- Department of Hepatobiliary Surgery, Tianjin Medical University General Hospital, Tianjin, China
Intraductal papillary neoplasm of the bile duct (IPNB) is a rare bile duct tumor characterized by intraductal papillary or villous neoplasms covered by neoplastic epithelium with fine fibrovascular stalks in the dilated bile ducts (1). Its true etiology remains unknown. Herein, we report two cases of IPNB that underwent surgical resection. The first case was a 66-year-old male who complained of upper abdominal pain for three years. We found obstruction of the common bile duct and dilation of the intrahepatic and extrahepatic bile ducts after MRCP. Laparoscopic hepatic segmentectomy (S2, S3, S4), resection of the common bile duct, cholecystectomy, and hepaticojejunostomy were performed. The second case was a 67-year-old male with asymptomatic dilation of the intrahepatic duct. The patient underwent robot-assisted laparoscopic hepatic segmentectomy (S5, S6, S7, S8), resection of the common bile duct, hepaticojejunostomy and cholecystectomy.
Background
According to the 2019 World Health Organization (WHO) classification of digestive system tumors, intraductal papillary neoplasm of the bile duct (IPNB) is a rare tumor in the bile duct that is characterized by intraductal papillary or villous neoplasms covered by neoplastic epithelium with fine fibrovascular stalks in the dilated bile ducts (1, 2). IPNB is mainly reported in Far Eastern countries where hepatolithiasis and clonorchiasis are endemic (1). Based on a multicenter analysis, malignant features of tumors are observed in 43% of IPNB cases, and patient prognoses for malignant lesions are worse than for noninvasive lesions (3). Therefore, early identification and resection of lesions are significant, even in asymptomatic patients.
Here, we report two cases of surgical treatment of IPNB with a review of relevant literature; perhaps they could provide some information to understand this rare disease.
Case Presentation
Case 1
A 66-year-old man was referred to our hospital due to abdominal pain. Three years ago, he had presented with right upper quadrant abdominal pain, which worsened after meals, and the pain was more frequent in recent months. He had no noteworthy medical or family history. Physical examination was unremarkable.
