
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 26 September 2022
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.917353
This article is part of the Research Topic Novel Biomarkers for Predicting Response to Cancer Immunotherapy View all 69 articles
 Caiyun Nie1,2,3,4
Caiyun Nie1,2,3,4 Huifang Lv1,2,3,4
Huifang Lv1,2,3,4 Beibei Chen1,2,3,4
Beibei Chen1,2,3,4 Weifeng Xu1,2,3,4
Weifeng Xu1,2,3,4 Jianzheng Wang1,2,3,4
Jianzheng Wang1,2,3,4 Yingjun Liu5
Yingjun Liu5 Saiqi Wang1,2,3,4
Saiqi Wang1,2,3,4 Jing Zhao1,2,3,4
Jing Zhao1,2,3,4 Yunduan He1,2,3,4
Yunduan He1,2,3,4 Xiaobing Chen1,2,3,4*
Xiaobing Chen1,2,3,4*Objectives: The antitumor activity of nivolumab plus regorafenib in colorectal cancer from a phase Ib REGONIVO study is encouraging. The present study was conducted to evaluate the efficacy and safety of regorafenib or fruquintinib plus sintilimab as third-line or above therapy in patients with microsatellite stable (MSS) metastatic colorectal cancer.
Methods: Patients with MSS metastatic colorectal cancer who have failed from prior treatment and received regorafenib or fruquintinib plus sintilimab as third-line or above therapy from January 2019 to December 2020 were prospectively analyzed based on real-world clinical practice. The primary end point was progression free survival (PFS). Secondary end points included objective response rate (ORR), disease control rate (DCR), overall survival (OS), and safety.
Results: 42 patients received regorafenib plus sintilimab(RS), and the other 30 patients received fruquintinib plus sintilimab(FS). In the general population, the ORR and DCR were 13.9% and 70.8%, and the median PFS and OS was 4.2(95% CI=2.9-5.5) and 10.5 (95% CI=8.6-12.4) months, respectively. There were no statistically significant differences between RS and FS group in PFS (3.5(2.2-4.8) vs. 5.5(3.5-7.5) months, P=0.434) and OS (11.0(7.0-15.0) vs. 10.5(3.8-17.2) months, P=0.486). Subgroup analysis suggested that patients without liver metastasis responded well to this combination regimen (ORR: 21.4% vs. 9.1%) and obtained better OS (26(8.8-43.2) vs. 10.0(7.4-12.6) months, P=0.016). The incidence of Grade 3-4 adverse events (AEs) was 15.3% and the toxicities were generally tolerable and manageable.
Conclusions: Regorafenib or fruquintinib plus sintilimab as third-line or above therapy provide a feasible treatment regimen for MSS metastatic colorectal cancer with tolerated toxicity. Patients without liver metastasis may be the preferred population for this combination regimen.
Colorectal cancer (CRC) is the third most common cancer in men and the second most common cancer in women worldwide (1). In the past, the incidence of colorectal cancer in China was much lower than that in western countries. However, the incidence of colorectal cancer has increased rapidly in recent years, which has become the most common malignant tumor of digestive system. 80% of patients are in the advanced stage when they are diagnosed, which greatly affects the prognosis of colorectal cancer. At present, the level of diagnosis and treatment of metastatic colorectal cancer has made great progress, precision therapy guided by genetic status detection and differentiation of primary tumor sites(left vs. right) has become the main treatment strategy for colorectal cancer (2–4).
Compared with other solid tumors, immunotherapy for colorectal cancer is relatively backward (5). Until 2015, the KEYNOTE-016 study opened the immunotherapy era of microsatellite instability-high(MSI-H) colorectal cancer (6). However, MSI-H tumors account for only about 5%, and the remaining 95% are microsatellite stable(MSS) type colorectal cancer. As a representative of “cold tumors”, immunotherapy seems to be helpless in MSS tumors, and many exploratory studies have failed (7, 8). The antitumor activity of nivolumab plus regorafenib in a colorectal cancer cohort from a phase Ib REGONIVO study is encouraging. The ORR of 25 patients was 36% (ORR of MSS patients was 33%), the median PFS was 7.9 months and the median OS was not reached (9). This is by far the most effective third-line treatment regimen for colorectal cancer. However, this is a phase Ib exploratory study with only 24 patients of MSS colorectal cancer.
Although regorafenib and fruquintinib improved prognosis in metastatic colorectal cancer, the objective response rates of regorafenib and fruquintinib monotherapy in the CORRECT and FRESCO studies were only 1.0% and 4.7%, respectively. In the phase Ib study REGONIVO announced at the 2019 ASCO meeting, the ORR of regorafenib combined with PD-1 antibody was as high as 33% in patients with MSS metastatic colorectal cancer, which were significantly higher than regorafenib and fruquintinib monotherapy. Multi-targeted antiangiogenic TKIs combined with immunotherapy have become a new treatment strategy for MSS colorectal cancer. Since then, a number of prospective single-arm studies explored the efficacy of TKIs combined with immunotherapy in the third-line treatment of MSS metastatic colorectal cancer, including REGONIVO (North America), REGOMUNE, REGOTORI, etc (10–12).
