
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 23 August 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.920047
This article is part of the Research Topic How to Select Patients with Thoracic Cancers for Immunotherapy-Chemotherapy or Immunotherapy-Angiogenesis Inhibitor Combinations? View all 24 articles
 Yajie Cheng1,2,3†
Yajie Cheng1,2,3† Bin Yang1,2,3,4†
Bin Yang1,2,3,4† Wen Ouyang1,2,3†
Wen Ouyang1,2,3† Chen Jie1,2,3
Chen Jie1,2,3 Wei Zhang1,2,3
Wei Zhang1,2,3 Gang Chen1,2,3
Gang Chen1,2,3 Junhong Zhang1,2,3
Junhong Zhang1,2,3 Jing Yu1,2,3*‡
Jing Yu1,2,3*‡ Conghua Xie1,2,3*‡
Conghua Xie1,2,3*‡Purpose: To evaluate the outcomes of immune checkpoint inhibitor (ICI)-based treatments versus classical chemotherapy for metastatic non-small cell lung cancer (NSCLC) patients who develop epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) resistance and to explore the population that may benefit from ICI-based therapy.
Materials and methods: All patients who had previously received EGFR-TKI therapy at two cancer centers in China and developed resistance to targeted therapies were included. Progression-free survival (PFS) and overall survival (OS) were utilized to evaluate the outcomes of the study cohort.
Results: A total of 132 patients were included. The median follow-up time for this cohort was 21.7 months (IQR, 14.8–28.8 months), calculated from the date of EGFR-TKI resistance. The median PFS and OS were 4.9 months (IQR, 2.8–9.2) and 13.5 months (IQR, 6.6–26.5 months), respectively. Multivariate analysis showed that ICI-based therapy could significantly improve OS when compared to the classic chemotherapy (hazard ratio [HR], 0.55; 95% CI, 0.34–0.88; P = 0.01) after adjusting for variables such as gender, age, mutation status, and brain or liver metastasis status. The combined modality of ICI plus chemotherapy could offer a long-term OS benefit in most subgroups, such as young (<65 years) patients, and those without secondary T790M mutations or absence of liver and brain metastases, and the populations with good Eastern Cooperative Oncology Group (ECOG) scores.
Conclusion: For patients presenting with EGFR-TKI resistance, ICI-based therapy could offer a more favorable survival than classical chemotherapy. The combination of ICI with chemotherapy may be the optimal modality for those with good ECOG PS scores.
Lung cancer is currently the most prevalent malignancy worldwide (1). In recent years, with the introduction of immune checkpoint inhibitors (ICIs), such as programmed cell death-1 (PD-1) and programmed cell death-ligand 1 (PD-L1) antibodies, the outcomes of metastatic non-small cell lung cancer (NSCLC) have greatly improved (2–4). However, the responses to immunotherapy seem to differ according to differences in the inherent immune microenvironment (5, 6). For example, NSCLC patients without epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genetic aberrations (EGFR−/ALK−) seem to benefit from immunotherapy, while the response to immunotherapy seems to be poor in those who harbor EGFR-sensitive mutations and ALK rearrangements (EGFR+/ALK+) (7).
The tumor immune microenvironment (TME) may undergo changes as the disease progresses (8, 9). For example, one study found that NSCLC patients who developed resistance to first-generation EGFR tyrosine kinase inhibitors (TKIs) but did not have a secondary T790M mutation might benefit from ICI monotherapy due to an increase in PD-L1 expression and tumor mutation burden (10). Despite the benefits achieved, the results of ICI monotherapy after EGFR-TKI resistance were not yet satisfactory (11, 12).
Recently, a phase II study confirmed that ICI plus chemotherapy could be a promising second-line option for NSCLC patients developing EGFR-TKI resistance but without a secondary T790M mutation (13). However, a subgroup analysis of the IMpower150 showed that the combination of chemotherapy, bevacizumab, and ICI could only improve PFS but did not achieve an OS benefit when compared to bevacizumab plus chemotherapy (14). Therefore, we conducted this investigation of ICI-based therapy versus classic chemotherapy for those that developed EGFR-TKI resistance from two cancer centers in China and to explore the optimal treatment modality.
All metastatic NSCLC patients (n = 110), either squamous or adenocarcinoma, who had previously benefited from EGFR-TKI, including first- and third-generation drugs, and have developed resistance at the Department of Radiation and Medical Oncology, Zhongnan Hospital of Wuhan University, were included in this study. They did not receive chemotherapy before or during treatment with EGFR-TKI. The diagnostic criteria of EGFR-TKI resistance were based on radiological or pathological results. In order to match the number of patients who underwent immunotherapy and chemotherapy alone, we additionally included a subset of patients (n = 22) who underwent immunotherapy after EGFR-TKI resistance occurring at the Department of Hubei Cancer Hospital, between September 2018 and July 2020.
