
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 16 June 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.918580
This article is part of the Research Topic Digital Revolution in Oncology: How Digital Tools Transform the Evaluation and Management of Cancer Patients View all 24 articles
 Paola Chiara Rizzo1
Paola Chiara Rizzo1 Ilaria Girolami2
Ilaria Girolami2 Stefano Marletta1,3
Stefano Marletta1,3 Liron Pantanowitz4
Liron Pantanowitz4 Pietro Antonini1
Pietro Antonini1 Matteo Brunelli1
Matteo Brunelli1 Nicola Santonicco1
Nicola Santonicco1 Paola Vacca5
Paola Vacca5 Nicola Tumino5
Nicola Tumino5 Lorenzo Moretta5
Lorenzo Moretta5 Anil Parwani6
Anil Parwani6 Swati Satturwar6
Swati Satturwar6 Albino Eccher7*
Albino Eccher7* Enrico Munari8
Enrico Munari8Objective: Digital pathology with whole-slide imaging (WSI) has many potential clinical and non-clinical applications. In the past two decades, despite significant advances in WSI technology adoption remains slow for primary diagnosis. The aim of this study was to identify common pitfalls of WSI reported in validation studies and offer measures to overcome these challenges.
Methods: A systematic search was conducted in the electronic databases Pubmed-MEDLINE and Embase. Inclusion criteria were all validation studies designed to evaluate the feasibility of WSI for diagnostic clinical use in pathology. Technical and diagnostic problems encountered with WSI in these studies were recorded.
Results: A total of 45 studies were identified in which technical issues were reported in 15 (33%), diagnostic issues in 8 (18%), and 22 (49%) reported both. Key technical problems encompassed slide scan failure, prolonged time for pathologists to review cases, and a need for higher image resolution. Diagnostic challenges encountered were concerned with grading dysplasia, reliable assessment of mitoses, identification of microorganisms, and clearly defining the invasive front of tumors.
Conclusion: Despite technical advances with WSI technology, some critical concerns remain that need to be addressed to ensure trustworthy clinical diagnostic use. More focus on the quality of the pre-scanning phase and training of pathologists could help reduce the negative impact of WSI technical difficulties. WSI also seems to exacerbate specific diagnostic tasks that are already challenging among pathologists even when examining glass slides with conventional light microscopy.
Virtual microscopy (VM) using digital whole slide imaging (WSI) is a technology by which glass slides in pathology are digitally scanned at high-resolution for viewing on a computer screen. Ever since WSI scanners first became commercially available around two decades ago, progress in the technology of these devices has continually improved their image resolution, image quality, slide throughput, end-user software tools, and integration with laboratory information systems (1). Applications of WSI for clinical (e.g. telepathology, quantitative image analysis) and non-clinical (e.g. education and research) have markedly increased (2–6). Ample literature has been published demonstrating excellent concordance between utilizing WSI versus glass slides with traditional light microscopy (LM) to render diagnoses (7, 8). Nevertheless prior to implementing WSI for diagnostic use in clinical practice, several associations have recommended that such technology be validated by pathology laboratories for their intended use (9). Recently, the College of American Pathologists (CAP) updated their guideline providing recommendations for validating WSI for primary diagnosis (10). The validation process should “stress test” the WSI system in the appropriate clinical environment in order to assess that it allows pathologists to accurately diagnose cases, at least at the same level of accuracy as LM, and to identify and control for potential interfering artifacts or technological risks that could impair patient safety (10, 11).
Whilst published validation studies have largely focused on the success of WSI for specific clinical use cases, some of the “negative issues” that were encountered including technical failures or particular diagnostic difficulties were often under-reported. Furthermore, only few systematic analyses on this topic devoted to the tribulations of employing WSI in clinical practice have been performed. In the literature review undertaken by Goacher et al. from 2017, for example, the authors reported that there was in fact a slower time to diagnosis when using WSI compared with LM (7). The aim of this study was to systematically review the literature of published validation studies that evaluated the feasibility of WSI for diagnostic clinical use in pathology, recording and subsequently analyzing any technical and/or diagnostic problems encountered.
A systematic review of the literature was conducted according to the guideline for Preferred Reporting Items for a Systematic Review and Meta-Analysis (12).
