- 1Department of Obstetrics 1, Zhuzhou Central Hospital, Zhuzhou, China
- 2Department of Obstetrics and Gynecology, People’s Hospital of Leshan, Leshan, China
- 3Department of Obstetrics and Gynecology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
- 4Department of Pathology, Zhuzhou Central Hospital, Zhuzhou, China
- 5West China Second University Hospital, Sichuan University, Chengdu, China
Melanoma, also known as malignant melanoma, is a type of malignant tumour that originates from melanocytes in the basal layer of the epidermis. Primary malignant melanomas of the female genital tract are rare. Similarly, primary malignant melanoma of cervix, which originates from cervical melanocytes, is an extremely rare disease and the second most common type of female melanoma in women aged between 15 to 44 years worldwide. To date, primary malignant melanoma of the cervix is characterized by poor patient prognosis and little consensus exists regarding the best treatment therapy. The situation is worsened by lack of clinical studies with large samples. Notably, surgery remains the preferred treatment option for patients with primary malignant melanomas of the cervix. Current treatments are based on Federation International of Gynecology and Obstetrics(2018) staging with reference to National Comprehensive Cancer Network guidelines. This study is in order to find a more suitable treatment modality for primary malignant melanoma of cervix. Therefore, we first conducted an integrated analysis of case reports and series to assess the impact of various factors on the prognosis of such patients. In summary, this is the first pooled analysis including 149 cases of primary cervical melanoma. We found that patients who underwent radical hysterectomy-based surgery, those with non-metastatic lymph nodes and those who underwent lymphadenectomy had significantly higher survival rates. In patients who had RH-based surgery, survival rates at the 24m time point of those who did not add other treatments was higher than those who did, but for those who had total hysterectomy-based surgery, the addition of other treatments to prolong median survival may be considered. In the overall analysis, age and lymphadenectomy were associated with increased and reduced risk of death in these patients, respectively. Although there is no statistical difference, stage III&IV, TAH, lymphatic metastases increase the risk of death; whereas radical hysterectomy was associated with reduced risk of death. In the subgroup analysis, for patients who have undergone radical hysterectomy-based surgery, lymphadenectomy reduces the risk of death, while lymphatic metastases and complementary other treatments increase the risk of death. For patients who have undergone total hysterectomy-based surgery, complementary treatment reduces the risk of death. In conclusion, via summarizing previous reports, the recommended treatment procedure for PMMC are radical hysterectomy and lymphadenectomy. The addition of other treatment options for patients who undergoing RH-based surgery need further study.
Introduction
Melanoma, also known as malignant melanoma (MM), is a type of malignant tumour that originates from melanocytes in the basal layer of the epidermis (1). It is considered one of the most aggressive cancer diseases due to its high malignancy and treatment resistance (2). Notably, MM patients have a survival period of less than 5 years (3), although they have very good prognosis if the disease is detected in its early stages (4). Globally, disease accounts for approximately 0.03% of all newly diagnosed cancers (5). Previous studies have shown that the high mortality rate is due to the aggressive metastatic potential of melanoma cells (6). Previous estimates have shown that approximately 132,000 and 48,000 new malignant melanoma cases and deaths, respectively, occur each year (7). In fact, MM is ranked the fifth most common cancer in the world, and its incidence is on the rise (8). Primary MMs of the female genital tract are rare, accounting for only 3-7% of all mucosal melanomas (9). On the other hand, primary malignant melanoma of the cervix (PMMC) is an extremely rare disease that originates from cervical melanocytes (10). It is the second most common type of female melanoma in women aged between 15 to 44 years worldwide (11).
Melanoma is a highly malignant tumor with a poor prognosis (12), while that of PMMC worse (13). To date, little consensus has been reached regarding the best treatment therapy for PMMC. Lack of clinical studies involving large sample sizes has also contributed to scarcity of optimal treatment options. Nevertheless, surgery remains the preferred treatment option (13, 14). The current treatments are based on FIGO staging with reference to NCCN guidelines (15). This study is in order to find a more suitable treatment modality for PMMC. Therefore, we first conducted an integrated analysis of case reports and series to assess the impact of various factors on the prognosis of such patients.
Methods
Data Sources and Search Strategy
In this section, we describe a comprehensive analysis based on published case report data. Briefly, we searched three public databases, namely PubMed, EMBASE and the Cochrane Library, for case reports published from their inception until 31st January 2022. The search strategy involved the following terms: (((Cervixes) OR (Uterine Cervix)) OR (Cervix, Uterine)) OR (Cervix)) AND (Primary malignant melanoma). In addition, we performed a secondary search of the reference lists across relevant articles to identify additional eligible reports. Two independent reviewers (Aiping Min and Aizhen Fu) conducted the literature search, study selection and data extraction. Any discrepancy between them was resolved through consensus and arbitration by a third author (Meiyuan Huang).
Study Selection Criteria
All studies describing primary malignant melanoma of the cervix, with clinical information on the patients regardless of sample size, were included in this study. Conversely, articles that met the following criteria were excluded from the study: Primary melanoma of other genital tract origin, genital tract melanoma of undeterminable origin, reviews, books, as well as irrelevant. In cases where studies had overlapping data, we selected the study with the larger sample size.
Data Extraction
The following data were recorded: year of publication, patient’s age at diagnosis, patient’s symptoms, FIGO stage, whether surgery was performed, mode of surgery, presence or absence of lymphatic metastases, use of other treatment modalities and overall survival (OS) in months. If the patient’s clinical information was mentioned in another article, and the corresponding full text was not available, we marked the survival status with UNCERTAIN.
Outcomes and Statistical Analysis
Outcomes assessed were age and OS. The definition for survival time was based on data from the individual participants reported. Missing and unidentifiable data were specified as NA, thus were not included in the statistical analysis. Categorical data were presented as frequencies and percentages. On the other hand, continuous data that conformed to normal distribution were presented as means and standard deviations (SD), while non-normally distributed data were presented as medians (range). In addition, Kaplan-Meier survival curves were generated to estimate OS of patients, and differences across subgroups compared using the Log-rank test. Statistical analysis was performed using the SURVIVAL package implemented in R language (16). A multivariate analysis of these predictors of survival for overall and subgroups was conducted using a Cox proportional risk model. Data followed by P<0.05 were considered statistically significant.
