feature of malignant tumors. Anti-angiogenesis is considered to be an important therapeutic strategy for the treatment of various tumors, especially for advanced or metastatic cancer (6). Antiangiogenic drugs have shown good efficacy in a variety of solid tumors, including lung cancer, gastric cancer, colorectal cancer, gynecological tumors and so on (7–10). However, the value of antiangiogenic drugs in metastatic breast cancer is still controversial, including bevacizumab, ramucirumab, and et al (11–13). Bevacizumab is the first antiangiogenic drug used in metastatic breast cancer. Clinical studies have shown that bevacizumab can prolong PFS, but cannot improve OS, and may be accompanied by serious adverse reactions (14–16).
In recent years, small-molecule multi-targeted tyrosine kinase inhibitors (TKIs) have shown good antitumor activity and have become a new therapeutic strategy for many malignant tumors. Previous phase II clinical studies have confirmed the efficacy and safety of apatinib monotherapy in patients with metastatic triple-negative breast cancer (17). As a novel oral TKI, which can effectively inhibit vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor (PDGFR), fibroblast growth factor receptor (FGFR) and c-Kit, anlotinib has proven efficacy in many solid tumors (18–20). The present study was conducted to evaluate the efficacy and safety of anlotinib as third-line or above therapy for patients with HER-2 negative metastatic breast cancer based on real-world clinical practice.
Patients and methods
Patients population
From June 2018 to December 2020, patients with HER-2 negative metastatic breast cancer who received third-line or above therapy in Henan Provincial People’s Hospital were screened, and patients with HER-2 negative metastatic breast cancer who received anlotinib as third-line or above therapy were enrolled and analyzed for efficacy and safety. The main selection criteria included: 1) histopathological confirmed metastatic breast cancer; 2) HER-2 negative by immunohistochemistry or fluorescence in situ hybridization (FISH); 3) have received at least second-line system rescue treatment; 4) received anlotinib as third-line or above therapy; 5) at least one measurable lesion based on RECIST v1.1. The main exclusion criteria included: 1) received less than two cycles of anlotinib treatment and could not evaluate the efficacy; 2) efficacy evaluation and follow-up data are not available.
Study treatment
In this study, the patients received anlotinib monotherapy or combination therapy until disease progression, unacceptable toxicity or death. In the monotherapy regimen, anlotinib was given at a dose of 12mg as the initial dose once a day on d1 to d14 every three weeks. In the combination therapy regimen, anlotinib was given at a dose of 10 mg once a day on d1 to d14 every three weeks, and chemotherapy or immunotherapy were given simultaneously. Regarding chemotherapy, the regimen includes capecitabine and albumin bound paclitaxel. Capecitabine was provided as tablets and administered orally at a dose of 1000 mg/m2, bid, d1-d14, q21d. Albumin bound paclitaxel was administered intravenously at a dose of 100 mg/m2 on d1, q7d. With respect to immunotherapy, pembrolizumab was administered intravenously at a dose of 200 mg once every three weeks.
Efficacy and safety assessments