Electronic searches were carried out in the databases PubMed-MEDLINE and Embase until the 5th of December, 2021. No study type filters were used nor language restriction applied. References listed in all identified studies were also hand-searched to retrieve potential additional studies. Initial screening of articles by title/abstract was performed with the aid of the online systematic review web-app QRCI (13). Eligibility of published studies was determined independently by two reviewers with disagreement resolved through consensus. Inclusion criteria included the details of a validation study with a series of surgical pathology cases assessed with WSI and with LM, not only reporting concordance data but also noting any negative issues encountered during the validation process. Studies represented only by abstracts were excluded, as were reviews and published letters to the editor with no original data. Data extracted included: authors, year published, country of study, number and type of cases selected, critical issues reported divided ac-cording to issues pertaining to diagnostic and technical problems. Specific technical problems searched for included slide scan failures, delayed scan time, and difficulties related to viewing and navigating digital slides.
The search strategy identified a total of 1560 records, with only 45 suitable articles finally included in our analysis (Figure 1). Publication dates ranged from 2007 to 2021. Twenty (45%) of the included studies were from North American countries, nineteen (42%) were from European countries and six (13%) were from non-European and non-North American countries. In more than half of the included studies (n=24, 57%) the participating pathologists were experienced in digital pathology. The length of the washout period between LM and WSI diagnosis ranged from 7 days to 2 years, but 13 studies did not report any washout time. The number of cases in the included studies varied from 23 to 3222. Twenty-two studies included cases from various pathology subspecialties, while 23 selected a specific diagnostic field. The majority of Authors used Aperio scanners (n=18, 40%), followed by Ventana scanners (n=6, 13%), NanoZoomer (n=5, 11%), MIRAMAX (n=3, 7%), Philips (n=3, 7%), Pannoramic (n=2, 4%), DHistech (n=2, 4%), NAVIGO (n=1, 3%), Grandium Ocus (n=1, 1%), and OMYX (n=1, 3%). Twelve of the viewer systems used in these studies were APERIO (26%), 5 Ventana (11%), 3 Leica (7%), 3 DHistech (7%), 3 Philips (7%), 2 PathXL (4%), 2 Pannoramic (4%), 1 OMYX (3%), 1 Grandium Ocus (3%), and 1 CaloPix (3%). Only one study tested the use of tablets, specifically the iPad (14). Only one study tested the use of tablets, specifically the iPad (14).
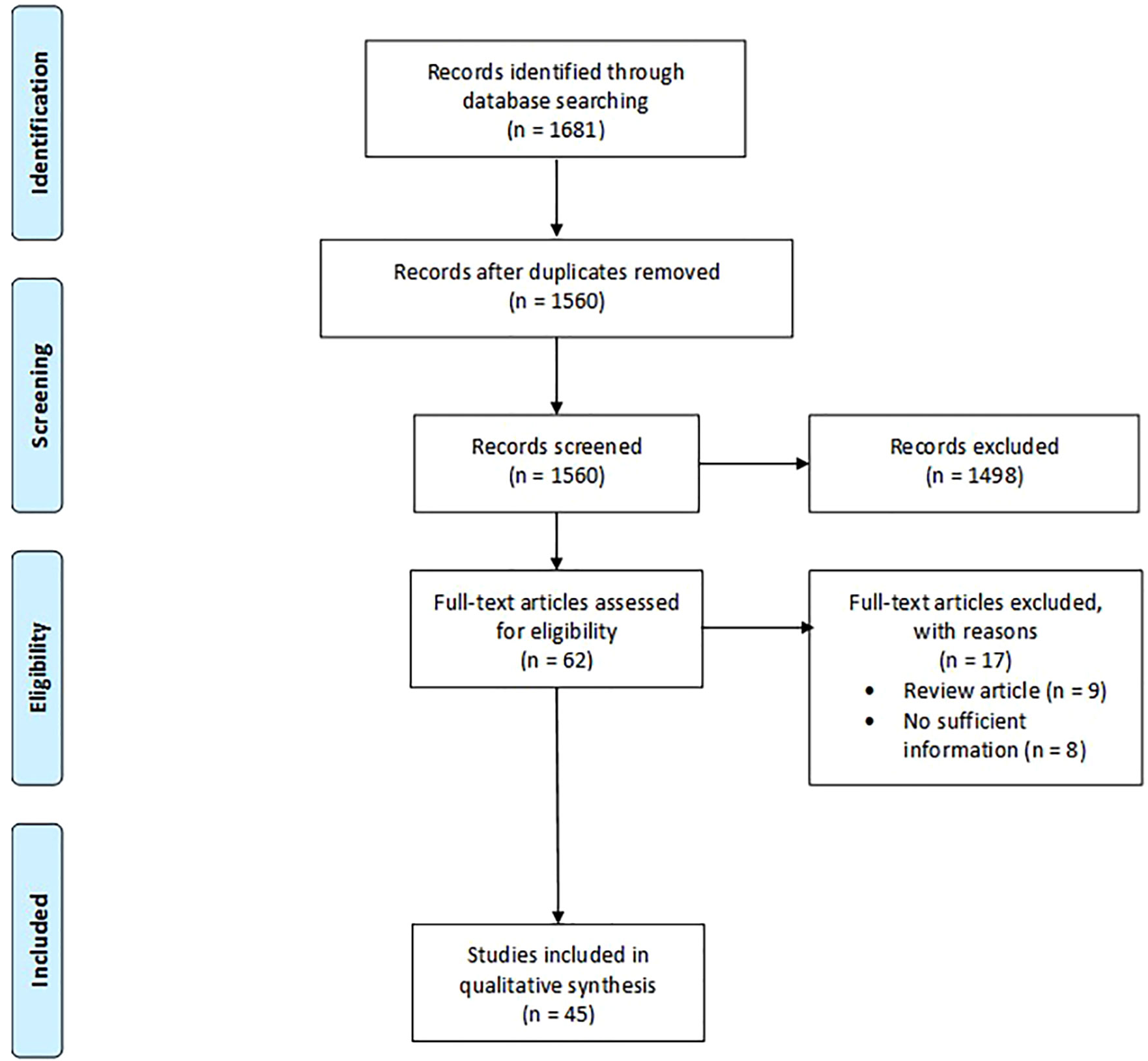
Figure 1 Search flow diagram. The diagram was designed according to the template of the PRISMA flow diagram from Page et al. (12) available at the PRISMA website (www.prismastatement.org).
