
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 14 July 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.934870
 Jitao Wang1,2†
Jitao Wang1,2† Zhanguo Zhang3†
Zhanguo Zhang3† Dong Shang4†
Dong Shang4† Jinlong Li2†
Jinlong Li2† Chengyu Liu2†
Chengyu Liu2† Peng Yu5
Peng Yu5 Mingguang Wang2
Mingguang Wang2 Dengxiang Liu2
Dengxiang Liu2 Hongrui Miao3
Hongrui Miao3 Shuang Li4
Shuang Li4 Biao Zhang4
Biao Zhang4 Anliang Huang4
Anliang Huang4 Yewei Zhang6*
Yewei Zhang6* Shubo Chen2*
Shubo Chen2* Xiaolong Qi1*
Xiaolong Qi1*Purpose: To determine the predictive value of portal hypertension (PH) for the development of post-hepatectomy liver failure (PHLF) in patients with hepatocellular carcinoma (HCC).
Patients and methods: This study enrolled a total of 659 patients with HCC that received hepatectomy as a first-line therapy. PH was classified as grade 0, 1, and 2 according to whether the indirect criteria for PH were met: 1) patients had obvious varicose veins and 2) splenomegaly was present and platelet count < 100 × 109/L. The effects of each variable on the occurrence of PHLF were assessed using univariate and multivariate analyses.
Results: PH grade 2 (odds ratio [OR] = 2.222, p = 0.011), higher age (OR = 1.031, p = 0.003), hepatitis C infection (OR = 3.711, p = 0.012), open surgery (OR = 2.336, p < 0.001), portal flow blockage (OR = 1.626, p = 0.023), major hepatectomy (OR = 2.919, p = 0.001), hyperbilirubinemia (≥ 17.2 μmol/L, OR = 2.113, p = 0.002), and high levels of alpha-fetoprotein (> 400n g/ml, OR = 1.799, p = 0.008) were significantly associated with PHLF occurrence. We performed a subgroup analysis of liver resection and found that the extent of liver resection and PH grade were good at distinguishing patients at high risk for PHLF, and we developed an easy-to-view roadmap.
Conclusion: PH is significantly related to the occurrence of PHLF in patients who underwent hepatectomy. Noninvasively assessing PH grade can predict PHLF risk.
Hepatocellular carcinoma (HCC) is one of the common malignant tumors. Currently, the preferred treatment for HCC is radical liver resection. However, since the successful completion of the world’s first elective liver resection by German physician Langenbueh in 1888, the continual development of surgical techniques, liver resection instruments, and perioperative management has significantly improved the safety of hepatectomy, and the indications for hepatectomy have also expanded (1, 2). The perioperative mortality rate after liver resection has been reported to exceed 10% (3). Post-hepatectomy liver decompensation increases the likelihood of post-hepatectomy liver failure (PHLF), which has been shown to be the leading cause of death after hepatectomy (4). The morbidity rate and poor clinical outcomes of PHLF have created serious social and public health problems. Therefore, prediction of high-risk patients for PHLF remains a significant concern that needs to be addressed urgently.
Portal hypertension (PH) is one of the high-risk factors for the development of PHLF (5). Most HCC patients undergoing liver resection are often in the stage of compensated cirrhosis. The severity of PH can predict the occurrence of PHLF (6). Hepatic venous pressure gradient (HVPG) measurement is the gold standard for the diagnosis of PH, but the invasiveness, high technical difficulty, and radiation exposure involved hinder its clinical application. The current indirect indicators of PH (presence of esophagogastric varices, platelet count <100 × 109/L, and spleen long diameter >12 cm) have been confirmed to predict the occurrence of PHLF (6–9). Previous studies were qualitative studies based on indirect criteria (i.e., presence or absence of PH) and could not further clarify the impact of preoperative PH severity on PHLF.
In previous guidelines for liver cancer, the presence of PH was specified as a contraindication for hepatectomy (10, 11). However, some studies have pointed out that even if PH is present before surgery, the poor prognosis after liver resection can be avoided when the extent of liver resection is reduced and treatment is timely (12, 13). Therefore, the relevance of PH as an independent factor in predicting complications such as PHLF and prognosis has been questioned (14). Several recent guidelines have modified the approach from considering PH as a contraindication for hepatectomy to a preoperative risk assessment that relies on a comprehensive evaluation system including PH, surgical approach, and extent of hepatectomy to select the best recipient (15, 16).
This study innovatively classified the severity of PH according to the preoperative clinical characteristic indicators and further clarified the predictive value of PH grading combined with the extent of hepatectomy in HCC patients with PHLF in a multicenter cohort of patients who underwent HCC resection.
This study was a multicenter retrospective study, analyzing patients who underwent hepatectomy in Xingtai People’s Hospital, Fifth Medical Center of PLA General Hospital, First Affiliated Hospital of Dalian Medical University, and Huazhong University of Science and Technology Hospital from 2012 to 2020. The inclusion criteria were as follows (1): age ≥ 18 years; (2) patients with HCC diagnosed according to histopathology who underwent hepatectomy; and (3) Child-Pugh A-B grade, and Barcelona Clinic Liver Cancer (BCLC) A-B stage. The exclusion criteria were as follows: (1) patients with vascular invasion, extrahepatic metastases, or metastatic liver cancer; (2) patients with a history of radiofrequency/microwave ablation, interventional chemotherapy, radiotherapy, or targeted or immune-related anti-cancer therapy; (3) patients with severe heart, lung or renal insufficiency or other systemic malignant tumors; (4) patients who underwent combined surgery with other organs during the same period; and (5) patients with incomplete clinical data or were lost to follow-up. This study was a retrospective case-control study. All patients and their families were fully informed and provided written consent before surgery, which complied with medical ethics regulations. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Xingtai People’s Hospital (IRB ID 2022-006). The researchers only retrospectively analyzed de-identified data from patients.
Hepatectomy and perioperative management are performed by experienced surgeons and nursing teams. In short, the surgeon choses to perform laparoscopy or laparotomy according to the location and size of the tumor, and completes the liver resection according to the standard procedures (17). Ultrasonic dissector or the clamp crushing technique were applied for parenchymal transection. At the second porta hepatis, the branches of the hepatic vein and the liver tissue were carried out by stapler hepatectomy. The Pringle method was used to block the blood flow into the liver when necessary. After checking for bleeding and bile leakage before closing, a biliary drain was placed.
The baseline data of patients were collected, including clinical baseline data (age, sex, height, and weight), etiology of liver disease, whether complicated with liver cirrhosis, laboratory tests (liver function, blood routine, and coagulation), PH clinical features (maximum spleen diameter and degree of esophagogastric varices), tumor status (size/number and extent), surgical conditions (surgical method, extent of resection, intraoperative blood loss, whether intraoperative blood transfusion was used, etc.), and PHLF situation.
