- 1Mini-Invasive Intervention Center, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University/Navy Medical University, Shanghai, China
- 2Department of Interventional Oncology, Fujian Medical University Cancer Hospital, Fuzhou, China
Background: The study aimed to assess the safety and efficacy of conversion therapy with portal vein embolization (PVE) and transcatheter arterial chemoembolization (TACE) in patients with large unresectable hepatocellular carcinoma (HCC) and ipsilateral portal vein tumor thrombus (PVTT).
Methods: This retrospective study evaluated consecutive patients with initially large (≥5 cm) unresectable HCC with ipsilateral PVTT who underwent PVE + TACE at our center between June 2016 and September 2020 (Group A). Clinically equivalent patients from three centers who were receiving tyrosine kinase inhibitors (TKIs) + TACE (Group B) were included. The survival times were evaluated and compared between the two therapeutic groups.
Results: In Group A (n = 33), the median tumor diameter was 14 cm (range, 5–18 cm) and 19 (57.6%) patients underwent radical resection 18–95 days after PVE. Radical liver resection was not performed because of inadequate hypertrophy (n = 11), pulmonary metastasis (n = 1), lack of consent for surgery (n = 1), and the rupture of the HCC (n = 1). There were no patients who underwent radical resection in Group B (n = 64) (P = 0.000). The mean and median overall survival (OS) were 736.5 days and 425.0 days in Group A and 424.5 days and 344.0 days in Group B, respectively. Compared with TKIs + TACE, treatment with PVE + TACE prolonged OS (P = 0.023).
Conclusions: This study shows that conversion therapy was safe and effective in patients with initially large unresectable HCC with ipsilateral PVTT treated with PVE + TACE. Moreover, PVE + TACE conferred more favorable outcomes than treatment with TKIs + TACE.
Introduction
Hepatocellular carcinoma (HCC) is the third cause of cancer-related deaths worldwide and the second cause in China (1, 2). HCC has a strong propensity to invade the adjacent vasculature (3). Portal vein tumor thrombosis (PVTT) is found in 44–62.2% of patients with HCC who have already lost the chance of radical resection; thus, PVTT is recognized as a major prognostic risk factor (4, 5). Even with the best supportive care, the overall survival (OS) of HCC patients with PVTT is only 2–4 months (6, 7). Many factors contribute to the poor prognosis of patients with portal vein invasion, such as more invasive tumor behavior, aggravation of portal hypertension, facilitation of tumor transfer throughout the liver parenchyma or distant metastasis, and decreased hepatopetal portal blood flow (8). The Barcelona Clinic Liver Cancer (BCLC) staging and management system, which is widely accepted and applied in western countries, classifies HCC patients with PVTT as having at least advanced HCC, and systemic therapy with tyrosine kinase inhibitors (TKIs) is recommended as the first-line therapy regardless of PVTT grades (9). Besides TKIs, more aggressive therapies have been used in the clinic, which may improve the prognosis of HCC patients with PVTT and prolong the survival time of patients, such as transarterial chemoembolization (TACE), radiotherapy, hepatic resection, liver transplantation, and various combination of therapies (10–13).
Hepatic resection remains the mainstay of the curative treatment of primary hepatic malignancies. Conversion therapy, which has become a topic of interest for treating advanced liver cancer, converts unresectable advanced HCC or potentially resectable HCC to resectable HCC (14). Conversion therapies for HCC mainly include systemic therapy (15), portal vein embolization (PVE), associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) (16), and TACE. PVE, for the first time reported by Makuuchi et al. (17), is a well-established procedure for inducing future liver remnant (FLR) hypertrophy. The indications for liver resection have been expanded by PVE, which can lower the risk of postoperative liver failure (18).
According to Cheng et al. (11), >90% of PVTT develops on the same side of the lobe of the main tumor, and the tumor thromboses progress in the portal vein with far-from-heart modes. Thus, a combination of PVE and TACE is a potential therapy option for large unresectable HCC with ipsilateral PVTT, in which surgery cannot be performed because of the small FLR and PVTT. This treatment protocol was based on the hypothesis that PVE not only induces FLR hypertrophy but also prevents PVTT from developing toward the main portal vein by mechanically embolizing the targeted portal vein. If a sufficient FLR is achieved and PVTT is successfully treated after PVE, radical surgery can be performed subsequently.
Here, we evaluated 33 patients with initially unresectable large (≥5 cm) HCC with ipsilateral PVTT who underwent PVE and TACE. The oncological results, including OS and progression-free survival (PFS), were compared with those of equivalent patients receiving TKIs + TACE. This study reports our experience with conversion therapy with PVE + TACE in an initially large unresectable HCC with ipsilateral PVTT. Additionally, we intend to evaluate the safety and efficacy of PVE and TACE in these sufferers. To our knowledge, this is the first study that focused on the conversion therapy of large unresectable HCC with ipsilateral PVTT using PVE + TACE.
Materials and Methods
Patients
This retrospective study included (a) patients diagnosed with initially large unresectable HCC with ipsilateral PVTT who received PVE + TACE at one center from June 2016 to September 2020, and (b) equivalent patients who received TKIs + TACE at the same center and two other centers. This study was conducted in conformity to the principles of the Declaration of Helsinki. The ethics committee of our hospital authorized the study protocol and waived the need for informed consent.
The criteria for inclusion were as follows: 1) over 18 years of age; 2) histopathologically or radiologically diagnosed HCC on the basis of the American Association for the Study of Liver Diseases criteria; 3) the HCC was unresectable because of PVTT and small standardized FLR (sFLR) (sFLR ≤40% and >30% in patients with cirrhosis, while patients with normal livers generally need ≤30% and >25%); 4) the size of the dominant tumor was ≥5 cm, and the ipsilateral PVTT was of grade Vp1, Vp2, or Vp3 and >1 cm from the main portal vein—according to the Japanese grading system for tumor emboli (19); 5) presence of PVTT spreading from the branches of intrahepatic portal vein (defined as low-attenuation intraluminal filling defect verified by contrast-enhanced computed tomography or magnetic resonance imaging); 6) Child–Pugh class A or B; 7) no extrahepatic invasion; 8) an Eastern Cooperative Oncology Group performance status score of 2 or less; and 9) no contraindication to TACE, PVE, or TKIs. Patients were excluded if they met any of the following criterion: 1) the dominant tumor size of <5 cm or contralateral PVTT; 2) left or right portal vein invasion of <1 cm from the main portal vein or PVTT with Vp4 grade as per the Japanese grading system; 3) other concurrent malignancies; 4) previous therapy for PVTT; 5) received other treatments (including radiofrequency ablation, iodine-125 seed implantation, radiotherapy, etc.) except for the aforementioned treatment for PVTT during the study; 6) sFLR ≤30% in patients with cirrhosis, while sFLR was ≤25% in patients with normal livers, or 7) lost to follow-up.
FLR volume was measured directly by computed tomography and total estimated liver volume (TELV) was calculated from the formula: TELV = −794.41 + 1,267.28 × BSA (body surface, square meters). Then, sFLR was obtained based on the formula: sFLR = FLR/TELV.
Thirty-three patients received PVE + TACE (Group A). Concomitantly, 64 clinically equivalent patients received TKIs + TACE from three centers (Group B).
Volumetric Assessment
The IQQA-Liver software (EDDA Technology Inc., Princeton, NJ, USA) was used to reconstruct 3-dimensional images and to measure liver volumes. A radical resection operation was considered safe and sufficient when the sFLR ratio was >40% in the cirrhotic liver or >30% in the normal liver.
TACE
The presence of tumor-feeding arteries was confirmed using digital subtraction angiography. Infusions of Lipiodol (5–20 ml), embolizing fluids, and/or microspheres were infused into the tumor-feeding arteries until the tumor blood flow slowed or stopped completely (Figure 1). Patients who underwent TACE were evaluated during follow-up every 4–6 weeks after the procedure. A repeat TACE was performed after confirming Child–Pugh class A or B status, the absence of any liver dysfunction (uncontrolled jaundice, intractable ascites, massive hematemesis, or severe hepatic encephalopathy), and a lesion that was not fully necrotic.
