
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 25 July 2022
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.918088
This article is part of the Research Topic Recent Advances in Drug Resistance in Colorectal Cancer View all 8 articles
 Xi Zhang1,2†
Xi Zhang1,2† Qing-hong Chen1†
Qing-hong Chen1† Ying Yang1†
Ying Yang1† Jing-xin Lin1†
Jing-xin Lin1† Yan-chun Li3†
Yan-chun Li3† Tian-yu Zhong1
Tian-yu Zhong1 Jie Chen1
Jie Chen1 Si-qi Wu1
Si-qi Wu1 Xiao-hu Chen1
Xiao-hu Chen1 Rui-si Zhou1
Rui-si Zhou1 Jia-man Lin1
Jia-man Lin1 Dong-qing Wang1
Dong-qing Wang1 Qiu-xing He1
Qiu-xing He1 Yan-ting You1
Yan-ting You1 Xing-hong Zhou1
Xing-hong Zhou1 Qiang Zuo4
Qiang Zuo4 Yan-yan Liu1
Yan-yan Liu1 Jing-ru Cheng3
Jing-ru Cheng3 Yi-fen Wu2*
Yi-fen Wu2* Xiao-shan Zhao1*
Xiao-shan Zhao1*Background: High serum uric acid (SUA) levels increase the risk of overall cancer morbidity and mortality, particularly for digestive malignancies. Nevertheless, the correlation between SUA level and clinical outcomes of the postoperative patients with colorectal cancer (CRC) treated by chemotherapy is unclear. This study aimed at exploring the relationship between baseline SUA level and progression-free survival (PFS), disease control rate (DCR), and safety in postoperative CRC patients receiving chemotherapy.
Patients and Methods: We conducted a retrospective study to evaluate the relationship between baseline SUA level and PFS, DCR, and incidence of serious adverse events of 736 postoperative CRC patients treated with FOLFOX, FOLFIRI or XELOX at our center.
Results: Data from our center suggested that high baseline SUA level is linked to poor PFS in non-metastatic CRC patients using FOLFOX (HR=2.59, 95%CI: 1.29-11.31, p=0.018) and in male patients using FOLFIRI (HR=3.77, 95%CI: 1.57-39.49, p=0.012). In patients treated by FOLFIRI, a high SUA is also linked to a low DCR (p=0.035). In patients using FOLFOX, high baseline SUA level is also linked to a high incidence of neutropenia (p=0.0037). For patients using XELOX, there is no significant correlation between SUA level and PFS, effectiveness, or safety.
Conclusions: These findings imply that a high SUA level is a promising biomarker associated with poor PFS, DCR and safety of postoperative CRC patients when treated with FOLFOX or FOLFIRI.
Colorectal cancer (CRC) is one of the most common cancers and ranks third in incidence and second in mortality among all the cancer types (1). Postoperative chemotherapy is critical for lowering the postoperative recurrence rate and extending the survival of CRC patients (2). Currently, the main systemic regimens used for postoperative chemotherapy in CRC are FOLFOX, FOLFIRI, and XELOX, but an increasing number of studies has reported the resistance to chemotherapeutic agents in postoperative CRC patients (3, 4). Uric acid (UA) belongs to the oxidative metabolites of purine nucleotides, and serum uric acid (SUA) is one of the most prevalent antioxidant molecules in human blood as a free radical scavenger and transition metal ion chelator (5, 6). SUA is primarily excreted from the body via the kidneys and gut (7). Elevated SUA has recognized pathogenic roles in respiratory and renal diseases (8, 9). In addition, clinical studies have shown that high SUA levels increase the risk of overall cancer morbidity and mortality, with digestive malignancies being particularly evident (10–13). UA is also reported to be one of the risk factors for the development of metabolic syndrome-associated colorectal adenomas (14). In patients with lung squamous cell carcinoma, esophageal cancer, gastric cancer, hepatitis B-associated liver cancer, breast cancer, acute myeloid leukemia, or diffuse large B-cell lymphoma, high SUA levels are associated with a poor prognosis (15–21). Furthermore, higher SUA levels prior to surgery are linked with a poorer prognosis in CRC patients (22). Elevated SUA levels are associated with metastasis in rectal cancer patients who have not received chemotherapy (23). SUA levels are significantly elevated in patients with metastatic CRC who responded well to bevacizumab chemotherapy, and this elevation is linked to better overall survival (24). SUA levels are not correlated with the prognosis of CRC patients receiving cetuximab chemotherapy (25). Elevated SUA before chemotherapy is associated with lower OS in patients with small cell lung cancer (26). Nonetheless, the impact of UA on the prognosis, efficacy and safety of CRC patients treated with chemotherapy after operation remains unclear. Thus, it’s urgent to elucidate the relationship between baseline SUA levels and their prognosis, disease control rate (DCR) and safety in patients treated with various chemotherapy regimens following CRC surgery.
We performed a retrospective study with the data of CRC postoperative patients treated with FOLFOX, FOLFIRI or XELOX in the Nanfang Hospital of Southern Medical University from November 2007 to April 2020. Medical practice data were collected by two independent investigators and assessed further by another investigator.
The inclusion criteria were as follows: (1) Patients who were diagnosed with CRC. (2) Patients received postoperative chemotherapy with FOLFOX, FOLFIRI or XELOX. (3) Patients had baseline SUA level after surgery.
The exclusion criteria were as follows: (1) Patients had events that significantly affected baseline SUA levels or were taking uric acid-lowering drugs. (2) Patients suffered from other malignant tumors. (3) Patients suffered from other diseases that seriously affect survival, such as uncontrolled hypertension, severe organ failure, other chronic diseases with long-term non-standard treatment, etc. (4) Patients with ECOG scores equal or above 3; (5) Patients detected baseline SUA 7 days or more later after the onset of chemotherapy. (6) Patients conducted imaging tests after the start of chemotherapy or 8 weeks before chemotherapy. (7) Patients with absence of baseline or endpoint imaging data.
High SUA level was defined as >420 mmol/L in men and >357 mmol/L in women while the non-high SUA level was defined as ≤420 mmol/L in men and ≤357 mmol/L in women.
The recruited patients treated with different chemotherapy regimens were followed-up. The primary end point of this study was progression-free survival (PFS), which was defined as the time from the first treatment with FOLFOX or FOLFIRI or XELOX following CRC surgery to the date of progression or death. If patients were still in a non-progressive state at the last follow-up, the end point of PFS was the date of the last follow-up. According to the Response Evaluation Criteria in Solid Tumors (RECIST) (version1.0), progression disease (PD) was defined as a 20% rise in the sum of the greatest diameter of the target lesions at baseline, or the occurrence of new lesions or confirmed advancement of non-target lesions (27).
DCR, that refers to the proportion of all non-progressive patients at the end of follow-up in all patients included in the trial, was used to assess the efficacy of various chemotherapy regimens.
We evaluated the safety of various chemotherapy regimens by the incidence of serious adverse events, which was defined as the proportion of patients with grade 3 or higher adverse events among all patients included in the study during the follow-up period. In this investigation, we recorded only adverse events with a grade of not less than three according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (28).
Categorical data were presented as number (%) and assessed by the Fisher’s exact test or the chi-square test when appropriate. Continuous data were presented as mean ± standard deviation (SD) and assessed by the student’s t-test with the GraphPad Prism (version 6.0). The log-rank test was used to compare survival curves of each group, and log-rank method was used to calculate hazard ratio (HR) and 95%CI.
A total of 736 patients treated with chemotherapy after CRC surgery were included in our clinical study after screening using inclusion and exclusion criteria, including 151 patients treated with FOLFOX, 45 patients treated with FOLFIRI, and 540 patients treated with FOLFOX. At baseline, there was no significant statistical difference between the HUA and non-HUA groups in terms of age, gender, primary tumor location, or tumor state (Table 1).
