
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Oncol., 15 July 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.937818
This article is part of the Research TopicEndoscopic Transorbital Surgery for Skull Base TumorsView all 6 articles
 Iacopo Dallan1
Iacopo Dallan1 Lodovica Cristofani-Mencacci1*
Lodovica Cristofani-Mencacci1* Giacomo Fiacchini1
Giacomo Fiacchini1 Mario Turri-Zanoni2
Mario Turri-Zanoni2 Wouter van Furth3
Wouter van Furth3 Matteo de Notaris4
Matteo de Notaris4 Miriana Picariello1
Miriana Picariello1 Enrico Alexandre1
Enrico Alexandre1 Christos Georgalas5
Christos Georgalas5 Luca Bruschini1
Luca Bruschini1Transorbital approaches are genuinely versatile surgical routes which show interesting potentials in skull base surgery. Given their “new” trajectory, they can be a very useful adjunct to traditional routes, even being a valid alternative to them in some cases, and add valuable opportunities in selected patients. Indications are constantly expanding, and currently include selected intraorbital, skull base and even intra-axial lesions, both benign and malignant. Given their relatively recent development and thus unfamiliarity among the skull base community, achieving adequate proficiency needs not only a personalized training and knowledge but also, above all, an adequate case volume and a dedicated setting. Current, but mostly future, applications should be selected by genetic, omics and biological features and applied in the context of a truly multidisciplinary environment.
The term “transorbital approaches” (TOAs) describes a very wide and heterogenous group of procedures. They all share one basic aspect, which is: the procedure is performed passing through the orbital space. The use of endoscopes as visualization tools introduces the concept of endoscopic-assisted transorbital surgery. Many transorbital routes are now available and, since their introduction in 2010 by Kris Moe (1), this “new” philosophy has gained increasing popularity. Initially described as ancillary alternatives to traditional routes, TOAs have evolved to the state of well-established surgical procedures, acting as valid alternative to traditional approaches for selected lesions (2). As evidence of the latter fact, when searching in Pubmed the terms “transorbital endoscopic”, the results show a consistent increase in publications in recent years, witnessing a growing and outstanding interest in this topic. In this paper, after reviewing current literature and retrospectively reviewing our 10-years experience in transorbital surgery, we present some considerations on three main aspects: actual possibilities, learning curve processes and future developments. As for any other surgical approach, also for TOAs there are pros and cons as there are good and bad indications, although their clear understanding is actually far from complete.
Since its first description (3), the use of endoscopes in orbital surgery has gained, with years, tremendous interest. In recent years TOAs have been used for the treatment of pathologies located within the orbit or adjacent to it (Figure 1) (1, 4–9) or even to target distant areas using the orbit as a corridor (10–21). Moreover, in the contest of multidisciplinary and modern surgery, TOAs can be performed as a single procedure or be part of a multiportal surgery. It is well established that selected patients can benefit from the potentially better exposure provided by a combination of approaches. Furthermore, as recent literature reveals, even intra-axial lesions of the temporal lobe have been managed via TOAs (21) and pre-clinical studies on neurovascular surgery have been conducted (22). From a surgical point of view, transorbital approaches have been demonstrated to offer equivalent exposure to traditional routes (23, 24), allowing a safe and effective management of selected lesions. Thus said, like any other approach, TOAs are obviously not suitable for all cases, and present its own pros and cons (Table 1). But certainly the orbit can be considered as a reliable port to overcome intrinsic limits of traditional routes, both endoscopic-assisted and not. Obviously TOAs present some intrinsic limitations. Among these, the possible compression of the orbital content necessary to gain adequate room for working; the need for a very careful management of eye surface and the not easy control in case of major bleeding (although this last concern is not typical of transorbital approaches rather than of endoscopic-assisted approaches). A recent systematic review reported a very wide range of pathologies managed via TOAs (2). The very large majority of this cohort are represented by spheno-orbital meningiomas, meckels’ cave schwannomas, inflammation/infection and csf leaks. In this respect, the long-term experience of the authors confirms these data. In Table 2 personal data and other groups’ experiences are summarized.