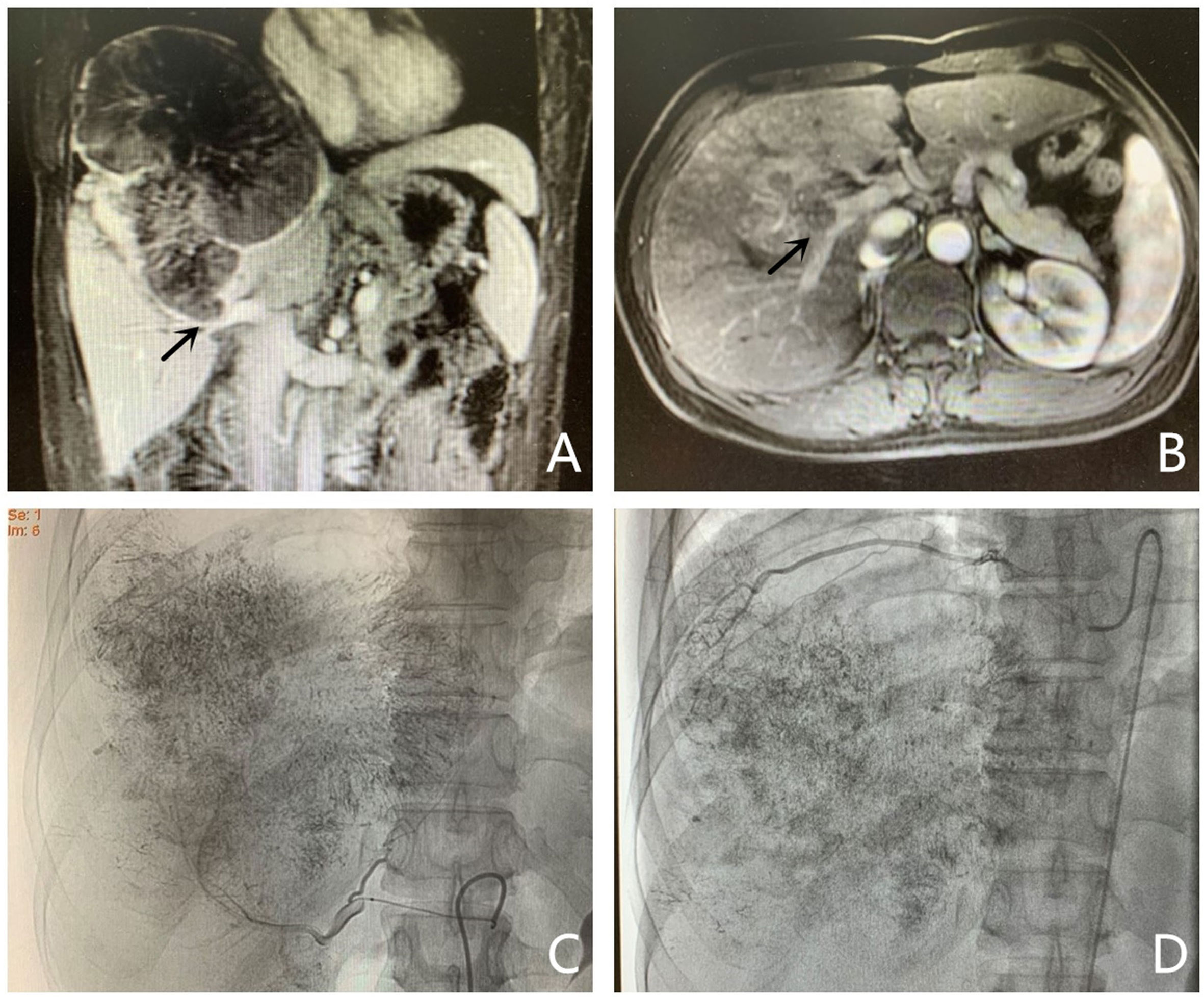
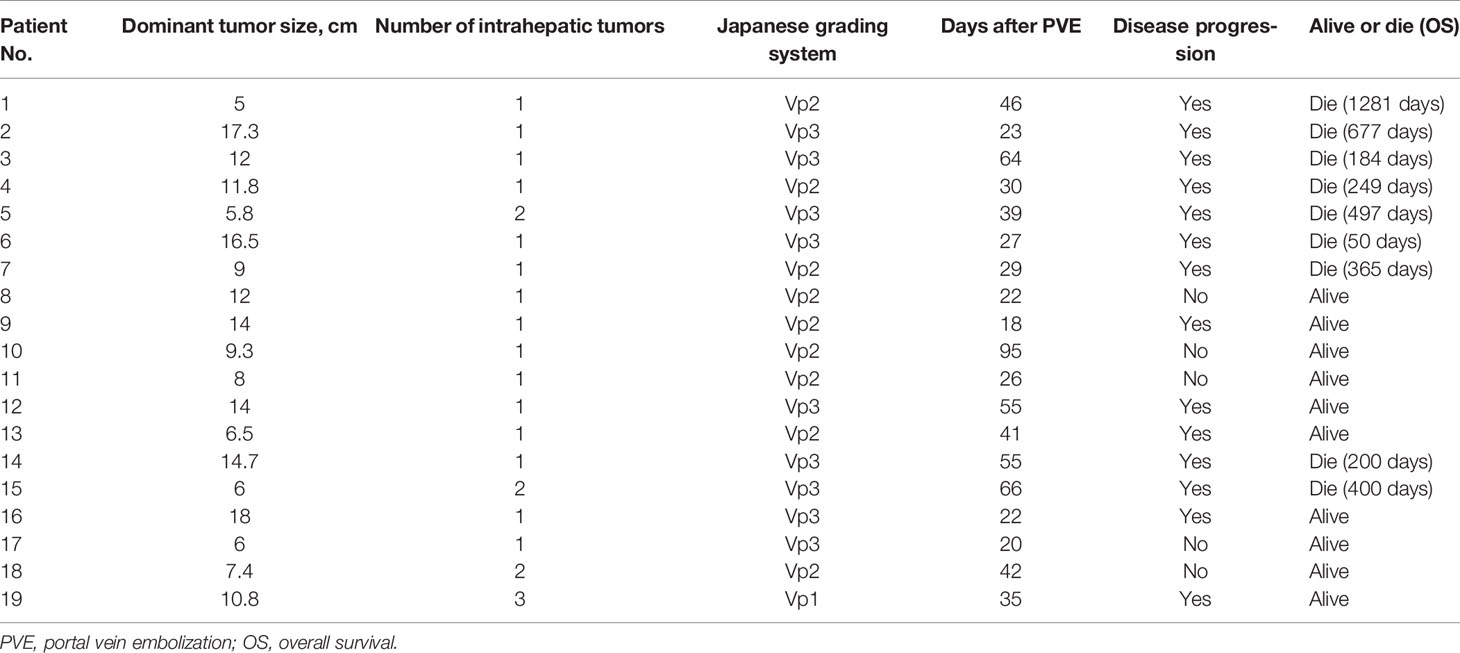
Figure 1 Photographs acquired by magnetic resonance imaging (MRI) and transarterial chemoembolization implemented in a 44-year-old man (PVE + TACE group). (A, B) Hepatocellular carcinoma with ipsilateral PVTT, with an intraluminal filling defect which was a low-attenuation and spread from the intrahepatic portal vein branches (black arrow), was detected in the right lobe on enhanced abdominal MRI before therapy. (C, D) Images of transarterial chemoembolization. Tumor-feeding arteries are confirmed using digital subtraction angiography. Lipiodol, chemotherapeutic agents, and microspheres were injected into the right hepatic (C) and right phrenic arteries (D).
PVE Procedure
To decrease the risk of PVTT spreading throughout the liver parenchyma or distant metastasis, there are three announcements about PVE for HCC patients with ipsilateral PVTT. First, a contralateral approach is the necessary prerequisite. Second, movements during PVE must be gentle. Third, in the case of migration of PVTT, suction through a catheter should be avoided when the catheter is positioned in the portal vein with PVTT. The contralateral approach provides a more favorable orientation for catheter control and flow-guided distal embolization. Under ultrasound guidance, a suitable branch of the portal vein was punctured by a needle, and portography was performed. The targeted portal vein was embolized using n-butyl cyanoacrylate (NBCA) mixed with iodized oil (n = 19), polyvinyl alcohol (n = 14), gelfoam (n = 6), and/or steel coil (n = 30) (Figure 2). All procedures were performed by the same physician. According to a previous report (20), after the embolization, the head end of the catheter was retracted to the hepatic parenchyma. The following day, the catheter was removed.
Figure 2 Images of portal vein embolization performed in a 44-year-old man (same patient as in Figure 1). Puncturing a suitable branch of the portal vein with the guidance of ultrasound. (A) Portography with a catheter placed into the main portal vein. The right portal vein is not completely visible; it appears as a thrombosis in the right portal vein and matched the images acquired by magnetic resonance imaging. (B) Portography with a catheter placed into the right portal vein. (C) Image showing embolizing of the targeted portal vein. (D) Portography after embolizing of the right portal vein. The targeted portal vein was embolized using an NBCA/Lipiodol mixture and coils.
