- 1Department of Dermatology, Yonsei University College of Dentistry, Seoul, South Korea
- 2Department of Oral Pathology, Yonsei University College of Dentistry, Seoul, South Korea
- 3BK21 FOUR Project, Yonsei University College of Dentistry, Seoul, South Korea
- 4Department of Dental Education, Yonsei University College of Dentistry, Seoul, South Korea
- 5Department of Oral and Maxillofacial Surgery, Yonsei University College of Dentistry, Seoul, South Korea
- 6Department of Pathology, Yanbian University Hospital, Yanji, China
- 7Oral Cancer Research Institute, Yonsei University College of Dentistry, Seoul, South Korea
Objectives: The concept of adequate surgical margins remains controversial in oral squamous cell carcinoma (OSCC) surgery. This study aimed to identify surgical margin-related indicators that might impact recurrence and survival of OSCC patients.
Materials and Methods: Histopathological examination was performed using hematoxylin-eosin-stained surgical margin tissue sections in 235 OSCC patients. Axin2 and Snail expression at the surgical margin was detected by immunohistochemistry. The impact of the Axin2-Snail cascade on tumorigenesis of the immortalized human oral keratinocyte (IHOK) line was investigated in vivo.
Results: The width and dysplasia of surgical margins were not significantly associated with the outcome of OSCC patients. In a multivariate analysis using variable clinicopathologic factors and with Axin2 and Snail expression as cofactors, higher age (hazard ratio [HR]:1.050; P=0.047), Axin2 (HR:6.883; P=0.014), and Snail abundance (HR:5.663; P=0.009) had independent impacts on worsened overall survival. Similarly, lesion site in retromolar trigone (HR:4.077; P=0.010), upper (HR:4.332; P=0.005) and lower gingiva (HR:3.545; P=0.012), presence of extranodal extension (HR:9.967; P<0.001), perineural invasion (HR:3.627; P=0.024), and Snail abundance (HR:3.587; P<0.001) had independent impacts on worsened recurrence-free survival. Furthermore, Axin2 knockdown induced decreased Snail expression and attenuated tumorigenesis in the IHOK line.
Conclusion: Histopathological examination of surgical margins may not be reliable to predict OSCC patient outcome. Molecular analysis may provide a more accurate risk assessment of surgical margins in OSCC. In particular, Axin2 and Snail are potential predictive biomarkers for the risk assessment of surgical margins in OSCC.
Introduction
As the most common histologic type of oral cancer, oral squamous cell carcinoma (OSCC) accounts for approximately 90% of oral cavity malignancies (1). More than 200000 new cases of OSCC are reported annually worldwide (2), and the five-year survival rate of patients is only ~50% (3). Despite good diagnostic and therapeutic strategies, local recurrence or distant metastasis occurs in 40–60% of OSCC patients (4, 5).
Surgical resection is the most accepted choice for OSCC therapy, and the characteristics of surgical margins in OSCC are considered a powerful predictive factor for both recurrence and patient survival (6–10). During OSCC surgery, surgeons should aim not only to secure sufficient resection margins, but also to maintain an acceptable physical appearance and the functions of adjacent organs of the oral cavity. Therefore, most OSCC patients have insufficient margins after surgical resection.
In OSCC surgery, the concept of marginal status (adequate or inadequate) remains controversial. Traditionally, the adequacy of margins was determined based on the width of the resection margin, defined as the distance from the histologically confirmed tumor edge to the inked margin of the specimen. The Royal College of Pathologists (PCRath)-issued guidelines are the most widely accepted criteria for histological OSCC resection margin status (11). In the PCRath scoring system, resection margins are divided into three categories based on the width of the resection margins: clear (width > 5 mm), close (width = 1–5 mm), and positive (width < 1 mm) margins. Generally, a clear margin is denoted as adequate, and both close and positive margins are considered inadequate. However, various factors, such as tissue shrinkage and mucosal elasticity, can influence the width of the resection margins postoperatively (12, 13). In particular, the degree of shrinkage varies according to many factors, including tissue component, lesion site, stage of tumors, status of keratinization, inflammation, and pathological processing (13). Thus, there is a considerable discrepancy in the width between the clinical and pathological resection margins. Therefore, it is difficult to represent the status of resection margins in patients with OSCC based only on the width of the pathological margins.
Research on the molecular characteristics of surgical margins is ongoing. In 1953, Slaughter et al. observed that histologically altered cells were present in tissues adjacent to cancers, and termed this phenomenon ‘field cancerization’ (14). Recently, researchers have found that ‘field cancerization’ is induced by various genetic alterations, such as gene mutation and amplification, as well as aberrant methylations; some of the histologically normal cells adjacent to cancer tissues also show genetic alterations (15–17). The aberrant expression of p53 and p16 in histologically normal surgical margins of OSCC has been highlighted by several investigators (15, 18). However, predictive biomarkers currently fall short of estimating the status of surgical margins of OSCC.
The crucial role of epithelial-to-mesenchymal transition (EMT), a well-known biological process, in both the invasion and metastasis of various cancers, including OSCC, has been well established (19). Moreover, the possible implications of EMT genes, such as axis inhibition protein 2 (Axin2) and Snail, in early-stage carcinogenesis was also highlighted in a previous study (20). Significantly increased Snail expression has been observed in early-stage endometrial carcinoma before invasion. In addition, overexpression of both Axin2 and Snail can be found in some precancerous lesions, including colorectal adenoma and oral leukoplakia (21–23). In our previous study, we also found that overexpression of Axin2 and Snail is a potential risk factor for the malignant conversion of oral leukoplakia (23). In this study, we determined the predictive value of Axin2 and Snail expression in the risk assessment of the surgical margin of OSCC and further evaluated the impact of the Axin2-Snail cascade on the tumorigenic activity of a spontaneously immortalized human oral keratinocyte (IHOK) line (24) in vivo. Histopathological risk assessment was also performed in our OSCC cohort based on the evaluation of the width and presence of epithelial dysplasia (ED) in the surgical margins.
Materials and Methods
Patients in the OSCC Cohort
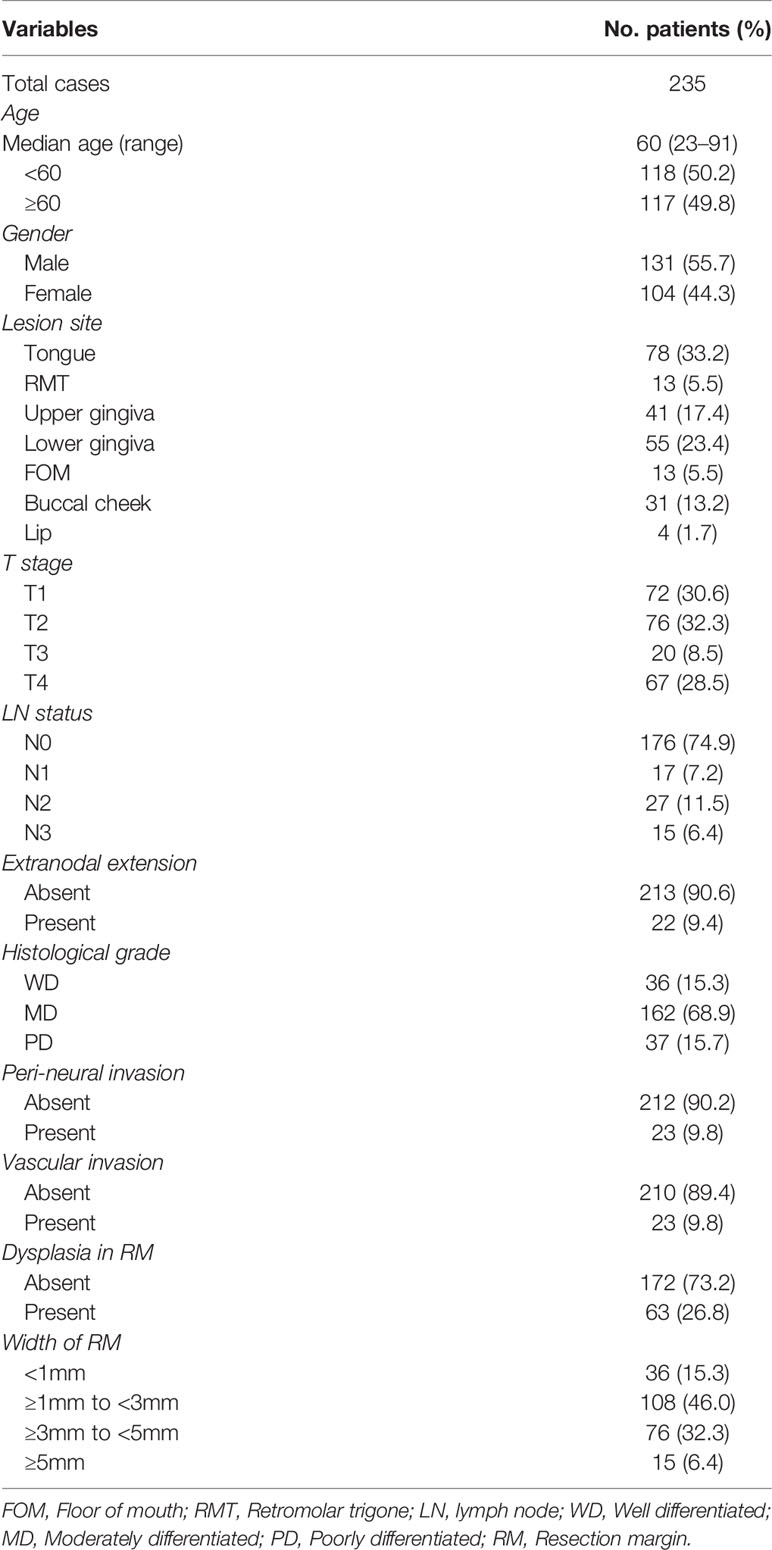
A total of 278 patients with OSCC who underwent surgery at the Department of Oral and Maxillofacial Surgery, Dental Hospital, Yonsei University Medical Center, between 2009 and 2018 were retrospectively reviewed; 43 patients with tumor extension to the surface of resection margin were excluded from this study. Tissue samples of the surgical resection margin were obtained from 235 OSCC patients from the Department of Oral Pathology: 58 (24.7%) patients with recurrence (median follow-up period: 12.2 months) and 177 (75.3%) patients without recurrence during follow-up (median follow-up: 36.6 months) (Figure 1). Patient characteristics are shown in Table 1. This study was approved by the Institutional Review Board of the Yonsei University College of Dentistry (2-2021-0098).