Based on the recommendations made by the BCLC guidelines, PH was diagnosed in this study based on indirect criteria (6–9), specifically: [1] obvious varicose veins and [2] splenomegaly and platelet count (PLT) <100 × 109/L. The PH grades were defined as grade 0: when neither feature is present; grade 1: when one feature is present; and grade 2: both features are present (18). The Child–Pugh score was based on criteria reported in the literature (19). The model for end-stage liver disease (MELD) score was calculated using the following formula (20): MELD = (0.957 × ln[creatinine, mg/dl] + 0.378 × ln[bilirubin, mg/dl] + 1.12 × ln[international normalized ratio] + 0.643) × 10. Major hepatectomy was defined as liver resection of at least three liver segments, and minor hepatectomy was defined as liver resection of less than three segments (16). Mortality was defined as death that occurred within 30 days of surgery.
In this study, the International Study Group of Liver Surgery (ISGLS) criteria (21) were used to diagnose PHLF on the premise of excluding biliary obstruction, the total bilirubin (TBil) level, and international normalized ratio (INR). PHLF was diagnosed when the TBil level and INR were elevated compared to preoperative levels on or after postoperative day 5, according to the normal limits of the local laboratory. According to the ISGLS criteria (22), PHLF class A is defined as transient deterioration of liver function that does not require a change in clinical management, and PHLF class B is defined as deviation from routine postoperative clinical management without invasive treatment, PHLF grade C was defined as a patient requiring invasive treatment.
R (The R Foundation, https://www.r-project.org/) software was used for statistical analyses. Statistical descriptions of enumeration data were expressed as numbers (percentage), and rates were compared using a chi-squared test or Fisher’s exact test. Statistical descriptions of measurement data were expressed as medians (interquartile range) and compared using a rank-sum test. Univariate and multivariate logistic regression analyses were performed to determine whether each variable was an independent risk factor of PHLF. Items with a P-value < 0.1 in the univariate analysis were included in the multivariate analysis. To balance the baseline data, the three groups were compared after propensity score matching (PSM) analysis using the R software to remove selection bias. P < 0.05 was considered statistically significant.
Data pertaining to patients with histopathologically confirmed HCC who underwent hepatectomy were extracted and reviewed from a multicenter HCC database according to the inclusion criteria. In total, 42 cases were excluded because of extrahepatic distant metastasis (n = 10), preoperative anticancer treatment (n = 4), lost to follow-up (n = 23), and incomplete clinical data (n = 5). The remaining 659 patients were included in the final analysis. Among them, there were 254 cases in the Fifth Medical Center of the PLA General Hospital, 235 cases in the Affiliated Hospital of Huazhong University of Science and Technology, 115 cases in the First Affiliated Hospital of Dalian Medical University, and 55 cases in the Xingtai People’s Hospital. The median age was 53 (46–60) years and 559 patients were male (84.8%). The overall incidence of PHLF was 27.5% (181/659), of which PHLF grade A was 21.5% (142/659), PHLF grade B+C was 5.9% (39/659). The 30-day mortality in our cohort was 0.3% (2/659). The number of cases with PH grade 0, 1, and 2 were 390, 193, and 76, respectively. The incidence of PHLF in each group was 24.6%, 26.42%, and 44.7%, respectively (P < 0.001; Figure 1). Liver cirrhosis status, etiology of liver disease, surgical method of liver resection, extent of liver resection, Child–Pugh score, prothrombin time (PT), and PLT were statistically significant among the PH groups (P < 0.05). There were no differences in other baseline data between groups (Table 1).
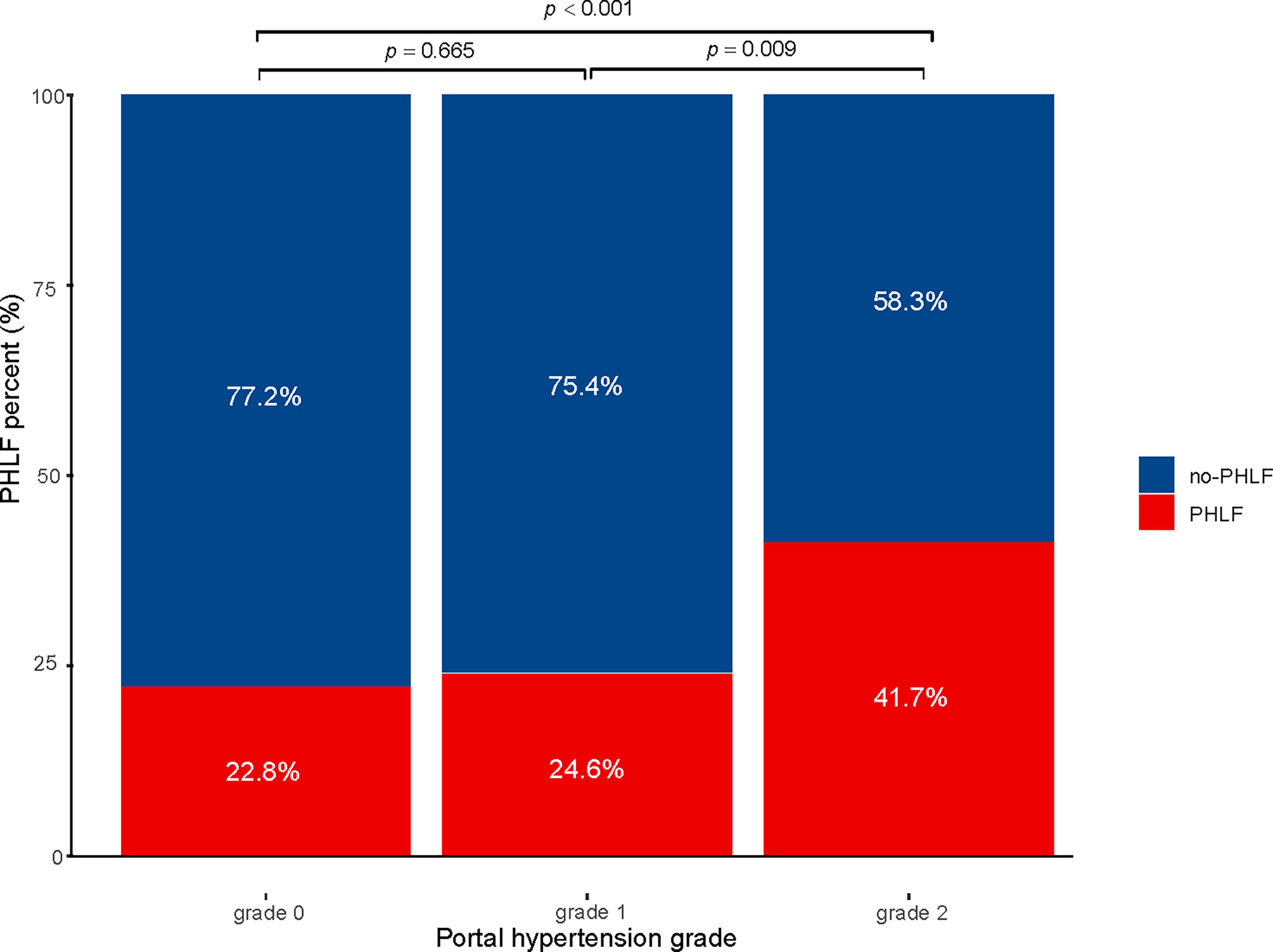
Figure 1 Incidence of PHLF with small-scale liver resection in patients with hepatocellular carcinoma PHLF, post hepatectomy liver failure.