Due to the limited sample size, the results of REGONIVO still need to be further verified. And simultaneously, the efficacy of regorafenib or fruquintinib plus novel immune checkpoint inhibitors (ICIs) has not been reported. The present study was conducted to evaluate the efficacy and safety of regorafenib or fruquintinib plus sintilimab as third-line or above therapy in patients with MSS metastatic colorectal cancer.
From January 2019 to December 2020, patients with MSS metastatic colorectal cancer who have failed from prior treatment and received regorafenib or fruquintinib plus sintilimab as third-line or above therapy from Henan Cancer Hospital were prospectively analyzed based on real-world clinical practice. Immunohistochemistry (IHC) staining of four kinds of MMR protein (MLH1,MSH2,MSH6,PMS2) or polymerase chain reaction (PCR) analysis of five microsatellite markers (BAT25,BAT26,D5S346,D2S123,D17S250) were used to determine MSS status of colorectal cancer patients.
In this study, the patients received regorafenib or fruquintinib in combination with PD-1 inhibitor sintilimab until disease progression, unacceptable toxicity or death. In the regorafenib plus sintilimab group (RS), sintilimab was administered intravenously at a dose of 200 mg once every three weeks, and regorafenib was given orally at a dose of 80 or 120 mg once a day on d1 to d21 every 28 days. In this study, we used regorafenib as the starting dose of 80 mg and adjusted to 120 mg after one week of use, which reduced from 120 to 80 mg in case of intolerable toxicity. In the fruquintinib plus sintilimab group(FS), sintilimab was given as the same dose and fruquintinib was given orally at a dose of 5 mg once a day on d1 to d21 every 4 weeks, which reduced from 4 mg in case of intolerable toxicity.
After treatment, all patients underwent imaging examination every two cycles (6 weeks) to evaluate the clinical efficacy. The efficacy evaluation criteria are RECIST version 1.1 response evaluation criteria in solid tumors, including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The objective response rate (ORR) was CR + PR, and the disease control rate (DCR) was CR+ PR and SD. Adverse events (AEs) were assessed according to the Common Terminology Criteria for Adverse Events, version 4.0.
Survival curves of patients were estimated by the Kaplan-Meier method and compared using the log-rank test. The follow-up deadline is January 31, 2022. Progression-free survival (PFS) was defined as starting regorafenib or fruquintinib plus sintilimab as third-line or above treatment to disease progression or death. Overall survival (OS) was defined as the period from the time of regorafenib or fruquintinib plus sintilimab as third-line or above treatment to patient death or last follow-up. Difference between groups were determined by Pearson’s chi squared test or Fisher’s exact test. Receiver operating characteristics (ROC) analysis was applied to determine the cut-off value of Mean Platelet Volume (MPV), Neutrophil-to-Lymphocyte Ratio(NLR), lactate dehydrogenase (LDH) and D-Dimer. Subgroup analysis of predictive factor for PFS and OS was carried out by Cox proportional hazards model. All the statistical descriptive analyses were performed with SPSS 22.0 software (SPSS Inc., IL, US) software. P<0.05 was considered significant.
A total of 72 patients with MSS metastatic colorectal cancer who have failed from prior treatment and received regorafenib or fruquintinib plus sintilimab as third-line or above therapy were included in the present study. Patient and treatment characteristics are summarized in Table 1. The median age was 57 years (range 32-78), with 36 female patients and 36 male patients. Primary tumor site in 54 patients were left colon, 16 patients had right colon cancer, and the other 2 patients were diagnosed as rectal cancer. Number of metastatic sites in 33(45.8%) patients were 1 or 2, and the other 39(54.2%) patients were 3 or more. The common metastatic sites included lymph node (65.3%), lung (62.5%), liver (61.1%) and peritoneum (27.8%). Regorafenib or fruquintinib plus sintilimab were given as third-line therapy in 39(54.2%) patients, and as fourth-line or above therapy in the other 33(45.8%) patients. All the patients included in the present study were confirmed as MSS status. KRAS, NRAS and BRAF gene were also detected. For KRAS, 32(44.4%) patients were wide type, 32(44.4%) patients were mutant. NRAS and BRAF gene in most patients were wide type(86.1% and 86.1%, respectively). Chemotherapy and targeted therapy are main prior treatment regimen. Chemotherapy regimens include FOLFOX and FOLFIRI. 60(83.3%) patients received anti-VEGF therapy with bevacizumab and 21(29.2%) patients received anti-EGFR therapy with cetuximab. A small proportion of patients(13.9%) had previously received regorafenib, and no patients received fruquintinib in prior therapy. Forty-two patients received regorafenib in combination with sintilimab and the other 30 patients received fruquintinib plus sintilimab. The baseline clinicopathological characteristics in the two groups were similar.
In the general population, CR was not observed, 10 patients achieved PR, 41 patients had SD and 21 patients had PD. The overall ORR and DCR were 13.9% (10/72) and 70.8% (51/72), respectively (Table 2). In the RS population, CR was not observed, 5 patients achieved PR, 20 patients had SD and 17 patients had PD. The overall ORR and DCR were 11.9% (5/42) and 59.5% (25/42), respectively. In the FS group, CR was not observed, 5 patients achieved PR, 21 patients had SD and 4 patients had PD. The overall ORR and DCR were 16.7% (5/30) and 86.7% (26/30), respectively. The patients in FS group had higher DCR than RS population (P=0.012), but there was no statistical difference in ORR between the two groups. Meanwhile, the ORR and DCR in patients with different tumor site (left colon vs. right colon), KRAS status (wide type vs. mutant) and metastatic site (with liver metastasis vs. without liver metastasis) were also analyzed, no statistical differences were found between groups. 13.9% of patients have used regorafenib in the previous treatment, there were no statistically significant differences in ORR and DCR between patients with and without prior regorafenib therapy.