Results
Search Results and Eligible Studies
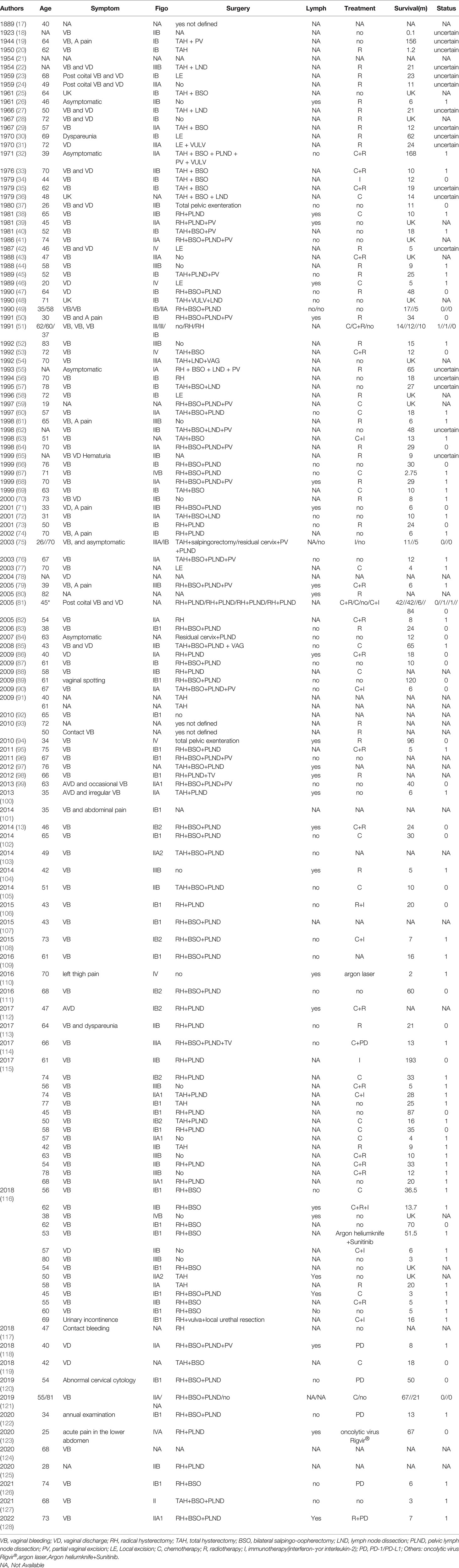
The aforementioned search strategy resulted in a total of 233 articles across the screened databases. Finally, 113 articles, containing 149 cases, were found to be eligible and therefore included in the analysis. A summary of the main features of the included studies is outlined in Table 1, whereas the procedure for literature search and selection is shown in Figure 1.
Patients’ Clinicopathological Characteristics
A total of 149 patients with PMMC were recruited in this study. Seven cases with missing data were excluded. The remaining cases had a median age of 58 years at diagnosis. Among the 149 patients, 126 (126/149) were suffered from vaginal bleeding or vaginal discharge, 2 due to contact bleeding, 7 asymptomatic, one each for acute pain in the lower abdomen, dyspareunia, left thigh pain, urinary incontinence, and 11 because of unknown reasons. FIGO staging guidelines stratified 56 patients into stage I, 45 into stage II, 18 into stage III, 8 in stage IV and 22 for whom staging information was not available. Notably, 7 patients had no surgical information, while 20 had no surgery and 7(4.6%) only had local excision. On the other hand, 77.1% (115/149) of patients were treated with surgery, 46.9%(70/149) patients had surgery based on radical hysterectomy (RH), 2 of whom had total pelvic exenteration, 28.2%(42/149) patients had surgery based on total hysterectomy (TAH), while 3 had surgery but not defined at all. In addition, 25 and 38 exhibited presence and absence of lymph node metastases, respectively, while corresponding information was not available in the remaining patients. For the 25 patients with lymph node metastases, lymphadenectomy was performed in 17 cases. Furthermore, 79 and 60 patients underwent and did not undergo lymphatic resection, respectively, with the rest of the cases lacking corresponding information. Finally, 68 patients received whereas 37 did not receive treatment other than surgery, which included radiotherapy, chemotherapy, immunotherapy(interferon-γ or interleukin-2), PD/PD-1 inhibitors, oncolytic virus Rigvir®, argon laser, Argon heliumknife. Among the 68 patients, 18 patients received chemotherapy, 6 patients received chemotherapy+immunotherapy, 1 patients received chemotherapy+PD, 14 patients received chemotherapy+radiotherapy,1 patients received chemotherapy+radiotherapy+immunotherapy,3 patients received immunotherapy, 2 patients received others(oncolytic virus Rigvir®, argon laser, Argon heliumknife) treatment,4 patients received PD, 17 patients received radiotherapy, 1 patients received radiotherapy+immunotherapy, 1 patients received radiotherapy+PD.
Patient Prognosis and Survival Rates
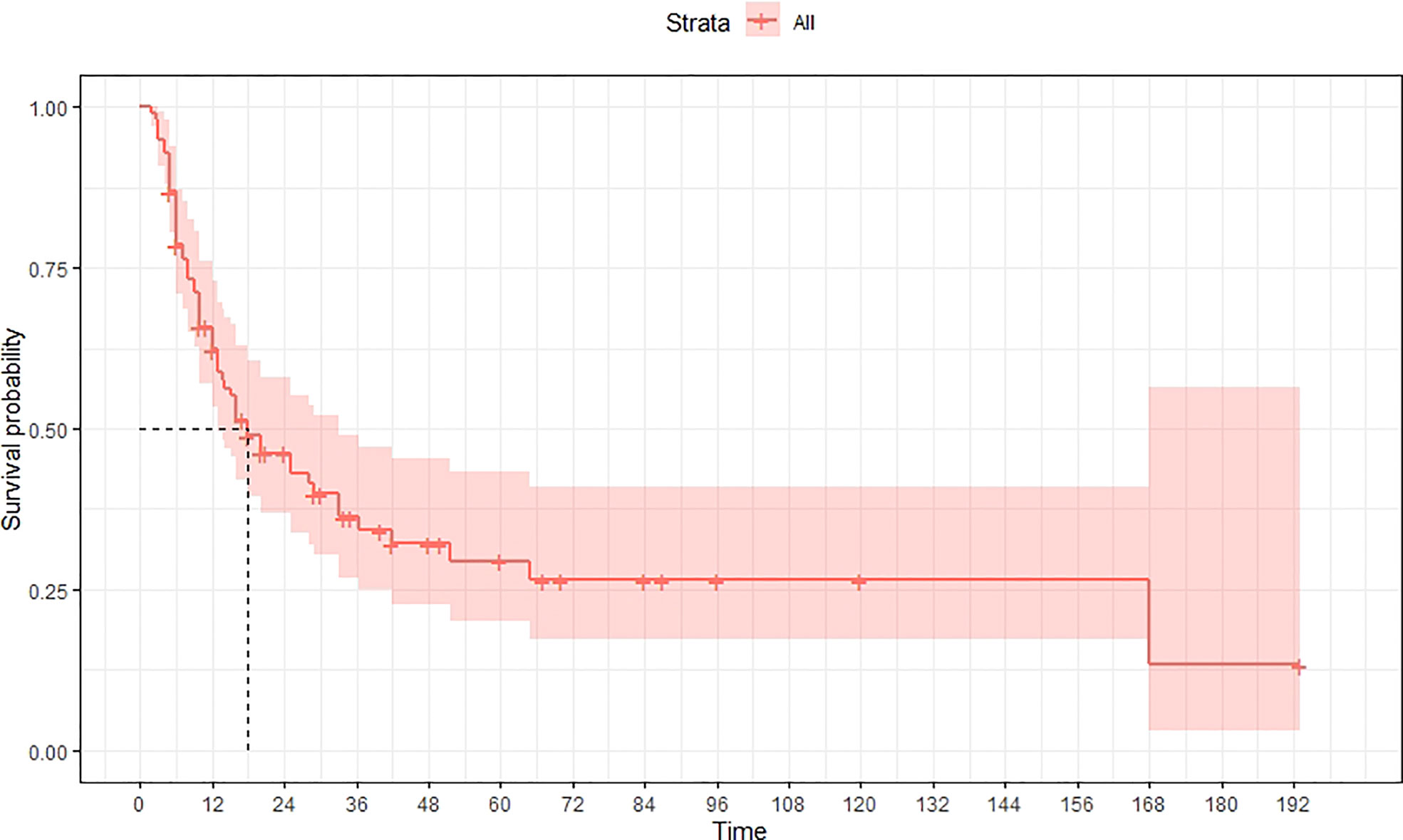
