
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 13 July 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.916091
This article is part of the Research Topic Clinicopathological Factors and Staging in Gastrointestinal Cancers View all 74 articles
Purpose: The aim of this study is to investigate the survival benefit of adjuvant chemotherapy in patients with colon cancer with the solitary tumor deposit (TD).
Methods: The primary study outcomes used in this study were colon cancer–specific survival (CSS) and overall survival (OS). The differences of the distribution of categorical variables in patients with colon cancer with the solitary TD according to adjuvant chemotherapy administration were tested using the Pearson’s chi-square test. The Kaplan–Meier method was utilized to evaluate CSS and OS. Hazard ratio (HR) and 95% confidence interval (CI) were calculated on the basis of Cox regression models to assess the prognostic value of different demographic and clinicopathological characteristics.
Results: A total of 877 patients with TanyN1cM0 colon cancer with solitary TD were identified in our analysis. It was found that OS (75.4% vs. 42.8% for 5-year OS rate, p < 0.001) and CSS (82.9% vs. 69.3% for 5-year CSS rate, p < 0.001) of patients with colon cancer with adjuvant chemotherapy administration were significantly better than those without adjuvant chemotherapy administration. Multivariate Cox survival analyses revealed that the overall and colon cancer–specific mortality risks of patients with adjuvant chemotherapy administration were decreased by 64.4% (HR = 0.356, 95% CI = 0.265–0.479, p < 0.001) and 57.4% (HR = 0.426, 95% CI = 0.286–0.634, p < 0.001) compared with those without adjuvant chemotherapy administration, respectively.
Conclusions: Adjuvant chemotherapy administration could significantly improve OS and CSS in patients with colon cancer with the solitary TD. This is the first study to investigate and demonstrate the survival benefit of adjuvant chemotherapy in patients with colon cancer with the solitary TD.
Colorectal cancer is now the third most common cancer in the world and the second most common cause of cancer death (1). The tumor, node, metastasis (TNM) staging is the most generally used system for the diagnosis and treatment of colorectal cancer, and it has been continuously developed with the continuous progress of tumor treatment; the latest eight edition of the TNM staging system was published in 2017 (2, 3).
In the eighth edition of TNM staging, tumor deposit (TD) is defined as tumor foci located in the pericolonic or perirectal fat, away from the tumor invasion front, in the lymphatic drainage area of the tumor, with no identifiable residual lymph node tissue. In addition, the presence of TD is classified as N1c in the absence of lymph node metastasis (LNM) and TD has no impact on tumor stage in the presence of LNM. Previous studies have shown that TD is an independent and strong prognostic factor in patients with colorectal cancer after surgery, regardless of lymph node status (4–6). More importantly, many studies have shown that the number of TDs is independently associated with poor outcomes in patients with colorectal cancer and suggested adding TDs to the number of LNMs (7–9). A meta-analysis found that TD was a stronger prognostic factor than LNM or extramural vascular invasion for patients with colorectal cancer with liver, lung, and peritoneal metastases (10). In addition, TD is also significantly associated with higher local recurrence and worse prognosis in patients diagnosed with rectal adenocarcinoma (11, 12).
To date, however, no studies have evaluated the role of adjuvant chemotherapy in patients with colon cancer with solitary TD although adjuvant chemotherapy is routinely used in patients with N1c colorectal cancer. Recently, Korean researchers retrospectively analyzed the effect of adjuvant chemotherapy in 281 patients with colon cancer and found that adjuvant chemotherapy did not provide clear survival benefit for patients with colon cancer with solitary LNM (13). Therefore, it is reasonable to question the survival benefit of adjuvant chemotherapy in patients with colon cancer with solitary TD.
Patients with solitary TD are a very small group of people in colon cancer; therefore, a large cancer database is utilized in this study. The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database is the largest cancer database in the United States, containing information on patient’s age, sex, tumor stage, tumor site, tumor grade, treatment, and prognosis of malignant tumors in 18 population-based cancer registries, covering about one-third of the population in the country. We then extract the patient data from the SEER database (https://seer.cancer.gov). Because patient identification information has been removed in this database, ethics committee approval and patient informed consent are not required in this study.
As shown in Figure 1, we first selected patients diagnosed with colon cancer in the cancer database from 2010 to 2016, and patients who met the following criteria were further confirmed: treated with surgery, adenocarcinoma, without distant metastasis, and tumor grade is known. Finally, patients with TanyN1cM0 colon cancer with the solitary TD were selected for the analysis of this study.
The primary study outcomes used in this study were colon cancer–specific survival (CSS) and overall survival (OS). CSS and OS were defined as the time between the cancer diagnosis and death due to colon cancer and death from any cause, respectively. Patients’ demographic and clinicopathological characteristics were included as follows: T staging (T1, T2, T3, and T4), age at diagnosis (≤65 and >65 years old), gender (male and female), tumor location [right-sided colon (from caecum to transverse colon) and left-sided colon (from splenic flexure to rectosigmoid junction)], and tumor grade (grade I/II and grade III/IV).
The differences of the distribution of categorical variables in patients with colon cancer with the solitary TD according to adjuvant chemotherapy administration were tested using the Pearson’s chi-square test. The Kaplan–Meier method was used to evaluate CSS and OS. The log-rank test was also carried out to assess whether the differences of CSS or OS rates between subgroups were statistically significant. Hazard ratio (HR) and 95% confidence interval (CI) were calculated on the basis of Cox regression models to assess the prognostic value of different demographic and clinicopathological characteristics. Statistically significant levels were two-tailed, and a p-value less than 0.05 was considered significant. Statistical analyses were conducted using the Statistical Package for the Social Sciences version 23.0 software (IBM Corporation, Chicago, IL, USA).
