- 1Department of Urology, Medical Center-University of Freiburg, Freiburg, Germany
- 2Faculty of Medicine, University of Freiburg, Freiburg, Germany
Despite decades of research and successful improvements in diagnosis and therapy, prostate cancer (PC) remains a major challenge. In recent years, it has become clear that PC stem cells (PCSCs) are the driving force in tumorigenesis, relapse, metastasis, and therapeutic resistance of PC. In this minireview, we discuss the impact of PCSCs in the clinical practice. Moreover, new therapeutic approaches to combat PCSCs are presented with the aim to achieve an improved outcome for patients with PC.
Introduction
Prostate cancer (PC) is the most common cancer and the second leading cause of cancer death in men from industrial countries. More than 1.41 million new cases and more than 375,000 deaths by this tumor are expected worldwide every year (1).
If the tumor is limited to the prostate, a good chance of cure is the surgical resection [radical prostatectomy (RP)] or radiation of the organ. Both treatment options are associated with adverse effects, such as incontinence and sexual dysfunction, which negatively affect the quality of life. The prerequisite for cure is the complete removal of the tumor. If residual tumor cells persist, the tumor may soon relapse and begin to metastasize. Overall, biochemical failure after RP in node-negative patients occurs in approximately 15%–40% of cases within 5 years and is independent of the surgical approach [reviewed in (2)]. The only potentially curative treatment for patients with local recurrence at the earliest sign of biochemical failure is salvage radiation therapy, preferably for PSA levels <0.2 ng/ml (3). In case of metastasized tumors, treatment options include androgen deprivation therapy (ADT) and chemotherapy (4, 5). However, chemo- and castration-resistant PC commonly develop and mainly contribute to therapy failure and mortality (6, 7).
One model that explains heterogeneity, tumor-initiating capability, and therapeutic resistance of tumors is the cancer stem cell (CSC) hypothesis. The CSC hypothesis postulates that cancer cells are hierarchically organized and form different heterogeneous subpopulations within a tumor. CSCs are on top of the hierarchy and represent cancer cells with stem cell-like properties, such as self-renewal, pluripotency, and plasticity, that evolve during the lifetime of a tumor (8, 9). Different factors are discussed that might foster the emergence of CSCs, like de-differentiation through genetic and epigenetic alterations, clonal expansion, and adaptation through epithelial–mesenchymal transition (EMT) as well as transdifferentiation under the influence of the tumor microenvironment or under therapeutic pressure [reviewed in (10)]. CSCs were found to be the driving force in tumor progression, metastasis, and therapeutic resistance, and new strategies are being developed to identify and treat them (11). Our minireview describes clinical aspects of prostate CSCs (PCSCs) and new therapeutic options, aiming to achieve a cure for hitherto incurable stages of the disease.
Prostate Cancer Stem Cells
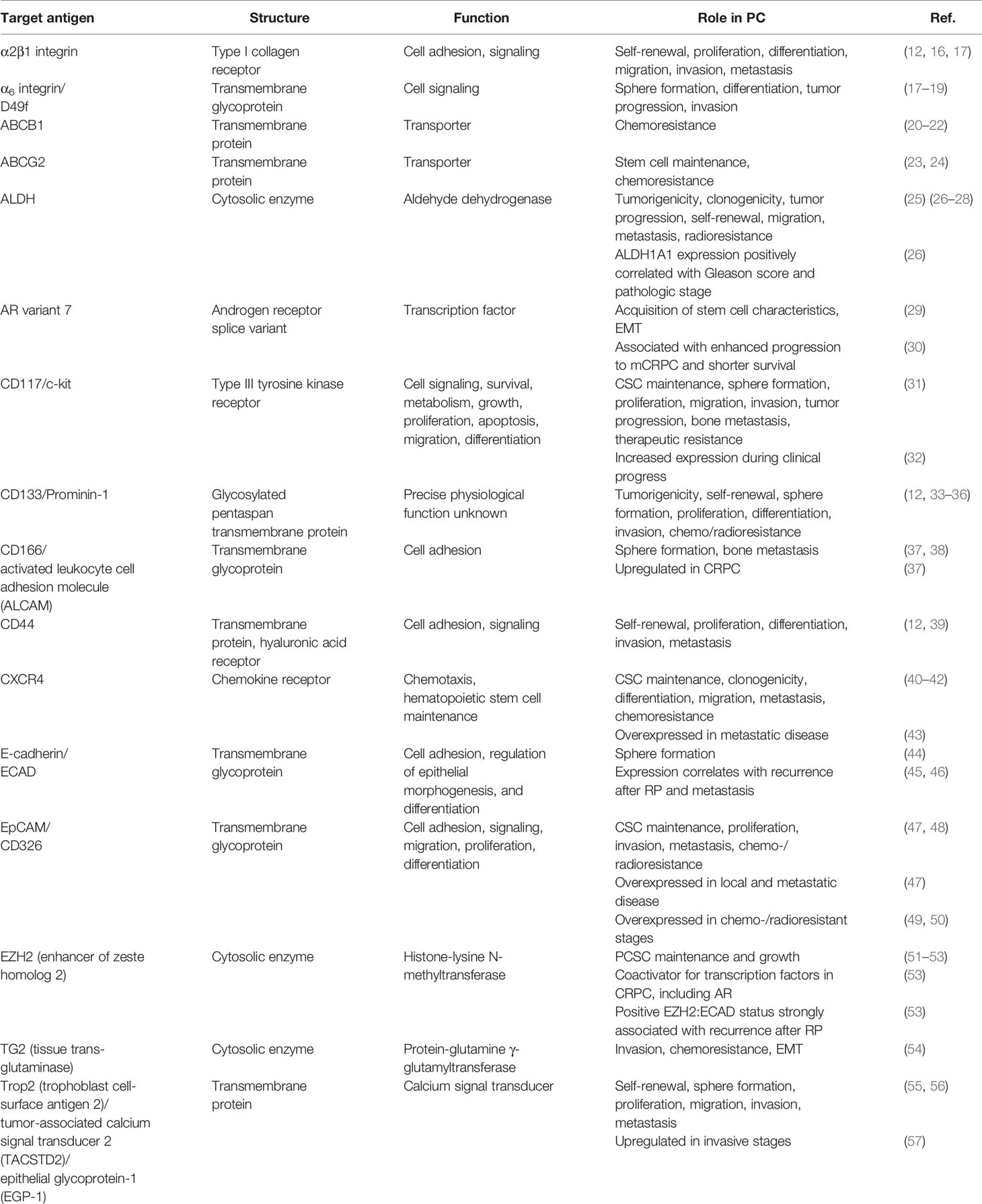
PC cells with stem cell characteristics, such as self-renewal, pluripotency, and plasticity, were isolated from patients undergoing RP for the first time in 2005 (12). PCSCs can also be obtained from established PC cell lines, especially of metastatic origin (12–14) and are characterized by sphere formation under non-adherent culture conditions, high clonogenicity, high rate of self-renewal, and the ability to form phenotypically mixed populations of non-clonogenic cells (12, 15). Different antigens, which are involved in various signaling pathways of tumorigenesis, metastasis, and therapeutic resistance of PC, were identified to characterize PCSCs (Table 1) (58).