A total of 99 patients had information on survival time and status, of whom 39 survived (5-193 months) while 60 died. Corresponding information was not available for 32 patients, whereas the survival status of 18 patients could not be determined owing to a lack of full text. We generated Kaplan–Meier survival curves to evaluate OS of the patients, and obtained a median OS of 18 months in the cohort (Figure 2). Next, we employed log-rank tests under stratified covariates (FIGO stage, Surgery or not, the extent of surgery, Lymphatic metastases or not, Lymphadenectomy or not, Supplementary treatment or not), to explore underlying factors that may affect patient prognosis. Results indicated that prognosis of patients significantly decreased with stage progression (P=0.00069; Figure 3). Notably, patients who did not have surgery had significantly worse prognosis compared to those who had RH-based and TAH-based surgery (P<0.0001), while those who had RH-based surgery had better prognosis than those who had TAH-based surgery (Figure 4). Moreover, patients with non-lymphatic metastases had higher median survival times than those with lymphatic metastases, albeit with no statistical significance (P=0.056; Figure 5). Patients who underwent lymphadenectomy exhibited better prognosis than those who did not undergo the procedure (P<0.0001; Figure 6). Next, we compared surgical results (RH+TAH, RH, TAH) with and without other treatments, and found that in patients who underwent surgery(RH+TAH), there was no statistically significant differences in patient prognosis between groups with or without the addition of other treatment modalities (P=0.81; Figure 7). However, patients who added chemotherapy and others treatments(oncolytic virus Rigvir®, argon laser, Argon heliumknife) had significantly longer median OS than those who added the remaining treatment modalities. The exception to this was in patients who added radiotherapy or immunotherapy, where survival rates at the 60m time point were significantly higher than others (Figure 8). Moreover, in patients who had RH-based surgery, we found survival rates at the 24m time point of those who did not add other treatments was higher than those who did (Figure 9; P=0.18). In order to exclude possible confounding effects due to inhomogeneous distribution of characteristics between patients with RH and patients with RH+T, we tested if the stage, LS, LM are the same (Supplementary Table). The results found no significant difference between the two groups for Stage, LS, but a significant difference for LM. Similarly, patients who had chemotherapy or other treatments (oncolytic virus Rigvir®, argon laser, Argon heliumknife) exhibited significantly better median OS than those who had the remaining treatments. The exception was observed in patients who underwent radiotherapy, immunotherapy or with no other treatment added, whose survival rates at the 54m were significantly higher than others (Figure 10; P=0.14). In patients who had TAH-based surgery, we found no statistically significant differences in prognosis between the group with or without addition of other treatment modalities. However, the former group had higher median survival times than the latter (Figure 11; P=0.066). Furthermore, patients who added chemotherapy+radiotherapy exhibited significantly longer OS than those who added the remaining treatment modalities. The median survival time for TAH-based patients with the addition of other treatment modalities was higher than for those who had only TAH surgery (Figure 12; P=0.39).
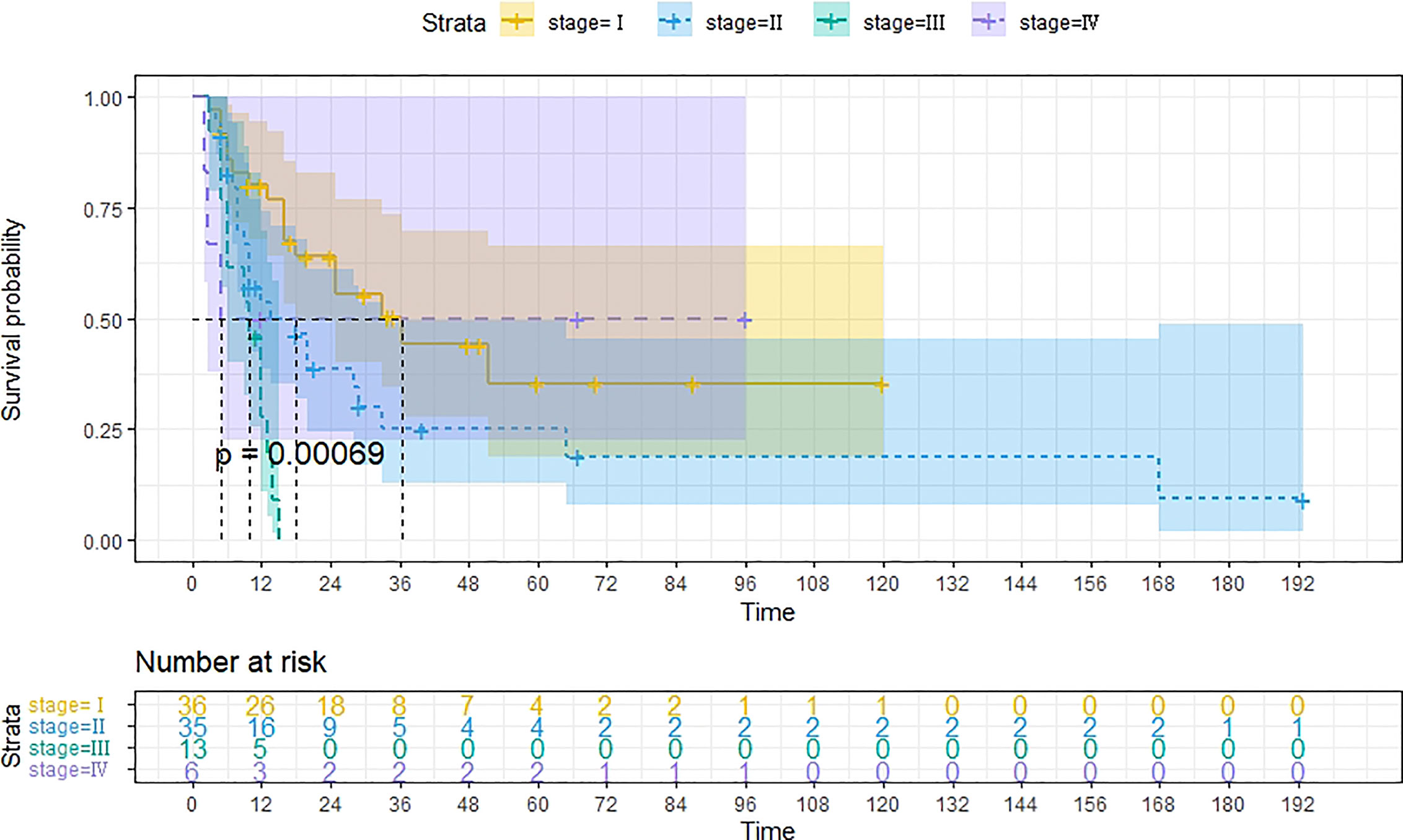
Figure 3 Kaplan-Meier survival curves for OS in patients of different FIGO stage. The median OS for FIGO I, II, III, IV are 36m, 18m, 10m, 5m respectively.
