
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol., 26 May 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.916681
This article is part of the Research TopicNovel Modalities in Cancer Diagnostics and TherapeuticsView all 18 articles
The incidence of lung cancer is high and about 75% of the patients with lung cancer are found in the middle and advanced stage, which has a limited treatment strategy. Non-small cell lung cancer (NSCLC) accounts for about 85% of all lung cancers. In this article, we delineate the treatment process of a middle-aged male patient with advanced-stage lung cancer to explain the significance of individualized chemotherapy combined with immunotherapy and surgery. This patient has extensive bone metastasis with PS scores of 2. After nine cycles of preoperative neoadjuvant chemotherapy, surgery, and two cycles of postoperative adjuvant chemotherapy, the patient achieved complete response (CR) and his PS score was 0. Although there is a standard chemotherapy regimen for lung adenocarcinoma, the treatment effect varies because of individual differences. Comprehensive analysis of the characteristics of patients through a variety of means to develop a precise individualized chemotherapy plan will be a major direction of lung cancer treatment in the future. Additionally, surgical treatment for advanced lung cancer patients after chemotherapy can effectively reduce the primary lesion and prolong the survival time of patients.
Lung cancer is a major health problem worldwide. About 2.1 million people are diagnosed with lung cancer and 1.8 million people die of lung cancer every year (1). Lung cancer is the leading cause of cancer death in the world. So far, there is still no effective method to screen for lung cancer, but studies have shown that annual low-dose chest CT (LDCT) can reduce lung cancer mortality by 20% and overall mortality by 6.7% compared with chest X-ray (CXR) (2). The US Preventive Services Task Force suggests that adults, aged 50 to 80, who have been smoking for 20 years, are still smoking, or have given up smoking for more than 15 years should be screened for lung cancer by LDCT (3). Lung cancer includes non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Adenocarcinoma is the most common pathological type in NSCLC (4), and squamous cell carcinoma ranks second (5). In recent years, the incidence of squamous-cell carcinoma has decreased significantly, which might be related to the lower smoking rate in high-income countries and changes in the composition of cigarettes (6).
Once lung cancer is suspected, diagnosis and staging must be made, because the treatment of lung cancer depends on its subtype and stage. The 5-year survival rates of NSCLC patients with stage I, stage II to stage III, and stage IV were 80%, 13-60%, and 0-10% respectively (7). Surgical resection is the standard treatment in stages I, II, and some IIIA (8). Adjuvant chemotherapy can improve the survival rate of stage II, IIA, or IB patients by 5% - 10%, but it also has side effects (9). For early NSCLC patients who are not suitable for surgery, stereotactic ablation radiotherapy (SABR) can be considered (10). Platinum-based chemotherapy (such as cisplatin and carboplatin) 2-drug regimen is standard for patients with stage IV NSCLC. Although chemotherapy is still indispensable in the treatment of lung cancer, targeted therapy for specific gene mutations has made progress in the past few years (11). The first-generation targeted drugs (gefitinib and erlotinib) and second-generation targeted drugs (afatinib and dacomitinib) for EGFR mutations can significantly improve progression-free survival time (PFS) and overall survival (OS) compared with double platinum chemotherapy (12–17). Crizotinib, a drug targeting ALK mutations, shows better survival than chemotherapy as the first-and second-line therapy in a phase III trial (18–20). In addition, targeted therapy against ROS1, BRAF, NTRK, MET, RET, KRAS, HER2, and other genes has achieved good results in clinical trials.
Compared with the second-line chemotherapy for NSCLC, patients with anti-PD-1 and anti-PD-L1 antibodies always maintain a higher survival rate, which has become an important treatment for primary NSCLC (21–24). In NSCLC, two recognized active immune checkpoints are the CTLA-4 and PD-1 axes. CTLA-4 is usually expressed on CD4 and CD8 positive T lymphocytes and inhibits T cell activation. PD-1 is expressed in T cells, B cells, and NK cells and regulates central and peripheral immune tolerance. The expression of PD-L1 in tumor cells leads to immune escape (25). In the study of non-squamous NSCLC, such as the phase 3 KEYNOTE-189 trial (26–28), PD-1 or PD-L1 antibody combined with platinum chemotherapy is better than chemotherapy alone. Now, patients whose PD-L1 expression is 50% or more can use pembrolizumab or atezolizumab monotherapy, chemotherapy plus immunotherapy, or dual-drug immunotherapy with or without chemotherapy (8). For patients with PD-L1 expression of less than 50%, chemotherapy combined with PD-1 or PD-L1 inhibitors is the standard treatment (8). However, rare toxic effects associated with immunotherapy can happen at any point. It has been reported that immune checkpoint inhibitors (ICIs) can be used for two years (22). If properly treated, immune-related side effects are usually transient, but in some cases, they can be fatal. However, there is a problem with drug resistance in both targeted therapy and immunotherapy. Dealing with the problem of drug resistance is critical for lung cancer treatment.