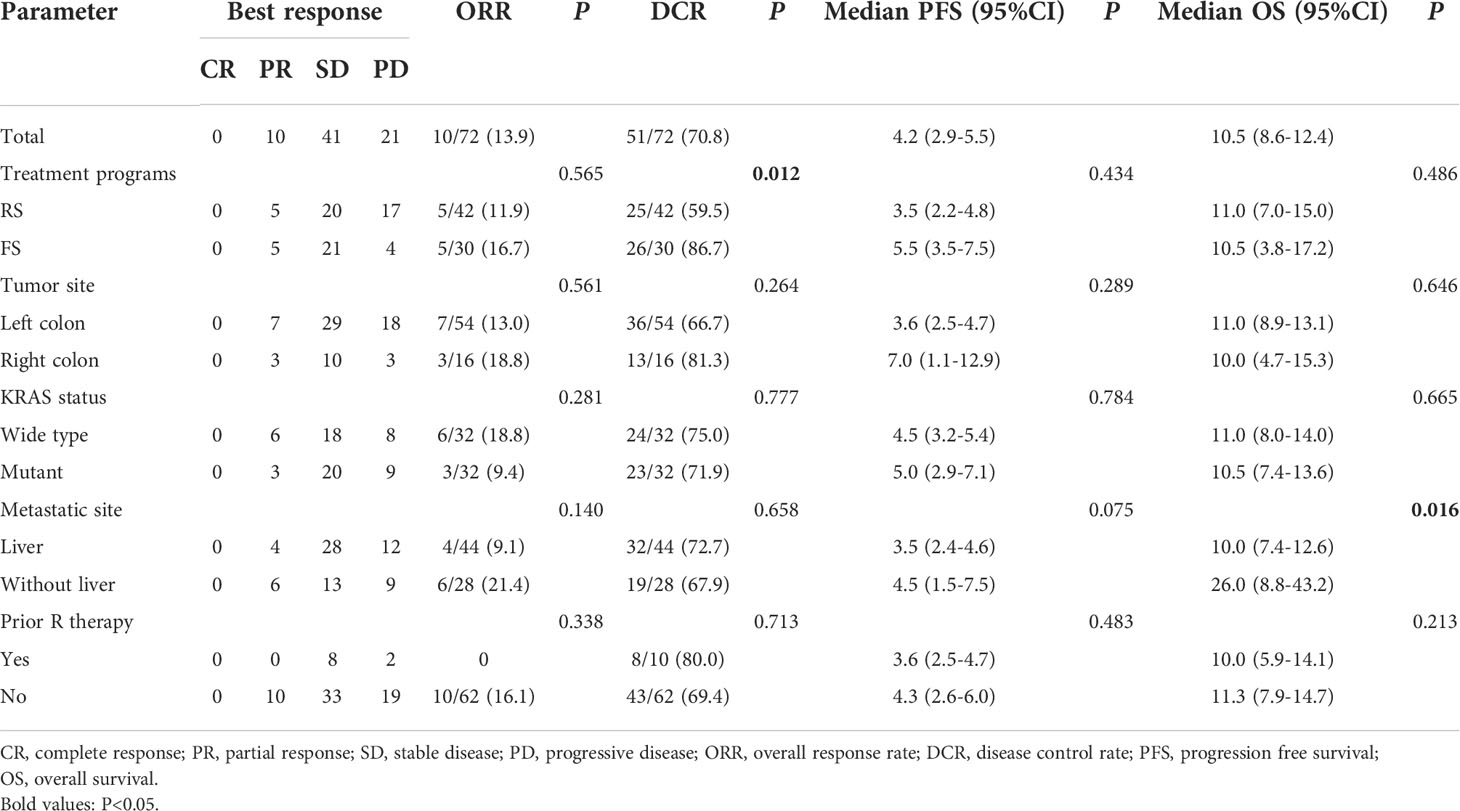
Table 2 Efficacy of regorafenib or fruquintinib plus sintilimab in metastatic MSS colorectal cancer.
In the general population, the median PFS and median OS were 4.2 (95% CI= 2.9-5.5) and 10.5 (95% CI= 8.6-12.4) months, respectively (Figures 1A, B). The median PFS were 3.5 (95% CI= 2.2-4.8) and 5.5 (95% CI= 3.5-7.5) months in the RS and FS population, respectively (P = 0.434; Figure 1C). The median OS in the two groups were 11.0 (95% CI=7.0-15.0) months and 10.5 (95% CI=3.8-17.2) months, respectively (P = 0.486; Figure 1D). Simultaneously, the median PFS and OS in patients with different tumor site (left colon vs. right colon), KRAS status (wide type vs. mutant), metastatic site (with liver metastasis vs. without liver metastasis) and prior regorafenib therapy (Yes vs. No) were also compared, no statistical differences were found between groups with different tumor site, KRAS status and with or without prior regorafenib therapy (Figure 2). However, although no statistical difference exists in median PFS between patients with liver metastasis or without liver metastasis (3.5(2.4-4.6) vs. 4.5(1.5-7.5) months, P=0.075), the median OS in patients without liver metastasis was significantly better than patients with liver metastasis (26.0(8.8-43.2) vs. 10.0(7.4-12.6) months, P=0.016, Figure 2).