Univariate analysis showed that PH grade 2, advanced age, positive hepatitis B viral DNA (> 500 copies), hepatitis C virus (HCV) infection, laparotomy, intraoperative blockade of portal blood flow, extensive hepatectomy, intraoperative bleeding (> 400 mL), multiple intrahepatic tumors, larger tumor diameter, alanine transaminase (ALT) level ≥ 40 U/L, aspartate transaminase (AST) level ≥ 40 U/L, albumin level < 35 g/L, TBil level ≥ 17.2 µmol/L, red blood cell count < 3.5×1012/L, alpha-fetoprotein (AFP) ≥ 400 ng/mL, Child–Pugh class B, and higher MELD scores were significantly associated with postoperative PHLF (Table 2).
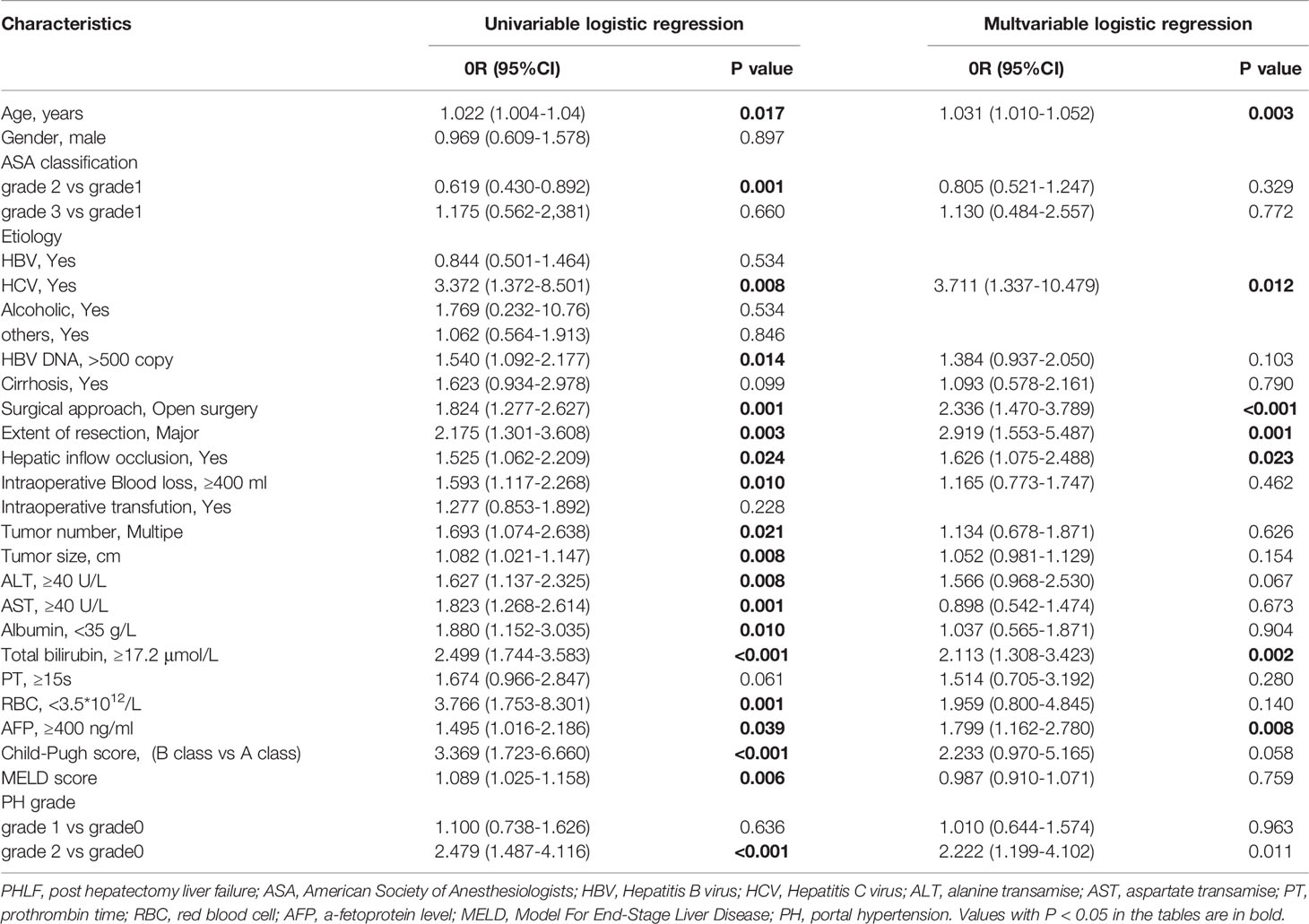
Table 2 Factors Associated with Post Hepatectomy Liver Failure at Univariate and Multivariate Logistic Regression Analyses.
Multivariate regression analysis revealed that PH grade 2 [odds ratio [OR] 2.222; 95% confidence interval [CI], 1.199-4.102], age (OR, 1.031; 95% CI, 1.012-1.052), HCV infection (OR, 3.711; 95% CI, 1.337-10.479), laparotomy (OR, 2.336; 95% CI, 1.470-3.789), hepatic blood loss (OR, 1.626; 95% CI, 1.075-2.488), extensive hepatectomy (OR, 2.919; 95% CI, 1.553-5.487), TBil level ≥ 17.2 µmol/L (OR, 2.113; 95% CI, 1.308-3.423), and AFP level > 400 ng/mL (OR, 1.799; 95% CI, 1.162-2.780) were independent risk factors for postoperative PHLF (Table 2).
To balance differences in baseline variables among the three PH grade groups, we performed propensity score matching (PSM). After PSM, there were no statistically significant differences in baseline variables other than PLT (one of the defining criteria for PH grades) among the three subgroups (Table 3). Further univariate and multivariate analyses reconfirmed that PH grade 2 was an independent risk factor for PHLF (Table 4).