Enhanced computed tomography (CT) at our hospital revealed interruption of the intrapancreatic common bile duct (Figure 1B) and dilatation of the extrahepatic and intrahepatic bile duct, which was especially obvious in the left lobe of the liver (Figure 1A). To evaluate dilatation of the bile duct, magnetic resonance cholangiopancreatography (MRCP) was performed. MRCP showed dilatation of the extrahepatic and intrahepatic bile ducts, irregular dilatation and thickening of the bile duct in the left lobe of the liver (Figures 1C–H). At the same time, there were localized nodular prominences in the bile duct lumen. Laboratory values on admission were as follows: tumor markers, alpha-fetoprotein (AFP) 2. 6 ng/mL (normal range 0.00-8.78 ng/mL), ferritin (Fer) 153.96 ng/mL (normal range 21.80–274. 66 ng/mL), carcinoembryonic antigen (CEA) 2. 57 ng/mL (normal range 0.00–5. 00 ng/mL), and cancer antigen 19-9 (CA199) 47. 85 U/mL (normal range 0.00–37.0 U/mL). A routine blood examination was not abnormal. The level of γ-glutamyltranspeptidase (γ-GTP) was elevated, 109 U/L (normal range 7–49 U/L) (Table 1).
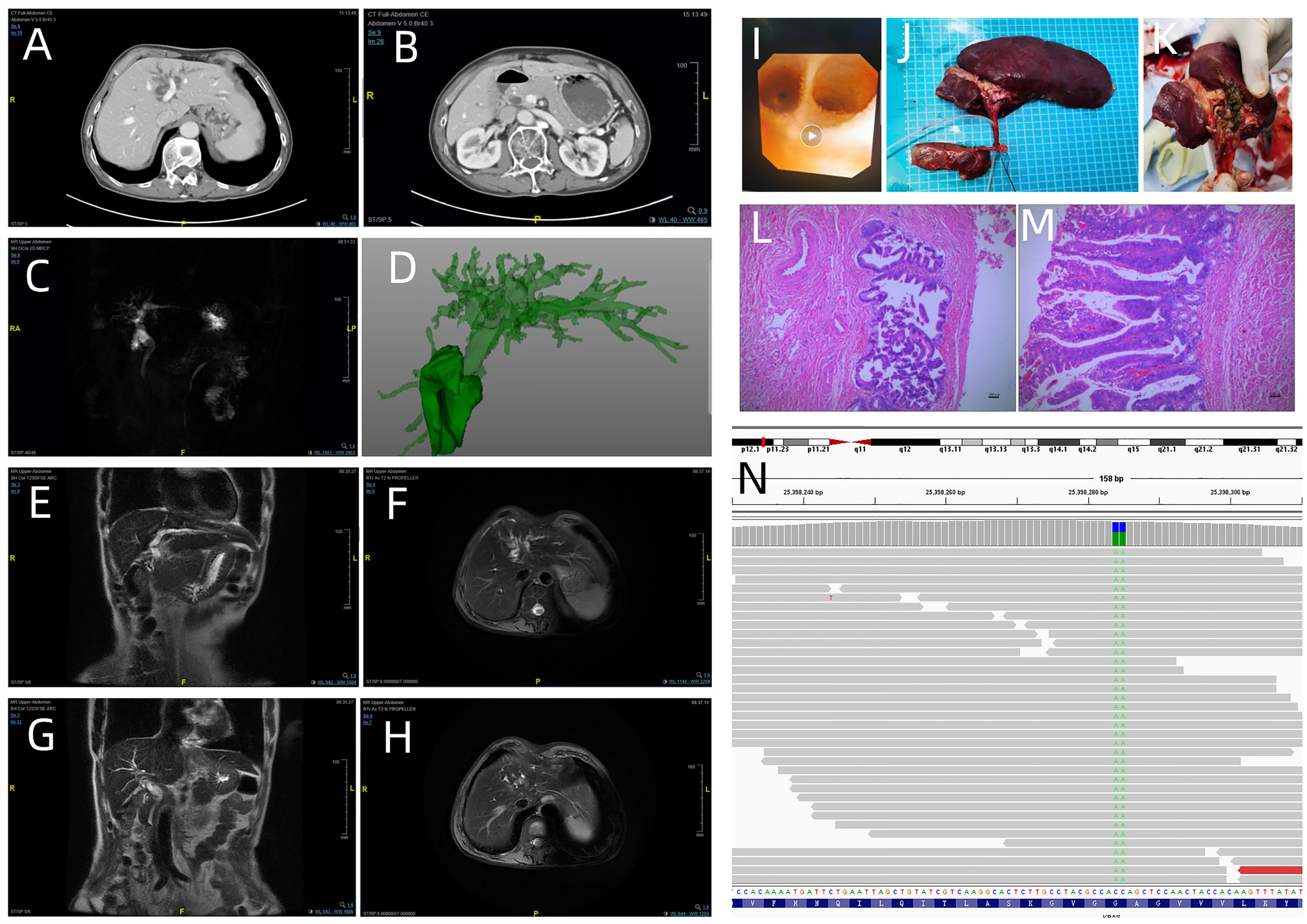
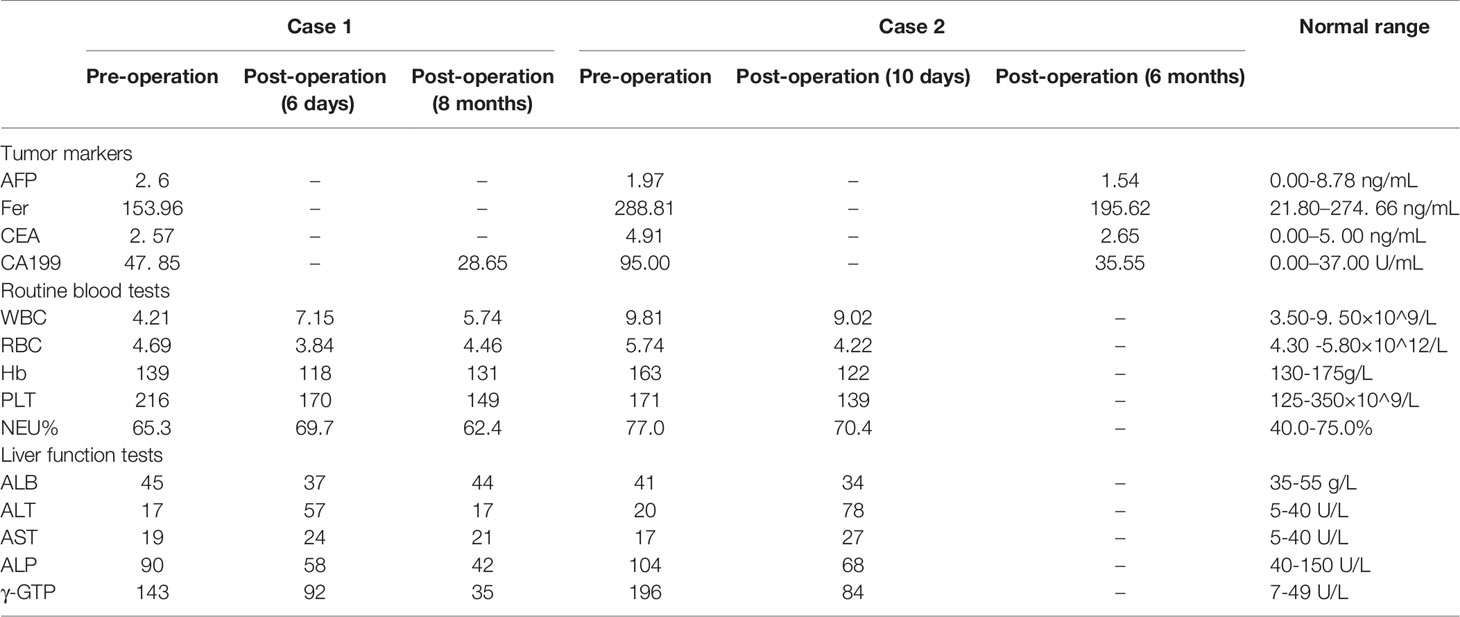
Figure 1 Enhanced computed tomography showed dilatation of the intrahepatic bile duct in the left lobe of the liver (A) and common bile duct (B); (C) (MRCP) and (D) (CT three dimensional reconstruction) revealed the dilatation of extrahepatic and intrahepatic bile duct; (E) (coronal view) and (F) (axial view). Irregular dilatation and thickening of the bile duct are visible in the left hepatic lobe; (G) (coronal view) and (H) (axial view) show dilatation of the bile duct in the right left hepatic lobe. (I) Intraoperative cholangioscopy findings: biliary papillomatosis in the left hepatic duct. (J, K) Macroscopic findings: the bile duct is filled with greenish- yellow mucus- like sludge. (L: magnification, x40 and M: magnification, x100; hematoxylin and eosin staining) Microscopic findings: the intrahepatic bile duct, common bile duct and cystic duct were lined by papillary growth neoplasia with high-grade intraepithelial neoplasia. (N) Next-generation sequencing results revealed the mutation of KRAS codon 12 (p.G12F).