After treatment, imaging examinations were performed after every two cycles of treatment in all patients to evaluate the clinical efficacy. The efficacy evaluation criteria are RECIST version 1.1 response evaluation criteria in solid tumors. According to RECIST version 1.1 response evaluation criteria, the efficacy assessment is divided into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The objective response rate (ORR) was defined as CR + PR, and the disease control rate (DCR) was CR+ PR and SD. The toxicity was assessed according to the Common Terminology Criteria for Adverse Events, version 4.0.
Statistical analysis
All the statistical analyses were performed with SPSS 22.0 software (SPSS Inc., IL, US) software. Survival curves of patients were estimated by the Kaplan-Meier method and compared using the log-rank test. The follow-up deadline is March 15, 2022. Progression-free survival (PFS) was defined as starting anlotinib monotherapy or combination therapy as third-line or above treatment to disease progression or death. Overall survival (OS) was defined as the period from the time of anlotinib monotherapy or combination therapy as third-line or above therapy to patient death or last follow-up. Difference between groups were determined by Pearson’s chi squared test or Fisher’s exact test. P<0.05 was considered significant.
Results
Patient and treatment characteristics
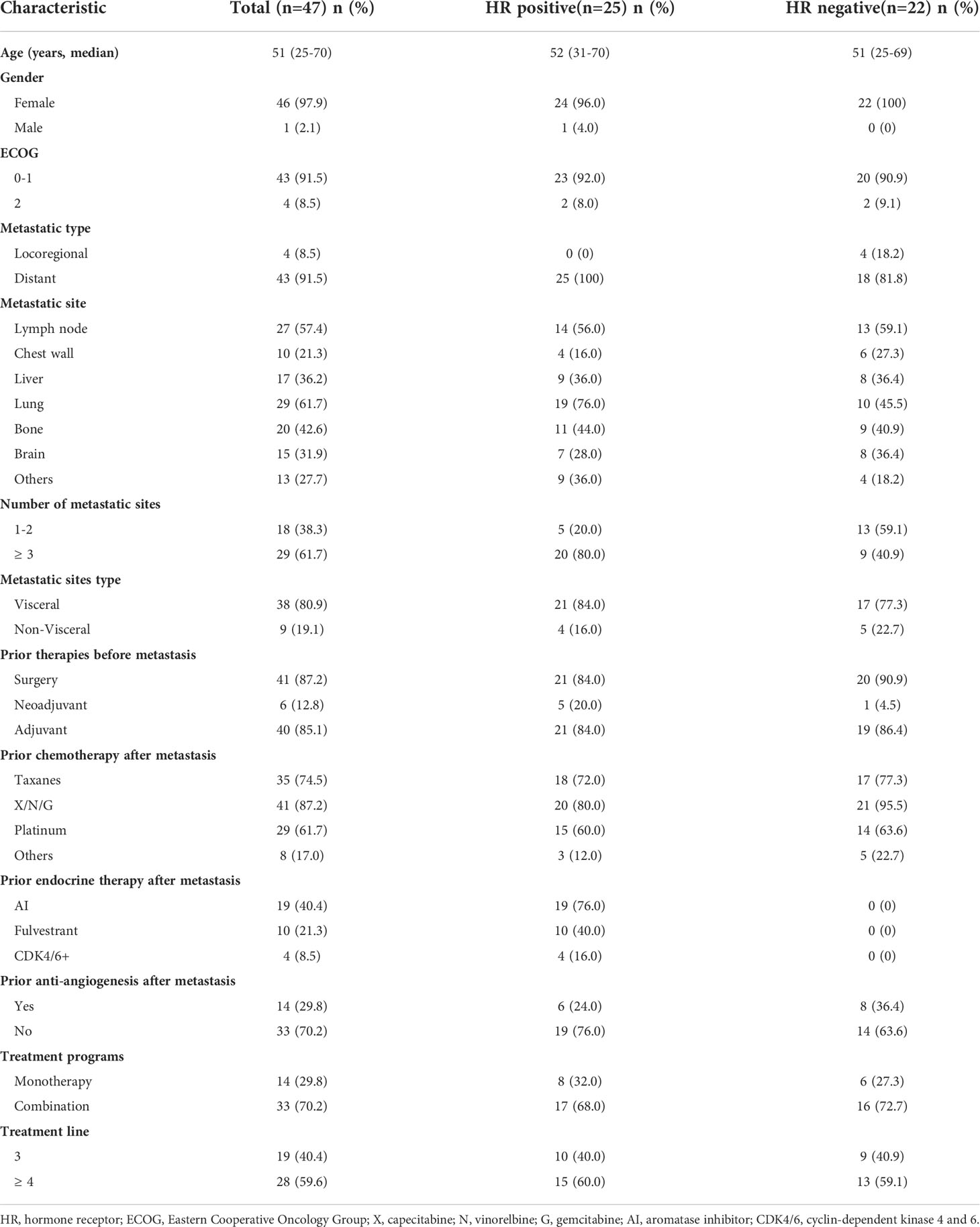
A total of 47 patients with HER-2 negative metastatic breast cancer who received anlotinib as third-line or above therapy were included. Patient and treatment characteristics are summarized in Table 1. The median age was 51 years (range 25-70), with 46 female patients and 1 male patients. In total, 25 (53.2%) patients were hormone receptor positive/HER2-negative, and 22 (46.8%) patients were TNBC. Most patients (43, 91.5%) were ECOG PS 0-1, and the other 4 (8.5%) patients were ECOG PS 2. All patients were diagnosed as recurrent and metastatic breast cancer. The common metastatic sites included lymph node (57.4%), chest wall (21.3%), liver (36.2%), lung (61.7%), bone (42.6%) and brain (31.9%). Number of metastatic sites in 18 (38.3%) patients were 1 or 2, and the other 29 (61.7%) patients were 3 or more. Thirty-eight patients (80.9%) had visceral metastasis, and the other 9 patients (19.1) had non-visceral metastasis. Six patients were in advanced stage at the time of initial diagnosis, and the other 41 patients were diagnosed as recurrent breast cancer. All the 41 patients had undergone previous breast surgery, and forty of these patients had received adjuvant therapy.
During rescue therapy stage, 74.5% of patients had received taxanes treatment, 87.2% had received X/N/G treatment, including capecitabine, vinorelbine and gemcitabine, and 61.7% had received platinum treatment. For HR positive breast cancer patients, 76.0% of patients had received AI endocrine therapy, 40.0% had received fulvestrant endocrine therapy, and only 4 patients had received cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor treatment. In the general population, 14 (29.8%) patients had received anti-angiogenesis therapy, including bevacizumab or apatinib. All the patients included in this study had received at least two lines of rescue therapy, so anlotinib was given as third-line in 19 (40.4%) patients, and as forth-line or above therapy in 28 (59.6%) patients. 14 (29.8%) patients received anlotinib monotherapy, and the other 33 (70.2%) patients received anlotinib combination therapy. In the combination group, chemotherapy and immunotherapy were used in 26 and 7 patients, respectively.
Efficacy
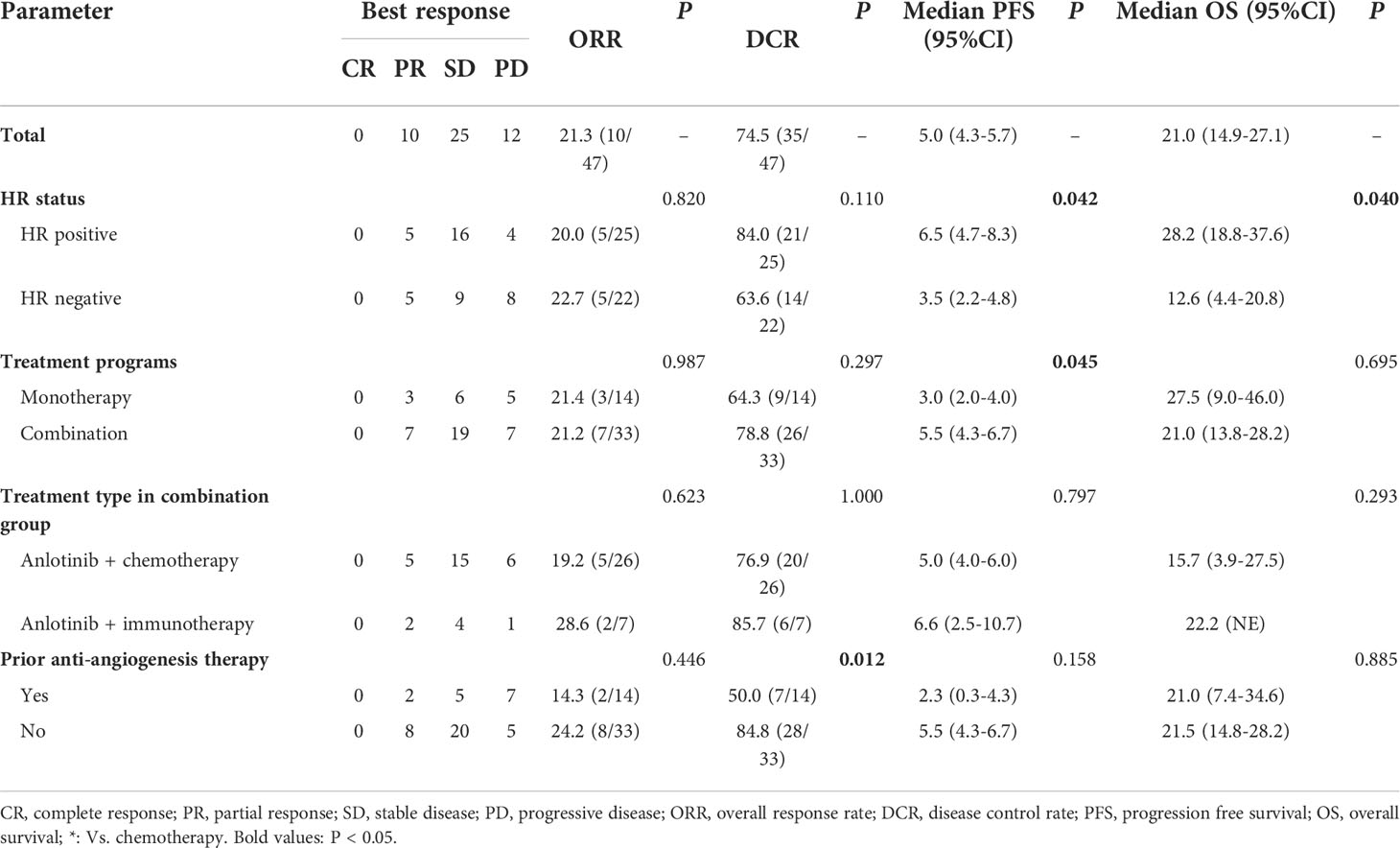