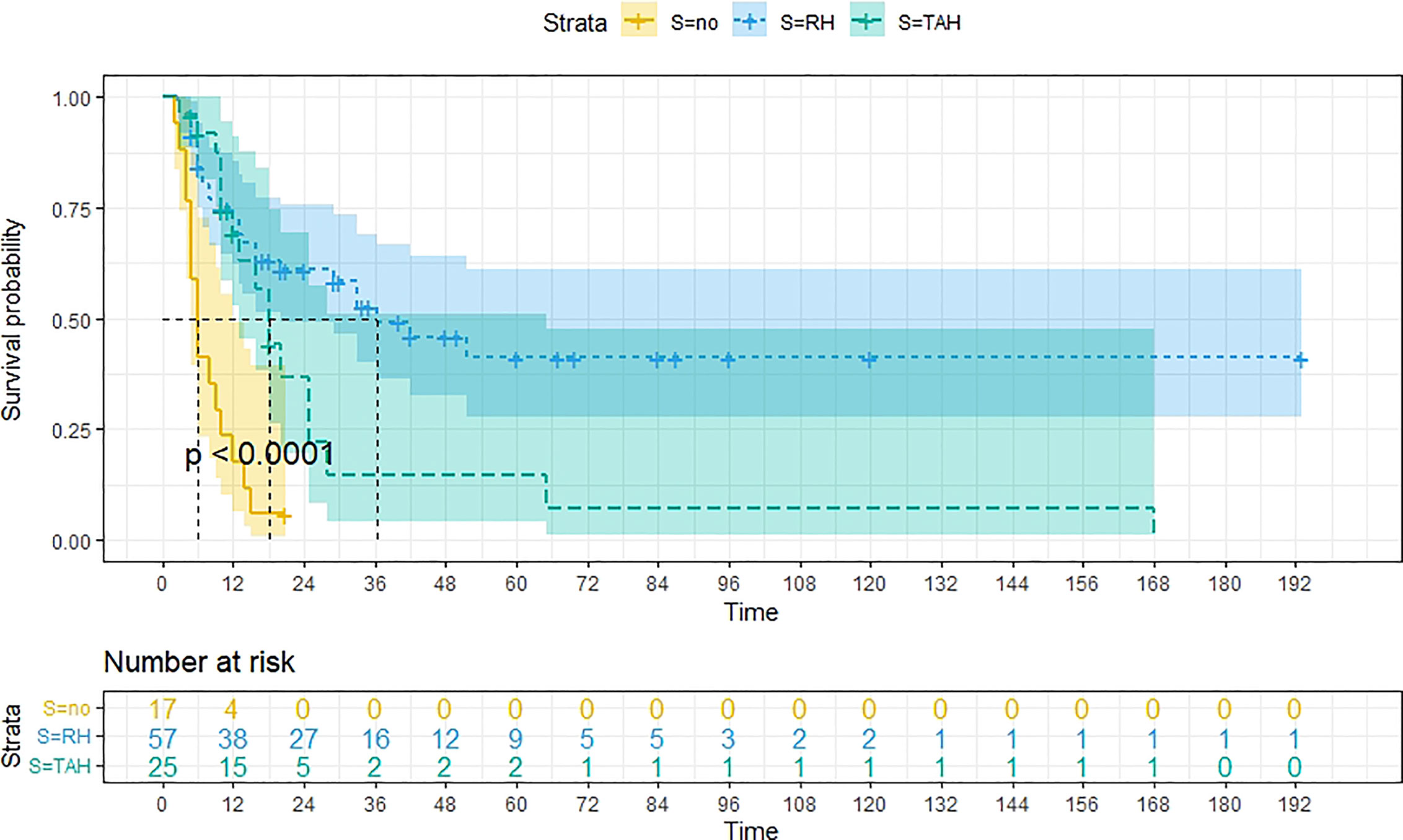
Figure 4 Kaplan-Meier survival curves for OS in patients of no surgery, TAH-based surgery, RH-based surgery. The median OS for them are 6m, 18m, 36m, respectively.