After the relevant preoperative examination was completed, laparoscopic hepatic segmentectomy (S2, S3, S4), resection of the common bile duct, cholecystectomy, and hepaticojejunostomy were performed. On intraoperative cholangioscopy, we found the left hepatic duct filled with mucus and papillary protrusions adhering to the surface of the left hepatic duct (Figure 1I). No abnormalities were detected in the common bile duct. Laparoscopic hepatic segmentectomy (S2, S3, S4), resection of the common bile duct, cholecystectomy, and hepaticojejunostomy were performed. The bile duct was filled with greenish -yellow mucus -like sludge (Figures 1J, K). Microscopically, the intrahepatic bile duct, common bile duct and cystic duct were lined by papillary growth neoplasia with high-grade intraepithelial neoplasia (Figures 1L, M). Gene detection was performed using DNA extracted from paraffin-embedded bile ducts. Gene detection revealed KRAS codon 12 (p. G12F) mutation, and the mutation frequency was 62.9% (Figure 1N).
The postoperative course was uneventful, and the patient was discharged from the hospital on the 7th postoperative day. The patient had no recurrence and no complications for 8 months after surgery.
Case 2
A 67-year-old male with diabetes mellitus was admitted to our hospital due to finding dilatation of extrahepatic and intrahepatic bile ducts by accident during a routine medical examination 15 days ago. The patient was asymptomatic. He did not feel abdominal pain or abdominal distension. The patient did not have a history of viral hepatitis or alcoholic liver disease. There were no remarkable findings during the physical examination.
Dynamic upper abdominal computed tomography (CT) showed thickening of the distal common bile duct wall, luminal stenosis and dilatation of extrahepatic and intrahepatic bile duct, which was especially pronounced in the right lobe of the liver with a diameter of approximately 15 mm at its widest point (Figures 2A, B). Magnetic resonance cholangiopancreatography (MRCP) revealed that the common hepatic duct and intrahepatic bile duct were dilated (Figures 2C, D, F). The bile duct wall showed high intensity on diffusion-weighted imaging (Figures 2E). Laboratory test results included tumor markers, alpha-fetoprotein (AFP) 1. 97 ng/mL (normal range 0.00-8.78 ng/mL), ferritin (Fer) 288.81 ng/mL (normal range 21.80–274. 66 ng/mL), carcinoembryonic antigen (CEA) 4. 91 ng/mL (normal range 0.00–5. 00 ng/mL), and cancer antigen 19-9 (CA199) 95.00 U/mL (normal range 0.00–37.0 U/mL). Routine blood tests revealed a white blood cell count of 9.81×109/L (normal range 3.50-9. 50×109/L) and a neutrophilic granulocyte percentage of 77.0% (normal range 40.0-75.0%). The level of γ-glutamyltranspeptidase (γ-GTP) was elevated, 196 U/L (normal range 7–49 U/L) (Table 1). The diagnosis of intraductal papillary neoplasm of the bile duct (IPNB) was suspected based on the imaging and laboratory findings.

Figure 2 (A, B) Enhanced computed tomography demonstrated thickening of the distal common bile duct wall, luminal stenosis, dilatation of extrahepatic and intrahepatic bile duct, especially more evident in the right lobe of liver; (C) (MRCP) and (D) (CT three dimensional reconstruction) revealed the dilatation of extrahepatic and intrahepatic bile duct; (E) (diffusion-weighted image) The bile duct showed high intensity; (F) (coronal view) showed the dilatation of intrahepatic bile duct in the right lobe of liver; Intraoperative cholangioscopy revealed the common bile duct wall was full of the white flocculus (G) and rough (H); Grossly, the lumen of intrahepatic bile duct was full of yellow, gelatinous, sticky mucous masses (I, J); abdominal computed tomography (CT) showed homogeneous liver parenchyma without bile duct dilation (K); Microscopically, Intraductal papillary neoplasm of the bile duct(IPNB)with low-grade intraepithelial neoplasia, focal high-grade intraepithelial neoplasia; A large amount of lymphocytes aggregated portal area with extensive bile duct proliferation (L: magnification, x40 and M: magnification, x100; hematoxylin and eosin staining).