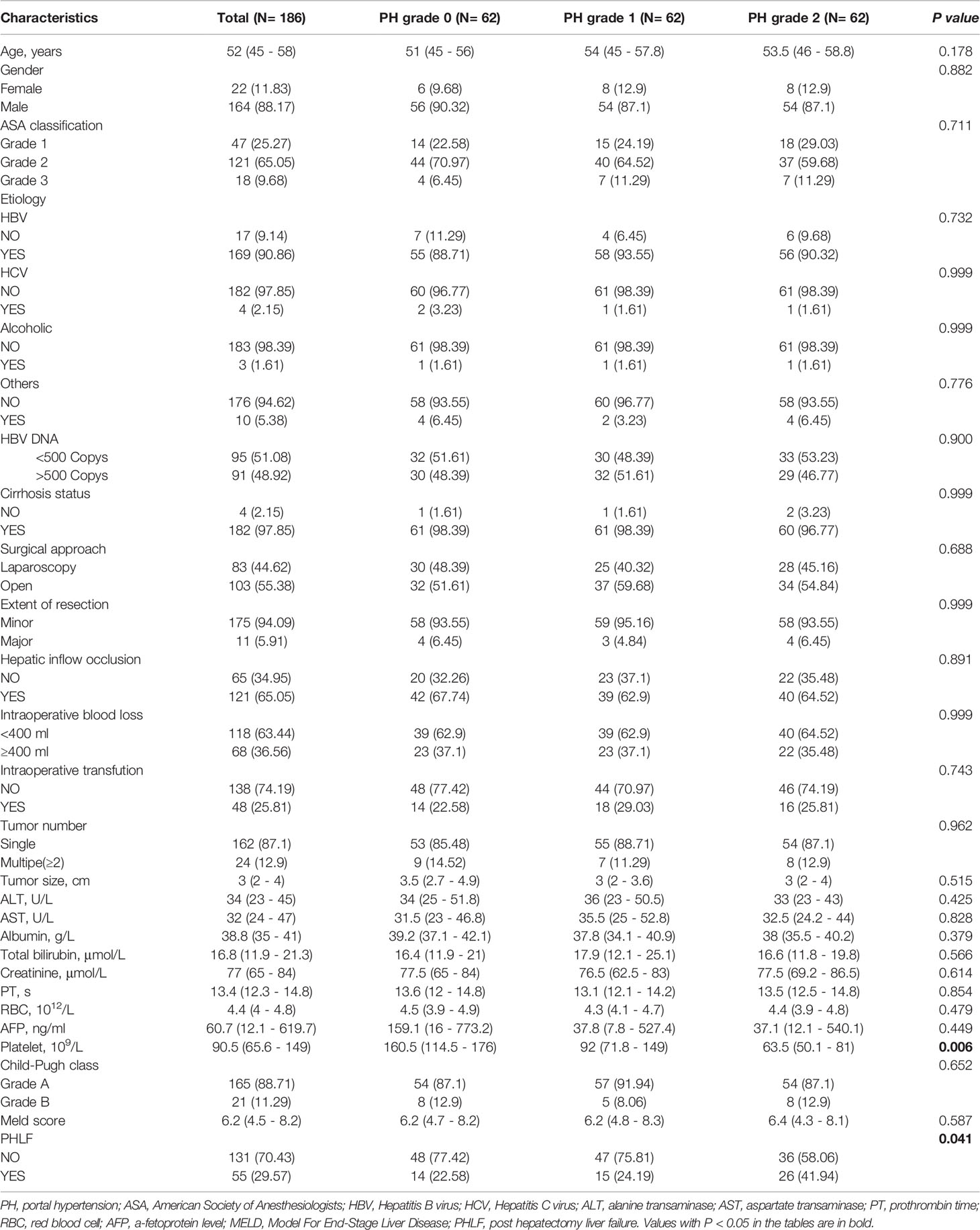
Table 3 Comparisons of Characteristics between different portal hypertension grades after Propensity Score Matching.
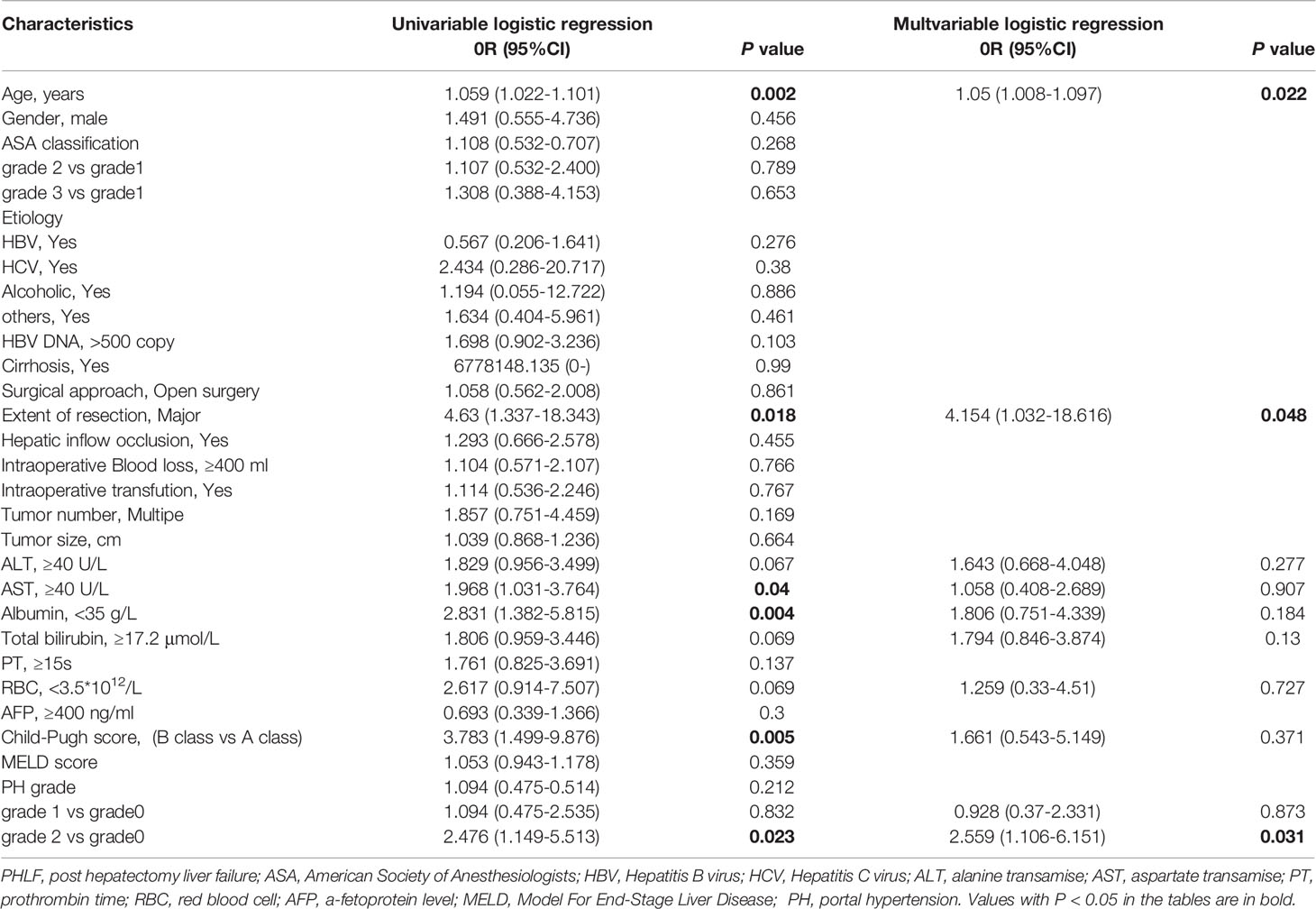
Table 4 Factors Associated with Post Hepatectomy Liver Failure at Univariate and Multivariate Logistic Regression Analyses after Propensity Score Matching.
