- 1Graduate School, Beijing University of Chinese Medicine, Beijing, China
- 2Oncology Department of Integrative Medicine, China-Japan Friendship Hospital, Beijing, China
Background: Immunotherapies represented by immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment. A large part of the population has both cancer and psoriasis but is usually excluded from ICI clinical trials because of the dysregulated activation of the immune system. This is the first study to evaluate the safety and efficacy of ICI therapy in patients with cancer and preexisting psoriasis.
Methods: PubMed, EMBASE, Cochrane, and MEDLINE databases were searched from inception through February 2022. Observational studies on patients with cancer and confirmed psoriasis before ICI initiation were included. Outcomes included the incidence of psoriasis flares, de novo immune-related adverse events (irAEs), discontinuation rate due to flare/de novo irAEs, and efficacy of ICI therapy. Clinical manifestations, management, and outcomes for adverse events (AEs) were systematically reviewed. All pooled analyses were based on a random-effects model using Stata software. Meta-regression and subgroup analyses were performed to identify sources of heterogeneity.
Results: Twelve studies involving 191 patients were included. The pooled incidence of psoriasis flares was 45.0% (95% CI: 31.1%-58.9%, I2 = 71.7%) and 44.9% (95% CI: 29.0%–60.7%, I2 = 71.8%) for de novo irAEs. The tumor type, psoriasis subtype, ICI class, and country were the main sources of heterogeneity. Grade 3–4 flares occurred in 10.8% (95% CI: 5.3%–16.3%) of patients, and about 16.6% (95% CI: 10.7%–22.5%) of patients experienced grade 3–4 de novo irAEs. The estimated incidence of ICI discontinuation due to AE was 18.5% (95% CI: 6.1%–30.8%, I2 = 68.7%). The median times to develop flare and de novo irAEs were 44 and 63 days, respectively. Endocrinopathies and colitis were the most common de novo irAEs. Conventional therapy is effective for most AEs. The estimated objective response rate (ORR) of ICIs was 38.1% (95% CI: 11.8%–64.3%, I2 = 81.7%), and the disease control rate (DCR) was 64.5% (95% CI: 55.3%–73.8%, I2 = 0).
Conclusions: The flare of patients with cancer and preexisting psoriasis treated with ICI therapy is frequent, but the incidence of de novo irAEs and the efficacy of ICI therapy are comparable to those of the general population. Most AEs are mild and manageable with conventional therapy, which required discontinuation of ICI therapy in 18.5%.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022320646
Introduction
Cancer is the second leading cause of death in the United States, with 608,570 cancer-related deaths reported in 2021 (1). Recently, immunotherapies represented by immune checkpoint inhibitors (ICIs) have revolutionized the management of cancer. Nevertheless, the prevalence of immune-related adverse events (irAEs) and their clinical efficacy remain alarming.
Psoriasis is a common autoimmune disease (AID) characterized by chronic T cell-mediated inflammation. It predominantly affects the skin (psoriasis) and joints [psoriatic arthritis (PsA)]. Psoriasis lesions manifest mainly as plaques, whereas other manifestations include guttate, flexural, erythrodermic, pustular palmoplantar, nail psoriasis, and arthritis of PsA (2). Notably, a significant number of patients suffer from both cancer and psoriasis. In a large register-based analysis of 2,10,509 lung cancer patients, 2.8% of the patients also had comorbid psoriasis (3). In a recent meta-analysis (4), the prevalence of cancer in patients with psoriasis was 4.78% (95% CI: 4.02%-5.59%), which was not trivial.
Blocking antibodies against programmed cell death receptor-1 (PD-1), programmed cell death ligand-1 (PD-L1), and cytotoxic T lymphocyte-associated molecule-4 (CTLA-4) checkpoint molecules are the most widely used type of ICIs (5). In the past, ICI therapy was thought to trigger severe autoimmune manifestations. This concern stems from the fact that both checkpoint molecules contribute to self-tolerance maintenance. CTLA-4 inhibitors interrupt the interaction of CTLA-4 with B7 co-stimulatory molecules and decrease regulatory T cell (Treg)-suppressive function, while PD-1/PD-L1 inhibitors block the PD-1/PD-L1 pathway (6, 7). Both can restore T-cell activation. ICIs can also activate helper T cells 1 and 17, which produce interleukin-17 (IL-17) and play a crucial role in the pathogenesis of various AIDs (8). Moreover, previous immunosuppressive treatments are thought to compromise ICI efficacy (9).
Hence, patients with preexisting psoriasis were always excluded from the initial clinical trials. However, the latest National Comprehensive Cancer Network (NCCN) guidelines demonstrated that these patients are still potential users of ICIs. More trials recently have included patients who do not require systemic therapy (10). Because of the few studies that have reported patients with both cancer and preexisting AIDs covering a small sample of psoriasis patients, fewer details of ICI therapy in patients with psoriasis are known. Hitherto, the safety and efficacy of ICI therapy in this population are still inconclusive.
To provide evidence for clinical decisions, this study aimed to estimate the prevalence of adverse events (AEs) and the response rate of ICI therapy and to review the clinical manifestations, management, and outcomes of these AEs in patients with cancer and preexisting psoriasis.
Methods
Protocol and Registration
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, and the protocol has been registered with the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42022320646).
Literature Search and Eligibility Criteria
We searched PubMed, EMBASE, Cochrane library databases, and MEDLINE without language restriction from database inception through February 9, 2022.
The search terms mainly included cancer, tumor, immune checkpoint inhibitor, psoriasis, and pre-existing. The specific electronic search strategy is provided in Supplementary Material 1.
The inclusion criteria were observational studies (cohort studies, case–control studies, or case series, prospective or retrospective, excluding single case reports) that reported the incidence of irAEs or the efficacy of ICI therapy. The study population included patients with cancer and a confirmed diagnosis of psoriasis (including PsA and other subtypes) before ICI therapy (anti-PD-1/anti-PD-L1/anti-CTLA-4 or combination). The outcomes of patients with psoriasis were reported separately.
We excluded a) studies that only included patients with pre-immunotherapy-induced psoriasis, b) the treatment regimen involved therapies other than ICI (such as targeted therapy, chemotherapy, radiation therapy, or other immunotherapies), c) duplicated studies, and d) studies in which the full text cannot be obtained.
Study Selection and Data Extraction
Two reviewers (YY and YZ) independently participated in the primary screening of the titles and abstracts, full-text reading, and final decisions. Discrepancies and disagreements were resolved through discussion. If the disagreement could not be resolved, a third-party (HC) expert was consulted.
The data were extracted independently by four reviewers (YY, YZ, XZ, and KT) using the pretested electronic form (Supplementary Table 1) and then cross-checked. The primary outcomes included the incidence of psoriasis flares, de novo irAEs, discontinuation due to flare/de novo irAEs, and efficacy of ICI therapy. Efficacy was measured using objective response rate (ORR) and disease control rate (DCR) [complete response (CR), partial response (PR), and stable disease (SD); ORR, CR + PR; DCR, CR + PR + SD].
The Risk of Bias Assessment and Publication Bias
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) instrument was used to assess the risk of bias, as no appropriate assessment tool was applied. STROBE consists of 22 items to evaluate observational studies. Each item would be labeled as “Yes” or “No,” and total fractions were expressed as a percentage of this value. The risk of bias was scored as follows: 0%–25%, high risk; 25%–50%, medium to high risk; 50%–75%, low to medium risk; and 75%–100%, low risk. Eligibility was evaluated by two reviewers (KT and JL), and disagreements were resolved by a third reviewer (HC). The possibility of publication bias was assessed using Egger’s test, which can be seen in funnel plots.
Data Synthesis and Analysis
To start with, meta-analyses were performed by calculating the incidence and response rate with 95% CI based on the random-effects model according to heterogeneity.
Heterogeneity was assessed using the I2 statistic. When I2 was greater than 50%, a meta-regression analysis was performed to identify the sources of heterogeneity. Second, subgroup analysis was carried out according to the types of cancer, class of ICIs, psoriasis subtypes, etc. For comparing differences in the incidence between the subgroups, we used the chi-square test, continuity correction chi-square test, or Fisher’s exact test.
The data were recorded in Microsoft Excel and analyzed using Stata 15.0. Statistical significance was defined as a two-sided p-value of <0.05.