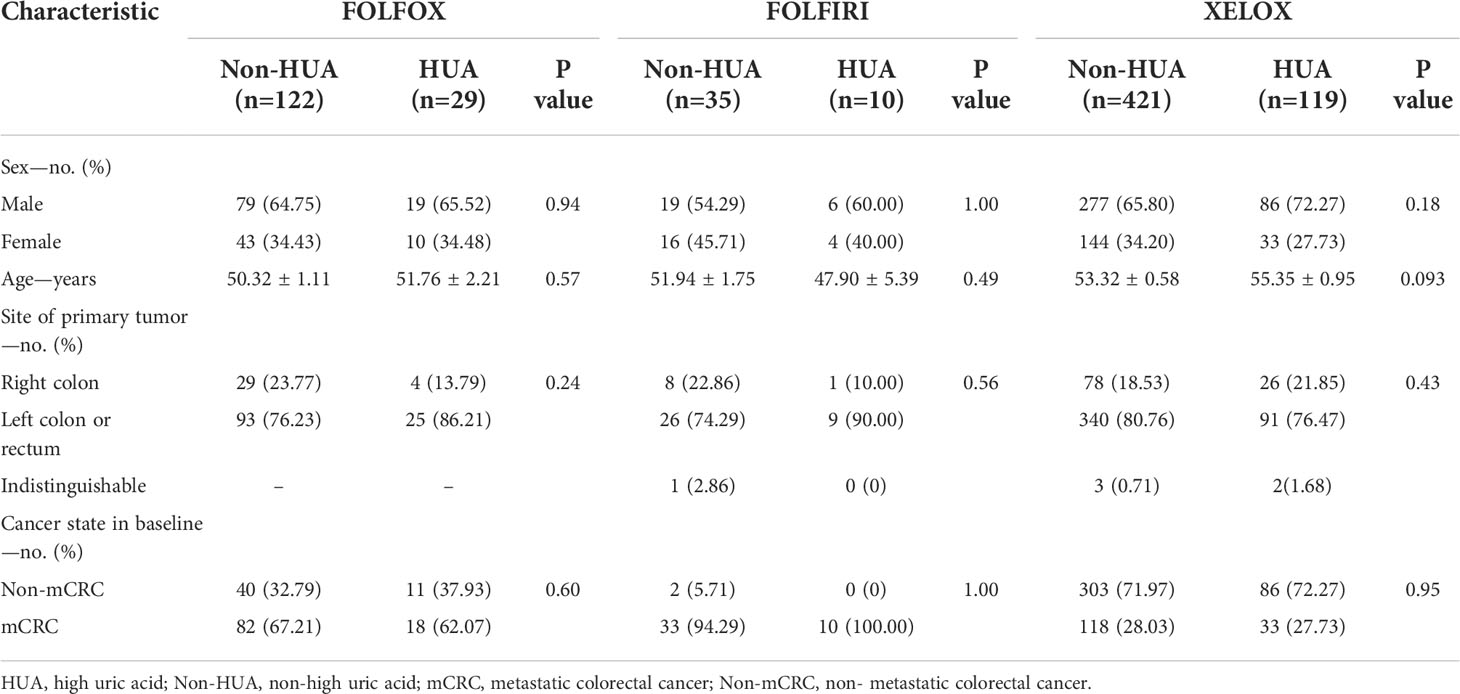
Table 1 Baseline characteristics of colorectal cancer patients treated with postoperative chemotherapy.
In all patients treated with FOLFOX after operation, higher SUA levels were associated with shorter PFS (median PFS: 19.07 vs 29.42 months in the non-HUA group; HR: 1.31, 95%CI= (0.70-2.69), p=0.37, Figure 1). Nonetheless, this association was significant in non-metastatic CRC (non-mCRC) patients (median PFS: 19.07 vs 48.79 months in the non-HUA group; HR: 2.59, 95%CI= (1.29-11.31), p=0.018, Figure 2A) but not in metastatic CRC (mCRC) patients (median PFS: 16.24 vs 7.86 months in the non-HUA group; HR: 0.60, 95%CI= (0.27-1.47), p=0.29, Figure 2B). In the subgroup analysis of gender, higher SUA levels were associated with shorter PFS in both men(median PFS: 19.07 vs 29.42 months in the non-HUA group; HR: 1.55, 95%CI= (0.71-3.93), p=0.24, Figure 2C) and women (median PFS: 22.09 vs 57.40 months in the non-HUA group; HR: 1.04, 95%CI= (0.34-3.18), p=0.95, Figure 2D), although the association was not significant in both genders.
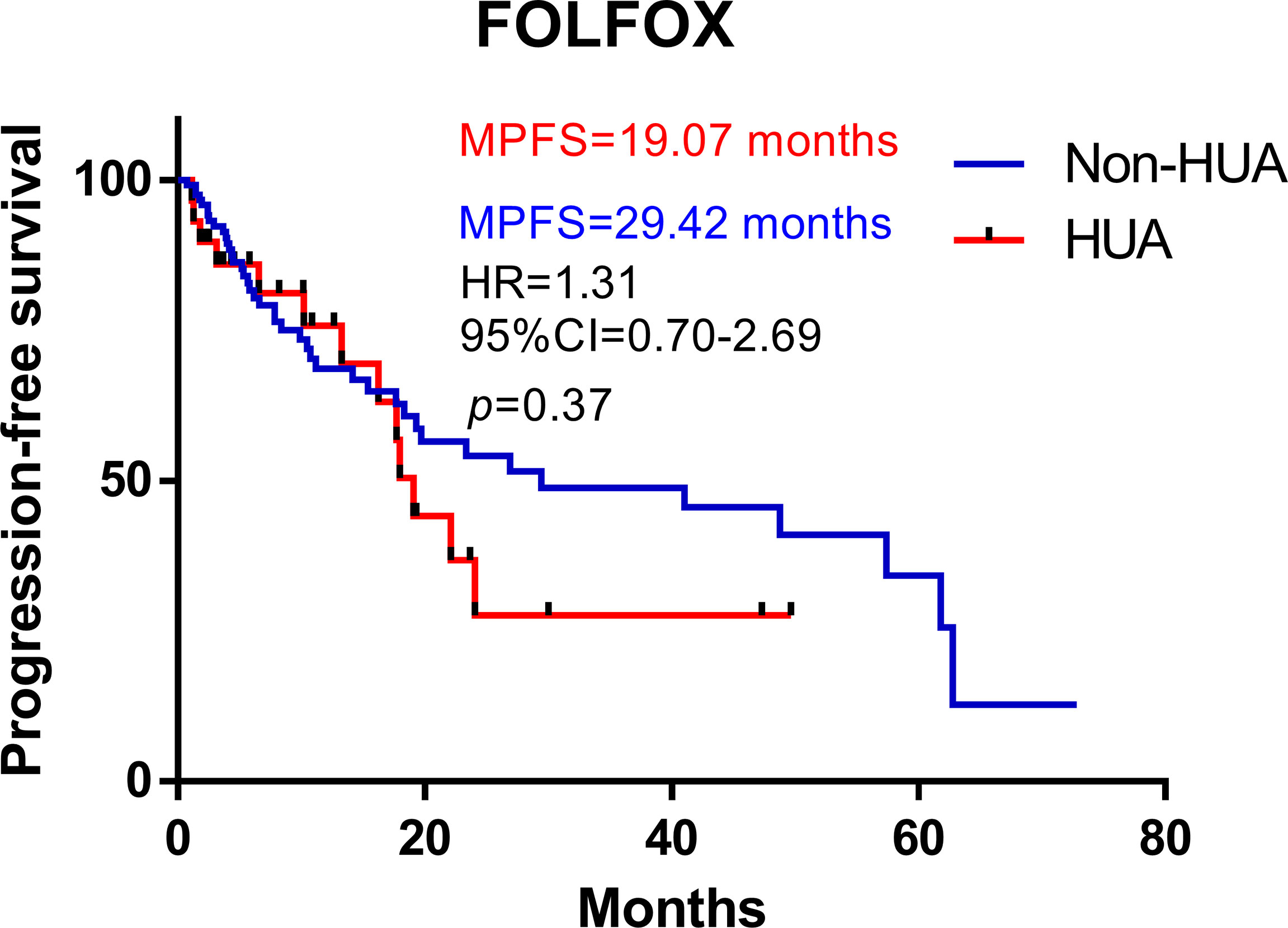
Figure 1 Kaplan-Meier plots comparing the PFS of the different baseline SUA level CRC patients treated with FOLFOX. HUA, high uric acid, Non-HUA, non-high uric acid, MPFS, median PFS.