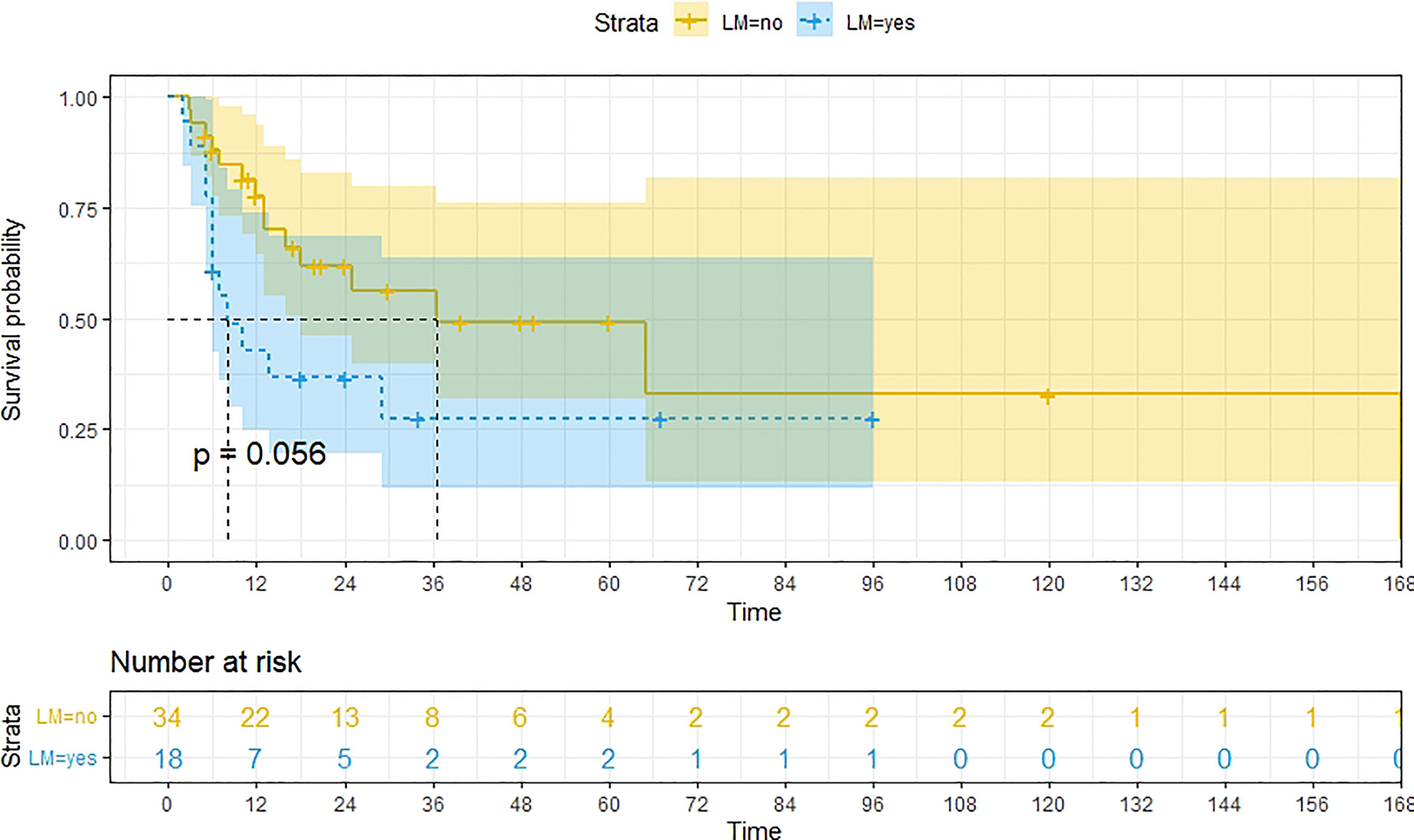
Figure 5 Kaplan-Meier survival curves for OS in patients of no lymphatic metastases and Lymphatic metastases. The median OS for them are 37m, 8m respectively.
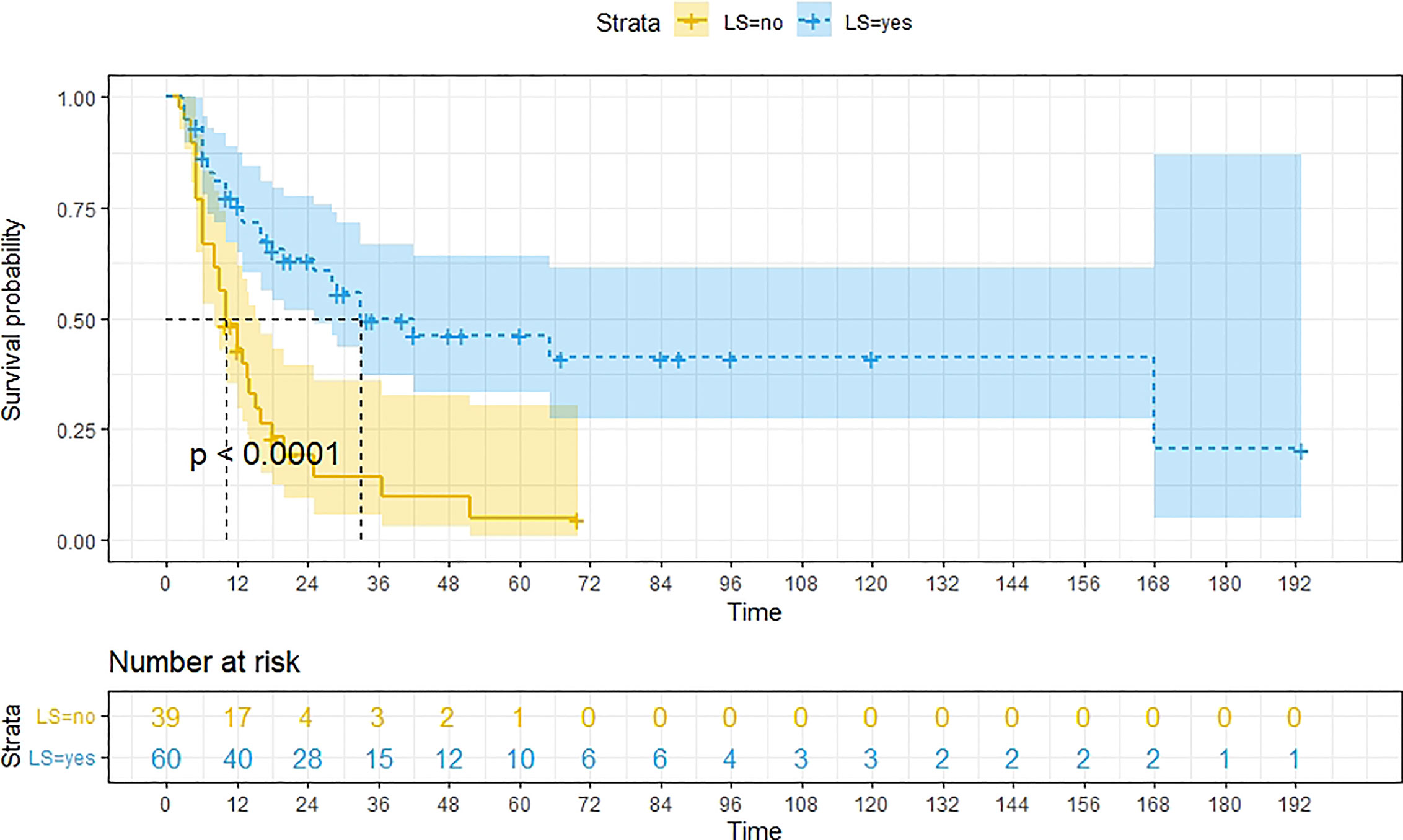
Figure 6 Kaplan-Meier survival curves for OS in patients of no Lymphadenectomy and Lymphadenectomy. The median OS for them are10m, 33m respectively.