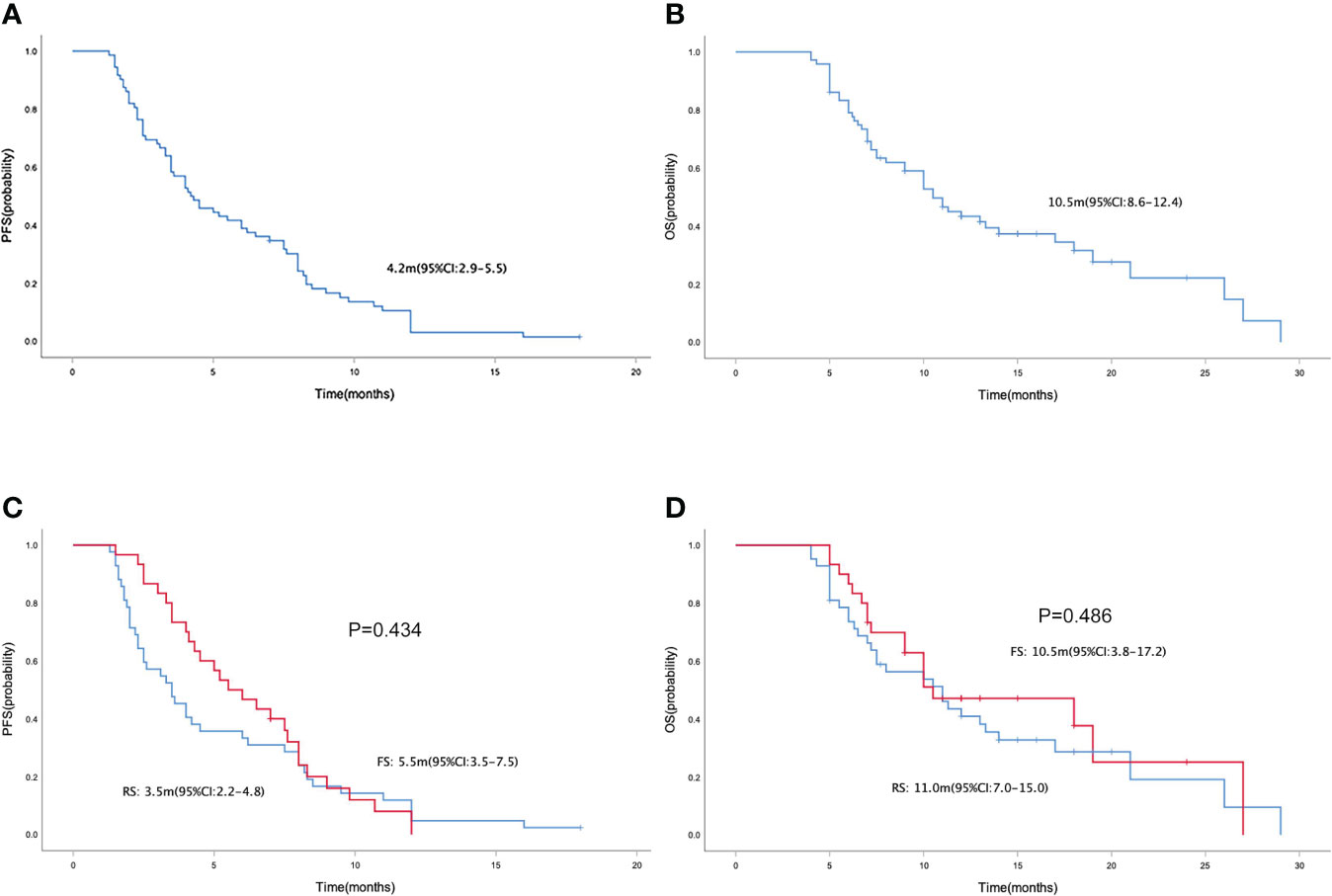
Figure 1 Kaplan-Meier curve of PFS (A) and OS (B) in the general population. Kaplan-Meier curve of PFS (C) and OS (D) in the regorafenib plus sintilimab(RS), and fruquintinib plus sintilimab(FS) group.
