
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 14 July 2022
Sec. Hematologic Malignancies
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.929012
This article is part of the Research Topic Linking Cellular Metabolism to Hematological Malignancies View all 14 articles
Classical Hodgkin lymphoma (cHL) is the most common type of HL that occurs mainly in people aged between 15–30 and over 55 years. Although its general prognosis is favorable, 10%–30% of patients with cHL will ultimately develop relapsed or refractory disease (r/r cHL). Improving the cure rate of r/r cHL has proven to be challenging. Some novel agents, such as brentuximab vedotin and immune checkpoint inhibitors, which have been used in conventional regimens for patients with r/r cHL in the past decade, have been shown to have good curative effects. This paper reviews the conventional regimens for patients with r/r cHL and focuses on the newest clinical trials and treatment measures to prolong prognosis and reduce adverse events. The evaluation of prognosis plays a vital role in analyzing the risk of relapse or disease progression; thus, finding new predictive strategies may help treat patients with r/r cHL more efficaciously.
Hodgkin lymphoma (HL) is a frequent hematological malignancy, with 8,830 new cases reported in the United States in 2021 (1). Classical HL (cHL) is one of the most common types of HL and is defined as a B lymphatic cell malignancy characterized by the presence of malignant Hodgkin and Reed–Sternberg (HRS) cells in the tumor microenvironment (2). It occurs mainly in people aged between 15–30 and over 55 years, with an incidence of approximately three newly diagnosed cases per 100,000 individuals per year (3, 4). cHL is mainly divided into stages I–II and stages III–IV, the former of which is further classified into three subgroups, namely, stages IA–IIA (favorable), stages I–II (unfavorable with non-bulky disease), and stages I–II (unfavorable with bulky disease) (5). Patients with different stages may have different therapeutic choices. However, the prognosis of patients with HL is generally favorable, with approximately 80% of young adults cured of the disease after receiving initial standard chemotherapeutic treatment (3). However, 10%–30% of patients ultimately develop relapsed or refractory cHL (r/r cHL) disease (3). Patients with r/r cHL, especially those aged 60 years or older, have a much poorer prognosis, with a reported 3-year progression-free survival (PFS) rate of 50% and an overall survival (OS) rate of 68% (6). In addition, the toxic effects of the therapeutic approach on the number of years lost from productive life are remarkable. The common adverse events of conventional chemotherapeutic agents, such as peripheral neuropathy (7), also negatively affect the prognosis. Therefore, finding novel agents and therapeutic modalities is still of great significance in improving the overall cure rates and prolonging PFS and OS in patients with r/r cHL. Herein, we introduce the current chemotherapies used in clinical practice and novel treatments that show improved prognosis in the latest clinical trials to provide a more comprehensive understanding of r/r cHL treatment and promising directions for future research in this field.
Combined modality therapy (chemotherapy and radiotherapy) remains a top priority for patients with newly diagnosed cHL. For stage I–II patients aged 18–60, two cycles of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) followed by positron emission tomography/computed tomography (PET/CT) evaluation are routinely used as the primary treatment. Further decisions regarding combined therapy are based on lymphoma remission status assessed by PET/CT. The escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone in escalated doses) regimen is also widely used in early-stage young adults as an additional therapy. For stage III–IV patients, apart from the ABVD regimen, escalated BEACOPP or brentuximab vedotin (BV) combined with AVD (doxorubicin, vinblastine, and dacarbazine) is recommended as the primary treatment in certain cases (8). However, the prognosis is significantly poorer for a small proportion of patients who may have a limited response to first-line therapy or relapse. Therefore, salvage treatment, high-dose therapy, and autologous stem cell transplantation (HDT-ASCT) play a key role in prolonging PFS and OS.
Patients with r/r cHL are recommended to be treated with salvage regimens, which mainly include dexamethasone, cisplatin, high-dose cytarabine (DHAP); etoposide, methylprednisolone, high-dose cytarabine, cisplatin (ESHAP); ifosfamide, carboplatin, etoposide (ICE); gemcitabine, vinorelbine, liposomal doxorubicin (GVD); BV; and a combination of bendamustine and nivolumab (5). A brief summary of the newly reported clinical trials on salvage systemic therapies is listed in Table 1. If PET/CT re-evaluation indicates a complete response (CR) or partial response (PR), additional therapy with HDT-ASCT would be feasible and effective if not contraindicated. Radiation should also be considered for selected sites that have not previously been irradiated. Response to salvage treatment is an important predictor of prognosis in patients with r/r cHL since individuals achieving metabolic CR after salvage treatment have a higher chance of being cured (9). In contrast, patients who experience disease progression after salvage treatment may benefit from further systemic therapy with or without radiation, and if they respond, autologous or allogeneic hematopoietic stem cell transplantation (HSCT) is also favorable. However, half of the patients who undergo HSCT cannot be cured, and their prognosis is poor (10). Therefore, maintenance therapy, such as BV and other novel modalities, is vital for patients at a high risk of relapse. For those who underwent multiple-line therapy and still suffered from disease relapse, regimens such as chimeric antigen receptor (CAR) T-cell therapy (11) may offer sustained relief.
cHL in patients over 60 years of age is highly linked to poorer prognosis since these patients generally suffer from inferior efficacy but great toxicity, owing to comorbidity and geriatric fitness (12). The management of older patients should be individualized and usually requires clinical judgment. Indeed, choosing agents with mild toxicities and at suitable doses remains challenging when aiming to improve the OS and PFS of this group of patients (5). ACCRU, as a multicenter phase II trial, reported the combination of BV and nivolumab as first-line therapy in older patients, and 22 out of 46 (48%) individuals achieved complete response, although grade 3–5 adverse events occurred in 80% of the patients (13). However, for older patients with r/r cHL, no consensus has been reached regarding the suggested treatment regimens.
Regimen options for pediatric cHL patients are distinct from those for adult patients, and the treatment of pediatric patients is highly based on risk stratification (14, 15). OEPA (vincristine, etoposide, prednisone, and doxorubicin), AVPC (doxorubicin, vincristine, prednisone, and cyclophosphamide), and ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, cyclophosphamide, and prednisone) regimens are conventionally used in underage patients as first-line systemic therapy (16) but are rarely used in adult patients. In contrast, for adolescents with suspected r/r cHL, available clinical trials are favored if the biopsy site is positive. Otherwise, re-induction therapy is required to avoid relapse or prolong PFS. HDT-ASCT, radiotherapy, and maintenance therapy are optional based on the metabolic condition of patients. Subsequent therapy was considered when relapse occurred after maintenance treatment. Re-induction and subsequent therapy are similar to those in adults, such as DHAP, IGEV (ifosfamide, gemcitabine, vinorelbine), ICE, nivolumab (17), BV (18), and the combination of BV with bendamustine, gemcitabine, or nivolumab.