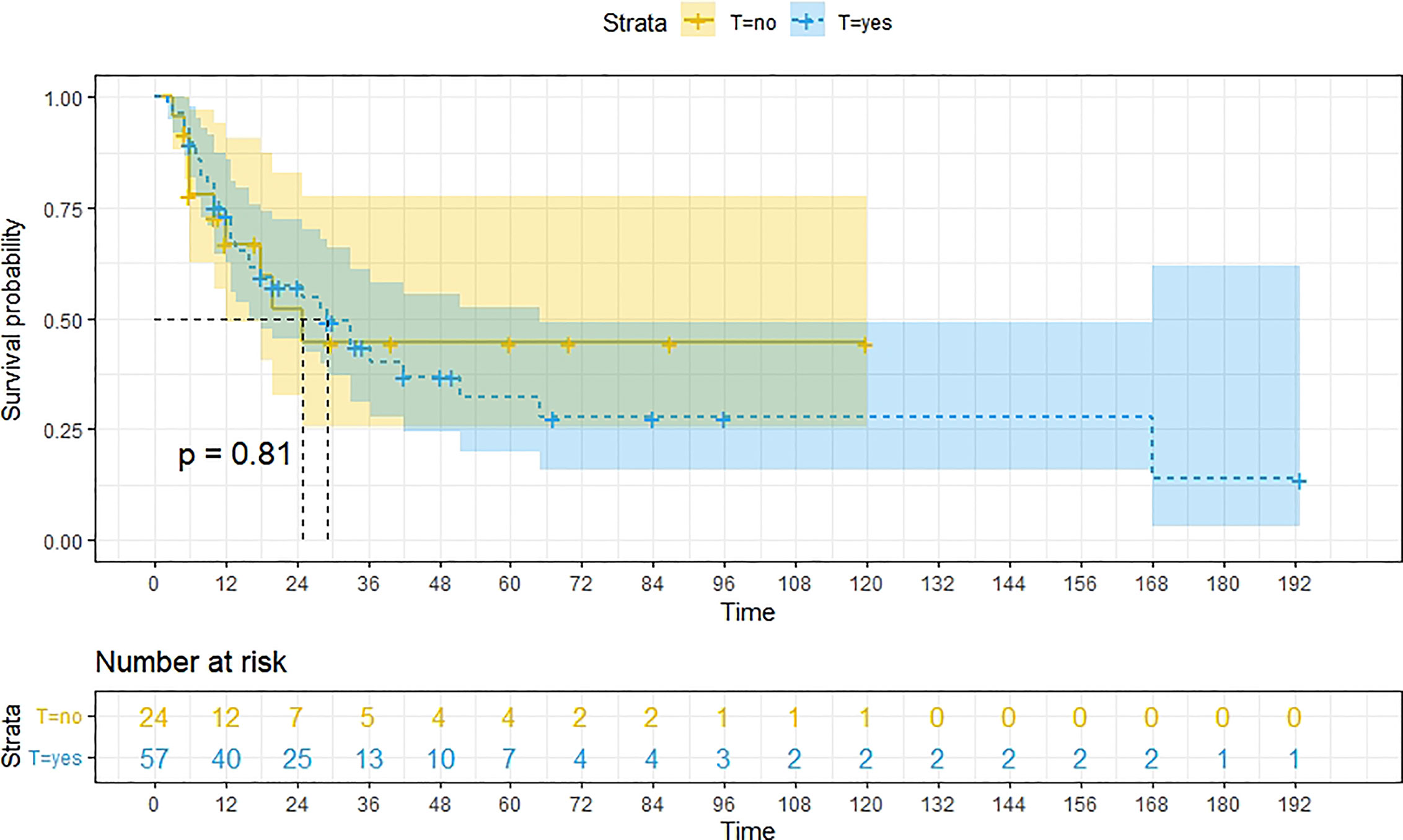
Figure 7 Kaplan-Meier survival curves for OS in patients of surgery (RH+TAH) with and without other treatments. There was little difference in median OS between these two groups.
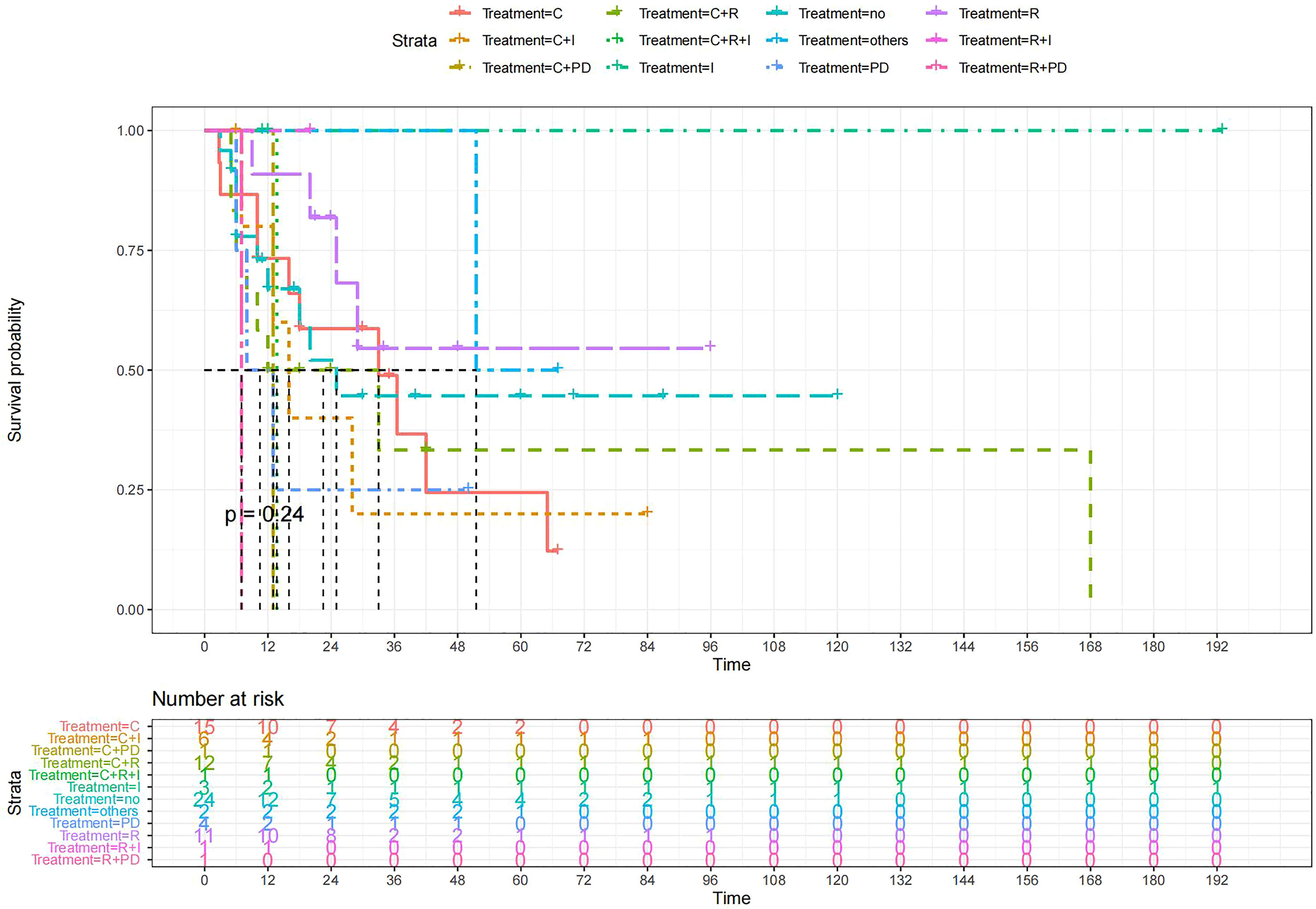
Figure 8 Kaplan-Meier survival curves for OS in patients of surgery (RH+TAH) plus various treatments vs. no other treatments. The median OS was significantly longer for patients who added chemotherapy and other treatments(oncolytic virus Rigvir®, argon laser, Argon heliumknife) than for those who added the remaining treatment modalities (Except for radiotherapy, Survival rates of this group at the 54m time point were higher for patients than other groups.) C: chemotherapy R: radiotherapy I: immunotherapy (interferon-γ or interleukin-2) PD : PD-1/PD-L1 Others: oncolytic virus Rigvir®, argon laser, Argon heliumknife+Sunitinib no:no other treatment.