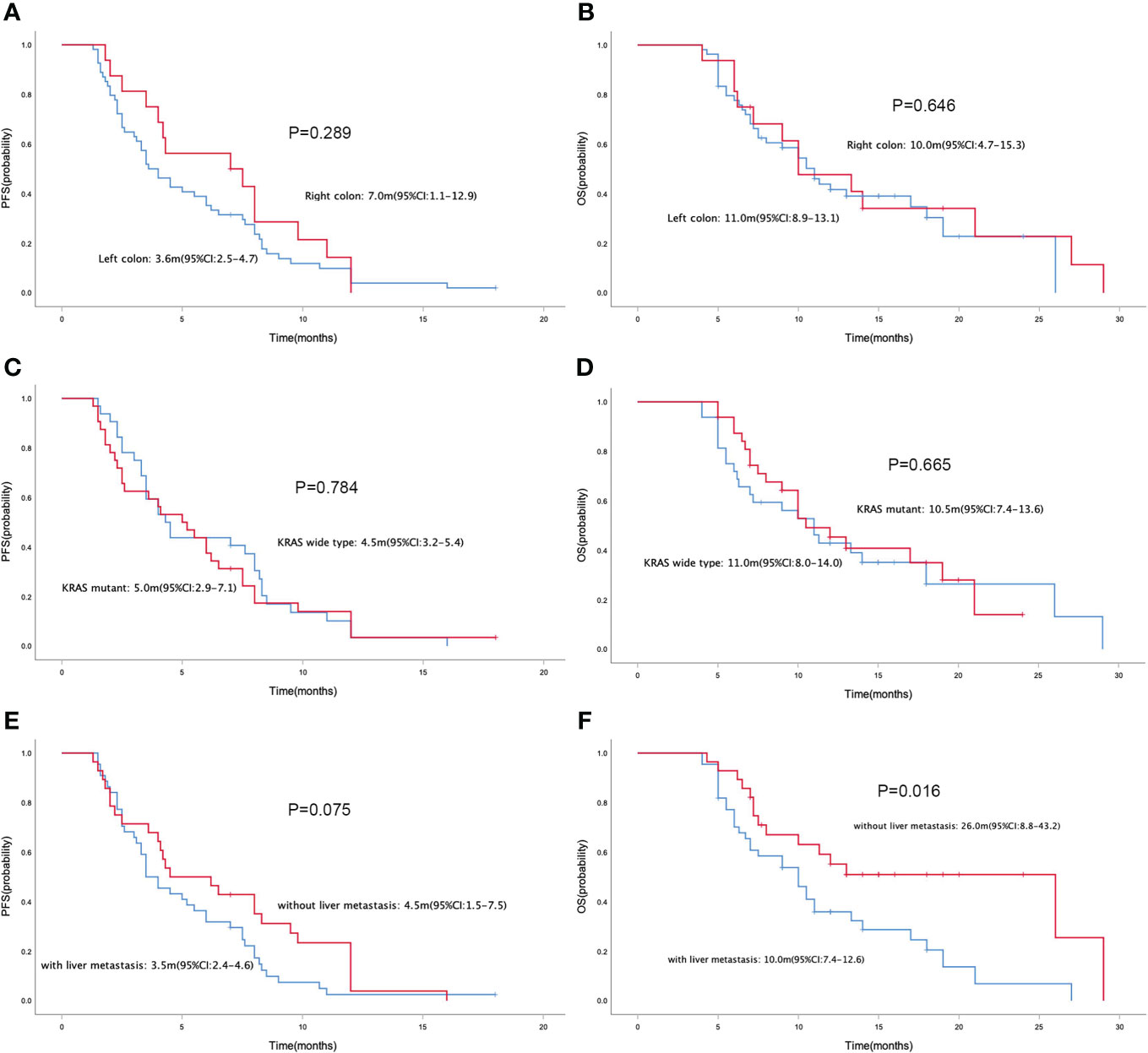
Figure 2 Kaplan-Meier curve of PFS (A) and OS (B) in patients with different primary tumor site (left colon vs. right colon). Kaplan-Meier curve of PFS (C) and OS (D) in patients with different KRAS status (wide type vs. mutant). Kaplan-Meier curve of PFS (E) and OS (F) in patients with liver metastasis or without liver metastasis.
The present study also performed univariate analysis to evaluate the predictive value of clinicopathologic factors for PFS and OS, including sex (male vs. female), age (<65 vs. ≥65), treatment program (RS vs. FS), primary tumor site (left colon vs. right colon), liver metastasis (with vs. without), KRAS status (wide type vs. mutant), MPV (<9.9 vs. ≥9.9), NLR (<2.15 vs. ≥2.15), LDH (<312 vs. ≥312) and D-Dimer (<0.89 vs. ≥0.89). None of the above factors were found to be predictive factors for PFS. For OS, only with or without liver metastasis was confirmed to be a potential predictive factor (P=0.021, Table 3). We compared NLR and LDH between colorectal cancer patients with and without liver metastasis and no statistical difference was found in NLR between the two groups (P=0.330). However, the baseline LDH levels in patients with liver metastasis were significantly higher than those without liver metastasis (median level: 278 U/L vs. 218 U/L, P=0.000, Figure 3).
Most of the adverse events were grade 1-2 in severity and the incidence of Grade 3-4 AEs was 15.3% (Table 4). No unexpected side effects or treatment-related death were observed. The dose reduction and treatment interruptions as a result of serious adverse events occurred in 28 (38.9%) and 33 (45.8%) patients, respectively. The most common treatment-related hematological AEs were increased ALT/AST (n=13, 18.1%), anemia (n=11, 15.3%), decreased white blood count (n=6, 8.3%), hyperbilirubinemia (n=5,6.9%), and decreased platelet (n=4, 5.6%). Non-hematological treatment-related AEs were fatigue (n=23, 31.9%), decreased appetite (n=22, 30.6%), secondary hypertension (n=17, 23.6%), hypothyroidism (n=15, 20.8%), oral mucositis (n=14, 19.4%), diarrhea (n=10, 13.9%), hand-foot syndrome (n=8, 11.1%), proteinuria (n=4, 5.6%), rash (n=4, 5.6%), pneumonitis (n=3, 4.2%), pyrexia (n=2, 2.8%). Grade 3-4 AEs were decreased platelet (n=1, 1.4%), increased ALT/AST (n=2, 2.8%), secondary hypertension (n=4, 5.6%), hand-foot syndrome (n=2, 2.8%), rash (n=1, 1.4%) and pneumonitis (n=1, 1.4%).
