- 1Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 2Department of Pathology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 3Department of Oncology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 4Department of Research and Development, Foundation Medicine, Inc., Cambridge, MA, United States
- 5Institute of Clinical Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 6Department of Biochemistry and Molecular Biology, College of Medicine, National Cheng Kung University, Tainan, Taiwan
Telisotuzumab vedotin is a MET-targeting antibody–drug conjugate that has demonstrated a good treatment response in patients with EGFR wild-type MET-overexpressing non-squamous non-small cell lung cancer. However, patients have been reported to acquire resistance to this drug, and the subsequent therapy has not been standardized. Here, we present a case of a 56-year-old woman diagnosed with KIF5B-MET fusion-positive non-small cell lung cancer who had a durable response to capmatinib after acquired resistance to telisotuzumab vedotin.
Introduction
Telisotuzumab vedotin, previously named ABBV-399, is an antibody-drug conjugate, which comprises a human MET-targeting antibody, ABT-700, and a cytotoxic microtubule inhibitor, monomethyl auristatin E, through a valine-citrulline linker (1). Preliminary results from a phase 2 trial demonstrated that telisotuzumab vedotin yielded an objective response rate of 53.8% in patients with epidermal growth factor receptor (EGFR) wild-type MET-overexpressing non-squamous non-small cell lung cancer (NSCLC) (2). However, the subsequent treatment strategy after acquiring resistance to telisotuzumab vedotin remains under investigation. Here, we present the case of a patient with KIF5B-MET fusion-positive advanced NSCLC who exhibited a durable response to capmatinib after acquiring resistance to telisotuzumab vedotin. To the best of our knowledge, this is the first case report to describe the clinical benefit of capmatinib in patients with NSCLC with MET fusion.
Case presentation
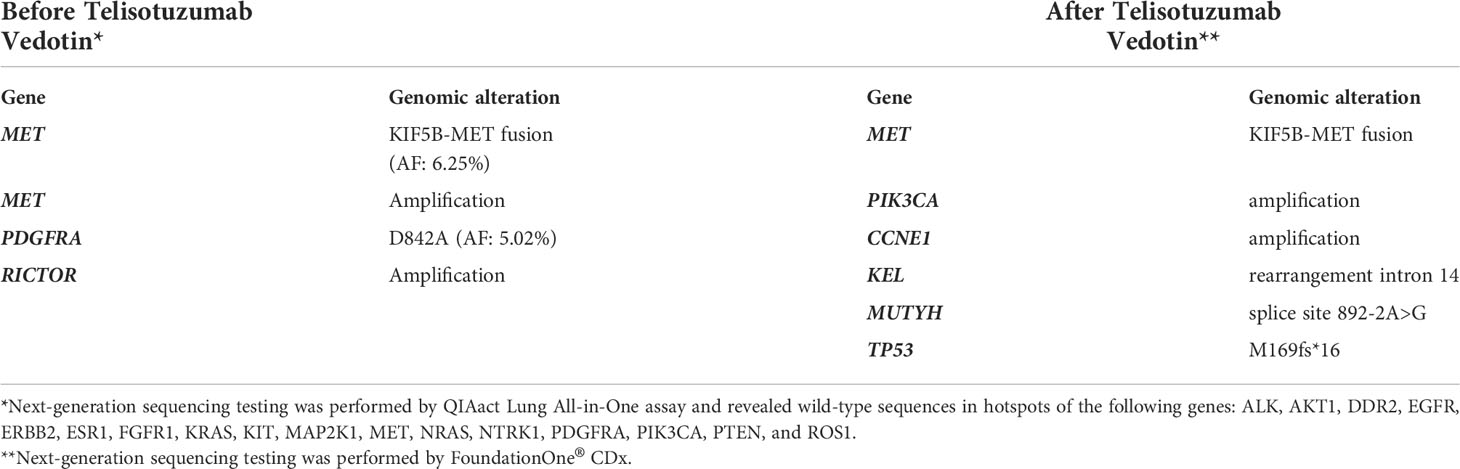
In October 2019, a 56-year-old woman was diagnosed with poorly differentiated stage IIIC pulmonary adenocarcinoma. The tumor involved the right upper lobe and mediastinal lymph nodes. Polymerase chain reaction analysis revealed wild-type EGFR, and the immunohistochemical staining for ALK and ROS1 was negative. Concurrent chemoradiotherapy followed by 12-month durvalumab consolidation was administered, and the tumor showed significant shrinkage. Eleven months after completion of durvalumab therapy, the patient experienced disease progression with enlargement of the left axillary lymph node. Sonography-guided biopsy revealed an adenocarcinoma. Next-generation sequencing (NGS) by the QIAact Lung All-in-One assay revealed KIF5B-MET fusion (Figure 1A), PDGFRA mutation, and RICTOR amplification (Table 1). She was then enrolled in a phase 2 clinical trial with telisotuzumab vedotin, and subsequent chest computed tomography revealed partial regression of the axillary lymph node. During the administration of telisotuzumab vedotin, the patient experienced grade 2 blurred vision and grade 1 pneumonitis.