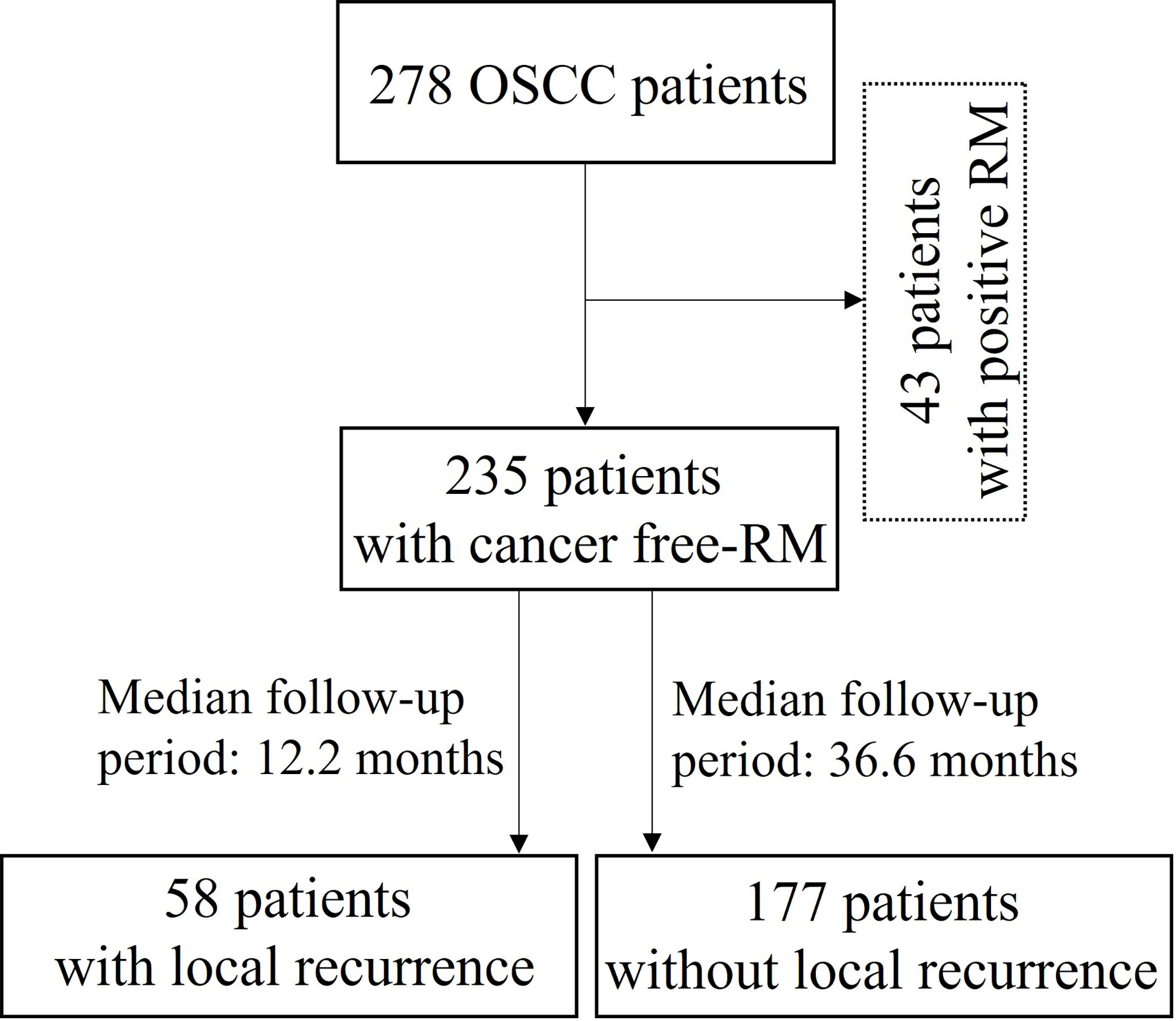
Figure 1 Flow diagram for the selection and outcome of patients with oral squamous cell carcinoma (OSCC: Oral squamous cell carcinoma; RM: Resection margin).
Immunohistochemical Staining
Immunohistochemistry was performed on 4-μm sections of paraffin-embedded tissue specimens of surgical margins obtained from 235 patients with OSCC. Axin2 (Rabbit polyclonal IgG, working dilution 1:500; ab32197, Abcam, Cambridge, UK) and Snail antibodies (Rabbit polyclonal IgG, working dilution 1:1000) were used as primary antibodies. The Snail antibody was prepared as previously described (25). Endogenous peroxidase activity was inhibited using a mixture of H2O2 and methanol (1:40), and antigen retrieval was performed by the pressure-cooking method using citrate buffer (pH 6.0, Sigma-Aldrich, Darmstadt, Germany) for the deparaffinized tissue sections. The Real Envision HRP Rabbit/Mouse detection system (Dako, Glostrup, Denmark) was used as a secondary antibody, and immunoreactivity of the tissue sections against Axin2 and Snail was visualized using 3,3′-diaminobenzidine.
The histoscore for the immunoreactivity of both Axin2 and Snail in the surgical margins of OSCC was calculated according to the staining intensity and percentage of positive cells using the weighted histoscore method (26). Patients were subdivided into two groups according to the histoscore: low (histoscore 0–100) and high (histoscore 101–300) expression groups.
Cell Culture and Establishment of Axin2 Knockdown IHOK Line
IHOKs were cultured in a medium composed of Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, USA) and Ham’s nutrient mixture F-12 (Ham’s F12; Gibco BRL, USA) at a ratio of 3:1. The medium was supplemented with 10% Tet-approved FBS (HyClone Laboratories, Inc., Logan, UT, USA), 1% penicillin/streptomycin (Sigma-Aldrich), 0.01 µg/mL cholera toxin (Sigma-Aldrich), 0.04 µg/mL hydrocortisone (Sigma-Aldrich), 0.5 µg/mL insulin, 0.5 µg/mL apo-transferrin(Sigma-Aldrich), and 0.2 µg/mL 3′-5-triodo-1-thyroine (Sigma-Aldrich). The pLKO-Tet-shAxin2 vector was constructed using the pLKO-Tet-On vector (Addgene, Cambridge, MA, USA) and Axin2 oligo (5’-ACCACCACTACATCCACCA-3’). The Axin2 knockdown IHOK line was constructed by transfection of the pLKO-Tet-shAxin2 vector as well as treatment with doxycycline (5 μg/mL, Sigma-Aldrich).