Colorectal cancer is a highly heterogeneous disease, and stratified therapy based on genetic testing is currently the main strategy for third-line treatment of metastatic colorectal cancer. For MSI-H colorectal cancer, immunotherapy is preferred recommended treatment regimen (13–15). However, for the vast majority of patients with MSS type, single-agent chemotherapy and immunotherapy are almost ineffective. The international multicenter phase 3 clinical CORRECT study for the first time confirmed the OS benefit of regorafenib in refractory advanced colorectal cancer. The results showed that the median OS of the regorafenib group reached 6.4 months, which was significantly longer than that of the placebo control group (16). The FRESCO study evaluated the efficacy and safety of fruquintinib as third-line or later therapy in 416 patients with metastatic CRC. The results showed that the median OS of patients in the fruquintinib group was 9.3 months, which was 2.7 months longer than that in the placebo group, and the median PFS was extended from 1.8 months in the placebo group to 3.7 months (17). Based on the above clinical trials, small molecule tyrosine kinase inhibitors including regorafenib and fruquintinib are the standard third-line treatment options for colorectal cancer recommended in the current guidelines.
In addition to monotherapy, regorafenib and fruquintinib combined with immunotherapy has become a new treatment strategy. Most studies obtained consistent findings with the REGONIVO study, however in REGONIVO (North America) trial, the ORR of regorafenib combined with nivolumab was 7% in patients with MSS metastatic colorectal cancer, PFS and OS were 1.8 and 11.9 months respectively, which were worse than previous studies. Therefore, the ideal drug selection, dosage, benefit population for TKIs combined with immunotherapy in metastatic colorectal cancer still need to be further explored. Our present study evaluated the efficacy of regorafenib and fruquintinib plus sintilimab as third-line or above therapy in patients with MSS metastatic colorectal cancer, the overall ORR and DCR reached 13.9% and 70.8%, respectively. Although the ORR in our study is lower than previous clinical trials, it is worth noting that 54.2% patients received regorafenib or fruquintinib plus sintilimab as third-line therapy, and as fourth-line or above therapy in the other 45.8% patients. At the same time, the DCR was 70.8%, and median PFS and OS reached 4.2 and 10.5 months respectively, so regorafenib or fruquintinib plus sintilimab therapy still achieved a good therapeutic effect in such a relatively late-line patient population.
MSS-type colorectal cancer has been referred to as a “cold tumor” due to the low response to single-agent immunotherapy. Combination immunotherapy, including chemotherapy, targeted therapy or other immunomodulatory agents, to change it from “cold tumor” to “hot tumor”, is being actively explored. Multi-targeted antiangiogenic TKIs, including regorafenib and fruquintinib achieved better effect. Immunosuppressive cells such as regulatory T cells (Tregs) and tumor associated macrophages (TAMs) exist in the tumor microenvironment of patients with MSS colorectal cancer, which can suppress T cell activity (18–20). Basic research has shown that regorafenib can relieve the immunosuppression of Treg and TAM cells on T cells by inhibiting CSF1R and VEGFR to enhance the efficacy of immunotherapy (21, 22). Several previous retrospective studies have compared the efficacy of regorafenib and fruquintinib in combination with immunotherapy, and the results are inconsistent (23, 24). In our present study, except for DCR, fruquintinib was superior to regorafenib(86.7% vs. 59.5%, P=0.012), there were no significant differences in ORR, PFS and OS between the two groups.
However, not all MSS colorectal cancer patients respond well to this combination therapy mode, which means that it is necessary to further explore effective biomarkers and stratify the patient population to improve the survival benefit of patients. Subgroup analysis of predictive factors for PFS and OS demonstrated that the clinical benefit of this regimen was not related with sex, age, treatment program, primary tumor site, KRAS status, MPV, NLR, LDH and D-Dimer. However, patients without liver metastasis responded well to this combination regimen(ORR: 21.4% vs. 9.1%), and meantime although no statistical difference exists in median PFS between patients with liver metastasis or without liver metastasis, the median OS in patients without liver metastasis was significantly better than patients with liver metastasis. Liver is a common metastatic site of colorectal cancer, and liver metastasis is also the main cause of death in patients with colorectal cancer. Metastasis site may be a predictor of immunotherapy efficacy (25). In colorectal cancer, patients with liver metastasis have a suboptimal response to immunotherapy and have a poor prognosis (26). The REGOTORI study evaluated the efficacy of regorafenib plus toripalimab in patients with metastatic colorectal cancer, the ORR of patients with liver metastases was lower than that of patients without liver metastases (8.7% and 30.0%, respectively). Our present study yielded consistent findings that patients without liver metastasis responded well to this combination regimen and benefited more. Recent studies have shown that liver metastases suppress systemic antitumor immune responses and suppress immunotherapy efficacy by reducing systemic CD8+ T cells (27). LDH might be an indirect sign of activated tumor angiogenesis and immunosuppression, and our study also found that patients with liver metastasis had higher levels of LDH. For MSS metastatic colorectal cancer patients with liver metastasis, it is necessary to explore more effective treatment options.