In recent years, the diagnosis and treatment of lung cancer have made some progress, but the effect is still not satisfactory. Individualized treatment of lung cancer has gradually become a newer trend. In this article, we present a case of a middle-aged male patient with advanced lung adenocarcinoma who underwent a descending surgery after 9 cycles of individualized chemotherapy combined with targeted immunotherapy and continued adjuvant chemotherapy until the condition reached complete response (CR). Here we will elaborate on the choice of treatment and the significance of surgery for patients with advanced lung cancer, to emphasize the importance of individualized therapy.
The patient, a 69-year-old male, coughed intermittently with white mucous sputum after catching a cold for more than five months, developed left chest pain for more than two months, and felt wheezing and these symptoms aggravated after activity. Chest CT in the out-patient clinic suggested a tumor in the right lower hilum and multiple military nodules in both lungs and pleura. These findings indicated that central lung cancer should be considered. Pathological consultation revealed adenocarcinoma of the lung. Genetic examination showed EGFR exon20 insertion mutation. Carboplatin plus pemetrexed treatment was not effective, so he was transferred to our hospital. There is nothing special about past history, personal history, and family history. The patient was hoarse, suffocated, and in a wheelchair. PS scores: 2 points. On admission, tumor markers: CA125:93.8U/ml (normal value: 0-24U/ml) and CEA:1.74ng/ml (normal value: 0-5U/ml). Chest CT suggested that the space-occupying lesion in the lower lobe of the right lung was consistent with the manifestation of lung cancer (Figure 1). PET/CT: soft tissue mass was seen near the right hilum, FDG uptake increased unevenly, SUVmax9.2; left atlas, right 9th rib, left 10th rib, right humerus and sacrum showed multiple abnormal increased FDG uptake, SUVmax11.6. It was suggested that the hypermetabolic mass adjacent to the right hilum should be considered as the residual metabolic activity of the tumor after the treatment. Brain MRI: no abnormality. Pathological stage: T4N3M1c, stage IVB. Immunohistochemistry of drug sensitivity showed: BRCA-1 (-), ERCC-1 (+), TS (-), MSH-2 (+++), MSH-6 (+++),VEGF(-),PD-L1 (TPS=0). Combined with the examination results, we decided to carry out the first and second cycles of chemotherapy combined with targeted immunotherapy. The specific regimens were as follows: bevacizumab 500mg + pemetrexed 900mg + carboplatin 500mg + durvalumab 1000mg.However, the effect was not good, and the myelosuppression was obvious, so the adjusted regimen was bevacizumab 600mg + pemetrexed 900mg + nedaplatin 100mg + durvalumab 1000mg. After the third cycle of chemotherapy combined with targeted immunotherapy, the patients had few adverse reactions, so we continued to carry on the 4th-9th cycles. After nine cycles of chemotherapy, the re-examination of chest CT (Figure 2) and PET/CT showed that the metabolic activity of most of the films in the lower lobe of the right lung increased, SUVmax6.6, significantly reduced from the previous range, and the activity decreased (Figure 3). However, there was no change compared with the recent, and the tumor markers did not decrease, so we judged that drug resistance occurred. The relevant examination indexes were in accordance with the surgical indications, so a right lower lobe lobectomy was performed. Examination of postoperative freezing specimens showed: invasive lung adenocarcinoma in the lower lobe of the right lung, with two foci, one moderately differentiated (acinar type 70%, papillary type 20%, solid type 10%), and the other poorly differentiated (solid type 90%, acinar type 10%). The tumor surrounded the bronchial wall and there was a tumor thrombus inside blood vessels. Immunohistochemical results showed: CK7 (+), CK20 (+), TTF-1 (+), NapsinA (weak +), CK5 (-), P40 (-), ALK (D5F3) (-), Ki-67 (hot spot index 15%), P53 (wild type), MLH1 (+), MSH2 (+), MSH6 (+), PMS2 (+), PD-1 (UMAB199) (TILS:20%), NTRK (-). The cutting edge was negative, that is, R0 resection. The patients recovered well after the surgery (Figure 4) and received two cycles of postoperative adjuvant chemotherapy combined with targeted immunotherapy. The specific regimens were as follows: bevacizumab 600mg + pemetrexed 900mg + nedaplatin 100mg + durvalumab 1000mg. The tumor marker CA125:6.6 U/ml, CEA:2.0ng/ml, was significantly lower than that before treatment (Figure 5). Chest CT (Figure 6) suggested that the right pleural effusion decreased significantly after surgery. After two weeks of postoperative treatment, patients with mass elimination, negative lymph nodes, negative bone metastasis, normal tumor markers, PS score: 0, and no indication of progression, had reached CR, so we suspended chemotherapy, used immune maintenance therapy alone, requiring the patient to return regularly. It took one year from the beginning of the treatment to the time that the patient reached CR.