This retrospective study was approved by the Department of Radiation and Medical Oncology, Zhongnan Hospital of Wuhan University ethics committee (2021050K). Waiver of informed consent was approved for the aggregated data.
For patients who developed resistance to first-generation EGFR-TKI and had a secondary T790M mutation, the third-generation EGFR-TKI, osimertinib, would be preferred, while patients who were resistant to first-generation EGFR-TKI but do not have secondary T790M mutations, or those who have been resistant to both first- and third-generation EGFR-TKI, would be treated with chemotherapy or chemotherapy combined with ICI. The chemotherapy regimen after EGFR-TKI resistance (first- or third generation) was pemetrexed (500 mg/m2, Q3 weeks) in combination with cisplatin (75 mg/m2, Q3 weeks) or carboplatin (AUC 5), which was changed to pemetrexed (500 mg/m2, Q3 weeks) monotherapy after 4 cycles of doublet chemotherapy (intravenously).
Treatment options for patients receiving ICI-based therapies included ICI monotherapy or a combination of ICI with chemotherapy. ICI monotherapy was administered to patients with PS score >1 or those who were intolerant to chemotherapy. The chemotherapy regimens for combined ICIs were pemetrexed (500 mg/m2, Q3 weeks) plus cisplatin (75 mg/m2, Q3 weeks) or carboplatin (AUC 5). Patients receiving ICI in combination with chemotherapy would enter ICI maintenance therapy after 4 cycles of combined treatments. The details of ICI-based therapy are shown in Supplementary Table S1.
The mutation status of EGFR in all patients was detected by the next-generation sequencing technology (NGS) based on tumor biopsy specimens. Patients with atypical EGFR mutations were defined as those who harbored concomitant mutations or uncommon EGFR mutations. Treatment response was defined according to the Response Evaluation Criteria in Solid Tumors (RECIST) v.1.1. Overall survival (OS) was calculated from the date of immunotherapy or chemotherapy to the date of death from any cause or the date of final follow-up. Progression-free survival (PFS) was defined as the period from the date of immunotherapy or chemotherapy initiation to the date of disease progression or death from any cause or final follow-up.
Patients would undergo a comprehensive review after every two cycles of therapy, including imaging evaluations and laboratory tests, such as blood count, biochemical analyses (coagulation profile, and hepatic and renal function), thyroid function, and tumor marker tests.
OS and PFS were evaluated using the Kaplan–Meier method. The log-rank statistic is approximately distributed as a chi-square test statistic with degree of freedom corresponding to the number of comparison groups minus 1. Multivariate Cox proportional hazard analysis was performed to determine the association of different covariates with OS and PFS. All analyses were carried out using SPSS Statistics, version 20.0 (IBM Corp., Armonk, NY). Statistical significance was at P ≤ 0.05. The P values were derived from a two-tailed test.
From September 2018 to July 2020, a total of 132 metastatic NSCLC patients who developed EGFR-TKI resistance were included in our study. Their median age was 57 years (interquartile range [IQR], 52–64 years). In terms of treatment modality, 54.5% of patients received ICI-based therapy compared to 45.5% of patients who received chemotherapy alone. Those who received chemotherapy alone were not subsequently treated with ICI because of the accessibility of the medication and their disease. Their median number of treatment cycles was similar, at 6 and 7 cycles, respectively. The ICI-based treatment group showed a longer duration (≥12 months) of EGFR-TKI treatment and a higher proportion of T790M mutations as compared to the chemotherapy group. Their baseline characteristics are presented in Table 1.
The median follow-up time was 21.7 months (IQR, 14.8–28.8 months) as of 11 October 2021. The median PFS of the study cohort was 4.9 months (IQR, 2.8–9.2 months), and the PFS at 1 year was 19.0% (95% confidence interval [CI], 12.6–26.3) (Supplementary Figure S1A). Univariate analysis showed that ICI-based therapy has a similar PFS in comparison to chemotherapy alone (P = 0.19, Figure 1A). Multivariate analysis demonstrated that having good Eastern Cooperative Oncology Group (ECOG) scores and absence of brain metastases contained a lower risk of progression; however, ICI-based therapy was not significantly linked to progression improvement (HR, 0.75; 95% CI, 0.49–1.13; P = 0.17) (Table 2).