BV is a CD30-directed antibody–drug conjugate (ADC) that links an antineoplastic agent, monomethyl auristatin E (MMAE), to a monoclonal antibody that can direct MMAE to CD30-positive lymphoma cells (19). BV was initially approved for patients who failed ASCT or at least two prior lines of chemotherapy by the US Food and Drug Administration (FDA) in 2011, based on its great drug efficacy in a phase II clinical trial, with an overall response rate (ORR) of 75% (20). BV is now commonly used as salvage or post-ASCT maintenance therapy for patients with r/r cHL, but it has also been proven to be effective and recommended in patients with stage III/IV cHL as first-line therapy when combined with the AVD modality (8). Notably, severe adverse events of BV are negligible due to a high incidence rate of 32%, among which the most common and severe one is neutropenia (21). The latest update of the ECHELON-1 study illustrated that the BV plus AVD regimen could significantly improve the 5-year PFS compared to the standard ABVD regimen for the first-line treatment of patients with stage III/IV cHL (82.2% vs. 75.3%, p = 0.0017) (22). Another phase II trial further investigated the outcomes of BV-AVD treatment in patients with non-bulky stage I/II cHL, concluding a favorable PFS (94%) and OS (97%) during a median follow-up of 38 months (23).
For salvage treatment, BV monotherapy administered prior to ASCT in the treatment of r/r cHL yielded a CR rate (CRR) of 24%–35% (24, 25), which is not a favorable outcome, probably due to BV chemoresistance (26). In contrast, BV combined therapy has been intensively studied and demonstrated to markedly improve CR (25, 27, 28). Clinical trials of BV combined with conventional salvage chemotherapy have been widely performed over the last decade, including the combination of BV with ICE, DHAP, and ESHAP. The concurrent treatment with BV and ICE produced a high CRR of 74% and improved post-HSCT outcomes (29). Bendamustine is a purine analog with antitumor activity by damaging DNA and inducing cell apoptosis and has been approved for the treatment of chronic lymphocytic leukemia and non-HL. The combination of BV and bendamustine (BVB) as salvage therapy has shown a high CRR of 73.6% (30). BVB therapy has been widely researched in developed countries and has displayed impressive outcomes as a second-line treatment, with a CR of over 70% (30). In comparison, the CR of BVB combined therapy in a middle-income setting in India was lower at 62% (31). BVB is also highly active in patients with prior BV exposure since the PFS duration was similar to that of patients that had not received BV before (32). Another retrospective analysis indicated that BVB salvage therapy is highly effective in children and young adults under 30, with a high CR of 79% (33). A phase II transplant BRaVE trial recruited 55 patients with r/r cHL and administered BV-DHAP combined therapy as the first salvage treatment. CR was achieved in 81% of the patients before HDT-ASCT (34), which is a high rate compared to BV in combination with bendamustine (73.6%) (30) and ICE (74%) (29).
For maintenance therapy, the phase 3 AETHERA trial demonstrated the efficacy of BV in patients with a high risk of relapse after auto-HSCT treatment. The 5-year PFS was 59% in BV-treated patients compared with 41% in the placebo group [hazard ratio (HR), 0.521; 95% CI, 0.379–0.717] (35). Maintenance therapy with BV is comparatively safe, and 90% of the patients are diagnosed with peripheral neuropathy, which is the most common adverse event of BV (35, 36). The AETHERA trial showed that BV is an effective post-ASCT maintenance agent. However, since the AETHERA trial excluded individuals who had previously received BV, the efficacy of BV in this group remains unclear. A recent multicenter retrospective study of 105 cases, including both BV-naive and BV-exposed patients, reported 3‐year PFS and OS rates of 54% and 71%, respectively, for BV-naive patients and 77% and 96%, respectively, for BV-exposed patients (37).
Immune checkpoint inhibitors (ICIs) have been widely used to treat hematologic malignancies, including cHL. Anti-PD-1 agents have been widely used to treat cHL. Tumor cells expressing PD-L1 and PD-L2 can escape the immune surveillance of mature cytotoxic T cells via the PD-1 pathway, which is one of the most critical mechanisms that leads to cHL. 9p24 Genomic amplification of this.1, which may cause overexpression of PD-1, PD-L1, and PD-L2 in malignant HRS cells, is prevalent in this disease (38). PD-L1 blockers can restore immunoactivity in the tumor microenvironment (TME) and suppress the viability of HRS cells that express PD-1, PD-L1, and PD-L2. Nivolumab and pembrolizumab, which are both fully human IgG4 monoclonal PD-1 antibodies, were approved by the FDA in 2016 and 2017, respectively, as treatment options for patients with r/r cHL. The safety and efficacy of these two agents are not yet weighted, but healthcare insurance data suggest that the pembrolizumab cohort had a lower hospitalization rate than the nivolumab cohort (39). To date, finding novel and highly effective anti-PD-1 agents remains a major challenge worldwide. In China, several anti-PD-1 agents have been approved by the National Medical Products Administration for use in the treatment of r/r cHL from 2018 to 2022, including sintilimab (40, 41), tislelizumab (42, 43), camrelizumab (44–50), penpulimab (51), and zimberelimab (52). A summary of these agents is provided in Table 2.
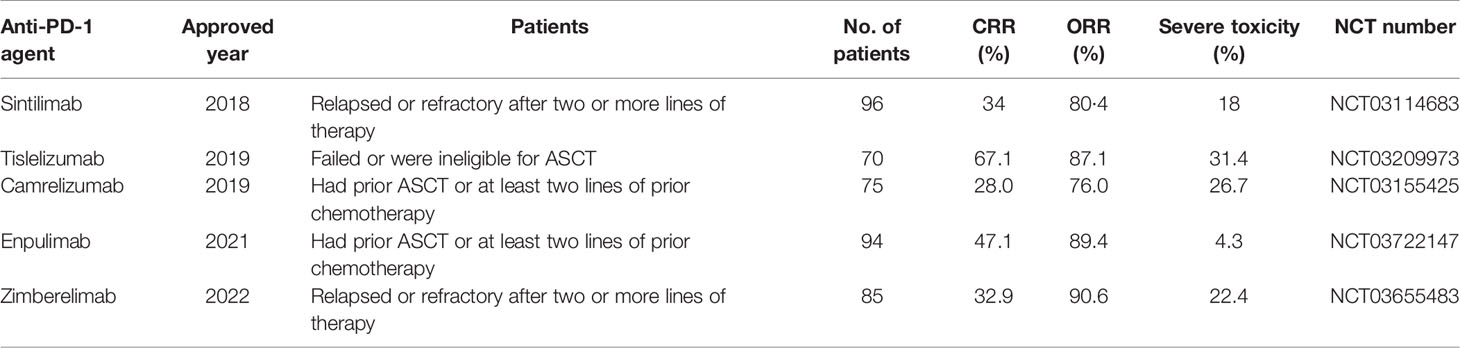
Table 2 Anti-PD-1 antibodies approved by the National Medical Products Administration in China for r/r cHL.
The side effects of ICIs are non-specific, including infection, inflammation, pyrexia, digestive symptoms, etc. Notably, the side effect of nivolumab is statistically higher than that of pembrolizumab, and neutropenia is the most common side effect of nivolumab (53).
Nivolumab was the first ICI approved by the FDA to treat patients with r/r cHL. CheckMate 205 showed a durable response to nivolumab monotherapy in r/r cHL in 2018 (54), and the latest real-life experience in Turkey is consistent with this result in heavily pretreated r/r cHL (55). Analysis of cohort D of CheckMate 205 suggested that nivolumab combined with the AVD regimen (N-AVD) significantly increased ORR and CRR. The ORR and CRR at the end of nivolumab monotherapy were 69% and 18%, respectively, whereas, after two combination cycles of N-AVD, the figures increased to 90% and 51%, respectively (53). The randomized phase II NIVAHL trial explored the efficacy of N-AVD in an earlier stage of cHL as first-line therapy, which also resulted in an attractively high CR (56).