Results
Study Selection and Characteristics
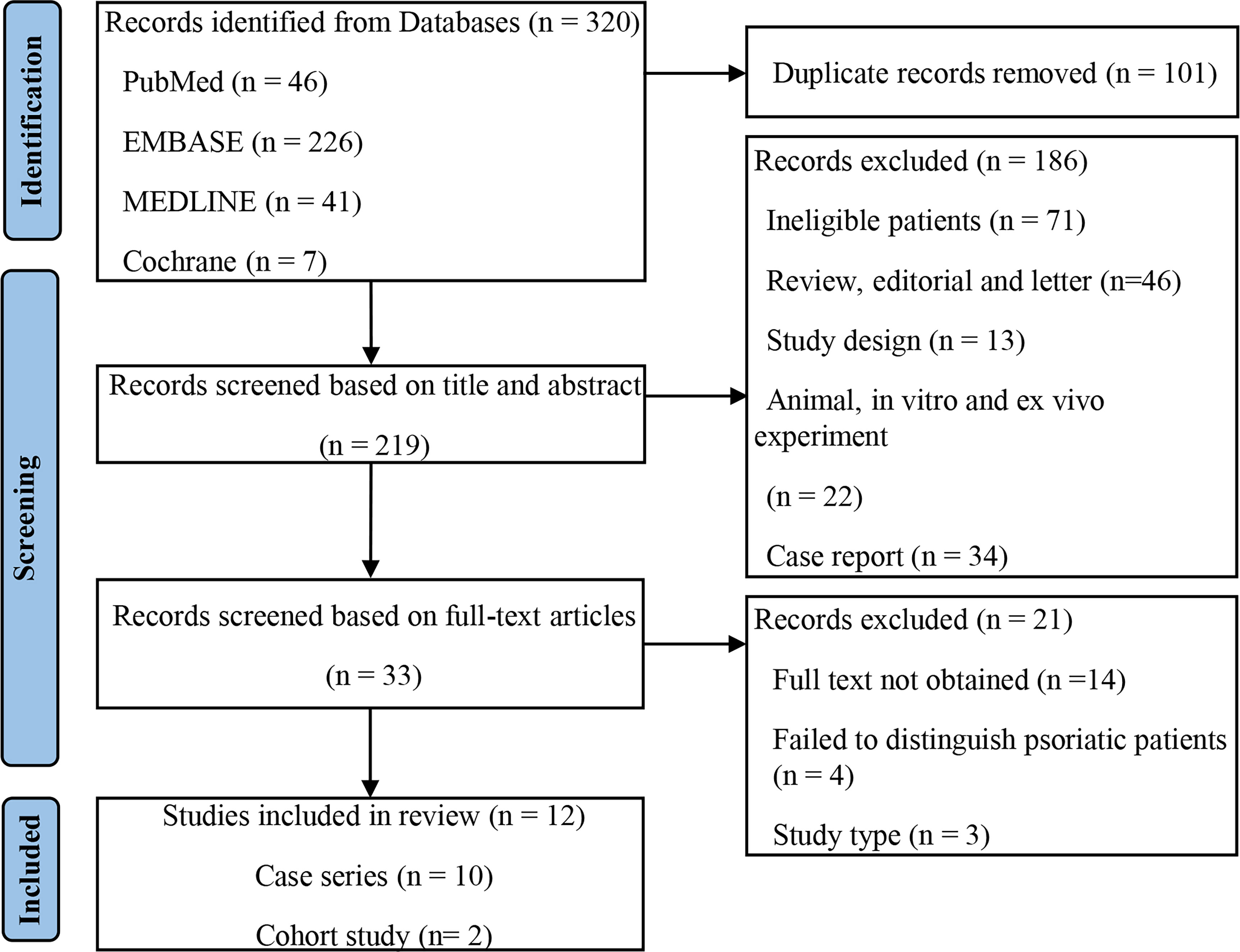
A total of 320 records were retrieved from the primary search, and 219 remained after adjusting for duplicates. Screening and eligibility assessment of the titles and abstracts was performed, and 33 studies were included. Finally, 12 studies (11–22) met the eligibility criteria, and the selection flowchart is shown in Figure 1.
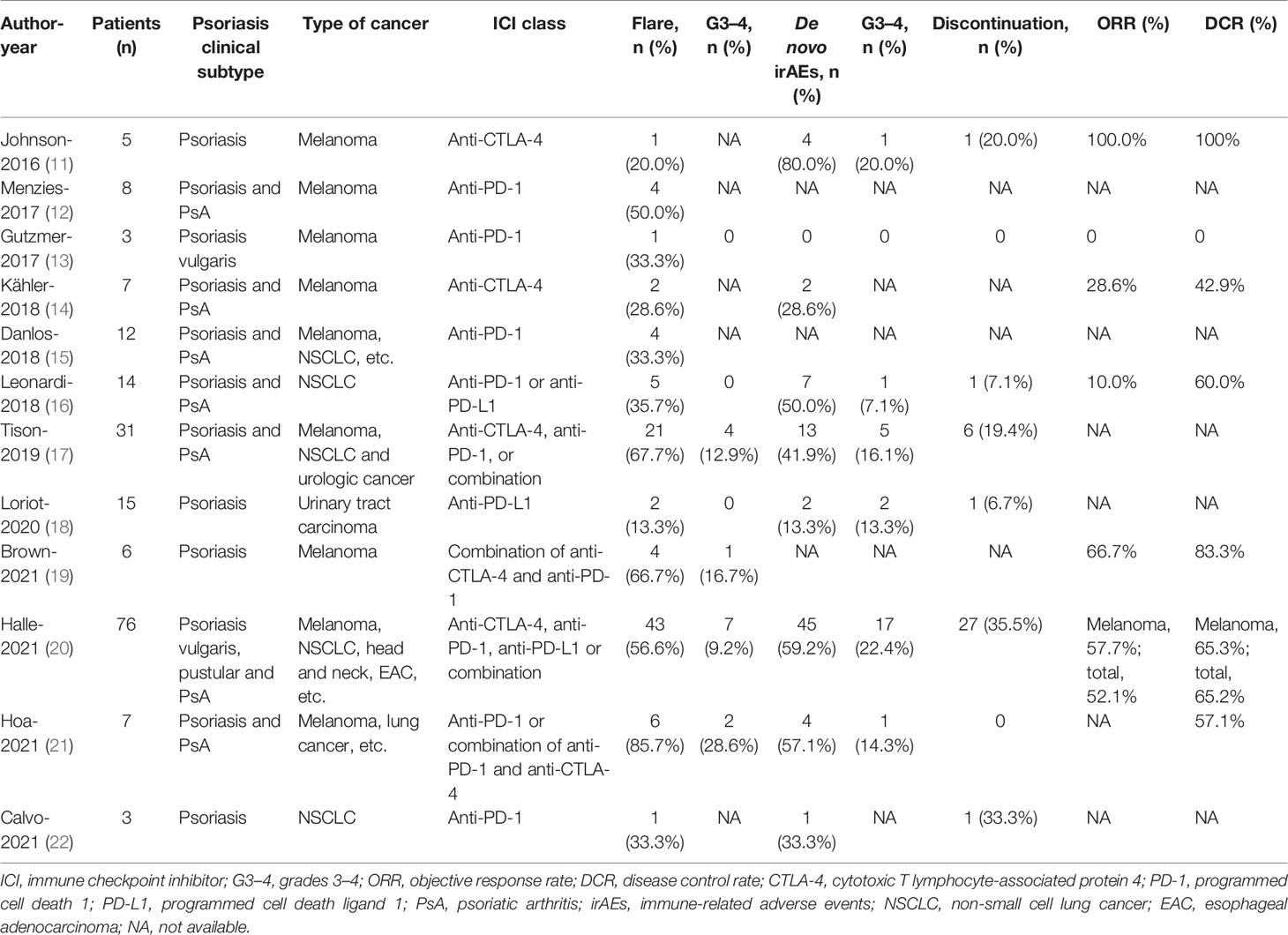
All studies (11–22) were published in English. The publication dates ranged from 2016 to 2021. Two studies (15, 18) were prospective. Eleven studies (11–14, 16–22) were multicenter, and the majority of these studies were conducted in the United States, Australia, France, Germany, and Spain. A total of 191 participants were included, with four studies (11, 18, 19, 21) including patients with psoriasis, whereas the remaining eight studies (12–17, 20, 22) included patients with psoriasis and PsA. One study (20) targeted psoriatic patients only, whereas 11 studies (11–19, 21, 22) included participants with cancer and any AIDs. Five studies (11–14, 19) included patients with melanoma, two studies (16, 22) included patients with non-small cell cancer (NSCLC), one study (18) focused on urologic cancer, and the remaining (11–15, 17, 19–21) were mixed. The classes of ICIs included the following: anti-PD-1 (12, 13, 15, 17, 22), anti-PD-L1 (17, 18) anti-CTLA-4 (11, 14), and combination (16, 19–21). All of these studies have reported the safety and efficacy of ICI therapy. The median follow-up ranged from 4.7 to 25.1 months. The study characteristics are described in Table 1, and more details can be found in Supplementary Table 2.
Psoriasis Flare, Clinical Manifestation, and Management
A flare was defined as a recurrent or worsening of prior psoriasis syndrome, which was reported in all 12 studies (11–22), involving 191 participants. The pooled meta-analysis showed that the incidence of flares was 45.0% (95% CI: 31.1%–58.9%) based on a random-effects model with significant heterogeneity (I2 = 71.7%, p < 0.001; Figure 2A).