Intraoperative cholangioscopy was performed to evaluate the common bile duct. The common bile duct wall was rough and full of white flocculus (Figures 2G, H). Therefore, the patient underwent robot-assisted laparoscopic hepatic segmentectomy (S5, S6, S7, S8), resection of the common bile duct, hepaticojejunostomy and cholecystectomy. Grossly, the intrahepatic bile duct lumen was filled with yellow, gelatinous, sticky mucous masses. The diameter of the largest one was 18 mm. (Figures 2I, J) Pathologic findings were as follows: Intraductal papillary neoplasm of the bile duct (IPNB) with low-grade intraepithelial neoplasia, focal high-grade intraepithelial neoplasia; a large number of lymphocytes aggregated in the portal area with extensive bile duct proliferation (Figures 2L, M). The patient refused gene detection for financial reasons.
The patient’s postoperative course was uneventful. The patient was discharged from our department 13 days after surgery. The patient was followed up for 6 months after the operation, and there were no signs of recurrence. (Figure 2K).
Discussion
Intraductal papillary neoplasm of the bile duct (IPNB) is a rare bile duct tumor characterized by intraductal papillary or villous neoplasms covered by neoplastic epithelium with fine fibrovascular stalks in the dilated bile ducts (1).
IPNB is mainly found in patients in Eastern countries, such as Japan, and Korea, where hepatolithiasis and clonorchiasis, which are known to be major risk factors for IPNB, are endemic (4). IPNB shows a slight male predominance, and most patients are between 50 and 70 years of age is reported (4, 5).
However, the pathogenesis and nature of IPNB are still unclear. It is likely caused by cholestasis, biliary tract infection, and biliary tract cancer. Studies have found a mechanism for biliary tract cancer due to the progression of chronic inflammation to multistage carcinogenesis and eventually hyperplasia-dysplasia-carcinoma (6). Furthermore, chronic inflammation induces the production of reactive oxygen species and reactive nitrogen species, resulting in DNA damage, which plays an important role in carcinogenesis (7). IPNB symptoms include recurrent and intermittent abdominal pain, cholangitis, and jaundice. However, some patients are asymptomatic (3, 8). Histological types of IPNB have been classified into the following four types: gastric, intestinal, pancreaticobiliary, oncocytic, and IPMN, depending on morphologic appearance and mucin staining properties (9). GNAS and KRAS mutations detected in 50% and 46.2% of IPNBs are common in IPNBs. KRAS plays an important role in regulating cell growth and differentiation (10), A recent study has shown that KRAS mutation was detected in one third of intrahepatic cholangiocarcinoma (ICC) and also one third of biliary intraepithelialneoplasia (BilINs) associated with hepatolithiasis (11). Moreover, the KRAS mutation rate in high-grade IPNBs, and invasive IPNBs is significantly higher than low- and intermediate-grade IPNBs. So KRAS mutation may contribute to the pathogenesis of IPNBs (12, 13). Research shows that the 5-year survival rate of patients undergoing R0 resection is 59.7%. However, it is still lower than that for patients with benign diseases and better than intrahepatic cholangiocarcinoma (14, 15).
Surgical resection is the first line treatment for patients with IPNB without distant metastasis because of recurrent cholangitis and obstructive jaundice (1, 16). Liver transplantation and pancreaticoduodenectomy can be theoretically regarded as the only curative treatment. However, liver transplantation is unsuitable for patients with advanced tumor invasion or positive lymph nodes (4). Recently, new approaches, such as RFA and APC, have been helpful for patients who are not candidates for surgery due to age or physical condition (17, 18).
To further investigate the clinical features and prognosis of IPNB, we gathered case reports of IPNB in PubMed (17–55). We searched PubMed for “Intraductal papillary neoplasm of the bile duct” and “case report” during 2002 to 2021. Case reports were included if they reported any aspect of the clinicopathological or surgical characteristics of patients with IPNB confirmed by pathology. Most patients were more than 45 years of age, with a median age of 69 years. Only two patients were younger than 45 years of age. Males slightly predominated the case reports. The geographic distribution of patients was not different from other reviews, with IPNB mainly in Asia. There was only one patient found in Africa. However, the patients was Japanese. The tumor size ranged from 10.6-130. 0 mm, with a median size of 52. 9 mm. IPNB can develop anywhere along the biliary tree, with 25.0% in the right lobe of the liver, 32.5% in the left lobe of the liver, 12.5% in the CBD, 17.5% in the extrahepatic duct and liver and 12.5% in other locations, including the whole liver or gallbladder. The common radiologic findings for IPNB are bile duct dilatation, intraductal mass and cystic lesion. Among all cases, 28 included information on tumor markers, the CA 19-9 of 10 cases was elevated, and 18 were not elevated. The type of surgery depended on various anatomical locations of the tumor and the physical qualifications of the patients. Hepatectomy, which accounted for 40%, was the main surgical approach. Twenty-five percent of patients underwent hepatectomy, extrahepatic bile duct resection, and hepaticojejunostomy. Five percent of patients underwent hepatectomy and segmental pancreatectomy. A total of 7.5% of patients underwent pancreatoduodenectomy. As a palliative treatment, endoscopic treatment is necessary for patients with poor physical conditions. It is worth mentioning that RFA and APC accounted for 7.5% of promising therapeutic strategies and are expected to bring new approaches for IPNB (Table 2). IPNB lesions frequently contain invasive components. Twenty-three cases (57.5%) contained evidence of high- grade cholangiocarcinoma, while 17 cases (42.5%) ranged from no invasion to medium-grade cholangiocarcinoma. Immunohistochemical data were available for 26 cases, from which we found that CK7 (42.3%), MUC5AC (53.8%) and MUC6 (53.8%) expression was common in the IPNB. Based on the recurrence-free survival (RFS) reported in 23 cases, we found that the range of RFS was 4.0-39.0 months, with a median of 14.0 months (Table 3).