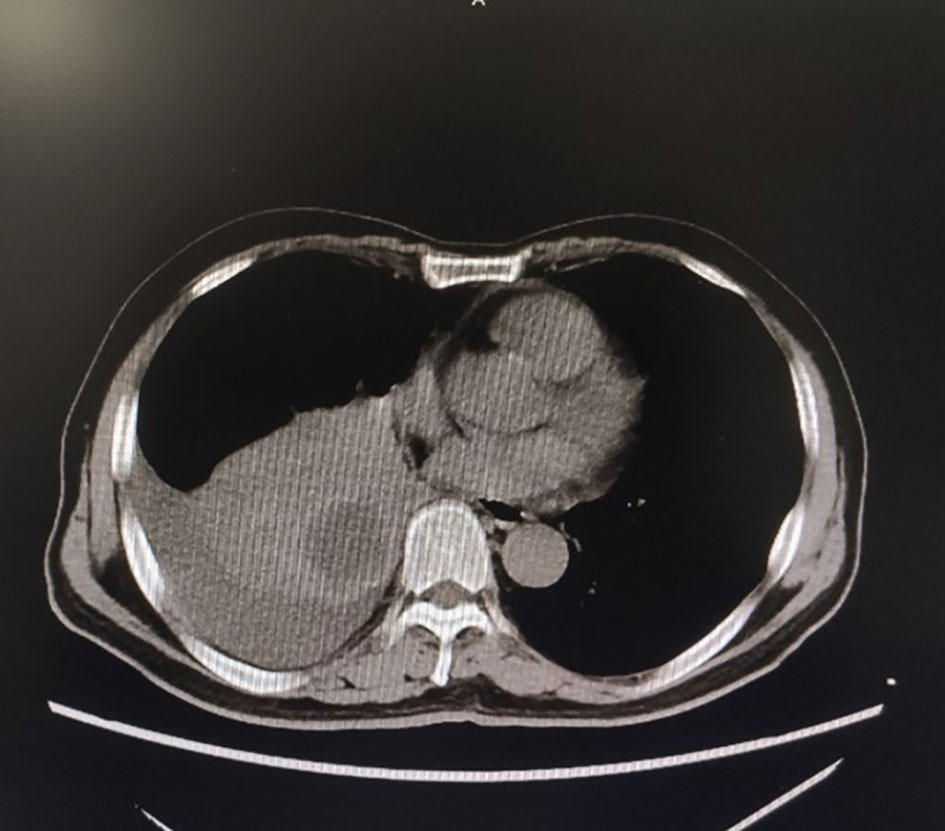
Figure 1 Chest CT for the first time in our hospital showed a space-occupying mass in the lower lobe of the right lung.
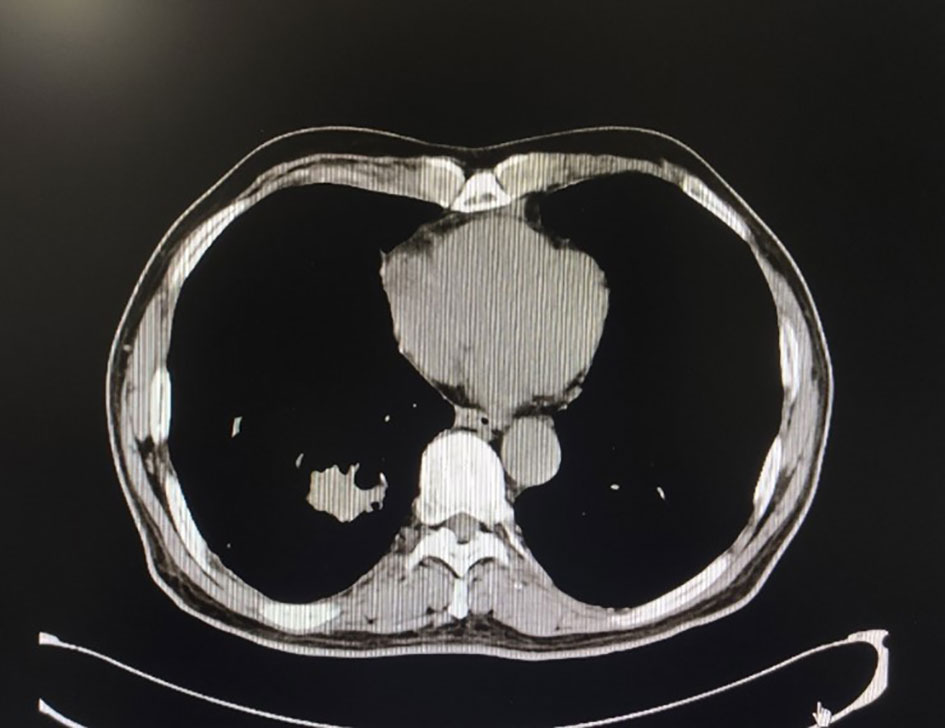
Figure 2 Chest CT after nine cycles of treatment, the space-occupying mass in the lower lobe of the right lung was significantly reduced.