Hepatectomy
The tumor was considered resectable if (1) R0 resection could be achieved with sufficient remnant liver volume and function, sFLR >40% in cirrhotic liver and >30% in normal liver, (2) liver function was Child–Pugh stage A or B, (3) there was no extrahepatic metastasis, and (4) no contraindications for hepatectomy existed.
TKI Therapy and Combination Therapy
TACE combined with TKIs was recommended for patients after assessing its clinical effects, potential adverse events, and costs. Sorafenib, lenvatinib, regorafenib, and apatinib were also recommended. If the recommendation was accepted, TKIs were administered 3–7 days after the first TACE procedure. Sorafenib was administered twice daily, at a dose of 0.4 g. Regorafenib was administered at a dose of 0.16 g once daily. Apatinib was administered at a dose of 0.75 g once daily. Lenvatinib was administered at doses of 8 mg (less than 60 kg) or 12 mg (greater than or equal to 60 kg) once a day. The administration of TKIs was discontinued for three days leading up to the TACE procedure. Therapeutic administration was continued only after any effects of TACE (pyrexia, nausea, or vomiting) subsided. Drug reduction and interruption of drug-related adverse events were permitted.
Safety Assessment
Adverse events (AEs) were graded based on the Common Terminology Criteria for Adverse Events (version 5.0) and logged during follow-ups at intervals of 1–2 months.
Follow-Up and Assessment
The outpatient follow-up was conducted every 1–2 months. The censoring date was 5 May 2021. The OS and PFS of the two treatment groups were compared. The definition of OS was the interval from the first TACE session to death or last follow-up. As advanced HCC with PVTT progresses quickly, PVTT progression can induce portal hypertension and deterioration of liver function. The emergence of tumor spread, liver function deterioration, and esophageal and gastric variceal bleeding were indicators of disease progression. The definition of PFS was the interval from the day of the first TACE session to the occurrence of at least one of the aforementioned events or death.
Statistical Analysis
A Student’s t-test was used to compare the continuous variables between the treatment category and baseline characteristics, and Fisher’s exact or χ2 test was employed to compare categorical variables. The Kaplan–Meier method was used to estimate survival curves, and the log-rank test was used to analyze differences. Using the Cox regression model, independent prognostic factors, which were correlated with OS identified by univariate analyses, were confirmed through multivariate analyses. SPSS, version 25.0 (SPSS Inc., Chicago, IL, USA), was employed for statistical analyses, with the statistical significance set at P <0.05.
Results
Patient Characteristics
A total of 357 patients underwent PVE. As shown in Figure 3, 152 patients did not have HCC, and 172 patients did not satisfy the enrollment criteria. Thirty-three patients received PVE + TACE (Group A), and 64 patients received TKIs + TACE from three centers (Group B). The baseline characteristics of the two treatment groups are shown in Table 1.
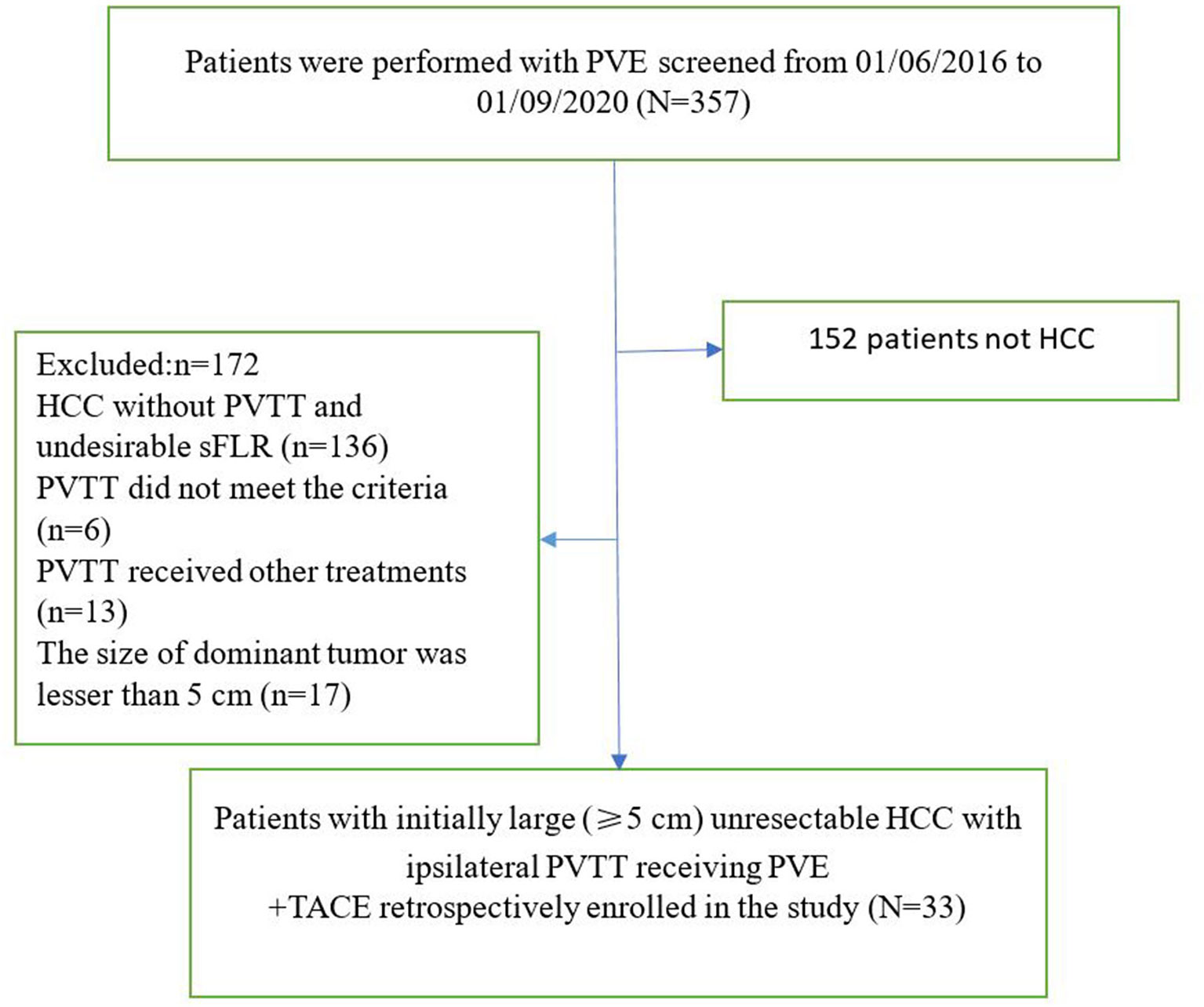
Figure 3 The flow diagram of patients (PVE + TACE group). PVE, portal vein embolization; HCC, hepatocellular carcinoma; PVTT, portal vein tumor thrombus; sFLR, standized future liver remnant; TACE, transarterial chemoembolization.