On further review of the literature, we could conclude that IPNB mainly behaves as in imaging solid mass, cystic lesion and dilation of the bile duct in imaging. Surgical resection is the major treatment for patients in fine condition, and interventional therapies such as RFA and APC are good choices for palliative treatments. Although most IPNB cases are high-grade intraepithelial neoplasia or invasive carcinoma in microscopically, surgical resection could make patients get satisfactory prognosis.
Conclusion
In summary, we report two cases of IPNB, a rare tumor of the hepatobiliary system, and analyze published case reports about IPNB. Since IPNB has a high potential for transforming into an invasive lesion, R0 surgical resection is preferred. At the same time, RFA and APC may be new palliative approaches for treating IPNB.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author Contributions
BL designed the case report. ZL, QL, and WT participated in the operation and management of the patients. BL, ZM, and ML prepared radiological and histology figures. BL, ZL reviewed the literature and drafted the article. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ohtsuka M, Shimizu H, Kato A, Yoshitomi H, Furukawa K, Tsuyuguchi T, et al. Intraductal Papillary Neoplasms of the Bile Duct. Int J Hepatol (2014) 2014:459091. doi: 10.1155/2014/459091
2. Nakanuma Y, Basturk O, Esposito I, Klimstra DS, Komuta M, Zen Y. Intraductal Papillary Neoplasm of the Bile Ducts. In: WHO Classification of Tumours. Digestive System Tumours, fifth ed. Lyon: IARC (2019). p. 279–82.
3. Gordon-Weeks AN, Jones K, Harriss E, Smith A, Silva M. Systematic Review and Meta-Analysis of Current Experience in Treating IPNB: Clinical and Pathological Correlates. Ann Surg (2016) 263(4):656–63. doi: 10.1097/SLA.0000000000001426
4. Wan XS, Xu YY, Qian JY, Yang XB, Wang AQ, He L, et al. Intraductal Papillary Neoplasm of the Bile Duct. World J Gastroenterol (2013) 19(46):8595–604. doi: 10.3748/wjg.v19.i46.8595
5. Wu X, Li B, Zheng C, Chang X, Zhang T, He X, et al. Intraductal Papillary Neoplasm of the Bile Duct: A Single-Center Retrospective Study. J Int Med Res (2018) 46(10):4258–68. doi: 10.1177/0300060518792800
6. Terada T, Nakanuma Y, Ohta T, Nagakawa T. Histological Features and Interphase Nucleolar Organizer Regions in Hyperplastic, Dysplastic and Neoplastic Epithelium of Intrahepatic Bile Ducts in Hepatolithiasis. Histopathology (1992) 21(3):233–40. doi: 10.1111/j.1365-2559.1992.tb00381.x
7. Aishima S, Kubo Y, Tanaka Y, Oda Y. Histological Features of Precancerous and Early Cancerous Lesions of Biliary Tract Carcinoma. J Hepatobiliary Pancreat Sci (2014) 21(7):448–52. doi: 10.1002/jhbp.71
8. Park HJ, Kim SY, Kim HJ, Lee SS, Hong GS, Byun JH, et al. Intraductal Papillary Neoplasm of the Bile Duct: Clinical, Imaging, and Pathologic Features. AJR Am J Roentgenol (2018) 211(1):67–75. doi: 10.2214/AJR.17.19261
9. Wang X, Cai YQ, Chen YH, Liu XB. Biliary Tract Intraductal Papillary Mucinous Neoplasm: Report of 19 Cases. World J Gastroenterol (2015) 21(14):4261–7. doi: 10.3748/wjg.v21.i14.4261
10. Xiao HD, Yamaguchi H, Dias-Santagata D, Kuboki Y, Akhavanfard S, Hatori T, et al. Molecular Characteristics and Biological Behaviours of the Oncocytic and Pancreatobiliary Subtypes of Intraductal Papillary Mucinous Neoplasms. J Pathol (2011) 224(4):508–16. doi: 10.1002/path.2875
11. Hsu M, Sasaki M, Igarashi S, Sato Y, Nakanuma Y. KRAS and GNAS Mutations and P53 Overexpression in Biliary Intraepithelial Neoplasia and Intrahepatic Cholangiocarcinomas. Cancer (2013) 119(9):1669–74. doi: 10.1002/cncr.27955
12. Sasaki M, Matsubara T, Nitta T, Sato Y, Nakanuma Y. GNAS and KRAS Mutations are Common in Intraductal Papillary Neoplasms of the Bile Duct. PloS One (2013) 8(12):e81706. doi: 10.1371/journal.pone.0081706