Western Blot Analysis
Western blot analysis was performed in both the pLKO-Tet-ShAxin2-transfected stable IHOK line with or without doxycycline treatment and tumor nodules obtained from the animal study. Total protein extraction was performed with lysis buffer (RIPA buffer; Cell Signaling Technology, Inc., MA, USA), and a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis system was used for protein resolution. After the proteins were transferred to polyvinylidene difluoride membranes (EMD Millipore, MA, USA), blocking was performed using 5% non-fat milk. Primary antibodies against Axin2 (Abcam), Snail, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Cell Signaling Technology) were used for western blot analysis, and the related signal was developed using an enhanced chemiluminescence detection system (Pierce Biotechnology, IL, USA).
Tumor Growth in Mice
Ten female 6-week-old BALB/c-nu/nu mice (BALB/c Slc-nu/nu; Japan SLC, Inc., Hamamatsu, Japan) were randomized into two groups (n=5 per group). A total of 5 × 105 pLKO-Tet-ShAxin2-transfected IHOK cells were subcutaneously injected into the two groups of mice. For the experimental group, cells were pretreated with 5 μg/mL doxycycline for 48 h before cell injection. After cell injection, the experimental group was administered doxycycline at 10 mg/kg body weight by intraperitoneal injection for five consecutive days per week for 28 days. The control group was administered phosphate-buffered saline (pH 7.4) according to the same schedule as that of the experimental group. The tumor volume was determined every 4 days and calculated by measuring the length and width of the tumor nodules (27). At the end of the study period, all mice were sacrificed, and the tumor nodules were removed. All procedures for the animal study were performed according to protocols approved by the Animal Care and Use Committee of the College of Medicine of Yonsei University. The animal research program used in the present study was accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Statistical Analysis
The difference in the volume of tumor nodules between groups was assessed using the Mann–Whitney U test. The Chi-square test, Kaplan–Meier analysis, and Cox regression analysis were performed to estimate the clinicopathological relevance of surgical margin characteristics in the OSCC cohort. Results were considered statistically significant at P < 0.05. The SPSS 25 statistical package (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Histopathologic Assessment for Resection Margins in the OSCC Cohort
Histopathologic assessment was performed independently by two pathologists by evaluating the width and presence of epithelial dysplasia in the hematoxylin-eosin-stained surgical margin of OSCC patients. In this study, margins of 1, 3, and 5 mm were set as the commonly used margin cut-offs to specify adequate safety margins based on previous literature (9, 28–30). In our cohort, 36 (15.3%), 108 (46.0%), 76 (32.3%), and 15 (6.4%) patients showed <1 mm, ≥1 mm to <3 mm, ≥3 mm to <5 mm, and ≥5 mm resection margin widths, respectively. Dysplastic surgical margins were found in 63 (26.8%) patients, and which 6 (9.5%) patients showed severe dysplasia in our cohort. According to the Kaplan–Meier analysis, neither the width of the surgical margin nor margins with dysplasia was significantly associated with both recurrence-free and overall survival of the patients in our cohort (Figure 2).
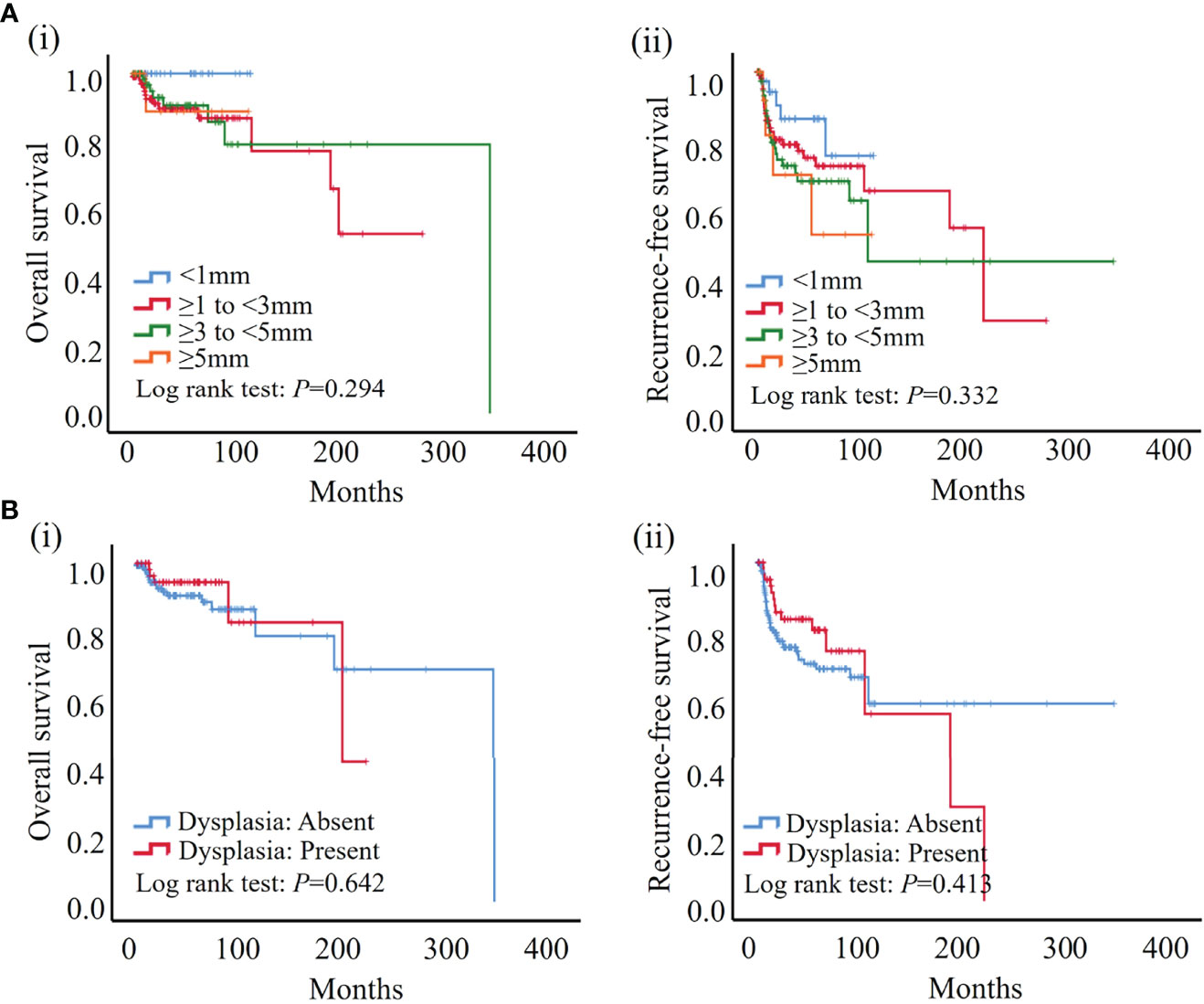
Figure 2 According to the results of the Kaplan–Meier analysis, neither width nor dysplasia was significantly associated with patient outcome. (A) No significant association was found between width of surgical margin and both overall (i) and recurrence-free survival (ii) in our cohort. (B) No significant association was found between dysplasia of surgical margin and both overall (i) and recurrence-free survival (ii) in our cohort.
Axin2 and Snail Are Potential Novel Biomarker(s) in the Risk Assessment of Surgical Margins in OSCC
Cytoplasmic Axin2 expression was found in epithelial cells of resection margins in OSCC, and tissue immunoreactivity against Axin2 was high in 60 (Axin2-high, 25.5%) and low in 175 (Axin2-low, 74.5%) surgical margin tissue samples. In contrast, Snail expression was found in both the nucleus and cytoplasm of epithelial cells in surgical margins of OSCC, and the immunoreactivity against Snail was high in 54 (Snail-high, 23.0%) and low in 181 (Snail-low, 77.0%) surgical margin tissue samples. Representative expression patterns for low or high immunoreactivity for both Axin2 and Snail in the surgical margins are shown in Figure 3A. Interestingly, Snail-high expression was detected more often in resection margins with Axin2-high expression (30, 50.0%) than in resection margins with Axin2-low expression (24, 13.7%) (P<0.001) (Figure 3B). Consistent with previous studies, Axin2-mediated Snail stabilization may also be present in the resection margins of OSCC (25).
