- 1Department of Radiation Oncology, Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Haikou, China
- 2Department of Radiation Oncology, Xiamen Cancer Center, Xiamen Key Laboratory of Radiation Oncology, The First Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, China
- 3Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, China
Purpose: To evaluate whether adjuvant radiotherapy (RT) after breast-conserving surgery (BCS) was associated with better survival among elderly (≥70 years) breast cancer patients with T1-2N0 and estrogen receptor (ER) positive disease.
Methods: We included patients who met the inclusion criteria between 2010 and 2014 from the Surveillance, Epidemiology, and End Results program. Patients were subdivided into three groups based on surgery and RT: BCS alone, BCS plus RT, and refusal of RT. The primary outcomes were breast cancer-specific survival (BCSS) and overall survival (OS). Chi-squared tests, Kaplan–Meier method, and Multivariate Cox regression analysis were used for statistical analysis. Propensity score matching (PSM) was performed to minimize the potential selection bias.
Results: A total of 26586 patients were included in this analysis. The median follow-up was 66 months. Of these patients, 15591 (58.6%) patients received RT, RT was recommended but not performed due to patient refusal for 1270 (4.8%) patients, and RT was not recommended for 9725 (36.6%) patients. The 5-year BCSS was 98.3% for patients receiving RT, 97.1% for patients refusal of RT, and 96.4% for patients not recommended RT (P<0.001). The 5-year OS was 88.6% for patients receiving RT, 77.6% for patients who refused RT, and 72.1% for patients not recommended RT (P<0.001). Multivariate Cox regression analyses showed that patients who received adjuvant RT after BCS had significantly better BCSS (hazard ratio [HR] 0.523, 95%confidence interval [CI] 0.447-0.612, P<0.001) and OS (HR 0.589, 95%CI 0.558-0.622, P<0.001) compared to those without RT. A total of 7721 pairs of patients were matched successfully between those with and without RT using PSM. The results also showed that patients who received RT after BCS had significantly better BCSS (HR 562, 95%CI 0.467-0.676, P<0.001) and OS (HR 0.612, 95%CI 0.0.575-0.652, P<0.001) compared to those without RT.
Conclusions: These data suggest that individual counseling is important for treatment decision-making in elderly breast cancer patients with T1-2N0 and ER-positive disease. Given the relatively lower toxicity of modern RT techniques, adjuvant RT should be recommended in patients with high life expectancy.
Introduction
Breast cancer (BC) is the most common malignancy in women worldwide (1). The probability of BC diagnosis increases with age. The majority of BC occurs in women aged ≥60 years, and 42% of patients were aged ≥70 years at BC diagnosis (2, 3). BC in the elderly is often indolent with positive expression of estrogen receptor (ER) and progesterone receptor (PR) (4). Because patients in the elderly are often excluded from clinical trials, limited data is available for determining the decision on escalation and de-escalation of treatment in this population (5).
Despite an increasing incidence of BC in the elderly, the optimal management of BC in the elderly remains unclear, and the treatment in the elderly mainly refers to their younger counterpart. In clinical practice, treatment decisions for elderly patients with BC are based individually on the assessment of comorbidities, functional status, expected tolerance, and life expectancy (6). In elderly BC patients with T1-2N0 and ER-positive disease, the role of postoperative radiotherapy (RT) in those treated with breast-conserving surgery (BCS) has been controversial in the last two decades.
Several prospective studies have shown that adjuvant RT after BCS may substantially decrease the local recurrence risk, while no benefit of BC-specific death and overall survival (OS) with the addition of RT to BCS among BC patients with aged ≥70 years and ER-positive disease (7, 8). The National Comprehensive Cancer Network guidelines allow for the use of BCS plus endocrine therapy without adjuvant RT in women aged ≥70 years with nodal negative disease and ER-positive, T1 BC (9). However, there were also approximately 60% of patients receive RT after BCS in clinical practice (10). Moreover, although the information on BC death was not analyzed, several population-based studies have shown that the receipt of postoperative RT was associated with a better OS compared to those without RT (11, 12). Therefore, the importance and necessity of RT after BCS in elderly BC remains considerably controversial. In recent years, great progress has been made in the endocrine therapy of ER-positive BC (13). In light of this, our study aimed to investigate whether adjuvant RT after BCS was associated with better survival among elderly (≥70 years) BC patients with T1-2N0 and ER-positive disease in the contemporary model of endocrine therapy.