13. Xian ZH, Qin C, Cong WM. KRAS Mutation and Immunohistochemical Profile in Intraductal Papillary Neoplasm of the Intrahepatic Bile Ducts. Pathol Res Pract (2018) 214(1):105–11. doi: 10.1016/j.prp.2017.10.017
14. Luvira V, Pugkhem A, Bhudhisawasdi V, Pairojkul C, Sathitkarnmanee E, Luvira V, et al. Long-Term Outcome of Surgical Resection for Intraductal Papillary Neoplasm of the Bile Duct. J Gastroenterol Hepatol (2017) 32(2):527–33. doi: 10.1111/jgh.13481
15. Kawarada Y, Yamagiwa K, Das BC. Analysis of the Relationships Between Clinicopathologic Factors and Survival Time in Intrahepatic Cholangiocarcinoma. Am J Surg (2002) 183(6):679–85. doi: 10.1016/S0002-9610(02)00853-X
16. Krawczyk M, Ziarkiewicz-Wróblewska B, Podgórska J, Grzybowski J, Gierej B, Krawczyk P, et al. Intraductal Papillary Neoplasm of the Bile Duct - A Comprehensive Review. Adv Med Sci (2021) 66(1):138–47. doi: 10.1016/j.advms.2021.01.005
17. Arai J, Kato J, Toda N, Kurokawa K, Shibata C, Kurosaki S, et al. Long-Term Survival After Palliative Argon Plasma Coagulation for Intraductal Papillary Mucinous Neoplasm of the Bile Duct. Clin J Gastroenterol (2021) 14(1):314–8. doi: 10.1007/s12328-020-01199-0
18. Natov NS, Horton LC, Hegde SR. Successful Endoscopic Treatment of an Intraductal Papillary Neoplasm of the Bile Duct. World J Gastrointest Endosc. (2017) 9(5):238–42. doi: 10.4253/wjge.v9.i5.238
19. Nakagawa T, Arisaka Y, Ajiki T, Fujikura K, Masuda A, Takenaka M, et al. Intraductal Tubulopapillary Neoplasm of the Bile Duct: A Case Report and Review of the Published Work. Hepatol Res (2016) 46(7):713–8. doi: 10.1111/hepr.12604
20. Kunovsky L, Kala Z, Svaton R, Moravcik P, Mazanec J, Husty J, et al. Mucinous Cystic Neoplasm of the Liver or Intraductal Papillary Mucinous Neoplasm of the Bile Duct? A Case Report and a Review of Literature. Ann Hepatol (2018) 17(3):519–24. doi: 10.5604/01.3001.0011.7397
21. Apostolopoulos P, Ekmektzoglou KA, Paraskeva K, Dimopoulos K, Paparaskeva K, Alexandrakis G. Intraductal Papillary Neoplasm of the Bile Duct : Case Report and Review of the Literature. Acta Gastroenterol Belg. (2018) 81(1):97–9.
22. Nam NH, Taura K, Kanai M, Fukuyama K, Uza N, Maeda H, et al. Unexpected Metastasis of Intraductal Papillary Neoplasm of the Bile Duct Without an Invasive Component to the Brain and Lungs: A Case Report. World J Gastroenterol (2020) 26(3):366–74. doi: 10.3748/wjg.v26.i3.366
23. Tsujimae M, Sakai A, Masuda A, Inomata N, Masuda S, Gonda M, et al. A Case in Which an Intraductal Papillary Neoplasm of the Bile Duct Was Surgically Resected 12 Years After the Initial Diagnosis. Intern Med (2020) 59(22):2879–83. doi: 10.2169/internalmedicine.4891-20
24. Peeters K, Delvaux P, Huysentruyt F. Intraductal Papillary Neoplasm of the Bile Duct: A Case Report. Acta Chir Belg. (2017) 117(4):260–3. doi: 10.1080/00015458.2016.1258785
25. Aliyev V, Yasuchika K, Hammad A, Tajima T, Fukumitsu K, Hata K, et al. A Huge Intraductal Papillary Neoplasm of the Bile Duct Treated by Right Trisectionectomy After Right Portal Vein Embolization. Ann Hepatobiliary Pancreat Surg (2018) 22(2):150–5. doi: 10.14701/ahbps.2018.22.2.150
26. Ma Z, Zhao F, Pan J, Lin G, Chen B, Fu W. Cystic Intraductal Papillary Neoplasms With Infiltrating Carcinoma of the Intrahepatic Bile Duct: A Case Report. Med (Baltimore) (2020) 99(3):e18758. doi: 10.1097/MD.0000000000018758
27. Tan Y, Milikowski C, Toribio Y, Singer A, Rojas CP, Garcia-Buitrago MT. Intraductal Papillary Neoplasm of the Bile Ducts: A Case Report and Literature Review. World J Gastroenterol (2015) 21(43):12498–504. doi: 10.3748/wjg.v21.i43.12498
28. Dutta S, Upadhyay P, Jain A, Nachiappa Ganesh R, Nelamangala Ramakrishnaiah VP. Intraductal Papillary Neoplasm of the Bile Duct: A Rare Case of Intrahepatic Space-Occupying Lesion. Cureus (2021) 13(2):e13063. doi: 10.7759/cureus.13063