In the general population, CR was not observed, 10 patients achieved PR, 25 patients had SD and 12 patients had PD. The overall ORR and DCR were 21.3% (10/47) and 74.5% (35/47), respectively (Table 2). In HR positive breast cancer patients, CR was not observed, 5 patients achieved PR, 16 patients had SD and 4 patients had PD. The overall ORR and DCR were 20.0% (5/25) and 84.0% (21/25), respectively. In HR negative breast cancer patients, CR was not observed, 5 patients achieved PR, 9 patients had SD and 8 patients had PD. The overall ORR and DCR were 22.7% (5/22) and 63.6% (14/22), respectively. The ORR in anlotinib monotherapy and combination therapy group were 21.4% and 21.2%, respectively. The DCR in anlotinib monotherapy and combination therapy group were 64.3% and 78.8%, respectively. In anlotinib combination therapy group, the ORR and DCR in anlotinib plus chemotherapy group were 19.2% and 76.9%, and 28.6% and 85.7% in anlotinib plus immunotherapy group, respectively. There were no statistically significant differences in ORR and DCR with respect to HR status (positive vs. negative), treatment programs (monotherapy vs. combination) treatment type in combination group (chemotherapy vs. immunotherapy), metastatic sites type (visceral vs. non-visceral), number of metastatic sites (1-2 vs. ≥ 3) and prior chemotherapy after metastasis (with taxanes vs. without taxanes, Supplementary Table 1). The patients who did not received prior anti-angiogenesis therapy had higher DCR than patients who had received prior anti-angiogenesis therapy (84.8% vs. 50.0%, P=0.012), but there was no statistical difference in ORR between the two groups (24.2% vs. 14.3%, P=0.446, Table 2). There were no significant differences in ORR and DCR among different chemotherapy and immunotherapy medications in the combination group (Supplementary Table 1).
In the general population, the median PFS and median OS were 5.0 (95% CI= 4.3-5.7) and 21.0 (95% CI= 14.9-27.1) months, respectively (Figures 1A, B). The median PFS were 6.5 (95% CI= 4.7-8.3) and 3.5 (95% CI= 2.2-4.8) months in the HR positive and negative population, respectively (P = 0.042; Figure 2A). The median OS in the two groups were 28.2 (95% CI=18.8-37.6) months and 12.6 (95% CI=4.4-20.8) months, respectively(P = 0.040; Figure 2B). The PFS and OS were better in HR positive patients than HR negative patients. In the meantime, patients who received anlotinib combination therapy obtained better PFS than those who received anlotinib monotherapy (5.5m vs. 3.0m, P=0.045; Figure 2C). However, the OS was not statistically different between the two groups (21.0m vs. 27.5m, P=0.695; Figure 2D). There were no statistically significant differences in PFS and OS with respect to treatment type in combination group (plus chemotherapy vs. immunotherapy; Figures 2E, F) and prior anti-angiogenesis therapy (Yes vs. No; Figures 2G, H). There were also no significant differences in PFS and OS in metastatic sites type (visceral vs. non-visceral), and prior chemotherapy after metastasis (with taxanes vs. without taxanes). However, the median OS in patients with 1-2 metastatic sites was better than in those with ≥ 3 metastatic sites (Supplementary Table 1). And simultaneously, the median PFS in patients with 1-2 metastatic sites was longer, but no significant difference was found. No significant differences were found in treatment type in combination group among anlotinib plus capecitabine, nab-paclitaxel or pembrolizumab (Supplementary Table 1).