Nivolumab combined with BV has shown great outcomes as a second-line regimen, reporting an ORR of 82% and CR of 61% in r/r cHL (57). Another phase I study explored BV combined with nivolumab, nivolumab, and ipilimumab and found a similar ORR of 76% and CRR of 57% in the BV-nivolumab group and a high ORR of 82% and CRR of 73% in the triplet group (58). The next phase II study will compare these two modalities as a bridge to HCST (58). A comparison of the efficacy of nivolumab monotherapy and nivolumab in combination with ICE (NICE) multiagent therapy was conducted in a phase II trial (NCT03016871). Of the 42 evaluable patients, 34 received monotherapy, and nine received NICE therapy. The ORR and CRR in the NICE group were noticeably higher than those in the single-agent group (ORR 81% versus 93%; CRR 71% versus 91%) (59), although the number of samples was limited.
The ORR of anti-PD-1 agents was initially high. However, long-term treatment with nivolumab may decrease the drug efficacy and lead to chemoresistance. Nivolumab as salvage treatment without ASCT consolidation displayed a markedly higher relapse rate than those with ASCT consolidation (62.2% versus 0%), based on a retrospective analysis (60).
Pembrolizumab is another commonly used anti-PD-1 antibody approved by the FDA that has shown great PFS improvement (61–63). The phase III clinical trial of the KEYNOTE-204 study reported pembrolizumab as a preferred agent compared to BV in treating r/r cHL individuals who were ineligible for ASCT or had relapsed after ASCT. The median PFS reached 13.2 months for pembrolizumab versus 8.3 months for BV (p = 0.0027), although the ORR and CR did not show statistical significance ([65.6% (57.4–73.1) versus 54.2% (46.0–62.3) and 25% versus 24%, respectively) (64). Furthermore, pembrolizumab can improve health-related quality of life compared to BV in patients with r/r cHL (65). The duration of pembrolizumab response was observed when BV monotherapy was ineffective in the KEYNOTE-013 study (NCT01953692), and it was found that some patients may benefit from long-term pembrolizumab treatment since the duration of response was not reached in a 4-year follow-up (66).
Despite the remarkable efficacy of pembrolizumab as monotherapy, combinations of pembrolizumab and other agents are more widely suggested. A phase II study reported that pembrolizumab followed by AVD as first-line therapy was effective and well-tolerated in untreated patients with advanced stage cHL. Similarly, for r/r cHL patients, pembrolizumab also showed some favorable outcomes (67). A retrospective analysis reported a pembrolizumab-BV regimen in 10 patients with multirefractory cHL, and the final CR reached 80%. Seven out of 10 proceeded to ASCT directly, although the number of patients was limited, and further studies on two-drug therapy are of great significance (68). GVD, another commonly used salvage regimen of r/r cHL with a CRR of less than 50% (69), combined with pembrolizumab (pembro-GVD), was studied in a phase II clinical trial (NCT03618550) to evaluate its efficacy and safety. After two to four cycles of the pembro-GVD regimen, 95% of the patients achieved CR. Moreover, 36 out of 38 patients underwent HDT-ASCT, and no relapses were observed during a median follow-up of 13.5 months (70). AFM13, a CD30/CD16A-bispecific antibody that can stimulate innate immune cells, combined with pembrolizumab, has been reported to display good tolerance and an ORR of 88% in patients with r/r cHL (71); however, further investigation is needed.
Sintilimab is a highly selective PD-1 blocker that revealed an ORR of 80.4% in heavily pretreated cHL patients from 18 hospitals in China in a single-arm phase II trial (NCT03114683) (41). A new case was reported in which one cHL-HIV patient, who failed ABVD and GDP chemotherapeutic regimens, showed CR after receiving sintilimab for nine cycles, with acceptable adverse events (40).
Tislelizumab is a humanized IgG4 monoclonal antibody that binds PD-1 with high affinity. A phase II trial in China evaluated the efficacy and safety of r/r cHL in patients who failed or were ineligible for ASCT, reporting an ORR of 87.1% and CR of 62.9% after a median of 9.8 months of follow-up (43). The latest update revealed that the PFS and OS rates were 40.8% and 84.8%, respectively, after a 3-year follow-up.
Camrelizumab is an anti-PD-1 agent that has shown good efficacy against various advanced malignancies (44). It achieved a high rate of objective response (76.0%; 95% CI, 64.7–85.1) in cHL patients failing or ineligible for ASCT (45), and the median PFS was 22.5 months and the 36-month OS was 82.7%, according to the latest updates (46). However, some patients still have progressive disease or relapse after receiving anti-PD-1 agents, probably due to the overexpression of PD-1 in T cells in the microenvironment or upregulation of PD-L1 in HRS cells (47). Decitabine is a DNA methyltransferase inhibitor with the potential to reduce camrelizumab resistance (50). Therapy with camrelizumab plus decitabine markedly improved the CRR (71%) in patients with r/r cHL compared with camrelizumab monotherapy (32%) (48), and after a median follow-up of 34.5 months, the complete remission rate reached 79% with dual therapy (49). Further study of this combined therapy was conducted in two phase II trials (NCT02961101 and NCT03250962) in r/r cHL patients with PD-1 resistance, and the ORRs in two separate cohorts were 52% and 68%, respectively, with a longer PFS compared to PD-1 monotherapy (50). This suggests a high synergistic antitumor activity and long-term benefit of the camrelizumab plus decitabine combination. However, grade 3–4 adverse events of leukocytopenia occur more frequently with this combined therapy than with camrelizumab monotherapy.
Clinical Trials of potential effective novel agents in the treatment of r/r cHL under recruiting are summarized in Table 3. Although conventional therapeutic regimens, BV, and ICIs have greatly improved the prognosis, heavily chemoresistant patients still have a limited choice of effective drugs. Moreover, the high rate of adverse events among the aforementioned regimens remains a major obstacle, especially in adolescents and older patients. Therefore, novel agents that prolong PFS and OS and reduce adverse events are of great significance.
Since BV and ICIs are increasingly used in either the first-line or second-line treatment of cHL, the efficacy of these drugs may not be as effective as post-HCST maintenance therapy. Radioimmunotherapy is a novel targeted approach that may reduce the risk of relapse after HSCT in patients with radiosensitivity. 90Y-basiliximab/DOTA is a specially designed agent that conjugates basiliximab (anti-CD25 antibody) and 1,4,7,10-tetraazacyclododecane tetraacetic acid (DOTA) and radiolabels 90Y for therapeutic dosing. 90Y-basiliximab/DOTA was administered in combination with the BEAM regimen (carmustine, etoposide, cytarabine, and melphalan) as maintenance therapy, and the estimated 5-year PFS and OS rates were 68% and 95%, respectively (72). Further evaluation of this approach is ongoing in phase II trials.