As shown in Figure 1, a total of 877 patients with TanyN1cM0 colon cancer with solitary TD were identified in our analysis, among which 405 (46.2%) patients received adjuvant chemotherapy and 472 (53.8%) patients did not receive adjuvant chemotherapy; 29 (3.3%) patients were diagnosed with stage T1, 80 (9.1%) patients were diagnosed with stage T2, 567 (64.7%) patients were diagnosed with stage T3, and 201 (22.9%) patients were diagnosed with stage T4; 332 (37.9%) patients were less than 65 years old, and 545 (62.1%) patients were more than 65 years old; 453 (51.7%) patients were male, and 424 (48.3%) patients were female; 489 (55.8%) patients were diagnosed with right-sided colon cancer, and 388 (44.2%) patients were diagnosed with left-sided colon cancer; 727 (82.9%) patients were diagnosed with grade I/II colon cancer, and 150 (17.1%) patients were diagnosed with grade III/IV colon cancer.
Patients’ demographic and clinicopathological characteristics according to adjuvant chemotherapy administration were shown in Table 1. There were no differences in T staging, gender, tumor grade, and number of lymph nodes examined between the two subgroups. Younger age (56.5% vs. 21.8% for ≤ 65 years old, p < 0.001) and left-sided colon cancer (48.6% vs. 40.5% for left-sided colon cancer, p < 0.015) were more likely to be associated with the adjuvant chemotherapy administration.
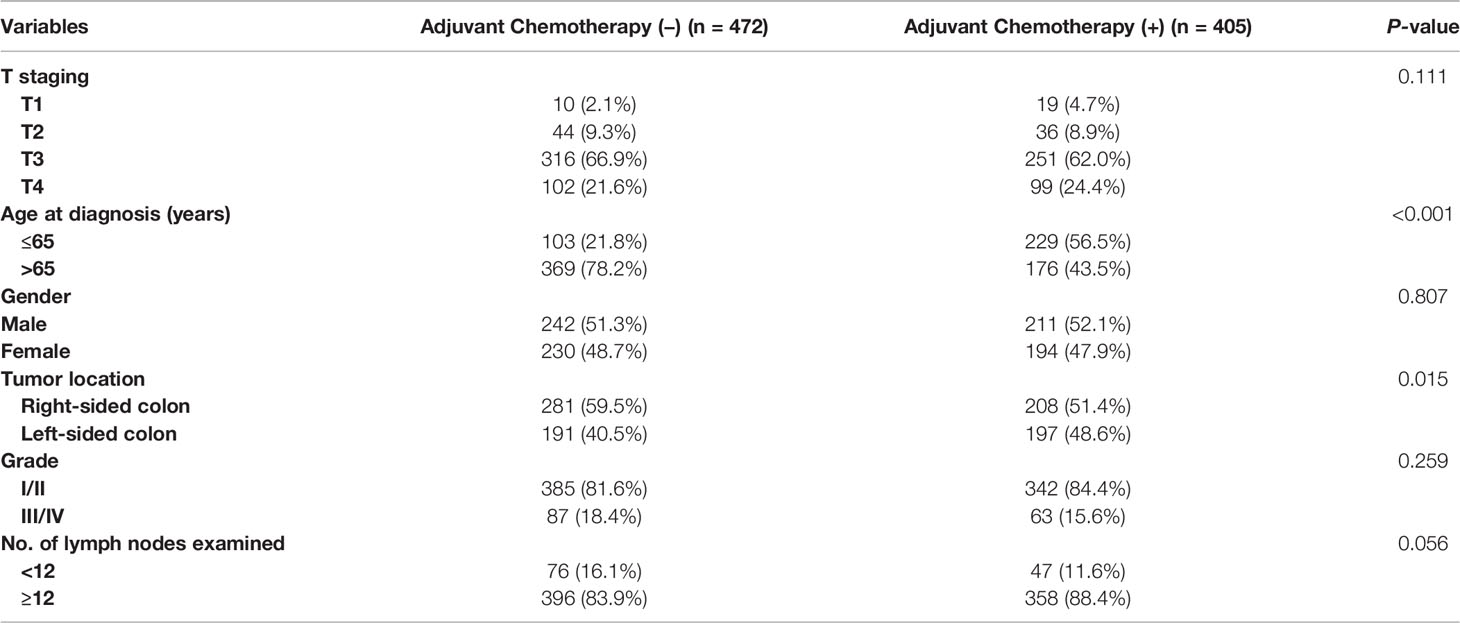
Table 1 Clinicopathologic characteristics of colon cancer with solitary tumor deposit according to adjuvant chemotherapy administration.
As shown in Figure 2, the CSS and OS curves of patients with colon cancer with the solitary TD were generated using the Kaplan–Meier method. It was found that OS of patients with colon cancer with the solitary TD with adjuvant chemotherapy administration was significantly better than those without adjuvant chemotherapy administration (75.4% vs. 42.8% for 5-year OS rate, p < 0.001) and that CSS of patients with colon cancer with the solitary TD with adjuvant chemotherapy administration was significantly better than those without adjuvant chemotherapy administration (82.9% vs. 69.3% for 5-year CSS rate, p < 0.001).