PCSCs might originate from normal prostate stem cells, normal prostate progenitor cells, or differentiated normal prostate cells after genetic and epigenetic alterations or changes in the tumor microenvironment (15). It was found that activation of the proto-oncogene MYC, loss of the tumor suppressor PTEN, or mutations in the repair genes BRCA2, ATM, and CHEK2, induce genomic instability and drive progression and heterogeneity of PC (10, 59, 60). Polson and colleagues showed a high frequency of the TMPRSS2:ERG gene fusion not only in differentiated PC cells but also in PCSC (61). When the transcription factor ERG comes under the control of the prostate-specific, androgen-regulated TMPRSS2 gene promoter, enhanced ERG expression is found. Since enhanced EGR expression can influence differentiation, self-renewal, and maintenance of SCs, it is discussed that the TMPRSS2: ERG fusion might also play a decisive role in the emergence and maintenance of PCSCs (62).
PCSCs can evolve from basal or luminal epithelial cells after oncogenic transformation (11). Recent findings from a single-cell sequencing study in mice suggest that differentiated luminal cells that survived castration can contribute to prostate regeneration by acquisition of stem cell-like regenerative properties (63). There is also evidence that PCSCs might come from the basal cell layer, because tissue-derived tumor-initiating cells in immunocompromised mice expressed basal markers (such as p63), but did not express the androgen receptor (AR) or markers of luminal differentiation (PSA and PAP) (64). Moreover, there might be a loss of basal cells and expansion of luminal cells during PC tumorigenesis (65). For example, Choi and colleagues demonstrated that inactivation of the tumor suppressor PTEN induced the differentiation of basal cells to transformation-competent luminal cells (66).
PCSCs can differentiate into PC progenitor cells (PCPCs) or differentiated PC cells (DPCCs), which leads to the typical formation of heterogeneous prostate tumors with increasing grading that is determined by the Gleason score. Interestingly, Castellon et al. found highest expression of the stem cell markers CD133, CD44, and ABCG2 in medium-grade Gleason biopsies compared to lower- or higher-grade biopsies or lymph-node and bone metastases (67). This suggests that PCSCs reach a significant number at stages, in which the tumor seems to be confined to the gland and in which surgical treatment or radiation is usually with curative intention. However, many PC patients develop biochemical relapse despite local therapy (68, 69). It is therefore assumed that PCSCs remain in the surgical or radiation area or have already entered blood circulation and colonized lymph nodes or other organs due to their ability to migrate and persist in extra-prostatic tissues (67). Number and signatures of PCSCs in local tumors are therefore discussed as prognostic factors for PC recurrence (70). For example, significantly enhanced expression of the stem cell markers SOX2, OCT4, KLF4, and ABCG2 in recurrent PC tissues in comparison to non-recurrent PC tissues was found after RP (71).
Radiation is a therapeutic option for local disease, recurrence, or advanced PC. However, radioresistance of PC cells is an obstacle for successful radiation therapy. A subpopulation of PC cells with CSC characteristics was found after radiation that was marked by enhanced PI3K/Akt/mTOR signaling (72). PCSCs therefore appear to contribute to the formation of radioresistant tumors.
PC cells metastasize preferentially in lymph nodes, liver, and bones (73). The spine, pelvis, and ribs are the most frequently observed sites of bone metastasis in PC. This distribution is often multifocal, and preferable involvement of the axial skeleton suggests an affinity to the red bone marrow. It seems that the bone marrow is particularly suitable as a metastatic site for PC cells, because it is strongly supplied with a low blood flow rate. In addition, it seems that the bone marrow, which harbors the hematopoietic stem cells, forms a suitable niche for disseminated PCSCs. About 10% of patients already harbor bone metastases at the time of diagnosis and 70%–80% of patients, who relapse after RP, fatally progress to advanced disease with bone metastases. This confirms that there are already subpopulations of PC cells in early-diagnosed, local prostate tumors with stem-cell like properties that are able to disseminate and colonize distant organs. Metastatic PC cells are marked by a high expression of integrins that promote their adherence to a broad spectrum of proteins of the bone extracellular matrix, and release factors (FGFs, IGFs, VEGF, or Wnt pathway-related factors, originally involved in bone formation and maintenance) for persistence [reviewed in (74)]. Castellon and colleagues found only low expression of PCSC markers in metastases from lymph nodes and bone, but explained this phenomenon with prevalence of PCPCs and DPCCs (67).
PCSCs are marked by low or lack of androgen receptor (AR) expression (75) and, as a result, by a missing or reduced PSA release. Therefore, they might escape a PSA screening and androgen receptor expression and measurable PSA values might only be detected when the metastatic PCSCs have already differentiated and expanded. This could be an explanation for the often observed discrepancy between detection of biochemical recurrence (defined by a rising PSA profile) and already existing progressive disease (76).
First-line treatment of advanced PC is ADT. In general, tumors initially respond well to ADT; however, the therapeutic effect of ADT only lasts for a limited period of 12–33 months. At that point, ADT-resistant PC cells emerge and form castration-resistant tumor lesions (77). There is growing evidence that PCSCs contribute to this phenomenon. Since PCSCs were found to be AR-negative and have the ability to grow androgen independently, they might have a survival benefit when treated with ADT (78). Indeed, whereas AR+/PSA+ tumor cells form the main cell population in untreated androgen-sensitive tumors, enrichment of AR-/lo and PSA-/lo cells was found in untreated higher grades of the disease and in CRPC (79). Moreover, genes associated with CSCs, like OCT4, SOX2, NANOG, BMI1, BMP2, CD44, SOX9, and ALDH1, were found to be upregulated in enzalutamide-resistant cells (80). There is evidence that truncated AR variants, which lack the ligand binding domain, but retained transcriptional activity, play a decisive role. In particular, the variant AR-V7 might be involved in EMT and promotes the emergence of PCSCs (81).
Re-programming of PC cells to stem-like cells during ADT was demonstrated in a recent study. After androgen depletion over 90 days, a re-differentiation to a stem-like phenotype was observed in LNCaP cells, which was marked by growth as floating spheres and enhanced expression of CD133, ALDH1A1, and the multidrug resistance protein transporter ABCB1A. Interestingly, ABCB1A expression in the re-differentiated stem-like cells was associated with enhanced resistance against docetaxel and 2-hydroxyflutamide (20). This provides a rationale that chemoresistance may already be induced in prostate tumors during ADT and reinforces the medical guidelines recommending chemotherapy in hormone-naïve PC (82).
An example for transdifferentiation is the emergence of neuroendocrine PC cells (NEPCCs) in about 25% of patients after treatment with ADT (83). NEPCCs are hormone-refractory and secrete peptide hormones and growth factors for paracrine stimulation of surrounding cells in the microenvironment (83). It was found that loss of PTEN concurrently with inactivation of the tumor suppressors TP53 and Rb1 caused plasticity of PC cells with enhanced metastatic potential and conversion from adenocarcinoma to neuroendocrine PC [reviewed in (84)].
PCSCs also contribute to chemoresistance of PC. Docetaxel (DTX)-resistant cells showed enhanced expression of CD44 and CD133, leading to enhanced migration and invasion (85–87). Moreover, enhanced activity of Notch signaling was found, which promoted DTX resistance by upregulation of ABCC1 (88).