ADC is a group of agents that connect small molecule bioactive drugs to monoclonal antibodies; hence, the antibodies can transport bioactive drugs to target cells directly. Because of the huge success of BV as an ADC in the treatment of cHL, novel ADCs against other key receptors are being investigated in both preclinical and clinical studies (73).
CD25 is a receptor of interleukin-2 (IL-2) that is abundantly distributed on the surface of both hematological tumor cells and Treg cells. Camidanlumab tesirine is a novel agent that conjugates anti-CD25 antibody to a pyrrolobenzodiazepine dimer and causes cell death by damaging the DNA structure. A phase I study assessed the efficacy of camidanlumab tesirine in patients with r/r cHL who had no available therapies. The median duration of response was 7.2 months, and the ORR was 71% (74, 75). A later phase II study demonstrated that 18 (38.3%) and 20 (42.6%) of 47 heavily treated patients attained CR and PR, respectively (76), showing that camidanlumab tesirine is a promising ADC agent next to BV.
CD123 is an alpha subunit of the interleukin 3 receptor (IL3RA) that is enriched in acute myeloid leukemia and cHL cells. IL3RA-directed ADC, such as BAY-943, seems to be effective in suppressing cHL development. BAY-943 has demonstrated noticeable antiproliferative efficacy in HL cell lines and xenograft models (77), and further clinical trials of BAY-943 are warranted.
CAR-T-cell immunotherapy has been increasingly studied in a wide range of cancers. Engineered T cells express CARs on the cell surface so that they can recognize and eliminate cells expressing specific target antigens. CAR-T therapy targeting the CD30 antigen (CD30.CAR-T) can be effective in hematological malignancies, including cHL (78). Adoptive transfer of CD30.CAR-T preceded by fludarabine-based lymphodepletion chemotherapy was performed in 41 heavily pretreated patients with r/r cHL who developed chemoresistance to the aforementioned agents, including BV and IPIs, with an ORR of 72%, CR of 59%, 1-year PFS of 36%, and OS of 94% (11). Although individuals that achieve CR after CD30.CAR-T could experience relapse again, possibly due to insufficient persistence of CAR-Ts, the combination of CD30.CAR-Ts and ICIs may improve their PFS, and further clinical trials are needed (11).
New combination regimens of standard chemotherapeutic agents have also been tested in an attempt to expand the range of options and achieve a higher prognosis. For example, gemcitabine and bendamustine are used in different conventional regimens, while their concurrent combination was first reported in a phase I/II study as late-line therapy for heavily pretreated patients, showing a well-tolerated and effective result (ORR 69%; CRR 46%) but a potentially high pulmonary toxicity risk (79).
Novel chemotherapeutic agents are also being researched in both preclinical and clinical studies. Lenalidomide is an immunomodulatory drug used to treat myelodysplastic syndrome, and a low dose displays some activity in patients with r/r cHL who fail or are ineligible for ASCT, with 64% of the patients remaining stable and 11% achieving a PR. However, it is not suggested for post-ASCT treatment (80, 81). A phase I/II clinical trial combined lenalidomide with the mTOR inhibitor temsirolimus in patients with r/r cHL and reported an ORR of 80% and a CR of 35% (82), which is encouraging. Trabectedin is a tetrahydroisoquinoline alkaloid with antitumor activity targeting both TME and HRS cells in cHL. Preclinical studies have demonstrated that trabectedin induces DNA skeleton cleavage and cancer cell necrosis by binding to the DNA structure and blocking the transcription level of stress-induced proteins. It also reduced HRS cell secretion of important factors and reduced immunosuppressive immunocytes in the TME, indicating that trabectedin can be a rational candidate in patients with r/r cHL (83). Furthermore, the combination of trabectedin and the CCR5 antagonist maraviroc enhanced DNA damage and antitumor activity (84).
cHL is a generally curable disease that occurs mainly in those aged 15–30 and over 55 years (3). However, a small group of patients are refractory to treatment or suffer a relapse after receiving primary systemic therapy and HDT-ASCT. The prognosis for these patients is notably poor. In this review, we briefly summarized the current treatment principles and conventional regimens and provided an update on the latest clinical trials of leading cHL agents (BV and ICIs) and novel potential drugs that may be used in the near future. In addition, prognosis evaluation plays a vital role in directing the next treatment strategy and analyzing the risk of relapse or disease progression. The International Prognostic Score (IPS) is composed of seven potential risk factors [albumin < 4 g/dl, hemoglobin < 10.5 g/dl, male, age ≥ 45 years, stage IV disease, leukocytosis, and lymphocytopenia (5)] and is intensively applied for risk classification. PET/CT is currently the most effective method to assess treatment efficacy and predict individual prognosis. The Deauville criteria is the most commonly used scale to evaluate the degree of lymphoma remission. However, when comparing different clinical trials, ORR and CRR may be based on different criteria, among which the Lugano 2014 classification and 2007 revised response criteria are frequently used. Although the guidelines for adult patients with cHL are mature, the treatment of certain groups of patients (including pediatric, older, and HIV-related individuals) (85) remains controversial. Further studies should focus on these population groups.
YZ, TW, and WW designed and analyzed the manuscript and were responsible for the funding acquisition and manuscript writing—review and editing. ZX, LM, ZL, and JZ supervised the study and drafted the manuscript. All authors reviewed and approved the manuscript.
This work was financially supported by the Support Program for Science and Technology Department of Sichuan Province (2021YFS0228, 2021YFS0230) and the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC21033).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. Dores GM, Curtis RE, Dalal NH, Linet MS, Morton LM. Cause-Specific Mortality Following Initial Chemotherapy in a Population-Based Cohort of Patients With Classical Hodgkin Lymphoma, 2000-2016. J Clin Oncol (2020) 38(35):4149–62. doi: 10.1200/jco.20.00264
3. Ansell SM. Hodgkin Lymphoma: 2018 Update on Diagnosis, Risk-Stratification, and Management. Am J Hematol (2018) 93(5):704–15. doi: 10.1002/ajh.25071
4. Brice P, de Kerviler E, Friedberg JW. Classical Hodgkin Lymphoma. Lancet (2021) 398(10310):1518–27. doi: 10.1016/s0140-6736(20)32207-8
5. NCCN Clinical Practice Guidelines in Oncology, Hodgkin Lymphoma (Version 2.2022 — February 23, 2022). Available at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1439.