Subgroup analysis was performed according to the extent of liver resection. In the small-scale liver resection cohort, the incidence of postoperative PHLF in patients with PH grade 0, 1, and 2 was 24.6%, 26.4, and 44.7%, respectively (Figure 1). In patients in the extensive liver resection cohort, the trend was more obvious, and the incidence of PHLF in patients with PH grade 0, 1, and 2 was 35.7%, 60%, and 100%, respectively (Figure 2). We stratified patients at risk of developing PHLF according to the extent of liver resection and grade of PH and developed a surgeon-friendly roadmap for PHLF prediction (Figure 3).
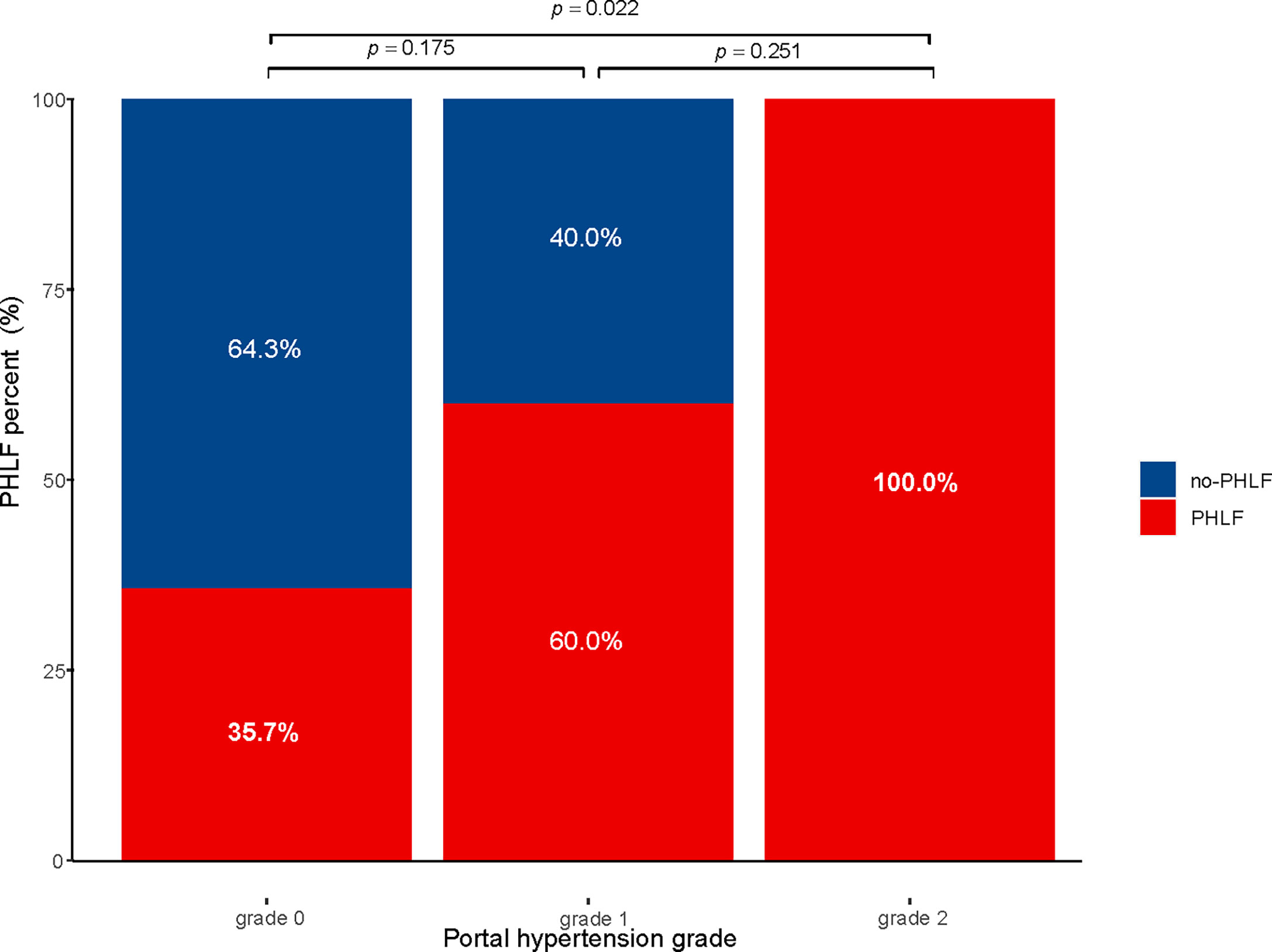
Figure 2 Incidence of PHLF with extensive liver resection in patients with hepatocellular carcinoma PHLF, post hepatectomy liver failure.