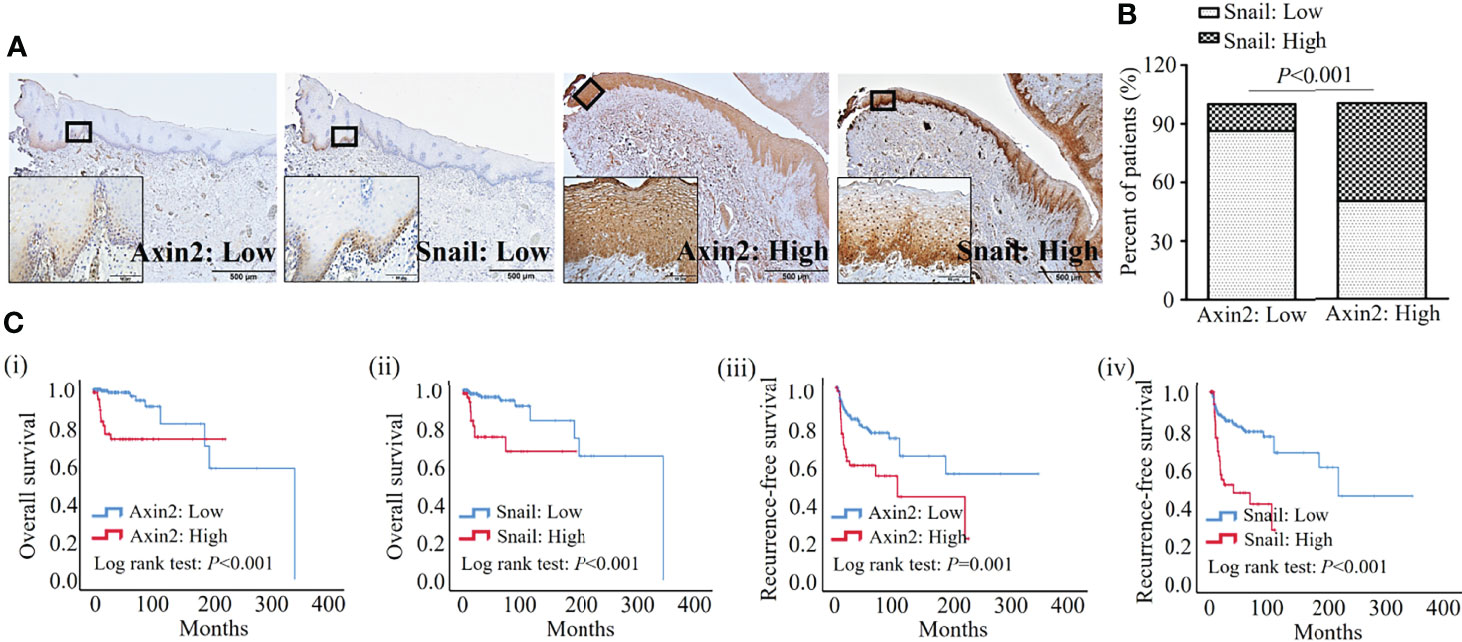
Figure 3 Clinicopathologic significance of Axin2 and Snail expression in surgical margins from the OSCC cohort. (A) Representative expression patterns for low or high levels of Axin2 and Snail in surgical margins of OSCC; (B) Axin2 and Snail expression showed a significant correlation in surgical margins of OSCC; (C) Axin2 and Snail expression in surgical margins showed significant associations with overall and recurrence-free survival in OSCC patients.
In our cohort, no significant association was found between the width or presence of dysplasia in resection margins and both Axin2 and Snail expression (Table 2). Meanwhile, according to Kaplan-Meir analysis, both Axin2 and Snail abundance was significantly associated with both overall (both P<0.001) and recurrence-free survival (P=0.001 and P<0.001) in our cohort (Figure 3C). In a multivariate analysis using variable clinicopathologic factors and Axin2 and Snail as cofactors, higher age (Hazard ratio, HR: 1.050; 95% confidence interval, CI: 1.001–1.102; P=0.047), Axin2 (HR: 6.883; 95% CI: 1.467–32.284; P=0.014), and Snail abundance (HR: 5.663; 95% CI: 1.555–20.619; P=0.009) had independent impacts on worsened overall survival of the patients, and lesion site in retromolar trigone (HR: 4.077; 95% CI: 1.409–11.791; P=0.010), upper– (HR: 4.332; 95% CI: 1.549–12.114; P=0.005) and lower gingiva (HR: 3.545; 95% CI: 1.315–9.555; P=0.012), presence of extranodal extension (HR: 9.967; 95% CI: 2.841–34.964; P<0.001), perineural invasion (HR: 3.627; 95% CI: 1.189–11.059; P=0.024), and Snail abundance (HR: 3.587; 95% CI: 1.839–6.998; P<0.001) in resection margins had independent impacts on worsened recurrence-free survival of OSCC patients (Table 3). However, there are no significant association between the width or presence of dysplasia in resection margins and both Axin2 and Snail expression (Table 2).
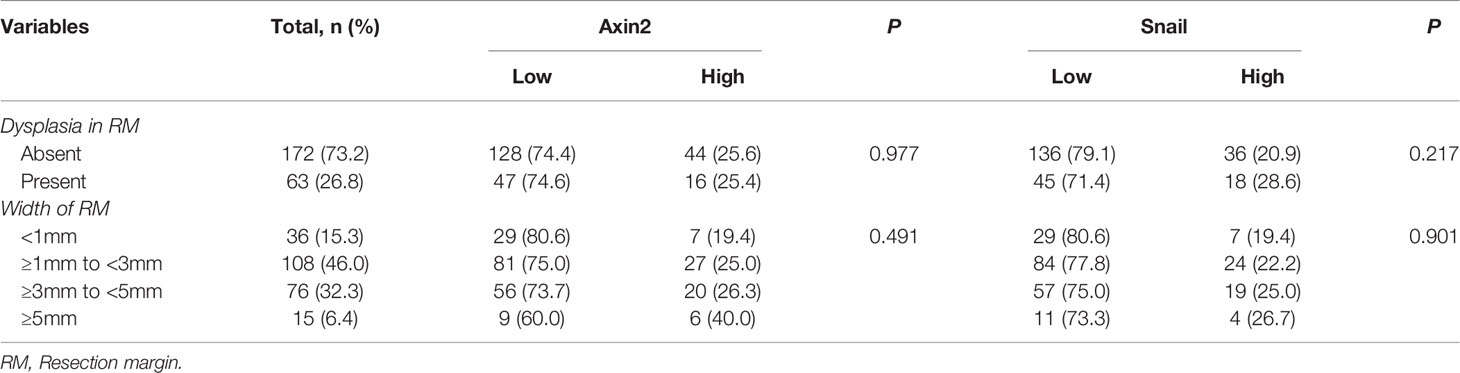
Table 2 Association between status of surgical margin and both Axin2 and Snail expression in 235 OSCC surgical margins.