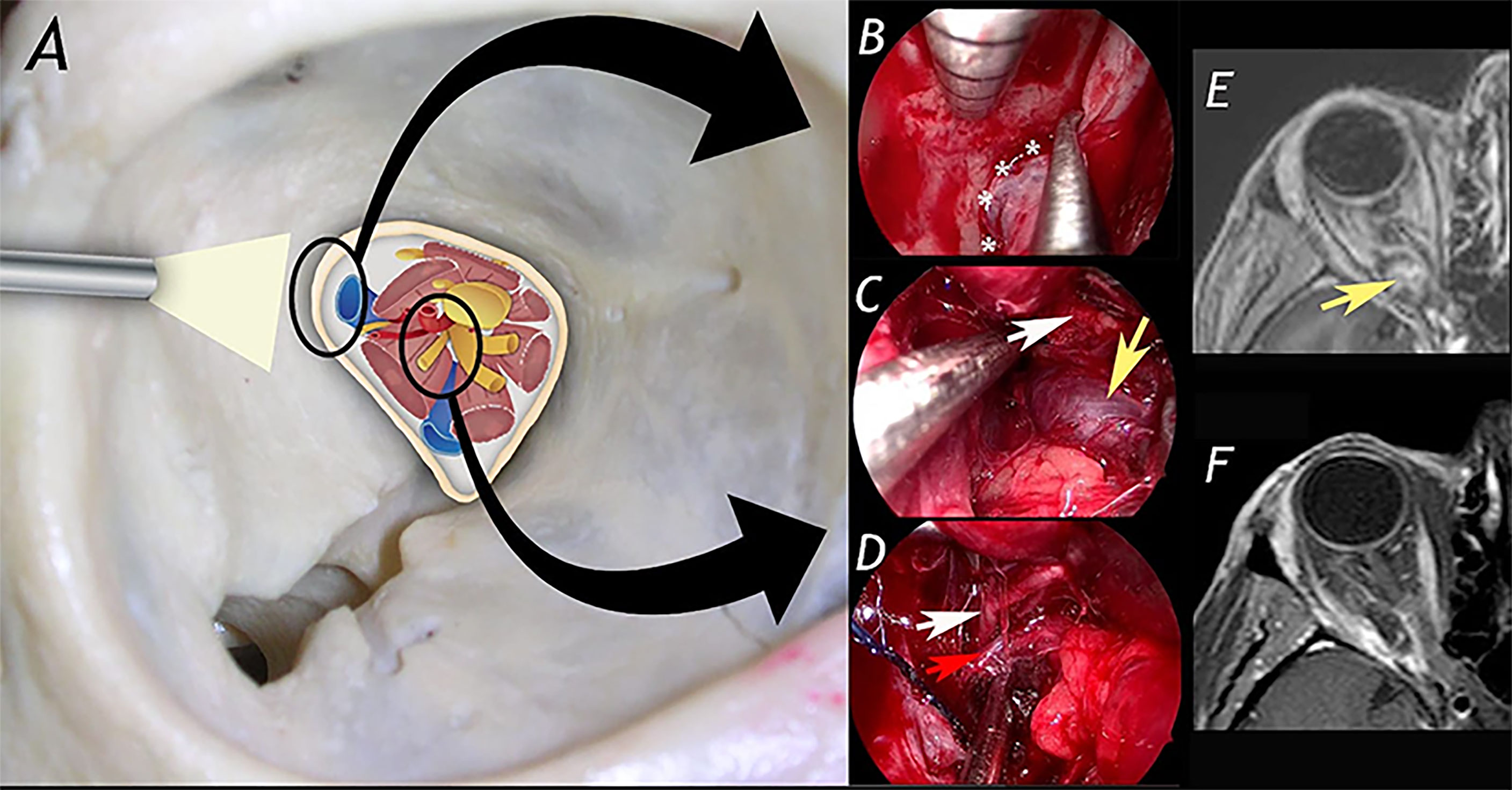
Figure 1 A 42-year-old woman affected by right orbital apex cavernous hemangioma (with progressive worsening of right visual field) was treated via superior eyelid endoscopic-assisted approach with complete resection of the lesion and no significant morbidity. (A) schematic drawing showing anatomical structures in the orbital apex. In (B–D) surgical steps of the procedure. (B): exposure of the lateral aspect of the superior orbital fissure. (C): identification of the cavernous hemangioma. (D): dissection of the lesion from superior division of III cranial nerve and ophthalmic artery. (E, F) show pre- and post-operative MRI. White asterisks: lateralaspect of superior orbital fissure; white arrow: superior division of the oculomotor nerve; yellow arrow: cavernous hemangioma; red arrow: ophthalmic artery.