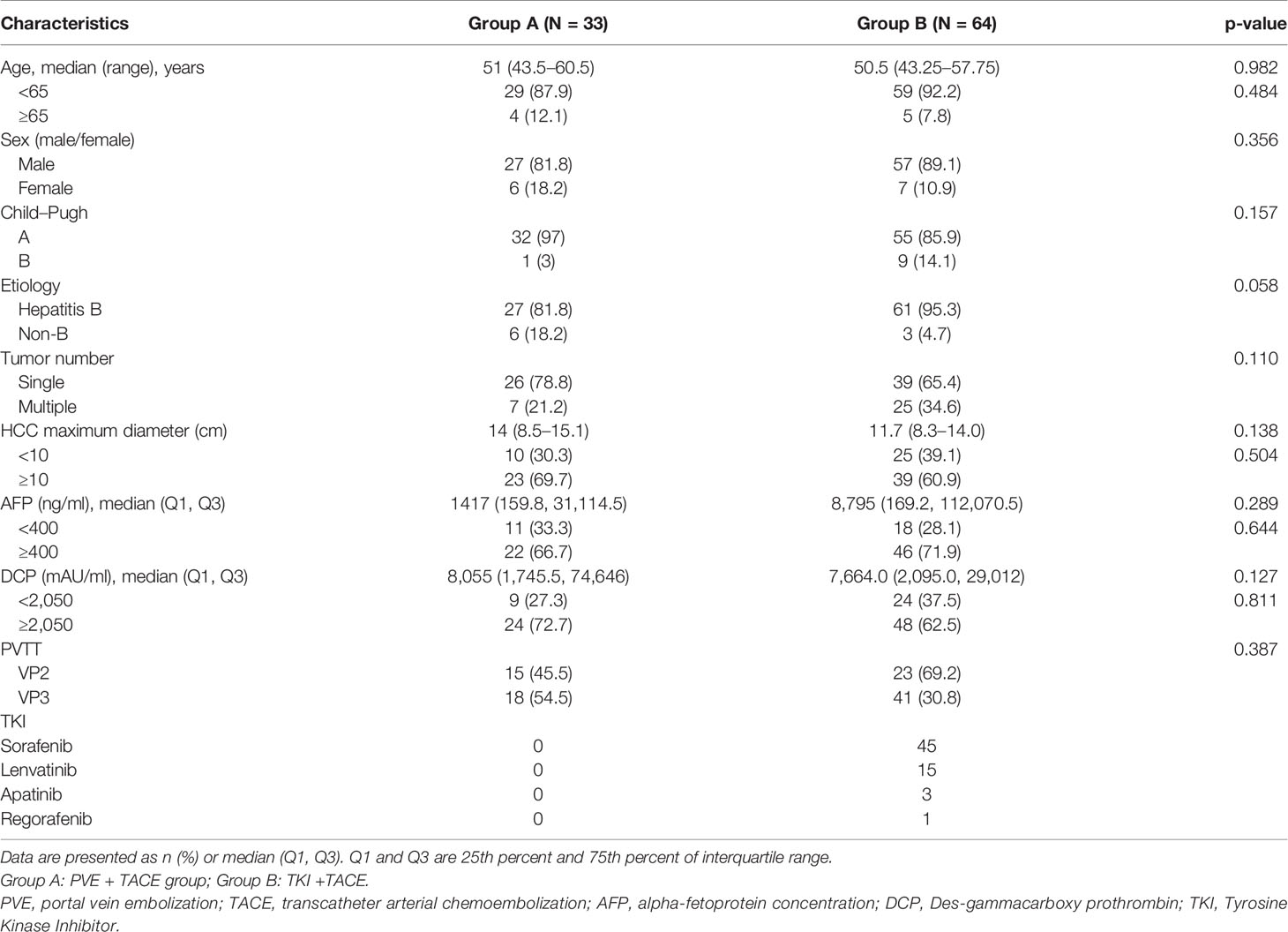
Table 1 Baseline demographic and clinical characteristics of patients with large unresectable HCC with ipsilateral PVTT.
FLR Hypertrophy and Surgery Rate
In the PVE + TACE group, mean sFLR increased from 29.7% before PVE to 35.9% (P =0.000) after PVE, respectively. In Group A, 19 patients (57.6%) underwent radical resection through laparotomy 18–95 days after PVE (Table 2). In one patient, because of the rupture and bleeding of the large HCC, an emergency surgery was performed 5 days after PVE and 1 day after the first TACE procedure. Liver resection was not performed because of a small FLR in 11 patients, because of pulmonary metastasis in one patient, and because of lack of consent for surgery in one patient (Figures 4, 5). In the TKIs + TACE group, no patient underwent radical resection (P =0.000).
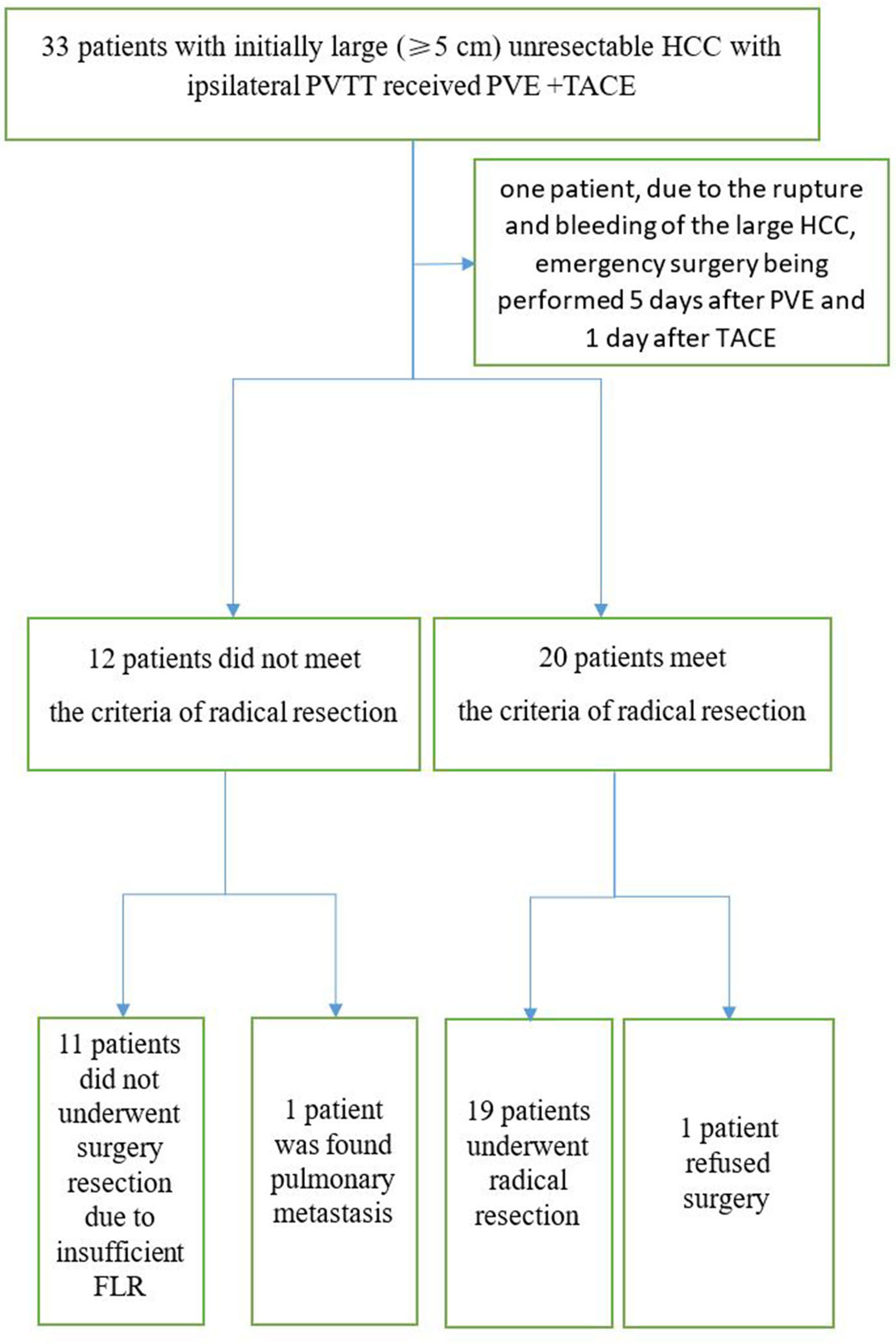
Figure 4 Consolidated Standards of Reporting Trials diagram including all 33 patients who entered the study. PVE, Portal vein emboliaztion; PVTT, Portal vein tumor thrombus; TACE, Transarterial chemoembolization.