29. Hokuto D, Nomi T, Yasuda S, Yoshikawa T, Ishioka K, Yamada T, et al. Long-Term Observation and Treatment of a Widespread Intraductal Papillary Neoplasm of the Bile Duct Extending From the Intrapancreatic Bile Duct to the Bilateral Intrahepatic Bile Duct: A Case Report. Int J Surg Case Rep (2017) 38:166–71. doi: 10.1016/j.ijscr.2017.07.031
30. Anjum MR, Dasari BVM, Menon S. Multiple Intraductal Papillary Neoplasms of the Bile Ducts: A Case Report. VideoGIE (2020) 6(1):22–3. doi: 10.1016/j.vgie.2020.08.008
31. Hachiya H, Kita J, Shiraki T, Iso Y, Shimoda M, Kubota K. Intraductal Papillary Neoplasm of the Bile Duct Developing in a Patient With Primary Sclerosing Cholangitis: A Case Report. World J Gastroenterol (2014) 20(42):15925–30. doi: 10.3748/wjg.v20.i42.15925
32. Kitahama T, Yamane H, Mohri K, Fukuoka E, Yoshida T, Yamagishi T, et al. A Case of Intraductal Papillary Neoplasm of the Bile Duct Accompanied by Intraductal Papillary Mucinous Neoplasm of the Pancreas and Hepatocellular Carcinoma. Clin J Gastroenterol (2021) 14(5):1536–43. doi: 10.1007/s12328-021-01461-z
33. Kakisaka T, Kamiyama T, Yokoo H, Nakanishi K, Wakayama K, Tsuruga Y, et al. An Intraductal Papillary Neoplasm of the Bile Duct Mimicking a Hemorrhagic Hepatic Cyst: A Case Report. World J Surg Oncol (2013) 11:111. doi: 10.1186/1477-7819-11-111
34. Tsuchida K, Yamagata M, Saifuku Y, Ichikawa D, Kanke K, Murohisa T, et al. Successful Endoscopic Procedures for Intraductal Papillary Neoplasm of the Bile Duct: A Case Report. World J Gastroenterol (2010) 16(7):909–13. doi: 10.3748/wjg.v16.i7.909
35. Tominaga K, Kamimura K, Sakamaki A, Terai S. Intraductal Papillary Neoplasm of the Bile Duct: A Rare Liver Tumor Complicated by Malignancy. Hepatol (2017) 66(5):1695–7. doi: 10.1002/hep.29266
36. Shimoda T, Yoshida H, Hirakata A, Makino H, Yokoyama T, Maruyama H, et al. Surgical Resection of Cystic Intraductal Papillary Adenocarcinoma of the Bile Duct: Report of a Case. J Nippon Med Sch (2013) 80(3):234–9. doi: 10.1272/jnms.80.234
37. Tajima S, Ohata A, Koda K, Maruyama Y. Intraductal Papillary Neoplasm of the Bile Duct, Gastric Type, Arising in the Intrapancreatic Common Bile Duct Could Progress to Colloid Carcinoma: Report of a Case. Int J Clin Exp Pathol (2015) 8(5):5848–55.
38. Kang GH, Moon HS, Lee ES, Kim SH, Sung JK, Lee B, et al. A Case of Colloid Carcinoma Arising in Association With Intraductal Papillary Neoplasm of the Liver. Korean J Gastroenterol (2012) 60(6):386–90. doi: 10.4166/kjg.2012.60.6.386
39. Hasebe T, Sawada K, Hayashi H, Nakajima S, Takahashi H, Hagiwara M, et al. Long-Term Growth of Intrahepatic Papillary Neoplasms: A Case Report. World J Gastroenterol (2019) 25(36):5569–77. doi: 10.3748/wjg.v25.i36.5569
40. Okamoto T, Nakamura K, Fukuda K. Mucin-Producing Bile Duct Tumor Treated Successfully With Endoscopic Ultrasound-Guided Hepaticogastrostomy. Clin J Gastroenterol (2020) 13(5):812–7. doi: 10.1007/s12328-020-01123-6
41. Mo A, Brat G, Spolverato G, Pawlik TM. Intraductal Papillary Mucinous Neoplasm of the Liver: GI Image. J Gastrointest Surg (2015) 19(4):792–4. doi: 10.1007/s11605-015-2750-2
42. Lee JH, Kim HS, Park JH, Park JS. Intraductal Papillary Mucinous Neoplasm of the Biliary Tract With Cardiac Metastasis: A Case Report. Med (Baltimore) (2021) 100(3):e24310. doi: 10.1097/MD.0000000000024310
43. Nanashima A, Sumida Y, Tomoshige K, Takeshita H, Shibata K, Sawai T, et al. A Case of Intraductal Papillary Neoplasm of the Bile Duct With Stromal Invasion. Case Rep Gastroenterol (2008) 2(3):314–20. doi: 10.1159/000154818
44. Cocca S, Grande G, Reggiani Bonetti L, Magistri P, Di Sandro S, Di Benedetto F, et al. Common Bile Duct Lesions - How Cholangioscopy Helps Rule Out Intraductal Papillary Neoplasms of the Bile Duct: A Case Report. World J Gastrointest Endosc. (2020) 12(12):555–9. doi: 10.4253/wjge.v12.i12.555