Generally speaking, surgeons spend most of their professional life acquiring new surgical skills and learning new surgical procedures, the real value of which will be judged by time. As a matter of fact, understanding the surgical anatomy of TOAs requires a certain eclecticism and dedicated training. It is well established that, when learning a new procedure, performance tends to improve with experience. Graphically plotting performance against experience produces what is called a “learning curve” (36). This model applies across the full spectrum of medical science and procedures; however, with the advent of technically demanding minimally invasive techniques, surgery in particular is where there are specific and potentially dramatic implications. As demonstrated in colorectal cancer surgery, surgical experience and case-volume, combined with technological resources, are good prognostic factors for the patients’ outcome. Therefore, before reaching an adequate proficiency (which means being skilled in doing or using something), several obstacles have to be overcome. The most important of these obstacles is probably the volume of cases. In other words, the number of cases performing a specific procedure seems to be critical (37). The real problem is that this number is not known in skull base surgery. As well underlined by Snyderman, the learning curve in endoscopic skull base surgery has to deal with issues of knowledge of endoscopic anatomy, quality of instrumentation, 2-D visualization and team dynamics (37). Furthermore, the quality of the learning experience is of paramount importance. Different situations offer different information to the surgeon. All these factors can be important in determining the final outcome (than means a proper management of the patient). Historical data seem to confirm that, in respect to pituitary surgery, proficiency can be achieved after 20-50 cases (38–41). But these numbers do not consider a lot of factors. If we transpose these considerations to TOAs we easily understand that several concerns can be raised. First, as TOAs are a “recent” approach, there are a lot of controversies regarding their correct indications. An honest rethinking of our 10-year experience makes us perfect witnesses to this aspect. In all honesty and with our actual experience and knowledge, looking retrospectively we would have not managed with these approaches some of our early-experience patients (Figure 2). Unfortunately we consider this a price to be paid in the early phase of any given procedure/treatment. On the other hand, the low number of possible candidates to TOAs procedures and the validity of the coded traditional approaches, make the patient-selection process difficult. In detail, if we consider as possible candidates for TOAs intraorbital pathologies and skull base lesions (mostly spheno-orbital meningiomas and meckels’ cave tumors), we can only collect a small cohort of patients. From an epidemiological point of view, these pathologies are extremely rare. Estimated incidence of sphenoid ridge meningioma (SOM) is about 1, 5 cases per 100000 person-years (42). Trigeminal schwannomas account for less than 0.4% of intracranial tumors. And obviously, not all these cases can eventually be considered good candidates for a TOA. Indeed, if we change perspective and check current existing medical literature, our doubts are confirmed (31, 43–49). Case series on SOM are normally collected over decades and seldom include more than 50 patients. Numbers of trigeminal schwannomas are even less. Furthermore, current literature includes patient series often covering multiple decades, while surgical techniques have improved over the years. These numbers open a serious debate: how can we deal with a proper learning curve in TOAs given the number of cases available? Which is the number of cases to be performed before achieving technical proficiency? From our point of view, confidence with these approaches can be achieved after at least 50 cases (although we have no clear and objective data, our experience of more than 200 TOAs seems to be a sound base for balanced considerations). Obviously, this is only our feeling. Furthermore, we do feel that this proficiency can be maintained only if at least 20 cases a year are performed.