6. Evens AM, Advani RH, Helenowski IB, Fanale M, Smith SM, Jovanovic BD, et al. Multicenter Phase II Study of Sequential Brentuximab Vedotin and Doxorubicin, Vinblastine, and Dacarbazine Chemotherapy for Older Patients With Untreated Classical Hodgkin Lymphoma. J Clin Oncol (2018) 36(30):3015–22. doi: 10.1200/jco.2018.79.0139
7. Straus DJ, Długosz-Danecka M, Alekseev S, Illés Á, Picardi M, Lech-Maranda E, et al. Brentuximab Vedotin With Chemotherapy for Stage III/IV Classical Hodgkin Lymphoma: 3-Year Update of the ECHELON-1 Study. Blood (2020) 135(10):735–42. doi: 10.1182/blood.2019003127
8. Ramchandren R, Advani RH, Ansell SM, Bartlett NL, Chen R, Connors JM, et al. Brentuximab Vedotin Plus Chemotherapy in North American Subjects With Newly Diagnosed Stage III or IV Hodgkin Lymphoma. Clin Cancer Res (2019) 25(6):1718–26. doi: 10.1158/1078-0432.Ccr-18-2435
9. Moskowitz CH, Matasar MJ, Zelenetz AD, Nimer SD, Gerecitano J, Hamlin P, et al. Normalization of Pre-ASCT, FDG-PET Imaging With Second-Line, non-Cross-Resistant, Chemotherapy Programs Improves Event-Free Survival in Patients With Hodgkin Lymphoma. Blood (2012) 119(7):1665–70. doi: 10.1182/blood-2011-10-388058
10. Smith SD, Moskowitz CH, Dean R, Pohlman B, Sobecks R, Copelan E, et al. Autologous Stem Cell Transplant for Early Relapsed/Refractory Hodgkin Lymphoma: Results From Two Transplant Centres. Br J Haematol (2011) 153(3):358–63. doi: 10.1111/j.1365-2141.2011.08616.x
11. Ramos CA, Grover NS, Beaven AW, Lulla PD, Wu MF, Ivanova A, et al. Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma. J Clin Oncol (2020) 38(32):3794–804. doi: 10.1200/jco.20.01342
12. Orellana-Noia VM, Isaac K, Malecek MK, Bartlett NL, Voorhees TJ, Grover NS, et al. Multicenter Analysis of Geriatric Fitness and Real-World Outcomes in Older Patients With Classical Hodgkin Lymphoma. Blood Adv (2021) 5(18):3623–32. doi: 10.1182/bloodadvances.2021004645
13. Cheson BD, Bartlett NL, LaPlant B, Lee HJ, Advani RJ, Christian B, et al. Brentuximab Vedotin Plus Nivolumab as First-Line Therapy in Older or Chemotherapy-Ineligible Patients With Hodgkin Lymphoma (ACCRU): A Multicentre, Single-Arm, Phase 2 Trial. Lancet Haematol (2020) 7(11):e808–15. doi: 10.1016/s2352-3026(20)30275-1
14. Johnston RL, Mottok A, Chan FC, Jiang A, Diepstra A, Visser L, et al. A Gene Expression-Based Model Predicts Outcome in Children With Intermediate-Risk Classical Hodgkin Lymphoma. Blood (2022) 139(6):889–93. doi: 10.1182/blood.2021011941
15. Parikh RR, Kelly KM, Hodgson DC, Hoppe BS, McCarten KM, Karolczuk K, et al. Patterns of Initial Relapse From a Phase 3 Study of Response-Based Therapy for High-Risk Hodgkin Lymphoma (AHOD0831): A Report From the Children’s Oncology Group. Int J Radiat Oncol Biol Phys (2022) 112(4):890–900. doi: 10.1016/j.ijrobp.2021.10.152
16. NCCN Clinical Practice Guidelines in Oncology, Pediatric Hodgkin Lymphoma (Version 1.2022 — April 8, 2022). Available at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1439.
17. Davis KL, Fox E, Merchant MS, Reid JM, Kudgus RA, Liu X, et al. Nivolumab in Children and Young Adults With Relapsed or Refractory Solid Tumours or Lymphoma (ADVL1412): A Multicentre, Open-Label, Single-Arm, Phase 1-2 Trial. Lancet Oncol (2020) 21(4):541–50. doi: 10.1016/s1470-2045(20)30023-1
18. Metzger ML, Link MP, Billett AL, Flerlage J, Lucas JT Jr., Mandrell BN, et al. Excellent Outcome for Pediatric Patients With High-Risk Hodgkin Lymphoma Treated With Brentuximab Vedotin and Risk-Adapted Residual Node Radiation. J Clin Oncol (2021) 39(20):2276–83. doi: 10.1200/jco.20.03286
19. Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, et al. Cac10-vcMMAE, an Anti-CD30-Monomethyl Auristatin E Conjugate With Potent and Selective Antitumor Activity. Blood (2003) 102(4):1458–65. doi: 10.1182/blood-2003-01-0039
20. Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a Pivotal Phase II Study of Brentuximab Vedotin for Patients With Relapsed or Refractory Hodgkin’s Lymphoma. J Clin Oncol (2012) 30(18):2183–9. doi: 10.1200/JCO.2011.38.0410
21. Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, et al. Brentuximab Vedotin as Consolidation Therapy After Autologous Stem-Cell Transplantation in Patients With Hodgkin’s Lymphoma at Risk of Relapse or Progression (AETHERA): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet (2015) 385(9980):1853–62. doi: 10.1016/s0140-6736(15)60165-9
22. Straus DJ, Długosz-Danecka M, Connors JM, Alekseev S, Illés Á, Picardi M, et al. Brentuximab Vedotin With Chemotherapy for Stage III or IV Classical Hodgkin Lymphoma (ECHELON-1): 5-Year Update of an International, Open-Label, Randomised, Phase 3 Trial. Lancet Haematol (2021) 8(6):e410–21. doi: 10.1016/s2352-3026(21)00102-2
23. Abramson JS, Arnason JE, LaCasce AS, Redd R, Barnes JA, Sokol L, et al. Brentuximab Vedotin, Doxorubicin, Vinblastine, and Dacarbazine for Nonbulky Limited-Stage Classical Hodgkin Lymphoma. Blood (2019) 134(7):606–13. doi: 10.1182/blood.2019001272
24. Chen R, Palmer JM, Martin P, Tsai N, Kim Y, Chen BT, et al. Results of a Multicenter Phase II Trial of Brentuximab Vedotin as Second-Line Therapy Before Autologous Transplantation in Relapsed/Refractory Hodgkin Lymphoma. Biol Blood Marrow Transplant (2015) 21(12):2136–40. doi: 10.1016/j.bbmt.2015.07.018
25. O’Connor OA, Lue JK, Sawas A, Amengual JE, Deng C, Kalac M, et al. Brentuximab Vedotin Plus Bendamustine in Relapsed or Refractory Hodgkin’s Lymphoma: An International, Multicentre, Single-Arm, Phase 1-2 Trial. Lancet Oncol (2018) 19(2):257–66. doi: 10.1016/s1470-2045(17)30912-9
26. Chen R, Herrera AF, Hou J, Chen L, Wu J, Guo Y, et al. Inhibition of MDR1 Overcomes Resistance to Brentuximab Vedotin in Hodgkin Lymphoma. Clin Cancer Res (2020) 26(5):1034–44. doi: 10.1158/1078-0432.Ccr-19-1768