In order to summarize the pitfalls documented in the various validation studies, we categorized all the discordances into two main groups: technical issues and diagnostic issues (Table 1).
Sixteen (36%) studies reported about technical issues only, eight (18%) reported on diagnostic issues only, and 21 (46%) reported both on technical and diagnostic issue. Among the technical issues described, nine studies (20%) reported failures in scanning glass slides, 19 studies (42%) considered WSI more time consuming than LM, and nine studies (20%) reported specifically the need for higher magnification (better image resolution) with WSI to more easily view and navigate cases. Other technical issues reported were: lack of focus (n=8, 18%), suboptimal navigation tools (n=2, 4%), need for polarization (n=2, 4%), and lack of adequate color fidelity for special stain or immunohistochemical staining (n=3, 7%). In addition, some validation studies (n=7, 15%) reported difficulty related to image storage.
Concerning diagnostic issues when using WSI, in eight studies (18%) grading of dysplasia represented the most common problem encountered. Furthermore, six (13%) studies re-ported challenges in assessing mitotic count, four (9%) studies reported general misdiagnosis, while three (7%) studies reported discordant diagnoses related to the identification of microorganisms. In three (7%) studies the authors mentioned there was lack of diagnostic confidence, and in two (4%) that pathologists experienced difficulty interpreting the invasive component of tumors.
The characteristics of each of the included studies are extensively detailed in Supplementary Table S1.
Digital pathology has been increasingly deployed in many institutions (15, 16). Nevertheless, problems encountered when using WSI for routine pathology diagnosis still remain. A critical appraisal of such issues is important to understand and hopefully re-solve. Our systematic review identified 45 articles that specifically reported problems experienced with WSI usage for primary diagnosis during a validation process.
As expected, technical issues when validating WSI were the most frequently reported. The most commonly mentioned technical issue involved scan failures with the need for re-scan slides and the consequent prolonging of turn-around-time (TAT). When combined with the reported experience by participating pathologists that it took them longer to evaluate digital slides in order to establish a diagnosis, switching to WSI for primary diagnosis has the potential to delay TAT. This drawback would need to be offset by some of the other workflow benefits of digital pathology such as decreased time for slide distribution, quicker archival image retrieval, in addition to ensuring faster network connections, better workstations and improved viewing software.