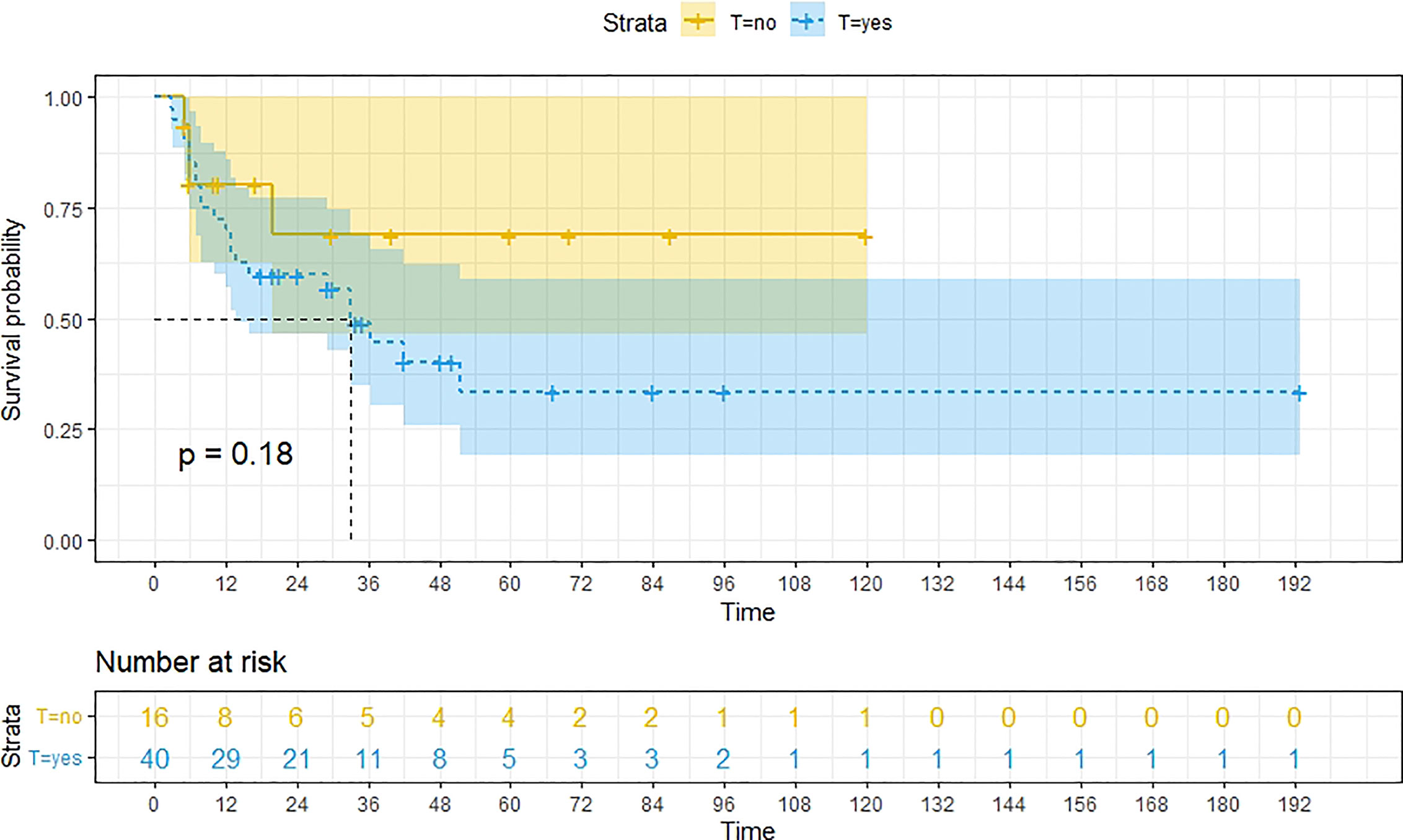
Figure 9 Kaplan-Meier survival curves for OS in patients of RH-based surgery with and without other treatments. Survival rates at the 24m, 36m, 48m and 60m time points were significantly higher for patients who did not add other treatment modalities than for those who did.