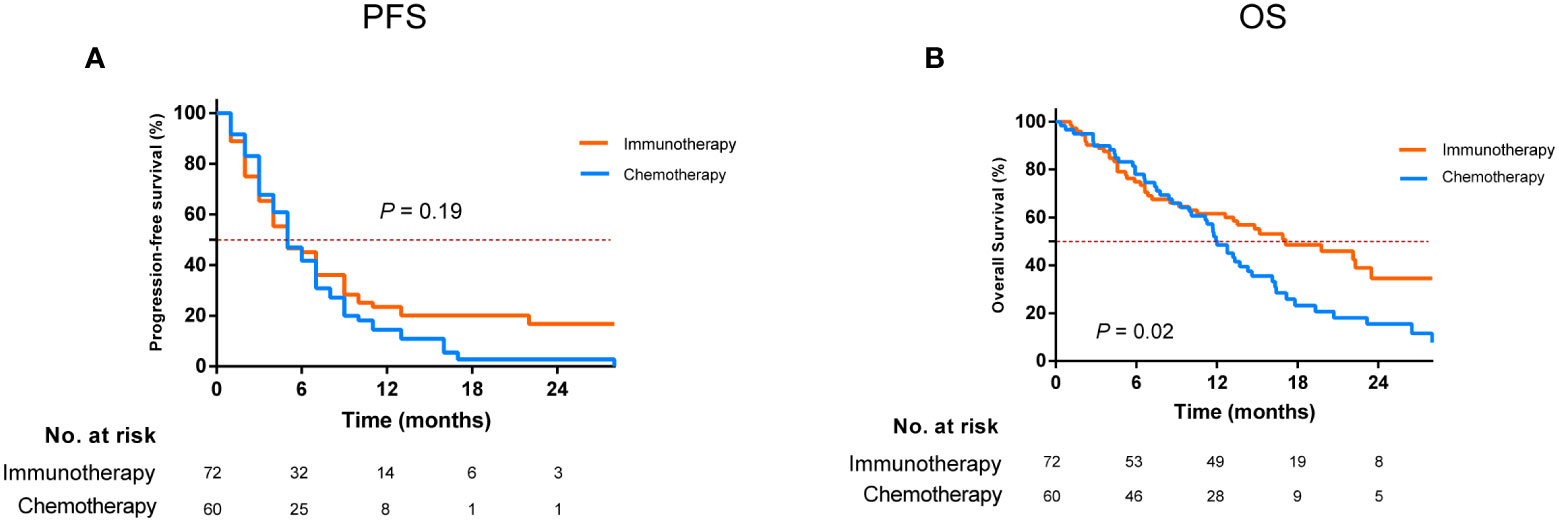
Figure 1 Comparison of progression-free survival (A) and overall survival (B) for patients who developed EGFR-TKI resistance treated with immunotherapy and chemotherapy alone. EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
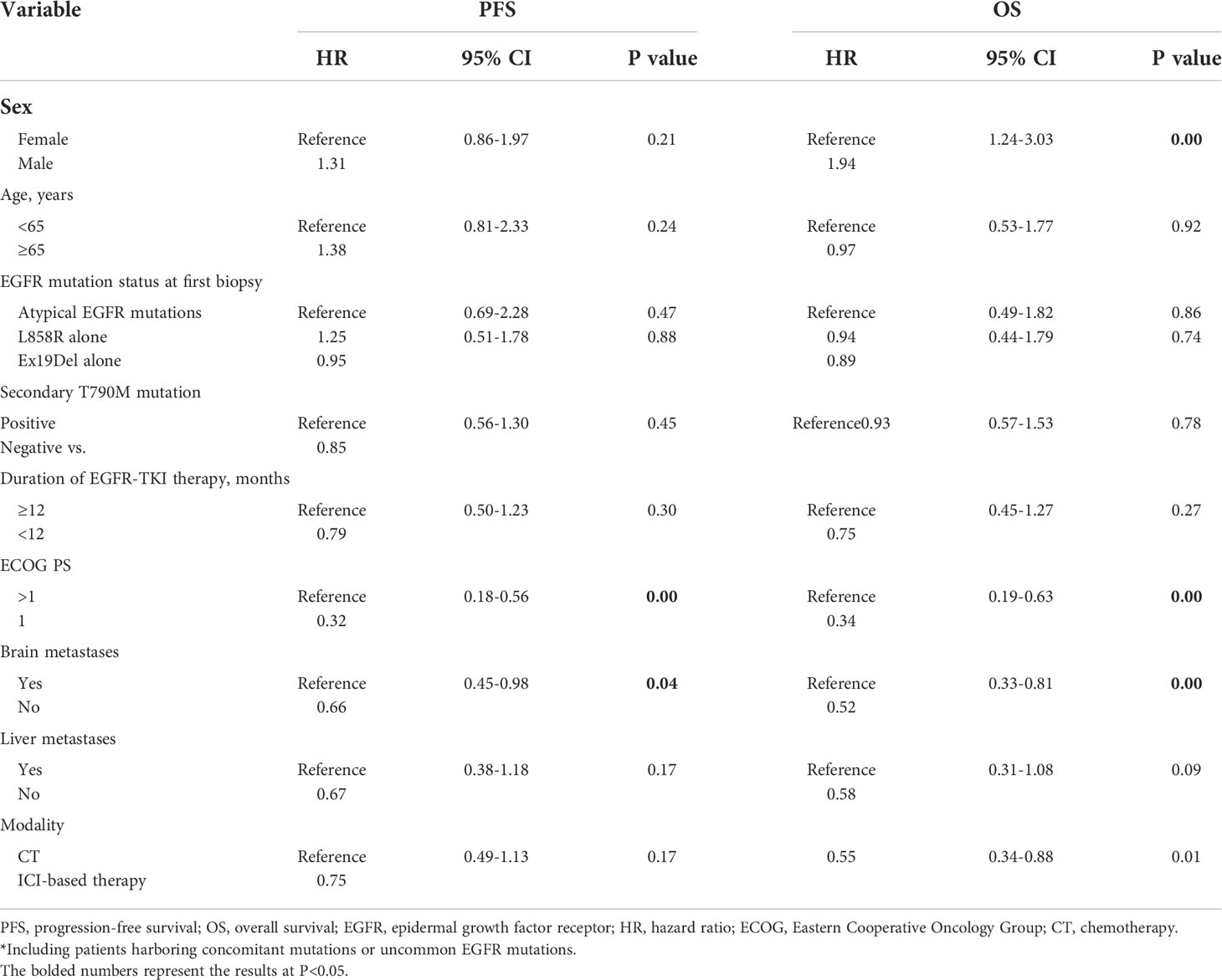
Table 2 Multivariable analysis of PFS and OS in patients who received ICI-based therapy and chemotherapy after developing EGFR-TKI resistance.
The median OS was 13.5 months (IQR, 6.6–26.5 months), with 1- and 2-year OS of 55.4% (95% CI, 46.4%–63.6%) and 25.8% (95% CI, 16.9%–35.5%), respectively (Supplementary Figure S1B). ICI-based therapy could show a significant OS advantage over chemotherapy alone, which could achieve a median OS of 17.1 and 12.0 months, respectively (P = 0.02, Figure 1B). Multivariate analysis confirmed that ICI-based therapy was an independent contributor for improving OS (HR, 0.55; 95% CI, 0.34–0.88; P = 0.01). Meanwhile, female, having a good ECOG PS scores, and without brain metastases were also independent predictors for harboring the better OS (Table 2).
To determine the optimal mode of ICI-based therapy, we then performed a comparison of different treatment subgroups. We found that for EGFR-TKI-resistant patients, ICI plus chemotherapy resulted in the maximum improvement in OS relative to chemotherapy alone, yielding a corresponding median OS of 19.7 and 12.0 months, respectively (P = 0.02, Figure 2B); however, it only slightly prolonged median PFS from 5.0 to 5.2 months (P = 0.08, Figure 2A).