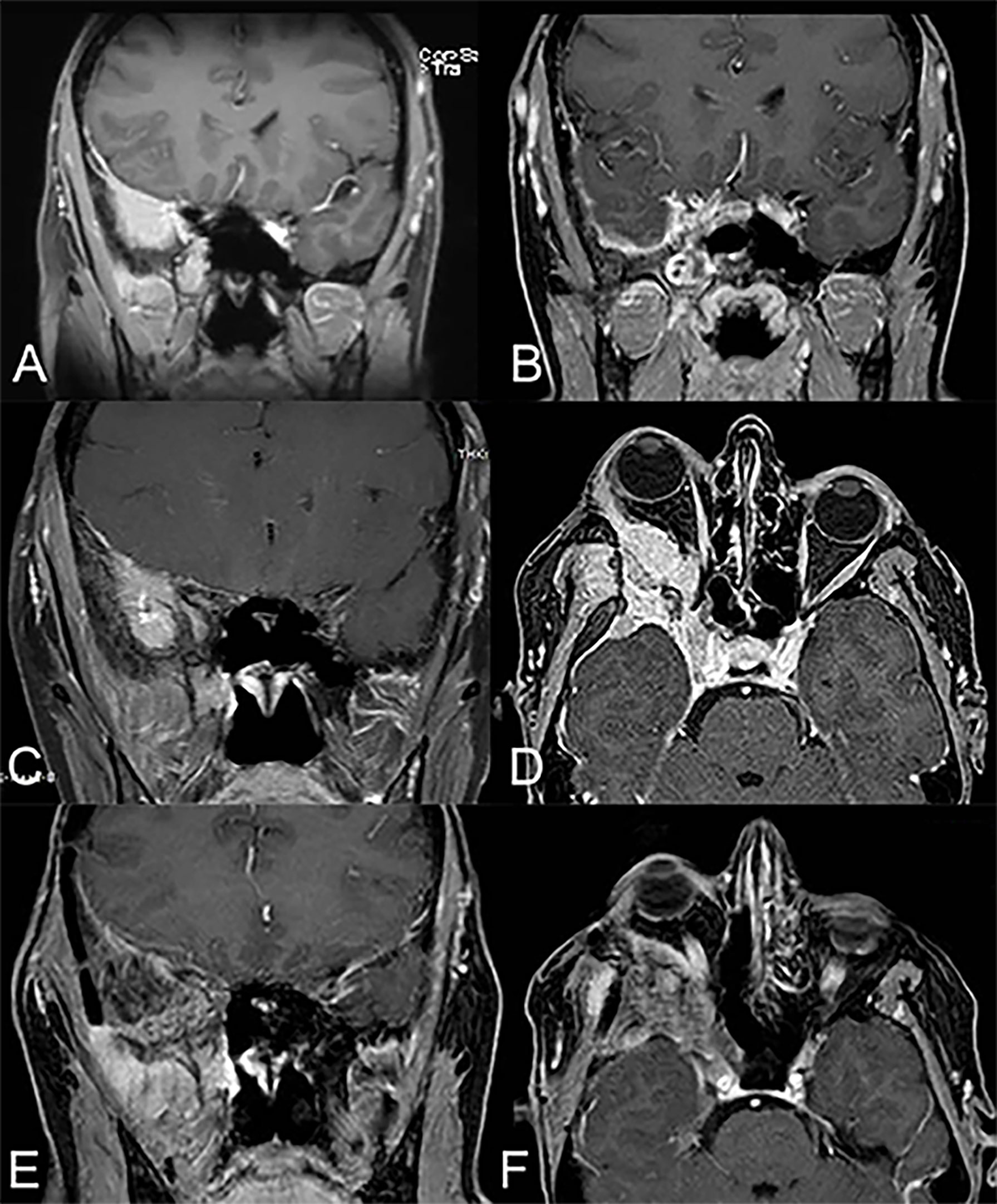
Figure 2 A 48-year-old woman affected by right spheno-temporal meningioma (pre-operative MRI depicted in (A)) was submitted to endoscopic-assisted resection via combined trans-nasal and trans-orbital corridors, obtaining gross total resection (post-operative MRI depicted in (B)). During the follow-up, 5 years after the primary treatment, she developed right proptosis and periorbital pain and the MRI documented a recurrence of the meningioma (MRI in panel (C) involving also the transorbital corridor (MRI in panel (D). The patient was then submitted to revision surgery via transcranial approach (right frontotemporal orbitozygomatic craniotomy). The MRI performed two years after revision surgery were clear from macroscopic recurrence of disease (E, F), with partial resolution of the right orbital pain and proptosis of the patient.
It’s clear that the evolution of surgery, and, in this contest, of TOAs, is still underway. But to what end? On one hand surgeons and hospitals are committed to deliver to the patient always the best possible outcome. But, on the other one, is surgery necessarily the right approach? Everyone talking about future applications of any given or medical procedure needs to be open-minded and, to some extent, provocative. In this respect, we strongly feel that, to best serve our patients, surgeons need to reevaluate their isolated position within the medical profession. If surgeons continue to work within an isolated medical arena, they may risk missing break-event moments and fundamental knowledge. Everyone knows that falling in love with any given procedure, mastering it and getting popularity from it, is very easy. As Ulysses, we, as surgeons, need to be tied to the boat and think of our targets, in order not to surrender to sirens’ voices. For decades and decades surgeons had had a surgery-based approach. In recent years something has changed. Despite this, there’s still a long way to go. Understanding biological, psychological and economical aspects and not limiting our perspective only to surgery will be a game changer. To do this we really need to act, synergistically, in a truly multidisciplinary environment. This multidisciplinary and disease–centered approach will facilitate the development of novel techniques. This will be easier to achieve if both surgical and nonsurgical specialists are involved in the global-procedural management of the patient. In other words, disease-based practices will facilitate a focus on outcomes and patient necessities. In this respect technological evolutions and refinements, such as integrated suites with all facilities available, are a step forward. There is no doubt that surgeons are still missing technologies and real-time clinical data to improve decision-making processes. These are critical in the setting of high-pressure and highly variable situations which happen constantly during any skull base surgery. More specifically, in transorbital procedures, sophisticated autostatic orbital retractors, real-time visual function checking systems and dedicated instrumentations able to increase the identification and dissections of noble structures (e.g. small vessels and nerves) will increase the safety and consequently the efficacy of this kind of procedures. Furthermore, pre-operative functional studies will greatly help in the correct indication of the patients. But only targeted therapies, that imply a truly personalized treatment, based on genetics, omics, and so on, will lead the way to the future.