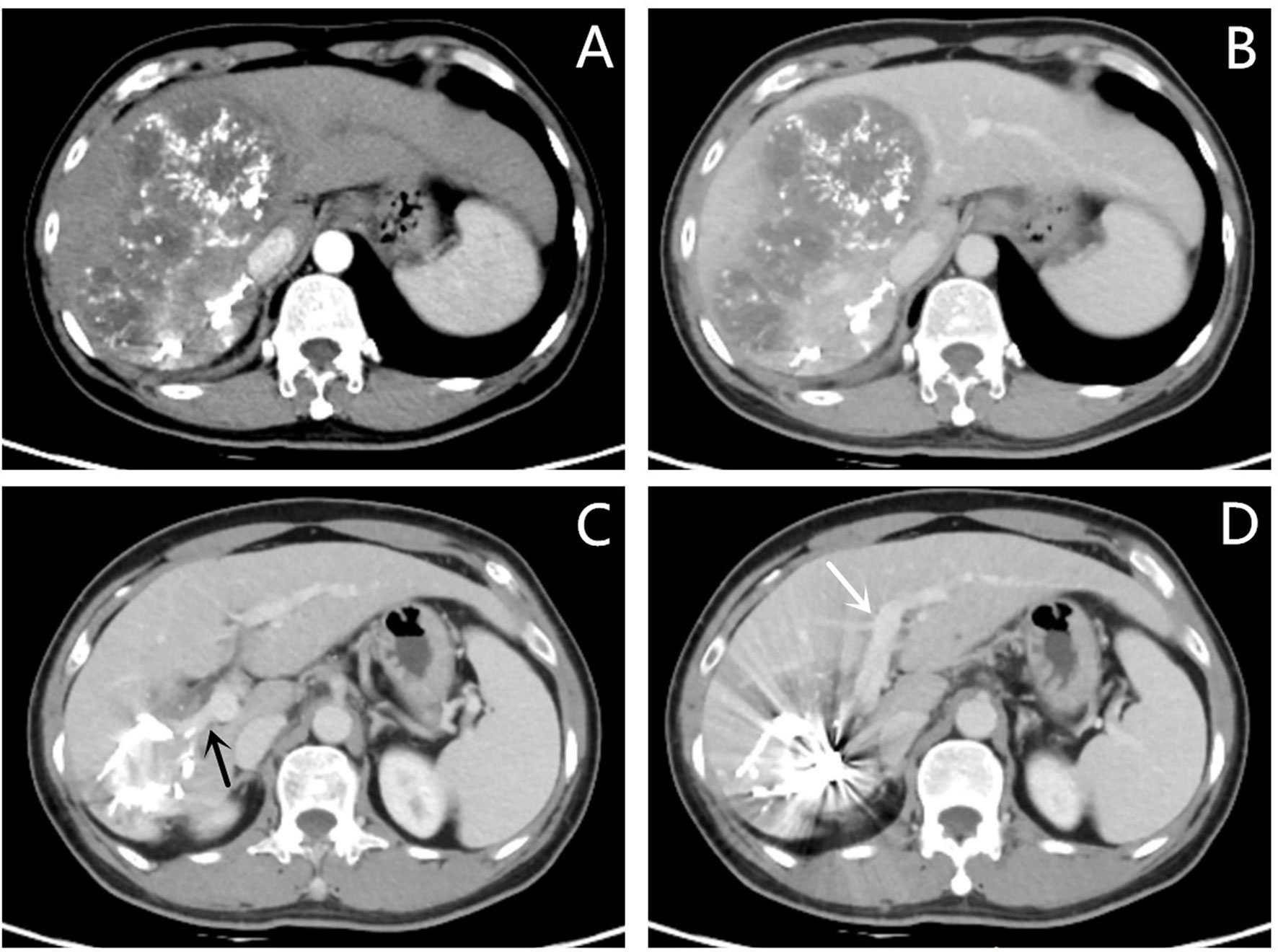
Figure 5 Images of follow-up contrast-enhanced CT of the same patient as in Figures 1, 2 after PVE and three sessions of transarterial chemoembolization. (A, B) Hepatic arterial phase (A) and portal vein phase (B) showing that the lesion is fully necrotic. (C) Images of the portal vein phase showing a clear initial part of the right portal vein (black arrow). (D) Images of the portal vein phase showing a clear left portal vein (white arrow). Compared with Figure 1, the left lobe of the liver proliferated significantly after PVE.
Number of TACE Sessions
Patients in Group A had an average of 2.3 (range, 1–6) TACE sessions and those in Group B had an average of 2.44 sessions (range, 1–13; P = 0.70).
Treatment-related Complications
PVE-related postoperative reactions and TACE-related post-chemoembolization syndrome (pyrexia, nausea, emesis, loss of appetite, and abdominal pain) occurred in nearly all patients. All symptoms are alleviated within a few days of TACE or PVE.
The most common AEs were decreased albumin (16 patients, 48.5%), elevated aspartate transaminase (14 patients, 42.4%), and decreased platelet count (13 patients, 39.4%) in the PVE + TACE group. Grade 3 adverse events included decreased platelet count (two patients, 6.1%), elevated aspartate transaminase level (one patient, 3.0%), and elevated total bilirubin level (one patient, 3.0%). Grade 4 adverse events included rupture and bleeding of the large HCC (1 patient, 3.0%).
OS Analysis
The median follow-up times in Groups A and B were 777.0 and 805.0 days, respectively (p = 0.220). More patients died in Group B than in Group A during the follow-up period [53 (82.8%) vs. 19 (57.6%); P = 0.013]. The mean and median OS were 736.5 days [95% confidence interval (CI) 507.8–965.3 days] and 425.0 days (95% CI, 96.5–753.5 days) in Group A and 424.5 days (95% CI, 319.0–530.1 days) and 344.0 days (95% CI, 251.6–436.4 days) in Group B, respectively. There was a significant difference in OS between Groups B and A (P = 0.023) (Figure 6A). Compared with TKIs + TACE, the treatment consisting of PVE + TACE prolonged OS.
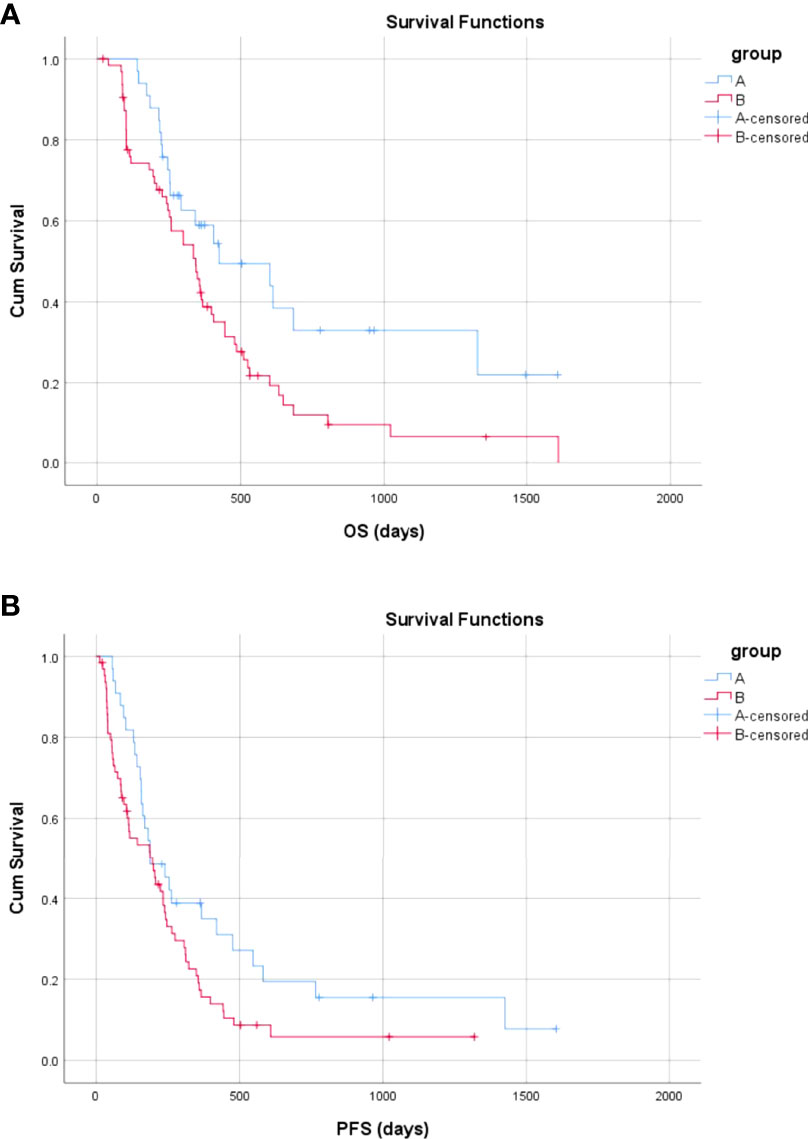
Figure 6 Overall and progression-free survival in Group A (transarterial chemoembolization with portal vein embolization) and Group B (transarterial chemoembolization with molecular targeted therapy). (A) The overall survival in Group A and Group B (B) The progression-free survival in Group A and Group B.
In Group A, 19 patients underwent radical resection 18–95 days after PVE (Group ASur), and 13 patients did not undergo radical surgery, and one patient underwent emergency surgery 1 day after the first TACE in Group A (Group ANoSur). The mean and median OS for patients in Group ASur were 881.4 days (95% CI, 571.5–1191.3 days) and 684.0 days (95% CI, 0.0–1442.6 days), respectively. There was a significant difference in OS between Group B and Group ASur (P = 0.009) (Figure 7A). Compared with TKIs + TACE, treatment consisting of PVE + TACE + sequential radical resection prolonged OS.