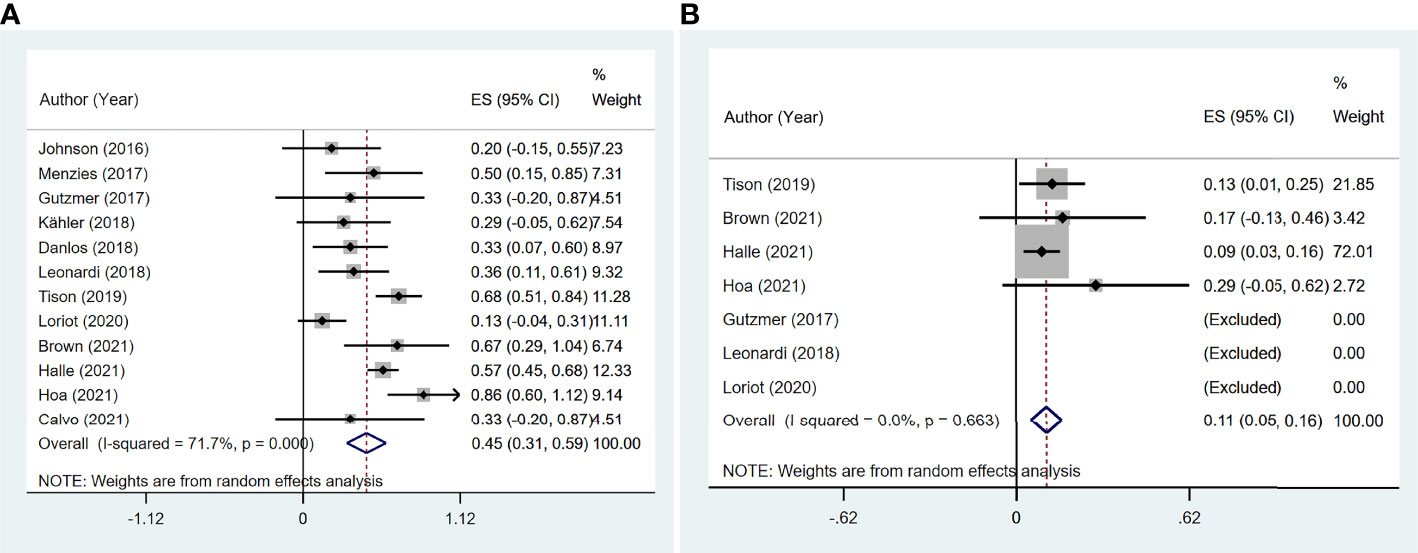
Figure 2 The pooled incidence of flare in patients with cancer and preexisting psoriasis. (A) The incidence of flare for any grades. (B) The incidence of flare for grades 3–4.
In the meta-regression, the study design showed statistical significance (p = 0.027, 95% CI: −57.8%–3.5%; Supplementary Table 3).
Further subgroup analysis revealed heterogeneity in the mixed tumor type (I2 = 66.6%, p = 0.032) and mixed psoriasis subtype (I2 = 60.0%, p = 0.020). The incidence of flare was 39.5% (95% CI: 22.8%–56.2%) in melanoma and 35.3% (95% CI: 12.6%–58.0%) in NSCLC in the subgroup analysis based on cancer types (Supplementary Figure 1).
According to the class of ICIs, the incidence of flares was 24.5% (95% CI: 0.3%–48.7%) for anti-CTLA-4, 27.0% (95% CI: 15.8%–38.3%) for anti-PD-1/PD-L1, and 65.6% (95% CI: 53.7%–77.5%) for the mixed group (Figure 3). There was no difference in incidence between the anti-CTLA-4 group and anti-PD-1/PD-L1 group χ2 = 0.03, p = 0.954).
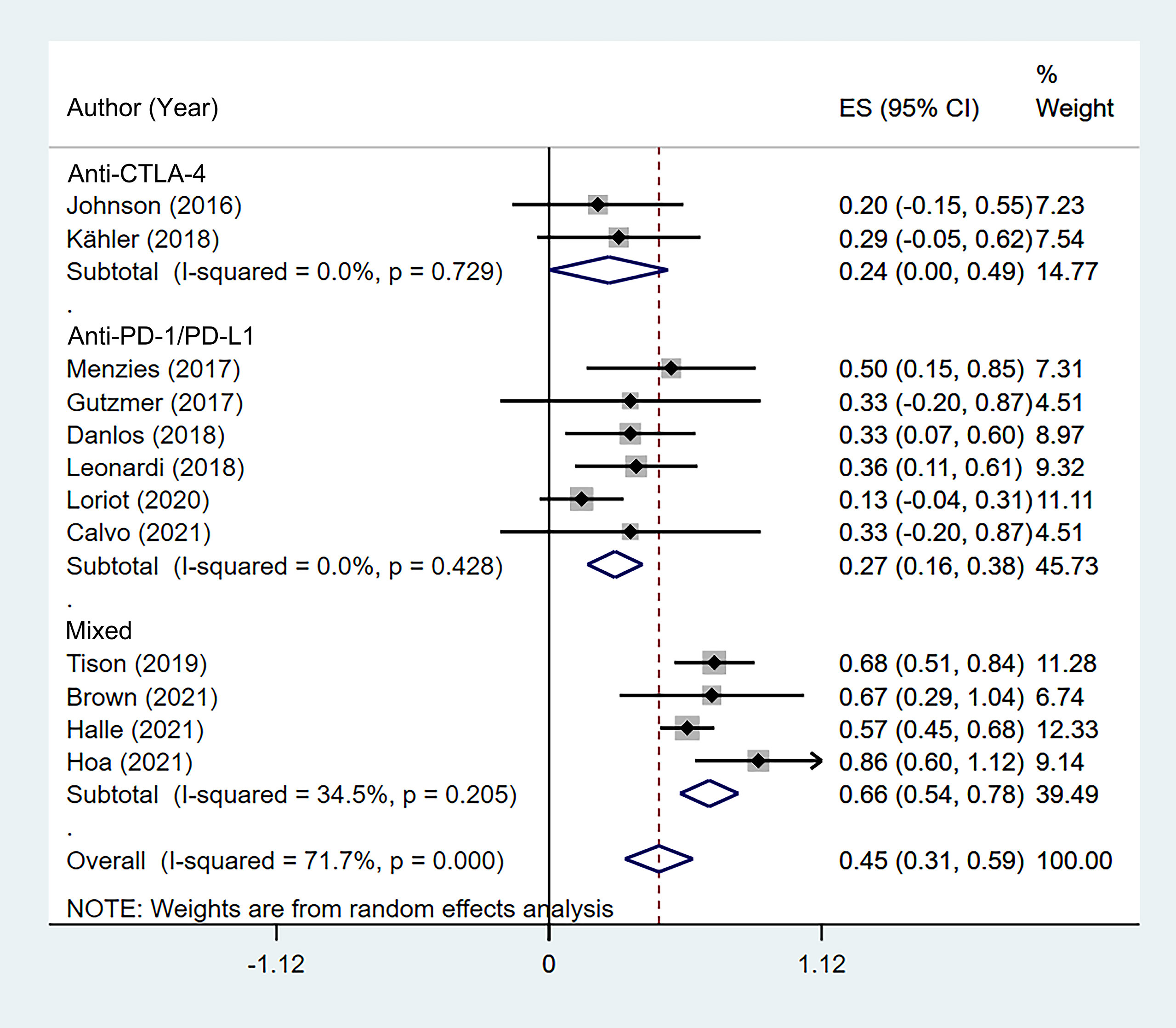
Figure 3 Subgroup analysis of the pooled incidence of flare in patients with cancer and preexisting psoriasis based on the class of immune checkpoint inhibitors (ICIs).
In terms of clinical manifestations, 12 patients (11, 14, 16, 21) and a 76-scale case series (20) of flare onset time were reported, with a median time of 44 days, ranging from 4 to 725 days. The pooled incidence of grade 3–4 flares was 10.8% (95% CI: 5.3%–16.3%, I2 = 0, p = 0.663; Figure 2B).
Topical steroids and vitamin D analogs (including calcipotriol and calcitriol) were used to treat the majority of the patients. Eighty patients received phototherapy, six patients used acitretin additively, and a few patients with severe flare syndrome were given an oral steroid, methotrexate, apremilast, or biologic agents in addition. The majority of the flares were controlled.
De novo Immune-Related Adverse Event, Clinical Manifestation, and Management
The incidence of de novo irAEs was available for nine studies (11, 13, 14, 16–18, 20–22), involving 161 participants. The incidence of de novo irAEs varied from 0% to 80.0%, with a pooled result of 44.9% (95% CI: 29.0%–60.7%). However, significant heterogeneity was found between studies (I2 = 71.8%, p = 0.001; Figure 4A).
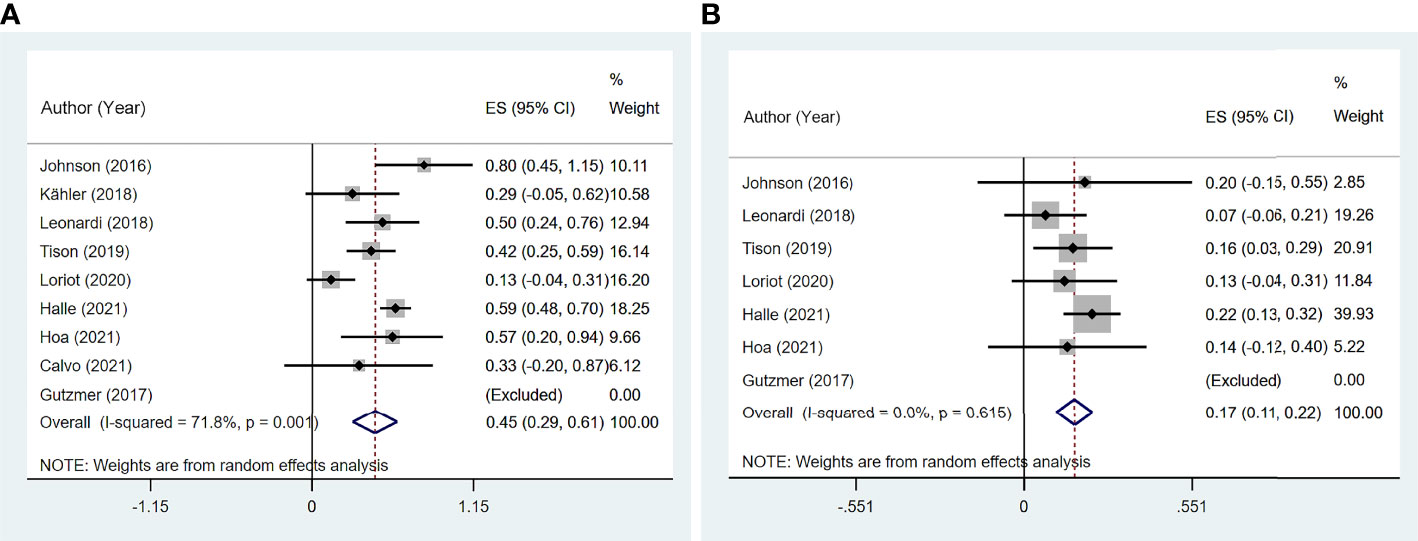
Figure 4 The pooled incidence of de novo immune-related adverse event (irAE) in patients with cancer and preexisting psoriasis. (A) The incidence of flare for any grades. (B) The incidence of de novo irAE for grades 3–4.