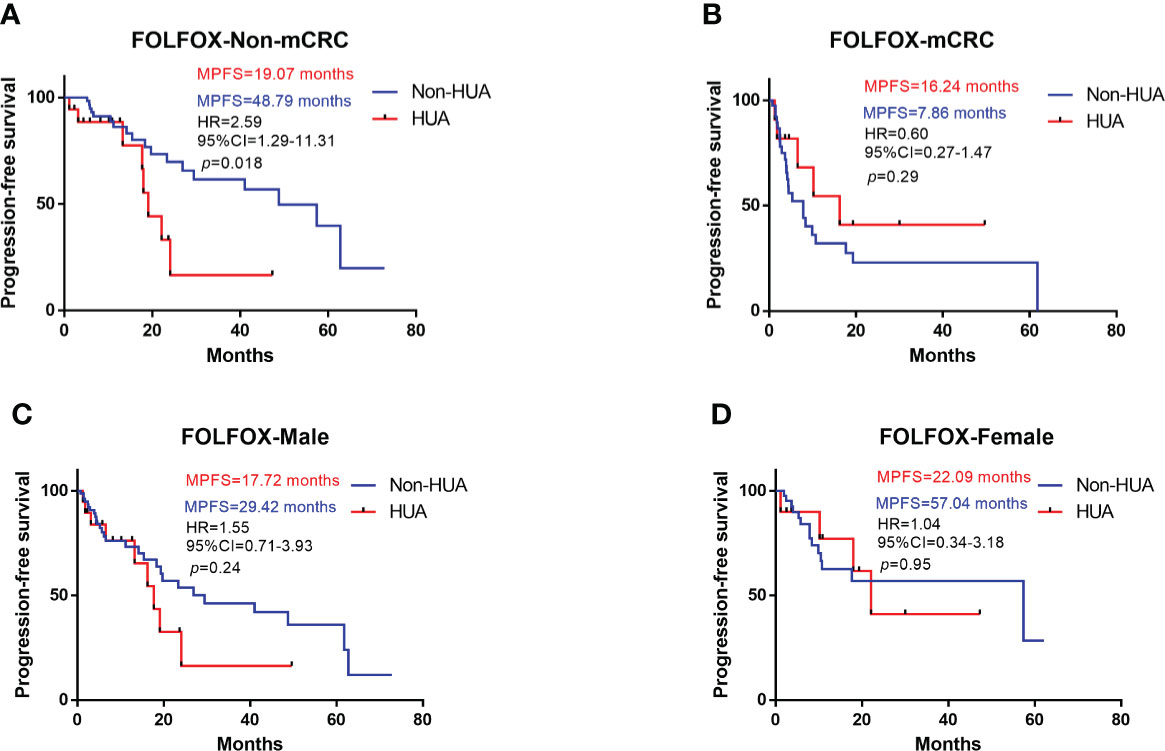
Figure 2 Subgroup analysis for PFS of patients treated with FOLFOX. HUA, high uric acid, Non-HUA, non-high uric acid, MPFS, median PFS. (A) PFS of the non-mCRC patients treated with FOLFOX. (B) PFS of the mCRC patients treated with FOLFOX. (C) PFS of male CRC patients treated with FOLFOX. (D) PFS of female CRC patients treated with FOLFOX.
In all patients treated with FOLFIRI following surgery, higher SUA levels were associated with shorter PFS (median PFS: 6.24 vs 7.00 months in the non-HUA group; HR: 1.90, 95%CI= (0.76-6.24), p=0.15, Figure 3). This association, however, was only significant in male patients (median PFS: 3.52 vs 8.91 months in the non-HUA group; HR: 3.77, 95%CI= (1.57-39.49), p=0.012, Figure 4A), but not in female patients (median PFS: 9.47 vs 6.15 months in the non-HUA group; HR: 0.52, 95%CI= (0.12-1.66), p=0.28, Figure 4B).
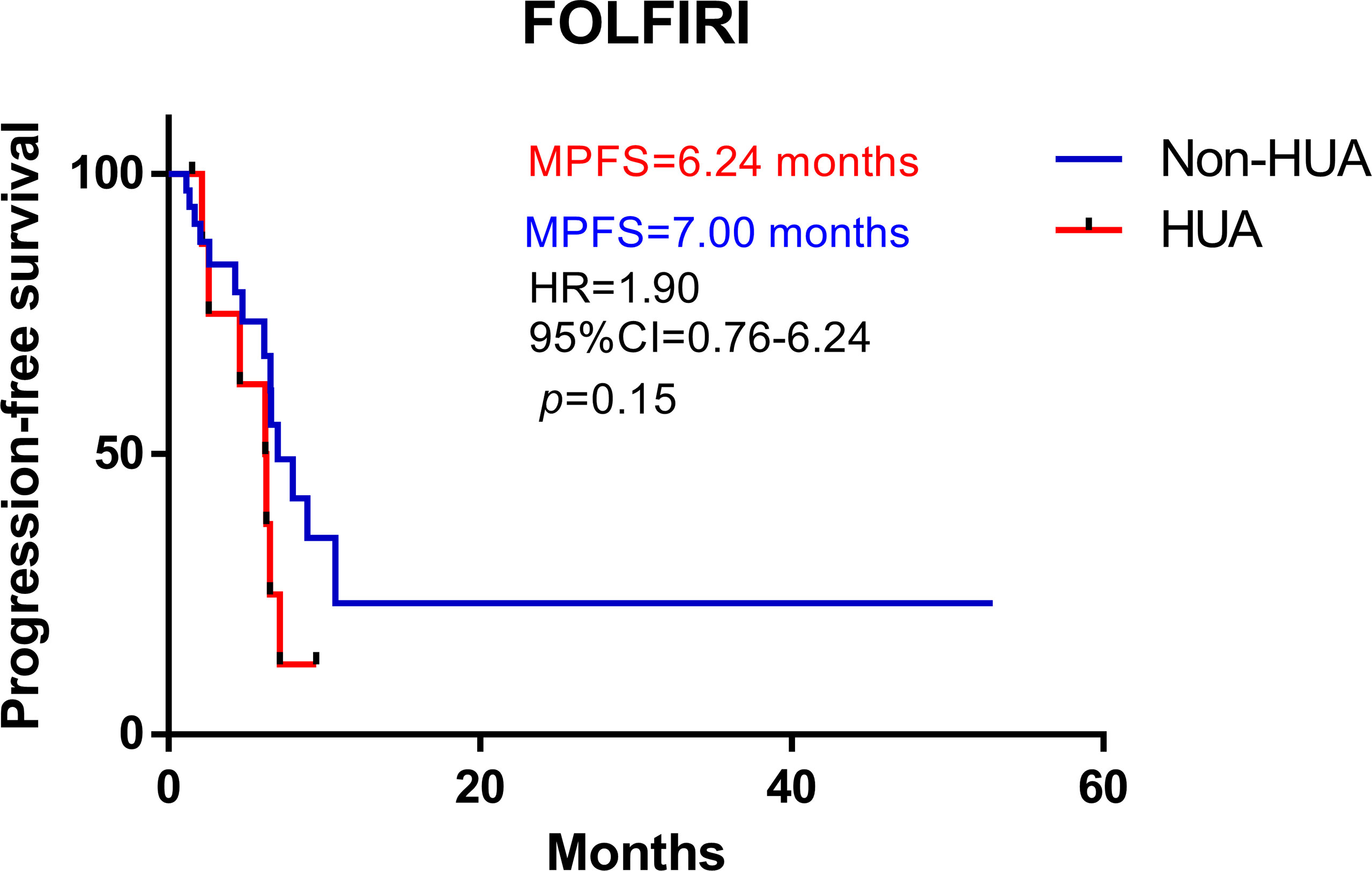
Figure 3 Kaplan-Meier plots comparing the PFS of the different baseline SUA level CRC patients treated with FOLFIRI. HUA, high uric acid, Non-HUA, non-high uric acid, MPFS, median PFS.