13.9% of patients have used regorafenib in the previous treatment, there were no statistically significant differences in ORR, DCR, median PFS and median OS between patients with prior regorafenib therapy and without prior regorafenib therapy. This suggests that regorafenib or fruquintinib plus sintilimab remains an optional treatment strategy for patients who have failed previous regorafenib therapy.
The toxicity profile of this combination regimen was tolerable and was comparable with previous studies (28–30). The REGONIVO study demonstrated that combination of regorafenib 80 mg plus nivolumab had a manageable safety profile and encouraging antitumor activity. In this study, the dose reduction as a result of serious adverse events occurred in 28 (38.9%) patients. With this dose adjustment strategy, the treatment was well tolerated in patients. Our study has several strengths and limitations, because it is an observational study and the number of patients included is not large. Future validation clinical trials would be needed to confirm the value of regorafenib or fruquintinib plus sintilimab as third-line or above therapy in MSS metastatic colorectal cancer.
In conclusion, these data confirm that regorafenib or fruquintinib plus sintilimab as third-line or above therapy provide a feasible treatment regimen for MSS metastatic colorectal cancer with tolerated toxicity. Patients without liver metastases may be the preferred population for this combination regimen.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by This study was carried out in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of the Affiliated Cancer Hospital of Zhengzhou University (KY-0192). The patients/participants provided their written informed consent to participate in this study.
CN and XC designed the research, analyzed the data and drafted the paper. CN, HL, BC, WX, JW and YL were mainly responsible for data collection and analysis. CN, SW, JZ and YH were primarily responsible for statistical analysis. All authors contributed to the article and approved the submitted version.
This work was supported by Medical Science and Technique Foundation of Henan Province (No. 212102310623), Medical Science and Technique Foundation of Henan Province (No. SB201901101), Young and Middle-aged Health and Technology Innovation Leading Talent Project of Henan Province (No. YXKC2020008), 1000 Talents Program of Central plains (No. 204200510023) and the Sate Key Laboratory of Esophageal Cancer Prevention & Treatment (No. Z2020000X).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Coupez D, Hulo P, Touchefeu Y, Denis MG, Bennouna J. KRAS mutations in metastatic colorectal cancer: from a de facto ban on anti-EGFR treatment in the past to a potential biomarker for precision medicine. Expert Opin Biol Ther (2021) 21(10):1325–34. doi: 10.1080/14712598.2021.1967318
3. Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: Retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol (2017) 3(2):194–201. doi: 10.1001/jamaoncol.2016.3797
4. Cremolini C, Antoniotti C, Lonardi S, Bergamo F, Cortesi E, Tomasello G, et al. Primary tumor sidedness and benefit from FOLFOXIRI plus bevacizumab as initial therapy for metastatic colorectal cancer Retrospective analysis of the TRIBE trial by GONO. Ann Oncol (2018) 29(7):1528–34. doi: 10.1093/annonc/mdy140
5. He R, Lao Y, Yu W, Zhang X, Jiang M, Zhu C. Progress in the application of immune checkpoint inhibitor-based immunotherapy for targeting different types of colorectal cancer. Front Oncol (2021) 11:764618. doi: 10.3389/fonc.2021.764618
6. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med (2015) 372(26):2509–20. doi: 10.1056/NEJMoa1500596
7. Chen EX, Jonker DJ, Loree JM, Kennecke HF, Berry SR, Couture F, et al. Effect of combined immune checkpoint inhibition vs best supportive care alone in patients with advanced colorectal cancer: The Canadian cancer trials group CO.26 study. JAMA Oncol (2020) 6(6):831–8. doi: 10.1001/jamaoncol.2020.0910
8. Mettu NB, Ou FS, Zemla TJ, Halfdanarson TR, Lenz HJ, Breakstone RA, et al. Assessment of capecitabine and bevacizumab with or without atezolizumab for the treatment of refractory metastatic colorectal cancer: A randomized clinical trial. JAMA Netw Open (2022) 5(2):e2149040. doi: 10.1001/jamanetworkopen.2021.49040
9. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: An open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). J Clin Oncol (2020) 38(18):2053–61. doi: 10.1200/JCO.19.03296
10. Wang C, Chevalier D, Saluja J, Sandhu J, Lau C, Fakih M. Regorafenib and nivolumab or pembrolizumab combination and circulating tumor DNA response assessment in refractory microsatellite stable colorectal cancer. Oncologist. (2020) 25(8):e1188–94. doi: 10.1634/theoncologist.2020-0161