Targeting PCSCs
Current treatments against PC, like ADT, chemotherapy, and radiotherapy, aim to remove bulk tumors, but do not seem to affect resistant PCSCs effectively. Therefore, research is increasingly being conducted into new therapeutic approaches against PCSCs. Such approaches comprise the targeting of PCSC-associated pathways, the targeting of the PCSC microenvironment, and immunotherapies.
Targeting PCSC-Associated Signaling Pathways
The Hedgehog (Hh), Wnt, Notch, and NF-kB pathways, which regulate proliferation, survival, metastasis, apoptosis, recurrence, and therapeutic resistance, were identified to be associated with PCSCs (11, 89–91). Different strategies have therefore been developed to target these pathways by inhibitors or RNA silencing (90, 92–94). Specific inhibitors against the Hh pathway (Sonidegib, GANT-61, and GDC-0449), the Wnt pathway (3289-8625, LGK974, Foxy-5, and OMP-54F28), the Notch pathway (RO4929097), and the NFkB pathway [bortezomib, PS1145, BMS345541, Aspyrin, 17-(allylamino)-17-demethoxygeldanamycin, and BKM120] are tested in preclinical and clinical trials with the intention of attacking PCSCs for an improved therapeutic outcome (90).
The PI3K/AKT/mTOR pathway is associated with PC progression and ADT resistance (95). Suppression of this pathway is therefore discussed to restore sensitivity against ADT, chemotherapy, and radiation (96, 97). In a recent study, an enhanced sensitivity of LNCaP cells against paclitaxel was determined after siRNA knockdown of the stem cell marker CD133. Mechanistically, an induction of the tumor suppressor PTEN accompanied by a decrease of AKT and c-myc oncogenes was found (33). Chang and colleagues were able to restore radiosensitivity and to induce apoptosis in radioresistant PCSCs by the use of the dual PI3K/mTOR inhibitor BEZ235 (72). Marhold and colleagues found elevated HIF1α levels and an enhanced HIF target gene expression in PCSCs under hypoxic conditions. This was accompanied by drug resistance to selective mTOR inhibitors. The authors therefore proposed a deregulation of the PI3K/AKT/mTOR pathway through HIF1α for quiescence and maintenance of PCSCs by attenuating CSC metabolism and growth via mTOR signaling and promoting survival by AKT signaling (98). Since hypoxia often prevails in the tumor microenvironment, targeting the HIF1α pathway might damage PCSCs while sparing normal stem cells.
ABC transporters were found to contribute to drug resistance of PSCs (99) and PCSCs (67). Liu and colleagues examined that the intracellular domain of NOTCH1, called ICN1, directly binds to the promoter region of ABCC1 and that inhibition of NOTCH1 with shRNA decreased ABCC1 expression and restored chemosensitivity of PCSCs (88). ABCG2 was found to play a decisive role in ADT-resistant PSCs by efflux of intracellular androgens. When ABCG2 was blocked by the inhibitor Ko143, an increasing nuclear AR level was observed followed by enhanced AR regulated gene expression and increased differentiation with ADT-sensitive luminal phenotype (23). Future experiments have to prove whether targeted differentiation is a new strategy to sensitize PCSCs to conventional therapies.
Targeting the PCSC Microenvironment
Multiple signaling pathways exist between epithelial cells, stromal cells, and the extracellular matrix of the prostate tumor microenvironment to support tumor progression from the primary site to regional lymph nodes and distant metastases. For example, the CSC niche was found to induce Hh, Wnt, NF-κB, Notch, or PI3K/AKT/mTOR signaling in CSCs (100). Therefore, targeting of these pathways aims to disrupt the interaction between the microenvironment and the tumor cells in order to stop tumor spread [reviewed in (100, 101)].
Since PCSCs are also dependent on a microenvironment, called the PCSC niche, for the maintenance of their stemness properties, research is ongoing to investigate whether targeting of the tumor microenvironment might also lead to damage of PCSCs (11). The monoclonal antibody bevacizumab can be used to target the vascular endothelial growth factor (VEGF) and to reduce the tumor neovasculature for disruption of CSC niches. Bevacizumab-resistant PCSCs were found to have Rac1-mediated ERK activation, and Rac1 inhibition or P-Rex1 downregulation increased their sensitivity to bevacizumab (102). The CXCL12/CXCR4 chemokine pathway was also found to be activated in CD44/CD133-positive PCSCs and to affect cell adhesion, clonal growth, and tumorigenicity. The use of the CXCR4 antagonist AMD3100 inhibited sphere formation and restored the chemosensitivity of PCSCs (103). Since CD44 associates with the extracellular matrix hyaluronic acid (HA) (104), HA-coated liposomes containing cabazitaxel were generated for the inhibition of migration and the triggering of apoptosis in CD44-positive PC cells (105).
Immunotherapy of PCSCs
PCSCs show enhanced expression of cell surface markers that can serve as target antigens for new immunotherapeutic approaches (Table 1). Recently, chimeric antigen receptor (CAR)-modified T-cell therapy targeting CSC-associated tumor antigens emerged as a new therapeutic approach for the treatment of CSCs (106). Zhu et al. demonstrated that anti-C133 CAR-T therapy leads to toxicity of patient-derived glioblastoma CSCs in vitro and in an orthotropic tumor model in vivo (107). Another study by Deng et al. showed that CAR T cells targeting the CSC marker EpCAM reduced PC progression in preclinical models (49). Currently, there is only one completed phase I/II clinical study of CD133-directed CAR T cells for the treatment of relapsed and/or chemotherapy refractory advanced hepatocellular carcinoma (NCT04427449) (108). However, several clinical trials using CAR-T cells targeting CSC surface markers are in the recruiting stage, representing a promising therapeutic option for the treatment of PCSCs in the future (106).
A further immunotherapeutic approach includes the use of dendritic cells preloaded with the PCSC-associated antigens CD44 and EpCAM for the activation of cytokine-induced killer T cells. This led to high cytotoxicity against PCSCs in vitro and antitumor activity in vivo in PCSC-derived xenograft models (109). Ma and colleagues generated aptamer-based liposomes loaded with curcumin to target CD133-positive PC cells and found antitumor activity in a PC mouse xenograft model (110). Interestingly, CD133 is expressed on both CSCs and differentiated tumor cells, but seems to be differentially folded and glycosylated, and therefore presents different target epitopes (111). Since different antibodies recognize different glycosylated CD133 epitopes (112), evaluation of glycosylation patterns of markers in PCSCs and differentiated PC cells could lead to the development of antibodies specifically directed against PCSCs in the future. Other strategies comprise the targeting of multiple antigens to enhance PCSC specificity or the targeting of potentially relevant splice variants.