Newer scanners with higher throughput capacity and reduced image acquisition time have further helped overcome TAT issues. The quality of pre-imaging factors can also help re-duce the aforementioned limitation of delayed TAT. For example, striving to produce uniform histological sections without folds and clean, dry slides without artifacts such as air bubbles are important to reduce the probability of scan failures. Such pre-imaging measures are especially important for the digitization of cytology slides (17, 18), where thick smears, three dimensional cell groups and obscuring material make it harder for scanners without z-stacking capability to achieve optimal focus. For some studies, the technical difficulty noted when viewing digital slides was related to the monitor and input device (specifically, computer mouse) used. Hanna et al. (19) suggested trying different input devices instead of a conventional mouse to circumvent problems with digital slide navigation. Similarly, Brunelli et al. (14) tested the use of a tablet to improve WSI navigation. Although a validation study should not suffice for official training of end-users, spending more time training pathologists to better use WSI and allowing them to become more familiar with this technology can certainly improve their ease with utilizing WSI. Alassiri et al. (20) showed that at the beginning of their validation study participating pathologists were slow-er in assessing cases with WSI, but by the end of their validation process they experienced no notable time difference when reading cases with either WSI or LM modalities.
The other important category of shortcomings with WSI that emerged in published validation studies was concerned with performing certain challenging diagnostic tasks. The most frequently reported were misinterpretation regarding grading of dysplasia, in a variety of settings including gastrointestinal biopsies (21–23) and melanocytes atypia (24). Such errors were related to both downgrading or upgrading lesions (25–30). Apart from the cited discordance related to interpreting gastrointestinal dysplasia, other challenging diagnostic areas that were reported in validation studies included urothelial dysplasia, cervical dysplasia, grading of ovarian and endometrial cancers, in situ lesions of the breast, and brain pathology. However, in most studies, especially the most recent publications, overall diagnostic concordance was above the cutoff of 95% recommended in the CAP guidelines for WSI vali-dation, and discordances with a potential impact on clinical management were often lower than 3% (8, 10, 21, 29–31). Another frequently reported area of discordance, as well source of dissatisfaction among pathologists, when using WSI relates to counting mitoses, such as is required in grading meningiomas (32) or breast carcinoma (33, 34) or when diagnosing malignancy in a melanocytic lesion (35). Other less frequently reported. but still relevant, reported diagnostic difficulties with WSI were the detection of microorganisms (19), discriminating single inflammatory cell types in dermatopathology and hematopathology (24, 36–38), assessment of tumor budding and tumor pattern of invasion in colon cancer (39), and overestimation of steatosis and fibrosis in liver cases (40). In general, pathologists reported lower diagnostic confidence when signing out with WSI. Similar considerations have also been observed in the setting of pediatric pathology (41), where WSI showed to be at least as reliable as LM, fully satisfying the CAP guidelines. Even in this setting the few reported discrepancies concerned subtle morphological features, such as identification of Candida spores and hypha, likely linked to the difficult of the case rather than to the classic or digital method of evaluation of the slides. Many of these diagnostic concerns are being addressed with improvements in WSI technology (e.g. incorporating higher resolution cameras and objectives into scanners) and leveraging artificial intelligence (AI) to apply algorithms for specific (narrow) tasks such as counting mitoses, screening slides for microorganisms, and standardizing the grading of dysplasia or cancer. Development and deployment of these technologies are foreseeably going to increase in the near future, further allowing pathologists to benefit from digital supports to proficiently reach proper histological diagnoses for both adults and pediatric patients.
Additional collateral problems were also reported in published validation studies, which were mainly of a technical and institution’s organizational nature. Some authors reported problems related to the storage of WSI cases, given the huge size of WSI files and consequent high demand this has on information technology (IT) infra-structure for image management (31, 37, 42, 43). Lastly, Ordi et al. (44) and Al-Janabi et al. (43) reported about the cultural barrier of pathologists, including their concerns about legal is-sues and resistant mindset to accept WSI over more familiar LM for routine primary diagnosis. However, since then we have witnessed increased regulatory approval of WSI solutions, such as clearances issued by the Food and Drug Administration (FDA) in the USA (45, 46) for primary diagnosis which has helped increase overall confidence in the adoption of WSI. Moreover, with the rapid shift experienced towards using digital pathology to permit re-mote signing-out during the Coronavirus Disease 2019 (COVID-19) pandemic many pre-pandemic skeptics have since been convinced about the value of digital pathology (47).