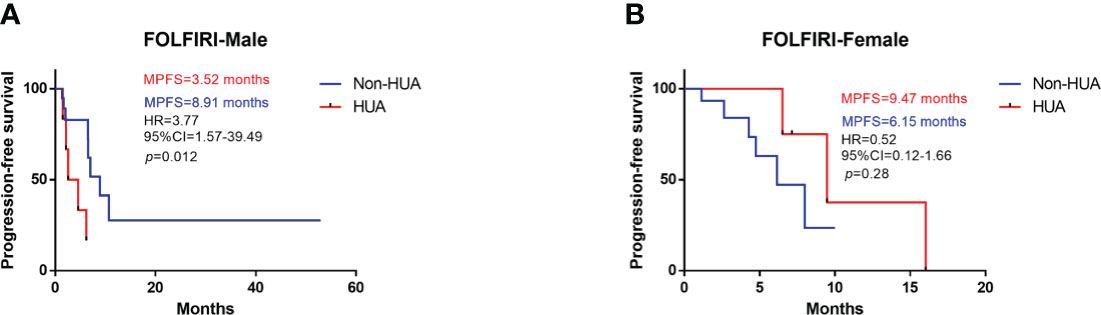
Figure 4 Subgroup analysis for PFS of patients treated with FOLFIRI. HUA, high uric acid, Non-HUA, non-high uric acid, MPFS, median PFS. (A) PFS of male CRC patients treated with FOLFIRI. (B) PFS of female CRC patients treated with FOLFRI.
Survival analysis suggests higher SUA levels were correlated with shorter PFS in all patients treated with XELOX chemotherapy after surgery (HR: 1.07, 95%CI= (0.74-1.56), p=0.72, Figure 5). Subgroup analysis revealed no significant difference in PFS between HUA group and non-HUA group in men, women, mCRC patients or non-metastatic CRC patients (Figure 6).
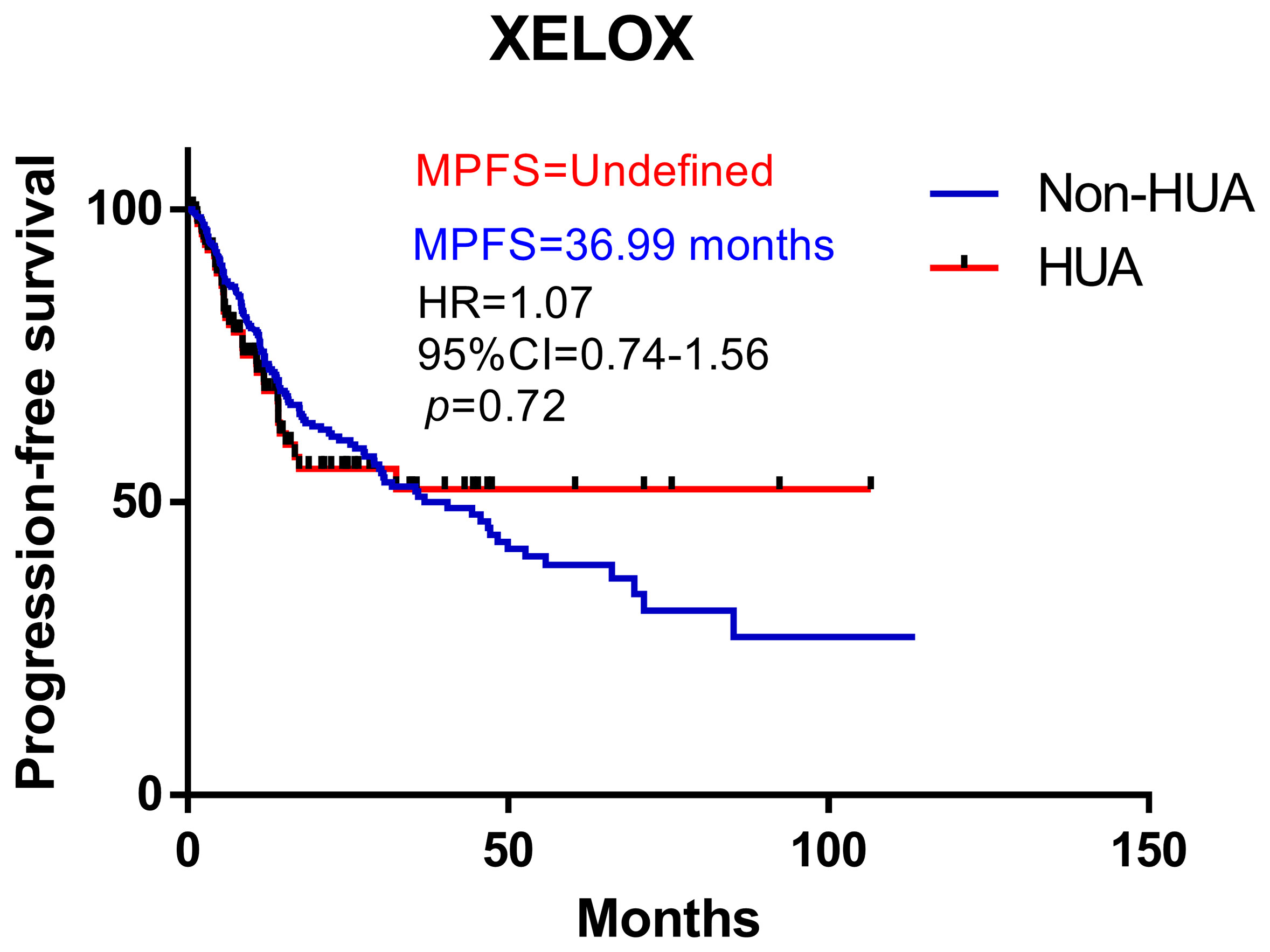
Figure 5 Kaplan-Meier plots comparing the PFS of the different baseline SUA level CRC patients treated with XELOX. HUA, high uric acid, Non-HUA, nonhigh uric acid, MPFS, median PFS.