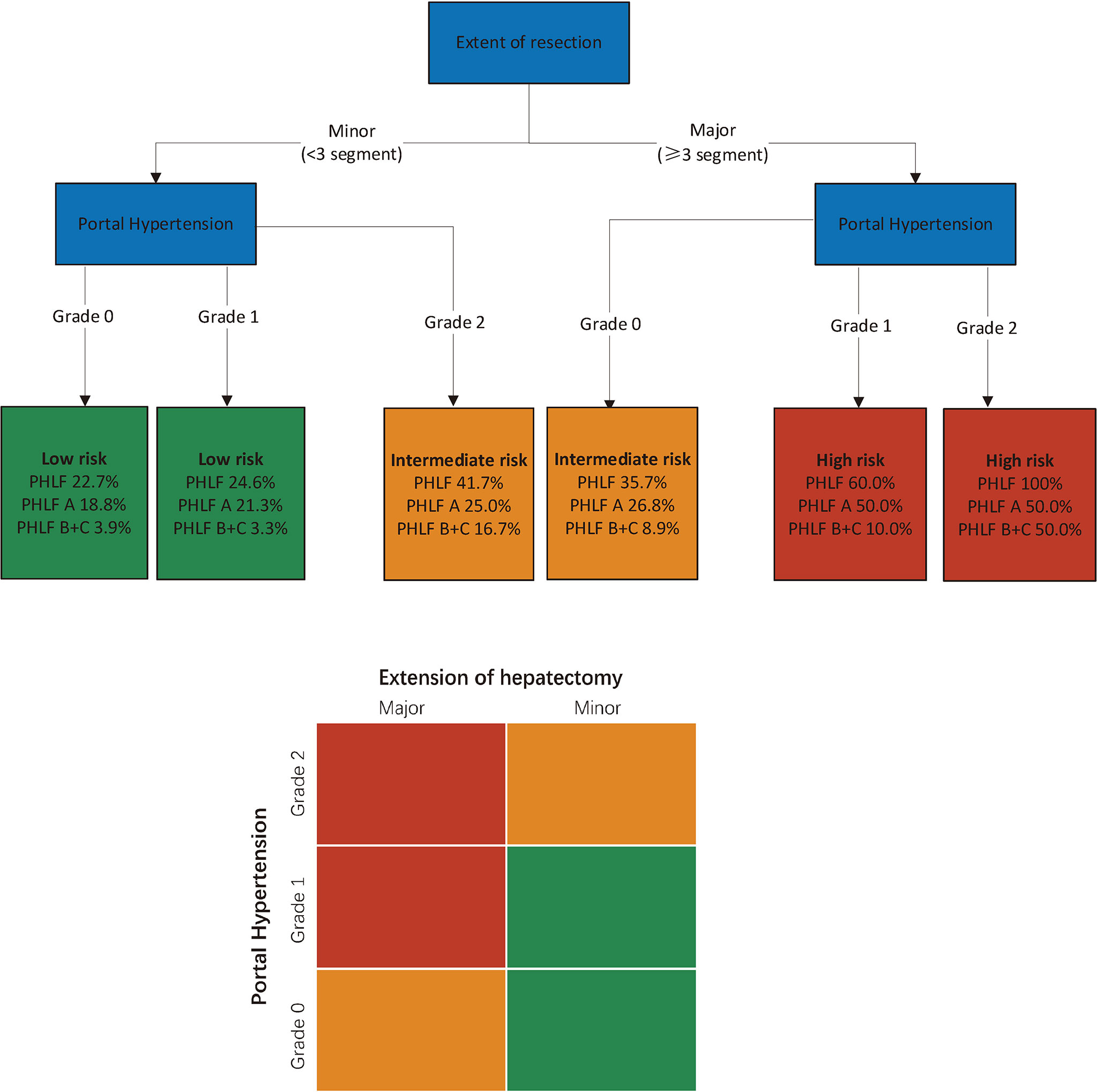
Figure 3 Multi-parametric assessment of the risk of PHLF in patients with hepatocellular carcinoma. Simplified decisional algorithm identifying high (red), intermediate (yellow) and low (green) risk of PHLF, according to a hierarchic interaction of the two independent risk factors: portal hypertension, and extent of resection. Major hepatectomy, hepatectomy range at least 3 liver segments; minor hepatectomy, hepatectomy range less than 3 segments. PHLF, post hepatectomy liver failure.
Our study showed that the severity of PH as assessed by noninvasive PH grade assessed by preoperative noninvasive methods is a useful predictive marker of PHLF in patients with HCC who underwent liver resection. High-risk PHLF patients can be well identified according to the extent of liver resection and PH grade.
PHLF refers to a group of clinical syndromes characterized by abnormal liver function, ascites, jaundice, and hepatic encephalopathy after hepatectomy. Its primary mechanism involves liver resection that leads to serious dysfunction of liver synthesis, decomposition, enzyme metabolism, and biotransformation (23, 24). As one of the serious complications after hepatectomy, PHLF has been proven to be the leading cause of death after hepatectomy (4, 24). In a previous large clinical experiment, > 70% of all deaths in patients with indications for liver resection met the criteria for PHLF, and the fatality rate of patients with PHLF was >50% (25). Therefore, prediction of high-risk patients for PHLF remains an urgent clinical issue.
There is no unified and standardized definition for the diagnosis of PHLF. Currently, the main diagnostic criteria include the “50-50” and ISGLS criteria. The “50-50 criteria,” which predicts PHLF based on changes in PT and TBil, was first proposed by Balzan in 2005 after a statistical analysis of the clinical data of 775 patients who underwent liver resection (26). This method allows for easy calculation; the diagnostic specificity reaches 97.7%, but its sensitivity is only 69.6%, limiting its wide application. ISGLS redefined PHLF in 2011 and predicts PHLF based on whether the liver’s ability to maintain synthesis, excretion, and detoxification deteriorates after hepatectomy. The specific diagnostic criteria are to detect the levels of TBil and INR on the 5th postoperative day or later. PHLF can be diagnosed when TBil and INR levels are elevated compared to preoperative levels (21). The PHLF definition of ISGLS allows for calculation and comparison, making it widely used and gradually becoming the standardized definition of PHLF (24, 27, 28). Therefore, this study used the ISGLS-PHLF standard.
Previous studies have shown that the severity of PH is an independent predictor of decompensation and increased mortality (29). The EASL guidelines point out that, different from non-cirrhotic patients who are the first choice for surgical treatment, preoperative PH in patients with liver cirrhosis is an important influencing factor for the choice of surgical resection (16). With improvements in operator ability, more refined management of PH evaluation of patients during the perioperative period, and consideration of factors such as resection volume and liver function, the presence of PH is no longer a contraindication for hepatectomy. However, PH still seriously affects the prognosis and is inextricably linked to the occurrence of PHLF (6). Considering that HVPG has not yet been widely adopted in clinical practice, PH is still currently considered as a surrogate standard. Previous studies have qualitatively determined whether the presence of PH predicts postoperative complications in patients with HCC (6, 30, 31). A single-center study of 190 patients with Child-Pugh A HCC demonstrated that PH severity independently predicted the risk of PHLF(OR 3 24, 95% CI 1.38 to 7.65; P = 0.007) (32). However, because this study was developed based on a single center, so its generalizability needs to be verified in multiple centers. In addition, in the study, only 190 patients with HCC were enrolled, and all patients underwent laparotomy. Our multicenter study enrolled more HCC patients from different regions in China, and 42.3% (279/659) of the patients underwent laparoscopic surgery.
This study divided PH into three grades according to the clinical characteristics. Our study found that as the grade of PH increased, HCC patients were more likely to have liver cirrhosis, lower platelet levels, and worse liver function and coagulation function, and were more likely to undergo small-scale liver resection. This is consistent with the clinical characteristics of this population. The results showed that regardless of resection size, the PH grade positively correlated with the incidence of postoperative PHLF (p = 0.002). The same finding was also made in the subgroup analysis based on the extent of liver resection. Multivariate regression analysis showed that PH grade was an independent risk factor for PHLF.