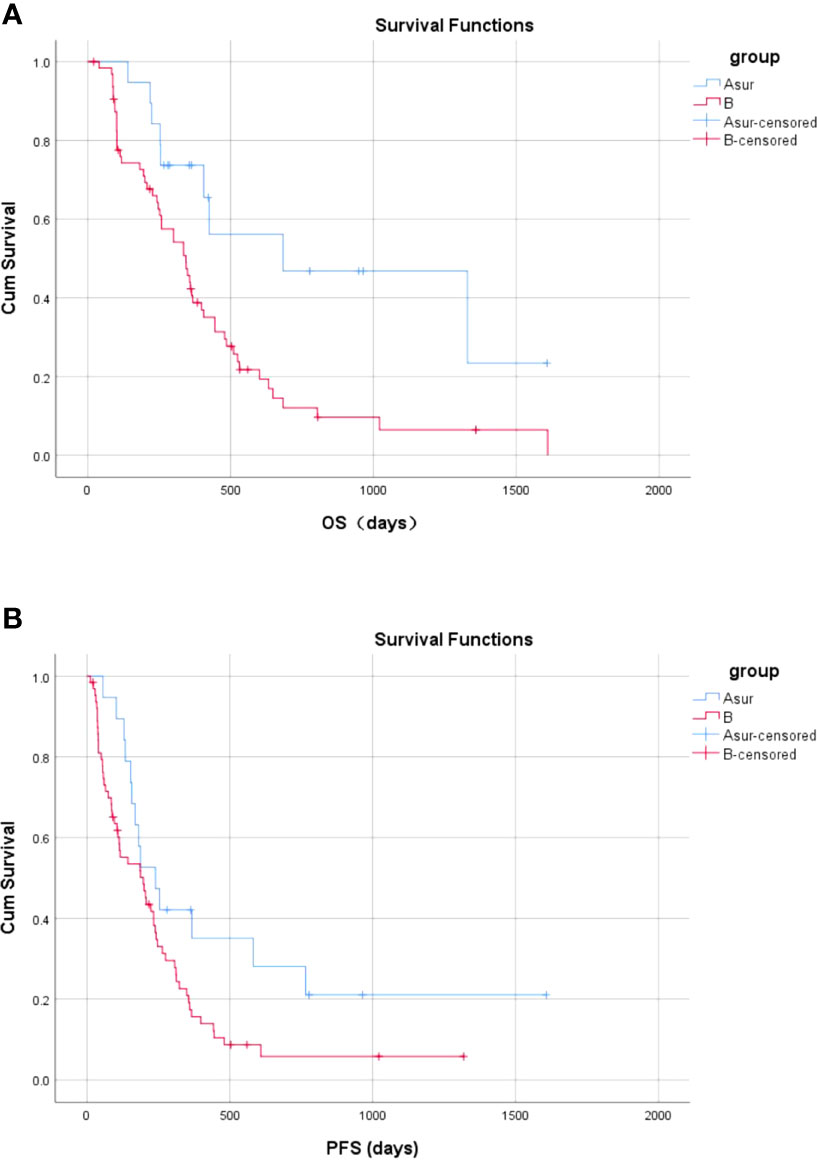
Figure 7 Overall and progression-free survival between Group ASur and Group B (A) The OS in Group ASur and Group B (B) The PFS in the no-operative Group ASur and Group B.
The mean and median OS for patients in Group ANoSur were 501.2 days (95% CI, 239.1–763.2 days) and 292.0 days (95% CI, 131.0–453.0 days), respectively. There was no significant difference in OS between Group B and Group ANoSur (P = 0.610) (Figure 8A).
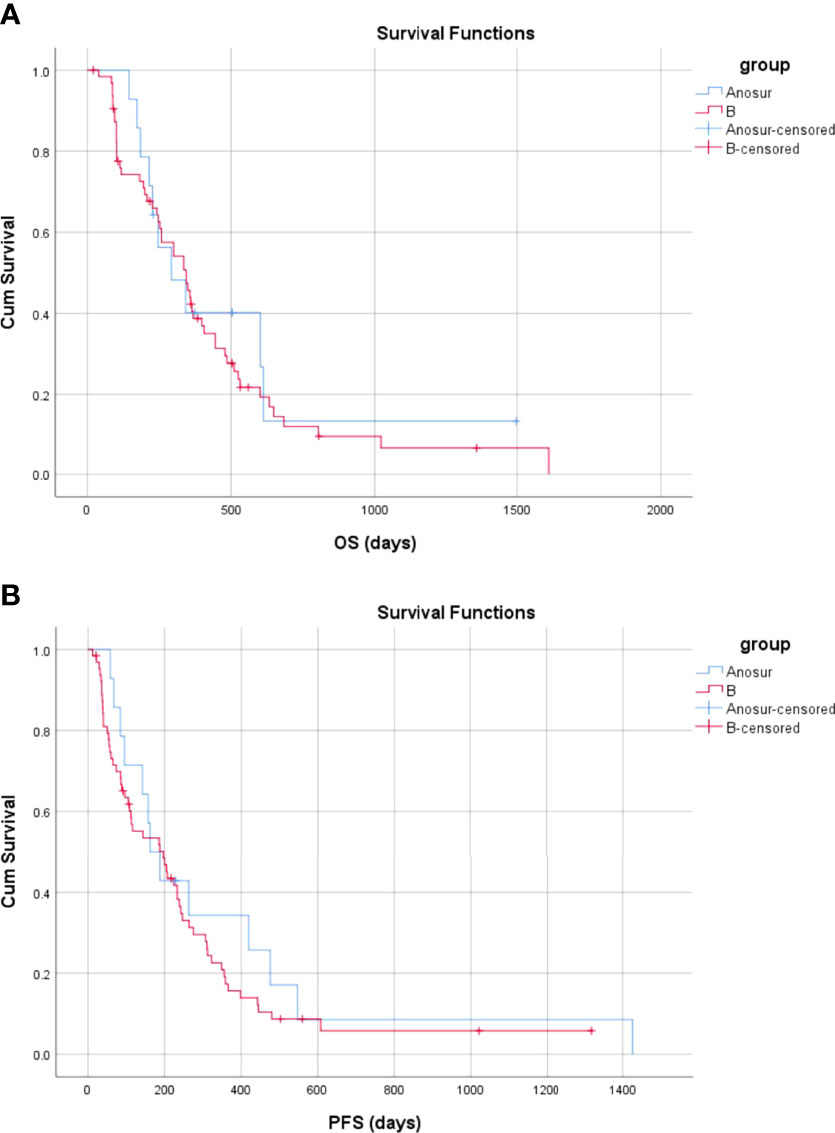
Figure 8 Overall and progression-free survival between Group ANoSur and Group B (A) The OS in Group ANoSur and Group B (B) The PFS in the no operative Group ANoSur and Group B.
PFS Analysis
The mean and median PFS were 449.6 days (95% CI, 271.8–627.3 days) and 188.0 days (95% CI, 94.8–281.2 days) in Group A and 255.2 days (95% CI, 175.9–334.5 days) and 197.0 days (95% CI, 99.9–294.1 days) in Group B, respectively. There was a significant difference in PFS between Group B and Group A (P = 0.047) (Figure 6B). Compared with TKIs + TACE, the treatment comprised of PVE + TACE prolonged PFS.
The mean and median PFS were 551.6 days (95% CI, 277.9–825.3 days) and 240.0 days (95% CI, 136.2–343.8 days), respectively, in Group ASur. There was a significant difference in PFS between Group B and Group ASur (P = 0.037) (Figure 7B). Compared with TKIs + TACE, treatment comprised of PVE + TACE + sequential radical resection prolonged PFS.
The mean and median PFS were 336.4 days (95% CI, 124.8–548.1 days) and 162.0 days (95% CI, 105.2–218.8 days) in Group ANoSur, respectively. There was no significant difference in PFS between Group B and Group ANoSur (P = 0.425) (Figure 8B).
Prognostic Factors for OS
The independent prognostic factors that contributed to OS were confirmed using the Cox regression model. Univariate analysis revealed that OS was correlated with treatment options (P = 0.025), maximum tumor diameter <10 cm (P = 0.017), and alpha-fetoprotein <400 (P = 0.015). Multivariate analysis was performed and maximum tumor diameter <10 cm [HR = 0.538 (95% CI, 0.325–0.890); P = 0.016] and treatment options (PVE + TACE) [HR = 0.582 (95% CI, 0.399–1.001); P = 0.050] were identified as independent prognostic factors for OS (Table 3).