Meta-regression showed significant heterogeneity between US studies and other studies (p = 0.010, 95% CI: 8.8%–65.4%; Supplementary Table 4).
Significant heterogeneity was found in the psoriasis (excluded PsA) group (I2 = 82.2%, p = 0.004), melanoma group (I2 = 76.9%, p = 0.038), anti-CTLA-4 group (I2 = 76.9%, p = 0.038), and anti-PD-1/PD-L1 group (I2 = 62.7%, p = 0.069) based on subgroup analysis. In addition, subgroup analysis indicated that the incidence of de novo irAEs was 54.0% (95% CI: 3.6%–104.4%) in melanoma and 46.8% (95% CI: 23.3%–70.3%) in NSCLC (Supplementary Figure 2).
According to the class of ICIs, the incidence of de novo irAEs was 54.0% (95% CI: 3.6%–104.4%) for anti-CTLA-4, 30.3% (95% CI: 3.2%–57.4%) for anti-PD-1/PD-L1, and 53.3% (95% CI: 41.4%–65.2%) for the mixed group (Figure 5). There was no difference in incidence between the anti-CTLA-4 group and anti-PD-1/PD-L1 group (χ2 = 0.998, p = 0.318).
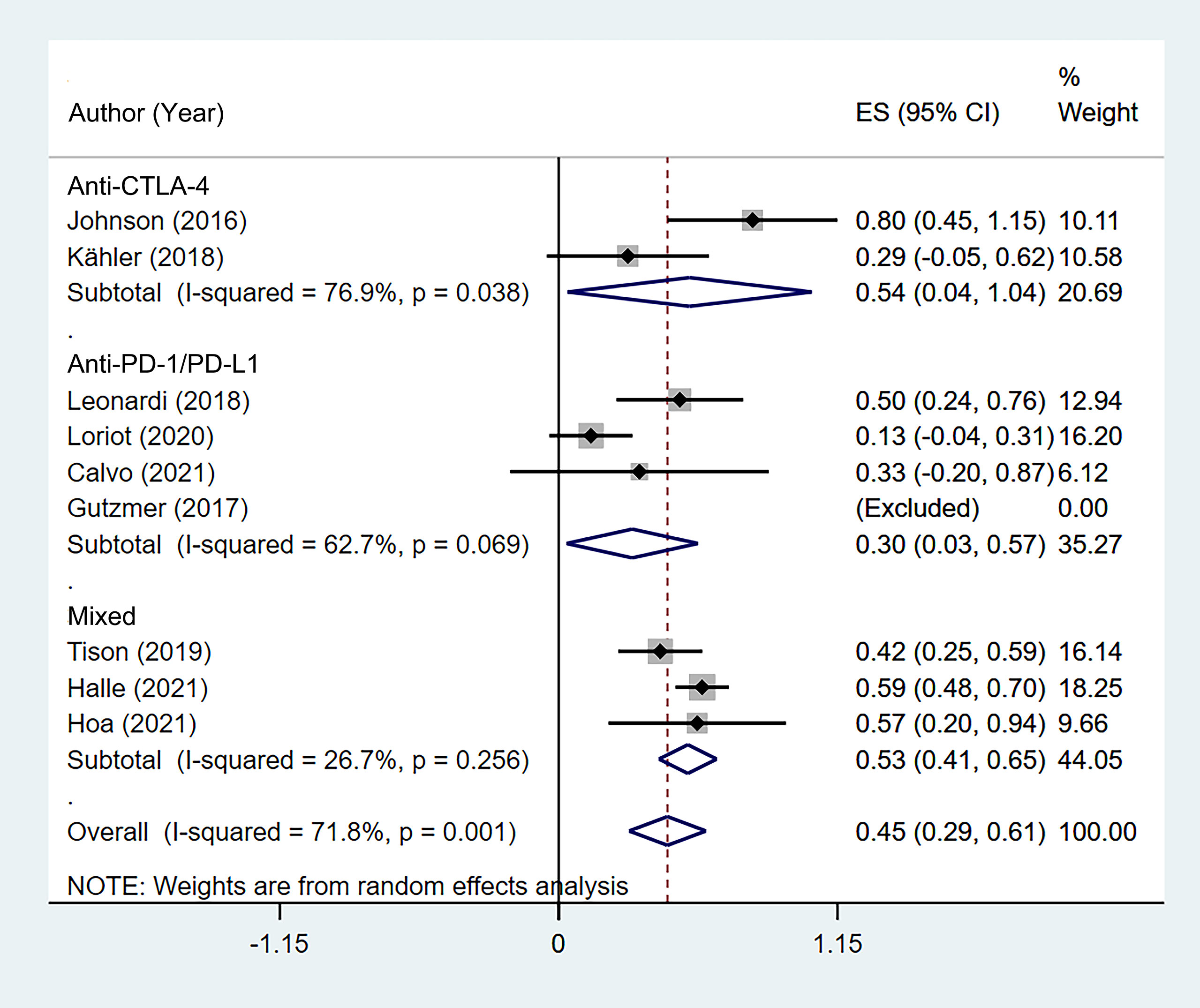
Figure 5 Subgroup analysis of the pooled incidence of de novo immune-related adverse event (irAE) in patients with cancer and preexisting psoriasis based on the class of immune checkpoint inhibitors (ICIs).
Of the 170 cases from six studies (11, 14, 16, 19–21) that reported the specific types of de novo irAEs, 26 patients had endocrinopathies (including thyroiditis and hypophysitis), 23 suffered colitis, 14 suffered hepatitis, 13 had toxicities excluding psoriasis, and 9 suffered arthralgias excluding PsA. Based on the 12 cases, the median onset time was 63 days, with a range of 21 to 270 days. The pooled incidence of grade 3–4 de novo irAEs was 16.6% (95% CI: 10.7%–22.5%, I2 = 0, p = 0.615; Figure 4B). Most of the patients received steroids and symptomatic therapy. However, the outcomes of de novo irAEs have rarely been documented.
Discontinuation Due to Flare/De Novo Immune-related Adverse Event and Rechallenge
Seven studies (11, 13, 16–20) reported the discontinuation rate of ICI therapy due to flare or de novo irAEs. The summary rate was 18.5% (95% CI: 6.1%–30.8%) according to a random-effects analysis. However, significant heterogeneity was noted (I2 = 68.7%, p = 0.007; Figure 6). One patient with delayed treatment of colitis died.
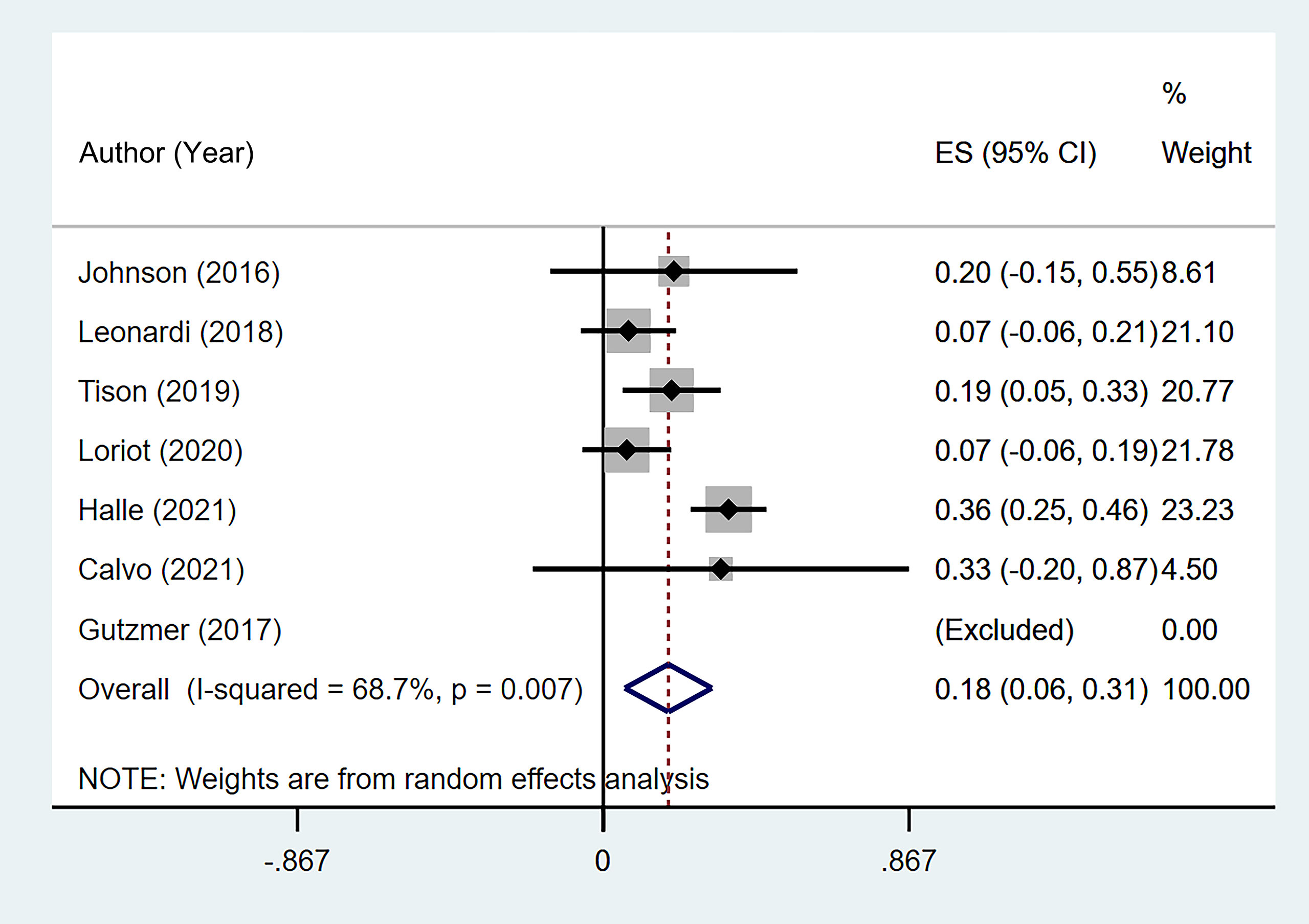
Figure 6 The pooled incidence of immune checkpoint inhibitor (ICI) therapy discontinuation due to flare/de novo immune-related adverse event (irAE) in patients with cancer and preexisting psoriasis.