Conclusions
PCSCs were identified as the driving force in PC. There is emerging knowledge about the role of PCSCs, and new therapeutic approaches aim to achieve an improved therapeutic outcome. Selective and effective targeting of PCSCs, however, remains challenging, since cellular plasticity and intra- as well as inter-tumoral heterogeneity drive tumor progression and therapeutic resistance against conventional therapies (113, 114). From a clinical perspective, the understanding of the interactions between PCSCs, differentiated PC cells, and the TME is of utmost importance, but these interactions are very difficult to reproduce in vitro. Furthermore, some CSC markers (e.g., CD133 and ALDH) are expressed not only on malignant cells but also on healthy stem cells causing on-target/off-tumor toxicity (115, 116). Therefore, treatment side effects can be hindrances for a successful therapy of PCSCs. In the future, the characterization of PCSCs using (single-cell) genomics and proteomics could lead to improved prognosis and more individualized therapy for patients with PC to probably achieve complete cure of advanced hitherto incurable stages of the disease.
Author Contributions
PW drafted the manuscript and designed the figure. IW provided the data for the table. IW, CG, and PW wrote, discussed and reviewed the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the German Research foundation (Grant No. WO2178/3-1 to PW). We acknowledge support by the Open Access Publication Fund of the University of Freiburg.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADT, androgen deprivation therapy; AR, androgen receptor; ABCG2, ATP-binding cassette sub-family G member 2 transporter; CSC, cancer stem cell; CRPC, castration-resistant prostate cancer; DPCC, differentiated prostate cancer cells; EMT, epithelial–mesenchymal transition; NEPCC, neuroendocrine prostate cancer cell; PAP, prostatic acid phosphatase; PCPC, prostate cancer progenitor cells; PCSC, prostate cancer stem cell; RP, radical prostatectomy; PSA, prostate-specific antigen.
References
1. Gandaglia G, Leni R, Bray F, Fleshner N, Freedland SJ, Kibel A, et al. Epidemiology and Prevention of Prostate Cancer. Eur Urol Oncol (2021) 4(6):877–92. doi: 10.1016/j.euo.2021.09.006
2. Yossepowitch O, Briganti A, Eastham JA, Epstein J, Graefen M, Montironi R, et al. Positive Surgical Margins After Radical Prostatectomy: A Systematic Review and Contemporary Update. Eur Urol (2014) 65(2):303–13. doi: 10.1016/j.eururo.2013.07.039
3. Tendulkar RD, Agrawal S, Gao T, Efstathiou JA, Pisansky TM, Michalski JM, et al. Contemporary Update of a Multi-Institutional Predictive Nomogram for Salvage Radiotherapy After Radical Prostatectomy. J Clin Oncol (2016) 34(30):3648–54. doi: 10.1200/jco.2016.67.9647
4. Litwin MS, Tan HJ. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA (2017) 317(24):2532–42. doi: 10.1001/jama.2017.7248
5. Teo MY, Rathkopf DE, Kantoff P. Treatment of Advanced Prostate Cancer. Annu Rev Med (2019) 70:479–99. doi: 10.1146/annurev-med-051517-011947
6. Ehsani M, David FO, Baniahmad A. Androgen Receptor-Dependent Mechanisms Mediating Drug Resistance in Prostate Cancer. Cancers (Basel) (2021) 13(7) :1534. doi: 10.3390/cancers13071534
7. Mansinho A, Macedo D, Fernandes I, Costa L. Castration-Resistant Prostate Cancer: Mechanisms, Targets and Treatment. Adv Exp Med Biol (2018) 1096:117–33. doi: 10.1007/978-3-319-99286-0_7
8. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem Cells, Cancer, and Cancer Stem Cells. Nature (2001) 414(6859):105–11. doi: 10.1038/35102167
9. Kreso A, Dick JE. Evolution of the Cancer Stem Cell Model. Cell Stem Cell (2014) 14(3):275–91. doi: 10.1016/j.stem.2014.02.006
10. Lin CJ, Lo UG, Hsieh JT. The Regulatory Pathways Leading to Stem-Like Cells Underlie Prostate Cancer Progression. Asian J andrology (2019) 21(3):233–40. doi: 10.4103/aja.aja_72_18
11. Skvortsov S, Skvortsova II, Tang DG, Dubrovska A. Concise Review: Prostate Cancer Stem Cells: Current Understanding. Stem Cells (Dayton Ohio) (2018) 36(10):1457–74. doi: 10.1002/stem.2859
12. Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective Identification of Tumorigenic Prostate Cancer Stem Cells. Cancer Res (2005) 65(23):10946–51. doi: 10.1158/0008-5472.can-05-2018
13. Miki J, Rhim JS. Prostate Cell Cultures as In Vitro Models for the Study of Normal Stem Cells and Cancer Stem Cells. Prostate Cancer Prostatic Dis (2008) 11(1):32–9. doi: 10.1038/sj.pcan.4501018
14. Di Stefano C, Grazioli P, Fontanella RA, De Cesaris P, D’Amore A, Regno M, et al. Stem-Like and Highly Invasive Prostate Cancer Cells Expressing CD44v8-10 Marker Originate From CD44-Negative Cells. Oncotarget (2018) 9(56):30905–18. doi: 10.18632/oncotarget.25773
15. Li JJ, Shen MM. Prostate Stem Cells and Cancer Stem Cells. Cold Spring Harbor Perspect Med (2019) 9(6):a030395. doi: 10.1101/cshperspect.a030395
16. Sottnik JL, Daignault-Newton S, Zhang X, Morrissey C, Hussain MH, Keller ET, et al. Integrin Alpha2beta 1 (α2β1) Promotes Prostate Cancer Skeletal Metastasis. Clin Exp metastasis (2013) 30(5):569–78. doi: 10.1007/s10585-012-9561-6
17. Hall CL, Dubyk CW, Riesenberger TA, Shein D, Keller ET, van Golen KL. Type I Collagen Receptor (Alpha2beta1) Signaling Promotes Prostate Cancer Invasion Through RhoC GTPase. Neoplasia (2008) 10(8):797–803. doi: 10.1593/neo.08380
18. Rabinovitz I, Nagle RB, Cress AE. Integrin Alpha 6 Expression in Human Prostate Carcinoma Cells Is Associated With a Migratory and Invasive Phenotype In Vitro and In Vivo. Clin Exp metastasis (1995) 13(6):481–91. doi: 10.1007/bf00118187
19. Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and Functional Characterization of Murine Prostate Stem Cells. Proc Natl Acad Sci U S A (2007) 104(1):181–6. doi: 10.1073/pnas.0609684104
20. Sánchez BG, Bort A, Vara-Ciruelos D, Díaz-Laviada I. Androgen Deprivation Induces Reprogramming of Prostate Cancer Cells to Stem-Like Cells. Cells (2020) 9(6):1441. doi: 10.3390/cells9061441
21. Seo HK, Lee SJ, Kwon WA, Jeong KC. Docetaxel-Resistant Prostate Cancer Cells Become Sensitive to Gemcitabine Due to the Upregulation of ABCB1. Prostate (2020) 80(6):453–62. doi: 10.1002/pros.23946
22. Lombard AP, Lou W, Armstrong CM, D’Abronzo LS, Ning S, Evans CP, et al. Activation of the ABCB1 Amplicon in Docetaxel- and Cabazitaxel-Resistant Prostate Cancer Cells. Mol Cancer Ther (2021) 20(10):2061–70. doi: 10.1158/1535-7163.mct-20-0983
23. Sabnis NG, Miller A, Titus MA, Huss WJ. The Efflux Transporter ABCG2 Maintains Prostate Stem Cells. Mol Cancer research: MCR. (2017) 15(2):128–40. doi: 10.1158/1541-7786.mcr-16-0270-t
24. Wang L, Stadlbauer B, Lyu C, Buchner A, Pohla H. Shikonin Enhances the Antitumor Effect of Cabazitaxel in Prostate Cancer Stem Cells and Reverses Cabazitaxel Resistance by Inhibiting ABCG2 and ALDH3A1. Am J Cancer Res (2020) 10(11):3784–800 eCollection 2020.