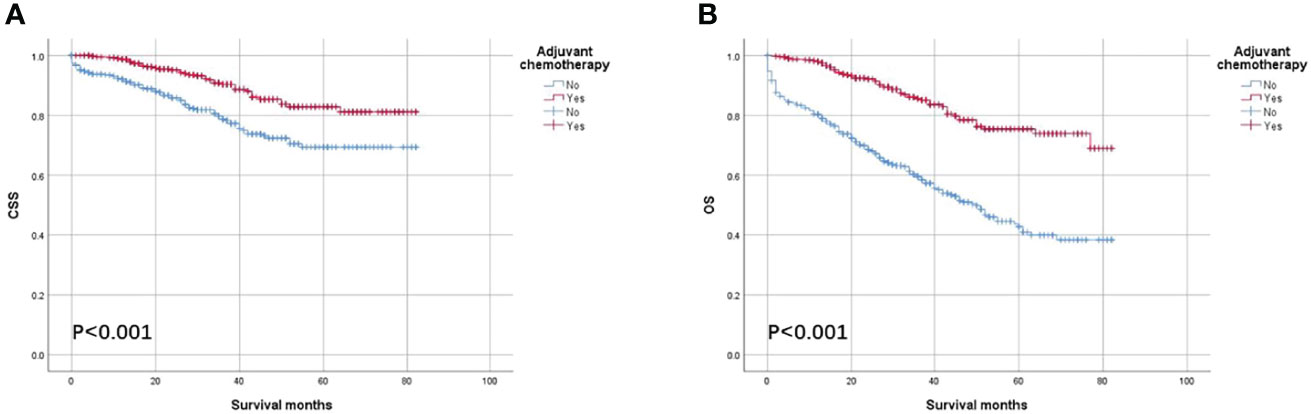
Figure 2 Kaplan–Meier survival curves comparing (A) CSS and (B) OS according to the use of adjuvant chemotherapy in all patients with colon cancer with solitary tumor deposit.
Multivariate Cox survival analyses revealed that OS was significantly associated with T staging (HR = 2.018, 95% CI = 1.545–2.634, p < 0.001; Table 2), number of lymph nodes examined (HR = 0.587, 95% CI = 0.434–0.794, p = 0.001; Table 2), and age at diagnosis (HR = 1.987, 95% CI = 1.446–2.730, p < 0.001; Table 2) in all patients with colon cancer with solitary TD. Moreover, the overall mortality risk of all patients with adjuvant chemotherapy administration was decreased by 64.4% compared with those without adjuvant chemotherapy administration (HR = 0.356, 95% CI = 0.265–0.479, p < 0.001; Table 2). Multivariate Cox survival analyses revealed that CSS was significantly associated with T staging (HR = 3.497, 95% CI = 2.430–5.031, p < 0.001; Table 2), and number of lymph nodes examined (HR = 0.487, 95% CI = 0.323–0.734, p = 0.001; Table 2) in all patients with colon cancer with the solitary TD. Moreover, colon cancer–specific mortality risk of patients with adjuvant chemotherapy administration was decreased by 57.4% compared with those without adjuvant chemotherapy administration (HR = 0.426, 95% CI = 0.286–0.634, p < 0.001; Table 2).
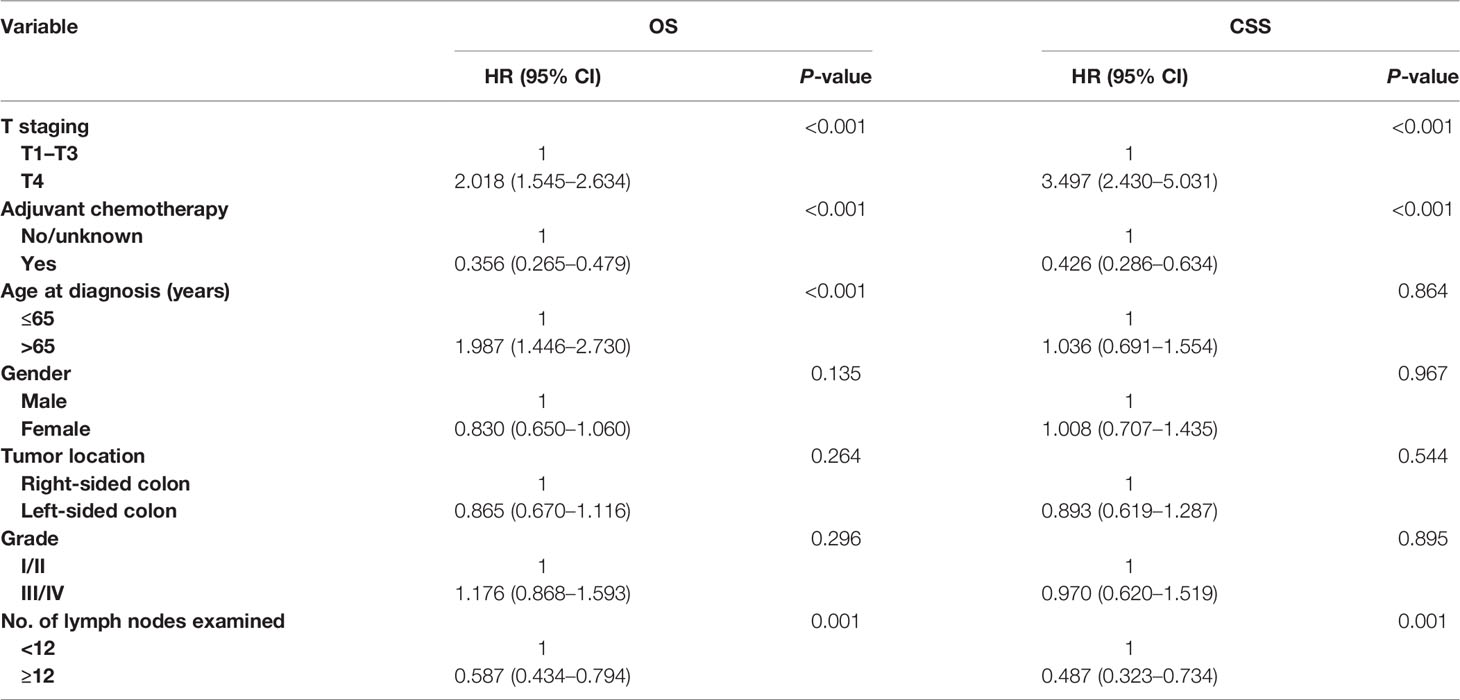
Table 2 Multivariate Cox regression analysis of prognostic factors for OS and CSS in colon cancer with solitary tumor deposit.