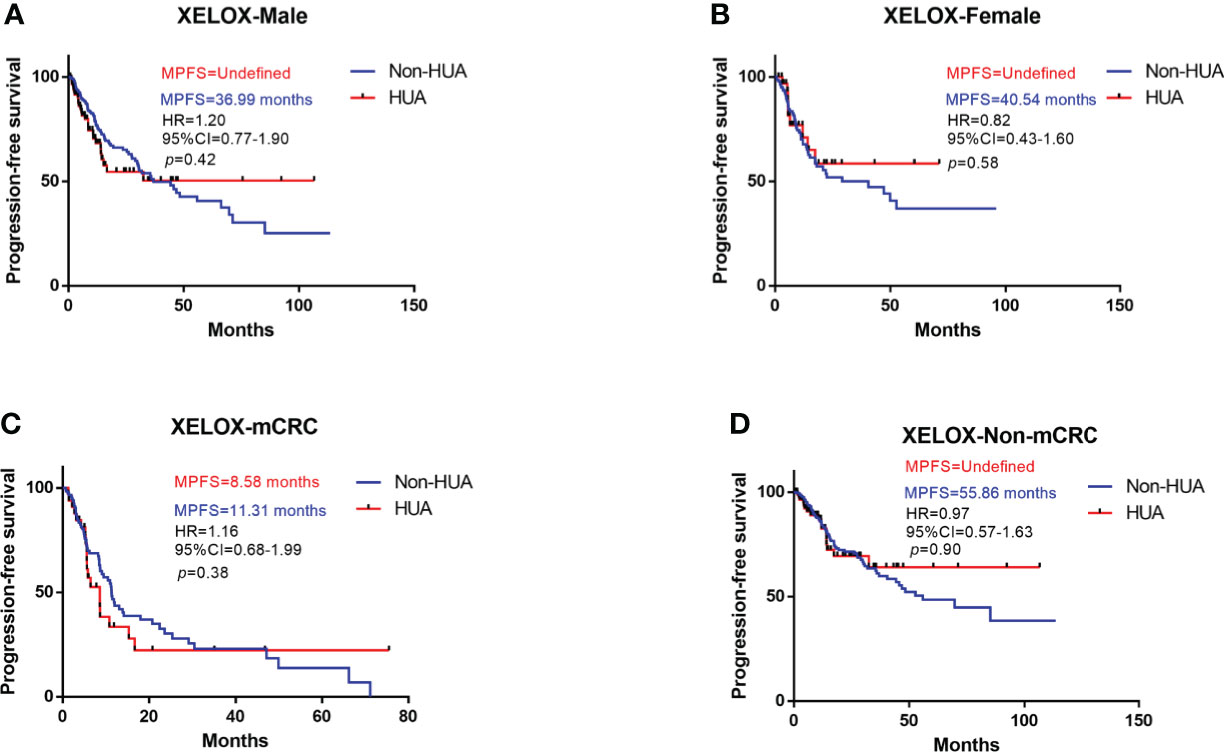
Figure 6 Subgroup analysis for PFS of patients treated with XELOX. HUA, high uric acid, Non-HUA, non-high uric acid, MPFS, median PFS. (A) PFS of male CRC patients treated with XELOX. (B) PFS of female CRC patients treated with XELOX. (C) PFS of the mCRC patients treated with XELOX. (D) PFS of the non-mCRC patients treated with XELOX.
Patients receiving FOLFOX chemotherapy after surgery had a larger DCR in the non-HUA group (65.57%) than in the HUA group (55.17%), but no significant difference in DCR was identified between the two groups (p=0.30, Table 2). In terms of DCR in males, women, mCRC patients, and non-metastatic CRC patients, subgroup analysis based on gender and cancer state revealed no significant difference between non-HUA and HUA groups in men, women, metastatic patients, or non-metastatic patients (p>0.05, Table 2).

Table 2 Relationship between SUA levels and disease control rate in patients using FOLFOX following colorectal cancer surgery.
Patients who received FOLFIRI chemotherapy following surgery had a significantly greater DCR in the non-HUA group (60.00%) than the HUA group (20.00%) (p=0.035, Table 3). In terms of DCR in male and female patients, subgroup analysis based on gender indicated no significant difference between non-HUA and HUA groups (p>0.05, Table 3).
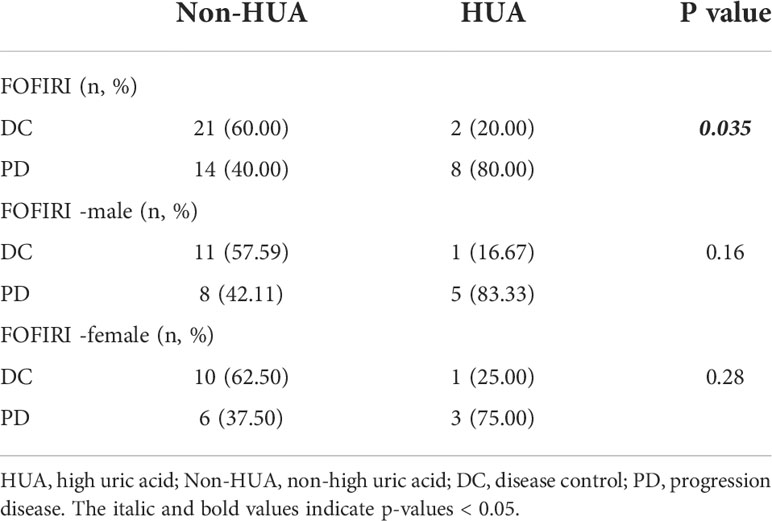
Table 3 Relationship between SUA levels and disease control rate in patients using FOLFIRI following colorectal cancer surgery.
There was no significant difference in DCR between the non-HUA group and the HUA group of patients receiving XELOX after surgery (p>0.05, Table 4). Subgroup analysis based on gender and cancer state suggests no significant difference between non-HUA group and HUA group in terms of DCR in men, women, metastatic patients, or non-metastatic patients (p>0.05, Table 4).
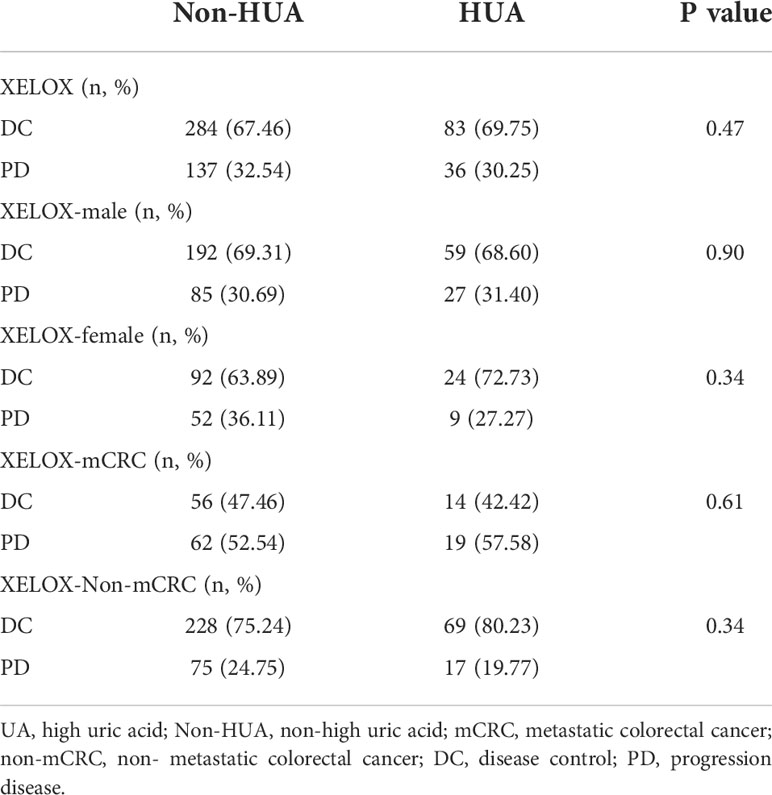
Table 4 Relationship between SUA levels and disease control rate in patients using XELOX following colorectal cancer surgery.