There is a significant positive correlation between PH grade and PH after hepatectomy, and these phenomena may be caused by many complex and interacting factors. In terms of pathology, patients with liver cirrhosis have different mechanisms leading to inflammation and necrosis of liver tissue and the formation of fibrotic nodules, resulting in distorted and closed hepatic vascular structure. This histological state results in increased resistance to portal blood flow, which can lead to PH (33). We noted that the incidence of postoperative PHLF reached 100% in patients with PH grade 2 and 60% in patients with PH grade 1 who underwent extensive liver resection. Under similar portal pressure levels, extensive resection is more traumatic and requires higher liver function reserve; the risk of postoperative PHLF is thus greatly increased. This suggests that for patients with severe PH, adequate liver function and systemic status evaluation should be performed before undertaking extensive liver resection and consideration should be given to improving the patient’s general condition to improve the prognosis before surgery or other treatment. This conclusion is consistent with the EASL guidelines for surgical treatment of liver cancer with cirrhosis (16). On the other hand, similar conclusions could be drawn from the small-scale liver resection subgroup. Surgical resection is not an absolute contraindication even if with severe PH. Under the premise of adequate preoperative evaluation and preparation, small-scale liver resection is feasible, but it requires more careful perioperative management to effectively maintain the patient prognosis. Hepatectomy remains the relatively preferred treatment modality for those patients.
The use of liver resection extent and PH grade to assess the risk of PHLF is a practical method that can facilitate individual treatment decisions for patients with HCC based on the potential risk of PHLF. Moreover, the evaluation of PH grades for patients with HCC undergoing elective liver resection only requires common parameters, such as routine blood tests and preoperative computed tomography or gastroscopy, which is more practical for primary medical institutions. A roadmap for assessing PHLF based on PH grade and extent of liver resection is a useful tool for optimizing patient selection for liver resection and improving current treatment strategies.
Our study has some limitations. First, this is a multicenter retrospective study in China with a high proportion of patients with HBV infection. More patients with HCC with other etiologies of liver disease need to be prospectively included in the future to confirm our conclusions. Second, PH is indirectly diagnosed by clinical criteria and not by HVPG. Whether PH ratings accurately reflect HVPG values has not been fully validated. Finally, first, considering the retrospective nature of our study, part of data was incompletely recorded. Owing to the lack of other perioperative complications other than PHLF and long-term follow-up data, the relationship between PH grade and other perioperative complications and overall survival of patients with HCC who underwent hepatectomy requires additional studies. A large-scale, prospective multicenter study with external verification cohorts remains needed.
PH is an independent risk factor for predicting the occurrence of PHLF, and the noninvasive methods of assessing PH grade may be a useful predictor of PHLF in patients with HCC after hepatectomy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of Xingtai People’s Hospital (IRB ID 2022-006). The patients/participants provided their written informed consent to participate in this study.
JW and XQ contributed to conception and design of the study. ZZ, DS, JL, BZ, AH, and PY carried out the data curation. JL and CL performed the statistical analysis. MW, DL, HM and SL carried out the methodology. JW and YZ wrote the manuscript. YZ and SC carried out the project administration. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Teh SH, Nagorney DM, Stevens SR, Offord KP, Therneau TM, Plevak DJ, et al. Risk Factors for Mortality After Surgery in Patients With Cirrhosis. Gastroenterology (2007) 132(4):1261–9. doi: 10.1053/j.gastro.2007.01.040
2. Reverter E, Cirera I, Albillos A, Debernardi-Venon W, Abraldes JG, Llop E, et al. The Prognostic Role of Hepatic Venous Pressure Gradient in Cirrhotic Patients Undergoing Elective Extrahepatic Surgery. J Hepatol (2019) 71(5):942–50. doi: 10.1016/j.jhep.2019.07.007
3. Filmann N, Walter D, Schadde E, Bruns C, Keck T, Lang H, et al. Mortality After Liver Surgery in Germany. Br J Surg (2019) 106(11):1523–9. doi: 10.1002/bjs.11236
4. Golse N, Joly F, Combari P, Lewin M, Nicolas Q, Audebert C, et al. Predicting the Risk of Post-Hepatectomy Portal Hypertension Using a Digital Twin: A Clinical Proof of Concept. J Hepatol (2021) 74(3):661–9. doi: 10.1016/j.jhep.2020.10.036
5. Fu J, Chen Q, Yu Y, You W, Ding Z, Gao Y, et al. Impact of Portal Hypertension on Short- and Long-Term Outcomes After Liver Resection for Intrahepatic Cholangiocarcinoma: A Propensity Score Matching Analysis. Cancer Med (2021) 10(20):6985–97. doi: 10.1002/cam4.4222
6. Berzigotti A, Reig M, Abraldes JG, Bosch J, Bruix J. Portal Hypertension and the Outcome of Surgery for Hepatocellular Carcinoma in Compensated Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology. (2015) 61(2):526–36. doi: 10.1002/hep.27431
7. Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, et al. Incidence, Prevalence, and Clinical Significance of Abnormal Hematologic Indices in Compensated Cirrhosis. Clin Gastroenterol Hepatol (2009) 7(6):689–95. doi: 10.1016/j.cgh.2009.02.021
8. Bosch J, Garcia-Pagán JC, Berzigotti A, Abraldes JG. Measurement of Portal Pressure and its Role in the Management of Chronic Liver Disease. Semin Liver Dis (2006) 26(4):348–62. doi: 10.1055/s-2006-951603
9. de Franchis R, Baveno VF. Revising Consensus in Portal Hypertension: Report of the Baveno V Consensus Workshop on Methodology of Diagnosis and Therapy in Portal Hypertension. J Hepatol (2010) 53(4):762–8. doi: 10.1016/j.jhep.2010.06.004
10. European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J Hepatol (2012) 56(4):908–43. doi: 10.1016/j.jhep.2011.12.001