As shown in Figure 3, the CSS and OS curves of patients with T1–T3 colon cancer with the solitary TD were generated using the Kaplan–Meier method. It was found that OS of patients with T1–T3 colon cancer with the solitary TD with adjuvant chemotherapy administration was significantly better than those without adjuvant chemotherapy administration (80.5% vs. 48.1% for 5-year OS rate, p < 0.001; Figure 3A) and that CSS of patients with colon cancer with the solitary TD with adjuvant chemotherapy administration was significantly better than those without adjuvant chemotherapy administration (88.7% vs. 76.2% for 5-year CSS rate, p < 0.001; Figure 3B).
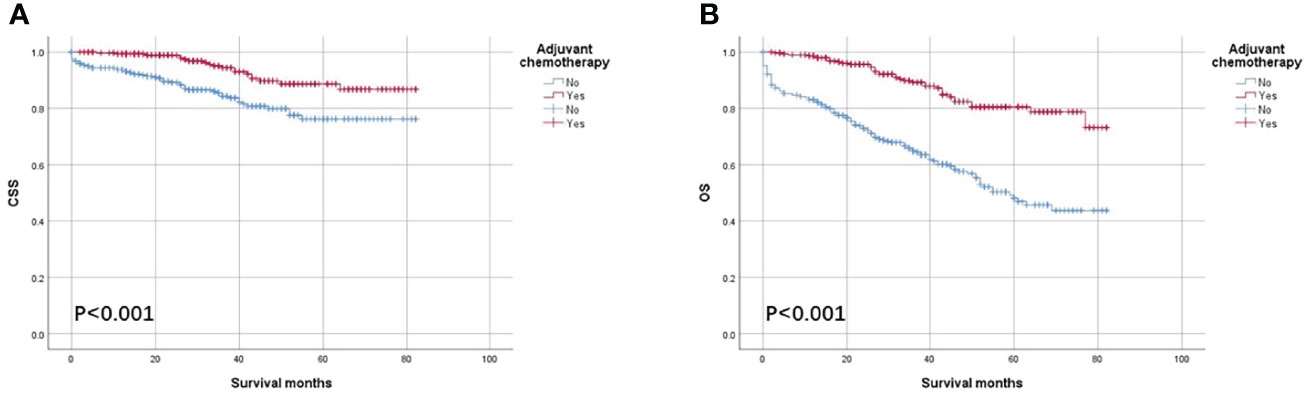
Figure 3 Kaplan–Meier survival curves comparing (A) CSS and (B) OS according to the use of adjuvant chemotherapy in patients with T1–T3 colon cancer with solitary tumor deposit.
Multivariate Cox survival analyses revealed that OS was significantly associated with age at diagnosis (HR = 2.681, 95% CI = 1.804–3.983, p < 0.001; Table 3), and number of lymph nodes examined (HR = 0.602, 95% CI = 0.412–0.879, p = 0.009; Table 3) in patients with T1–T3 colon cancer with the solitary TD. Moreover, the overall mortality risk of patients with adjuvant chemotherapy administration was decreased by 65.4% compared with those without adjuvant chemotherapy administration (HR = 0.346, 95% CI = 0.239–0.500, p < 0.001; Table 3). Multivariate Cox survival analyses revealed that colon cancer–specific mortality risk of patients with adjuvant chemotherapy administration was decreased by 57.7% compared with those without adjuvant chemotherapy administration (HR = 0.423, 95% CI = 0.246–0.725, p = 0.002; Table 3).
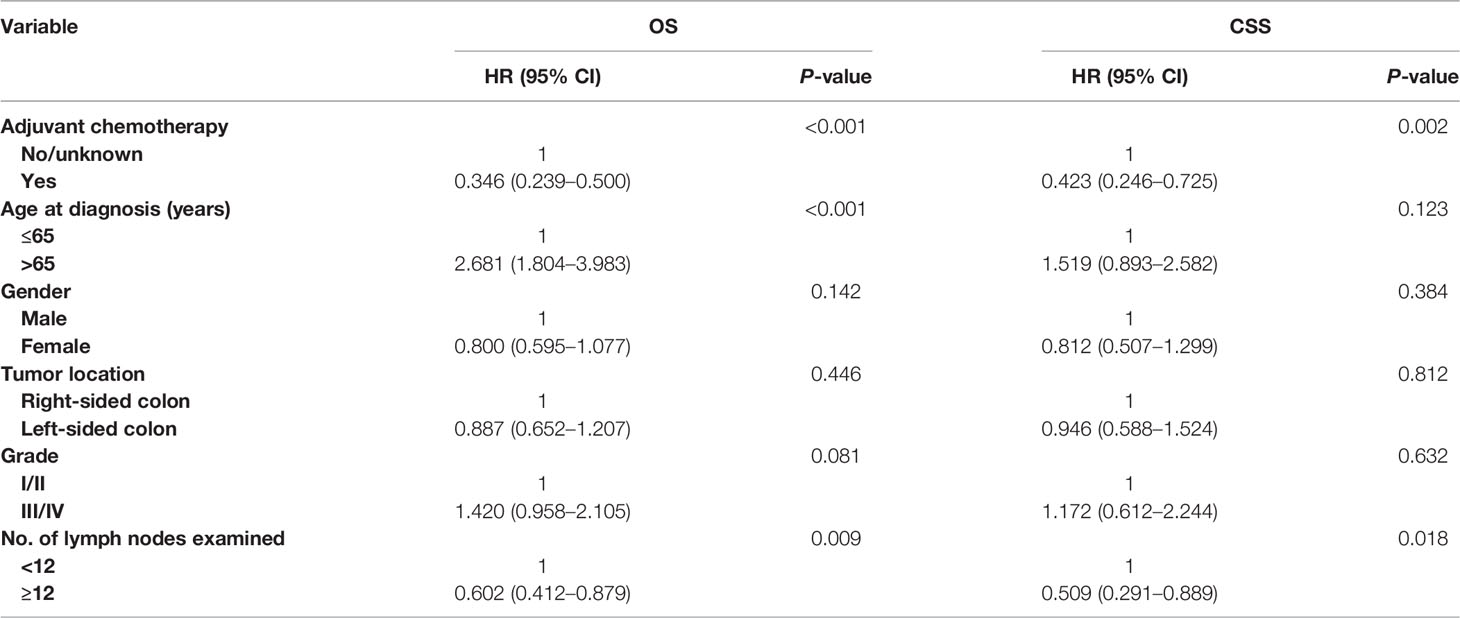
Table 3 Multivariate Cox regression analysis of prognostic factors for OS and CSS in T1–T3 colon cancer with solitary tumor deposit.