11. Cousin S, Cantarel C, Guegan JP, Gomez-Roca C, Metges JP, Adenis A, et al. Regorafenib-avelumab combination in patients with microsatellite stable colorectal cancer (REGOMUNE): A single-arm, open-label, phase II trial. Clin Cancer Res (2021) 27(8):2139–47. doi: 10.1158/1078-0432.CCR-20-3416
12. Wang F, He MM, Yao YC, Zhao X, Wang ZQ, Jin Y, et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med (2021) 2(9):100383. doi: 10.1016/j.xcrm.2021.100383
13. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol (2017) 18(9):1182–91. doi: 10.1016/S1470-2045(17)30422-9
14. Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-Deficient/Microsatellite instability-high metastatic colorectal cancer. J Clin Oncol (2018) 36(8):773–9. doi: 10.1200/JCO.2017.76.9901
15. Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-Instability-High advanced colorectal cancer. N Engl J Med (2020) 383(23):2207–18. doi: 10.1056/NEJMoa2017699
16. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet (9863) 2013:381. doi: 10.1016/S0140-6736(12)61900-X
17. Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: The FRESCO randomized clinical trial. JAMA (2018) 319(24):2486–96. doi: 10.1001/jama.2018.7855
18. Chen L, Jiang X, Li Y, Zhang Q, Li Q, Zhang X, et al. How to overcome tumor resistance to anti-PD-1/PD-L1 therapy by immunotherapy modifying the tumor microenvironment in MSS CRC. Clin Immunol (2022) 237:108962. doi: 10.1016/j.clim.2022.108962
19. Kim CW, Chon HJ, Kim C. Combination immunotherapies to overcome intrinsic resistance to checkpoint blockade in microsatellite stable colorectal cancer. Cancers (Basel) (2021) 13(19):4906. doi: 10.3390/cancers13194906
20. Tian S, Wang F, Zhang R, Chen G. Global pattern of CD8(+) T-cell infiltration and exhaustion in colorectal cancer predicts cancer immunotherapy response. Front Pharmacol (2021) 12:715721. doi: 10.3389/fphar.2021.715721
21. Bai H, Wang J, Phan CU, Chen Q, Hu X, Shao G, et al. Cyclodextrin-based host-guest complexes loaded with regorafenib for colorectal cancer treatment. Nat Commun (2021) 12(1):759. doi: 10.1038/s41467-021-21071-0
22. Doleschel D, Hoff S, Koletnik S, Rix A, Zopf D, Kiessling F, et al. Regorafenib enhances anti-PD1 immunotherapy efficacy in murine colorectal cancers and their combination prevents tumor regrowth. J Exp Clin Cancer Res (2021) 40(1):288. doi: 10.1186/s13046-021-02043-0
23. Zhang Q, Chen M, Wang Z, Qi C, Cao Y, Zhang J, et al. Efficacy and safety comparison of regorafenib and fruquintinib in metastatic colorectal cancer-an observational cohort study in the real world. Clin Colorectal Cancer (2022) 21 (3):e152–61. doi: 10.1016/j.clcc.2022.01.007
24. Sun L, Huang S, Li D, Mao Y, Wang Y, Wu J. Efficacy and safety of fruquintinib plus PD-1 inhibitors versus regorafenib plus PD-1 inhibitors in refractory microsatellite stable metastatic colorectal cancer. Front Oncol (2021) 11:754881. doi: 10.3389/fonc.2021.754881
25. Bilen MA, Shabto JM, Martini DJ, Liu Y, Lewis C, Collins H, et al. Sites of metastasis and association with clinical outcome in advanced stage cancer patients treated with immunotherapy. BMC Cancer. (2019) 19(1):857. doi: 10.1186/s12885-019-6073-7
26. Wang C, Fakih M. Targeting MSS colorectal cancer with immunotherapy: are we turning the corner? Expert Opin Biol Ther (2021) 21(10):1347–57. doi: 10.1080/14712598.2021.1933940
27. Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med (2021) 27(1):152–64. doi: 10.1038/s41591-020-1131-x
28. Zhou H, Wang Y, Lin Y, Cai W, Li X, He X. Preliminary efficacy and safety of camrelizumab in combination with XELOX plus bevacizumab or regorafenib in patients with metastatic colorectal cancer: A retrospective study. Front Oncol (2021) 11:774445. doi: 10.3389/fonc.2021.774445
29. Yang K, Han L, Wu S, Qu X, Li Q, Zhao C, et al. Real-world outcomes of regorafenib combined with immune checkpoint inhibitors in patients with advanced or metastatic microsatellite stable colorectal cancer: A multicenter study. Cancer Immunol Immunother (2022) 71(6):1143–451. doi: 10.1007/s00262-021-03083-3
Keywords: microsatellite stable, immunotherapy, targeted therapy, liver metastasis, colorectal cancer
Citation: Nie C, Lv H, Chen B, Xu W, Wang J, Liu Y, Wang S, Zhao J, He Y and Chen X (2022) Microsatellite stable metastatic colorectal cancer without liver metastasis may be preferred population for regorafenib or fruquintinib plus sintilimab as third-line or above therapy:A real-world study. Front. Oncol. 12:917353. doi: 10.3389/fonc.2022.917353
Received: 29 April 2022; Accepted: 12 September 2022;
Published: 26 September 2022.
Edited by:
Jinghua Pan, Jinan University, ChinaReviewed by:
Nikola Živković, University of Niš, SerbiaCopyright © 2022 Nie, Lv, Chen, Xu, Wang, Liu, Wang, Zhao, He and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaobing Chen, emx5eWNoZW54YjA4MDdAenp1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.