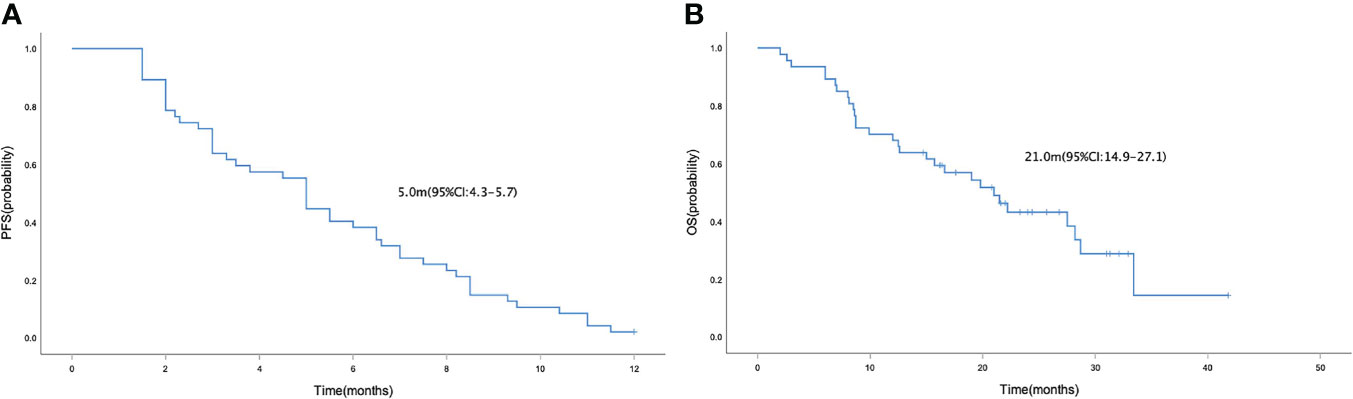
Figure 1 Kaplan-Meier curve of PFS (A) and OS (B) in the general population who received anlotinib as third-line or above therapy for patients with HER-2 negative metastatic breast cancer.
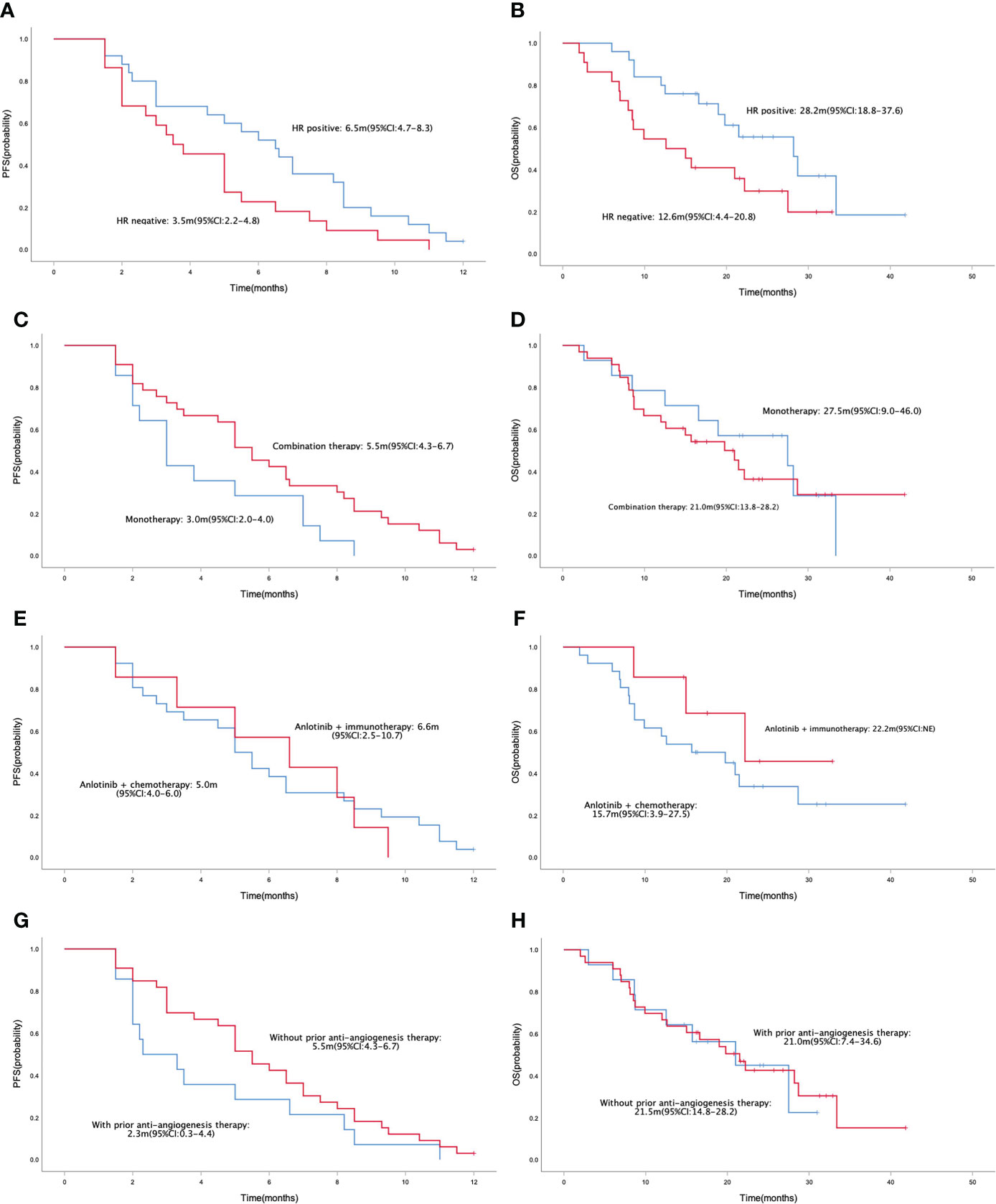
Figure 2 Kaplan-Meier curve of PFS (A) and OS (B) in patients with different HR status (HR positive vs. HR negative). Kaplan-Meier curve of PFS (C) and OS (D) in patients with different treatment programs (monotherapy vs. combination therapy). Kaplan-Meier curve of PFS (E) and OS (F) in patients with different treatment type in combination group (anlotinib + chemotherapy vs. anlotinib + immunotherapy). Kaplan-Meier curve of PFS (G) and OS (H) in patients with or without prior anti-angiogenesis therapy.