Digital pathology with WSI is nowadays a reality in many laboratories, but there are still some negative aspects that may restrain an even wider spread adoption of WSI. When reviewing the literature for validation studies highlighting these conflicting aspects, we found both some technical and diagnostic critical issues still remain of concern. The majority of technical points could be reasonably overcome by further improvement of technology and dedicated training of pathologists. Likewise, the diagnostic issues are mainly represented by subtle tasks which yield per se an unsatisfactory reproducibility among pathologists with conventional glass slides as well. In the near future, the development of dedicated and more objective AI tools could be of aid to further support pathologists in reducing the gap between LM and WSI in order to increase the efficiency of the diagnostic process and ultimately improve patients’ management and care.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conceptualization, IG, SM, LP and AE. Data curation, PR, IG, SM and AE. Formal analysis, PR, IG and SM. Methodology, IG, LP and AE. Project administration, AE and EM. Supervision, AE and EM. Validation, PA, MB, NS, PV, NT, LM, and EM. Writing – original draft, PRC, IG and SM. Writing – review & editing, PA, MB, NS, PV, NT, LM, AP, SS, AE and EM. All authors contributed to the article and approved the submitted version.
This work was supported by grants from Associazione Italiana Ricerca sul Cancro (AIRC) Investigator Grant ID 19920 (LM); Special Program Metastatic disease: the key unmet need in oncology 5 per mille 2018, ID 21147 (LM).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.918580/full#supplementary-material
1. Hanna MG, Reuter VE, Samboy J, England C, Corsale L, Fine SW, et al. Implementation of Digital Pathology Offers Clinical and Operational Increase in Efficiency and Cost Savings. Arch Pathol Lab Med (2019) 143:1545–55. doi: 10.5858/arpa.2018-0514-OA
2. Santonicco N, Marletta S, Pantanowitz L, Fadda G, Troncone G, Brunelli M, et al. Impact of Mobile Devices on Cancer Diagnosis in Cytology. Diagn Cytopathol (2022) 50:34–45. doi: 10.1002/dc.24890
3. Eccher A, Fontanini G, Fusco N, Girolami I, Graziano P, Rocco EG, et al. Digital Slides as an Effective Tool for Programmed Death Ligand 1 Combined Positive Score Assessment and Training: Lessons Learned From the “Programmed Death Ligand 1 Key Learning Program in Head-And-Neck Squamous Cell Carcinoma. J Pathol Inform (2021) 12:1. doi: 10.4103/jpi.jpi_63_20
4. Eccher A, Neil D, Ciangherotti A, Cima L, Boschiero L, Martignoni G, et al. Digital Reporting of Whole-Slide Images is Safe and Suitable for Assessing Organ Quality in Preimplantation Renal Biopsies. Hum Pathol (2016) 47:115–20. doi: 10.1016/j.humpath.2015.09.012
5. Pantanowitz L, Wiley CA, Demetris A, Lesniak A, Ahmed I, Cable W, et al. Experience With Multimodality Telepathology at the University of Pittsburgh Medical Center. J Pathol Inform (2012) 3:45. doi: 10.4103/2153-3539.104907
6. Fraggetta F, Garozzo S, Zannoni GF, Pantanowitz L, Rossi ED. Routine Digital Pathology Workflow: The Catania Experience. J Pathol Inform (2017) 8:51. doi: 10.4103/jpi.jpi_58_17
7. Goacher E, Randell R, Williams B, Treanor D. The Diagnostic Concordance of Whole Slide Imaging and Light Microscopy: A Systematic Review. Arch Pathol Lab Med (2017) 141:151–61. doi: 10.5858/arpa.2016-0025-RA