Figure 1 Next-generation sequencing of tissue before and after Telisotuzumab Vedotin. (A) The RNA-based NGS by QIAact Lung All-in-One assay revealed a KIF5B-MET (K24;M15) fusion. (B) The DNA-based NGS by FoundationOne® CDx after Telisotuzumab Vedotin still revealed KIF5B-MET fusion. The data was provided by the Department of Research and Development, Foundation Medicine Inc. (C) The diagram of KIF5B-MET fusion. NGS, next-generation sequencing.
However, 8 months later, the patient experienced disease progression again with new-onset left chest wall metastasis. Re-biopsy showed adenocarcinoma, and NGS using FoundationOne® CDx revealed KIF5B-MET fusion without other oncogenic driver mutations (Figure 1B and Table 1), which implies a potential response to MET tyrosine kinase inhibitor (TKI). Thus, capmatinib was administered, and further imaging showed a dramatic response after 3 months of therapy. The therapeutic response was determined based on the radiographic evidence according to Response Evaluation Criteria in Solid Tumors version 1.1 (3). The patient has been on capmatinib for more than nine months, and no significant adverse events have developed. The treatment course is summarized in Figure 2.
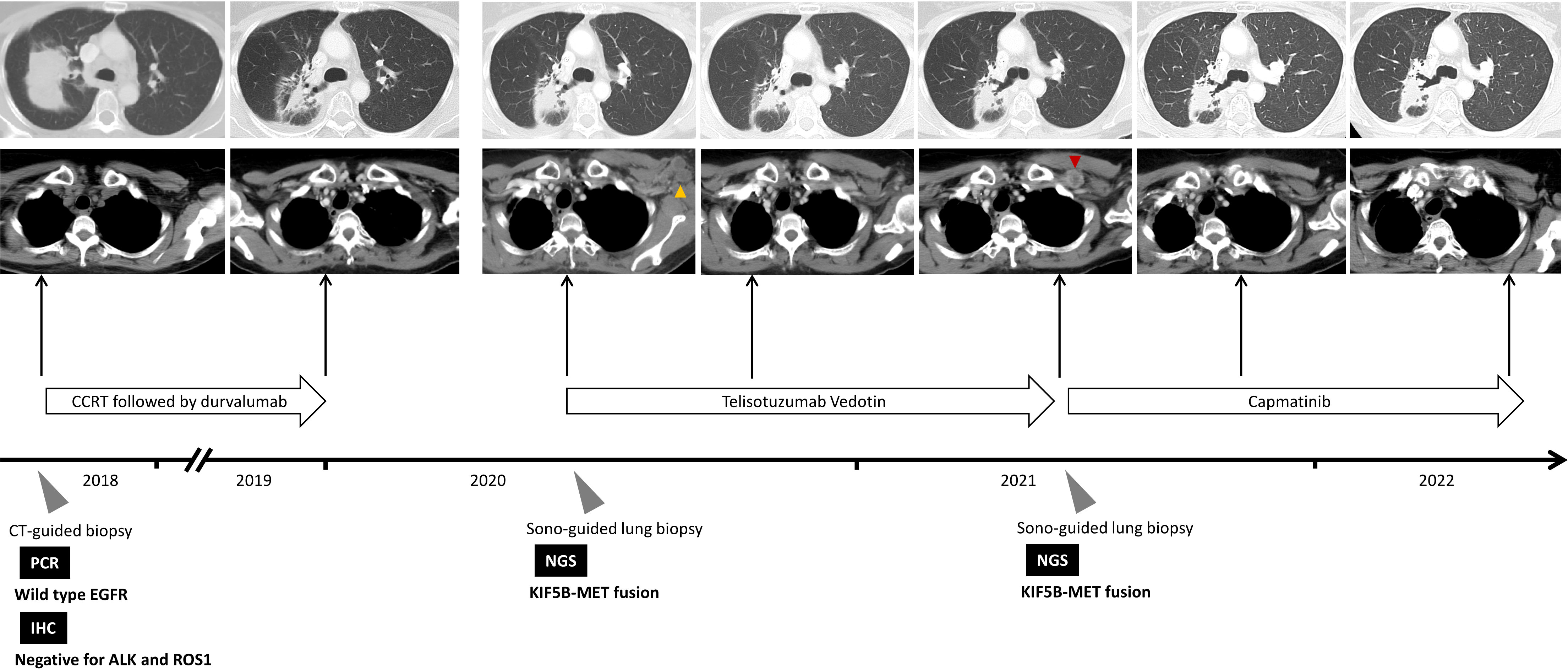
Figure 2 Summary of treatment courses mentioned in this case report. The yellow arrowhead indicate the target lesion at disease progression after consolidation therapy with durvalumab, whereas the red arrowhead indicates the target lesion at disease progression after the use of Telisotuzumab Vedotin. ALK, anaplastic lymphoma kinase; CCRT, concurrent chemoradiotherapy; CT, computed tomography; EGFR, epidermal growth factor receptor; IHC, immunohistochemical; NGS, next-generation sequencing; PCR, polymerase chain reaction; ROS1, ROS proto-Oncogene 1.
Discussion
MET fusion is a rare oncogenic driver mutation in NSCLC, which comprises only 0.5% of the total number of patients with lung cancer (4, 5). This may be underestimated if the genomic test is carried out using DNA-based NGS (6). Several fusion of MET gene partners have been described in lung adenocarcinomas, including KIF5B, HLA-DRB1, UBE2H, CD47, ATXN7L1, SPECC1L, and CAV1 (7, 8). Similar to the MET exon 14 skipping mutation, MET fusion induces ligand-independent activation of downstream signaling pathways, resulting in cellular proliferation, survival, migration, and angiogenesis (6). In addition, depending on the breakpoint of each fusion gene, MET fusion might also result in overexpression of MET protein