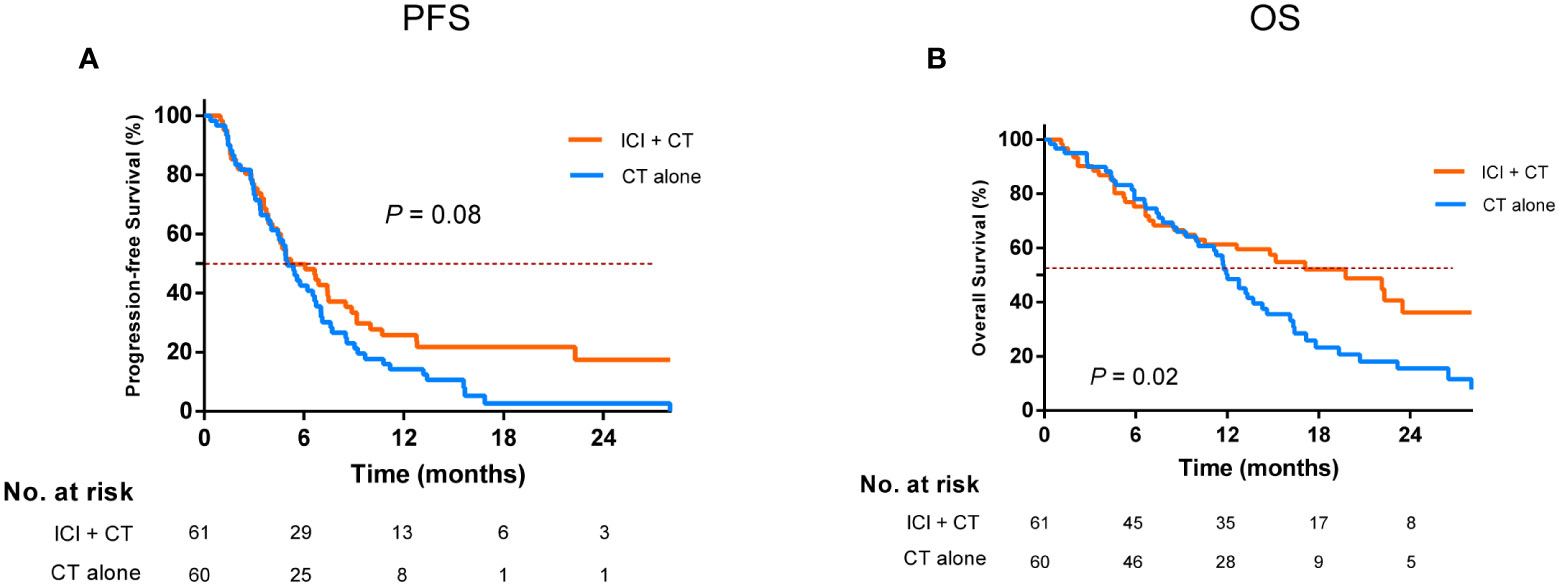
Figure 2 Comparison of progression-free survival (A) and overall survival (B) in the ICI combination chemotherapy versus the chemotherapy alone after EGFR-TKI resistance. EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; ICI, immune checkpoint inhibitor; CT, chemotherapy.
Furthermore, we concluded that patients who were younger (<65 years), have no T790M secondary mutations, or have good ECOG PS scores and those without brain or liver metastases were all the beneficiaries of the ICI-chemotherapy combination modality (Table 3).
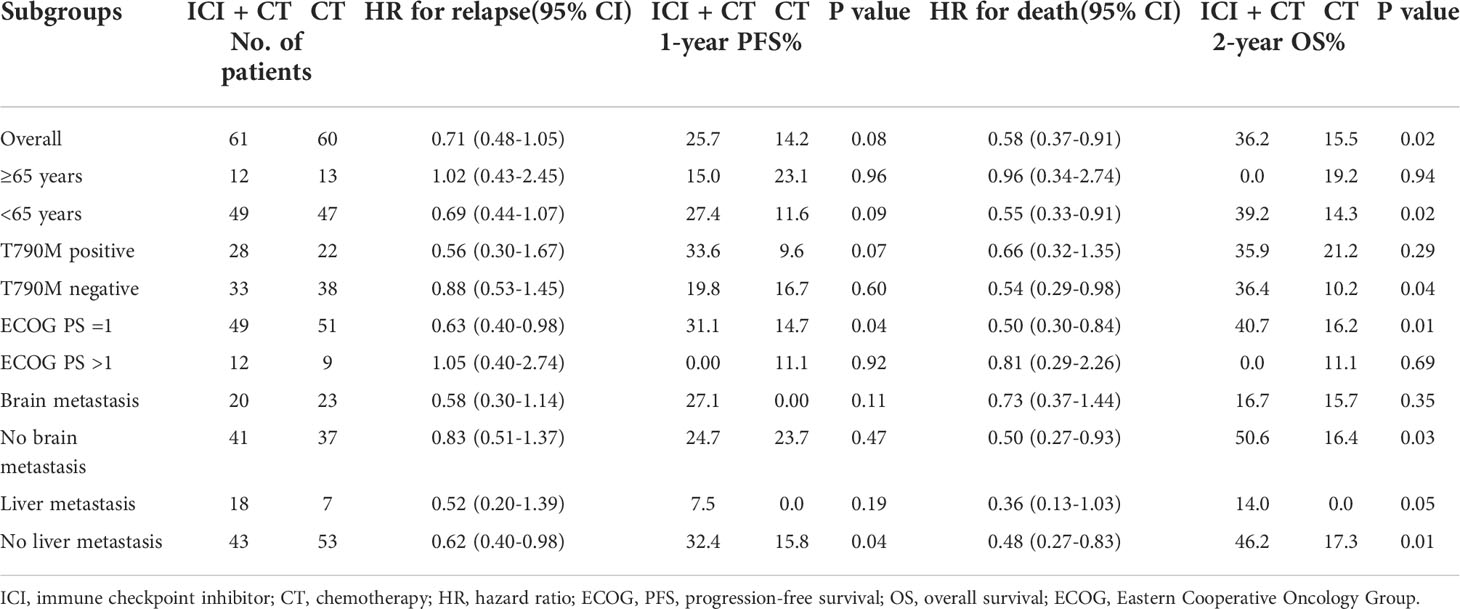
Table 3 Subgroup analysis of the outcome of patients receiving ICI in combination with chemotherapy and chemotherapy alone.