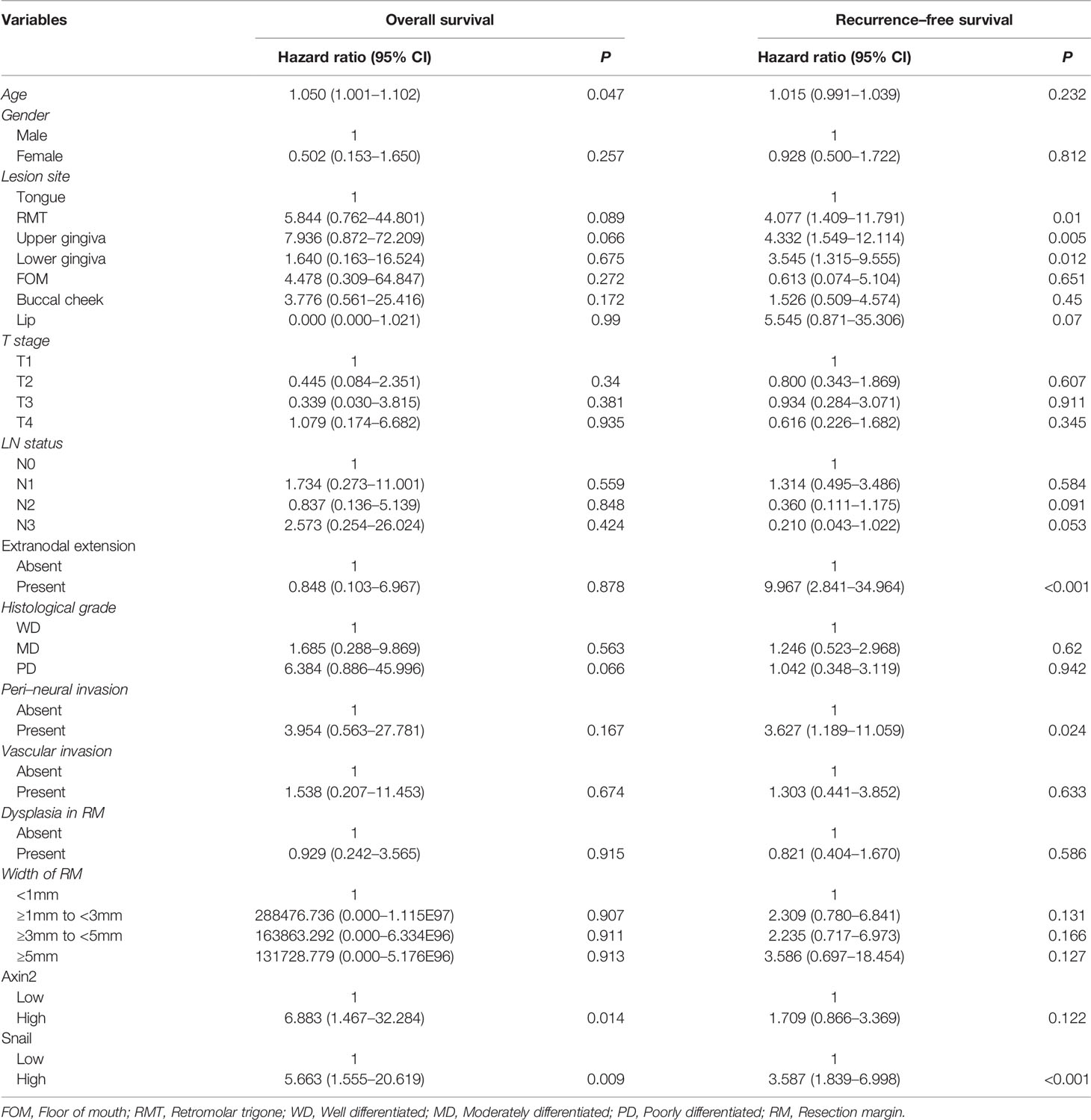
Table 3 Multivariable Cox-regression analysis for risk factors of overall and recurrence-free survival of 235 OSCC patients.
Axin2–Snail Cascade May Strongly Influence the Tumorigenesis of IHOK Line in Vivo
The volume of the tumor nodules was significantly lower in the doxycycline-treated group than in the control group (P=0.032) (Figure 4A). Western blot analysis revealed decreased Axin2 and Snail expression both in the pLKO-Tet-shAxin2 vector-transfected IHOK line and tumor nodules from groups treated with doxycycline compared with that in controls (Figure 4B). The Axin2–Snail cascade may strongly influence tumorigenic activity in the IHOK line.
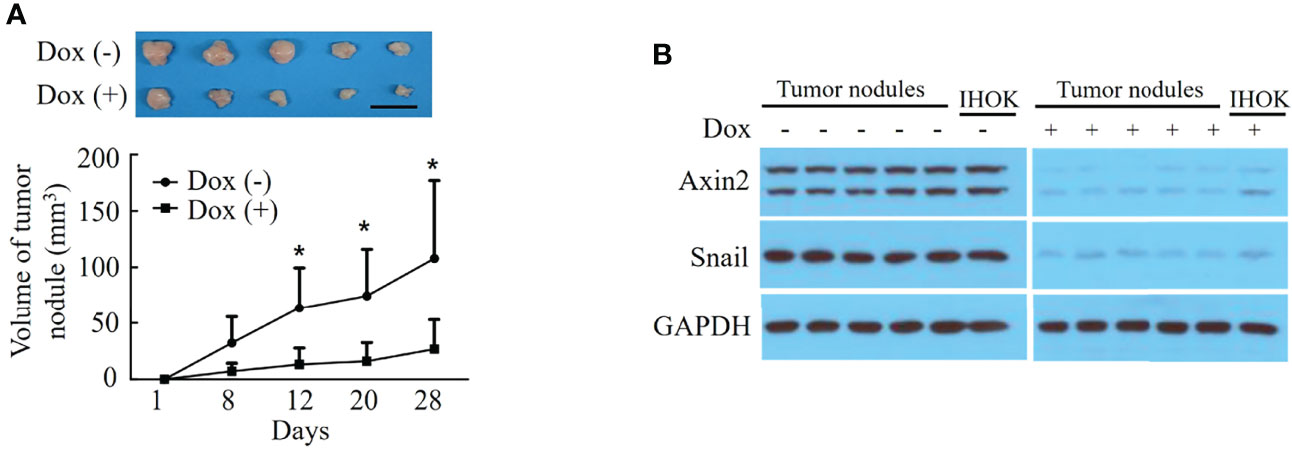
Figure 4 Knockdown of Axin2 attenuates tumorigenic activity in the IHOK line. (A) The volume of the tumor nodules was significantly lower in the doxycycline-treated group than in the control group (*P=0.032, Scale bar: 1cm). (B) Western blot analysis revealed decreased Axin2 and Snail expression both in the pLKO-Tet-shAxin2 vector-transfected IHOK line and tumor nodules from groups treated with doxycycline compared with that in controls.
Discussion
Risk assessment for surgical margins is necessary for the post-operative clinical management of patients with OSCC. In current clinical practice, width and dysplasia are routine histopathologic risk assessment points for surgical margins of OSCC.
Archiving additional histologically normal tissues at least 5 mm beyond the tumor tissue is generally considered the ‘gold standard’ in OSCC surgery. However, owing to the anatomical restrictions of the oral cavity, surgeons cannot achieve a sufficient width of the surgical margin in most patients with OSCC during surgery (30–32). In addition, the width of the margins is shortened by various side effects after surgery, including shrinkage and mucosal elasticity; therefore, pathologic surgical margins larger than 5 mm were found in only a minority of patients with OSCC, according to previous retrospective cohort studies, and in agreement with the present findings (32–34). Moreover, many studies have shown that 5-mm cut offs are not significant in patient outcome, and some investigators have explained that the width of certain safety margins may also be less than 5 mm. Therefore, it may be inappropriate to use 5-mm resection margins in surgical specimens as the cut-off distance for demarcation between adequate and inadequate margins.
Many previous studies have investigated the prognostic relevance of the width of surgical margins and have recommended varying margin cut-offs ranging from 1 to 5 mm. However, these margin cut-offs may not be universally applicable to all OSCC cohorts (33, 35). In our cohort, the width of the surgical margin did not show any significant association with the outcome of OSCC patients when the patients were subdivided into four groups based on 1-, 3-, and 5-mm cut-offs. The width of the surgical margin alone may lack evidence-based background to predict patient outcomes.
ED is considered a high-risk lesion for malignant transformation in the oral cavity. However, opinions regarding the risk of surgical margins involving ED in OSCC lack uniformity. Some investigators have observed that dysplastic surgical margins have a negative impact on patient outcomes, regardless of severity (36). However, other investigators have shown that the impact of mild or moderate dysplastic surgical margins is comparable to that of clear margins on patient outcome (37). In our cohort, we found that dysplastic resection margins were not significantly associated with overall and recurrence-free survival in patients with OSCC. According to the results of a survey by the American Head and Neck Society members, resection margins involving dysplasia were regarded as a ‘positive margin’ in 17%, ‘negative margin’ in 76%, and ‘variation in situation’ in 7% of responders (38). Our department policy is to consider severe dysplasia as the tumor itself and re-reset it until clear margins are obtained as much as possible, so only a limited number of severe dysplastic margins were included in our cohort. The difference in management practice between the organization and the subjectivity in diagnosis of dysplasia between observers would largely influence the predictive value of the dysplastic margin in the outcome of OSCC patients.