27. Advani RH, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, et al. Brentuximab Vedotin in Combination With Nivolumab in Relapsed or Refractory Hodgkin Lymphoma: 3-Year Study Results. Blood (2021) 138(6):427–38. doi: 10.1182/blood.2020009178
28. Garcia-Sanz R, Sureda A, de la Cruz F, Canales M, Gonzalez AP, Pinana JL, et al. Brentuximab Vedotin and ESHAP is Highly Effective as Second-Line Therapy for Hodgkin Lymphoma Patients (Long-Term Results of a Trial by the Spanish GELTAMO Group). Ann Oncol (2019) 30(4):612–20. doi: 10.1093/annonc/mdz009
29. Lynch RC, Cassaday RD, Smith SD, Fromm JR, Cowan AJ, Warren EH, et al. Dose-Dense Brentuximab Vedotin Plus Ifosfamide, Carboplatin, and Etoposide for Second-Line Treatment of Relapsed or Refractory Classical Hodgkin Lymphoma: A Single Centre, Phase 1/2 Study. Lancet Haematol (2021) 8(8):e562–71. doi: 10.1016/s2352-3026(21)00170-8
30. LaCasce AS, Bociek RG, Sawas A, Caimi P, Agura E, Matous J, et al. Brentuximab Vedotin Plus Bendamustine: A Highly Active First Salvage Regimen for Relapsed or Refractory Hodgkin Lymphoma. Blood (2018) 132(1):40–8. doi: 10.1182/blood-2017-11-815183
31. Radhakrishnan VS, Bajaj R, Raina V, Kumar J, Bhave SJ, Sukumaran Nair RK, et al. Relapsed Refractory Hodgkin Lymphoma and Brentuximab Vedotin-Bendamustine Combination Therapy as a Bridge to Transplantation: Real-World Evidence From a Middle-Income Setting and Literature Review. Front Oncol (2021) 11:796270. doi: 10.3389/fonc.2021.796270
32. Moretti M, Liberati AM, Rigacci L, Puccini B, Pulsoni A, Gini G, et al. Brentuximab Vedotin and Bendamustine Produce Long-Term Clinical Benefit in Patients With Relapsed or Refractory Classical Hodgkin Lymphoma: A Multicenter Real-Life Experience. Clin Lymphoma Myeloma Leuk (2022) 22(3):198–204. doi: 10.1016/j.clml.2021.09.018
33. McMillan A, O’Neill AT, Townsend W, Lambert J, Virchis A, Shah R, et al. The Addition of Bendamustine to Brentuximab Vedotin Leads to Improved Rates of Complete Metabolic Remission in Children, Adolescents and Young Adults With Relapsed and Refractory Classical Hodgkin Lymphoma: A Retrospective Single-Centre Series. Br J Haematol (2021) 192(3):e84–7. doi: 10.1111/bjh.17274
34. Kersten MJ, Driessen J, Zijlstra JM, Plattel WJ, Morschhauser F, Lugtenburg PJ, et al. Combining Brentuximab Vedotin With Dexamethasone, High-Dose Cytarabine and Cisplatin as Salvage Treatment in Relapsed or Refractory Hodgkin Lymphoma: The Phase II HOVON/LLPC Transplant BRaVE Study. Haematologica (2021) 106(4):1129–37. doi: 10.3324/haematol.2019.243238
35. Moskowitz CH, Walewski J, Nademanee A, Masszi T, Agura E, Holowiecki J, et al. Five-Year PFS From the AETHERA Trial of Brentuximab Vedotin for Hodgkin Lymphoma at High Risk of Progression or Relapse. Blood (2018) 132(25):2639–42. doi: 10.1182/blood-2018-07-861641
36. Nademanee A, Sureda A, Stiff P, Holowiecki J, Abidi M, Hunder N, et al. Safety Analysis of Brentuximab Vedotin From the Phase III AETHERA Trial in Hodgkin Lymphoma in the Post-Transplant Consolidation Setting. Biol Blood Marrow Transplant (2018) 24(11):2354–9. doi: 10.1016/j.bbmt.2018.05.026
37. Massaro F, Pavone V, Stefani PM, Botto B, Pulsoni A, Patti C, et al. Brentuximab Vedotin Consolidation After Autologous Stem Cell Transplantation for Hodgkin Lymphoma: A Fondazione Italiana Linfomi Real-Life Experience. Hematol Oncol (2022) 40(1):31–9. doi: 10.1002/hon.2939
38. Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O’Donnell E, et al. Integrative Analysis Reveals Selective 9p24.1 Amplification, Increased PD-1 Ligand Expression, and Further Induction via JAK2 in Nodular Sclerosing Hodgkin Lymphoma and Primary Mediastinal Large B-Cell Lymphoma. Blood (2010) 116(17):3268–77. doi: 10.1182/blood-2010-05-282780
39. Laliberté F, Raut M, Yang X, Germain G, Nahar A, Desai KD, et al. Real-World Healthcare Resource Utilization in Patients With Classical Hodgkin Lymphoma Treated With Pembrolizumab and Nivolumab in the USA. Target Oncol (2021) 16(1):85–94. doi: 10.1007/s11523-020-00778-y
40. Shi Y, Li Q, Zhang W, Nan Y, Yang T, Liang X, et al. Sintilimab as Salvage Treatment in an HIV Patient With Relapsed/Refractory Hodgkin: A Case Report. Ann Palliat Med (2020) 9(4):2414–9. doi: 10.21037/apm-20-1333
41. Shi Y, Su H, Song Y, Jiang W, Sun X, Qian W, et al. Safety and Activity of Sintilimab in Patients With Relapsed or Refractory Classical Hodgkin Lymphoma (ORIENT-1): A Multicentre, Single-Arm, Phase 2 Trial. Lancet Haematol (2019) 6(1):e12–9. doi: 10.1016/s2352-3026(18)30192-3
42. Song Y, Gao Q, Zhang H, Fan L, Zhou J, Zou D, et al. Tislelizumab for Relapsed/Refractory Classical Hodgkin Lymphoma: 3-Year Follow-Up and Correlative Biomarker Analysis. Clin Cancer Res (2022) 28(6):1147–56. doi: 10.1158/1078-0432.Ccr-21-2023
43. Song Y, Gao Q, Zhang H, Fan L, Zhou J, Zou D, et al. Treatment of Relapsed or Refractory Classical Hodgkin Lymphoma With the Anti-PD-1, Tislelizumab: Results of a Phase 2, Single-Arm, Multicenter Study. Leukemia (2020) 34(2):533–42. doi: 10.1038/s41375-019-0545-2
44. Wang CY, Sheng CC, Ma GL, Xu D, Liu XQ, Wang YY, et al. Population Pharmacokinetics of the Anti-PD-1 Antibody Camrelizumab in Patients With Multiple Tumor Types and Model-Informed Dosing Strategy. Acta Pharmacol Sin (2021) 42(8):1368–75. doi: 10.1038/s41401-020-00550-y
45. Song Y, Wu J, Chen X, Lin T, Cao J, Liu Y, et al. A Single-Arm, Multicenter, Phase II Study of Camrelizumab in Relapsed or Refractory Classical Hodgkin Lymphoma. Clin Cancer Res (2019) 25(24):7363–9. doi: 10.1158/1078-0432.Ccr-19-1680