Whether TOAs will get or not a sound value for patients is still a matter of discussion. Certainly this “corridor” has expanded the armamentarium of skull base surgeons. The rapidly growing number of publications on this topic reflects a vivid interest. But similarly to what happened in the gold rush, not all the participants can be lucky and not all that shines is gold.
One thing is certain: surgery will not stay the same. As in the 1900s, technology and knowledge will catch up with imagination and the evolution of surgery and medicine will continue. The real revolution will be moving from a surgical-based approach to a truly disease-based approach. Because that’s the worthy goal: to deliver consistently superior patient outcomes regardless of surgeon skills, training or location.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
ID made substantial contributions to conception and design. He participated in drafting the article and revising it critically for important intellectual content. LC-M made substantial contributions to acquisition of data and analysis; participated in drafting the article and revising it critically for important intellectual content. MP made substantial contributions and participated in revising the article critically for important intellectual content. GF made substantial contributions and participated in revising the article critically for important intellectual content. MT-Z participated in revising the article critically for important intellectual content and he gave final approval of the version to be submitted. WF participated in revising the article critically for important intellectual content and he gave final approval of the version to be submitted. MN participated in revising the article critically for important content and he gave final approval of the version to be submitted. CG-H participated in revising the article critically for important content. He participated in drafting the article and he gave final approval of the version to be submitted. LB made substantial contributions to conception and design. He participated in drafting the article and gave final approval of the version to be submitted. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Moe KS, Bergeron CM, Ellenbogen RG. Transorbital neuroendoscopic surgery. Neurosurgery (2010) 67(3 Suppl Operative):ons16–28. doi: 10.1227/01.NEU.0000373431.08464.43
2. Vural A, Carobbio ALC, Ferrari M, Rampinelli V, Schreiber A, Mattavelli D, et al. Transorbital endoscopic approaches to the skull base: A systematic literature review and anatomical description. Neurosurg Rev (2021) 44(5):2857–78. doi: 10.1007/s10143-020-01470-5
3. Prabhakaran VC, Selva D. Orbital endoscopic surgery. Indian J Ophthalmol (2008) 56(1):5–8. doi: 10.4103/0301-4738.37587
4. Balakrishnan K, Moe KS. Applications and outcomes of orbital and transorbital endoscopic surgery. Otolaryngol Head Neck Surg (2011) 144(5):815–20. doi: 10.1177/0194599810397285
5. Lim JH, Sardesai MG, Ferreira M Jr, Moe KS. Transorbital neuroendoscopic management of sinogenic complications involving the frontal sinus, orbit, and anterior cranial fossa. J Neurol Surg B Skull Base (2012) 73(6):394–400. doi: 10.1055/s-0032-1329617
6. Lyson T, Sieskiewicz A, Rogowski M, Mariak Z. Endoscopic lateral orbitotomy. Acta Neurochir (Wien) (2014) 156(10):1897–900. doi: 10.1007/s00701-014-2205-7
7. Kong DS, Young SM, Hong CK, Kim YD, Hong SD, Choi JW, et al. Clinical and ophthalmological outcome of endoscopic transorbital surgery for cranioorbital tumors. J Neurosurg (2018) 131(3):667–75. doi: 10.3171/2018.3.JNS173233
8. Dallan I, Locatelli D, Turri-Zanoni M, Battaglia P, Lepera D, Galante N, et al. Transorbital endoscopic assisted resection of a superior orbital fissure cavernous haemangioma: A technical case report. Eur Arch Otorhinolaryngol (2015) 272(12):3851–6. doi: 10.1007/s00405-015-3556-2
9. Dallan I, Castelnuovo P, Turri-Zanoni M, Fiacchini G, Locatelli D, Battaglia P, et al. Transorbital endoscopic assisted management of intraorbital lesions: Lessons learned from our first 9 cases. Rhinology (2016) 54(3):247–53. doi: 10.4193/Rhino15.237
10. Almeida JP, Ruiz-Treviño AS, Shetty SR, Omay SB, Anand VK, Schwartz TH. Transorbital endoscopic approach for exposure of the sylvian fissure, middle cerebral artery and crural cistern: An anatomical study. Acta Neurochir (Wien) (2017) 159(10):1893–907. doi: 10.1007/s00701-017-3296-8
11. Alqahtani A, Padoan G, Segnini G, Lepera D, Fortunato S, Dallan I, et al. Transorbital transnasal endoscopic combined approach to the anterior and middle skull base: A laboratory investigation. Acta Otorhinolaryngol Ital (2015) 35(3):173–9.