In three studies (16, 20, 21), twenty-two patients were rechallenged with ICI therapy after flares or de novo irAEs. Leonardi et al. (16) reported that a patient suspended anti-PD-1/PD-L1 treatment for about 1 month because of grade 2 thyroiditis, but the symptom persisted after the rechallenge. Twenty patients in the study by Halle et al. (20) received a rechallenge, involving 13 patients with flares and 7 patients with de novo irAEs. One of the nine patients who received the same class of ICI flared. One of 11 patients who changed the ICIs class flared. The flare grades were 1–2 without discontinuation. One patient developed grade 3 colitis and grade 2 rash after rechallenge with anti-CTLA-4. Hoa et al. (21) reported one patient who discontinued ICI for 2 weeks due to a flare and then resumed it without any further AEs.
Clinical Efficacy of Immune Checkpoint Inhibitor Therapy
Six studies (11, 13, 14, 16, 19, 20) reported ORR, and seven studies (11, 13, 14, 16, 19–21) reported DCR among 97 patients. The pooled ORR was 38.1% (95% CI: 11.8%–64.3%) with significant heterogeneity (I2 = 81.7%, p = 0.001). The summary DCR was 64.5% (95% CI: 55.3%–73.8%), with low heterogeneity (I2 = 0, p = 0.537; Figure 7).
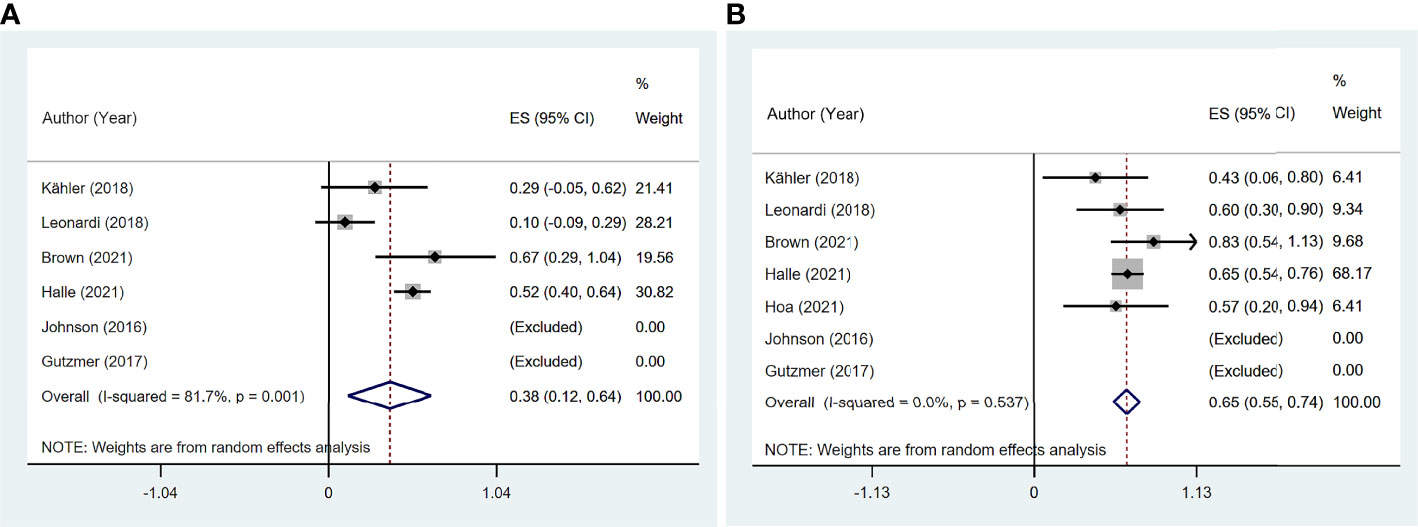
Figure 7 The pooled efficacy in immune checkpoint inhibitor (ICI)-treated patient with preexisting psoriasis. (A) Objective response rate (ORR). (B) Disease control rate (DCR).
Four studies (11, 13, 14, 19) reported 17 patients with preexisting psoriasis and advanced melanoma (Figure 8) corresponding to five with CR, two with PR, two with SD, and eight with PD. The pooled ORR was 46.6% (95% CI: 9.3%–83.9%), and the pooled DCR was 64.6% (95% CI: 25.0%–104.1%).
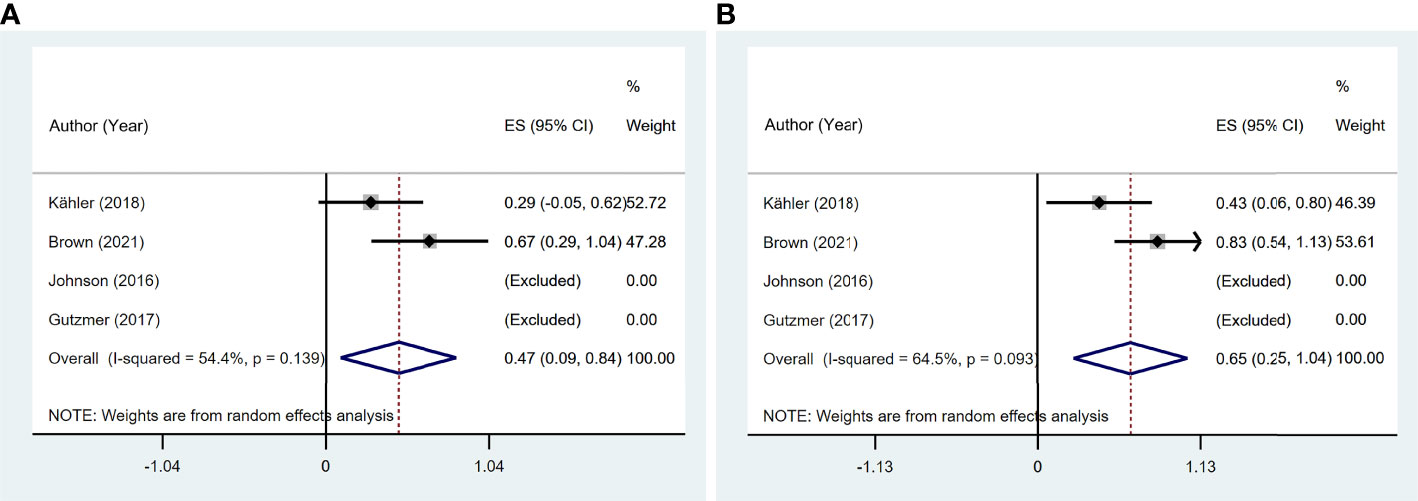
Figure 8 The pooled efficacy in immune checkpoint inhibitor (ICI)-treated patients with preexisting psoriasis and melanoma. (A) Objective response rate (ORR). (B) Disease control rate (DCR).