In patients treated with FOLFOX following surgery, grade 3 or higher adverse events were recorded, including liver failure, neutropenia, intestinal obstruction, and thrombocytopenia. The HUA group had a greater incidence of neutropenia than the non-HUA group (p=0.0037, Table 5). In addition, the incidence of liver failure and intestinal obstruction were higher in the HUA group than in the non-HUA group and the incidence of thrombocytopenia was lower than in the non-HUA group, but there was no significant difference between the two groups in the incidence of adverse events mentioned above (p>0.05, Table 5).
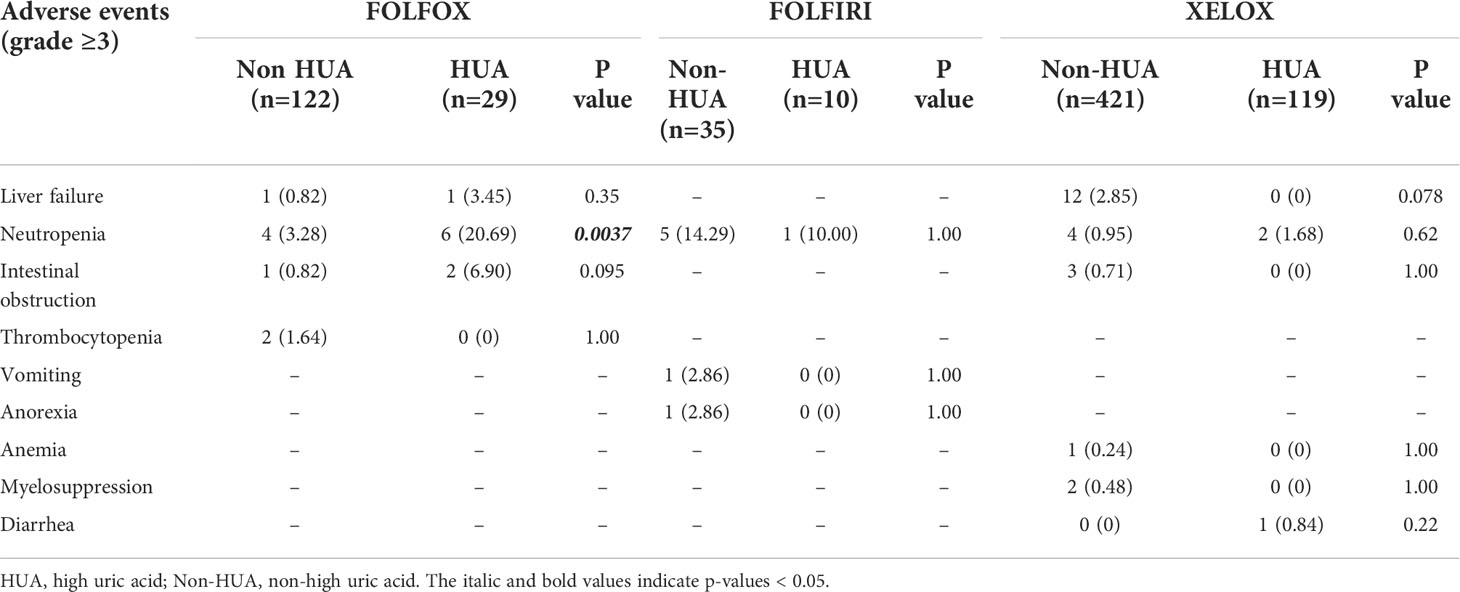
Table 5 Relationship between baseline SUA levels and safety in colorectal cancer patients treated with postoperative chemotherapy.
Grade three or higher adverse events, including neutropenia, vomiting, and anorexia were observed in patients receiving FOLFIRI chemotherapy after surgery. But there was no significant difference in the occurrence of these adverse events between HUA patients and non-HUA individuals (p>0.05, Table 5).
In patients treated with XELOX chemotherapy after surgery, we observed grade 3 or higher adverse events, including neutropenia, intestinal obstruction, anemia, myelosuppression, and diarrhea. In the HUA group, the incidence of neutropenia and diarrhea was higher than in the non-HUA group, while the incidence of liver failure, intestinal obstruction, anemia, and bone marrow suppression was lower than in the non-HUA group, but there was no significant difference in the incidence of these adverse events between the two groups (p>0.05, Table 5).
This is the first study to show a linkage between the baseline SUA levels and prognosis, effectiveness, and safety in patients treated with chemotherapy following CRC resection. Other study shows that patients with higher SUA levels were found to have a higher percentage and shorter duration of brain metastases, as well as a lower overall survival rate in non-small cell lung cancer patients (15). While CRC patients in stage IIIA/IIIB who have a high SUA level may have early metastasis independent of all variables, implying uric acid is associated with CRC metastasis (29). In line with these findings, we found that non-metastasis CRC patients who were treated with FOLFOX following CRC surgery had shorter PFS if they had high baseline SUA than those had low baseline SUA. FOLFIRI is primarily used for mCRC patients who are refractory to oxaliplatin chemotherapy, and it has been shown to improve quality of life and prolong survival in these patients (30–32). As a result, 43 patients with mCRC and only 2 patients with non-metastatic CRC were enrolled in FOLFIRI chemotherapy after surgery. Our data show that the DCR of patients treated with FOLFIRI after CRC was considerably greater in those with high baseline SUA levels than in those with low baseline SUA levels. This may be related to the fact that uric acid promotes the CRC progression or reduces the drug sensitivity to 5-FU. In addition, we found that the PFS of men with high SUA levels on this regimen were significantly shorter than those with low SUA levels; while in women, there was no statistically significant difference in PFS with different SUA levels. We hypothesize that the above discrepancies in different genders are due to the effect of estrogen on the uric acid levels. Estrogen may promote uric acid excretion by regulating uric acid transport-related proteins (33, 34). By suppressing xanthine oxidase and maintaining a stable nutrient metabolism, estrogen can lower uric acid generation (35, 36). Therefore, for female patients with higher SUA levels, estrogen may reduce UA to a level below the threshold that promotes CRC progression or reduces the sensitivity of CRC to 5-FU, leading to a non-significant difference in DCR and prognosis.
When SUA level is elevated, both in the state of soluble high uric acid and crystalline uric acid, ROS production and IL-1β formation can be increased, resulting in oxidative stress and inflammatory response and thus promoting tumor progression (37–39). Increased ROS is associated to an increase in matrix metalloproteinase (MMP) in the tumor microenvironment, which is directly related to tumor invasion and migration (40, 41). In addition, elevated ROS accelerates angiogenesis by inducing the production of angiogenic factors (42).Therefore, ROS production and IL-1β may be responsible for shortening PFS in patients with high SUA levels. As a capecitabine-based regimen, XELOX differs from the regularly used 5-FU-based chemotherapy regimen of FOLFOX and FOLFIRI. As an oral fluorouracil agent, capecitabine is slightly different from 5-FU that directly targets tumor cells and it has low bioavailability. Capecitabine enhances the concentration of 5-Fu in tumor tissues by utilizing the different activities of thymine phosphorylase in tumor tissues and normal tissues (43). Studies have shown that the average concentration of 5-FU in primary colorectal tumors is 3.2 times higher than that in adjacent normal tissues, and the mean ratio of 5-FU concentration in liver metastasis to normal tissues is 1.4. The average 5-FU concentration ratio of colorectal tumor tissue to plasma is over 20, while that of other tissues are between 8 and 10 (44). Based on our data, there was no significant difference in prognosis or DCR between patients with varying SUA levels who received XELOX following surgery. This discrepancy could be due to the fact that the concentration of 5-Fu in CRC tissues in XELOX patients was higher than in FOLFOX or FOLFIRI patients, masking the reduction in CRC sensitivity to 5-FU induced by elevated uric acid.