The ICI-based treatment had similar treatment-related toxicities compared to chemotherapy alone, the most common of which included neutropenia, anemia, and fatigue, with incidences of 58.3% (N = 42) versus 61.7% (N = 37), 48.6% (N = 35) versus 65.0% (N = 39), and 19.4% (N = 14) versus 25% (N = 15), respectively.
Grade 3 or higher toxicities occurred mainly in chemotherapy-containing regimens (ICI plus chemotherapy or chemotherapy alone), with neutropenia being the most common at 7.4% (N = 9), followed by thrombocytopenia at 9.1% (N = 11). For those treated with ICI monotherapy, no grade 3 or higher toxicities were observed.
A patient developed G3 dermatitis after receiving two cycles of ICI plus chemotherapy. After discontinuation and symptomatic management, the severity of the dermatitis returned to G1.
To date, ICI-based therapy is regarded as a promising second-line option for metastatic NSCLC with EGFR-TKI resistance, but the optimal modality is still under investigation. Our investigation has shown that ICI-based therapy is a superior treatment to conventional chemotherapy, and ICI combined with chemotherapy should be the recommended treatment for those with good ECOG PS scores, without secondary T790M mutations, and without initial brain metastases or liver metastases.
Previous studies have confirmed a lack of response to ICIs in metastatic NSCLC patients with EGFR mutations, and one of the potential mechanisms could be the low expression of PD-L1 or absence of infiltrating T cells in the TME (6, 7, 15). However, as tumors continue to evolve, the TME may change accordingly and, therefore, EGFR-TKI resistance might enhance the response to ICIs (8, 9, 13). As reported from the EGFR+/ALK+ cohort in the ATLANTIC study, the use of durvalumab monotherapy could provide a favorable outcome in EGFR+/ALK+ patients, with a median PFS and OS of 1.9 and 13.3 months, respectively, if PD-L1 expression is greater than 25% (16, 17). In the present study, patients receiving immunotherapy achieved PFS and OS of 4.9 and 17.1 months, respectively, which seemed to be superior to those reported from ATLANTIC, even when PD-L1 expression could not be clarified. In this cohort, more than 90% of ICI modalities were ICI combined with chemotherapy, which would be a possible reason for the better prognosis.
Previous studies have shown that the combination of cytotoxic chemotherapy agents and immunotherapy could increase the possibility of de novo antigen cross-presentation in tumor tissues (18), downregulate the expression of immunosuppressive cells (19), enhance the infiltration of effector T cells (20), and ultimately, improve the response to immunotherapy (4, 21–23). Notably, an important recent single-arm phase II study showed that in EGFR-TKI-resistant NSCLC, the regime of ICI combined with chemotherapy could result in a favorable objective remission rate (ORR, 50%), PFS (7 months), and OS (23.5 months) (13). A retrospective study has also identified the value of ICI combination chemotherapy in metastatic NSCLC after the advent of EGFR-TKI resistance (24). In our study, ICI plus chemotherapy resulted in a PFS of 5.2 months and an OS of 19.7 months, respectively. Our data and the results of that prospective study may suggest that even in EGFR-TKI-resistant populations, the combination of chemotherapy and ICI could provide a good treatment response.
Previous literature has reported that chemotherapy alone could be the best modality when resistance to EGFR-TKI occurred (25). In this study, we compared head-to-head the outcomes of ICI-based therapy and chemotherapy alone and confirmed a significant prognostic advantage of ICI-based therapy, which was mainly reflected in the OS benefit (26, 27).