Risk of Bias Assessment and Publication Bias
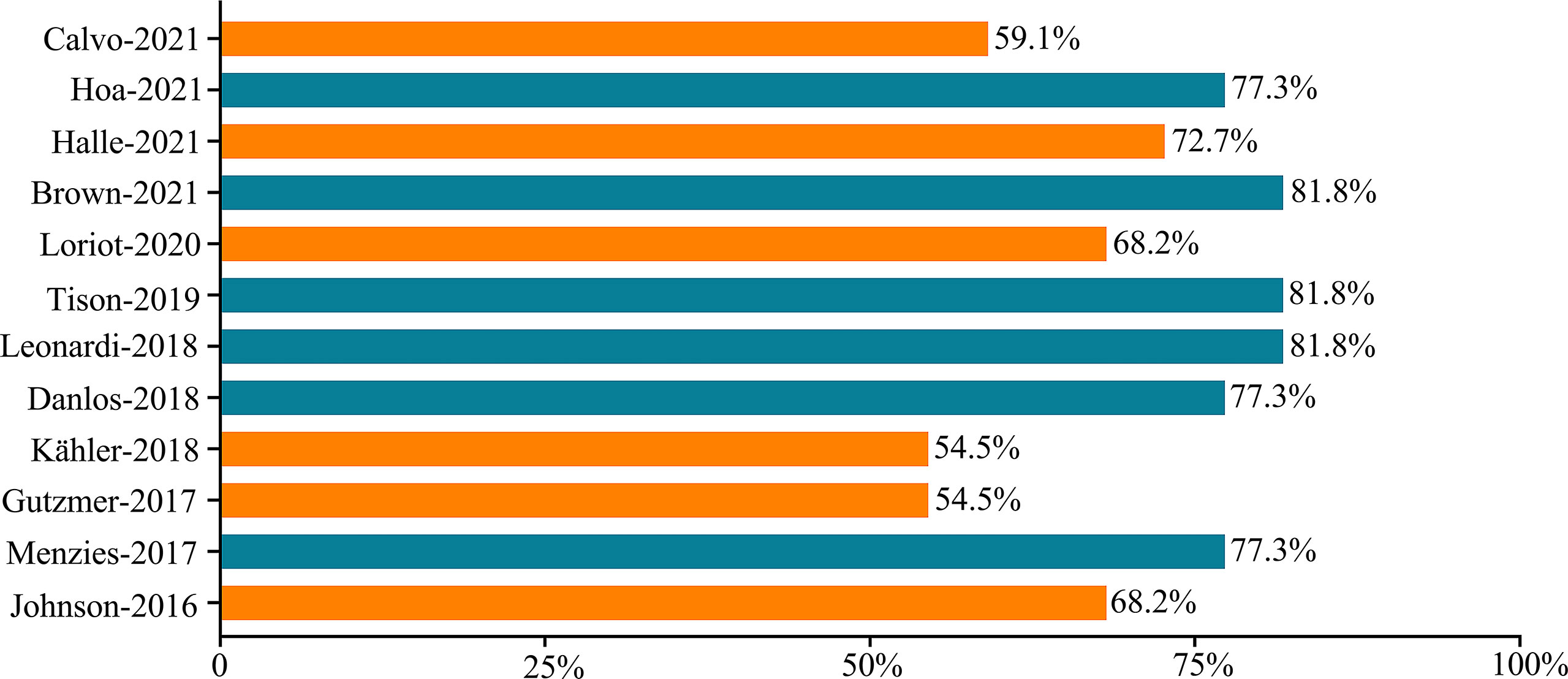
Figure 9 shows the risk of bias scored points, which ranged from 12/22 (54.5%) to 18/22 (81.9%) points. Six studies were classified as low risk, while the other six studies were classified as low to medium risk (Supplementary Table 5).
There was no evidence of publication bias for safety outcomes based on Egger’s test (all p > 0.05; Figure 10).
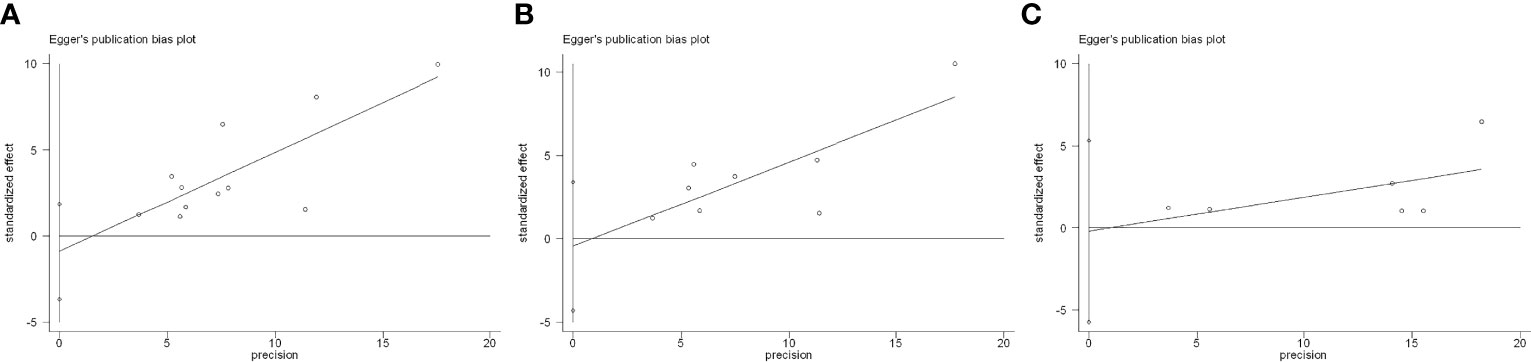
Figure 10 Egger’s test for included studies. (A) Flare. (B) De novo immune-related adverse event (irAE). (C) Discontinuation.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to demonstrate the safety, efficacy, and AE management of patients with cancer and preexisting psoriasis treated with ICIs. This study comprehensively indicated a relatively high incidence of flare (45.0%) but a comparable incidence of de novo irAE (44.9%) and efficacy of ICIs (ORR, 38.1%; DCR, 64.5%) in those patients. In addition, the majority of AEs were mild and manageable with conventional therapy in our study. The discontinuation rate of flare/de novo irAEs was approximately 18.5%.
The summarized incidence of flares in our study was higher than the 35% reported by a previous meta-analysis involving patients with any AIDs (24). Admittedly, psoriasis seems to be more predisposed to flares during ICI therapy. Studies conducted by Kähler et al. (14), Tison et al. (17), and Halle et al. (20) noted that flare occurrence was more frequent in patients with psoriasis and rheumatoid arthritis patients than in other AIDs. ICIs disrupt the CTLA-4 (25) and PD-1/PD-L1 pathways, activate the T cells, and decrease Tregs. They can activate the immune system and promote secondary overexpression of proinflammatory cytokines mediated by Th17 and Th1 lymphocytes (26) with elevated levels of proinflammatory cytokines, such as interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) (27). Off-target inflammation and autoimmunity exacerbate previous psoriasis (28). However, as co-receptors, the expression and regulation of PD-1 and CTLA-4 differ between numerous AIDs (29). This may explain the high exacerbation rate of psoriasis attributed to the above stronger connections and more mechanisms between the pathways and psoriatic patients than other AIDs.
Most flares were grades 1–2 and could be managed by conventional therapy; only 10.8% of the patients experienced grade 3–4 flares in our data. Different treatments have been used to target skin symptoms as well as extracutaneous issues (such as arthralgia). Most of the patients in our study prioritized topical therapy, with topical corticosteroids and vitamin D analogs being the most commonly used to effectively control the flare, while the severe flares needed systemic therapies and phototherapy. There was no significant preference for immunosuppressants; however, acitretin (30) and apremilast (31) were the most common drugs according to the included studies. Nevertheless, recent strategy guidelines (32) recommended specific selective immunosuppressant drugs, including anti-interleukin-12 (anti-IL-12), interleukin-23 (IL-23), and anti-IL-17 blockade as the priority treatment. We found that systemic steroids were rarely used, owing to earlier concerns that they would intervene with ICI therapy (23), albeit no definitive conclusion has been drawn.
The incidence of ICI therapies varied from 66% to 90% for irAEs of any grade and 14%–43% of grade 3 or higher severity in the general population included in previous clinical trials (33–37). Compared with our results, the incidence (44.9% for any grades) and severity (16.6% for grades 3–4) of de novo irAEs did not seem to increase in the targeted population. Concerns about the higher risk of irAEs in patients with preexisting psoriasis have been raised over the few years. However, we now believe it may in part be explained by the lack of distinction between flare and de novo irAEs in the past (38). Recent studies (39, 40) also demonstrated that there was no sign of a higher incidence of de novo irAEs as compared to the general population.
Moreover, we found that endocrinopathies and colitis were the most common irAEs in patients with preexisting psoriasis. Likewise, Yamaguchi et al. (41) suggested the highest incidence rate (38.3%) of endocrine-related irAEs in patients with a history of AIDs. In other words, endocrine-related syndromes and diarrhea deserve more attention and close monitoring during ICI therapy in those patients. Although the correlation between the class of ICIs and irAE types has been recognized, anti-PD-1 is associated with a high incidence of pneumonia, hypothyroidism, arthralgia, and vitiligo, while anti-CTLA-4 is more inclined to induce colitis, hypophysis, and rashes. Further studies of the relationship between preexisting psoriasis and the subtype of underlying irAEs are warranted. What is more, greater research into AID-related antibodies (Abs) is needed to predict irAEs such as anti-thyroglobulin (Tg) and anti-thyroid peroxidase (TPO) Abs (42, 43).
This study suggested that the median onset was 44 days to flare and 63 days to de novo irAEs from the commencement of ICI, which supports the occurrence of flares early during the ICI therapy period. Clarifying the onset of both flare and de novo irAEs is crucial for early identification and timely management to minimize the severity. The class of ICIs and types of irAEs that influence the onset time of irAEs have been identified in earlier investigations (44, 45). The skin-related adverse events were regarded to occur the earliest, always within the first two cycles (46), which explains the findings in this study. The findings of Nikolaou et al. (47) supported that patients with prior history of psoriasis suffered from immune-related psoriasis earlier than the general population (5.4 vs. 12.2 months, p < 0.05). Our findings also corroborated the conclusion drawn by Ramos-Casals et al. (48) that most irAEs occur within 2–16 weeks of ICI initiation.
In consideration of the relation between the safety and class of ICIs, for anti-CTLA-4 and anti-PD-1/PD-L1, the pooled incidence of flares was 24.5% and 27.0%, and the pooled incidence of de novo irAEs was 54.0% and 30.3%, respectively. There was no significant difference between groups in incidence in our results, although the earlier study found that the type of irAE was different among different ICI classes; anti-CTLA-4 is more susceptible to inducing cutaneous irAE as compared to anti-PD-1/PD-L1 (45).