Safety
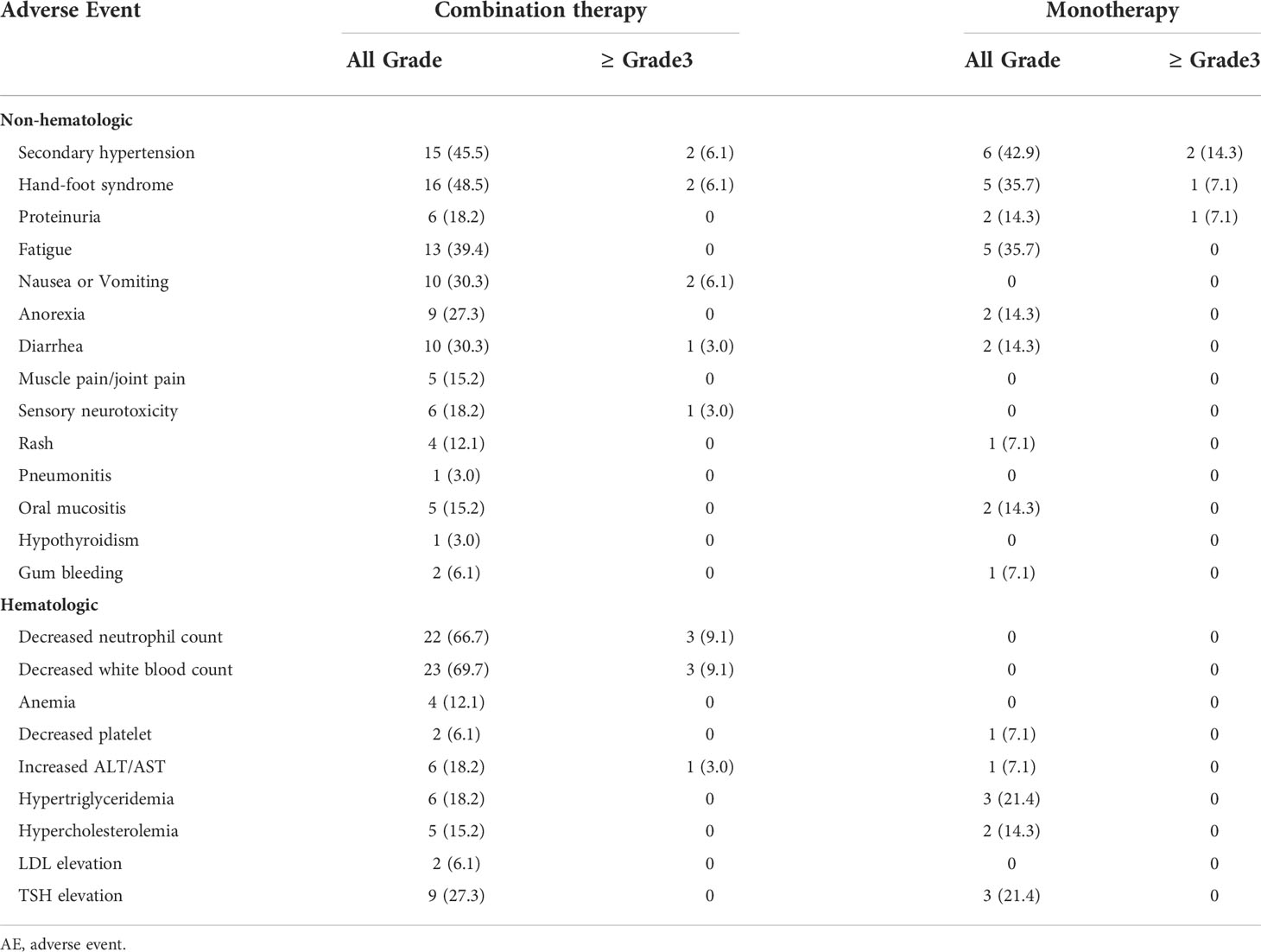
Most of the adverse events in patients received anlotinib therapy were grade 1-2 in severity, and no unexpected side effects or treatment-related death occurred (Table 3). The incidence of Grade 3-4 AEs was 31.9%. In anlotinib monotherapy group, Non-hematological treatment-related AEs were secondary hypertension (n=6, 42.9%), hand-foot syndrome (n=5, 35.7%), proteinuria (n=2, 14.3%), fatigue (n=5, 35.7%), anorexia (n=2, 14.3%), diarrhea (n=2, 14.3%), rash (n=1, 7.1%), oral mucositis (n=2, 14.3%) and gum bleeding (n=1, 7.1%). Hematological AEs were decreased platelet, increased ALT/AST, dyslipidemia and TSH elevation. The incidence of adverse reactions was generally low. Grade 3-4 AEs were secondary hypertension (n=2,14.3%), hand-foot syndrome (n=1, 7.1%), and proteinuria (n=1,7.1%). The dose of anlotinib was reduced from 12 mg to 10 mg due to adverse reactions in 2 patients. The remaining patient could tolerate the treatment by suspending the medication.
In anlotinib combination therapy group, the incidence of treatment-related hematological and non-hematological AEs was both higher than those of the anlotinib monotherapy group, which is related to the concurrent chemotherapy and immunotherapy. Grade 3-4 AEs were secondary hypertension (n=2,6.1%), hand-foot syndrome (n=2,6.1%), nausea or Vomiting (n=2,6.1%), diarrhea (n=1,3.0%), sensory neurotoxicity (n=1,3.0%), decreased neutrophil count (n=3,9.1%), decreased white blood count (n=3,9.1%) and increased ALT/AST (n=1,3.0%). No patient had an anlotinib dose reduction due to adverse reactions.