25. Chen X, Li Q, Liu X, Liu C, Liu R, Rycaj K, et al. Defining a Population of Stem-Like Human Prostate Cancer Cells That Can Generate and Propagate Castration-Resistant Prostate Cancer. Clin Cancer Res (2016) 22(17):4505–16. doi: 10.1158/1078-0432.ccr-15-2956
26. Li T, Su Y, Mei Y, Leng Q, Leng B, Liu Z, et al. ALDH1A1 is a Marker for Malignant Prostate Stem Cells and Predictor of Prostate Cancer Patients’ Outcome. Laboratory Investigation;. J Tech Methods pathology. (2010) 90(2):234–44. doi: 10.1038/labinvest.2009.127
27. van den Hoogen C, van der Horst G, Cheung H, Buijs JT, Lippitt JM, Guzmán-Ramírez N, et al. High Aldehyde Dehydrogenase Activity Identifies Tumor-Initiating and Metastasis-Initiating Cells in Human Prostate Cancer. Cancer Res (2010) 70(12):5163–73. doi: 10.1158/0008-5472.can-09-3806
28. Gorodetska I, Offermann A, Püschel J, Lukiyanchuk V, Gaete D, Kurzyukova A, et al. The Distinct Role of ALDH1A1 and ALDH1A3 in the Regulation of Prostate Cancer Metastases. bioRxiv (2021) 443223. doi: 10.1101/2021.05.08.443223
29. Chen Y, Lan T. Molecular Origin, Expression Regulation, and Biological Function of Androgen Receptor Splicing Variant 7 in Prostate Cancer. Urol Int (2021) 105(5-6):337–53. doi: 10.1159/000510124
30. Sobhani N, Neeli PK, D’Angelo A, Pittacolo M, Sirico M, Galli IC, et al. AR-V7 in Metastatic Prostate Cancer: A Strategy Beyond Redemption. Int J Mol Sci (2021) 22(11):5515. doi: 10.3390/ijms22115515
31. Harris KS, Shi L, Foster BM, Mobley ME, Elliott PL, Song CJ, et al. CD117/c-Kit Defines a Prostate CSC-Like Subpopulation Driving Progression and TKI Resistance. Sci Rep (2021) 11(1):1465. doi: 10.1038/s41598-021-81126-6
32. Wiesner C, Nabha SM, Dos Santos EB, Yamamoto H, Meng H, Melchior SW, et al. C-Kit and its Ligand Stem Cell Factor: Potential Contribution to Prostate Cancer Bone Metastasis. Neoplasia (2008) 10(9):996–1003. doi: 10.1593/neo.08618
33. Aghajani M, Mokhtarzadeh A, Aghebati-Maleki L, Mansoori B, Mohammadi A, Safaei S, et al. CD133 Suppression Increases the Sensitivity of Prostate Cancer Cells to Paclitaxel. Mol Biol Rep (2020) 47(5):3691–703. doi: 10.1007/s11033-020-05411-9
34. Glumac PM, LeBeau AM. The Role of CD133 in Cancer: A Concise Review. Clin Trans Med (2018) 7(1):18. doi: 10.1186/s40169-018-0198-1
35. Rowehl RA, Crawford H, Dufour A, Ju J, Botchkina GI. Genomic Analysis of Prostate Cancer Stem Cells Isolated From a Highly Metastatic Cell Line. Cancer Genomics proteomics (2008) 5(6):301–10.
36. Barzegar Behrooz A, Syahir A, Ahmad S. CD133: Beyond a Cancer Stem Cell Biomarker. J Drug targeting. (2019) 27(3):257–69. doi: 10.1080/1061186x.2018.1479756
37. Jiao J, Hindoyan A, Wang S, Tran LM, Goldstein AS, Lawson D, et al. Identification of CD166 as a Surface Marker for Enriching Prostate Stem/Progenitor and Cancer Initiating Cells. PLoS One (2012) 7(8):e42564. doi: 10.1371/journal.pone.0042564
38. Hansen AG, Arnold SA, Jiang M, Palmer TD, Ketova T, Merkel A, et al. ALCAM/CD166 is a TGF-β-Responsive Marker and Functional Regulator of Prostate Cancer Metastasis to Bone. Cancer Res (2014) 74(5):1404–15. doi: 10.1158/0008-5472.can-13-1296
39. Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, et al. Highly Purified CD44+ Prostate Cancer Cells From Xenograft Human Tumors are Enriched in Tumorigenic and Metastatic Progenitor Cells. Oncogene (2006) 25(12):1696–708. doi: 10.1038/sj.onc.1209327
40. Mochizuki H, Matsubara A, Teishima J, Mutaguchi K, Yasumoto H, Dahiya R, et al. Interaction of Ligand-Receptor System Between Stromal-Cell-Derived Factor-1 and CXC Chemokine Receptor 4 in Human Prostate Cancer: A Possible Predictor of Metastasis. Biochem Biophys Res Commun (2004) 320(3):656–63. doi: 10.1016/j.bbrc.2004.06.013
41. Domanska UM, Timmer-Bosscha H, Nagengast WB, Oude Munnink TH, Kruizinga RC, Ananias HJ, et al. CXCR4 Inhibition With AMD3100 Sensitizes Prostate Cancer to Docetaxel Chemotherapy. Neoplasia (2012) 14(8):709–18. doi: 10.1593/neo.12324
42. Dubrovska A, Elliott J, Salamone RJ, Telegeev GD, Stakhovsky AE, Schepotin IB, et al. CXCR4 Expression in Prostate Cancer Progenitor Cells. PLoS One (2012) 7(2):e31226. doi: 10.1371/journal.pone.0031226
43. Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, et al. Role of High Expression Levels of CXCR4 in Tumor Growth, Vascularization, and Metastasis. FASEB J (2004) 18(11):1240–2. doi: 10.1096/fj.03-0935fje
44. Bae KM, Su Z, Frye C, McClellan S, Allan RW, Andrejewski JT, et al. Expression of Pluripotent Stem Cell Reprogramming Factors by Prostate Tumor Initiating Cells. J Urol (2010) 183(5):2045–53. doi: 10.1016/j.juro.2009.12.092
45. Rhodes DR, Sanda MG, Otte AP, Chinnaiyan AM, Rubin MA. Multiplex Biomarker Approach for Determining Risk of Prostate-Specific Antigen-Defined Recurrence of Prostate Cancer. J Natl Cancer Inst (2003) 95(9):661–8. doi: 10.1093/jnci/95.9.661
46. Putzke AP, Ventura AP, Bailey AM, Akture C, Opoku-Ansah J, Celiktaş M, et al. Metastatic Progression of Prostate Cancer and E-Cadherin Regulation by Zeb1 and SRC Family Kinases. Am J Pathol (2011) 179(1):400–10. doi: 10.1016/j.ajpath.2011.03.028
47. Ni J, Cozzi P, Hao J, Beretov J, Chang L, Duan W, et al. Epithelial Cell Adhesion Molecule (EpCAM) Is Associated With Prostate Cancer Metastasis and Chemo/Radioresistance via the PI3K/Akt/mTOR Signaling Pathway. Int J Biochem Cell Biol (2013) 45(12):2736–48. doi: 10.1016/j.biocel.2013.09.008