The fundamental underlying concern is whether the risk of flare and de novo irAE and the immunosuppressive treatment for psoriasis, flare, and de novo irAE would reduce the efficacy of ICI therapy. The calculated ORR and DCR were 38.1% and 64.5%, respectively, which were comparable to those of previous phase III clinical trials on melanoma (49, 50) and NSCLC (51, 52). As for advanced melanoma, the summarized ORR was 46.6%, and the DCR was 64.6%. There was no significant difference between the ORR and DCR in the earlier trials, which ranged from 17% to 46% and 42% to 63% (53–56). Research on this complex issue has always been controversial, but the majority of studies have indicated that AID patients have at least the same response rate as the general cancer population (57, 58).
In general, we concentrate on psoriasis rather than all AIDs to minimize the heterogeneity across all AIDs and directly guide clinical decision-making. These findings are consistent with the NCCN guidelines and contribute to the current body of evidence.
There are several limitations. First, most of the included patients had clinically inactive diseases. Severe psoriasis had been excluded by clinicians, resulting in an inherent selection bias. Second, significant heterogeneity between the included studies is inevitable for various cancer types, psoriasis subtypes, and classes of ICIs across studies. Third, most included studies were retrospective, and psoriatic patients only comprised a small percentage of included patients; therefore, many crucial parameters such as psoriasis area and severity index (PASI) scores, previous treatment, and the disease activity were not available. Fourth, the sample size of the included population was small, and few studies were included in each subgroup based on ICI class. Hence, the reliability of these results is limited. Moreover, few studies have reported the subsequent management of AEs, such as rechallenge. Finally, few patients may be included in multiple studies. Therefore, these results must be viewed with caution toward clinical application.
Conclusions
This study revealed that flares are frequent in patients with cancer and preexisting psoriasis treated with ICIs, but with a comparable incidence of de novo irAEs and clinical efficacy to the general population. Most AEs are mild and managed with conventional therapies. Patients with preexisting psoriasis should not be deprived of access to ICI therapy. Further prospective trials including those patients are required to provide distinct clinical care guidelines.
Author Contributions
YY, YZ, and HC designed the study. YY, YZ, and HC screened and selected the records. YY, YZ, XZ, and KT extracted and analyzed the data. YY and JL contributed to the manuscript draft. XZ, JZ, and HC revised the manuscript critically. All authors approved the manuscript version to be published.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81873396), which was led by HC.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the authors for their contributions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.934093/full#supplementary-material
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. Gottlieb AB, Germino R, Herrera V, Meng X, Merola JF. Exploration of the Product of the 5-Point Investigator's Global Assessment and Body Surface Area (IGA × BSA) as a Practical Minimal Disease Activity Goal in Patients With Moderate-To-Severe Psoriasis. Dermatology (2019) 235(4):348–54. doi: 10.1159/000499925
3. Khan SA, Pruitt SL, Xuan L, Gerber DE. Prevalence of Autoimmune Disease Among Patients With Lung Cancer: Implications for Immunotherapy Treatment Options. JAMA Oncol (2016) 2(11):1507–8. doi: 10.1001/jamaoncol.2016.2238
4. Vaengebjerg S, Skov L, Egeberg A, Loft ND. Prevalence, Incidence, and Risk of Cancer in Patients With Psoriasis and Psoriatic Arthritis: A Systematic Review and Meta-Analysis. JAMA Dermatol (2020) 156(4):421–9. doi: 10.1001/jamadermatol.2020.0024
5. Marin-Acevedo JA, Kimbrough EO, Lou Y. Next Generation of Immune Checkpoint Inhibitors and Beyond. J Hematol Oncol (2021) 14(1):45. doi: 10.1186/s13045-021-01056-8
6. Rowshanravan B, Halliday N, Sansom DM. CTLA-4: A Moving Target in Immunotherapy. Blood (2018) 131(1):58–67. doi: 10.1182/blood-2017-06-741033
7. Sunshine J, Taube JM. PD-1/PD-L1 Inhibitors. Curr Opin Pharmacol (2015) 23:32–8. doi: 10.1016/j.coph.2015.05.011
8. Geisler AN, Phillips GS, Barrios DM, Wu J, Leung DYM, Moy AP, et al. Immune Checkpoint Inhibitor-Related Dermatologic Adverse Events. J Am Acad Dermatol (2020) 83(5):1255–68. doi: 10.1016/j.jaad.2020.03.132
9. Drakaki A, Dhillon PK, Wakelee H, Chui SY, Shim J, Kent M, et al. Association of Baseline Systemic Corticosteroid Use With Overall Survival and Time to Next Treatment in Patients Receiving Immune Checkpoint Inhibitor Therapy in Real-World US Oncology Practice for Advanced non-Small Cell Lung Cancer, Melanoma, or Urothelial Carcinoma. Oncoimmunology (2020) 9(1):1824645. doi: 10.1080/2162402X.2020.1824645
10. Rzeniewicz K, Larkin J, Menzies AM, Turajlic S. Immunotherapy Use Outside Clinical Trial Populations: Never Say Never? Ann Oncol (2021) 32(7):866–80. doi: 10.1016/j.annonc.2021.03.199
11. Johnson DB, Sullivan RJ, Ott PA, Carlino MS, Khushalani NI, Ye F, et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol (2016) 2(2):234–40. doi: 10.1001/jamaoncol.2015.4368
12. Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, et al. Anti-PD-1 Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders or Major Toxicity With Ipilimumab. Ann Oncol (2017) 28(2):368–76. doi: 10.1093/annonc/mdw443
13. Gutzmer R, Koop A, Meier F, Meier F, Hassel JC, Terheyden P, et al. Programmed Cell Death Protein-1 (PD-1) Inhibitor Therapy in Patients With Advanced Melanoma and Preexisting Autoimmunity or Ipilimumab-Triggered Autoimmunity. Eur J Cancer (2017) 75:24–32. doi: 10.1016/j.ejca.2016.12.038
14. Kähler KC, Eigentler TK, Gesierich A, Heinzerlinget L, Loquai C, Meier F, et al. Ipilimumab in Metastatic Melanoma Patients With Pre-Existing Autoimmune Disorders. Cancer Immunol Immunother (2018) 67(5):825–34. doi: 10.1007/s00262-018-2134-z
15. Danlos FX, Voisin AL, Dyevre V, Michot JM, Poytier E, Taillade L, et al. Safety and Efficacy of Anti-Programmed Death 1 Antibodies in Patients With Cancer and Pre-Existing Autoimmune or Inflammatory Disease. Eur J Cancer (2018) 91:21–9. doi: 10.1016/j.ejca.2017.12.008
16. Leonardi GC, Gainor JF, Altan M, Kravets S, Danhlberg S, Gedmintas L, et al. Safety of Programmed Death-1 Pathway Inhibitors Among Patients With Non-Small-Cell Lung Cancer and Preexisting Autoimmune Disorders. J Clin Oncol (2018) 36(19):1905–12. doi: 10.1200/JCO.2017.77.0305
17. Tison A, Quéré G, Misery L, Funck-Brentano E, Danlos F, Routier E, et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis Rheumatol (2019) 71(12):2100–11. doi: 10.1002/art.41068
18. Loriot Y, Sternberg CN, Castellano D, Oosting SF, Dumez H, Huddart R, et al. Safety and Efficacy of Atezolizumab in Patients With Autoimmune Disease: Subgroup Analysis of the SAUL Study in Locally Advanced/Metastatic Urinary Tract Carcinoma. Eur J Cancer (2020) 138:202–11. doi: 10.1016/j.ejca.2020.07.023
19. Brown LJ, Weppler A, Bhave P, Allayous C, Patrinely jr JR, Ott P, et al. Combination Anti-PD1 and Ipilimumab Therapy in Patients With Advanced Melanoma and Pre-Existing Autoimmune Disorders. J Immunother Cancer (2021) 9(5):e002121. doi: 10.1136/jitc-2020-002121
20. Halle BR, Betof Warner A, Zaman FY, Haydon A, Bhave P, Dewan AK, et al. Immune Checkpoint Inhibitors in Patients With Pre-Existing Psoriasis: Safety and Efficacy. J Immunother Cancer (2021) 9(10):e003066. doi: 10.1136/jitc-2021-003066
21. Hoa S, Laaouad L, Roberts J, Ennis D, Ye C, Jumaily KA, et al. Preexisting Autoimmune Disease and Immune-Related Adverse Events Associated With Anti-PD-1 Cancer Immunotherapy: A National Case Series From the Canadian Research Group of Rheumatology in Immuno-Oncology. Cancer Immunol Immunother (2021) 70(8):2197–207. doi: 10.1007/s00262-021-02851-5
22. Calvo V, Fernández MA, Collazo-Lorduy A, Franco F, Núñez B, Provencio M. Use of Immune Checkpoint Inhibitors in Patients With Solid Tumors and Pre-Existing Autoimmune or Inflammatory Disease: Real-World Data. Lung Cancer Manag (2021) 10(4):LMT51. doi: 10.2217/lmt-2021-0003
23. Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol (2018) 36(28):2872–8. doi: 10.1200/JCO.2018.79.0006