In our study, patients receiving FOLFOX chemotherapy for CRC experienced grade 3 or higher adverse effects, such as liver failure, neutropenia, intestinal obstruction, and thrombocytopenia. These events are common in people treated by FOLFOX (45–47). Neutropenia is a regular occurrence in patients during postoperative chemotherapy, which is also one of the serious adverse events of chemotherapy. Our data show that patients with high SUA levels had considerably higher incidences of neutropenia than those with low SUA levels. High concentrations of SUA increase intracellular oxidation (6). In addition, by activating calpain-1 and endoplasmic reticulum stress (ERS), excessive UA induces apoptosis in normal cells (48). As a result, we hypothesize that the increased prevalence of neutropenia in patients with high SUA levels could be linked to the fact that UA enhances the apoptotic action of chemotherapeutic medicines on neutrophils. There was no significant association between the occurrence of adverse events and blood UA levels in individuals who received FOLFIRI as postoperative chemotherapy. We speculate that this is due to the sample size being insufficient. For patients treated with XELOX, we have not observed significant association between the incidence of adverse events and SUA levels either, which could be due to the difference in the mechanism of action of capecitabine and 5-FU.
Our research has some limitations. We only focused on the values of baseline SUA, and failed to include the SUA levels after chemotherapy. Secondly, the current study was based on retrospective observations, future prospective studies with larger samples are needed to confirm our findings. Thirdly, the number of high SUA patients was much less than that of non-HUA patients, and this may lead to the inaccuracy of the statistics analysis. Further studies with larger sample size or multicenter studies are needed to validate our findings.
In summary, our findings imply that the baseline blood uric acid level is an important biomarker correlated with the clinical prognosis, DCR, and safety of postoperative chemotherapy for CRC. Elevated baseline SUA is associated with poor prognosis in non-metastatic CRC patients treated by FOLFOX and in male patients treated by FOLFIRI, low DCR in patients with FOLFIRI, and reduced safety in patients with FOLFOX.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Chinese Ethics Committee of Registering Clinical Trials. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
XZ, Q-hC, YY, J-xL, and Y-cL: conceptualization, data curation, formal analysis, investigation, software, and visualization. XZ: methodology and writing–original draft. T-yZ, JC, S-qW, X-hC, R-sZ, J-mL, D-qW, Q-xH, Y-tY, X-hZ, QZ, Y-yL, and J-rC: writing–review and editing. X-sZ and Y-fW: funding acquisition, project administration, resources, and supervision. All authors contributed to the article and approved the submitted version.
This work was supported by the Key Project of National Natural Science Foundation of China (81830117), the National Natural Science Foundation of China (81803877, 81873205), the Natural Science Foundation of Guangdong Province, China (2020B1515120063, 2021A1515110990), and the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-C-202001).
We are very grateful to Professor Hiu Yee Kwan from Hong Kong Baptist University for polishing our manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Gravalos C, Garcia-Escobar I, Garcia-Alfonso P, Cassinello J, D. Malon and A. Carrato: Adjuvant chemotherapy for stages II, III and IV of colon cancer. Clin Transl Oncol (2009) 11(8):526–33. doi: 10.1007/s12094-009-0397-8
3. Liu S, Wang J, Niu W, Liu E, Wang J, Peng C, et al. The beta6-integrin-ERK/MAP kinase pathway contributes to chemo resistance in colon cancer. Cancer Lett (2013) 328(2):325–34. doi: 10.1016/j.canlet.2012.10.004
4. Ahn JY, Lee JS, Min HY, Lee HY. Acquired resistance to 5-fluorouracil via HSP90/Src-mediated increase in thymidylate synthase expression in colon cancer. Oncotarget (2015) 6(32):32622–33. doi: 10.18632/oncotarget.5327
5. Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des (2005) 11(32):4145–51. doi: 10.2174/138161205774913255
6. Kang DH, Ha SK. Uric acid puzzle: Dual role as anti-oxidantand pro-oxidant. Electrolyte Blood Press (2014) 12(1):1–6. doi: 10.5049/EBP.2014.12.1.1
7. Battelli MG, Bortolotti M, L. Polito and A. Bolognesi: The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim Biophys Acta Mol Basis Dis (2018) 1864(8):2557–65. doi: 10.1016/j.bbadis.2018.05.003
8. Horsfall LJ, Nazareth I, Petersen I. Serum uric acid and the risk of respiratory disease: a population-based cohort study. Thorax (2014) 69(11):1021–6. doi: 10.1136/thoraxjnl-2014-205271
9. Kim IY, Lee DW, Lee SB, Kwak IS. The role of uric acid in kidney fibrosis: experimental evidences for the causal relationship. BioMed Res Int (2014) 2014:638732. doi: 10.1155/2014/638732
10. Yan S, Zhang P, Xu W, Liu Y, Wang B, Jiang T, et al. Serum uric acid increases risk of cancer incidence and mortality: A systematic review and meta-analysis. Mediators Inflammation (2015) 2015:764250. doi: 10.1155/2015/764250
11. Chen CJ, Yen JH, Chang SJ. Gout patients have an increased risk of developing most cancers, especially urological cancers. Scand J Rheumatol (2014) 43(5):385–90. doi: 10.3109/03009742.2013.878387
12. Wang W, Xu D, Wang B, Yan S, Wang X, Yin Y, et al. Increased risk of cancer in relation to gout: A review of three prospective cohort studies with 50,358 subjects. Mediators Inflammation (2015) 2015:680853. doi: 10.1155/2015/680853
13. Kuo CF, Luo SF, See LC, Chou IJ, Fang YF, Yu KH. Increased risk of cancer among gout patients: a nationwide population study. Joint Bone Spine (2012) 79(4):375–8. doi: 10.1016/j.jbspin.2011.09.011
14. Kim HJ, Kim JE, Jung JH, Kim ER, Hong SN, Chang DK, et al. Uric acid is a risk indicator for metabolic syndrome-related colorectal adenoma: Results in a Korean population receiving screening colonoscopy. Korean J Gastroenterol (2015) 66(4):202–8. doi: 10.4166/kjg.2015.66.4.202
15. Tanriverdi O, Cokmert S, Oktay E, Pilanci KN, Menekse S, Kocar M, et al. Prognostic significance of the baseline serum uric acid level in non-small cell lung cancer patients treated with first-line chemotherapy: a study of the Turkish descriptive oncological researches group. Med Oncol (2014) 31(10):217. doi: 10.1007/s12032-014-0217-z
16. Chen YF, Li Q, Chen DT, Pan JH, Chen YH, Wen ZS, et al. Prognostic value of pre-operative serum uric acid levels in esophageal squamous cell carcinoma patients who undergo R0 esophagectomy. Cancer biomark (2016) 17(1):89–96. doi: 10.3233/CBM-160621
17. Yang S, He X, Liu Y, Ding X, Jiang H, Tan Y, et al. Prognostic significance of serum uric acid and gamma-glutamyltransferase in patients with advanced gastric cancer. Dis Markers (2019) 2019:1415421. doi: 10.1155/2019/1415421
18. Yu M, Zhang C, Tang H, Xiao C. Correlation between serum oxidative stress level and serum uric acid and prognosis in patients with hepatitis b-related liver cancer before operation. J Healthc Eng (2022) 2022:1964866. doi: 10.1155/2022/1964866
19. Yue CF, Feng PN, Yao ZR, Yu XG, Lin WB, Qian YM, et al. High serum uric acid concentration predicts poor survival in patients with breast cancer. Clin Chim Acta (2017) 473:160–5. doi: 10.1016/j.cca.2017.08.027
20. Yamauchi T, Negoro E, Lee S, Takai M, Matsuda Y, Takagi K, et al. A high serum uric acid level is associated with poor prognosis in patients with acute myeloid leukemia. Anticancer Res (2013) 33(9):3947–51.