45. Ishida M, Seki K, Honda K, Kimura T, Katayama K, Hirose K, et al. Intraductal Mucinous Tumors Occurring Simultaneously in the Liver and Pancreas. J Gastroenterol (2002) 37(12):1073–8. doi: 10.1007/s005350200181
46. Yokode M, Hanada K, Shimizu A, Minami T, Hirohata R, Abe T, et al. Intracholecystic Papillary Neoplasm of the Gallbladder Protruding Into the Common Bile Duct: A Case Report. Mol Clin Oncol (2019) 11(5):488–92. doi: 10.3892/mco.2019.1919
47. Hong MY, Yu DW, Hong SG. Intraductal Papillary Mucinous Neoplasm of the Bile Duct With Gastric and Duodenal Fistulas. World J Gastrointest Endosc. (2014) 6(7):328–33. doi: 10.4253/wjge.v6.i7.328
48. Kinoshita M, Asaoka T, Eguchi H, Hanaki T, Iwagami Y, Akita H, et al. A Case of Intraductal Papillary Neoplasm of the Bile Duct That Developed 38 Years After Choledochoduodenostomy With Invasive Adenocarcinoma and Lymph Node Metastasis. Surg Case Rep (2019) 5(1):93. doi: 10.1186/s40792-019-0651-4
49. Kung JWC, Parks RW, Ireland HM, Kendall TJ, Church NI. Intraductal Papillary Neoplasm of the Bile Duct: The Role of Single-Operator Cholangioscopy. VideoGIE (2017) 3(2):55–7. doi: 10.1016/j.vgie.2017.10.006
50. Parekh R, Krol G, Piraka C, Batra S. A Rare Case of Intraductal Papillary Mucinous Neoplasm of the Biliary Duct in a Patient With Prostate Adenocarcinoma. Case Rep Gastroenterol (2016) 10(3):743–8. doi: 10.1159/000450539
51. Carrafiello G, Bertolotti E, Sessa F, Cafaro T, Dionigi G, Genovese E, et al. Intraductal Papillary Mucinous Tumor of Bile Ducts Radiologic and Pathologic Features: A Case Report. cases J (2008) 1(1):319. doi: 10.1186/1757-1626-1-319
52. Fujita M, Wakui N, Yamauchi Y, Takeda Y, Sato T, Ueki N, et al. A Case of Branch Duct Type Intraductal Papillary Neoplasm of the Bile Duct Treated by Open Surgery After 11 Years of Follow-Up. Mol Clin Oncol (2013) 1(6):965–9. doi: 10.3892/mco.2013.160
53. Baltagiannis EG, Kalyvioti C, Glantzouni A, Batistatou A, Tzimas P, Glantzounis GK. Intrahepatic Intraductal Papillary Cystic Neoplasm of the Bile Duct: A Case Report. Ann Med Surg (Lond) (2021) 63:102167. doi: 10.1016/j.amsu.2021.02.013
54. Tang W, Qiu JG, Wei XF, Xiao H, Deng X, Wang SD, et al. Endoscopic Endoluminal Radiofrequency Ablation and Single-Operator Peroral Cholangioscopy System (SpyGlass) in the Diagnosis and Treatment of Intraductal Papillary Neoplasm of the Bile Duct: A Case Report and Literature Review. Front Med (Lausanne) (2021) 8:675720. doi: 10.3389/fmed.2021.675720
55. Baterdene N, Hwang S, Lee JW, Jung MJ, Shin H, Seo HK, et al. Surgical Treatment of Mucin-Producing Cholangiocarcinoma Arising From Intraductal Papillary Neoplasm of the Intrahepatic Bile Duct: A Report of 2 Cases. Korean J Hepatobiliary Pancreat Surg (2016) 20(3):137–43. doi: 10.14701/kjhbps.2016.20.3.137
Keywords: surgical treatment, intraductal papillary neoplasm of the bile duct, mutation, clinical features, prognosis
Citation: Li B, Liu Z, Meng Z, Li M, Tian W and Liu Q (2022) Surgical Treatment of Intraductal Papillary Neoplasm of the Bile Duct: A Report of Two Cases and Review of the Literature. Front. Oncol. 12:916457. doi: 10.3389/fonc.2022.916457
Received: 09 April 2022; Accepted: 16 May 2022;
Published: 23 June 2022.
Edited by:
Sanjit Mukherjee, National Institutes of Health (NIH), United StatesReviewed by:
Asit Kumar Manna, The University of Utah, United StatesAbhisek Bhattacharya, National Institutes of Health (NIH), United States
Copyright © 2022 Li, Liu, Meng, Li, Tian and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quanyan Liu, c3Bzc2xxeUB2aXAuMTI2LmNvbQ==
 Binjie Li
Binjie Li Zhiqiang Liu
Zhiqiang Liu Zhuo Meng
Zhuo Meng Mingyang Li
Mingyang Li Weijun Tian
Weijun Tian Quanyan Liu
Quanyan Liu