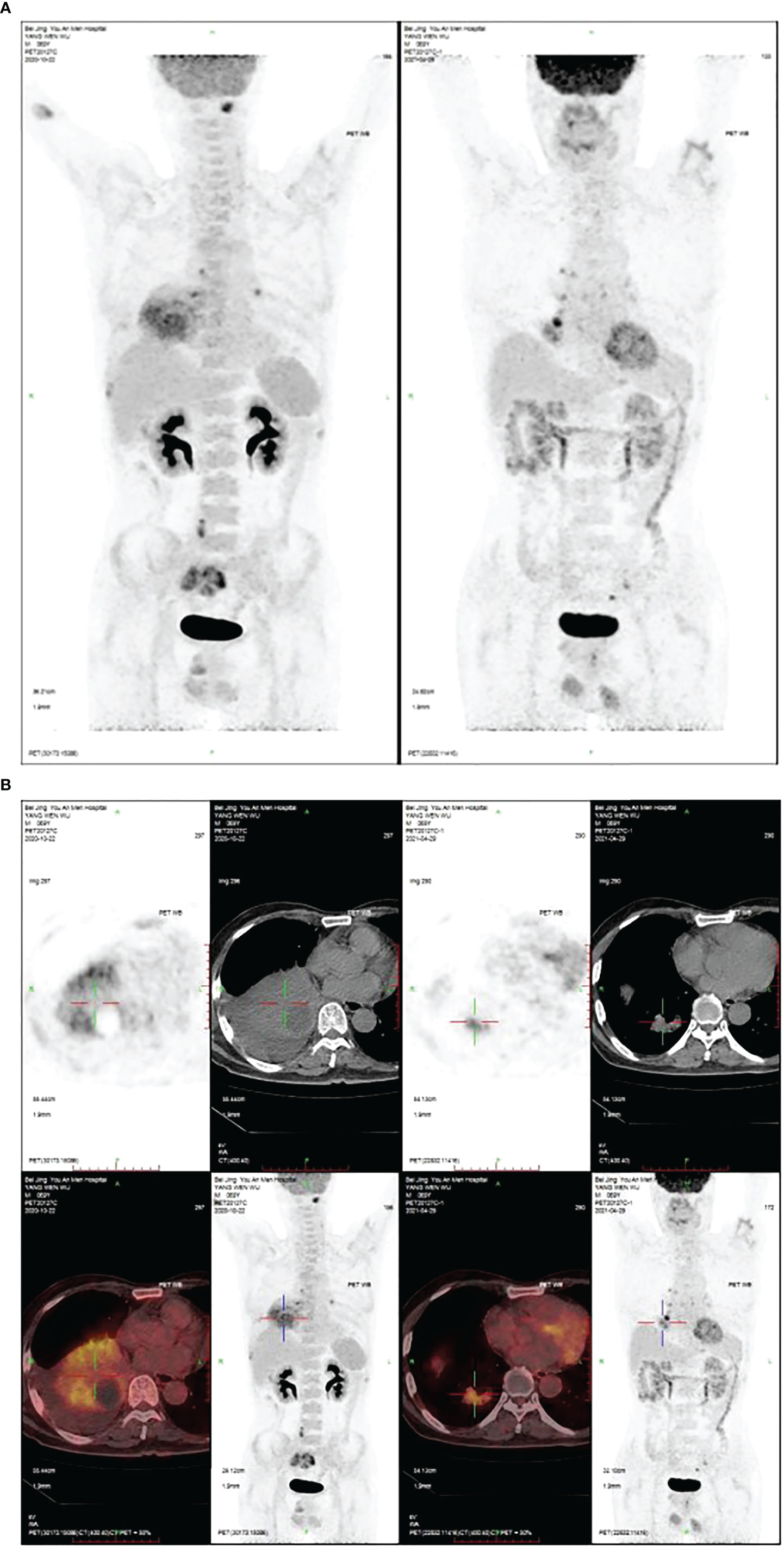
Figure 3 (A) After nine cycles of treatment, the PET/CT of the hand and chest showed the space-occupying mass in the lower lobe of the right lung was significantly reduced. (B) The PET/CT of hand and chest after nine cycles of treatment. The space-occupying mass in the lower lobe of the right lung was significantly reduced.