on the cell surface. In a study conducted by Gow et al., there were two NSCLC cases with the KIF5B-MET rearrangement, which is a fusion between exons 1-24 of KIF5B and exons 15-21 of MET. The loss of exon 14 in MET results in a lack of the juxtamembrane domain of the MET protein, leading to its overexpression during immunohistochemical staining, which is secondary to failure of ubiquitin-dependent protein degradation (4). Our patient also harbored a KIF5B-MET rearrangement with a fusion between exons 1-24 of KIF5B and exons 15-21 of MET (Figure 1C), which is associated with MET protein overexpression and a potentially higher response rate to telisotuzumab vedotin (2). In addition, the KIF5B-MET rearrangement also contain the exon 1-15 of the KIF5B, which preserves the kinesin motor and coiled-coil domains that mediate homodimerization and subsequent activation of MET signaling pathway (9). The KIF5B is also an active promoter, which had been reported to activate and enhance the downstream oncogenic pathway of ALK and RET (10, 11).
The therapeutic strategy after acquiring resistance to telisotuzumab vedotin remains to be standardized. In our case report, NGS of the re-biopsied tumor revealed KIF5B-MET fusion, and no other oncogenic driver mutation was discovered, which implies that the tumor might be responsive to targeted therapy. In a in vivo study, the lung cancer xenograft model of KIF5B-MET fusion exhibited a good treatment response to crizotinib, a type Ia MET-TKI (4). Several case reports also demonstrated a good treatment response to crizotinib among NSCLC patients with de noo MET fusion (5, 8, 12–15). In addition, MET fusion could also be a resistance mechanism in patients with EGFR-mutant NSCLC who experience disease progression after treatment with EGFR-TKI. The combination of EGFR-TKIs and MET-TKIs can provide clinical benefits (5, 16, 17). Recently, targeted therapies with type Ib MET-TKIs, including tepotinib and capmatinib, have demonstrated their effectiveness and have been approved to treat patients with MET exon 14 skipping mutation-positive NSCLC (18). Capmatinib has a higher potency for MET protein binding based on an in vitro study (19); however, there are no reports on capmatinib treatment in patients with MET fusion. In the present case report, our patient experienced significant tumor shrinkage after receiving capmatinib, which implies that capmatinib could be a potential salvage therapy for patients with NSCLC with MET fusion and acquired resistance to telisotuzumab vedotin.