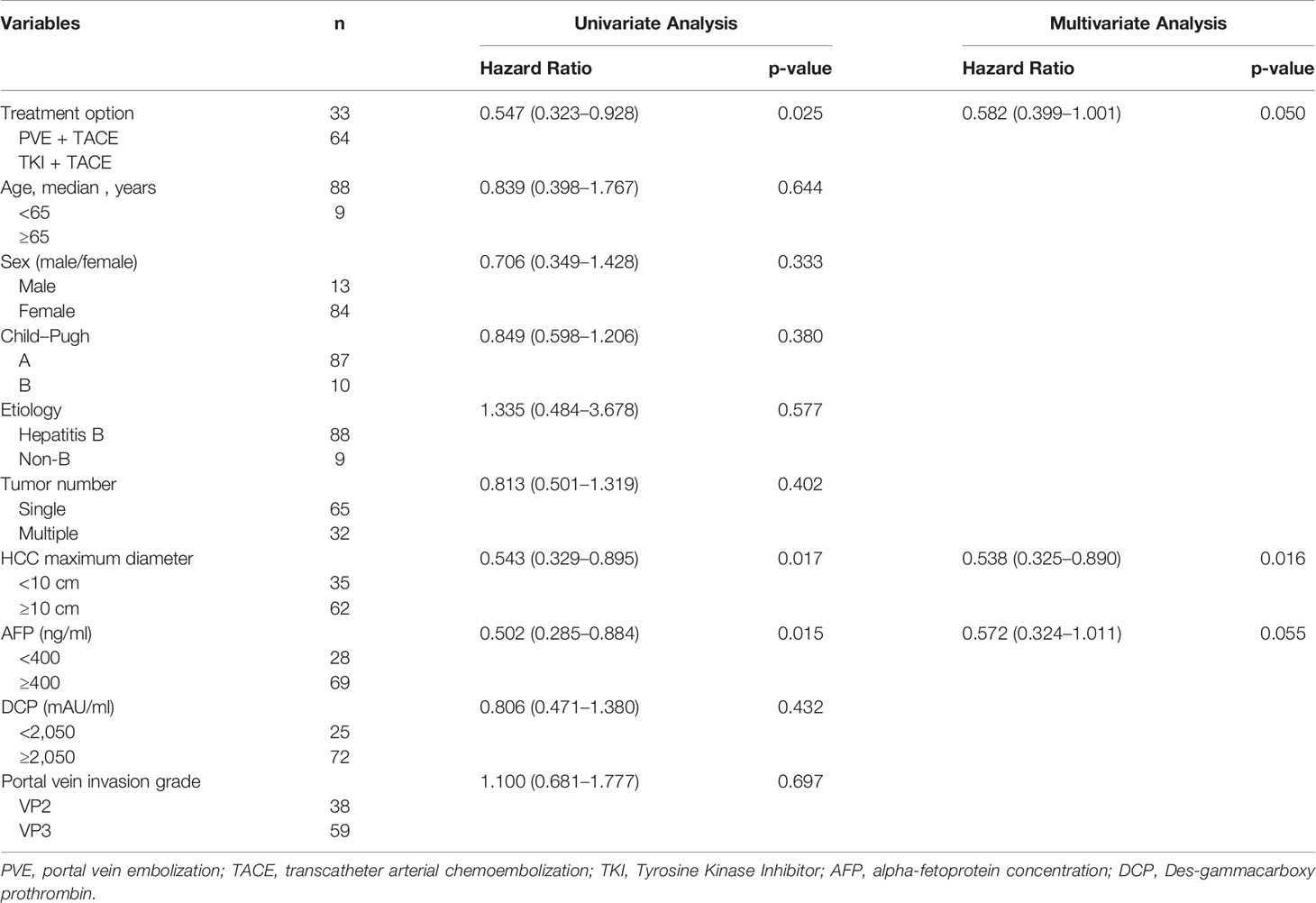
Table 3 Univariate and multivariate analysis of predictors associated with overall survival in total cohort.
Subsequent Treatment
Patients with tumor progression underwent subsequent treatment. In Group A, three patients had TKIs and programmed cell death protein-1 (PD-1) inhibitors added to their treatment regimen. In Group B, seven patients had a PD-1 inhibitor added to their primary treatment.
Discussion
This retrospective study investigated and evaluated the safety and efficacy of conversion therapy with PVE + TACE in patients with an initially large unresectable HCC with ipsilateral PVTT. Additionally, survival time was compared with the equivalent patients from three centers who were receiving TKIs + TACE.
Compared with patients who received TACE with TKIs (Group B), those treated with TACE and PVE (Group A) had a longer OS (736.5 days vs 424.5 days; P = 0.023) and PFS (449.6 days vs 255.2 days; P = 0.047). In Group A, 19 (57.6%) patients underwent radical resection after PVE, and one patient had an opportunity to undergo radical resection but refused surgery. On evaluation of the group of patients in Group A that did not undergo radical resection (Group ANoSur), we found that TACE with PVE in Group ANoSur did not prolong OS (501.2 days vs. 424.5 days; P = 0.610) and PFS (336.4 days vs. 255.2 days; P = 0.425) compared with TACE and TKIs in Group B. However, compared with TACE and TKIs in Group B, TACE with PVE in Group ASur prolonged OS (881.4 days vs. 424.5 days; P = 0.009) and PFS (551.6 days vs 255.2 days; P = 0.037). It can be speculated that radical resection after PVE + TACE in Group A can prolong OS and PFS, but PVE + TACE alone without sequential radical resection cannot prolong OS and PFS compared with TKIs + TACE by mechanically embolizing the targeted portal vein. In other words, the possible reason for this finding was that radical resection, the opportunity for which was provided by PVE through inducing FLR hypertrophy, prolonged OS and PFS. The results suggest that PVE + TACE is a feasible conversion therapy for patients with initially large (>5 cm) unresectable HCC with PVTT to achieve successful resection with a potential long survival time.
Besides surgical resection, targeted therapy (sorafenib and lenvatinib as the first-line treatments; regorafenib, apatinib, cabozantinib, and ramucirumab as the second-line therapies); TACE; radiation therapy; and liver transplantation have been recommended and practiced in HCC patients with PVTT. According to the Asia-Pacific region study, sorafenib monotherapy has only prolonged the survival time for 2–3 months (21). According to Jeong et al. (22), the real-world practical effect of sorafenib monotherapy in HCC with PVTT may be worse because of the selection bias and the median survival time was only 3.1 months. In addition, immunotherapy has developed greatly, and targeted therapy combined with immunotherapy might be a promising treatment strategy. Zhu et al. (15) reported 10 initially unresectable patients (15.9%) underwent R0 resection after TKI and anti-PD-1 antibody combinations, and one of them received PVE due to insufficient FLR. In HCC patients with PVTT, TACE alone had more survival benefit compared to supportive care, which has been confirmed by retrospective and prospective studies (23–25). However, the effect of TACE alone is still unsatisfactory. TACE combined with other therapies may gain a survival benefit and has been recommended as a new therapeutic strategy. With the aid of precision radiotherapy technology, external radiotherapy has been applied to more and more HCC patients with PVTT (26, 27). Compared with external radiotherapy, internal radiotherapy including iodine-125 seed implantation and transarterial radioembolization is more invasive. In comparison with hepatic resection, liver transplantation resolves the lesion and restores liver function simultaneously. Though the indication is expanding, in most research, PVTT is still considered as an absolute contraindication to liver transplantation (28–30).
Hepatic resection is the mainstay of curative treatment for patients with HCC (9). However, resecting HCC with macrovascular invasion is not recommended by the BCLC guidelines (5), while surgical resection is recommended by some Asian guidelines or consensus for patients with PVTT, and highly selected patients meeting these criteria acquire R0 resection (11). A large retrospective study indicated that liver resection could result in survival benefits as long as the PVTT is limited to Vp1–Vp3 (31). However, in this study, before treatment, the tumors were unresectable in all patients because of PVTT and insufficient FLR. Thus, sufficient FLR must be gained before performing curative resection. Besides PVE, ALPPS can also induce FLR hypertrophy (27). In theory, not a surgery-like ALPPS, PVE is an interventional, minimally invasive procedure, which could cause less damage and perioperative complications and is often more acceptable in advanced HCC patients. However, the safety and effectiveness of PVE and ALPPS in large HCC patients with ipsilateral PVTT should be studied by future research.
This study has some limitations. First, selection bias could not be eliminated in this study because of its retrospective character. Second, the number of samples in this study was small. Third, the type of TKI administered was not the same for all patients in Group B. Fourth, the follow-up period was relatively short. Fifth, with the advent of targeted therapy and immunotherapy, conversion therapy for HCC with ipsilateral PVTT in the experimental group excluded TKIs and anti-PD-1 antibodies. Prospective research with a large sample size is essential to verify the efficacy of conversion therapy with PVE, TACE, TKIs, and anti-PD-1 antibodies in HCC patients with ipsilateral PVTT.