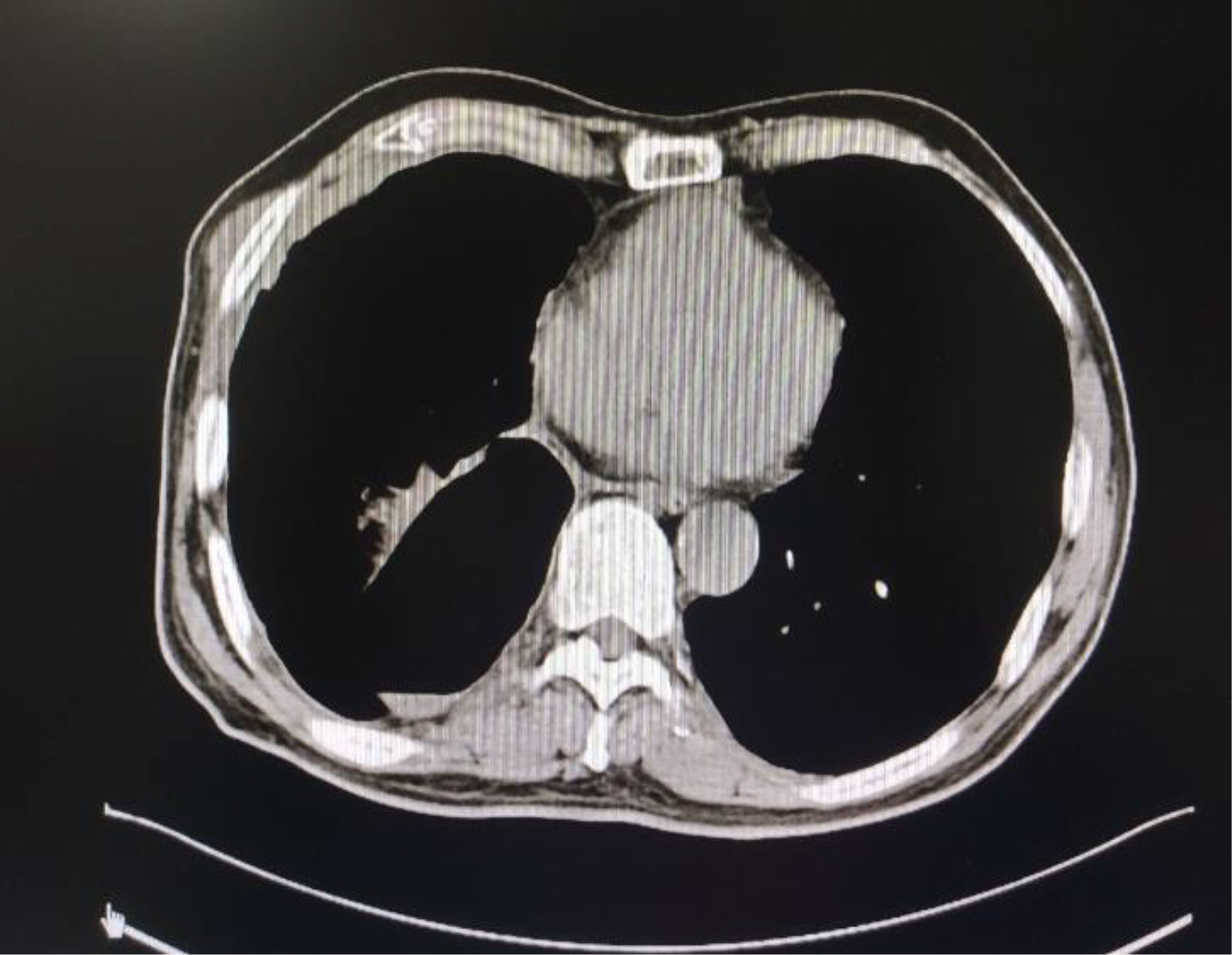
Figure 6 Chest CT after lung surgery and after two cycles of postoperative treatment. The right pleural effusion decreased significantly.
Non-small cell lung cancer accounts for about 85% of lung cancer (29). With the understanding of gene mutation in NSCLC, the emergence of new drugs (30), and the use of immune checkpoint inhibitors, the treatment of NSCLC has improved. For most patients, chemotherapy is still an important part of systemic treatment, but for about 50% of advanced NSCLC patients (31), molecular targeted therapy or immunotherapy instead of chemotherapy is the standard first-line treatment.
According to the immunohistochemical results of the patients, it is suggested that carboplatin, nedaplatin, and pemetrexed can be used and immunotherapy is effective. Therefore, the external hospital regime: carboplatin combined with pemetrexed is selected first, but because of obvious myelosuppression, nedaplatin is used instead, and the effect is remarkable. Although immunohistochemistry indicates the effectiveness of the drug, it is still necessary to flexibly adjust the treatment regimen according to the patient’s condition, to maximize the effect of chemotherapeutic drugs and minimize the adverse reactions, which reflects the necessity of individualized chemotherapy. The nine cycles of chemotherapy before surgery can be carried out smoothly because we provide patients with adequate support treatment and the patient’s systemic condition and immunity are maintained at a good level. Because drug resistance develops after nine cycles of chemotherapy, and the tumor mass becomes small which is in line with surgical indication, surgery is considered. After the operation, the patient has no progress in metastatic focus. In our view, surgery not only removes the primary lesion, but also reduces the possibility of metastasis and recurrence. Postoperative pathology shows tumor heterogeneity and positive vascular thrombus, which provides a reference for further consolidation of chemotherapy.