Materials and methods
Patients
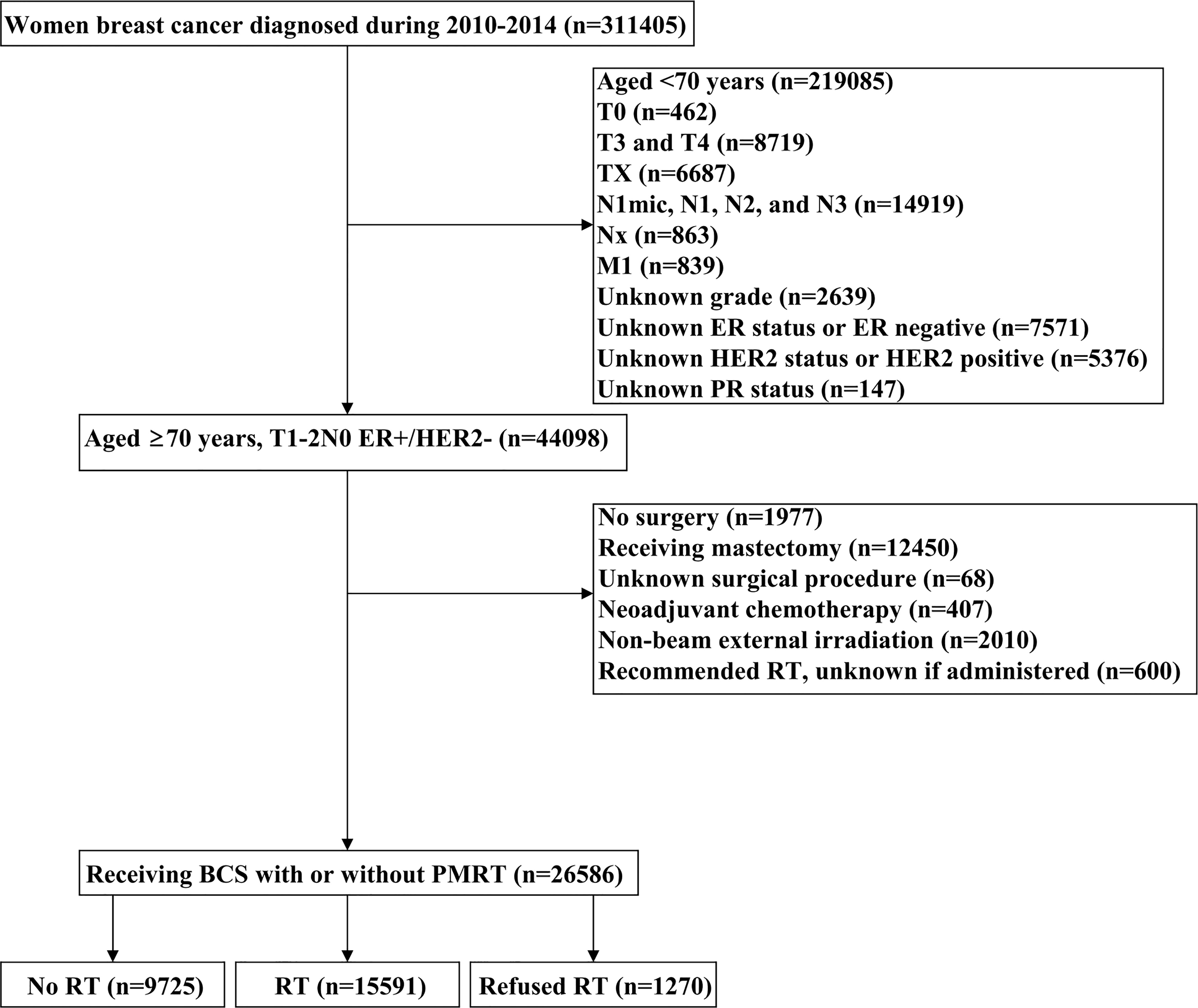
Patients diagnosed with BC between 2010 and 2014 were identified from the Surveillance, Epidemiology, and End Results (SEER) program (14). We included patients who met the following criteria: 1) T1-2N0 BC receiving BCS with or without postoperative RT; 2) aged ≥70 years with ER-positive and human epidermal growth factor receptor 2 (HER2) negative; 3) available for histology. The patient selection flowchart has listed in Figure 1. Patients who were diagnosed with metastatic disease at BC diagnosis, who received non-beam irradiation, and who received systemic therapy before BCS were excluded. This study used a public de-identified SEER database and the institutional review board approval was waived.
Measures
We extracted the following data from the SEER program in the analysis: age, race, tumor grade, histology, tumor (T) stage, PR status, chemotherapy use, and RT use. To reduce bias in this retrospective analysis, data were specifically extracted to divide patients into three groups: patients who were recommended to receive RT but ultimately did not receive it due to patients refusal; patients who received adjuvant RT; and patients for whom RT was not recommended and not given. The primary endpoints in the current study were 5-year breast cancer-specific survival (BCSS) and OS. BCSS was defined as the time from the BC diagnosis to the death of BC, and OS was defined as the time from the BC diagnosis to the death related to any cause. The events of locoregional recurrence or distant metastasis were unavailable in the SEER database. Therefore, survival endpoints regarding locoregional recurrence or distant metastasis could not be evaluated in our study.