In summary, our case report highlights that patients with MET fusion could potentially respond to capmatinib treatment. More importantly, it could be used as salvage therapy for patients with acquired resistance to telisotuzumab vedotin. Further prospective clinical trials are warranted to validate these results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was reviewed and approved by The Review Board and Ethics Committee of National Cheng Kung University Hospital. The patient provided their written informed consent to participate in this study.
Author contributions
C-YL, S-HW, and P-LS had full access to data in this case report and takes responsibility for the integrity and accuracy of data analysis. Y-LC and C-LH contributed to the genomic data analysis. C-TL, S-YW, C-LH, DP, P-LS, and C-CL contributed to the scientific review and final approval of this manuscript. All authors contributed to the article and approved the submitted version.
Funding
The present study was funded by grant no. MOST 110-2314-B-006-102, MOST 111-2314-B-006 -092-MY3 from the Ministry of Science and Technology, Taiwan, and grant no. NCKUH-11102019 from National Cheng Kung University Hospital, Taiwan.
Acknowledgments
The patient involved in this case report gave his informed consent authorizing use and disclosure of his health information. We also thank the Molecular Medicine Core Laboratory, Clinical Medicine Research Center, National Cheng Kung University Hospital for technical support, experimental design, and data analysis with the GeneReader NGS system.
Conflict of interest
DP is employed by Foundation Medicine Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang J, Anderson MG, Oleksijew A, Vaidya KS, Boghaert ER, Tucker L, et al. ABBV-399, a c-met antibody–drug conjugate that targets both MET–amplified and c-Met–overexpressing tumors, irrespective of MET pathway dependence. Cancer Res (2017) 23(4):992–1000. doi: 10.1158/1078-0432.CCR-16-1568
2. Camidge DR, Moiseenko F, Cicin I, Horinouchi H, Filippova E, Bar J, et al. OA15. 04 telisotuzumab vedotin (teliso-v) monotherapy in patients with previously treated c-met+ advanced non-small cell lung cancer. J Thorac Oncol (2021) 16(10):S875. doi: 10.1016/j.jtho.2021.08.085
3. Tirkes T, Hollar MA, Tann M, Kohli MD, Akisik F, Sandrasegaran K. Response criteria in oncologic imaging: review of traditional and new criteria. Radiographics (2013) 33(5):1323–41. doi: 10.1148/rg.335125214
4. Gow CH, Liu YN, Li HY, Hsieh MS, Chang SH, Luo SC, et al. Oncogenic function of a KIF5B-MET fusion variant in non-small cell lung cancer. Neoplasia (2018) 20(8):838–47. doi: 10.1016/j.neo.2018.06.007
5. Plenker D, Bertrand M, de Langen AJ, Riedel R, Lorenz C, Scheel AH, et al. Structural alterations of MET trigger response to MET kinase inhibition in lung adenocarcinoma patients. Cancer Res (2018) 24(6):1337–43. doi: 10.1158/1078-0432.CCR-17-3001
6. Coleman N, Harbery A, Heuss S, Vivanco I, Popat S. Targeting un-MET needs in advanced non-small cell lung cancer. Lung Cancer (2022) 164:56–68. doi: 10.1016/j.lungcan.2021.12.016
7. Zhuo M, Liang Z, Yi Y, Wu N, Yang X, Zhong J, et al. Analysis of MET kinase domain rearrangement in NSCLC. Lung Cancer (2020) 145:140–3. doi: 10.1016/j.lungcan.2020.04.040
8. Zhu YC, Wang WX, Xu CW, Zhang QX, Du KQ, Chen G, et al. Identification of a novel crizotinib-sensitive MET–ATXN7L1 gene fusion variant in lung adenocarcinoma by next generation sequencing. Ann Oncol (2018) 29(12):2392–3. doi: 10.1093/annonc/mdy455