12. Bly RA, Su D, Hannaford B, Ferreira M Jr, Moe KS. Computer modeled multiportal approaches to the skull base. J Neurol Surg B Skull Base (2012) 73(6):415–23. doi: 10.1055/s-0032-1329623
13. Bly RA, Ramakrishna R, Ferreira M, Moe KS. Lateral transorbital neuroendoscopic approach to the lateral cavernous sinus. J Neurol Surg B Skull Base (2014) 75(1):11–7. doi: 10.1055/s-0033-1353363
14. Chen HI, Bohman LE, Emery L, Martinez-Lage M, Richardson AG, Davis KA, et al. Lateral transorbital endoscopic access to the hippocampus, amygdala, and entorhinal cortex: Initial clinical experience. ORL J Otorhinolaryngol Relat Spec (2015) 77(6):321–32. doi: 10.1159/000438762
15. Dallan I, Di Somma A, Prats-Galino A, Solari D, Alobid I, Turri-Zanoni M, et al. Endoscopic transorbital route to the cavernous sinus through the meningo-orbital band: a descriptive anatomical study. J Neurosurg (2017) 127(3):622–9. doi: 10.3171/2016.8.JNS16465
16. Dallan I, Sellari-Franceschini S, Turri-Zanoni M, de Notaris M, Fiacchini G, Fiorini FR, et al. Endoscopic transorbital superior eyelid approach for the management of selected spheno-orbital meningiomas: Preliminary experience. Oper Neurosurg (Hagerstown) (2018) 14(3):243–51. doi: 10.1093/ons/opx100
17. Di Somma A, Cavallo LM, de Notaris M, Solari D, Topczewski TE, Bernal-Sprekelsen M, et al. Endoscopic endonasal medial-to-lateral and transorbital lateral-to-medial optic nerve decompression: An anatomical study with surgical implications. J Neurosurg (2017) 127(1):199–208. doi: 10.3171/2016.8.JNS16566
18. Di Somma A, Andaluz N, Cavallo LM, de Notaris M, Dallan I, Solari D, et al. Endoscopic transorbital superior eyelid approach: Anatomical study from a neurosurgical perspective. J Neurosurg (2018) 129(5):1203–16. doi: 10.3171/2017.4.JNS162749
19. Dallan I, Cristofani-Mencacci L, Fiacchini G, Caniglia M, Sellari-Franceschini S, Berrettini S. When multidisciplinary surgical trans-orbital approaches should be considered to reach the skull base. Acta Otorhinolaryngol Ital (2021) 41(Suppl. 1):S59–66. doi: 10.14639/0392-100X-suppl.1-41-2021-06
20. Gerges MM, Godil SS, Younus I, Rezk M, Schwartz TH. Endoscopic transorbital approach to the infratemporal fossa and parapharyngeal space: A cadaveric study. J Neurosurg (2019) 1:1–12. doi: 10.3171/2019.7.JNS191743
21. Park HH, Roh TH, Choi S, Yoo J, Kim WH, Jung IH, et al. Endoscopic transorbital approach to mesial temporal lobe for intra-axial lesions: Cadaveric study and case series (SevEN-008). Oper Neurosurg (Hagerstown) (2021) 21(6):E506–15. doi: 10.1093/ons/opab319
22. Gillham HE, Lucke-Wold B, Dogan A, Cetas J, Cameron WE, Ciporen JN. Development of a cadaveric multiport model of posterior circulation aneurysm clipping for neurosurgery and otolaryngology residents. J Vis Exp (2021) 175):56809. doi: 10.3791/56809
23. Noiphithak R, Yanez-Siller JC, Revuelta Barbero JM, Otto BA, Carrau RL, Prevedello DM. Quantitative analysis of the surgical exposure and surgical freedom between transcranial and transorbital endoscopic anterior petrosectomies to the posterior fossa. J Neurosurg (2018) 131(2):569–77. doi: 10.3171/2018.2.JNS172334
24. Lima LR, Beer-Furlan A, Prevedello DM, Carrau RL, Servián-Duarte DA, Galarce MG, et al. Minimally invasive approaches to the lateral cavernous sinus and meckel's cave: Comparison of transorbital and subtemporal endoscopic techniques. World Neurosurg (2020) 141, e86–e96. doi: 10.1016/j.wneu.2020.04.180