Discussion
Angiogenesis plays a key role in the growth, proliferation and metastasis of a variety of solid tumors. Anti-angiogenic drugs can exert anti-tumor effects by targeting vascular endothelial growth factor (VEGF) and other signal factors, inhibiting their overexpression, and promoting the normalization of tumor blood vessels. As a recombinant humanized monoclonal antibody, which can target and bind with VEGF, reduce neovascularization and inhibit tumor growth, bevacizumab was the first angiogenesis inhibitor approved for clinical use. Currently, bevacizumab has shown promising antitumor activity in a variety of solid tumors, including metastatic colorectal cancer, non-small cell lung cancer (NSCLC), glioblastoma, renal cell carcinoma, ovarian and cervical cancer. However, the clinical value of bevacizumab in metastatic breast cancer remains controversial. E2100, AVADO and RIBBON-1 clinical studies have shown that first-line bevacizumab combined with chemotherapy significantly improved the PFS of metastatic breast cancer patients compared with chemotherapy alone, but did not bring about the benefit of OS (14–16). The AVF2119G, RIBBON-2, and TANIA studies evaluated the value of bevacizumab combined with chemotherapy in the second-line or above therapy of metastatic breast cancer (21–23). These studies demonstrated that adding bevacizumab on the basis of chemotherapy could not improve the PFS and OS of patients.
In addition to bevacizumab, small molecule tyrosine kinase inhibitors (TKIs) can not only act on VEGFR, but also inhibit other related tyrosine kinases, so as to inhibit angiogenesis. Numerous clinical trials confirmed that these TKIs show good efficacy in a variety of solid tumors and become a new anti-angiogenesis strategy (24, 25). Previous studies have explored the efficacy of sunitinib, sorafenib, and apatinib in metastatic breast cancer, and the results suggest that only apatinib shows better efficacy and safety (26, 27). A phase II study evaluated the efficacy and safety of apatinib monotherapy in heavily pretreated metastatic triple negative breast cancer (mTNBC). Median PFS and OS reached 3.3 and 10.6 months, respectively (17). As a novel oral TKI, anlotinib has been approved by the China Food and Drug Administration (CFDA) for advanced non-small cell lung cancer (NSCLC), soft tissue sarcoma and medullary thyroid cancer. Meanwhile, real-world studies have also shown that anlotinib is effective in multiple solid tumors. At present, the clinical efficacy of anlotinib in metastatic breast cancer has rarely been reported. A previous basic study showed that anlotinib inhibits the proliferation and induces apoptosis of MCF‐7 breast cancer cells by downregulating TFAP2C (28). At present, only one phase II clinical study with a small sample size has evaluated the clinical efficacy of anlotinib in metastatic breast cancer (29).
Our present study evaluated the efficacy of anlotinib monotherapy or combination therapy as third-line or above therapy in 47 patients with HER-2 negative metastatic breast cancer. To our knowledge, this is the largest real-world analysis to date investigating the efficacy and safety of anlotinib in breast cancer. The overall ORR and DCR were 21.3% and 74.5%, and median PFS and OS reached 5.0 and 21.0 months. It is worth noting that all the patients enrolled in the present study are heavily pretreated metastatic breast cancer, thirty-eight patients (80.9%) had visceral metastasis, and anlotinib was given as forth-line or above therapy in 59.6% patients. In such a population with a heavy tumor burden, anlotinib has still achieved good clinical efficacy as a late-line treatment manner. Regardless of the NCCN guidelines or the Chinese Society of Clinical Oncology (CSCO) guidelines, there is no standard recommended regimen for the third-line and above treatment of metastatic breast cancer. Therefore, there is no accepted standard treatment regimen to serve as the control group for this study. However, we can review the data of chemotherapy and targeted therapy in the third-line or above treatment of metastatic breast cancer in previous clinical studies. The 304 study is a phase III study, which explored the efficacy of eribulin versus vinorelbine in the treatment of recurrent/metastatic breast cancer. The median PFS in the eribulin-treated group was 2.8 months. The ASCENT study explored the efficacy of sacituzumab govitecan (SG) in the third-line or above treatment of metastatic breast cancer, a median PFS of 4.8 months and median OS of 12.1 months were obtained. Comparing these data, anlotinib showed better clinical efficacy. In TNBC population, the median PFS were 3.5 months and the median OS was 12.6 months, which is consistent with data from previous studies on apatinib in metastatic TNBC (median PFS and OS reached 3.3 and 10.6 months). Baseline clinicopathological characteristics of patients did not affect the clinical efficacy of anlotinib, including metastatic sites type and number of metastatic sites.
At present, the application of anti-angiogenic drugs in breast cancer is mainly in metastatic TNBC patients, and there is little exploration for hormone receptor-positive metastatic breast cancer. Endocrine therapy is an important treatment strategy for patients with HR positive metastatic breast cancer, especially the addition of targeted drugs such as CDK4/6 inhibitors, which significantly improves the prognosis of patients with HR positive metastatic breast cancer (30–32). However, we also face some difficulties in clinical practice. First, CDK4/6 inhibitors are currently not widely used due to drug availability and economic concerns. In the present study, only 4 patients had received CDK4/6 inhibitor treatment. Second, for endocrine resistant breast cancer, novel and feasible treatment strategies still need to be explored. In the present study, 25 HR positive metastatic breast cancer patients received anlotinib therapy. Although there was no statistically significant difference in ORR and DCR between HR-positive and HR-negative groups, the median PFS in HR-positive patients reached 6.5 months, which was significantly better than HR negative group. Our present study confirmed that anlotinib anti-angiogenesis therapy also provide an effective late-line treatment option for HR-positive metastatic breast cancer.
The optimal mode of administration of anlotinib has not been established. Unlike monoclonal antibodies anti-angiogenic drugs, which must be used in combination with chemotherapy or other drugs, small molecule TKIs monotherapy may also have objective clinical efficacy. In the present study, patients who received anlotinib monotherapy obtained no statistically significant ORR and DCR. But patients who received anlotinib combination therapy obtained better PFS than those who received anlotinib monotherapy. Anlotinib combination therapy is the preferred treatment mode, and for patients with poor physical condition, anlotinib monotherapy is also an optional treatment strategy. In anlotinib combination therapy group, except the combination with traditional chemotherapy drugs, some patients received anlotinib in combination with immune checkpoint inhibitors (ICIs) immunotherapy. Antiangiogenic therapy and immunotherapy both act on the tumor microenvironment, and previous preclinical studies have shown that immunotherapy combined with anti-angiogenic drugs synergistically inhibit tumor growth and metastasis (33). Some clinical trials also confirmed the value of antiangiogenic therapy in combination with immunotherapy (34, 35). However, the application of anlotinib combined with ICIs in breast cancer has not yet been reported, our present study shows that anlotinib combined with immunotherapy has also achieved good clinical efficacy in metastatic breast cancer. We also compared the efficacy of different chemotherapy and immunotherapy medications, and there were no significant differences among anlotinib plus capecitabine, nab-paclitaxel or pembrolizumab.