ED was traditionally divided into three-tier grades such as mild, moderate, and severe, based on the status of the dysplastic alterations such as architectural and cytological changes (39). However, the diagnosis of ED according to this system is very subjective and therefore, results in decreased reproducibility. Recently, World Health Organization suggested a new binary grading system of ED, which classified it into low-grade and high-grade ED, to overcome the limitations of its traditional grading system. The previous categories of ED namely, mild ED was included in low-grade ED, and moderate/severe ED and carcinoma in situ were included in high-grade ED (39, 40). Although the diagnostic reproducibility of ED and predictability for its malignant transformation are increased in the new binary grading system, there is still a need for investigating the molecular characteristics of ED to supplement the grading system (39, 41).
Resection margins of cancer tissues can harbor genetically altered cells during crosstalk with tumor cells or continued exposure to carcinogens. Genetic alterations in resection margins may be an important trigger for recurrence and poor prognosis in OSCC patients. However, it is difficult to detect by routine histopathological examination when genetically altered cells without predominant morphological change. Histopathological examination of surgical margins is not reliable for predicting the prognosis of OSCC, and molecular analysis is a suitable method to address this issue.
Axin2, as a target gene of the Wnt signaling pathway, was previously categorized as a tumor suppressor gene in a small fraction of colorectal cancers harboring APC gene mutations (42). However, recently, the oncogenic activities of Axin2 have been proposed in various types of cancers, such as breast, colorectal, and pancreatic cancers (43–45). Moreover, as a GSK-3 scaffolding protein, Axin2 is also known as an important participant in the stabilization of nuclear Snail because it mediates the nucleocytoplasmic shuttle for GSK-3, which can bind to and phosphorylate Snail, resulting in the subsequent degradation of Snail (Figure 5) (25). Snail, a transcriptional repressor of E-cadherin, is overexpressed in a variety of cancers and promotes the proliferation, invasion, and tumorigenesis of cancer cells (46, 47). Moreover, Axin2 and Snail expression were significantly associated with some of the classic poor prognostic indicators, such as lymph node metastasis, vascular invasion, and bone invasion in various types of cancers including OSCC (48–50). In our previous studies, we found that the Axin2–Snail axis is significantly related to malignant transformation of oral leukoplakia (23). Consistent with previous observations, we found that Axin2 knockdown induced decreased Snail expression as well as attenuated tumorigenic activity in IHOK cells. Moreover, Snail expression had a positive correlation with Axin2 expression in resection margins of OSCC, while both Axin2 and Snail were independent risk factors for overall survival of patients in our cohort when multiple clinicopathological factors, including age, sex, lesion site, histologic grade, T stage, N stage, extranodal extension, vascular invasion, perineural invasion, width, and dysplasia in resection margins were used as cofactors.
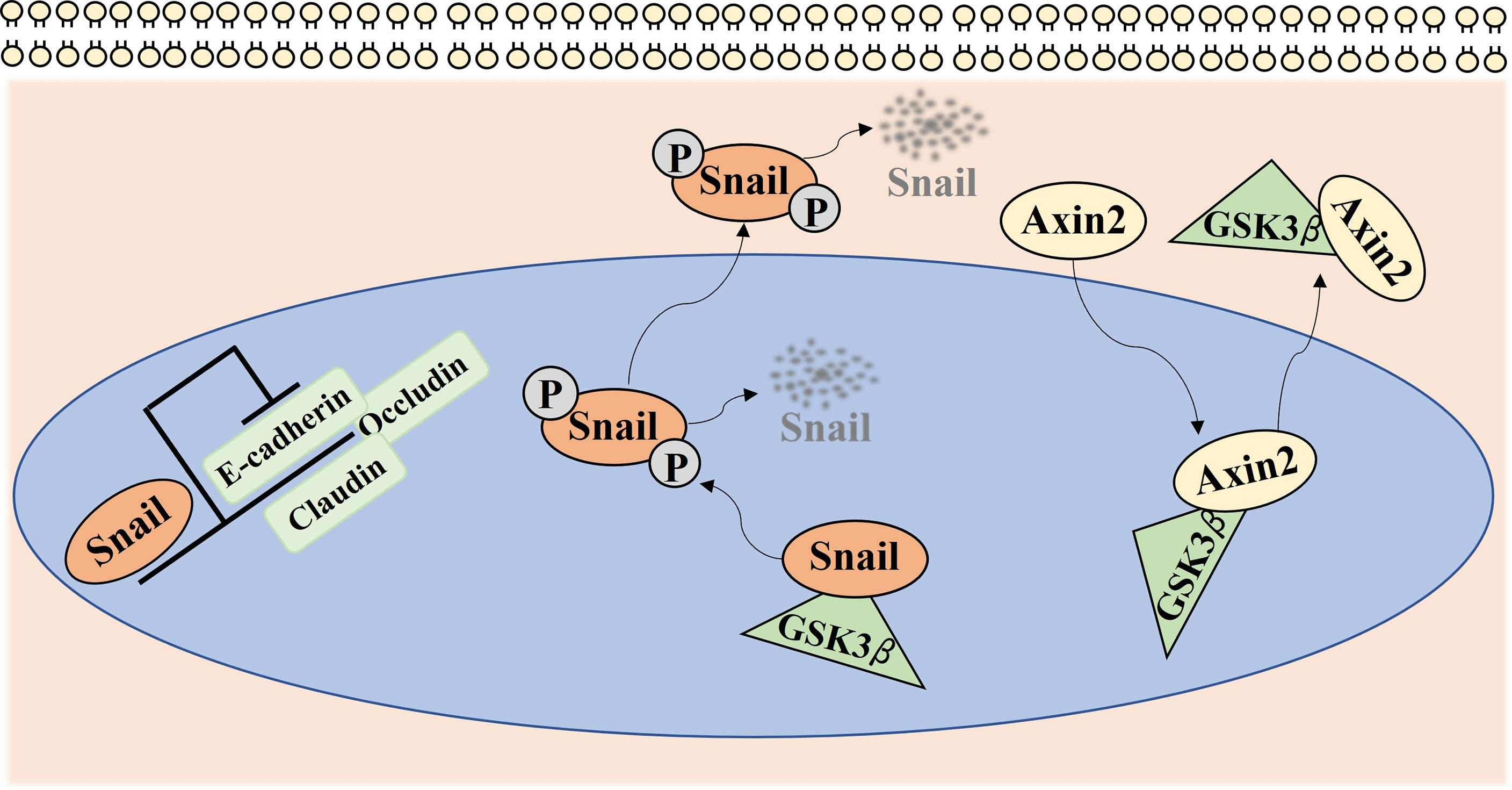
Figure 5 Axin2 expression implicates cancer progression via regulation of Snail mediated epithelial-mesenchymal transition. Snail, as a transcriptional repressor of many genes, such as E-cadherin, Claudin, and Occludin, mediates epithelial-mesenchymal transition in various types of cancers. GSK-3 can bind to and phosphorylate Snail, resulting in the subsequent degradation of Snail. As a GSK-3 scaffolding protein, Axin2 mediates the nucleocytoplasmic shuttle for GSK-3, resulting in the stabilization of nuclear Snail.
In summary, while some of the conventional clinical factors of OSCC, as well as Axin2 and Snail abundance in surgical margins, conferred a poor prognosis, both width and dysplasia, factors determined by histopathologic assessment for surgical margin, did not. Molecular analysis may provide a more accurate and objective assessment of surgical margins. Our results imply that the activation of EMT genes may be involved in the carcinogenesis cascade of surgical margins in OSCC. In particular, both Axin2 and Snail are possible biomarkers for resection margins for predicting outcomes in patients with OSCC.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Yonsei University College of Dentistry. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by Committee of the College of Medicine of Yonsei University.
Author Contributions
Methodology, MP and DH; Software, MP and K-YK; Validation, DK, WN, and EC; Formal analysis, MP and K-YK; Investigation: MP, DH, and K-YK; Writing-original draft preparation: MP and DH; Writing-review and editing, HK, HK, I-HC, and XZ; Supervision, I-HC, XZ. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1I1A1A01073437; No. 2021R1A2C1094413) and Yonsei University College of Dentistry Fund (No. 6-2019-0017).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks are given to all the teachers and classmates of the research group for their help.
Abbreviations
EMT, epithelial-to-mesenchymal transition; HR, hazard ratio; IHOK, immortalized human oral keratinocyte; OSCC, oral squamous cell carcinoma; RM, resection margin.