25. Dallan I, Cristofani-Mencacci L, Cambi C, Scarano M, Casani AP, Seccia V. Effectiveness of superior eyelid endoscopic-assisted approach in the management of selected orbital abscess: Considerations on 4 cases. Acta Otorhinolaryngol Ital (2020) 40(6):421–5. doi: 10.14639/0392-100X-N0679
26. Dallan I, Castelnuovo P, Locatelli D, Turri-Zanoni M, AlQahtani A, Battaglia P, et al. Multiportal combined transorbital transnasal endoscopic approach for the management of selected skull base lesions: Preliminary experience. World Neurosurg (2015) 84(1):97–107. doi: 10.1016/j.wneu.2015.02.034
27. Sellari-Franceschini S, Rocchi R, Marinò M, Bajraktari A, Mazzi B, Fiacchini G, et al. Rehabilitative orbital decompression for graves' orbitopathy: Results of a randomized clinical trial. J Endocrinol Invest (2018) 41(9):1037–42. doi: 10.1007/s40618-018-0847-7
28. Jeon C, Hong SD, Woo KI, Seol HJ, Nam DH, Lee JI, et al. Use of endoscopic transorbital and endonasal approaches for 360° circumferential access to orbital tumors. J Neurosurg (2020) 135, 1–10. doi: 10.3171/2020.6.JNS20890
29. Jeon C, Hong CK, Woo KI, Hong SD, Nam DH, Lee JI, et al. Endoscopic transorbital surgery for meckel's cave and middle cranial fossa tumors: Surgical technique and early results. J Neurosurg (2018) 1:1–10. doi: 10.3171/2018.6.JNS181099
30. Almeida JP, Omay SB, Shetty SR, Chen YN, Ruiz-Treviño AS, Liang B, et al. Transorbital endoscopic eyelid approach for resection of sphenoorbital meningiomas with predominant hyperostosis: report of 2 cases. J Neurosurg (2018) 128(6):1885–95. doi: 10.3171/2017.3.JNS163110
31. Park HH, Hong SD, Kim YH, Hong CK, Woo KI, Yun IS, et al. Endoscopic transorbital and endonasal approach for trigeminal schwannomas: A retrospective multicenter analysis (KOSEN-005). J Neurosurg (2020) 133(2):467–76. doi: 10.3171/2019.3.JNS19492
32. Lee MH, Hong SD, Woo KI, Kim YD, Choi JW, Seol HJ, et al. Endoscopic endonasal versus transorbital surgery for middle cranial fossa tumors: Comparison of clinical outcomes based on surgical corridors. World Neurosurg (2019) 122:e1491–504. doi: 10.1016/j.wneu.2018.11.090
33. Yoo J, Park HH, Yun IS, Hong CK. Clinical applications of the endoscopic transorbital approach for various lesions. Acta Neurochir (Wien) (2021) 163(8):2269–77. doi: 10.1007/s00701-020-04694-y
34. Zoia C, Bongetta D, Gaetani P. Endoscopic transorbital surgery for spheno-orbital lesions: How I do it. Acta Neurochir (Wien) (2018) 160(6):1231–3. doi: 10.1007/s00701-018-3529-5
35. Locatelli D, Restelli F, Alfiero T, Campione A, Pozzi F, Balbi S, et al. The role of the transorbital superior eyelid approach in the management of selected spheno-orbital meningiomas: In-depth analysis of indications, technique, and outcomes from the study of a cohort of 35 patients. J Neurol Surg B Skull Base (2020) 83(2):145–58. doi: 10.1055/s-0040-1718914
36. Hopper AN, Jamison MH, Lewis WG. Learning curves in surgical practice. Postgrad Med J (2007) 83(986):777–9. doi: 10.1136/pgmj.2007.057190
37. Snyderman CH, Fernandez-Miranda J, Gardner PA. Training in neurorhinology: The impact of case volume on the learning curve. Otolaryngol Clin North Am (2011) 44(5):1223–8. doi: 10.1016/j.otc.2011.06.014
38. Leach P, Abou-Zeid AH, Kearney T, Davis J, Trainer PJ, Gnanalingham KK. Endoscopic transsphenoidal pituitary surgery: evidence of an operative learning curve. Neurosurgery (2010) 67(5):1205–12. doi: 10.1227/NEU.0b013e3181ef25c5