8. Williams BJ, DaCosta P, Goacher E, Treanor D. A Systematic Analysis of Discordant Diagnoses in Digital Pathology Compared With Light Microscopy. Arch Pathol Lab Med (2017) 141:1712–8. doi: 10.5858/arpa.2016-0494-OA
9. Hanna MG, Pantanowitz L, Evans AJ. Overview of Contemporary Guidelines in Digital Pathology: What is Available in 2015 and What Still Needs to be Addressed? J Clin Pathol (2015) 68:499–505. doi: 10.1136/jclinpath-2015-202914
10. Evans AJ, Brown RW, Bui MM, Chlipala EA, Lacchetti C, Milner DA, et al. Validating Whole Slide Imaging Systems for Diagnostic Purposes in Pathology. Arch Pathol Lab Med (2022) 146:440–50. doi: 10.5858/arpa.2020-0723-CP
11. Pantanowitz L, Sinard JH, Henricks WH, Fatheree LA, Carter AB, Contis L, et al. Validating Whole Slide Imaging for Diagnostic Purposes in Pathology: Guideline From The College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med (2013) 137:1710–22. doi: 10.5858/arpa.2013-0093-CP
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
13. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-A Web and Mobile App for Systematic Reviews. Syst Rev (2016) 5:210. doi: 10.1186/s13643-016-0384-4
14. Brunelli M, Beccari S, Colombari R, Gobbo S, Giobelli L, Pellegrini A, et al. Ipathology Cockpit Diagnostic Station: Validation According to College of American Pathologists Pathology and Laboratory Quality Center Recommendation at the Hospital Trust and University of Verona. Diagn Pathol (2014) 9 Suppl 1:S12. doi: 10.1186/1746-1596-9-S1-S12
15. Fraggetta F, Caputo A, Guglielmino R, Pellegrino MG, Runza G, L’Imperio V. A Survival Guide for the Rapid Transition to a Fully Digital Workflow: The “Caltagirone Example. Diagnostics (Basel Switzerland) (2021) 11(10):1916. doi: 10.3390/diagnostics11101916
16. Retamero JA, Aneiros-Fernandez J, Del Moral RG. Complete Digital Pathology for Routine Histopathology Diagnosis in a Multicenter Hospital Network. Arch Pathol Lab Med (2020) 144:221–8. doi: 10.5858/arpa.2018-0541-OA
17. Eccher A, Girolami I. Current State of Whole Slide Imaging Use in Cytopathology: Pros and Pitfalls. Cytopathology (2020) 31:372–8. doi: 10.1111/cyt.12806
18. Girolami I, Pantanowitz L, Marletta S, Brunelli M, Mescoli C, Parisi A, et al. Diagnostic Concordance Between Whole Slide Imaging and Conventional Light Microscopy In Cytopathology: A Systematic Review. Cancer Cytopathol (2020) 128:17–28. doi: 10.1002/cncy.22195
19. Hanna MG, Reuter VE, Hameed MR, Tan LK, Chiang S, Sigel C, et al. Whole Slide Imaging Equivalency and Efficiency Study: Experience at a Large Academic Center. Mod Pathol an Off J United States Can Acad Pathol Inc (2019) 32:916–28. doi: 10.1038/s41379-019-0205-0
20. Alassiri A, Almutrafi A, Alsufiani F, Al Nehkilan A, Al Salim A, Musleh H, et al. Whole Slide Imaging Compared With Light Microscopy for Primary Diagnosis in Surgical Neuropathology: A Validation Study. Ann Saudi Med (2020) 40:36–41. doi: 10.5144/0256-4947.2020.36
21. Snead DRJ, Tsang Y-W, Meskiri A, Kimani PK, Crossman R, Rajpoot NM, et al. Validation of Digital Pathology Imaging for Primary Histopathological Diagnosis. Histopathology (2016) 68:1063–72. doi: 10.1111/his.12879
22. Gui D, Cortina G, Naini B, Hart S, Gerney G, Dawson D, et al. Diagnosis of Dysplasia in Upper Gastro-Intestinal Tract Biopsies Through Digital Microscopy. J Pathol Inform (2012) 3:27. doi: 10.4103/2153-3539.100149