Of the patients enrolled in this study, 14 patients had received prior antiangiogenic therapy, including bevacizumab and apatinib. Whether prior anti-angiogenic therapy will affect the therapeutic efficacy of anlotinib? Our present study showed that the patients who did not received prior anti-angiogenesis therapy had superior DCR (84.8% vs. 50.0%, P=0.012). And simultaneously, although there were no statistically significant differences in ORR, PFS, and OS between the two groups, the absolute values of ORR, PFS, and OS were also better in patients who did not received prior anti-angiogenesis therapy. Therefore, our study suggests that prior antiangiogenic therapy may affect the efficacy of anlotinib.
The most common anti-angiogenic drugs related adverse events are secondary hypertension, hand-foot syndrome, and proteinuria. In previous clinical studies, most of these toxicities can be alleviated by dose adjustment and suspension of administration. In the present study, most of the adverse events in patients received anlotinib therapy were grade 1-2 in severity. The incidence of secondary hypertension, hand-foot syndrome, and proteinuria was consistent with previous relevant clinical trials (36). Antiangiogenic drugs may increase the risk of bleeding. In this study, 3 patients had gum bleeding, but no serious bleeding events such as hemoptysis, gastrointestinal bleeding, and hematuria occurred.
Our present study has some several limitations, because it is an observational and exploratory study and the number of patients enrolled is not very large. The purpose of this study was to explore the clinical value of anti-angiogenesis therapy strategy, and therefore, the results of this study need future validation with large-scale prospective clinical trials. In this study, only a small number of patients received single drug treatment of anlotinib, and more patients received combination therapy. Therefore, this study confirmed the value of anlotinib based scheme as third-line or above therapy for patients with HER-2 negative metastatic breast cancer.
Conclusion
In conclusion, these data confirm that anlotinib monotherapy or combination therapy provide a viable third-line or above therapeutic strategy in patients with HER-2 negative metastatic breast cancer, a median PFS of 5.0 months was obtained with well tolerated toxicity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The ethics committee of the Henan Provincial People’s Hospital (2021-84). The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors contributed to the article and approved the submitted version. YS and HL designed the research, analyzed the data and drafted the paper. YS, ZL, YY and YH were mainly responsible for data collection and analysis. QC, CL, FZ and BN were primarily responsible for statistical analysis.
Funding
This work was supported by Medical Science and Technique Foundation of Henan Province (No. LHGJ20210055) and Beijing Medical Award Foundation Project (No.YXJL-2020-0941-0748).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.939343/full#supplementary-material
References
1. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin (2020) 70(3):145–64. doi: 10.3322/caac.21601
2. von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med (2019) 380(7):617–28. doi: 10.1056/NEJMoa1814017
3. von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol (2014) 15(7):747–56. doi: 10.1016/S1470-2045(14)70160-3
4. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. (2020) 396(10265):1817–28. doi: 10.1016/S0140-6736(20)32531-9
5. Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med (2018) 379(20):1926–36. doi: 10.1056/NEJMoa1810527
6. Ansari MJ, Bokov D, Markov A, Jalil AT, Shalaby MN, Suksatan W, et al. Cancer combination therapies by angiogenesis inhibitors; a comprehensive review. Cell Commun Signal (2022) 20(1):49. doi: 10.1186/s12964-022-00838-y
7. Li Y, Lin M, Wang S, Cao B, Li C, Li G. Novel angiogenic regulators and anti-angiogenesis drugs targeting angiogenesis signaling pathways: Perspectives for targeting angiogenesis in lung cancer. Front Oncol (2022) 12:842960. doi: 10.3389/fonc.2022.842960
8. Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol (2016) 34(13):1448–54. doi: 10.1200/JCO.2015.63.5995
9. Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: Retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol (2017) 3(2):194–201. doi: 10.1001/jamaoncol.2016.3797
10. Penson RT, Moore KM, Fleming GF, Braly P, Schimp V, Nguyen H, et al. A phase II study of ramucirumab (IMC-1121B) in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal carcinoma. Gynecol Oncol (2014) 134(3):478–85. doi: 10.1016/j.ygyno.2014.06.029
11. Saji S, Taira N, Kitada M, Takano T, Takada M, Ohtake T, et al. Switch maintenance endocrine therapy plus bevacizumab after bevacizumab plus paclitaxel in advanced or metastatic oestrogen receptor-positive, HER2-negative breast cancer (BOOSTER): a randomised, open-label, phase 2 trial. Lancet Oncol (2022) 23(5):636–49. doi: 10.1016/S1470-2045(22)00196-6
12. van Rossum AGJ, Mandjes IAM, van Werkhoven E, van Tinteren H, van Leeuwen-Stok AE, Nederlof P, et al. Carboplatin-cyclophosphamide or paclitaxel without or with bevacizumab as first-line treatment for metastatic triple-negative breast cancer (BOOG 2013-01). Breast Care (Basel) (2021) 16(6):598–606. doi: 10.1159/000512200
13. Vahdat LT, Layman R, Yardley DA, Gradishar W, Salkeni MA, Joy AA, et al. Randomized phase II study of ramucirumab or icrucumab in combination with capecitabine in patients with previously treated locally advanced or metastatic breast cancer. Oncologist (2017) 22(3):245–54. doi: 10.1634/theoncologist.2016-0265
14. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med (2007) 357(26):2666–76. doi: 10.1056/NEJMoa072113
15. Pivot X, Schneeweiss A, Verma S, Thomssen C, Passos-Coelho JL, Benedetti G, et al. Efficacy and safety of bevacizumab in combination with docetaxel for the first-line treatment of elderly patients with locally recurrent or metastatic breast cancer: results from AVADO. Eur J Cancer (2011) 47(16):2387–95. doi: 10.1016/j.ejca.2011.06.018
16. Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol (2011) 29(10):1252–60. doi: 10.1200/JCO.2010.28.0982
17. Hu X, Cao J, Hu W, Wu C, Pan Y, Cai L, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer (2014) 14:820. doi: 10.1186/1471-2407-14-820
18. Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: The ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol (2018) 4(11):1569–75. doi: 10.1001/jamaoncol.2018.3039
19. Cheng Y, Wang Q, Li K, Shi J, Liu Y, Wu L, et al. Anlotinib vs placebo as third- or further-line treatment for patients with small cell lung cancer: a randomised, double-blind, placebo-controlled phase 2 study. Br J Cancer (2021) 125(3):366–71. doi: 10.1038/s41416-021-01356-3
20. Huang NS, Wei WJ, Xiang J, Chen JY, Guan Q, Lu ZW, et al. The efficacy and safety of anlotinib in neoadjuvant treatment of locally advanced thyroid cancer: A single-arm phase II clinical trial. Thyroid (2021) 31(12):1808–13. doi: 10.1089/thy.2021.0307
21. Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol (2005) 23(4):792–9. doi: 10.1200/JCO.2005.05.098
22. Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O'Neill V, et al. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol (2011) 29(32):4286–93. doi: 10.1200/JCO.2010.34.1255
23. Vrdoljak E, Marschner N, Zielinski C, Gligorov J, Cortes J, Puglisi F, et al. Final results of the TANIA randomised phase III trial of bevacizumab after progression on first-line bevacizumab therapy for HER2-negative locally recurrent/metastatic breast cancer. Ann Oncol (2016) 27(11):2046–52. doi: 10.1093/annonc/mdw316
24. Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol (2021) 22(3):351–60. doi: 10.1016/S1470-2045(20)30702-6
25. Nie C, Lv H, Xing Y, Chen B, Xu W, Wang J, et al. The efficacy and safety of apatinib treatment for patients with advanced or recurrent biliary tract cancer: a retrospective study. BMC Cancer (2021) 21(1):189. doi: 10.1186/s12885-021-07907-4
26. Crown JP, Dieras V, Staroslawska E, Yardley DA, Bachelot T, Davidson N, et al. Phase III trial of sunitinib in combination with capecitabine versus capecitabine monotherapy for the treatment of patients with pretreated metastatic breast cancer. J Clin Oncol (2013) 31(23):2870–8. doi: 10.1200/JCO.2012.43.3391
27. Baselga J, Zamagni C, Gomez P, Bermejo B, Nagai SE, Melichar B, et al. RESILIENCE: Phase III randomized, double-blind trial comparing sorafenib with capecitabine versus placebo with capecitabine in locally advanced or metastatic HER2-negative breast cancer. Clin Breast Cancer (2017) 17(8):585–94.e4. doi: 10.1016/j.clbc.2017.05.006
28. Fang F, Yuan Q. Anlotinib inhibits the proliferation, migration and invasion, and induces apoptosis of breast cancer cells by downregulating TFAP2C. Oncol Lett (2022) 23(2):46. doi: 10.3892/ol.2021.13164
29. Hu N, Si Y, Yue J, Sun T, Wang X, Jia Z, et al. Anlotinib has good efficacy and low toxicity: a phase II study of anlotinib in pre-treated HER-2 negative metastatic breast cancer. Cancer Biol Med (2021) 18(3):849–59. doi: 10.20892/j.issn.2095-3941.2020.0463
30. Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol (2016) 17(4):425–39. doi: 10.1016/S1470-2045(15)00613-0
31. Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol (2017) 35(32):3638–46. doi: 10.1200/JCO.2017.75.6155
32. Lu YS, Im SA, Colleoni M, Franke F, Bardia A, Cardoso F, et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2- advanced breast cancer in MONALEESA-7: A phase III randomized clinical trial. Clin Cancer Res (2022) 28(5):851–9. doi: 10.1158/1078-0432.CCR-21-3032
33. Hu H, Chen Y, Tan S, Wu S, Huang Y, Fu S, et al. The research progress of antiangiogenic therapy, immune therapy and tumor microenvironment. Front Immunol (2022) 13:802846. doi: 10.3389/fimmu.2022.802846
34. Wu SY, Xu Y, Chen L, Fan L, Ma XY, Zhao S, et al. Combined angiogenesis and PD-1 inhibition for immunomodulatory TNBC: concept exploration and biomarker analysis in the FUTURE-C-Plus trial. Mol Cancer (2022) 21(1):84. doi: 10.1186/s12943-022-01536-6
35. Xia L, Peng J, Lou G, Pan M, Zhou Q, Hu W, et al. Antitumor activity and safety of camrelizumab plus famitinib in patients with platinum-resistant recurrent ovarian cancer: results from an open-label, multicenter phase 2 basket study. J Immunother Cancer (2022) 10(1):e003831. doi: 10.1136/jitc-2021-003831
Keywords: breast cancer, anlotinib, anti-angiogenesis, immunotherapy, safety
Citation: Shao Y, Luo Z, Yu Y, He Y, Liu C, Chen Q, Zhu F, Nie B and Liu H (2022) A real-world study of anlotinib as third-line or above therapy in patients with her-2 negative metastatic breast cancer. Front. Oncol. 12:939343. doi: 10.3389/fonc.2022.939343
Received: 09 May 2022; Accepted: 05 July 2022;
Published: 28 July 2022.
Edited by:
Prabhu Thirusangu, Mayo Clinic, United StatesReviewed by:
Fuhua Lin, Sun Yat-sen University Cancer Center (SYSUCC), ChinaYunwei Han, Affiliated Hospital of Southwest Medical University, China
Copyright © 2022 Shao, Luo, Yu, He, Liu, Chen, Zhu, Nie and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Liu, cnhsaXVodWlAenp1LmVkdS5jbg==
 Yingbo Shao1,2
Yingbo Shao1,2 Hui Liu
Hui Liu