As shown in Figure 4, the CSS and OS curves of patients with T4 colon cancer with solitary TD were generated using the Kaplan–Meier method. It was found that OS of patients with T4 colon cancer with the solitary TD with adjuvant chemotherapy administration was significantly better than those without adjuvant chemotherapy administration (56.9% vs. 24.0% for 5-year OS rate, p < 0.001; Figure 4A) and that CSS of patients with colon cancer with the solitary TD with adjuvant chemotherapy administration was significantly better than those without adjuvant chemotherapy administration (61.5% vs. 42.3% for 5-year CSS rate, p = 0.004; Figure 4B).
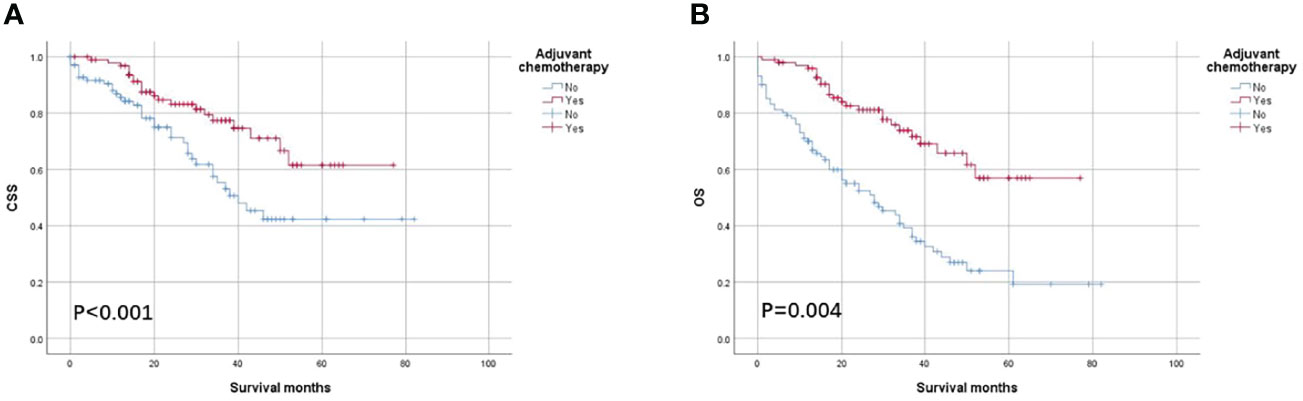
Figure 4 Kaplan–Meier survival curves comparing (A) CSS and (B) OS according to the use of adjuvant chemotherapy in patients with T4 colon cancer with solitary tumor deposit.
Multivariate Cox survival analyses revealed that the overall mortality risk of patients with adjuvant chemotherapy administration was decreased by 68.4% compared with those without adjuvant chemotherapy administration in patients with T4 colon cancer with solitary TD (HR = 0.316, 95% CI = 0.188–0.531, p < 0.001; Table 4). In addition, colon cancer–specific mortality risk of patients with adjuvant chemotherapy administration was reduced by 63.3% compared with those without adjuvant chemotherapy administration (HR = 0.367, 95% CI = 0.196–0.686, p = 0.002; Table 4).