23. Loughrey MB, Kelly PJ, Houghton OP, Coleman HG, Houghton JP, Carson A, et al. Digital Slide Viewing for Primary Reporting in Gastrointestinal Pathology: A Validation Study. Virchows Arch (2015) 467:137–44. doi: 10.1007/s00428-015-1780-1
24. Velez N, Jukic D, Ho J. Evaluation of 2 Whole-Slide Imaging Applications in Dermatopathology. Hum Pathol (2008) 39:1341–9. doi: 10.1016/j.humpath.2008.01.006
25. Campbell WS, Hinrichs SH, Lele SM, Baker JJ, Lazenby AJ, Talmon GA, et al. Whole Slide Imaging Diagnostic Concordance With Light Microscopy for Breast Needle Biopsies. Hum Pathol (2014) 45:1713–21. doi: 10.1016/j.humpath.2014.04.007
26. Bauer TW, Slaw RJ. Validating Whole-Slide Imaging for Consultation Diagnoses in Surgical Pathology. Arch Pathol Lab Med (2014) 138:1459–65. doi: 10.5858/arpa.2013-0541-OA
27. Bauer TW, Slaw RJ, McKenney JK, Patil DT. Validation of Whole Slide Imaging for Frozen Section Diagnosis in Surgical Pathology. J Pathol Inform (2015) 6:49. doi: 10.4103/2153-3539.163988
28. Thrall MJ, Wimmer JL, Schwartz MR. Validation of Multiple Whole Slide Imaging Scanners Based on the Guideline From the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med (2015) 139:656–64. doi: 10.5858/arpa.2014-0073-OA
29. Mukhopadhyay S, Feldman MD, Abels E, Ashfaq R, Beltaifa S, Cacciabeve NG, et al. Whole Slide Imaging Versus Microscopy for Primary Diagnosis in Surgical Pathology: A Multicenter Blinded Randomized Noninferiority Study of 1992 Cases (Pivotal Study). Am J Surg Pathol (2018) 42:39–52. doi: 10.1097/PAS.0000000000000948
30. Villa I, Mathieu M-C, Bosq J, Auperin A, Pomerol J-F, Lacroix-Triki M, et al. Daily Biopsy Diagnosis in Surgical Pathology: Concordance Between Light Microscopy and Whole-Slide Imaging in Real-Life Conditions. Am J Clin Pathol (2018) 149:344–51. doi: 10.1093/ajcp/aqx161
31. Tabata K, Mori I, Sasaki T, Itoh T, Shiraishi T, Yoshimi N, et al. Whole-Slide Imaging at Primary Pathological Diagnosis: Validation of Whole-Slide Imaging-Based Primary Pathological Diagnosis at Twelve Japanese Academic Institutes. Pathol Int (2017) 67:547–54. doi: 10.1111/pin.12590
32. Ammendola S, Bariani E, Eccher A, Capitanio A, Ghimenton C, Pantanowitz L, et al. The Histopathological Diagnosis of Atypical Meningioma: Glass Slide Versus Whole Slide Imaging for Grading Assessment. Virchows Arch (2021) 478:747–56. doi: 10.1007/s00428-020-02988-1
33. Williams BJ, Hanby A, Millican-Slater R, Nijhawan A, Verghese E, Treanor D. Digital Pathology for the Primary Diagnosis of Breast Histopathological Specimens: An Innovative Validation and Concordance Study on Digital Pathology Validation and Training. Histopathology (2018) 72:662–71. doi: 10.1111/his.13403
34. Davidson TM, Rendi MH, Frederick PD, Onega T, Allison KH, Mercan E, et al. Breast Cancer Prognostic Factors in the Digital Era: Comparison of Nottingham Grade Using Whole Slide Images and Glass Slides. J Pathol Inform (2019) 10:11. doi: 10.4103/jpi.jpi_29_18
35. Sturm B, Creytens D, Cook MG, Smits J, van Dijk MCRF, Eijken E, et al. Validation of Whole-Slide Digitally Imaged Melanocytic Lesions: Does Z-Stack Scanning Improve Diagnostic Accuracy? J Pathol Inform (2019) 10:6. doi: 10.4103/jpi.jpi_46_18
36. Bauer TW, Schoenfield L, Slaw RJ, Yerian L, Sun Z, Henricks WH. Validation of Whole Slide Imaging for Primary Diagnosis in Surgical Pathology. Arch Pathol Lab Med (2013) 137:518–24. doi: 10.5858/arpa.2011-0678-OA