Statistical analysis
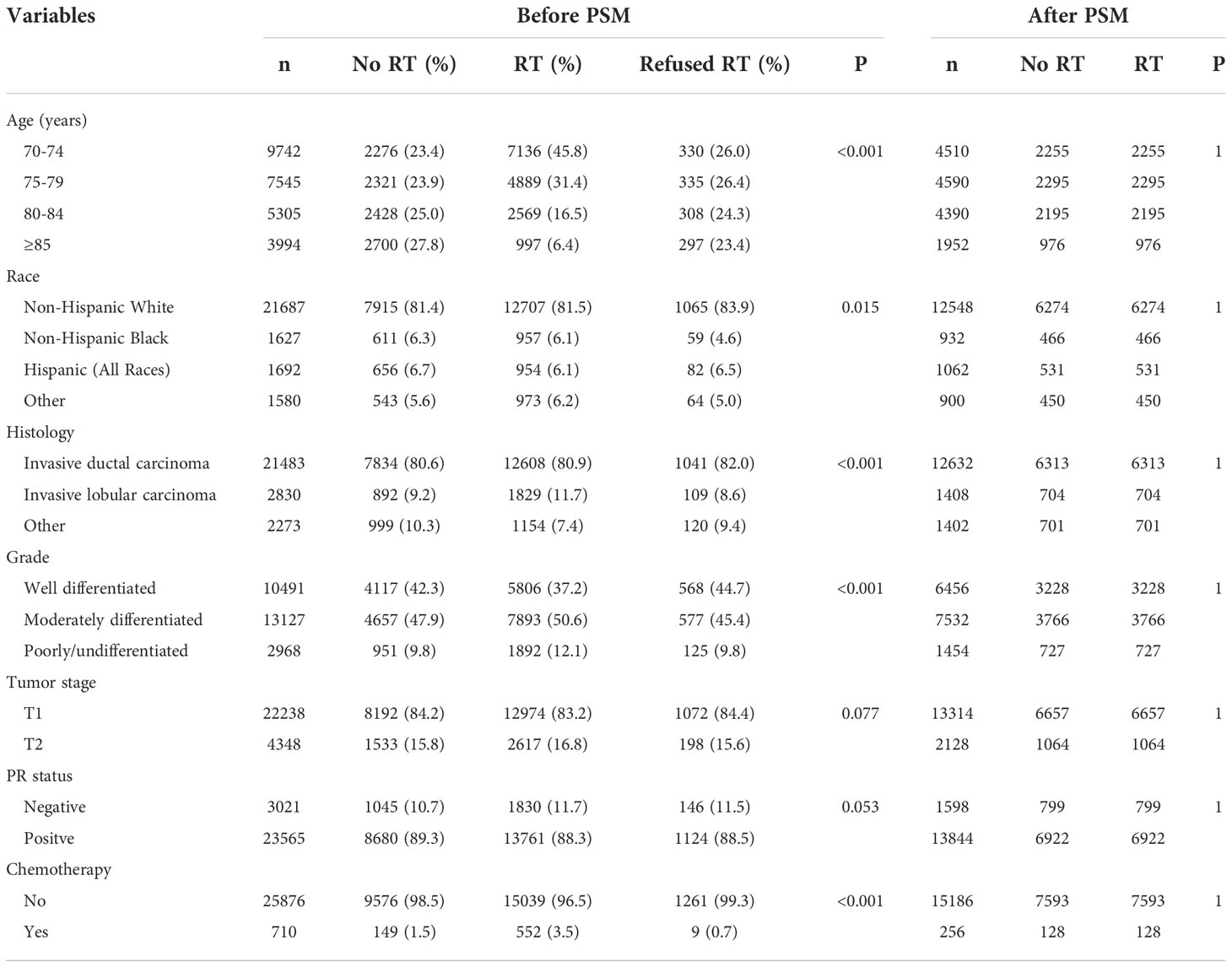
Descriptive characteristics of patients with and without RT after BCS were compared using Chi-squared tests for categorical variables. A 1:1 propensity score matching (PSM) between those with and without adjuvant RT after BCS was conducted to balance the potential confounders using the following variables: age, race, grade, histology, T stage, PR status, and chemotherapy use. Survival curves were generated using the Kaplan–Meier method and BCSS and OS were compared by the log-rank test. Multivariate Cox regression analyses were performed to analyze the independent factors associated with BCSS and OS. Covariates for adjustment included age at diagnosis, race, histology, tumor grade, T stage, PR status, and chemotherapy use. All statistical analyses were conducted using SPSS software (version 22.0; IBM Corporation, Armonk, NY, USA) or MedCalc Statistical Software version 18.2.1 (MedCalc Software bvba, Ostend, Belgium). All statistical tests were based on 2-sided probability and a P less than 0.05 was considered to be statistically significant.
Results
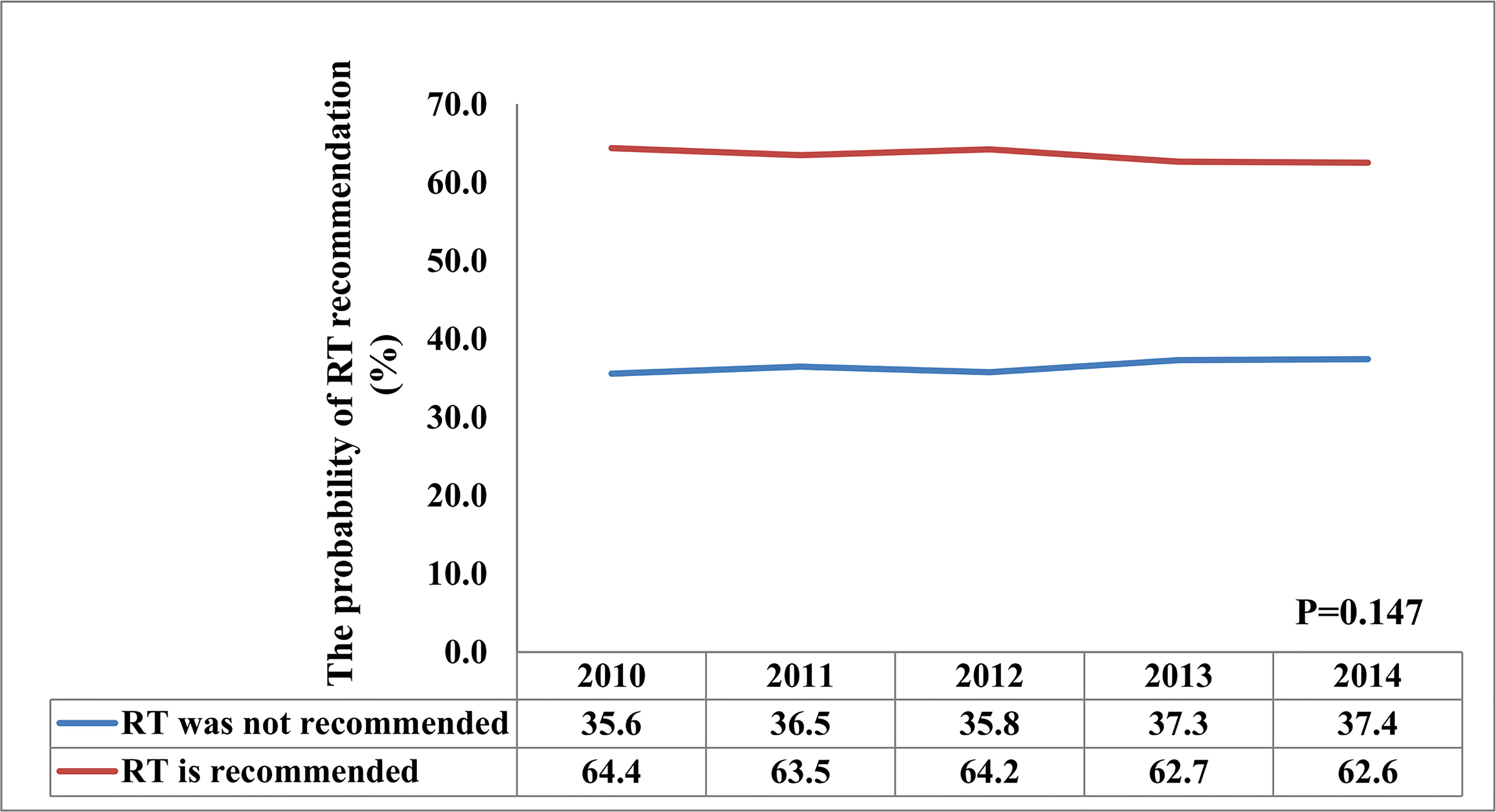
A total of 26586 patients who met our cohort definition were included in this study (Table 1). Of these patients, 15591 (58.6%) patients received RT, RT was recommended but not performed due to patient refusal for 1270 (4.8%) patients, and RT was not recommended for 9725 (36.6%) patients. There were no significant differences in the pattern of patients’ recommended RT over time (P=0.147) (Figure 2). The proportion of patients who did not recommend RT was 35.6% and had a slight increase to 37.4% in 2014, the proportion recommending RT was 64.4% in 2010 and had a slight decrease to 62.6% in 2014.