24. Xie W, Huang H, Xiao S, Fan Y, Deng X, Zhang Z. Immune Checkpoint Inhibitors Therapies in Patients With Cancer and Preexisting Autoimmune Diseases: A Meta-Analysis of Observational Studies. Autoimmun Rev (2020) 19(12):102687. doi: 10.1016/j.autrev.2020.102687
25. Liu S, Xu J, Wu J. The Role of Co-Signaling Molecules in Psoriasis and Their Implications for Targeted Treatment. Front Pharmacol (2021) 12:717042. doi: 10.3389/fphar.2021.717042
26. Sibaud V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am J Clin Dermatol (2018) 19(3):345–61. doi: 10.1007/s40257-017-0336-3
27. Bommarito D, Hall C, Taams LS, Corrigall VM. Inflammatory Cytokines Compromise Programmed Cell Death-1 (PD-1)-Mediated T Cell Suppression in Inflammatory Arthritis Through Up-Regulation of Soluble PD-1. Clin Exp Immunol (2017) 188(3):455–66. doi: 10.1111/cei.12949
28. Benhadou F, Mintoff D, Del Marmol V. Psoriasis: Keratinocytes or Immune Cells - Which Is the Trigger? Dermatology (2019) 235(2):91–100. doi: 10.1159/000495291
29. Adamczyk M, Krasowska D. PD1/PD-L1 Pathway in Psoriasis and Psoriatic Arthritis: A Review. Postepy Dermatol Alergol (2021) 38(6):925–30. doi: 10.5114/ada.2021.112274
30. Bonigen J, Raynaud-Donzel C, Hureaux J, Kramkimel N, Blom A, Jeudy G, et al. Anti-PD1-Induced Psoriasis: A Study of 21 Patients. J Eur Acad Dermatol Venereol (2017) 31(5):e254–7. doi: 10.1111/jdv.14011
31. Fattore D, Annunziata MC, Panariello L, Marasca C, Fabbrocini G. Successful Treatment of Psoriasis Induced by Immune Checkpoint Inhibitors With Apremilast. Eur J Cancer (2019) 110:107–9. doi: 10.1016/j.ejca.2019.01.010
32. Haanen J, Ernstoff MS, Wang Y, Menzies AM, Puzanov I, Grivas P, et al. Autoimmune Diseases and Immune-Checkpoint Inhibitors for Cancer Therapy: Review of the Literature and Personalized Risk-Based Prevention Strategy. Ann Oncol (2020) 31(6):724–44. doi: 10.1016/j.annonc.2020.03.285
33. Gu L, Khadaroo PA, Su H, Kong L, Chen L, Wang X, et al. The Safety and Tolerability of Combined Immune Checkpoint Inhibitors (Anti-PD-1/PD-L1 Plus Anti-CTLA-4): A Systematic Review and Meta-Analysis. BMC Cancer (2019) 19(1):559. doi: 10.1186/s12885-019-5785-z
34. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis. JAMA Oncol (2019) 5(7):1008–19. doi: 10.1001/jamaoncol.2019.0393
35. Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Prolonged Survival in Stage III Melanoma With Ipilimum Abadjuvant Therapy. N Engl J Med (2016) 375(19):1845−55. doi: 10.1056/NEJMoa1611299
36. Kennedy LB, Salama AKS. A Review of Cancer Immunotherapy Toxicity. CA Cancer J Clin (2020) 70(2):86–104. doi: 10.3322/caac.21596
37. Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front Pharmacol (2017) 8:49. doi: 10.3389/fphar.2017.00049
38. Dietz H, Weinmann SC, Salama AK. Checkpoint Inhibitors in Melanoma Patients With Underlying Autoimmune Disease. Cancer Manag Res (2021) 13:8199–208. doi: 10.2147/CMAR.S283217
39. Klavdianou K, Melissaropoulos K, Filippopoulou A, Daoussis D. Should We be Afraid of Immune Check Point Inhibitors in Cancer Patients With Pre-Existing Rheumatic Diseases? Immunotherapy in Pre-Existing Rheumatic Diseases. Mediterr J Rheumatol (2021) 32(3):218–26. doi: 10.31138/mjr.32.3.218
40. Coureau M, Meert AP, Berghmans T, Grigoriu B. Efficacy and Toxicity of Immune -Checkpoint Inhibitors in Patients With Preexisting Autoimmune Disorders. Front Med (Lausanne) (2020) 7:137. doi: 10.3389/fmed.2020.00137
41. Yamaguchi A, Saito Y, Okamoto K, Narumi K, Furugen A, Takekuma Y, et al. Preexisting Autoimmune Disease is a Risk Factor for Immune-Related Adverse Events: A Meta-Analysis. Support Care Cancer (2021) 29(12):7747–53. doi: 10.1007/s00520-021-06359-7
42. Izawa N, Shiokawa H, Onuki R, Hamaji K, Morikawa K, Saji H, et al. The Clinical Utility of Comprehensive Measurement of Autoimmune Disease-Related Antibodies in Patients With Advanced Solid Tumors Receiving Immune Checkpoint Inhibitors: A Retrospective Study. ESMO Open (2022) 7(2):100415. doi: 10.1016/j.esmoop.2022.100415
43. Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, Aiba T, et al. Profiling Preexisting Antibodies in Patients Treated With antiePD-1 Therapy for Advanced Nonesmall Cell Lung Cancer. JAMA Oncol (2019) 5(3):376–83. doi: 10.1001/jamaoncol.2018.5860
44. Apalla Z, Nikolaou V, Fattore D, Fabbrocini G, Freites-Martinez A, Sollena P, et al. European Recommendations for Management of Immune Checkpoint Inhibitors-Derived Dermatologic Adverse Events. The EADV Task Force 'Dermatology for Cancer Patients' Position Statement. J Eur Acad Dermatol Venereol (2022) 36(3):332–50. doi: 10.1111/jdv.17855
45. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and Class-Specific Patterns of Immune-Related Adverse Events of Immune Checkpoint Inhibitors: A Systematic Review. Ann Oncol (2017) 28(10):2377–85. doi: 10.1093/annonc/mdx286
46. Zhang L, Lu Y. Follow-Up Care for Patients Receiving Immune Checkpoint Inhibitors. Asia Pac J Oncol Nurs (2021) 8(6):596–603. doi: 10.4103/apjon.apjon-2129
47. Nikolaou V, Sibaud V, Fattore D, Sollena P, Ortiz-Brugués A, Giacchero D, et al. Immune Checkpoint-Mediated Psoriasis: A Multicenter European Study of 115 Patients From the European Network for Cutaneous Adverse Event to Oncologic Drugs (ENCADO) Group. J Am Acad Dermatol (2021) 84(5):1310–20. doi: 10.1016/j.jaad.2020.08.137
48. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-Related Adverse Events of Checkpoint Inhibitors. Nat Rev Dis Primers (2020) 6(1):38. doi: 10.1038/s41572-020-0160-6
49. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival With Ipilimumab in Patients With Metastatic Melanoma. N Engl J Med (2010) 363(12):711–23. doi: 10.1056/NEJMoa1003466
50. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J, Putkowski P, Lao CD, et al. Five-Year Survival With Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2019) 381(16):1535–46. doi: 10.1056/NEJMoa1910836
51. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab Versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic non-Small-Cell Lung Cancer (KEYNOTE-042): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet (2019) 393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7
52. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab Versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
53. Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab Versus Investigator-Choice Chemotherapy for Ipilimumab-Refractory Melanoma (KEYNOTE-002): A Randomised, Controlled, Phase 2 Trial. Lancet Oncol (2015) 16(8):908–18. doi: 10.1016/S1470-2045(15)00083-2
54. Robert C, Ribas A, Schachter J, Arance A, Grob J, Mortier L, et al. Pembrolizumab Versus Ipilimumab in Advanced Melanoma (KEYNOTE-006): Post-Hoc 5-Year Results From an Open-Label, Multicentre, Randomised, Controlled, Phase 3 Study. Lancet Oncol (2019) 20(9):1239–51. doi: 10.1016/S1470-2045(19)30388-2
55. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in Previously Untreated Melanoma Without BRAF Mutation. N Engl J Med (2015) 372(4):320–30. doi: 10.1056/NEJMoa1412082
56. Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob J, Rutkowski P, Cowey CL, et al. Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab Alone in Advanced Melanoma (CheckMate 067): 4-Year Outcomes of a Multicentre, Randomised, Phase 3 Trial. Lancet Oncol (2018) 19(11):1480–92. doi: 10.1016/S1470-2045(18)30700-9
57. Das S, Johnson DB. Immune-Related Adverse Events and Anti-Tumor Efficacy of Immune Checkpoint Inhibitors. J Immunother Cancer (2019) 7(1):306. doi: 10.1186/s40425-019-0805-8
58. Eggermont AMM, Kicinski M, Blank CU, Mandala M, Long GV, Atkinson V, et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol (2020) 6(4):519–27. doi: 10.1001/jamaoncol.2019.5570
Keywords: immune checkpoint inhibitor (ICI), immune-related adverse event (irAE), psoriasis, autoimmune disease (AID), programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), cytotoxic T lymphocyte-associated protein 4 (CTLA-4)
Citation: Yu Y, Zhou Y, Zhang X, Tan K, Zheng J, Li J and Cui H (2022) Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Psoriasis: A Systematic Review and Meta-Analysis of Observational Studies. Front. Oncol. 12:934093. doi: 10.3389/fonc.2022.934093
Received: 02 May 2022; Accepted: 20 June 2022;
Published: 15 July 2022.
Edited by:
John M. Kirkwood, University of Pittsburgh Hillman Cancer Center, United StatesReviewed by:
Matteo Carlino, Melanoma Institute Australia, AustraliaApril Salama, Duke University, United States
Copyright © 2022 Yu, Zhou, Zhang, Tan, Zheng, Li and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huijuan Cui, Y3VpaGoxOTYzQHNpbmEuY29t
 Yixuan Yu
Yixuan Yu Yang Zhou1
Yang Zhou1 Xu Zhang
Xu Zhang Jia Li
Jia Li