Cancer patients with specific gene mutations can benefit from targeted therapy. 69% of patients with advanced NSCLC may have potential operable molecular targets (32). Targeted therapy is effective for adenocarcinoma, most of these patients are young and have never smoked (33).. Platinum dual therapy with or without bevacizumab is the most common choice for advanced NSCLC patients who cannot be treated with targeted therapy, which is also the standard first-line treatment. The understanding of tumor immune patterns, including immune escape, makes a breakthrough in the treatment and lays a foundation for the development of treatment in the future. In this case, although VEGF (-), but for non-squamous non-small cell lung cancer, the vascular targeting drug bevacizumab can be used. Attention needs to by being paid to side effects such as hypertension, hemoptysis, albuminuria, and others. As early as 2015, the BEYOND study (34) for the Chinese population confirms that bevacizumab combined chemotherapy can notably prolong PFS, and median total survival time (mOS) compared with chemotherapy alone. Meanwhile, the bevacizumab combined treatment group significantly improved the objective remission rate (ORR) and disease control rate (DCR). In 2018, the State Drug Administration (NMPA) approves the first-line treatment of platinum-containing dual-drug chemotherapy combined with bevacizumab for advanced NSCLC. According to the 2021 Chinese Society of Clinical Oncology (CSCO) guidelines for the diagnosis and treatment of non-small cell lung cancer (35), platinum-containing dual-drug chemotherapy or platinum-containing dual-drug chemotherapy plus bevacizumab (lung squamous cell carcinoma) should be included as first-line treatment for patients with EGFR mutant NSCLC in stage IV, and bevacizumab should be given the first choice for patients with stage IV NSCLC without driving gene and NSCLC. Although this patient has an EGFR mutation, it is specific to exon 20 insertion mutation (EGFR20ins). According to NCCN’s latest guidelines on NSCLC (36): for EGFR20ins NSCLC patients, first-line treatment is chemotherapy combined with immunotherapy; when the disease progresses, targeted drugs, Amivantamab or Mobocertinib, are recommended. Because the effect of neoadjuvant therapy is better, we do not recommend patients take targeted drugs.
Immune checkpoint inhibitors (ICIs) are relatively new immunotherapy-based drugs. Different from traditional chemotherapy drugs, ICIs play a role in enhancing the natural tumor-killing response of the human body. Nivolumab and Pembrolizumab (PD-1 inhibitors), Atezolizumab and Durvalumab (PD-L1 inhibitors) have been approved by FDA in subsequent line therapy for advanced NSCLC patients without the sensitive mutation. They have been shown to improve the survival rate of advanced NSCLC. Although ICIs are not used in the guidelines for first-line treatment of advanced lung cancer patients with low expression of PD-L1, several new reports suggest that chemotherapy combined with immunotherapy as first-line treatment can effectively improve survival, regardless of PD-L1 expression level such as IMpower131、IMpower150. In one report (37), in the PD-L1 < 1% subgroup, the immune plus chemotherapy (I+C) regimen was more effective than chemotherapy in both OS and PFS. In the subgroup with PD-L1 ≥ 50%, the OS and PFS of the I+C regimen were also longer than those of chemotherapy. Therefore, for advanced NSCLC with different PD-L1 expressions, it is recommended to choose PD-1/PD-L1 inhibitors combined with chemotherapy in the first line and pay close attention to adverse events. Therefore, this patient chose chemotherapy combined with PD-L1 as the first choice. During the period of treatment, the tumor decreased significantly, and there were no immune-mediated adverse events in this patient. However, many factors must be considered in the treatment plan, such as therapeutic toxicity.
There is growing evidence that surgical resection is beneficial to survival in selective advanced patients. The surgery prolongs the survival time of some selected patients with stage IV NSCLC (38–40).. A national analysis (41) of long-term prognosis after surgery showed that the 5-year OS of patients with cT1-2, N0-1, M1 or cT3, N0, M1 was superior to non-operative treatment. These data supported surgical resection of specific advanced NSCLC patients. In a study (42) of a case with comprehensive treatment, the surgical prognoses of patients with stage IV NSCLC were analyzed, and the 1 -, 2-and 3-year OS rates were 75.9%, 59.1%, and 42.2% respectively. It is concluded that lung surgery may be a good choice for patients with IV stage NSCLC during comprehensive treatment. Surgical resection of malignant lesions can reduce the tumor load and restore the immune function of patients (43). Even in the case of pleural effusion (pleural dissemination or effusion is a contraindication for surgical treatment of NSCLC), the 5-years of OS after the surgery can reach 33.1%, and the patient recovers well after the surgery, and the primary tumor can be controlled (44). Many data suggest that radical surgery may be an option for symptom relief and further systematic treatment.
Lung cancer has strong temporal and spatial heterogeneity, which will affect its diagnosis and treatment. Understanding the heterogeneity of tumors may lead to new treatments, thus prolonging the survival time of patients with lung cancer in the future. It is the existence of tumor heterogeneity that makes individual differences in treatment methods. After a comprehensive evaluation of this patient, we worked out an accurate and individualized treatment plan; different treatment methods were given to different reactions due to individual differences in the process of treatment. The difference in pathological type and location of the patients determined the type of operation, which reflected the importance of individualized treatment. In short, the future treatment of lung cancer is inseparable from individualization.
The incidence and mortality of lung cancer are still high. Therefore, improving the therapeutic effect of lung cancer patients is an urgent task. In-depth study and understanding of tumor heterogeneity, continuing to find new treatment methods and individualized treatment for patients, will further improve the treatment outcome of lung cancer.
All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Nasim F, Sabath BF, Eapen GA. Lung Cancer. Med Clin North Am (2019) 103(3):463–73. doi: 10.1016/j.mcna.2018.12.006
3. Potter AL, Bajaj SS, Yang CJ. The 2021 USPSTF Lung Cancer Screening Guidelines: A New Frontier. Lancet Respir Med (2021) 9(7):689–91. doi: 10.1016/S2213-2600(21)00210-1
4. Curado M-P, Edwards B, Shin HR. Cancer Incidence in Five Continents Vol. Vol IX. Lyon: International Agency for Research on Cancer (2007).