References
1. Johnson NW, Jayasekara P, Amarasinghe AA. Squamous Cell Carcinoma and Precursor Lesions of the Oral Cavity: Epidemiology and Aetiology. Periodontol 2000 (2011) 57(1):19–37. doi: 10.1111/j.1600-0757.2011.00401.x
2. Pannone G, Santoro A, Papagerakis S, Lo Muzio L, De Rosa G, Bufo P. The Role of Human Papillomavirus in the Pathogenesis of Head & Neck Squamous Cell Carcinoma: An Overview. Infect Agent Cancer (2011) 6:4. doi: 10.1186/1750-9378-6-4
3. Liu W, Bao ZX, Shi LJ, Tang GY, Zhou ZT. Malignant Transformation of Oral Epithelial Dysplasia: Clinicopathological Risk Factors and Outcome Analysis in a Retrospective Cohort of 138 Cases. Histopathology (2011) 59(4):733–40. doi: 10.1111/j.1365-2559.2011.03938.x
4. Scully C, Porter S. ABC of Oral Health. Oral Cancer. BMJ (2000) 321(7253):97–100. doi: 10.1136/bmj.321.7253.97
5. Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and Fluorouracil Alone or With Docetaxel in Head and Neck Cancer. N Engl J Med (2007) 357(17):1705–15. doi: 10.1056/NEJMoa070956
6. Binahmed A, Nason RW, Abdoh AA. The Clinical Significance of the Positive Surgical Margin in Oral Cancer. Oral Oncol (2007) 43(8):780–4. doi: 10.1016/j.oraloncology.2006.10.001
7. Chen TC, Wang CP, Ko JY, Yang TL, Lou PJ. The Impact of Pathologic Close Margin on the Survival of Patients With Early Stage Oral Squamous Cell Carcinoma. Oral Oncol (2012) 48(7):623–8. doi: 10.1016/j.oraloncology.2012.01.015
8. Kurita H, Nakanishi Y, Nishizawa R, Xiao T, Kamata T, Koike T, et al. Impact of Different Surgical Margin Conditions on Local Recurrence of Oral Squamous Cell Carcinoma. Oral Oncol (2010) 46(11):814–7. doi: 10.1016/j.oraloncology.2010.08.014
9. Nason RW, Binahmed A, Pathak KA, Abdoh AA, Sandor GK. What is the Adequate Margin of Surgical Resection in Oral Cancer? Oral Surg Oral Med Oral Pathol Oral Radiol Endod (2009) 107(5):625–9. doi: 10.1016/j.tripleo.2008.11.013
10. Ow TJ, Myers JN. Current Management of Advanced Resectable Oral Cavity Squamous Cell Carcinoma. Clin Exp Otorhinolaryngol (2011) 4(1):1–10. doi: 10.3342/ceo.2011.4.1.1
11. Priya SR, D’Cruz AK, Pai PS. Cut Margins and Disease Control in Oral Cancers. J Cancer Res Ther (2012) 8(1):74–9. doi: 10.4103/0973-1482.95178
12. Gokavarapu S, Chander R, Parvataneni N, Puthamakula S. Close Margins in Oral Cancers: Implication of Close Margin Status in Recurrence and Survival of Pt1n0 and Pt2n0 Oral Cancers. Int J Surg Oncol (2014) 2014:545372. doi: 10.1155/2014/545372
13. Sarode SC, Sarode GS. A Novel ‘Microscopic Method’ of Shrinkage Calculation in the Pursuance of Shrinkage Based Histopathological Guidelines for Interpretation of Surgical Margins. Oral Oncol (2012) 48(6):e15–6. doi: 10.1016/j.oraloncology.2012.01.011
14. Slaughter DP, Southwick HW, Smejkal W. Field Cancerization in Oral Stratified Squamous Epithelium; Clinical Implications of Multicentric Origin. Cancer (1953) 6(5):963–8. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q
15. van Houten VM, Leemans CR, Kummer JA, Dijkstra J, Kuik DJ, van den Brekel MW, et al. Molecular Diagnosis of Surgical Margins and Local Recurrence in Head and Neck Cancer Patients: A Prospective Study. Clin Cancer Res (2004) 10(11):3614–20. doi: 10.1158/1078-0432.CCR-03-0631
16. Sorroche BP, Talukdar FR, Lima SCS, Melendez ME, de Carvalho AC, de Almeida GC, et al. DNA Methylation Markers From Negative Surgical Margins Can Predict Recurrence of Oral Squamous Cell Carcinoma. Cancers (Basel) (2021) 13(12):2915. doi: 10.3390/cancers13122915
17. Jelovac DB, Tepavcevic Z, Nikolic N, Ilic B, Eljabo N, Popovic B, et al. The Amplification of C-Erb-B2 in Cancer-Free Surgical Margins is a Predictor of Poor Outcome in Oral Squamous Cell Carcinoma. Int J Oral Maxillofac Surg (2016) 45(6):700–5. doi: 10.1016/j.ijom.2015.11.014
18. Bilde A, von Buchwald C, Dabelsteen E, Therkildsen MH, Dabelsteen S. Molecular Markers in the Surgical Margin of Oral Carcinomas. J Oral Pathol Med (2009) 38(1):72–8. doi: 10.1111/j.1600-0714.2008.00715.x
19. Jayanthi P, Varun BR, Selvaraj J. Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma: An Insight Into Molecular Mechanisms and Clinical Implications. J Oral Maxillofac Pathol (2020) 24(1):189. doi: 10.4103/jomfp.JOMFP_334_19
20. Puisieux A, Brabletz T, Caramel J. Oncogenic Roles of EMT-Inducing Transcription Factors. Nat Cell Biol (2014) 16(6):488–94. doi: 10.1038/ncb2976
21. Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, et al. Negative Feedback Loop of Wnt Signaling Through Upregulation of Conductin/Axin2 in Colorectal and Liver Tumors. Mol Cell Biol (2002) 22(4):1184–93. doi: 10.1128/MCB.22.4.1184-1193.2002
22. Kroepil F, Fluegen G, Totikov Z, Baldus SE, Vay C, Schauer M, et al. Down-Regulation of CDH1 is Associated With Expression of SNAI1 in Colorectal Adenomas. PloS One (2012) 7(9):e46665. doi: 10.1371/journal.pone.0046665
23. Zhang X, Kim KY, Zheng Z, Kim HS, Cha IH, Yook JI. Snail and Axin2 Expression Predict the Malignant Transformation of Oral Leukoplakia. Oral Oncol (2017) 73:48–55. doi: 10.1016/j.oraloncology.2017.08.004
24. Lee HJ, Guo HY, Lee SK, Jeon BH, Jun CD, Lee SK, et al. Effects of Nicotine on Proliferation, Cell Cycle, and Differentiation in Immortalized and Malignant Oral Keratinocytes. J Oral Pathol Med (2005) 34(7):436–43. doi: 10.1111/j.1600-0714.2005.00342.x
25. Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim NH, et al. A Wnt-Axin2-GSK3beta Cascade Regulates Snail1 Activity in Breast Cancer Cells. Nat Cell Biol (2006) 8(12):1398–406. doi: 10.1038/ncb1508
26. Zhang X, Zheng Z, Shin YK, Kim KY, Rha SY, Noh SH, et al. Angiogenic Factor Thymidine Phosphorylase Associates With Angiogenesis and Lymphangiogenesis in the Intestinal-Type Gastric Cancer. Pathology (2014) 46(4):316–24. doi: 10.1097/PAT.0000000000000094
27. Kunstfeld R, Wickenhauser G, Michaelis U, Teifel M, Umek W, Naujoks K, et al. Paclitaxel Encapsulated in Cationic Liposomes Diminishes Tumor Angiogenesis and Melanoma Growth in a “Humanized” SCID Mouse Model. J Invest Dermatol (2003) 120(3):476–82. doi: 10.1046/j.1523-1747.2003.12057.x
28. Chiou WY, Lin HY, Hsu FC, Lee MS, Ho HC, Su YC, et al. Buccal Mucosa Carcinoma: Surgical Margin Less Than 3 Mm, Not 5 Mm, Predicts Locoregional Recurrence. Radiat Oncol (2010) 5:79. doi: 10.1186/1748-717X-5-79