The median follow-up time was 66 months (range, 0-107 months). A total of 6551 patients had died, including 766 patients who died with BC. The 5-year BCSS and OS was 97.6% and 81.7%, respectively. The 5-year BCSS was 98.3% for patients receiving RT, 97.1% for patients refusal of RT, and 96.4% for patients not recommended RT (P<0.001, Figure 3A). The 5-year OS was 88.6% for patients receiving RT, 77.6% for patients who refused RT, and 72.1% for patients not recommended RT (P<0.001, Figure 3B). In univariate analyses, patients who received RT had a better BCSS compared to those who were not recommended RT (hazard ratio [HR] 2.244, 95%confidence interval [CI] 1.934-2.599, P<0.001) as did patients who refused RT (HR 1.946, 95%CI 1.429-2.650, P<0.001). Similar results were found regarding OS (no RT. vs. RT, HR 2.357, 95%CI 2.242-2.479, P<0.001; the refusal of RT vs. RT, HR 1.940, 95%CI 1.742-2.160, P<0.001). The year of diagnosis did not seem to be associated with survival outcomes.
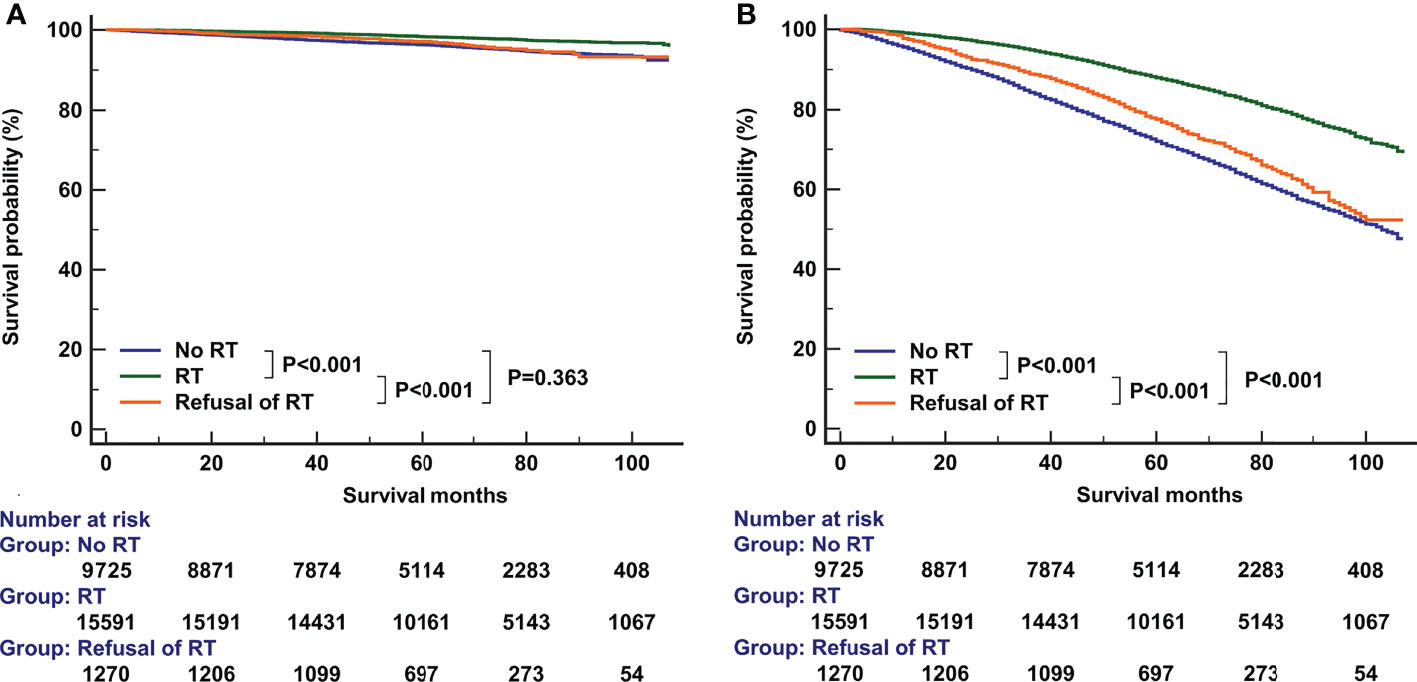
Figure 3 The effect of adjuvant radiotherapy on breast cancer-specific survival (A) and overall survival (B) before propensity score matching.