5. Travis WD, Brambilla E, Burke A, Marx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: Int Agency Res Cancer 4:78–9 (2015).
6. Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd Ed: American College of Chest Physicians Evidencebased Clinical Practice Guidelines. Chest (2013) 143(5 suppl):e1–29S. doi: 10.1378/chest.12-2345
7. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009
8. Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung Cancer. Lancet (2021) 398(10299):535–54. doi: 10.1016/S0140-6736(21)00312-3
9. Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ. Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group. J Clin Oncol (2008) 26:3552–59. doi: 10.1200/JCO.2007.13.9030
10. Tokes WA, Rusthoven CG. Surgery vs. SBRT in Retrospective Analyses: Confounding by Operability is the Elephant in the Room. J Thorac Dis (2018) 10(Suppl 17):S2007–10. doi: 10.21037/jtd.2018.05.40
11. Socinski MA, Evans T, Gettinger S, Hensing TA, VanDam Sequist L, Ireland B, et al. Treatment of Stage IV non-Small Cell Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest (2013) 143(5 Suppl):e341S–68S. doi: 10.1378/chest.12-2361
12. Mok TS, Wu Y-L, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or Carboplatinpaclitaxel in Pulmonary Adenocarcinoma. N Engl J Med (2009) 361:947–57. doi: 10.1056/NEJMoa0810699
13. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Final Overall Survival Results From a Randomised, Phase III Study of Erlotinib Versus Chemotherapy as First-Line Treatment of EGFR Mutation-Positive Advanced Non-Smallcell Lung Cancer (OPTIMAL, CTONG-0802). Ann Oncol (2015) 26:1877–83. doi: 10.1093/annonc/mdv276
14. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib Versus Standard Chemotherapy as First-Line Treatment for European Patients With Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (EURTAC): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol (2012) 13:239–46. doi: 10.1016/S1470-2045(11)70393-X
15. Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z, et al. First-Line Erlotinib Versus Gemcitabine/Cisplatin in Patients With Advanced EGFR Mutationpositive Non-Small-Cell Lung Cancer: Analyses From the Phase III, Randomized, Open-Label, ENSURE Study. Ann Oncol (2015) 26:1883–89. doi: 10.1093/annonc/mdv270
16. Yang JJ, Zhou Q, Yan HH, Zhang XC, Chen HJ, Tu HY, et al. A Phase III Randomised Controlled Trial of Erlotinib vs Gefitinib in Advanced Non-Small Cell Lung Cancer With EGFR Mutations. Br J Cancer (2017) 116:568–74. doi: 10.1038/bjc.2016.456
17. Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients With Metastatic Lung Adenocarcinoma With EGFR Mutations. J Clin Oncol (2013) 31:3327–34. doi: 10.1200/JCO.2012.44.2806
18. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic Lymphoma Kinase Inhibition in Non-Small-Cell Lung Cancer. N Engl J Med (2010) 363:1693–703. doi: 10.1056/NEJMoa1006448
19. Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, et al. Activity and Safety of Crizotinib in Patients With ALK-Positive Non-Small-Cell Lung Cancer: Updated Results From a Phase 1 Study. Lancet Oncol (2012) 13:1011–19. doi: 10.1016/S1470-2045(12)70344-3
20. Solomon BJ, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol (2018) 36:2251–58. doi: 10.1200/JCO.2017.77.4794
21. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N Engl J Med (2015) 372:2018–28. doi: 10.1056/NEJMoa1501824
22. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab Versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
23. Vokes EE, Ready N, Felip E, Horn L, Burgio MA, Antonia SJ, et al. Nivolumab Versus Docetaxel in Previously Treated Advanced Non-Small-Cell Lung Cancer (CheckMate 017 and CheckMate 057): 3-Year Update and Outcomes in Patients With Liver Metastases. Ann Oncol (2018) 29:959–65. doi: 10.1093/annonc/mdy041
24. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab Versus Docetaxel in Patients With Previously Treated Non-Small-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet (2017) 389:255–65. doi: 10.1016/S0140-6736(16)32517-X
25. Zarour HM. Reversing T-Cell Dysfunction and Exhaustion in Cancer. Clin Cancer Res (2016) 22:1856–64. doi: 10.1158/1078-0432.CCR-15-1849
26. Gadgeel SM, Garassino MC, Esteban E, Speranza G, Felip E, Hochmair MJ, et al. KEYNOTE-189: Updated OS and Progression After the Next Line of Therapy (PFS2) With Pembrolizumab (Pembro) Plus Chemo With Pemetrexed and Platinum vs Placebo Plus Chemo for Metastatic Nonsquamous NSCLC. Proc Am Soc Clin Oncol (2019) 37(suppl):9013. doi: 10.1200/JCO.2019.37.15_suppl.9013
27. Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol (2020) 38:1505–17. doi: 10.1200/JCO.19.03136
28. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab Plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
29. Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing Epidemiology of Small-Cell Lung Cancer in the United States Over the Last 30 Years: Analysis of the Surveillance, Epidemiologic, and End Results Database. J Clin Oncol (2006) 24(28):4539–44. doi: 10.1200/JCO.2005.04.4859
30. Jordan EJ, Kim HR, Arcila ME, Barron D, Chakravarty D, Gao J, et al. Prospective Comprehensive Molecular Characterization of Lung Adenocarcinomas for Efficient Patient Matching to Approved and Emerging Therapies. Cancer Discov (2017) 7(6):596–609. doi: 10.1158/2159-8290.CD-16-1337
31. Arbour KC, Riely GJ. Systemic Therapy for Locally Advanced and Metastatic Non-Small Cell Lung Cancer: A Review. JAMA (2019) 322(8):764–74. doi: 10.1001/jama.2019.11058
32. Tsao AS, Scagliotti GV, Bunn PA Jr, Carbone DP, Warren GW, Bai C, et al. Scientifi C Advances in Lung Cancer 2015. J Thorac Oncol (2016) 11:613–38. doi: 10.1016/j.jtho.2016.03.012
33. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, et al. Lung Cancer: Current Therapies and New Targeted Treatments. Lancet (2017) 389(10066):299–311. doi: 10.1016/S0140-6736(16)30958-8
34. Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S, et al. BEYOND: A Randomized, Double-Blind, Placebo-Con- Trolled, Multicenter, Phase IIIstudy of First-Line Carboplatin/Paclitaxel Plus Bevacizumab or Placebo in Chinese Patients With Advanced or Recurrent Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol (2015) 33(19):2197–204. doi: 10.1200/JCO.2014.59.4424
35. People's Medical Publishing House(PMPH). Guidelines Working Committee of the Chinese Society of Clinical Oncology the 2021 Chinese Society of Clinical Oncology (CSCO) Guidelines for the Diagnosis and Treatment of Non-Small Cell Lung Cancer (2021) Beijing: People's Medical Publishing House (PMPH)
36. NCCN.org. NCCN Clinical Practice Guidelines in Oncology, Non-Small Cell Lung Cancer, Version 6 (2021) Plymouth Meeting, USA: National Comprehensive Cancer Network (NCCN)
37. Liang H, Liu Z, Cai X, Pan Z, Chen D, Li C, et al. PD-(L)1 Inhibitors vs. Chemotherapy vs. Their Combination in Front-Line Treatment for NSCLC: An Indirect Comparison. Int J Cancer (2019) 145(11):3011–21. doi: 10.1002/ijc.32366
38. Hanagiri T, Takenaka M, Oka S, Shigematsu Y, Nagata Y, Shimokawa H, et al. Results of a Surgical Resection for Patients With Stage IV Non-Small-Cell Lung Cancer. Clin Lung Cancer (2012) 13:220–4. doi: 10.1016/j.cllc.2011.05.006
39. Kawano D, Takeo S, Katsura M, Tsukamoto S, Masuyama E, Nakaji Y. Surgical Treatment of Stage IV Non-Small Cell Lung Cancer. Interact Cardiovasc Thorac Surg (2012) 14:167–70. doi: 10.1093/icvts/ivr036
40. Collaud S, Stahel R, Inci I, Hillinger S, Schneiter D, Kestenholz P, et al. Survival of Patients Treated Surgically for Synchronous Single-Organ Metastatic NSCLC and Advanced Pathologic TN Stage. Lung Cancer (2012) 78:234–8. doi: 10.1016/j.lungcan.2012.09.011
41. Yang CJ, Gu L, Shah SA, Yerokun BA, D'Amico TA, Hartwig MG, et al. Long-Term Outcomes of Surgical Resection for Stage IV Non-Small-Cell Lung Cancer: A National Analysis. Lung Cancer (2018) 115:75–83. doi: 10.1016/j.lungcan.2017.11.021
42. Zhang C, Wang L, Li W, Huang Z, Liu W, Bao P, et al. Surgical Outcomes of Stage IV Non-Small Cell Lung Cancer: A Single-Center Experience. J Thorac Dis (2019) 11(12):5463–73. doi: 10.21037/jtd.2019.11.30
43. Jin WB, Liang CY, Peng YH, Zhou NK. Pleuropneumonectomy for Diffuse Pleural Metastasis in Primary Lung Cancer. J Cancer Res Ther (2013) 9:S92–7. doi: 10.4103/0973-1482.119115
Keywords: individualized treatment, NSCLC, surgery, chemotherapy, immunotherapy, targeted therapy
Citation: Sun Q, Li W, Liu T and Guo H (2022) Individualized Treatment for Advanced Non-Small Cell Lung Cancer: A Case Report and Literature Review. Front. Oncol. 12:916681. doi: 10.3389/fonc.2022.916681
Received: 09 April 2022; Accepted: 21 April 2022;
Published: 26 May 2022.
Edited by:
Dong-Hua Yang, St. John’s University, United StatesReviewed by:
Youping Deng, Rush University Medical Center, United StatesCopyright © 2022 Sun, Li, Liu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiqin Guo, Z3VvaHVpcWluQG1haWwuY2NtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.