Conclusively, the outcome of this study shows that conversion therapy with PVE + TACE could be a safe procedure for patients with large unresectable HCC with ipsilateral PVTT. Besides, the patients who underwent TACE + PVE had longer OS and PFS compared with TACE + TKIs.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Eastern Hepatobiliary Surgery Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
CH collected related papers and drafted the manuscript. NG and YY participated in the design of the review. SC was responsible for the supervision of the work. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by National Science Foundation of China (No.31971249).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, Morbidity, and Risk Factors in China and Its Provinces, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet (2019) 394:1145–58. doi: 10.1016/S0140-6736(19)30427-1
3. Cheung TK, Lai CL, Wong BC, Fung J, Yuen MF. Clinical Features, Biochemical Parameters, and Virological Profiles of Patients With Hepatocellular Carcinoma in Hong Kong. Aliment Pharmacol Ther (2006) 24(4):573–83. doi: 10.1111/j.1365-2036.2006.03029.x
4. Zhang ZM, Lai EC, Zhang C, Yu H, Liu Z, Wan B, et al. The Strategies for Treating Primary Hepatocellular Carcinoma With Portal Vein Tumor Thrombus. Int J Surg (2015) 20:8–16. doi: 10.1016/j.ijsu.2015.05.009
5. Yang T, Lin C, Zhai J, Shi S, Zhu M, Zhu N, et al. Surgical Resection for Advanced Hepatocellular Carcinoma According to Barcelona Clinic Liver Cancer (BCLC) Staging. J Cancer Res Clin Oncol (2012) 138(7):1121–9. doi: 10.1007/s00432-012-1188-0
6. Schöniger-Hekele M, Müller C, Kutilek M, Oesterreicher C, Ferenci P, Gangl A. Hepatocellular Carcinoma in Central Europe: Prognostic Features and Survival. Gut (2001) 48(1):103–9. doi: 10.1136/gut.48.1.103
7. Chan SL, Chong CC, Chan AW, Poon DM, Chok KS. Management of Hepatocellular Carcinoma With Portal Vein Tumor Thrombosis: Review and Update at 2016. World J Gastroenterol (2016) 22(32):7289–300. doi: 10.3748/wjg.v22.i32.7289
8. Liu PH, Huo TI, Miksad RA. Hepatocellular Carcinoma With Portal Vein Tumor Involvement: Best Management Strategies. Semin Liver Dis (2018) 38:242–51. doi: 10.1055/s-0038-1666805
9. Clinical Practice Guidelines EASL. Management of Hepatocellular Carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
10. Luo F, Li M, Ding J, Zheng S. The Progress in the Treatment of Hepatocellular Carcinoma With Portal Vein Tumor Thrombus. Front Oncol (2021) 11:635731. doi: 10.3389/fonc.2021.635731
11. Cheng S, Chen M, Cai J, Sun J, Guo R, Bi X, et al. Chinese Expert Consensus on Multidisciplinary Diagnosis and Treatment of Hepatocellular Carcinoma With Portal Vein Tumor Thrombus (2018 Edition). Liver Cancer (2020) 9:28–40. doi: 10.1159/000503685
12. Department of Medical Administration, National Health and Health Commission of the People's Republic of China. [Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2019 Edition)]. Zhonghua Gan Zang Bing Za Zhi (2020) 28:112–28. doi: 10.3760/cma.j.issn.1007-3418.2020.02.004
13. Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, et al. Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC Guidelines) 2019 Update. Hepatol Res (2019) 49:1109–13. doi: 10.1111/hepr.13411
14. Zhou H, Song T. Conversion Therapy and Maintenance Therapy for Primary Hepatocellular Carcinoma. Biosci Trends (2021) 15:155–60. doi: 10.5582/bst.2021.01091
15. Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Qu XD, et al. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma With Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody Combinations. Liver Cancer (2021) 10:320–9. doi: 10.1159/000514313
16. Wang Z, Peng Y, Hu J, Wang X, Sun H, Sun J, et al. Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy for Unresectable Hepatitis B Virus-Related Hepatocellular Carcinoma: A Single Center Study of 45 Patients. Ann Surg (2020) 271:534–41. doi: 10.1097/SLA.0000000000002942
17. Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, et al. Preoperative Portal Embolization to Increase Safety of Major Hepatectomy for Hilar Bile Duct Carcinoma: A Preliminary Report. Surgery (1990) 107:521–7.
18. Azoulay D, Castaing D, Krissat J, Smail A, Hargreaves GM, Lemoine A, et al. Percutaneous Portal Vein Embolization Increases the Feasibility and Safety of Major Liver Resection for Hepatocellular Carcinoma in Injured Liver. Ann Surg (2000) 232:665–72. doi: 10.1097/00000658-200011000-00008
19. Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, et al. Report of the 17th Nationwide Follow-Up Survey of Primary Liver Cancer in Japan. Hepatol Res (2007) 37:676–91. doi: 10.1111/j.1872-034X.2007.00119.x
20. He C, Ge N, Yang Y. Feasibility and Safety of Delayed Catheter Removal Technique in Percutaneous Trans-Hepatic Portal Vein Embolization. Technol Cancer Res Treat (2022) 21:15330338221075154. doi: 10.1177/15330338221075154
21. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region With Advanced Hepatocellular Carcinoma: A Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol (2009) 10:25–34. doi: 10.1016/S1470-2045(08)70285-7
22. Jeong SW, Jang JY, Shim KY, Lee SH, Kim SG, Cha SW, et al. Practical Effect of Sorafenib Monotherapy on Advanced Hepatocellular Carcinoma and Portal Vein Tumor Thrombosis. Gut Liver (2013) 7(6):696–703. doi: 10.5009/gnl.2013.7.6.696
23. Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, et al. Transarterial Chemoembolization can be Safely Performed in Patients With Hepatocellular Carcinoma Invading the Main Portal Vein and may Improve the Overall Survival. Radiology (2011) 258:627–34. doi: 10.1148/radiol.10101058
24. Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, et al. Transarterial Chemoembolization for Unresectable Hepatocellular Carcinoma With Portal Vein Tumor Thrombosis: A Prospective Comparative Study. Ann Surg Oncol (2011) 18:413–20. doi: 10.1245/s10434-010-1321-8
25. Niu ZJ, Ma YL, Kang P, Ou SQ, Meng ZB, Li ZK, et al. Transarterial Chemoembolization Compared With Conservative Treatment for Advanced Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: Using a New Classification. Med Oncol (2012) 29:2992–7. doi: 10.1007/s12032-011-0145-0
26. Klein J, Dawson LA. Hepatocellular Carcinoma Radiation Therapy: Review of Evidence and Future Opportunities. Int J Radiat Oncol Biol Phys (2013) 87:22–32. doi: 10.1016/j.ijrobp.2012.08.043
27. Hu Y, Qin T, Li S, Zhang T, Xue J. Efficacy and Safety of SBRT Combined With Camrelizumab and Apatinib in HCC Patients With PVTT: Study Protocol of a Randomized Controlled Trial. Front Oncol (2020) 10:1589. doi: 10.3389/fonc.2020.01589
28. Xu X, Lu D, Ling Q, Wei X, Wu J, Zhou L, et al. Liver Transplantation for Hepatocellular Carcinoma Beyond the Milan Criteria. Gut (2016) 65:1035–41. doi: 10.1136/gutjnl-2014-308513
29. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients With Cirrhosis. N Engl J Med (1996) 334:693–9. doi: 10.1056/NEJM199603143341104
30. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting Survival After Liver Transplantation in Patients With Hepatocellular Carcinoma Beyond the Milan Criteria: A Retrospective, Exploratory Analysis. Lancet Oncol (2009) 10:35–43. doi: 10.1016/S1470-2045(08)70284-5
Keywords: conversion therapy, initial unresectable, hepatocellular carcinoma (HCC), portal vein embolization (PVE), transcatheter arterial chemoembolization (TACE), tyrosine kinase inhibitors (TKIs)
Citation: He C, Ge N, Wang X, Li H, Chen S and Yang Y (2022) Conversion Therapy of Large Unresectable Hepatocellular Carcinoma With Ipsilateral Portal Vein Tumor Thrombus Using Portal Vein Embolization Plus Transcatheter Arterial Chemoembolization. Front. Oncol. 12:923566. doi: 10.3389/fonc.2022.923566
Received: 19 April 2022; Accepted: 18 May 2022;
Published: 23 June 2022.
Edited by:
Riccardo Memeo, Ospedale Generale Regionale F. Miulli, ItalyReviewed by:
Qinghe Tang, Tongji University, ChinaFeng Duan, First Affiliated Hospital of Chinese PLA General Hospital, China
Copyright © 2022 He, Ge, Wang, Li, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yefa Yang, eXlmZWhiaEAxNjMuY29t; Shiguang Chen, c2djaGVuMjA3QDE2My5jb20=
†These authors have contributed equally to this work
 Chengjian He
Chengjian He Naijian Ge1†
Naijian Ge1† Shiguang Chen
Shiguang Chen