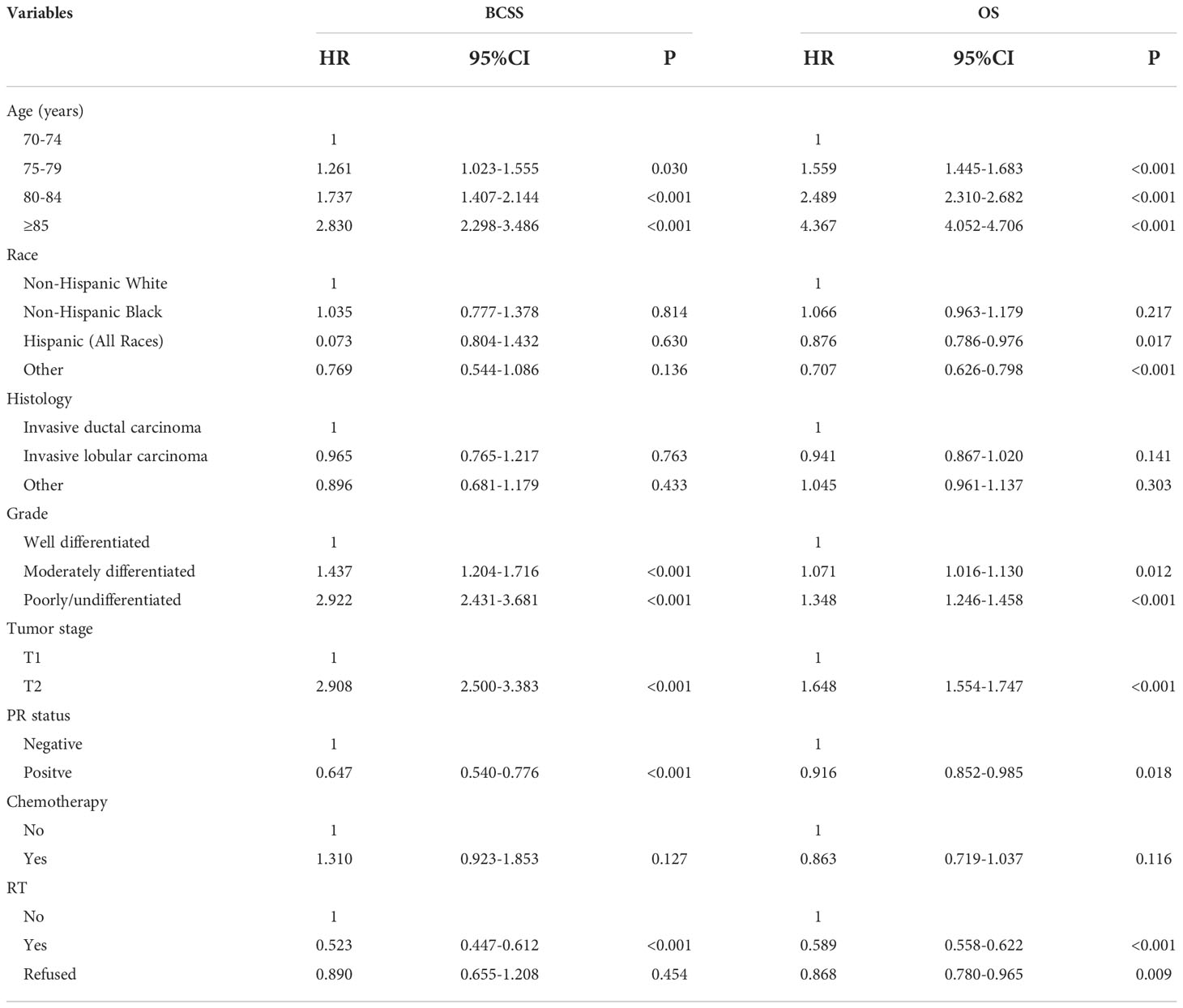
Multivariate Cox survival analyses were performed on the 26586 patients (Table 2). Patients who refused RT had comparable BCSS compared to those who did not receive RT (HR 0.890, 95%CI 0.655-1.208, P=0.454). Those who received RT had better BCSS compared to those who did not receive RT (HR 0.523, 95%CI 0.447-0.612, P<0.001). Regarding OS, patients who refused RT (HR 0.868, 95%CI 0.780-0.965, P=0.009) or patients who received RT (HR 0.589, 95%CI 0.558-0.622, P<0.001) had better OS compared to those who did not receive RT. Age at diagnosis, race, tumor grade, T stage, and PR status were also independent prognostic factors associated with survival outcomes.
PSM was used with the same dataset for the two cohorts of patients with (n=15591) and without (n=9725) adjuvant RT after BCS. Of the 9725 patients without RT, 7721 (79.4%) were matched successfully. Therefore, a total of 7721 pairs of patients were matched successfully (Table 1). The 5-year BCSS in those with and without RT was 98.1% and 96.8%, respectively (P<0.001, Figure 4A). The 5-year OS in those with and without RT was 85.4% and 76.3%, respectively (P<0.001, Figure 4B). The results of multivariate Cox survival analyses also showed that patients who received RT had better BCSS (HR 0.562, 95%CI 0.467-0.676, P<0.001) and OS (HR 0.612, 95%CI 0.575-0.652, P<0.001) compared to those who without RT (Table 3).