The lack of sufficient tissue samples for exploratory analysis is a limitation of this study. Therefore, we were only able to test a limited number of specimens for PD-L1 status prior to ICI treatment, and the results showed no significant difference in the proportion of patients with positive expression between the two groups. Therefore, further studies are needed to confirm whether PD-L1 status could predict the superiority of later-line ICI over chemotherapy. In addition, the heterogeneity of the immunotherapy regimens is also a shortcoming of this study. A series of published studies had shown that the ICI regimens in this study had a similar efficacy in NSCLC (23, 28, 29). Therefore, these inconsistent regimens might not significantly affect our outcomes.
ICI-based therapy is a promising option for NSCLC developing EGFR-TKI resistance. For those with good ECOG PS scores, no secondary T790M mutations, and without initial brain metastases or liver metastases, ICI combined with chemotherapy should be the optimal modality.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Zhongnan Hospital of Wuhan University ethics committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
YC and JY: data collection, conception and writing of the manuscript; OW: conception and review of the manuscript; BY: data collection and review of the manuscript; CJ, WZ, and GC: review of the manuscript; CX and JZ: advice and review of the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the National Natural Science Foundation of China (grant no. 81972852) and Health Commission of Hubei Province Scientific Research Project (grant no. WJ2021F108).
We thank Dr. Yong Yang (Fujian Union Hospital) for providing advice on study design.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.920047/full#supplementary-material
NSCLC, non-small cell lung cancer; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitors; ICI, immune checkpoint inhibitor; ECOG PS, Eastern Cooperative Oncology Group performance scores.
1. Siegel RL, Miller KD HE, Fuchs A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
2. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. KEYNOTE-024 investigators. pembrolizumab versus chemotherapy for PD-L1-Positive non-Small-Cell lung cancer. N Engl J Med (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
3. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. KEYNOTE-042 investigators. pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7
4. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. KEYNOTE-189 investigators. pembrolizumab plus chemotherapy in metastatic non-Small-Cell lung cancer. N Engl J Med (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
5. Takashima Y, Sakakibara-Konishi J, Hatanaka Y, Hatanaka KC, Ohhara Y, Oizumi S, et al. Clinicopathologic features and immune microenvironment of non-small-cell lung cancer with primary resistance to epidermal growth factor receptor tyrosine kinase inhibitors. Clin Lung Cancer (2018) 19:352–9. doi: 10.1016/j.cllc.2018.02.004
6. Bylicki O, Paleiron N, Margery J, Guisier F, Vergnenegre A, Robinet G, et al. Targeting the PD-1/PD-L1 immune checkpoint in EGFR-mutated or ALK-translocated non-small-cell lung cancer. Target Oncol (2017) 12:563–9. doi: 10.1007/s11523-017-0510-9
7. Liu SY, Dong ZY, Wu SP, Xie Z, Yan LX, Li YF, et al. Clinical relevance of PD-L1 expression and CD8+ T cells infiltration in patients with EGFR-mutated and ALK-rearranged lung cancer. Lung Cancer (2018) 125:86–92. doi: 10.1016/j.lungcan.2018.09.010
8. Isomoto K, Haratani K, Hayashi H, Shimizu S, Tomida S, Niwa T, et al. Impact of EGFR-TKI treatment on the tumor immune microenvironment in EGFR mutation-positive non-small cell lung cancer. Clin Cancer Res (2020) 26:2037–46. doi: 10.1158/1078-0432.CCR-19-2027
9. Fang Y, Wang Y, Zeng D, Zhi S, Shu T, Huang N, et al. Comprehensive analyses reveal TKI-induced remodeling of the tumor immune microenvironment in EGFR/ALK-positive non-small-cell lung cancer. Oncoimmunology (2021) 10:1951019. doi: 10.1080/2162402X.2021
10. Haratani K, Hayashi H, Tanaka T, Kaneda H, Togashi Y, Sakai K, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol (2017) 28:1532–9. doi: 10.1093/annonc/mdx183
11. Qiao M, Jiang T, Liu X, Mao S, Zhou F, Li X, et al. Immune checkpoint inhibitors in EGFR-mutated NSCLC: Dusk or dawn? J Thorac Oncol (2021) 16:1267–88. doi: 10.1016/j.jtho.2021.04.003