46. Wu J, Song Y, Chen X, Lin T, Cao J, Liu Y, et al. Camrelizumab for Relapsed or Refractory Classical Hodgkin Lymphoma: Extended Follow-Up of the Multicenter, Single-Arm, Phase 2 Study. Int J Cancer (2022) 150(6):984–92. doi: 10.1002/ijc.33852
47. Sasse S, Reddemann K, Diepstra A, Oschlies I, Schnitter A, Borchmann S, et al. Programmed Cell Death Protein-1 (PD-1)-Expression in the Microenvironment of Classical Hodgkin Lymphoma at Relapse During Anti-PD-1-Treatment. Haematologica (2019) 104(1):e21–4. doi: 10.3324/haematol.2018.196279
48. Nie J, Wang C, Liu Y, Yang Q, Mei Q, Dong L, et al. Addition of Low-Dose Decitabine to Anti-PD-1 Antibody Camrelizumab in Relapsed/Refractory Classical Hodgkin Lymphoma. J Clin Oncol (2019) 37(17):1479–89. doi: 10.1200/jco.18.02151
49. Liu Y, Wang C, Li X, Dong L, Yang Q, Chen M, et al. Improved Clinical Outcome in a Randomized Phase II Study of Anti-PD-1 Camrelizumab Plus Decitabine in Relapsed/Refractory Hodgkin Lymphoma. J Immunother Cancer (2021) 9(4):e002347-17. doi: 10.1136/jitc-2021-002347
50. Wang C, Liu Y, Dong L, Li X, Yang Q, Brock MV, et al. Efficacy of Decitabine Plus Anti-PD-1 Camrelizumab in Patients With Hodgkin Lymphoma Who Progressed or Relapsed After PD-1 Blockade Monotherapy. Clin Cancer Res (2021) 27(10):2782–91. doi: 10.1158/1078-0432.Ccr-21-0133
51. Song Y, Zhou K, Jin C, Qian Z, Hou M, Fan L, et al. A Phase II Study of Penpulimab, an Anti-PD-1 Antibody, in Patients With Relapsed or Refractoryclassic Hodgkin Lymphoma (cHL). J Clin Oncol (2021) 39(15_suppl):7529. doi: 10.1200/JCO.2021.39.15_suppl.7529
52. Lin N, Zhang M, Bai H, Liu H, Cui J, Ke X, et al. Efficacy and Safety of GLS-010 (Zimberelimab) in Patients With Relapsed or Refractory Classical Hodgkin Lymphoma: A Multicenter, Single-Arm, Phase II Study. Eur J Cancer (Oxford Engl 1990) (2022) 164:117–26. doi: 10.1016/j.ejca.2021.07.021
53. Ramchandren R, Domingo-Domènech E, Rueda A, Trněný M, Feldman TA, Lee HJ, et al. Nivolumab for Newly Diagnosed Advanced-Stage Classic Hodgkin Lymphoma: Safety and Efficacy in the Phase II CheckMate 205 Study. J Clin Oncol (2019) 37(23):1997–2007. doi: 10.1200/jco.19.00315
54. Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol (2018) 36(14):1428–39. doi: 10.1200/jco.2017.76.0793
55. Bekoz H, Ozbalak M, Karadurmus N, Paydas S, Turker A, Toptas T, et al. Nivolumab for Relapsed or Refractory Hodgkin Lymphoma: Real-Life Experience. Ann Hematol (2020) 99(11):2565–76. doi: 10.1007/s00277-020-04077-4
56. Bröckelmann PJ, Goergen H, Keller U, Meissner J, Ordemann R, Halbsguth TV, et al. Efficacy of Nivolumab and AVD in Early-Stage Unfavorable Classic Hodgkin Lymphoma: The Randomized Phase 2 German Hodgkin Study Group NIVAHL Trial. JAMA Oncol (2020) 6(6):872–80. doi: 10.1001/jamaoncol.2020.0750
57. Herrera AF, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, et al. Interim Results of Brentuximab Vedotin in Combination With Nivolumab in Patients With Relapsed or Refractory Hodgkin Lymphoma. Blood (2018) 131(11):1183–94. doi: 10.1182/blood-2017-10-811224
58. Diefenbach CS, Hong F, Ambinder RF, Cohen JB, Robertson MJ, David KA, et al. Ipilimumab, Nivolumab, and Brentuximab Vedotin Combination Therapies in Patients With Relapsed or Refractory Hodgkin Lymphoma: Phase 1 Results of an Open-Label, Multicentre, Phase 1/2 Trial. Lancet Haematol (2020) 7(9):e660–70. doi: 10.1016/s2352-3026(20)30221-0
59. Mei MG, Lee HJ, Palmer J, Chen RW, Tsai NC, Chen L, et al. Response-Adapted Anti-PD1 Based Salvage Therapy for Hodgkin Lymphoma With Nivolumab +/- ICE (NICE). Blood (2022) 139(25):3605–16. doi: 10.1182/blood.2022015423
60. Manson G, Mear JB, Herbaux C, Schiano JM, Casasnovas O, Stamatoullas A, et al. Long-Term Efficacy of Anti-PD1 Therapy in Hodgkin Lymphoma With and Without Allogenic Stem Cell Transplantation. Eur J Cancer (2019) 115:47–56. doi: 10.1016/j.ejca.2019.04.006
61. Armand P, Chen YB, Redd RA, Joyce RM, Bsat J, Jeter E, et al. PD-1 Blockade With Pembrolizumab for Classical Hodgkin Lymphoma After Autologous Stem Cell Transplantation. Blood (2019) 134(1):22–9. doi: 10.1182/blood.2019000215
62. Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J Clin Oncol (2017) 35(19):2125–32. doi: 10.1200/jco.2016.72.1316
63. Chen R, Zinzani PL, Lee HJ, Armand P, Johnson NA, Brice P, et al. Pembrolizumab in Relapsed or Refractory Hodgkin Lymphoma: 2-Year Follow-Up of KEYNOTE-087. Blood (2019) 134(14):1144–53. doi: 10.1182/blood.2019000324
64. Kuruvilla J, Ramchandren R, Santoro A, Paszkiewicz-Kozik E, Gasiorowski R, Johnson NA, et al. Pembrolizumab Versus Brentuximab Vedotin in Relapsed or Refractory Classical Hodgkin Lymphoma (KEYNOTE-204): An Interim Analysis of a Multicentre, Randomised, Open-Label, Phase 3 Study. Lancet Oncol (2021) 22(4):512–24. doi: 10.1016/s1470-2045(21)00005-x
65. Zinzani PL, Ramchandren R, Santoro A, Paszkiewicz-Kozik E, Gasiorowski R, Johnson NA, et al. Quality-Of-Life Analysis of Pembrolizumab vs Brentuximab Vedotin for Relapsed/Refractory Classical Hodgkin Lymphoma. Blood Adv (2022) 6(2):590–9. doi: 10.1182/bloodadvances.2021004970
66. Armand P, Kuruvilla J, Michot JM, Ribrag V, Zinzani PL, Zhu Y, et al. KEYNOTE-013 4-Year Follow-Up of Pembrolizumab in Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure. Blood Adv (2020) 4(12):2617–22. doi: 10.1182/bloodadvances.2019001367
67. Allen PB, Savas H, Evens AM, Advani RH, Palmer B, Pro B, et al. Pembrolizumab Followed by AVD in Untreated Early Unfavorable and Advanced-Stage Classical Hodgkin Lymphoma. Blood (2021) 137(10):1318–26. doi: 10.1182/blood.2020007400