9. Takeuchi K, Choi YL, Togashi Y, Soda M, Hatano S, Inamura K, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res (2009) 15(9):3143–9. doi: 10.1158/1078-0432.CCR-08-3248
10. Qian Y, Chai S, Liang Z, Wang Y, Zhou Y, Xu X, et al. KIF5B-RET fusion kinase promotes cell growth by multilevel activation of STAT3 in lung cancer. Mol Cancer (2014) 13:176. doi: 10.1186/1476-4598-13-176
11. Wong DW, Leung EL, Wong SK, Tin VP, Sihoe AD, Cheng L, et al. A novel KIF5B-ALK variant in nonsmall cell lung cancer. Cancer (2011) 117(12):2709–18. doi: 10.1002/cncr.25843
12. Kunte S, Stevenson J. A case of HLA-DRB1-MET rearranged lung adenocarcinoma with rapid response to crizotinib. Clin Lung Cancer (2021) 22(3):e298–300. doi: 10.1016/j.cllc.2020.05.005
13. Liang H, Zhou D, Dai L, Zhang M, Gao Z, Mu X. A novel c-Mesenchymal-Epithelial transition factor intergenic fusion response to crizotinib in a Chinese patient with lung adenocarcinoma: A case report. Front Oncol (2021) 11:727662. doi: 10.3389/fonc.2021.727662
14. Cho JH, Ku BM, Sun JM, Lee SH, Ahn JS, Park K, et al. KIF5B-MET gene rearrangement with robust antitumor activity in response to crizotinib in lung adenocarcinoma. J Thorac Oncol (2018) 13(3):e29–31. doi: 10.1016/j.jtho.2017.10.014
15. Davies KD, Ng TL, Estrada-Bernal A, Le AT, Ennever PR, Camidge DR, et al. Dramatic response to crizotinib in a patient with lung cancer positive for an HLA-DRB1-MET gene fusion. JCO Precis Oncol (2017) 2017:1. doi: 10.1200/PO.17.00117
16. Li Y, Wang K, Tian P, Li W. Acquired MET-DSTN fusion mediated resistance to EGFR-TKIs in lung adenocarcinoma and responded to crizotinib plus gefitinib: A case report. Clin Lung Cancer (2022) 23(1):e83–6. doi: 10.1016/j.cllc.2021.10.006
17. Liu J, Li X, Peng J. A novel CAV1-MET fusion in SCLC transformation responds to crizotinib and osimertinib treatment. J Thorac Oncol (2019) 14(6):e126–8. doi: 10.1016/j.jtho.2019.01.025
18. Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJ, et al. Capmatinib in MET exon 14–mutated or MET-amplified non–small-cell lung cancer. N Engl J Med (2020) 383(10):944–57. doi: 10.1056/NEJMoa2002787
Keywords: KIF5B-MET fusion, lung adenocarcinoma, capmatinib, telisotuzumab vedotin, case report
Citation: Lin C-Y, Wei S-H, Chen Y-L, Lee C-T, Wu S-Y, Ho C-L, Pavlick DC, Su P-L and Lin C-C (2022) Case report: Salvage capmatinib therapy in KIF5B-MET fusion-positive lung adenocarcinoma with resistance to telisotuzumab vedotin. Front. Oncol. 12:919123. doi: 10.3389/fonc.2022.919123
Received: 13 April 2022; Accepted: 25 July 2022;
Published: 11 August 2022.
Edited by:
Lele Song, Eighth Medical Center of the General Hospital of the Chinese People’s Liberation Army, ChinaReviewed by:
Anurag Mehta, Rajiv Gandhi Cancer Institute and Research Centre, IndiaTao Ren, Shanghai Sixth People’s Hospital, China
Copyright © 2022 Lin, Wei, Chen, Lee, Wu, Ho, Pavlick, Su and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Po-Lan Su, cG9sYW4uNzUwMzE3QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
‡ORCID: Po-Lan Su, orcid.org/0000-0002-8470-6590
 Chien-Yu Lin
Chien-Yu Lin Sheng-Huan Wei
Sheng-Huan Wei Yi-Lin Chen
Yi-Lin Chen Chung-Ta Lee
Chung-Ta Lee Shang-Yin Wu
Shang-Yin Wu Chung-Liang Ho2
Chung-Liang Ho2 Dean C. Pavlick
Dean C. Pavlick Po-Lan Su
Po-Lan Su Chien-Chung Lin
Chien-Chung Lin