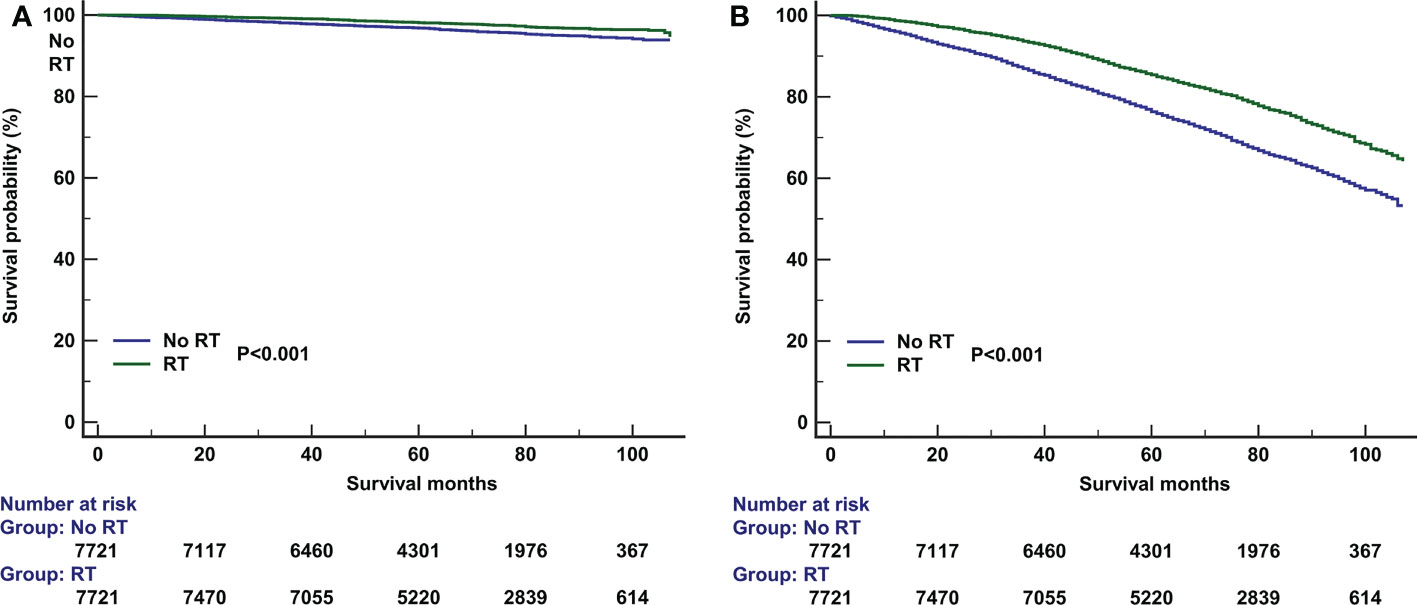
Figure 4 The effect of adjuvant radiotherapy on breast cancer-specific survival (A) and overall survival (B) after propensity score matching.
Discussion
In this study, using the data from the SEER program, we found that although the prospective studies have shown no survival benefit of RT after BCS in elderly BC patients, there were also approximately 60% of patients receiving RT after BCS in clinical practice. In addition, we also found that the addition of RT to BCS in elderly patients had a better BCSS and OS.
Since 2004, the findings from the GALGB 9343 trial supported that BCS plus endocrine therapy without RT yields similar OS and acceptable locoregional recurrence rate in women aged ≥70 years with T1N0 ER-positive BC compared to those received adjuvant RT after BCS (15). After that, the BCS rate increased over time for early-stage elderly BC (16), whereas the RT rate decreased after BCS over time. A prior SEER study found that approximately 68.6% of patients treated between 2000 and 2004 received RT after BCS, and there were 61.7% of patients received RT after BCS between 2005 and 2009 (P<0.001) (10). In our study, the proportion of RT recommendations after BCS did not have significant differences from 2010 to 2014 (P=0.147), and there were also 64.4% and 62.6% of patients recommended for RT after BCS in the year 2010 and 2014, respectively. Therefore, our findings suggest that the publication of the GALGB 9343 trial resulted in a small but significant decrease in RT delivery in elderly patients. However, the overall use of RT has shown a steady trend in recent years.
In this study, a total of 6551 patients died, while only 11.7% (n=766) of them died from BC. There was approximately 20-50% of patients had comorbidities at the BC diagnosis (12, 17, 18). Therefore, competing mortality is a major factor affecting survival in elderly BC patients. However, several studies have shown that comorbidities, education level, area of residence, receipt of endocrine therapy, and distance to the nearest RT clinic were not associated with the omission of RT after BCS in this population (14, 19). In clinical practice, age, marital status, and tumor grade were the main factors associated with RT decision-making in the elderly (14, 19). In our study, we also found that patients with younger age and higher tumor grade were more likely to receive RT. Several studies also showed that patients with younger age were more likely to receive chemotherapy and long-lasting endocrine therapy (20, 21). Although RT may lead to treatment-related acute and chronic toxicities and increase health care costs (22, 23), several studies have found that the use of RT did not negatively impact the quality of life of elderly patients (8, 24). With the progress of RT techniques and the altered RT fractionation (25, 26), lower RT-induced toxicity and a short course of RT may increase elderly patients’ compliance with RT.
Although approximately two-thirds of the elderly patients still receive RT after BCS in clinical practice, the exact role of RT in this population still needs to be further elucidated. A meta-analysis by Matuschek et al. including 3766 patients from five randomized trials found that the addition of RT to endocrine therapy improved local control but did not have an impact on OS (27). A study from Germany found that adjuvant RT did not improve recurrence-free survival (P=0.651) and OS (P=0.573) (28). However, the small number of patients in the above study may have influenced the statistical results due to the high survival rate and low recurrence rate in this population (n=950). Another study from Italy found that adjuvant RT had no effect on BC death and distant metastasis, and the 15-year local recurrence rate in patients without RT was significantly higher than that in patients with RT (14.6% vs. 0.8%, P= 0.004) (29). However, it should be noted that early patient enrollment, imprecise RT techniques, and insufficient endocrine therapy may impact the survival benefit of RT. Recently, two studies from the National Cancer Database (NCDB) showed a positive impact of RT in this population, they found that RT could improve the OS of patients, especially for those treated with endocrine therapy and RT (11, 12). However, the NCDB does not record BC-related deaths in patients. A large cohort (n=26279) study from the Ontario data also showed the addition of RT was associated with a lower recurrence (P<0.001) and death (P<0.001) even after accounting for age and comorbidities (30). In our study, we found that the addition of RT can not only improve OS but also improve BCSS in this patient subset before and after PSM. The participants in the clinical trial were highly selected and differed from those in routine clinical practice on several factors, including adherence to endocrine treatment, treatment facility and quality, follow-up frequency, etc. Therefore, our study raised the reconsideration for the omission of adjuvant RT after BCS in routine clinical practice.