11. Forner A, Llovet JM, Bruix J. Hepatocellular Carcinoma. Lancet (2012) 379(9822):1245–55. doi: 10.1016/S0140-6736(11)61347-0
12. Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, Ramacciato G, et al. Is Portal Hypertension a Contraindication to Hepatic Resection? Ann Surg (2009) 250(6):922–8. doi: 10.1097/SLA.0b013e3181b977a5
13. He W, Zeng Q, Zheng Y, Chen M, Shen J, Qiu J, et al. The Role of Clinically Significant Portal Hypertension in Hepatic Resection for Hepatocellular Carcinoma Patients: A Propensity Score Matching Analysis. BMC Cancer (2015) 15:263. doi: 10.1186/s12885-015-1280-3
14. Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, et al. Neither Multiple Tumors Nor Portal Hypertension are Surgical Contraindications for Hepatocellular Carcinoma. Gastroenterology (2008) 134(7):1908–16. doi: 10.1053/j.gastro.2008.02.091
15. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J Hepatol (2022) 76(3):681–93. doi: 10.1016/j.jhep.2021.11.018
16. Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul J-L, et al. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
17. Ariizumi SI, Katagiri S, Kotera Y, Yamashita S, Omori A, Kato T, et al. Improved Mortality, Morbidity, and Long-Term Outcome After Anatomical Hepatectomy With the Glissonean Pedicle Approach in Patients With Hepatocellular Carcinoma: 30 Years' Experience at a Single Institute. Ann Surg (2022) 275(5):947–54. doi: 10.1097/sla.0000000000004311
18. Choi JW, Chung JW, Lee DH, Kim H-C, Hur S, Lee M, et al. Portal Hypertension is Associated With Poor Outcome of Transarterial Chemoembolization in Patients With Hepatocellular Carcinoma. Eur Radiol (2018) 28(5):2184–93. doi: 10.1007/s00330-017-5145-9
19. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the Oesophagus for Bleeding Oesophageal Varices. Br J Surg (1973) 60(8):646–9. doi: 10.1002/bjs.1800600817
20. Freeman R. The New Liver Allocation System: Moving Toward Evidence-Based Transplantation Policy. Liver Transplantation (2002) 8(9):851–8. doi: 10.1053/jlts.2002.35927
21. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Calcium, ATP, and ROS: A Mitochondrial Love-Hate Triangle Posthepatectomy Liver Failure: A Definition and Grading by the International Study Group of Liver Surgery (ISGLS)Am J Physiol Cell PhysiolSurgery (2011) 149(5):713–24. doi: 10.1016/j.surg.2010.10.001
22. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy Liver Failure: A Definition and Grading by the International Study Group of Liver Surgery (ISGLS). HPB (Oxford) (2011) 13(8):528–35. doi: 10.1111/j.1477-2574.2011.00319.x
23. Prodeau M, Drumez E, Duhamel A, Vibert E, Farges O, Lassailly G, et al. An Ordinal Model to Predict the Risk of Symptomatic Liver Failure in Patients With Cirrhosis Undergoing Hepatectomy. J Hepatol (2019) 71(5):920–9. doi: 10.1016/j.jhep.2019.06.003
24. Søreide JA, Deshpande R. Post Hepatectomy Liver Failure (PHLF) - Recent Advances in Prevention and Clinical Management. Eur J Surg Oncol (2021) 47(2):216–24. doi: 10.1016/j.ejso.2020.09.001
25. Jara M, Reese T, Malinowski M, Valle E, Seehofer D, Puhl G, et al. Reductions in Post-Hepatectomy Liver Failure and Related Mortality After Implementation of the LiMAx Algorithm in Preoperative Work-Up: A Single-Centre Analysis of 1170 Hepatectomies of One or More Segments. HPB (Oxford). (2015) 17(7):651–8. doi: 10.1111/hpb.12424
26. Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The "50-50 Criteria" on Postoperative Day 5: An Accurate Predictor of Liver Failure and Death After Hepatectomy. Ann Surg (2005) 242(6):824–8. doi: 10.1097/01.sla.0000189131.90876.9e
27. Sultana A, Brooke-Smith M, Ullah S, Figueras J, Rees M, Vauthey J-N, et al. Prospective Evaluation of the International Study Group for Liver Surgery Definition of Post Hepatectomy Liver Failure After Liver Resection: An International Multicentre Study. HPB (Oxford) (2018) 20(5):462–9. doi: 10.1016/j.hpb.2017.11.007
28. Calthorpe L, Rashidian N, Benedetti Cacciaguerra A, Conroy PC, Hibi T, Hilal MA, et al. Using the Comprehensive Complication Index to Rethink theISGLS Criteria for Post-Hepatectomy Liver Failure in an International Cohortof Major Hepatectomies. Ann Surg (2021). [online ahead of print] doi: 10.1097/SLA.0000000000005338
29. Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, et al. Surgical Resection of Hepatocellular Carcinoma in Cirrhotic Patients: Prognostic Value of Preoperative Portal Pressure. Gastroenterology (1996) 111(4):1018–22. doi: 10.1016/s0016-5085(96)70070-7
30. Fu J, Chen Q, Yu Y, You W, Ding Z, Gao Y, et al. Impact of Portal Hypertension on Short- and Long-Term Outcomes After Liver Resection for Intrahepatic Cholangiocarcinoma: A Propensity Score Matching Analysis. Cancer Med (2021) 10(20):6985–97. doi: 10.1002/cam4.4222
31. Santambrogio R, Kluger MD, Costa M, Belli A, Barabino M, Laurent A, et al. Hepatic Resection for Hepatocellular Carcinoma in Patients With Child-Pugh's A Cirrhosis: Is Clinical Evidence of Portal Hypertension a Contraindication? HPB (Oxford) (2013) 15(1):78–84. doi: 10.1111/j.1477-2574.2012.00594.x
32. Chen X, Zhai J, Cai X, Zhang Y, Wei L, Shi L, et al. Severity of Portal Hypertension and Prediction of Postoperative Liver Failure After Liver Resection in Patients With Child-Pugh Grade A Cirrhosis. Br J Surg (2012) 99(12):1701–10. doi: 10.1002/bjs.8951
Keywords: non-invasive diagnosis, liver resection, complication, portal hypertension, post-hepatectomy liver failure
Citation: Wang J, Zhang Z, Shang D, Li J, Liu C, Yu P, Wang M, Liu D, Miao H, Li S, Zhang B, Huang A, Zhang Y, Chen S and Qi X (2022) Noninvasively Assessed Portal Hypertension Grade Predicts Post-Hepatectomy Liver Failure in Patients With HepatocellCarcinoma: A Multicenter Study. Front. Oncol. 12:934870. doi: 10.3389/fonc.2022.934870
Received: 03 May 2022; Accepted: 23 June 2022;
Published: 14 July 2022.
Edited by:
Andrea Laurenzi, Azienda Ospedaliero-Universitaria di Bologna (IRCCS), ItalyReviewed by:
Riccardo Memeo, Ospedale Generale Regionale F. Miulli, ItalyCopyright © 2022 Wang, Zhang, Shang, Li, Liu, Yu, Wang, Liu, Miao, Li, Zhang, Huang, Zhang, Chen and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolong Qi, cWl4aWFvbG9uZ0B2aXAuMTYzLmNvbQ==; Shubo Chen, Y3NiODE2MEAxMjYuY29t; Yewei Zhang, emhhbmd5ZXdlaUBuam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.