48. Yoshida GJ, Saya H. EpCAM Expression in the Prostate Cancer Makes the Difference in the Response to Growth Factors. Biochem Biophys Res Commun (2014) 443(1):239–45. doi: 10.1016/j.bbrc.2013.11.093
49. Deng Z, Wu Y, Ma W, Zhang S, Zhang YQ. Adoptive T-Cell Therapy of Prostate Cancer Targeting the Cancer Stem Cell Antigen EpCAM. BMC Immunol (2015) 16(1):1. doi: 10.1186/s12865-014-0064-x
50. Massoner P, Thomm T, Mack B, Untergasser G, Martowicz A, Bobowski K, et al. EpCAM is Overexpressed in Local and Metastatic Prostate Cancer, Suppressed by Chemotherapy and Modulated by MET-Associated miRNA-200c/205. Br J cancer. (2014) 111(5):955–64. doi: 10.1038/bjc.2014.366
51. Gorodetska I, Lukiyanchuk V, Peitzsch C, Kozeretska I, Dubrovska A. BRCA1 and EZH2 Cooperate in Regulation of Prostate Cancer Stem Cell Phenotype. Int J Cancer (2019) 145(11):2974–85. doi: 10.1002/ijc.32323
52. Li K, Liu C, Zhou B, Bi L, Huang H, Lin T, et al. Role of EZH2 in the Growth of Prostate Cancer Stem Cells Isolated From LNCaP Cells. Int J Mol Sci (2013) 14(6):11981–93. doi: 10.3390/ijms140611981
53. Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, et al. EZH2 Oncogenic Activity in Castration-Resistant Prostate Cancer Cells is Polycomb-Independent. Science (2012) 338(6113):1465–9. doi: 10.1126/science.1227604
54. Han AL, Kumar S, Fok JY, Tyagi AK, Mehta K. Tissue Transglutaminase Expression Promotes Castration-Resistant Phenotype and Transcriptional Repression of Androgen Receptor. Eur J Cancer (2014) 50(9):1685–96. doi: 10.1016/j.ejca.2014.02.014
55. Shen M, Liu S, Stoyanova T. The Role of Trop2 in Prostate Cancer: An Oncogene, Biomarker, and Therapeutic Target. Am J Clin Exp urology. (2021) 9(1):73–87. eCollection 2021.
56. Goldstein AS, Lawson DA, Cheng D, Sun W, Garraway IP, Witte ON. Trop2 Identifies a Subpopulation of Murine and Human Prostate Basal Cells With Stem Cell Characteristics. Proc Natl Acad Sci U S A (2008) 105(52):20882–7. doi: 10.1073/pnas.0811411106
57. Trerotola M, Ganguly KK, Fazli L, Fedele C, Lu H, Dutta A, et al. Trop-2 Is Up-Regulated in Invasive Prostate Cancer and Displaces FAK From Focal Contacts. Oncotarget (2015) 6(16):14318–28. doi: 10.18632/oncotarget.3960
58. Harris KS, Kerr BA. Prostate Cancer Stem Cell Markers Drive Progression, Therapeutic Resistance, and Bone Metastasis. Stem Cells Int (2017), 2017: 8629234. doi: 10.1155/2017/8629234
59. Taylor RA, Fraser M, Livingstone J, Espiritu SM, Thorne H, Huang V, et al. Germline BRCA2 Mutations Drive Prostate Cancers With Distinct Evolutionary Trajectories. Nat Commun (2017) 8:13671. doi: 10.1038/ncomms13671
60. Mateo J, Boysen G, Barbieri CE, Bryant HE, Castro E, Nelson PS, et al. DNA Repair in Prostate Cancer: Biology and Clinical Implications. Eur Urol (2017) 71(3):417–25. doi: 10.1016/j.eururo.2016.08.037
61. Polson ES, Lewis JL, Celik H, Mann VM, Stower MJ, Simms MS, et al. Monoallelic Expression of TMPRSS2/ERG in Prostate Cancer Stem Cells. Nat Commun (2013) 4:1623. doi: 10.1038/ncomms2627
62. Archer LK, Frame FM, Maitland NJ. Stem Cells and the Role of ETS Transcription Factors in the Differentiation Hierarchy of Normal and Malignant Prostate Epithelium. J Steroid Biochem Mol Biol (2017) 166:68–83. doi: 10.1016/j.jsbmb.2016.05.006
63. Karthaus WR, Hofree M, Choi D, Linton EL, Turkekul M, Bejnood A, et al. Regenerative Potential of Prostate Luminal Cells Revealed by Single-Cell Analysis. Science (2020) 368(6490):497–505. doi: 10.1126/science.aay0267
64. Maitland NJ, Frame FM, Polson ES, Lewis JL, Collins AT. Prostate Cancer Stem Cells: Do They Have a Basal or Luminal Phenotype? Hormones Cancer (2011) 2(1):47–61. doi: 10.1007/s12672-010-0058-y
65. Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a Cell of Origin for Human Prostate Cancer. Science (2010) 329(5991):568–71. doi: 10.1126/science.1189992
66. Choi N, Zhang B, Zhang L, Ittmann M, Xin L. Adult Murine Prostate Basal and Luminal Cells Are Self-Sustained Lineages That can Both Serve as Targets for Prostate Cancer Initiation. Cancer Cell (2012) 21(2):253–65. doi: 10.1016/j.ccr.2012.01.005
67. Castellón EA, Valenzuela R, Lillo J, Castillo V, Contreras HR, Gallegos I, et al. Molecular Signature of Cancer Stem Cells Isolated From Prostate Carcinoma and Expression of Stem Markers in Different Gleason Grades and Metastasis. Biol Res (2012) 45(3):297–305. doi: 10.4067/s0716-97602012000300011
68. Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (Prostate Specific Antigen) Recurrence Probability Following Radical Prostatectomy for Clinically Localized Prostate Cancer. J Urol (2003) 169(2):517–23. doi: 10.1097/01.ju.0000045749.90353.c7
69. Kestin LL, Vicini FA, Ziaja EL, Stromberg JS, Frazier RC, Martinez AA. Defining Biochemical Cure for Prostate Carcinoma Patients Treated With External Beam Radiation Therapy. Cancer (1999) 86(8):1557–66. doi: 10.1002/(sici)1097-0142(19991015)86:8<1557::aid-cncr24>3.0.co;2-2
70. Tsao T, Beretov J, Ni J, Bai X, Bucci J, Graham P, et al. Cancer Stem Cells in Prostate Cancer Radioresistance. Cancer Lett (2019) 465:94–104. doi: 10.1016/j.canlet.2019.08.020
71. Guzel E, Karatas OF, Duz MB, Solak M, Ittmann M, Ozen M. Differential Expression of Stem Cell Markers and ABCG2 in Recurrent Prostate Cancer. Prostate (2014) 74(15):1498–505. doi: 10.1002/pros.22867
72. Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, et al. Acquisition of Epithelial-Mesenchymal Transition and Cancer Stem Cell Phenotypes is Associated With Activation of the PI3K/Akt/mTOR Pathway in Prostate Cancer Radioresistance. Cell Death Dis (2013) 4(10):e875. doi: 10.1038/cddis.2013.407
73. Klusa D, Lohaus F, Furesi G, Rauner M, Benešová M, Krause M, et al. Metastatic Spread in Prostate Cancer Patients Influencing Radiotherapy Response. Front Oncol (2020) 10:627379. doi: 10.3389/fonc.2020.627379
74. Manna F, Karkampouna S, Zoni E, De Menna M, Hensel J, Thalmann GN, et al. Metastases in Prostate Cancer. Cold Spring Harbor Perspect Med (2019) 9(3). doi: 10.1101/cshperspect.a033688
75. Deng Q, Tang DG. Androgen Receptor and Prostate Cancer Stem Cells: Biological Mechanisms and Clinical Implications. Endocr Relat Cancer (2015) 22(6):T209–20. doi: 10.1530/erc-15-0217
76. Tourinho-Barbosa R, Srougi V, Nunes-Silva I, Baghdadi M, Rembeyo G, Eiffel SS, et al. Biochemical Recurrence After Radical Prostatectomy: What Does It Mean? Int Braz J Urol (2018) 44(1):14–21. doi: 10.1590/s1677-5538.ibju.2016.0656
77. Katzenwadel A, Wolf P. Androgen Deprivation of Prostate Cancer: Leading to a Therapeutic Dead End. Cancer Lett (2015) 367(1):12–7. doi: 10.1016/j.canlet.2015.06.021
78. Di Zazzo E, Galasso G, Giovannelli P, Di Donato M, Di Santi A, Cernera G, et al. Prostate Cancer Stem Cells: The Role of Androgen and Estrogen Receptors. Oncotarget (2016) 7(1):193–208. doi: 10.18632/oncotarget.6220
79. Qin J, Liu X, Laffin B, Chen X, Choy G, Jeter CR, et al. The PSA(-/Lo) Prostate Cancer Cell Population Harbors Self-Renewing Long-Term Tumor-Propagating Cells That Resist Castration. Cell Stem Cell (2012) 10(5):556–69. doi: 10.1016/j.stem.2012.03.009
80. Verma S, Shankar E, Kalayci FNC, Mukunda A, Alassfar M, Singh V, et al. Androgen Deprivation Induces Transcriptional Reprogramming in Prostate Cancer Cells to Develop Stem Cell-Like Characteristics. Int J Mol Sci (2020) 21(24). :9568 doi: 10.3390/ijms21249568
81. Kong D, Sethi S, Li Y, Chen W, Sakr WA, Heath E, et al. Androgen Receptor Splice Variants Contribute to Prostate Cancer Aggressiveness Through Induction of EMT and Expression of Stem Cell Marker Genes. Prostate (2015) 75(2):161–74. doi: 10.1002/pros.22901
82. Wolf P. Treatment of Metastatic Prostate Cancer After STAMPEDE. Trans Andrology Urology. (2017) 6(2):a030593:315–6. doi: 10.21037/tau.2017.02.01
83. Puca L, Vlachostergios PJ, Beltran H. Neuroendocrine Differentiation in Prostate Cancer: Emerging Biology, Models, and Therapies. Cold Spring Harbor Perspect Med (2019) 9(2):a030593. doi: 10.1101/cshperspect.a030593
84. Mei W, Lin X, Kapoor A, Gu Y, Zhao K, Tang D. The Contributions of Prostate Cancer Stem Cells in Prostate Cancer Initiation and Metastasis. Cancers (Basel). (2019) 11(4):434. doi: 10.3390/cancers11040434
85. Lai CJ, Lin CY, Liao WY, Hour TC, Wang HD, Chuu CP. CD44 Promotes Migration and Invasion of Docetaxel-Resistant Prostate Cancer Cells Likely via Induction of Hippo-Yap Signaling. Cells (2019) 8(4):295. doi: 10.3390/cells8040295
86. Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, et al. Suppression of Acquired Docetaxel Resistance in Prostate Cancer Through Depletion of Notch- and Hedgehog-Dependent Tumor-Initiating Cells. Cancer Cell (2012) 22(3):373–88. doi: 10.1016/j.ccr.2012.07.016
87. Sekino Y, Teishima J. Molecular Mechanisms of Docetaxel Resistance in Prostate Cancer. Cancer Drug resistance (Alhambra Calif). (2020) 3(4):676–85. doi: 10.20517/cdr.2020.37
88. Liu C, Li Z, Bi L, Li K, Zhou B, Xu C, et al. NOTCH1 Signaling Promotes Chemoresistance via Regulating ABCC1 Expression in Prostate Cancer Stem Cells. Mol Cell Biochem (2014) 393(1-2):265–70. doi: 10.1007/s11010-014-2069-4
89. Acikgoz E, Mukhtarova G, Alpay A, Avci CB, Bagca BG, Oktem G. Sonic Hedgehog Signaling is Associated With Resistance to Zoledronic Acid in CD133high/CD44high Prostate Cancer Stem Cells. Mol Biol Rep (2021) 48(4):3567–78. doi: 10.1007/s11033-021-06387-w
90. Leão R, Domingos C, Figueiredo A, Hamilton R, Tabori U, Castelo-Branco P. Cancer Stem Cells in Prostate Cancer: Implications for Targeted Therapy. Urol Int (2017) 99(2):125–36. doi: 10.1159/000455160
91. Ni J, Cozzi P, Hao J, Duan W, Graham P, Kearsley J, et al. Cancer Stem Cells in Prostate Cancer Chemoresistance. Curr Cancer Drug targets (2014) 14(3):225–40. doi: 10.2174/1568009614666140328152459
92. Chen K, Huang YH, Chen JL. Understanding and Targeting Cancer Stem Cells: Therapeutic Implications and Challenges. Acta pharmacologica Sinica (2013) 34(6):732–40. doi: 10.1038/aps.2013.27
93. Qin W, Zheng Y, Qian BZ, Zhao M. Prostate Cancer Stem Cells and Nanotechnology: A Focus on Wnt Signaling. Front Pharmacol (2017) 8:153. doi: 10.3389/fphar.2017.00153
94. Lee CH, Decker AM, Cackowski FC, Taichman RS. Bone Microenvironment Signaling of Cancer Stem Cells as a Therapeutic Target in Metastatic Prostate Cancer. Cell Biol toxicology (2020) 36(2):115–30. doi: 10.1007/s10565-019-09483-7
95. Edlind MP, Hsieh AC. PI3K-AKT-mTOR Signaling in Prostate Cancer Progression and Androgen Deprivation Therapy Resistance. Asian J andrology (2014) 16(3):378–86. doi: 10.4103/1008-682x.122876
96. Ibáñez E, Agliano A, Prior C, Nguewa P, Redrado M, González-Zubeldia I, et al. The Quinoline Imidoselenocarbamate EI201 Blocks the AKT/mTOR Pathway and Targets Cancer Stem Cells Leading to a Strong Antitumor Activity. Curr medicinal Chem (2012) 19(18):3031–43. doi: 10.2174/092986712800672076