Our study has OS benefits in those treated with RT, perhaps because we analyzed real-world data from a population-based cohort rather than highly selected cases in clinical trials. That hypothesis might indicate that the OS benefit with the addition of RT is apparent only in the cohort with a high sample size. The difference might also be attributable to the fact that published randomized trials tend to use stricter inclusion criteria and more extensive local surgical resection (31–35). However, the selection bias still could not be ruled out regarding the OS benefit of RT in our study. Despite the growing body of relevant evidence, many unanswered questions remain. Although high-quality evidence exists, the relatively short follow-up time and limited statistical power limit the results of prospective randomized trials for clinical practice. Studies with longer follow-up are required to detect significant differences in OS, but it is worth emphasizing that large retrospective population based-studies including ours have found early differences in OS (11, 12, 30). The large population-based studies not only provided real-world clinical evidence but may also compensate for any retrospective design-related drawbacks.
Several limitations should be acknowledged in our study. First, despite our best efforts to account for potential confounders with PSM, it is impossible to eliminate the risk of selection bias. Second, details regarding endocrine therapy were not recorded in the SEER program. However, the findings from a previous NCDB study included 113505 patients aged >65 years, the proportions of receiving endocrine therapy alone, RT alone, and RT plus endocrine therapy were 20.7%, 16.1%, and 63.2%, respectively (11). The BCSS of the patients in our study was 97.6%, therefore, we could assume that the majority of patients in our study received endocrine therapy. Third, the patterns of locoregional recurrence and distant metastasis were not recorded in the SEER database. Moreover, the median follow-up was only 66 months in this study because the information on HER2 status was not collected in the SEER until 2010, which was insufficient to assess the long-term survival of patients. Finally, age is closely related to comorbidities and frailty. However, comorbidity, frailty, and functional status were not recorded in the SEER database, and could not be evaluated in our study.
Conclusions
In conclusion, our findings suggest that individual counseling is important for treatment decision-making in elderly patients with early-stage BC. Given the relatively lower toxicity of modern RT techniques, adjuvant RT should be recommended in patients with high life expectancy. However, given that prospective clinical trials have failed to show an OS benefit with the addition of RT in this population, we caution using the retrospective data to draw a conclusion on OS. More studies are required to determine the optimal candidates for RT recommendation in this population.
Data availability statement
Publicly available datasets were analyzed in this study. We have got the permission to access the SEER database on purpose of research only (Reference number: 11025-Nov2016). This data can be found here: www.seer.cancer.gov.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
S-PY, PZ, and C-LL drafted the manuscript. S-GW acquired the datasets. S-GW and Z-YH conceived the study. S-GW conducted the statistical analyses. S-GW and S-PY participated in the study design. All authors contributed to the article and approved the submitted version.
Funding
This work was partly supported by the Social Development Projects of Key R & D Programs in Hainan Province (No. ZDYF2022SHFZ130), Commission Young and Middle-aged Talents Training Project of Fujian Health Commission (No. 2019-ZQNB-25), the Natural Science Foundation of Fujian Province (No. 2020J011240), and Natural Science Foundation of Hainan Province (No. 819QN345).
Acknowledgments
The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Yancik R, Ries LA. Cancer in older persons: an international issue in an aging world. Semin Oncol (2004) 31:128–36. doi: 10.1053/j.seminoncol.2003.12.024
3. Glaser R, Marinopoulos S, Dimitrakakis C. Breast cancer treatment in women over the age of 80: A tailored approach. Maturitas (2018) 110:29–32. doi: 10.1016/j.maturitas.2018.01.014
4. Syed BM, Green AR, Paish EC, Soria D, Garibaldi J, Morgan L, et al. Biology of primary breast cancer in older women treated by surgery: With correlation with long-term clinical outcome and comparison with their younger counterparts. Br J Cancer (2013) 108:1042–51. doi: 10.1038/bjc.2012.601
5. Luo J, Kesselheim AS. Underrepresentation of older adults in cancer trials. JAMA (2014) 311:965–6. doi: 10.1001/jama.2013.286454
6. Bouchardy C, Rapiti E, Blagojevic S, Vlastos AT, Vlastos G. Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol (2007) 25:1858–69. doi: 10.1200/JCO.2006.10.4208
7. Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long-term follow-up of CALGB 9343. J Clin Oncol (2013) 31:2382–7. doi: 10.1200/JCO.2012.45.2615
8. Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM, PRIME II investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol (2015) 16:266–73. doi: 10.1016/S1470-2045(14)71221-5
9. National comprehensive cancer network. breast cancer (Version 4. 2021) (2021). Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
10. Palta M, Palta P, Bhavsar NA, Horton JK, Blitzblau RC. The use of adjuvant radiotherapy in elderly patients with early-stage breast cancer: changes in practice patterns after publication of cancer and leukemia group b 9343. Cancer (2015) 121:188–93. doi: 10.1002/cncr.28937
11. Jhawar SR, Alpert N, Taioli E, Sayan M, Bazan J, Park KU, et al. Adjuvant radiation therapy alone is associated with improved overall survival compared to hormonal therapy alone in older women with estrogen receptor positive early stage breast cancer. Cancer Med (2020) 9:8345–54. doi: 10.1002/cam4.3443
12. Wang F, Meszoely I, Pal T, Mayer IA, Bailey CE, Zheng W, et al. Radiotherapy after breast-conserving surgery for elderly patients with early-stage breast cancer: A national registry-based study. Int J Cancer (2021) 148:857–67. doi: 10.1002/ijc.33265
13. Nagaraj G, Ma CX. Clinical challenges in the management of hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: A literature review. Adv Ther (2021) 38:109–36. doi: 10.1007/s12325-020-01552-2
14. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat database: Incidence - SEER research plus data, 18 registries, Nov 2020 Sub (2000-2018) - linked to county attributes - total U.S., 1969-2019 counties, national cancer institute, DCCPS, surveillance research program. Available at: www.seer.cancer.gov.