68. Massaro F, Meuleman N, Bron D, Vercruyssen M, Maerevoet M. Brentuximab Vedotin and Pembrolizumab Combination in Patients With Relapsed/Refractory Hodgkin Lymphoma: A Single-Centre Retrospective Analysis. Cancers (Basel) (2022) 14(4):982–9. doi: 10.3390/cancers14040982
69. Bartlett NL, Niedzwiecki D, Johnson JL, Friedberg JW, Johnson KB, van Besien K, et al. Gemcitabine, Vinorelbine, and Pegylated Liposomal Doxorubicin (GVD), a Salvage Regimen in Relapsed Hodgkin’s Lymphoma: CALGB 59804. Ann Oncol (2007) 18(6):1071–9. doi: 10.1093/annonc/mdm090
70. Moskowitz AJ, Shah G, Schöder H, Ganesan N, Drill E, Hancock H, et al. Phase II Trial of Pembrolizumab Plus Gemcitabine, Vinorelbine, and Liposomal Doxorubicin as Second-Line Therapy for Relapsed or Refractory Classical Hodgkin Lymphoma. J Clin Oncol (2021) 39(28):3109–17. doi: 10.1200/jco.21.01056
71. Bartlett NL, Herrera AF, Domingo-Domenech E, Mehta A, Forero-Torres A, Garcia-Sanz R, et al. A Phase 1b Study of AFM13 in Combination With Pembrolizumab in Patients With Relapsed or Refractory Hodgkin Lymphoma. Blood (2020) 136(21):2401–9. doi: 10.1182/blood.2019004701
72. Herrera AF, Palmer J, Adhikarla V, Yamauchi D, Poku EK, Bading J, et al. Anti-CD25 Radioimmunotherapy With BEAM Autologous Hematopoietic Cell Transplantation Conditioning in Hodgkin Lymphoma. Blood Adv (2021) 5(23):5300–11. doi: 10.1182/bloodadvances.2021004981
73. Dela Cruz Chuh J, Go M, Chen Y, Guo J, Rafidi H, Mandikian D, et al. Preclinical Optimization of Ly6E-Targeted ADCs for Increased Durability and Efficacy of Anti-Tumor Response. MAbs (2021) 13(1):1862452. doi: 10.1080/19420862.2020.1862452
74. Hamadani M, Collins GP, Caimi PF, Samaniego F, Spira A, Davies A, et al. Camidanlumab Tesirine in Patients With Relapsed or Refractory Lymphoma: A Phase 1, Open-Label, Multicentre, Dose-Escalation, Dose-Expansion Study. Lancet Haematol (2021) 8(6):e433–45. doi: 10.1016/s2352-3026(21)00103-4
75. Epperla N, Hamadani M. A New Target for Hodgkin Lymphoma - Camidanlumab Tesirine. Curr Hematol Malig Rep (2021) 16(1):19–24. doi: 10.1007/s11899-021-00604-w
76. Herrera AF, Carlo-Stella C, Collins GP, Maddocks KJ, Bartlett NL, Savage KJ, et al. Preliminary Results of a Phase 2 Study of Camidanlumab Tesirine (Cami), a Novel Pyrrolobenzodiazepine-Based Antibody-Drug Conjugate, in Patients With Relapsed or Refractory Hodgkin Lymphoma. Blood (2020) 136(Supplement 1):21–3. doi: 10.1182/blood-2020-137451
77. Kirchhoff D, Stelte-Ludwig B, Lerchen HG, Wengner AM, Ahsen OV, Buchmann P, et al. IL3RA-Targeting Antibody-Drug Conjugate BAY-943 With a Kinesin Spindle Protein Inhibitor Payload Shows Efficacy in Preclinical Models of Hematologic Malignancies. Cancers (Basel) (2020) 12(11):3464–80. doi: 10.3390/cancers12113464
78. Voorhees TJ, Zhao B, Oldan J, Hucks G, Khandani A, Dittus C, et al. Pretherapy Metabolic Tumor Volume is Associated With Response to CD30 CAR T Cells in Hodgkin Lymphoma. Blood Adv (2022) 6(4):1255–63. doi: 10.1182/bloodadvances.2021005385
79. Cohen JB, Wei L, Maddocks KJ, Christian B, Heffner LT, Langston AA, et al. Gemcitabine and Bendamustine is a Safe and Effective Salvage Regimen for Patients With Recurrent/Refractory Hodgkin Lymphoma: Results of a Phase 1/2 Study. Cancer (2020) 126(6):1235–42. doi: 10.1002/cncr.32640
80. Ma H, Cheng B, Montanari F, Lue JK, Deng C, Marchi E, et al. Low Dose Continuous Lenalidomide in Heavily Pretreated Patients With Relapsed or Refractory Classical Hodgkin Lymphoma: A Retrospective Case Series. Ther Adv Hematol (2020) 11:2040620720947340. doi: 10.1177/2040620720947340
81. Shea L, Watkins MP, Wan F, Cashen AF, Wagner-Johnston ND, Jacoby MA, et al. A Pilot Study of Lenalidomide Maintenance Therapy After Autologous Transplantation in Relapsed or Refractory Classical Hodgkin Lymphoma. Biol Blood Marrow Transplant (2020) 26(12):2223–8. doi: 10.1016/j.bbmt.2020.08.017
82. Major A, Kline J, Karrison TG, Fishkin PAS, Kimball AS, Petrich AM, et al. Phase I/II Clinical Trial of Temsirolimus and Lenalidomide in Patients With Relapsed and Refractory Lymphomas. Haematologica (2021) 107:1609–18. doi: 10.3324/haematol.2021.278853
83. Casagrande N, Borghese C, Favero A, Vicenzetto C, Aldinucci D. Trabectedin Overcomes Doxorubicin-Resistance, Counteracts Tumor-Immunosuppressive Reprogramming of Monocytes and Decreases Xenograft Growth in Hodgkin Lymphoma. Cancer Lett (2021) 500:182–93. doi: 10.1016/j.canlet.2020.12.015
84. Casagrande N, Borghese C, Aldinucci D. In Classical Hodgkin Lymphoma the Combination of the CCR5 Antagonist Maraviroc With Trabectedin Synergizes, Enhances DNA Damage and Decreases Three-Dimensional Tumor-Stroma Heterospheroid Viability. Haematologica (2022) 107(1):287–91. doi: 10.3324/haematol.2021.279389
85. Lievin R, Hendel-Chavez H, Baldé A, Lancar R, Algarte-Génin M, Krzysiek R, et al. Increased Production of B-Cell Activating Cytokines and Altered Peripheral B-Cell Subset Distribution During HIV-Related Classical Hodgkin Lymphoma. Cancers (Basel) (2021) 14(1):128–41. doi: 10.3390/cancers14010128
Keywords: novel agents, brentuximab vedotin, nivolumab, pembrolizumab, relapsed and refractory classical hodgkin lymphoma
Citation: Zhang Y, Xing Z, Mi L, Li Z, Zhu J, Wei T and Wu W (2022) Novel Agents For Relapsed and Refractory Classical Hodgkin Lymphoma: A Review. Front. Oncol. 12:929012. doi: 10.3389/fonc.2022.929012
Received: 26 April 2022; Accepted: 22 June 2022;
Published: 14 July 2022.
Edited by:
Zhizhuang Joe Zhao, University of Oklahoma Health Sciences Center, United StatesReviewed by:
Yi Shi, Nankai University, ChinaCopyright © 2022 Zhang, Xing, Mi, Li, Zhu, Wei and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenshuang Wu, d2Vuc2h1YW5nX3d1QDE2My5jb20=; Tao Wei, c3VyZ2VvbndlaUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.