97. Xia P, Xu XY. PI3K/Akt/mTOR Signaling Pathway in Cancer Stem Cells: From Basic Research to Clinical Application. Am J Cancer Res (2015) 5(5):1602–9. eCollection 2015
98. Marhold M, Tomasich E, El-Gazzar A, Heller G, Spittler A, Horvat R, et al. Hif1α Regulates mTOR Signaling and Viability of Prostate Cancer Stem Cells. Mol Cancer research: MCR. (2015) 13(3):556–64. doi: 10.1158/1541-7786.mcr-14-0153-t
99. Gangavarapu KJ, Azabdaftari G, Morrison CD, Miller A, Foster BA, Huss WJ. Aldehyde Dehydrogenase and ATP Binding Cassette Transporter G2 (ABCG2) Functional Assays Isolate Different Populations of Prostate Stem Cells Where ABCG2 Function Selects for Cells With Increased Stem Cell Activity. Stem Cell Res Ther (2013) 4(5):132. doi: 10.1186/scrt343
100. Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, et al. Targeting Cancer Stem Cell Pathways for Cancer Therapy. Signal transduction targeted Ther (2020) 5(1):8. doi: 10.1038/s41392-020-0110-5
101. Corn PG. The Tumor Microenvironment in Prostate Cancer: Elucidating Molecular Pathways for Therapy Development. Cancer Manage Res (2012) 4:183–93. doi: 10.2147/cmar.s32839
102. Goel HL, Pursell B, Shultz LD, Greiner DL, Brekken RA, Vander Kooi CW, et al. P-Rex1 Promotes Resistance to VEGF/VEGFR-Targeted Therapy in Prostate Cancer. Cell Rep (2016) 14(9):2193–208. doi: 10.1016/j.celrep.2016.02.016
103. Jung Y, Cackowski FC, Yumoto K, Decker AM, Wang J, Kim JK, et al. Cxcl12γ Promotes Metastatic Castration-Resistant Prostate Cancer by Inducing Cancer Stem Cell and Neuroendocrine Phenotypes. Cancer Res (2018) 78(8):2026–39. doi: 10.1158/0008-5472.can-17-2332
104. Xu H, Niu M, Yuan X, Wu K, Liu A. CD44 as a Tumor Biomarker and Therapeutic Target. Exp Hematol Oncol (2020) 9(1):36. doi: 10.1186/s40164-020-00192-0
105. Mahira S, Kommineni N, Husain GM, Khan W. Cabazitaxel and Silibinin Co-Encapsulated Cationic Liposomes for CD44 Targeted Delivery: A New Insight Into Nanomedicine Based Combinational Chemotherapy for Prostate Cancer. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2019) 110:803–17. doi: 10.1016/j.biopha.2018.11.145
106. Cui X, Liu R, Duan L, Cao D, Zhang Q, Zhang A. CAR-T Therapy: Prospects in Targeting Cancer Stem Cells. J Cell Mol Med (2021) 25(21):9891–904. doi: 10.1111/jcmm.16939
107. Zhu X, Prasad S, Gaedicke S, Hettich M, Firat E, Niedermann G. Patient-Derived Glioblastoma Stem Cells are Killed by CD133-Specific CAR T Cells But Induce the T Cell Aging Marker Cd57. Oncotarget (2015) 6(1):171–84. doi: 10.18632/oncotarget.2767
108. Dai H, Tong C, Shi D, Chen M, Guo Y, Chen D, et al. Efficacy and Biomarker Analysis of CD133-Directed CAR T Cells in Advanced Hepatocellular Carcinoma: A Single-Arm, Open-Label, Phase II Trial. Oncoimmunology (2020) 9(1):1846926. doi: 10.1080/2162402x.2020.1846926
109. Wang Z, Li Y, Wang Y, Wu D, Lau AHY, Zhao P, et al. Targeting Prostate Cancer Stem-Like Cells by an Immunotherapeutic Platform Based on Immunogenic Peptide-Sensitized Dendritic Cells-Cytokine-Induced Killer Cells. Stem Cell Res Ther (2020) 11(1):123. doi: 10.1186/s13287-020-01634-6
110. Ma Q, Qian W, Tao W, Zhou Y, Xue B. Delivery Of Curcumin Nanoliposomes Using Surface Modified With CD133 Aptamers For Prostate Cancer. Drug Des Devel Ther (2019) 13:4021–33. doi: 10.2147/dddt.s210949
111. Kemper K, Sprick MR, de Bree M, Scopelliti A, Vermeulen L, Hoek M, et al. The AC133 Epitope, But Not the CD133 Protein, is Lost Upon Cancer Stem Cell Differentiation. Cancer Res (2010) 70(2):719–29. doi: 10.1158/0008-5472.can-09-1820
112. Bidlingmaier S, Zhu X, Liu B. The Utility and Limitations of Glycosylated Human CD133 Epitopes in Defining Cancer Stem Cells. J Mol Med (Berlin Germany). (2008) 86(9):1025–32. doi: 10.1007/s00109-008-0357-8
113. Turdo A, Veschi V, Gaggianesi M, Chinnici A, Bianca P, Todaro M, et al. Meeting the Challenge of Targeting Cancer Stem Cells. Front Cell Dev Biol (2019) 7:16. doi: 10.3389/fcell.2019.00016
114. Paul R, Dorsey JF, Fan Y. Cell Plasticity, Senescence, and Quiescence in Cancer Stem Cells: Biological and Therapeutic Implications. Pharmacol Ther (2022) 231:107985. doi: 10.1016/j.pharmthera.2021.107985
115. Schuurhuis GJ, Meel MH, Wouters F, Min LA, Terwijn M, de Jonge NA, et al. Normal Hematopoietic Stem Cells Within the AML Bone Marrow Have a Distinct and Higher ALDH Activity Level Than Co-Existing Leukemic Stem Cells. PLoS One (2013) 8(11):e78897. doi: 10.1371/journal.pone.0078897
Keywords: prostate cancer, prostate cancer stem cells, prostate cancer stem cell hypothesis, prostate cancer stem cell antigens, prostate cancer stem cell therapy
Citation: Wolf I, Gratzke C and Wolf P (2022) Prostate Cancer Stem Cells: Clinical Aspects and Targeted Therapies. Front. Oncol. 12:935715. doi: 10.3389/fonc.2022.935715
Received: 04 May 2022; Accepted: 13 June 2022;
Published: 08 July 2022.
Edited by:
Pinuccia Faviana, University of Pisa, ItalyReviewed by:
Seema Chugh, University of Michigan, United StatesCarlo Catapano, Institute of Oncology Research (IOR), Switzerland
Copyright © 2022 Wolf, Gratzke and Wolf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philipp Wolf, cGhpbGlwcC53b2xmQHVuaWtsaW5pay1mcmVpYnVyZy5kZQ==
 Isis Wolf
Isis Wolf Christian Gratzke1,2
Christian Gratzke1,2 Philipp Wolf
Philipp Wolf