15. Hughes KS, Schnaper LA, Berry D, Cirrincione C, McCormick B, Shank B, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med (2004) 351:971–7. doi: 10.1056/NEJMoa040587
16. Bazan JG, Fisher JL, Park KU, Marcus EA, Bittoni MA, White JR. Assessing the impact of CALGB 9343 on surgical trends in elderly-women with stage I ER+ breast cancer: A SEER-based analysis. Front Oncol (2019) 9:621. doi: 10.3389/fonc.2019.00621
17. Minicozzi P, Van Eycken L, Molinie F, Innos K, Guevara M, Marcos-Gragera R, et al. Comorbidities, age and period of diagnosis influence treatment and outcomes in early breast cancer. Int J Cancer (2019) 144:2118–27. doi: 10.1002/ijc.31974
18. Shumway DA, Griffith KA, Hawley ST, Wallner LP, Ward KC, Hamilton AS, et al. Patient views and correlates of radiotherapy omission in a population-based sample of older women with favorable-prognosis breast cancer. Cancer (2018) 124:2714–23. doi: 10.1002/cncr.31378
19. Reyes SA, Williams AD, Arlow RL, de la Cruz LM, Anderson DN, Ugras S, et al. Changing practice patterns of adjuvant radiation among elderly women with early stage breast cancer in the united states from 2004 to 2014. Breast J (2020) 26:353–67. doi: 10.1111/tbj.13491
20. Smith-Graziani D, Lei X, Giordano SH, Zhao H, Karuturi M, Chavez-MacGregor M. Delayed initiation of adjuvant chemotherapy in older women with breast cancer. Cancer Med (2020) 9:6961–71. doi: 10.1002/cam4.3363
21. Meneveau MO, Keim-Malpass J, Camacho TF, Anderson RT, Showalter SL. Predicting adjuvant endocrine therapy initiation and adherence among older women with early-stage breast cancer. Breast Cancer Res Treat (2020) 184:805–16. doi: 10.1007/s10549-020-05908-8
22. Ali AA, Xiao H, Tawk R, Campbell E, Semykina A, Montero AJ, et al. Comparison of health utility weights among elderly patients receiving breast-conserving surgery plus hormonal therapy with or without radiotherapy. Curr Med Res Opin (2017) 33:391–400. doi: 10.1080/03007995.2016.1257983
23. Carvalho ÍT, Rezende ACP, Bernardo RJC. Pros and cons of radiotherapy omission in elderly breast cancer patients following breast conservative surgery. Transl Cancer Res (2020) 9:S236–41. doi: 10.21037/tcr.2019.10.39
24. Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al. A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. PRIME trial Health Technol Assess (2007) 11:1–149. doi: 10.3310/hta11310
25. Díaz Gavela AA, Vaquero Barrón B, Del Cerro Peñalver E, Couñago F. Breast radiotherapy in elderly women: myths, controversies, and current techniques in the adjuvant setting. Transl Cancer Res (2020) 9:S37–55. doi: 10.21037/tcr.2019.07.09
26. Pinzi V, Fariselli L, Jereczek-Fossa BA. Altered fractionation in radiation therapy for breast cancer in the elderly: are we moving forward? Transl Cancer Res (2020) 9:S217–27. doi: 10.21037/tcr.2019.09.39
27. Matuschek C, Bölke E, Haussmann J, Mohrmann S, Nestle-Krämling C, Gerber PA, et al. The benefit of adjuvant radiotherapy after breast conserving surgery in older patients with low risk breast cancer- a meta-analysis of randomized trials. Radiat Oncol (2017) 12:60. doi: 10.1186/s13014-017-0796-x
28. Stueber TN, Diessner J, Bartmann C, Leinert E, Janni W, Herr D, et al. Effect of adjuvant radiotherapy in elderly patients with breast cancer. PloS One (2020) 15:e0229518. doi: 10.1371/journal.pone.0229518
29. Martelli G, Boracchi P, Guzzetti E, Marano G, Lozza L, Agresti R, et al. Omission of radiotherapy in elderly patients with early breast cancer: 15-year results of a prospective non-randomised trial. Eur J Cancer (2015) 51:1358–64. doi: 10.1016/j.ejca.2015.04.018
30. Guidolin K, Lock M, Vogt K, McClure JA, Winick-Ng J, Vinden C, et al. Recurrence and mortality after breast-conserving surgery without radiation. Curr Oncol (2019) 26:380–8. doi: 10.3747/co.26.5225
31. Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C. Threats to applicability of randomised trials: exclusions and selective participation. J Health Serv Res Policy (1999) 4:112–21. doi: 10.1177/135581969900400210
32. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med (2002) 347:1233–41. doi: 10.1056/NEJMoa022152
33. Veronesi U, Salvadori B, Luini A, Greco M, Saccozzi R, del Vecchio M, et al. Breast conservation is a safe method in patients with small cancer of the breast. long-term results of three randomised trials on 1,973 patients. Eur J Cancer (1995) 31A:1574–9. doi: 10.1016/0959-8049(95)00271-j
34. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med (2002) 347:1227–32. doi: 10.1056/NEJMoa020989
Keywords: breast cancer, elderly, radiotherapy, breast-conserving surgery, SEER
Citation: Yang S-P, Tan L-L, Zhou P, Lian C-L, Wu S-G and He Z-Y (2022) The addition of radiotherapy to breast-conserving surgery improves survival for elderly patients with early breast cancer. Front. Oncol. 12:917054. doi: 10.3389/fonc.2022.917054
Received: 11 April 2022; Accepted: 28 October 2022;
Published: 23 November 2022.
Edited by:
Rosario Mazzola, Sacro Cuore Don Calabria Hospital (IRCCS), ItalyReviewed by:
Laszlo Mangel, Pécs University, HungaryUdhaya Kumar S, Vellore Institute of Technology, India
Copyright © 2022 Yang, Tan, Zhou, Lian, Wu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: San-Gang Wu, dW5vd3UxMjM0NUBob3RtYWlsLmNvbQ==; Zhen-Yu He, aGV6aHlAc3lzdWNjLm9yZy5jbg==
†These authors have contributed equally to this work
 Shi-Ping Yang
Shi-Ping Yang Lu-Lu Tan1†
Lu-Lu Tan1† Ping Zhou
Ping Zhou Chen-Lu Lian
Chen-Lu Lian San-Gang Wu
San-Gang Wu Zhen-Yu He
Zhen-Yu He