21. Prochazka KT, Melchardt T, Posch F, Schlick K, Deutsch A, Beham-Schmid C, et al. NCCN-IPI score-independent prognostic potential of pretreatment uric acid levels for clinical outcome of diffuse large b-cell lymphoma patients. Br J Cancer (2016) 115(10):1264–72. doi: 10.1038/bjc.2016.325
22. Ustuner MA, Dogan L. Relationship of preoperative serum uric acid level with survival in colorectal cancer. J Coll Phys Surg Pak (2020) 30(7):717–21. doi: 10.29271/jcpsp.2020.07.717
23. Yuan C, Xu XH, Wang XL, Xu L, Chen Z, Li YQ. Relationship between serum uric acid and metastatic and nonmetastatic rectal cancer patients with undergoing no chemotherapy. Med (Baltimore) (2016) 95(47):e5463. doi: 10.1097/MD.0000000000005463
24. Selcukbiricik F, Kanbay M, Solak Y, Bilici A, Kanitez M, Balik E, et al. Serum uric acid as a surrogate marker of favorable response to bevacizumab treatment in patients with metastatic colon cancer. Clin Transl Oncol (2016) 18(11):1082–7. doi: 10.1007/s12094-016-1485-1
25. Bohuslav M, Radomír H, Hana K, Petra H, Hana S. Serum uric acid and c-reactive protein in patients with metastatic colorectal carcinoma during combination therapy with high-dose folinic acid. Pteridines (2013) 23(1):8–13. doi: 10.1515/pteridines.2012.23.1.8
26. Sun J, Xing ZY, Yu SN, Chen J, Zha TT, Fan M, et al. [Correlation between susceptibility weighted imaging manifestation and serum cystatin c for delayed graft function]. Zhonghua Yi Xue Za Zhi (2016) 96(21):1682–6. doi: 10.3760/cma.j.issn.0376-2491.2016.21.014
27. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of Canada. J Natl Cancer Inst (2000) 92(3):205–16. doi: 10.1093/jnci/92.3.205
28. Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the common terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed) (2021) 112(1):90–2. doi: 10.1016/j.ad.2019.05.009
29. Cetin AO, Omar M, Calp S, Tunca H, Yimaz N, Ozseker B, et al. Hyperuricemia at the time of diagnosis is a factor for poor prognosis in patients with stage II and III colorectal cancer (Uric acid and colorectal cancer). Asian Pac J Cancer Prev (2017) 18(2):485–90. doi: 10.22034/APJCP.2017.18.2.485
30. Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol (2004) 22(2):229–37. doi: 10.1200/JCO.2004.05.113
31. Roque IFM, Sola I, Martin-Richard M, Lopez JJ, Bonfill CX. Second-line chemotherapy in advanced and metastatic CRC. Cochrane Database Syst Rev (2009) 2):CD006875. doi: 10.1002/14651858.CD006875.pub2
32. Sorbye H, Berglund A, Tveit KM, Ogreid D, Wanderas EH, Wentzel-Larsen T, et al. Secondary treatment and predictive factors for second-line chemotherapy after first-line oxaliplatin-based therapy in metastatic colorectal cancer. Acta Oncol (2007) 46(7):982–8. doi: 10.1080/02841860701261568
33. Zeng M, Chen B, Qing Y, Xie W, Dang W, Zhao M, et al. Estrogen receptor beta signaling induces autophagy and downregulates Glut9 expression. Nucleosides Nucleotides Nucleic Acids (2014) 33(7):455–65. doi: 10.1080/15257770.2014.885045
34. Ee PL, Kamalakaran S, Tonetti D, He X, Ross DD, Beck WT. Identification of a novel estrogen response element in the breast cancer resistance protein (ABCG2) gene. Cancer Res (2004) 64(4):1247–51. doi: 10.1158/0008-5472.can-03-3583
35. Budhiraja R, Kayyali US, Karamsetty M, Fogel M, Hill NS, Chalkley R, et al. Estrogen modulates xanthine dehydrogenase/xanthine oxidase activity by a receptor-independent mechanism. Antioxid Redox Signal (2003) 5(6):705–11. doi: 10.1089/152308603770380007
36. Stefanska A, Bergmann K, Sypniewska G. Metabolic syndrome and menopause: Pathophysiology, clinical and diagnostic significance. Adv Clin Chem (2015) 72:1–75. doi: 10.1016/bs.acc.2015.07.001
37. Braga TT, Forni MF, Correa-Costa M, Ramos RN, Barbuto JA, Branco P, et al. Soluble uric acid activates the NLRP3 inflammasome. Sci Rep (2017) 7 39884. doi: 10.1038/srep39884
38. Joosten L, Crisan TO, Bjornstad P, Johnson RJ. Asymptomatic hyperuricaemia: a silent activator of the innate immune system. Nat Rev Rheumatol (2020) 16(2):75–86. doi: 10.1038/s41584-019-0334-3
39. Cabau G, Crisan TO, Kluck V, Popp RA, Joosten L. Urate-induced immune programming: Consequences for gouty arthritis and hyperuricemia. Immunol Rev (2020) 294(1):92–105. doi: 10.1111/imr.12833
40. Burlaka AP, Ganusevich II, Gafurov MR, Lukin SM, Sidorik EP. Stomach cancer: Interconnection between the redox state, activity of MMP-2, MMP-9 and stage of tumor growth. Cancer Microenviron (2016) 9(1):27–32. doi: 10.1007/s12307-016-0182-5
41. Holmberg C, Ghesquiere B, Impens F, Gevaert K, Kumar JD, Cash N, et al. Mapping proteolytic processing in the secretome of gastric cancer-associated myofibroblasts reveals activation of MMP-1, MMP-2, and MMP-3. J Proteome Res (2013) 12(7):3413–22. doi: 10.1021/pr400270q
42. Prasad S, Gupta SC, Tyagi AK. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett (2017) 387:95–105. doi: 10.1016/j.canlet.2016.03.042
43. Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer (1998) 34(8):1274–81. doi: 10.1016/s0959-8049(98)00058-6
44. Schuller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, et al. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol (2000) 45(4):291–7. doi: 10.1007/s002800050043
45. Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med (2004) 350(23):2343–51. doi: 10.1056/NEJMoa032709
46. Uncu D, Aksoy S, Cetin B, Yetisyigit T, Ozdemir N, Berk V, et al. Results of adjuvant FOLFOX regimens in stage III colorectal cancer patients: retrospective analysis of 667 patients. Oncology (2013) 84(4):240–5. doi: 10.1159/000336902
47. Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol (2004) 15(3):460–6. doi: 10.1093/annonc/mdh095
Keywords: uric acid, colorectal cancer, prognosis, disease control rate, safety
Citation: Zhang X, Chen Q-h, Yang Y, Lin J-x, Li Y-c, Zhong T-y, Chen J, Wu S-q, Chen X-h, Zhou R-s, Lin J-m, Wang D-q, He Q-x, You Y-t, Zhou X-h, Zuo Q, Liu Y-y, Cheng J-r, Wu Y-f and Zhao X-s (2022) Baseline serum uric acid level is associated with progression-free survival, disease control rate, and safety in postoperative patients with colorectal cancer treated by FOLFOX, FOLFIRI, or XELOX. Front. Oncol. 12:918088. doi: 10.3389/fonc.2022.918088
Received: 12 April 2022; Accepted: 28 June 2022;
Published: 25 July 2022.
Edited by:
Ying Yuan, Zhejiang University, ChinaReviewed by:
Chenyang Ye, Zhejiang University, ChinaCopyright © 2022 Zhang, Chen, Yang, Lin, Li, Zhong, Chen, Wu, Chen, Zhou, Lin, Wang, He, You, Zhou, Zuo, Liu, Cheng, Wu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-fen Wu, d2d5Zl85OUAxMjYuY29t; Xiao-shan Zhao, emhhb3hzMDYwOUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.