29. Zanoni DK, Migliacci JC, Xu B, Katabi N, Montero PH, Ganly I, et al. A Proposal to Redefine Close Surgical Margins in Squamous Cell Carcinoma of the Oral Tongue. JAMA Otolaryngol Head Neck Surg (2017) 143(6):555–60. doi: 10.1001/jamaoto.2016.4238
30. Dik EA, Willems SM, Ipenburg NA, Adriaansens SO, Rosenberg AJ, van Es RJ. Resection of Early Oral Squamous Cell Carcinoma With Positive or Close Margins: Relevance of Adjuvant Treatment in Relation to Local Recurrence: Margins of 3 Mm as Safe as 5 Mm. Oral Oncol (2014) 50(6):611–5. doi: 10.1016/j.oraloncology.2014.02.014
31. Varvares MA, Poti S, Kenyon B, Christopher K, Walker RJ. Surgical Margins and Primary Site Resection in Achieving Local Control in Oral Cancer Resections. Laryngoscope (2015) 125(10):2298–307. doi: 10.1002/lary.25397
32. Smits RW, Koljenovic S, Hardillo JA, Ten Hove I, Meeuwis CA, Sewnaik A, et al. Resection Margins in Oral Cancer Surgery: Room for Improvement. Head Neck (2016) 38 Suppl 1:E2197–203. doi: 10.1002/hed.24075
33. Tasche KK, Buchakjian MR, Pagedar NA, Sperry SM. Definition of “Close Margin” in Oral Cancer Surgery and Association of Margin Distance With Local Recurrence Rate. JAMA Otolaryngol Head Neck Surg (2017) 143(12):1166–72. doi: 10.1001/jamaoto.2017.0548
34. Wong LS, McMahon J, Devine J, McLellan D, Thompson E, Farrow A, et al. Influence of Close Resection Margins on Local Recurrence and Disease-Specific Survival in Oral and Oropharyngeal Carcinoma. Br J Oral Maxillofac Surg (2012) 50(2):102–8. doi: 10.1016/j.bjoms.2011.05.008
35. Ch’ng S, Corbett-Burns S, Stanton N, Gao K, Shannon K, Clifford A, et al. Close Margin Alone Does Not Warrant Postoperative Adjuvant Radiotherapy in Oral Squamous Cell Carcinoma. Cancer (2013) 119(13):2427–37. doi: 10.1002/cncr.28081
36. Singh A, Mair M, Singhvi H, Ramalingam N, Bal M, Lamba K, et al. Incidence and Impact of Dysplasia at Final Resection Margins in Cancers of the Oral Cavity. Acta Otolaryngol (2020) 140(11):963–9. doi: 10.1080/00016489.2020.1785642
37. Chen TC, Chang HL, Yang TL, Lou PJ, Chang YL, Ko JY, et al. Impact of Dysplastic Surgical Margins for Patients With Oral Squamous Cell Carcinoma. Oral Oncol (2019) 97:1–6. doi: 10.1016/j.oraloncology.2019.07.015
38. Meier JD, Oliver DA, Varvares MA. Surgical Margin Determination in Head and Neck Oncology: Current Clinical Practice. The Results of an International American Head and Neck Society Member Survey. Head Neck (2005) 27(11):952–8. doi: 10.1002/hed.20269
39. Kujan O, Oliver RJ, Khattab A, Roberts SA, Thakker N, Sloan P. Evaluation of a New Binary System of Grading Oral Epithelial Dysplasia for Prediction of Malignant Transformation. Oral Oncol (2006) 42(10):987–93. doi: 10.1016/j.oraloncology.2005.12.014
40. Ranganathan K, Kavitha L. Oral Epithelial Dysplasia: Classifications and Clinical Relevance in Risk Assessment of Oral Potentially Malignant Disorders. J Oral Maxillofac Pathol (2019) 23(1):19–27. doi: 10.4103/jomfp.JOMFP_13_19
41. Zhang X, Han S, Han HY, Ryu MH, Kim KY, Choi EJ, et al. Risk Prediction for Malignant Conversion of Oral Epithelial Dysplasia by Hypoxia Related Protein Expression. Pathology (2013) 45(5):478–83. doi: 10.1097/PAT.0b013e3283632624
42. Liu W, Dong X, Mai M, Seelan RS, Taniguchi K, Krishnadath KK, et al. Mutations in AXIN2 Cause Colorectal Cancer With Defective Mismatch Repair by Activating Beta-Catenin/TCF Signalling. Nat Genet (2000) 26(2):146–7. doi: 10.1038/79859
43. Wu ZQ, Brabletz T, Fearon E, Willis AL, Hu CY, Li XY, et al. Canonical Wnt Suppressor, Axin2, Promotes Colon Carcinoma Oncogenic Activity. Proc Natl Acad Sci U S A (2012) 109(28):11312–7. doi: 10.1073/pnas.1203015109
44. Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, et al. Targeting Wnt-Driven Cancer Through the Inhibition of Porcupine by LGK974. Proc Natl Acad Sci U S A (2013) 110(50):20224–9. doi: 10.1073/pnas.1314239110
45. Jiang X, Hao HX, Growney JD, Woolfenden S, Bottiglio C, Ng N, et al. Inactivating Mutations of RNF43 Confer Wnt Dependency in Pancreatic Ductal Adenocarcinoma. Proc Natl Acad Sci U S A (2013) 110(31):12649–54. doi: 10.1073/pnas.1307218110
46. Smith BN, Burton LJ, Henderson V, Randle DD, Morton DJ, Smith BA, et al. Snail Promotes Epithelial Mesenchymal Transition in Breast Cancer Cells in Part via Activation of Nuclear ERK2. PloS One (2014) 9(8):e104987. doi: 10.1371/journal.pone.0104987
47. Yang X, Han M, Han H, Wang B, Li S, Zhang Z, et al. Silencing Snail Suppresses Tumor Cell Proliferation and Invasion by Reversing Epithelial-to-Mesenchymal Transition and Arresting G2/M Phase in non-Small Cell Lung Cancer. Int J Oncol (2017) 50(4):1251–60. doi: 10.3892/ijo.2017.3888
48. Olmeda D, Moreno-Bueno G, Flores JM, Fabra A, Portillo F, Cano A. SNAI1 is Required for Tumor Growth and Lymph Node Metastasis of Human Breast Carcinoma MDA-MB-231 Cells. Cancer Res (2007) 67(24):11721–31. doi: 10.1158/0008-5472.CAN-07-2318
49. Zhao G, Kim KY, Zheng Z, Oh Y, Yoo DS, Lee ME, et al. AXIN2 and SNAIL Expression Predict the Risk of Recurrence in Cutaneous Squamous Cell Carcinoma After Mohs Micrographic Surgery. Oncol Lett (2020) 19(3):2133–40. doi: 10.3892/ol.2020.11324
Keywords: OSCC, surgical margin, risk assessment, molecular markers, axin2, snail, recurrence, prognosis
Citation: Pei M, Han D, Kim K-Y, Kim DW, Nam W, Kim HJ, Cho ES, Kim HS, Cha I-H and Zhang X (2022) Risk Factors of Microscopically Tumor-Free Surgical Margins for Recurrence and Survival of Oral Squamous Cell Carcinoma Patients. Front. Oncol. 12:930988. doi: 10.3389/fonc.2022.930988
Received: 28 April 2022; Accepted: 13 June 2022;
Published: 07 July 2022.
Edited by:
Omar Kujan, University of Western Australia, AustraliaReviewed by:
Gayani Pitiyage, St George’s, University of London, United KingdomBruce Ashford, Illawarra Shoalhaven Local Health District (ISLHD), Australia
Ali-Farid Safi, Harvard University, United States
Copyright © 2022 Pei, Han, Kim, Kim, Nam, Kim, Cho, Kim, Cha and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: In-Ho Cha, Y2hhODc2NEB5dWhzLmFj; Xianglan Zhang, emhhbmd4aWFuZ2xhbkB5dWhzLmFj
 Meiling Pei
Meiling Pei Dawool Han
Dawool Han Ki-Yeol Kim
Ki-Yeol Kim Dong Wook Kim
Dong Wook Kim Woong Nam
Woong Nam Hyung Jun Kim
Hyung Jun Kim Eunae Sandra Cho2,3
Eunae Sandra Cho2,3 Hyun Sil Kim
Hyun Sil Kim Xianglan Zhang
Xianglan Zhang