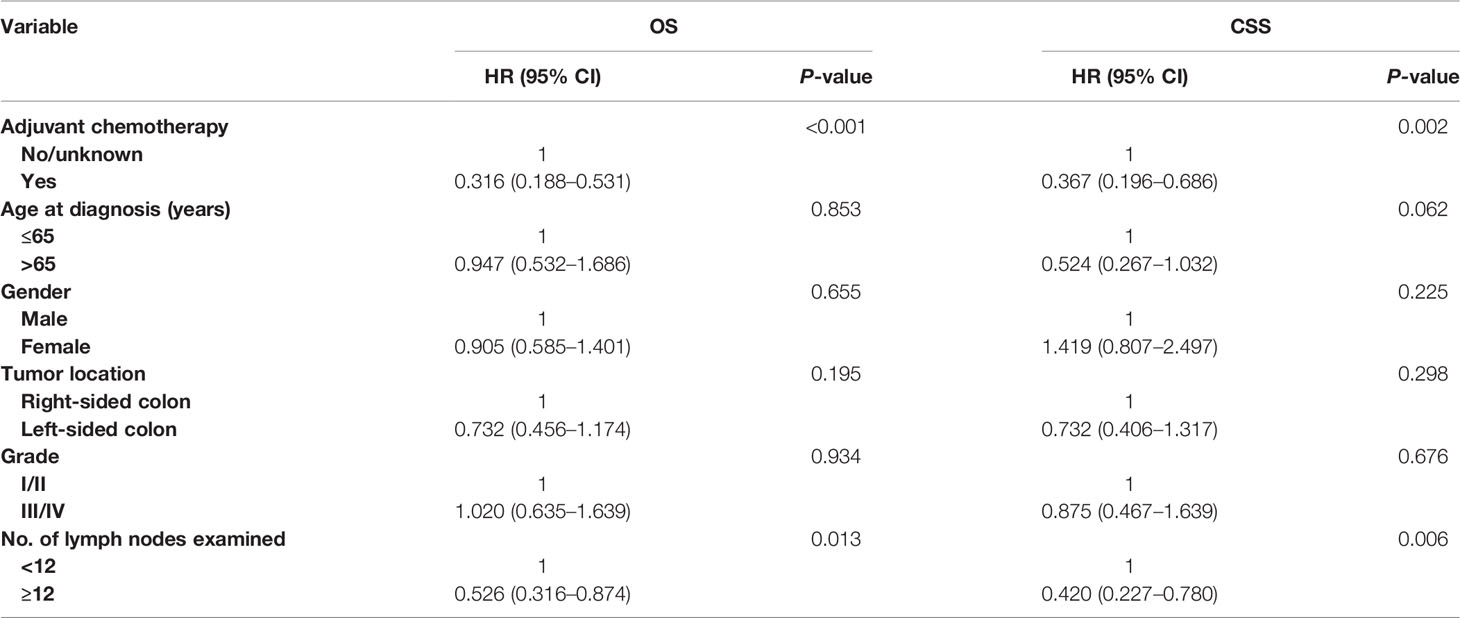
Table 4 Multivariate Cox regression analysis of prognostic factors for OS and CSS in T4 colon cancer with solitary tumor deposit.
TD was first described in 1935 and was considered as the deposit of carcinoma cells at a distance from the primary growth (14). This definition has, in fact, changed in colorectal cancer over time. TDs were first introduced in the fifth edition of the The American Joint Committee on Cancer (AJCC)/TNM staging in 1997, where tumor nodules larger than 3 mm in diameter with no histological evidence of residual LN were classified as regional lymph node metastases, whereas tumor nodules smaller than 3 mm were classified in the T category (15).The presence of TD was classified as N1c in the absence of LNM, and the N1c classification was first introduced in the seventh edition of the AJCC/TNM staging to allow us to better understand the clinical significance of TD.
Notably, TD was redefined in the new TNM classification (eighth edition) released in early 2017, and its incidence was reported in previous literature to be between 18% and 25% (12, 16–18). Many previous studies have revealed that the presence of TD was a manifestation of more aggressive tumor biology and should be further investigated by researchers. Gopal et al. reported a trend toward decreased tumor response to neoadjuvant chemoradiotherapy in patients with N1c rectal cancer, which resulted in worse survival and recurrence in this group of patients (19% vs. 10%, p = 0.092 for 5-year local recurrence rate and 3.1 vs. 11.2 years for OS, p = 0.027) (12). Wei et al. found that the presence of TD was an important independent prognostic factor for rectal cancer following preoperative treatment (HR = 2.41, 95% CI =1.24–4.69, p = 0.01) (19). Another study showed that among patients with right-sided colon adenocarcinoma, N1c patients had significantly lower survival compared with N0 patients (20). A higher recurrence rate was also reported in patients with TD-positive colorectal cancer compared with patients with TD-negative colorectal cancer (65.1% vs. 39.1%) (21).
The survival benefit of chemotherapy in patients with colorectal cancer with TD has been inconsistent in previous studies because it was not clear whether the pN1c category was or was not equal to LNM (9). It was reported that the presence and number of TDs did not affect the benefit of chemotherapy in stage III colorectal cancer (22). It has also been shown that patients with TD did not show a benefit in disease-free survival following chemotherapy (23). However, a recent study found that chemotherapy was independently associated with better prognosis in patients with colorectal cancer with TD (HR = 0.542, 95% CI = 0.501–0.586) (24). Despite that, patients with colon cancer with the presence of TD should receive adjuvant chemotherapy after radical surgery according to clinical guidelines for colorectal cancer. Therefore, it is necessary to explore the clinical significance of TD in adjuvant chemotherapy for patients with colon cancer.
Recently, Yeom et al. conducted a retrospective study in 281 patients with colon cancer and found that adjuvant chemotherapy did not provide clear survival benefit for patients with colon cancer with solitary LNM (84.1% vs. 83.3%, p = 0.490 for 5-year disease-free survival). Therefore, it is reasonable to question the survival benefit of adjuvant chemotherapy in patients with colon cancer with solitary TD. In this study, it was found that adjuvant chemotherapy administration could significantly improve OS (75.4% vs. 42.8% for 5-year OS rate, p < 0.001) and CSS (82.9% vs. 69.3% for 5-year CSS rate, p < 0.001) in all patients with colon cancer with the solitary TD. Multivariate Cox survival analyses also showed that adjuvant chemotherapy administration was a good prognosis factor: The overall mortality risk of all patients with adjuvant chemotherapy administration was decreased by 64.4% compared with those without adjuvant chemotherapy administration, and colon cancer–specific mortality risk of patients with adjuvant chemotherapy administration was decreased by 57.4% compared with those without adjuvant chemotherapy administration. The survival benefit conferred by adjuvant chemotherapy in patients with colon cancer with solitary TD in our study was better than survival benefit conferred by chemotherapy in patients with colorectal cancer with TD as reported by Chen et al. (24).