37. van den Brand M, Nooijen PTGA, van der Laan KD, de Bruin PC, van Leeuwen AMG, Leeuwis JW, et al. Discrepancies in Digital Hematopathology Diagnoses for Consultation and Expert Panel Analysis. Virchows Arch (2021) 478:535–40. doi: 10.1007/s00428-020-02907-4
38. Fertig RM, Gaudi S, Cervantes J, Maddy A, Sangueza O, Vu J, et al. Feasibility Study in Teledermatopathology: An Examination of the Histopathologic Features of Mycosis Fungoides and Spongiotic Dermatitis. J Cutan Pathol (2017) 44:919–24. doi: 10.1111/cup.13018
39. Hacking S, Nasim R, Lee L, Vitkovski T, Thomas R, Shaffer E, et al. Whole Slide Imaging and Colorectal Carcinoma: A Validation Study for Tumor Budding and Stromal Differentiation. Pathol Res Pract (2020) 216:153233. doi: 10.1016/j.prp.2020.153233
40. Cima L, Brunelli M, Parwani A, Girolami I, Ciangherotti A, Riva G, et al. Validation of Remote Digital Frozen Sections for Cancer and Transplant Intraoperative Services. J Pathol Inform (2018) 9:34. doi: 10.4103/jpi.jpi_52_18
41. Al-Janabi S, Huisman A, Nikkels PGJ, ten Kate FJW, van Diest PJ. Whole Slide Images for Primary Diagnostics of Paediatric Pathology Specimens: A Feasibility Study. J Clin Pathol (2013) 66:218–23. doi: 10.1136/jclinpath-2012-201104
42. Al-Janabi S, Huisman A, Nap M, Clarijs R, van Diest PJ. Whole Slide Images as a Platform for Initial Diagnostics in Histopathology in a Medium-Sized Routine Laboratory. J Clin Pathol (2012) 65:1107–11. doi: 10.1136/jclinpath-2012-200878
43. Al-Janabi S, Huisman A, Vink A, Leguit RJ, Offerhaus GJA, Ten Kate FJW, et al. Whole Slide Images for Primary Diagnostics in Dermatopathology: A Feasibility Study. J Clin Pathol (2012) 65:152–8. doi: 10.1136/jclinpath-2011-200277
44. Ordi J, Castillo P, Saco A, Del Pino M, Ordi O, Rodríguez-Carunchio L, et al. Validation of Whole Slide Imaging in the Primary Diagnosis of Gynaecological Pathology in a University Hospital. J Clin Pathol (2015) 68:33–9. doi: 10.1136/jclinpath-2014-202524
45. US Food & Drug Administration, in: FDA News Release: FDA Allows Marketing of First Whole Slide Imaging System for Digital Pathology (2017). Available at: https://www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-whole-slide-imaging-system-digital-pathology (Accessed April 10, 2022).
46. Image Technology News, in: Leica Biosystems Receives FDA Clearance for Aperio AT2 DX Digital Pathology System (2019). Available at: https://www.itnonline.com/content/leica-biosystems-receives-fda-clearance-aperio-at2-dx-digital-pathology-system (Accessed April 10, 2022).
Keywords: whole slide imaging, digital pathology, validation study, systematic (literature) reviews, artificial intelligence
Citation: Rizzo PC, Girolami I, Marletta S, Pantanowitz L, Antonini P, Brunelli M, Santonicco N, Vacca P, Tumino N, Moretta L, Parwani A, Satturwar S, Eccher A and Munari E (2022) Technical and Diagnostic Issues in Whole Slide Imaging Published Validation Studies. Front. Oncol. 12:918580. doi: 10.3389/fonc.2022.918580
Received: 12 April 2022; Accepted: 24 May 2022;
Published: 16 June 2022.
Edited by:
Konstanty Korski, Roche, SwitzerlandReviewed by:
Danielle Maracaja, Wake Forest University, United StatesCopyright © 2022 Rizzo, Girolami, Marletta, Pantanowitz, Antonini, Brunelli, Santonicco, Vacca, Tumino, Moretta, Parwani, Satturwar, Eccher and Munari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Albino Eccher, YWxiaW5vLmVjY2hlckBhb3ZyLnZlbmV0by5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.