39. Smith SJ, Eralil G, Woon K, Sama A, Dow G, Robertson I. Light at the end of the tunnel: the learning curve associated with endoscopic transsphenoidal skull base surgery. Skull Base (2010) 20(2):69–74. doi: 10.1055/s-0029-1238214
40. O'Malley BW Jr, Grady MS, Gabel BC, Cohen MA, Heuer GG, Pisapia J, et al. Comparison of endoscopic and microscopic removal of pituitary adenomas: single-surgeon experience and the learning curve. Neurosurg Focus (2008) 25(6):E10. doi: 10.3171/FOC.2008.25.12.E10
41. Eseonu CI, ReFaey K, Rincon-Torroella J, Garcia O, Wand GS, Salvatori R, et al. Endoscopic versus microscopic transsphenoidal approach for pituitary adenomas: Comparison of outcomes during the transition of methods of a single surgeon. World Neurosurg (2017) 97:317–25. doi: 10.1016/j.wneu.2016.09.120
42. Hanna EY, De Monte F. Comprehensive management of skull base tumors. Boca Raton: CRC Press (2008). 503p.
43. Belinsky I, Murchison AP, Evans JJ, Andrews DW, Farrell CJ, Casey JP, et al. Spheno-orbital meningiomas: An analysis based on world health organization classification and ki-67 proliferative index. Ophthalmic Plast Reconstr Surg (2018) 34(2):143–50. doi: 10.1097/IOP.0000000000000904
44. ZamanipoorNajafabadi AH, Genders SW, van Furth WR. Visual outcomes endorse surgery of patients with spheno-orbital meningioma with minimal visual impairment or hyperostosis. Acta Neurochir (Wien) (2021) 163(1):73–82. doi: 10.1007/s00701-020-04554-9
45. Freeman JL, Davern MS, Oushy S, Sillau S, Ormond DR, Youssef AS, et al. Spheno-orbital meningiomas: A 16-year surgical experience. World Neurosurg (2017) 99:369–80. doi: 10.1016/j.wneu.2016.12.063
46. Amirjamshidi A, Abbasioun K, Amiri RS, Ardalan A, Hashemi SM. Lateral orbitotomy approach for removing hyperostosingen plaque sphenoid wing meningiomas. description of surgical strategy and analysis of findings in a series of 88 patients with long-term follow up. Surg Neurol Int (2015) 6:79. doi: 10.4103/2152-7806.157074
47. Cárdenas Ruiz-Valdepeñas E, SimalJulián JA, Pérez Prat G, Arraez MA, Ambrosiani J, Martin Schrader I, et al. The quadrangular space, endonasal access to the meckel cave: Technical considerations and clinical series. World Neurosurg (2022) S1878-8750(22):00369–2. doi: 10.1016/j.wneu.2022.03.077
48. Raza SM, Donaldson AM, Mehta A, Tsiouris AJ, Anand VK, Schwartz TH. Surgical management of trigeminal schwannomas: Defining the role for endoscopic endonasal approaches. Neurosurg Focus (2014) 37(4):E17. doi: 10.3171/2014.7.FOCUS14341
Keywords: transorbital endoscopic surgery, orbital surgery, multiportal surgery, TOAs, learning curve, skull base surgery
Citation: Dallan I, Cristofani-Mencacci L, Fiacchini G, Turri-Zanoni M, van Furth W, de Notaris M, Picariello M, Alexandre E, Georgalas C and Bruschini L (2022) Endoscopic-assisted transorbital surgery: Where do we stand on the scott’s parabola? personal considerations after a 10-year experience. Front. Oncol. 12:937818. doi: 10.3389/fonc.2022.937818
Received: 06 May 2022; Accepted: 24 June 2022;
Published: 15 July 2022.
Edited by:
Doo-Sik Kong, Sungkyunkwan University, South KoreaReviewed by:
Raywat Noiphithak, Thammasat University Hospital, ThailandCopyright © 2022 Dallan, Cristofani-Mencacci, Fiacchini, Turri-Zanoni, van Furth, de Notaris, Picariello, Alexandre, Georgalas and Bruschini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lodovica Cristofani-Mencacci, bG9kb3ZpY2FjcmlzdG9mYW5pQHlhaG9vLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.