Considering that adjuvant chemotherapy had definite survival benefit in T4 colon cancer, we then performed subgroup analyses according to T stage, and all patients were divided into T1–T3 and T4 subgroups (25–27). Again, subgroup analyses showed that adjuvant chemotherapy had obvious survival benefit in both patients with T1–T3 and T4 colon cancer with solitary TD, which further confirmed the above results. In patients with T1–T3 colon cancer with solitary TD, the overall and colon cancer–specific mortality risks of patients with adjuvant chemotherapy administration were decreased by 65.4% (HR = 0.346, 95% CI = 0.239–0.500, p < 0.001) and 57.7% (HR = 0.423, 95% CI = 0.246–0.725, p = 0.002) compared with those without adjuvant chemotherapy administration, respectively; in patients with T4 colon cancer with solitary TD, the overall and colon cancer–specific mortality risks of patients with adjuvant chemotherapy administration were decreased by 68.4% (HR = 0.316, 95% CI = 0.188–0.531, p < 0.001) and 63.3% (HR = 0.367, 95% CI = 0.196–0.686, p = 0.002) compared with those without adjuvant chemotherapy administration, respectively.
Taken together, our study suggests that adjuvant chemotherapy is warranted and has an obvious survival benefit in patients with colon cancer with the solitary TD. To our knowledge, this is the first time that the efficacy of adjuvant chemotherapy in patients with colon cancer with the solitary TD is proposed and explored. We screened the target population from more than 150,000 patients with colon cancer and performed this retrospective analysis, which provided a high quality of evidence for the use of adjuvant chemotherapy in patients with colon cancer with solitary TD and gave us a better understanding of the clinical significance of TD in colon cancer.
However, our study has some shortcomings. First, this study has the potential selection bias because of the nature of retrospective studies. Second, the patients included in the study have a long longitudinal span from 2010 to 2016, which may have a potential impact on the results of the analysis. Finally, because of database limitations, some important tumor or patient characteristics that may affect the prognosis of patients with colon cancer were not analyzed in this study. In particular, this cancer database did not capture information on specific chemotherapy regimens, so we did not analyze the effect of a particular chemotherapy regimen on the prognosis of patients with colon cancer, which should be deemed as one limitation of the present study. Therefore, the efficacy of adjuvant chemotherapy in patients with colon cancer with the solitary TD still deserves special attention from researchers in the future.
Adjuvant chemotherapy administration could significantly improve OS and CSS in patients with colon cancer with the solitary TD. This is the first study to investigate and demonstrate the survival benefit of adjuvant chemotherapy in patients with colon cancer with the solitary TD.
The raw data supporting the conclusions of this article are available from the corresponding author on reasonable request.
QL and WY conceived the project and wrote the manuscript. QL, SS, and JL collected the data. WY, HZ, and SS performed the data analyses. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Weiser MR. AJCC 8th Edition: Colorectal Cancer. Ann Surg Oncol (2018) 25(6):1454–5. doi: 10.1245/s10434-018-6462-1
3. Liu Q, Luo D, Cai S, Li Q, Li X. Circulating Basophil Count as a Prognostic Marker of Tumor Aggressiveness and Survival Outcomes in Colorectal Cancer. Clin Trans Med (2020) 9(1):6. doi: 10.1186/s40169-019-0255-4
4. Lin Q, Wei Y, Ren L, Zhong Y, Qin C, Zheng P, et al. Tumor Deposit is a Poor Prognostic Indicator in Patients Who Underwent Simultaneous Resection for Synchronous Colorectal Liver Metastases. OncoTar Ther (2015) 8:233–40. doi: 10.2147/OTT.S71414
5. Zheng P, Chen Q, Li J, Jin C, Kang L, Chen D. Prognostic Significance of Tumor Deposits in Patients With Stage III Colon Cancer: A Nomogram Study. J Surg Res (2020) 245:475–82. doi: 10.1016/j.jss.2019.07.099
6. Yamano T, Semba S, Noda M, Yoshimura M, Kobayashi M, Hamanaka M, et al. Prognostic Significance of Classified Extramural Tumor Deposits and Extracapsular Lymph Node Invasion in T3-4 Colorectal Cancer: A Retrospective Single-Center Study. BMC Cancer (2015) 15:859. doi: 10.1186/s12885-015-1885-6
7. Wang S, Guan X, Ma M, Zhuang M, Ma T, Liu Z, et al. Reconsidering the Prognostic Significance of Tumour Deposit Count in the TNM Staging System for Colorectal Cancer. Sci Rep (2020) 10(1):89. doi: 10.1038/s41598-019-57041-2
8. Pei JP, Zhang CD, Fu X, Ba Y, Yue S, Zhao ZM, et al. A Modified Tumor-Node-Metastasis Classification for Stage III Colorectal Cancers Based on Treating Tumor Deposits as Positive Lymph Nodes. Front Med (2020) 7:571154. doi: 10.3389/fmed.2020.571154