12. Ng TL, Liu Y, Dimou A, Patil T, Aisner DL, Dong Z, et al. Predictive value of oncogenic driver subtype, programmed death-1 ligand (PD-L1) score, and smoking status on the efficacy of PD-1/PD-L1 inhibitors in patients with oncogene-driven non-small cell lung cancer. Cancer (2019) 125:1038–49. doi: 10.1002/cncr.31871
13. Jiang T, Wang P, Zhang J, Zhao Y, Zhou J, Fan Y, et al. Toripalimab plus chemotherapy as second-line treatment in previously EGFR-TKI treated patients with EGFR-mutant-advanced NSCLC: A multicenter phase-II trial. Signal Transduct Target Ther (2021) 15:355. doi: 10.1038/s41392-021-00751-9
14. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med (2019) 7:387–401. doi: 10.1016/S2213-2600(19)30084-0
15. Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: A retrospective analysis. Clin Cancer Res (2016) 15:22:4585–93. doi: 10.1158/1078-0432.CCR-15-3101
16. Garassino MC, Cho BC, Kim JH, Mazières J, Vansteenkiste J, Lena H, et al. ATLANTIC investigators. durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol (2018) 19:521–36. doi: 10.1016/S1470-2045(18)30144-X
17. Garassino MC, Cho BC, Kim JH, Mazières J, Vansteenkiste J, Lena H, et al. Final overall survival and safety update for durvalumab in third- or later-line advanced NSCLC: The phase II ATLANTIC study. Lung Cancer (2020) 147:137–42. doi: 10.1016/j.lungcan.2020.06.032
18. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ (2014) 21:15–25. doi: 10.1038/cdd.2013.67
19. Wang Z, Till B, Gao Q. Chemotherapeutic agent-mediated elimination of myeloid-derived suppressor cells. Oncoimmunology (2017) 6:e1331807. doi: 10.1080/2162402X.2017.1331807
20. Gameiro SR, Caballero JA, Higgins JP, Apelian D, Hodge JW. Exploitation of differential homeostatic proliferation of T-cell subsets following chemotherapy to enhance the efficacy of vaccine-mediated antitumor responses. Cancer Immunol Immunother (2011) 60:1227–42. doi: 10.1007/s00262-011-1020-8
21. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. KEYNOTE-407 investigators. pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med (2018) 379:2040–51. doi: 10.1056/NEJMoa1810865
22. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. IMpower150 study group. atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med (2018) 378:2288–301. doi: 10.1056/NEJMoa1716948
23. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): A randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med (2021) 9:305–14. doi: 10.1016/S2213-2600(20)30365-9
24. Tian T, Yu M, Li J, Jiang M, Ma D, Tang S, et al. Front-line ICI-based combination therapy post-TKI resistance may improve survival in NSCLC patients with EGFR mutation. Front Oncol (2021) 11:739090. doi: 10.3389/fonc.2021.739090
25. Wu JY, Shih JY, Yang CH, Chen KY, Ho CC, Yu CJ, et al. Second-line treatments after first-line gefitinib therapy in advanced non-small cell lung cancer. Int J Cancer (2010) 1:126:247–55. doi: 10.1002/ijc.24657
26. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol (2019) 37:537–46. doi: 10.1200/JCO.18.00149
27. Mouritzen MT, Carus A, Ladekarl M, Meldgaard P, Nielsen AWM, Livbjerg A, et al. Nationwide survival benefit after implementation of first-line immunotherapy for patients with advanced NSCLC-real world efficacy. Cancers (Basel) (2021) 28:13:4846. doi: 10.3390/cancers13194846
28. Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC: a randomized, double-blind, phase 3 study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol (2020) 15:1636–46. doi: 10.1016/j.jtho.2020.07.014
Keywords: EGFR-TKI resistance, EGFR-sensitive mutations, combined therapy, metastatic NSCLC, immunotherapy
Citation: Cheng Y, Yang B, Ouyang W, Jie C, Zhang W, Chen G, Zhang J, Yu J and Xie C (2022) Is ICI-based therapy better than chemotherapy for metastatic NSCLC patients who develop EGFR-TKI resistance? A real-world investigation. Front. Oncol. 12:920047. doi: 10.3389/fonc.2022.920047
Received: 14 April 2022; Accepted: 02 August 2022;
Published: 23 August 2022.
Edited by:
Herbert Yu, University of Hawaii, United StatesReviewed by:
Jinyi Lang, Sichuan Cancer Hospital, ChinaCopyright © 2022 Cheng, Yang, Ouyang, Jie, Zhang, Chen, Zhang, Yu and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Conghua Xie, Y2h4aWVfNjVAd2h1LmVkdS5jbg==; Jing Yu, eXVqaW5ncnRAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.