9. Delattre JF, Cohen R, Henriques J, Falcoz A, Emile JF, Fratte S, et al. Prognostic Value of Tumor Deposits for Disease-Free Survival in Patients With Stage III Colon Cancer: A Post Hoc Analysis of the IDEA France Phase III Trial (PRODIGE-GERCOR). J Clin Oncol (2020) 38(15):1702–10. doi: 10.1200/JCO.19.01960
10. Nagtegaal ID, Knijn N, Hugen N, Marshall HC, Sugihara K, Tot T, et al. Tumor Deposits in Colorectal Cancer: Improving the Value of Modern Staging-A Systematic Review and Meta-Analysis. J Clin Oncol (2017) 35(10):1119–27. doi: 10.1200/JCO.2016.68.9091
11. Wang Y, Zhang J, Zhou M, Yang L, Wan J, Shen L, et al. Poor Prognostic and Staging Value of Tumor Deposit in Locally Advanced Rectal Cancer With Neoadjuvant Chemoradiotherapy. Cancer Med (2019) 8(4):1508–20. doi: 10.1002/cam4.2034
12. Gopal P, Lu P, Ayers GD, Herline AJ, Washington MK. Tumor Deposits in Rectal Adenocarcinoma After Neoadjuvant Chemoradiation are Associated With Poor Prognosis. Modern Pathol (2014) 27(9):1281–7. doi: 10.1038/modpathol.2013.239
13. Yeom SS, Lee SY, Kim CH, Kim HR, Kim YJ. The Prognostic Effect of Adjuvant Chemotherapy in the Colon Cancer Patients With Solitary Lymph Node Metastasis. Int J colorectal Dis (2019) 34(8):1483–90. doi: 10.1007/s00384-019-03346-7
14. Gabriel WB, Dukes C, Bussey HJR. Lymphatic Spread in Cancer of the Rectum. BJS (British J Surgery) (1935) 23(90):395–413. doi: 10.1002/BJS.1800239017
15. Delattre JF, Selcen Oguz Erdogan A, Cohen R, Shi Q, Emile JF, Taieb J, et al. A Comprehensive Overview of Tumour Deposits in Colorectal Cancer: Towards a Next TNM Classification. Cancer Treat Rev (2022) 103:102325. doi: 10.1016/j.ctrv.2021.102325
16. Nagtegaal ID, Tot T, Jayne DG, McShane P, Nihlberg A, Marshall HC, et al. Lymph Nodes, Tumor Deposits, and TNM: Are We Getting Better? J Clin Oncol (2011) 9(18):2487–92. doi: 10.1200/JCO.2011.34.6429
17. Ueno H, Mochizuki H, Shirouzu K, Kusumi T, Yamada K, Ikegami M, et al. Multicenter Study for Optimal Categorization of Extramural Tumor Deposits for Colorectal Cancer Staging. Ann Surg (2012) 255(4):739–46. doi: 10.1097/SLA.0b013e31824b4839
18. Zhang LN, Xiao WW, Xi SY, OuYang PY, You KY, Zeng ZF, et al. Tumor Deposits: Markers of Poor Prognosis in Patients With Locally Advanced Rectal Cancer Following Neoadjuvant Chemoradiotherapy. Oncotarget (2016) 7(5):6335–44. doi: 10.18632/oncotarget.6656
19. Wei XL, Qiu MZ, Zhou YX, He MM, Luo HY, Wang FH, et al. The Clinicopathologic Relevance and Prognostic Value of Tumor Deposits and the Applicability of N1c Category in Rectal Cancer With Preoperative Radiotherapy. Oncotarget (2016) 7(46):75094–103. doi: 10.18632/oncotarget.12058
20. Jin M, Roth R, Rock JB, Washington MK, Lehman A, Frankel WL. The Impact of Tumor Deposits on Colonic Adenocarcinoma AJCC TNM Staging and Outcome. Am J Surg Pathol (2015) 39(1):109–15. doi: 10.1097/PAS.0000000000000320
21. Belt EJ, van Stijn MF, Bril H, de Lange-de Klerk ES, Meijer GA, Meijer S, et al. Lymph Node Negative Colorectal Cancers With Isolated Tumor Deposits Should be Classified and Treated as Stage III. Ann Surg Oncol (2010) 17(12):3203–11. doi: 10.1245/s10434-010-1152-7
22. Shi M, Zhang H, Yao G, Wu J, Zhu C, Zhang X, et al. The Role of Tumor Deposits in Predicting the Efficacy of Chemotherapy in Stage III Colon Cancer. Front Oncol (2020) 10:586603. doi: 10.3389/fonc.2020.586603
23. Li X, An B, Zhao Q, Qi J, Wang W, Zhang D, et al. Impact of Tumor Deposits on the Prognosis and Chemotherapy Efficacy in Stage III Colorectal Cancer Patients With Different Lymph Node Status: A Retrospective Cohort Study in China. Int J Surg (London England) (2018) 56:188–94. doi: 10.1016/j.ijsu.2018.06.029
24. Chen J, Zhang Z, Ni J, Sun J, Ren W, Shen Y, et al. Predictive and Prognostic Assessment Models for Tumor Deposit in Colorectal Cancer Patients With No Distant Metastasis. Front Oncol (2022) 12:809277. doi: 10.3389/fonc.2022.809277
25. Verhoeff SR, van Erning FN, Lemmens VE, de Wilt JH, Pruijt JF. Adjuvant Chemotherapy is Not Associated With Improved Survival for All High-Risk Factors in Stage II Colon Cancer. Int J Cancer (2016) 139(1):187–93. doi: 10.1002/ijc.30053
26. Kumar A, Kennecke HF, Renouf DJ, Lim HJ, Gill S, Woods R, et al. Adjuvant Chemotherapy Use and Outcomes of Patients With High-Risk Versus Low-Risk Stage II Colon Cancer. Cancer (2015) 121(4):527–34. doi: 10.1002/cncr.29072
Keywords: adjuvant chemotherapy, colon cancer, solitary, tumor deposit, survival
Citation: Lin Q, Zhou H, Shi S, Lin J and Yan W (2022) The Prognostic Value of Adjuvant Chemotherapy in Colon Cancer With Solitary Tumor Deposit. Front. Oncol. 12:916091. doi: 10.3389/fonc.2022.916091
Received: 08 April 2022; Accepted: 03 June 2022;
Published: 13 July 2022.
Edited by:
Qi Liu, Fudan University, ChinaReviewed by:
Paul Willemsen, Hospital Network Antwerp (ZNA), BelgiumCopyright © 2022 Lin, Zhou, Shi, Lin and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wangxin Yan, d2FuZ3hpbl9feUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.