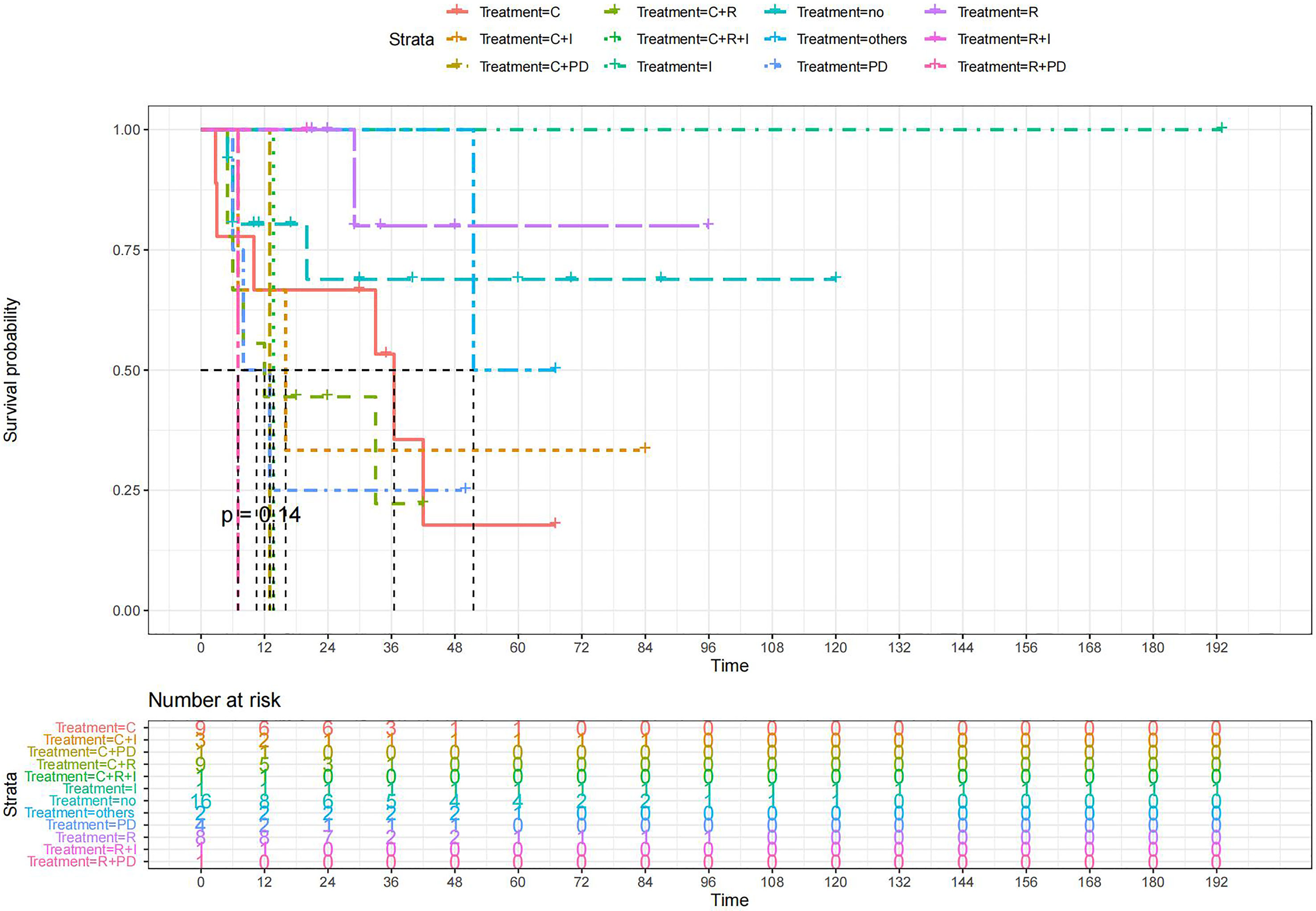
Figure 10 Kaplan-Meier survival curves for OS in patients of RH-based surgery plus various treatments vs. no other treatments. The median OS was significantly longer for patients who added chemotherapy and other treatments(oncolytic virus Rigvir®, argon laser, Argon heliumknife) than for those who added the remaining treatment modalities (Except for radiotherapy and no other treatment added, Survival rates of these two group at the 48m time point were significantly higher for patients than others.) C:chemotherapy R:radiotherapy I:immunotherapy(interferon-γ or interleukin-2) PD : PD-1/PD-L1 Others:oncolytic virus Rigvir®, argon laser, Argon heliumknife+Sunitinib no:no other treatment.
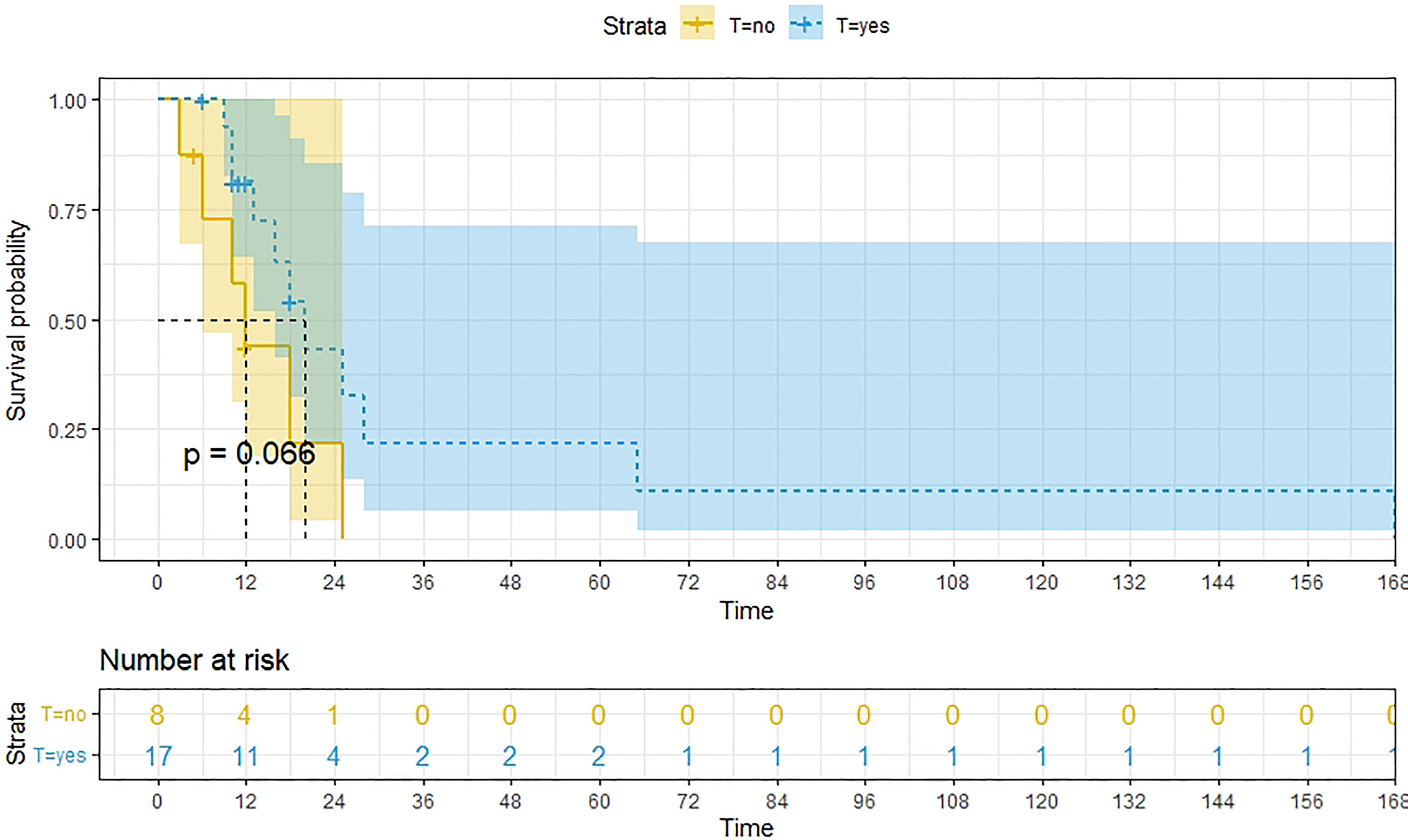
Figure 11 Kaplan-Meier survival curves for OS in patients of TAH-based surgery with and without other treatments. The median OS for them are 20m,12m respectively.
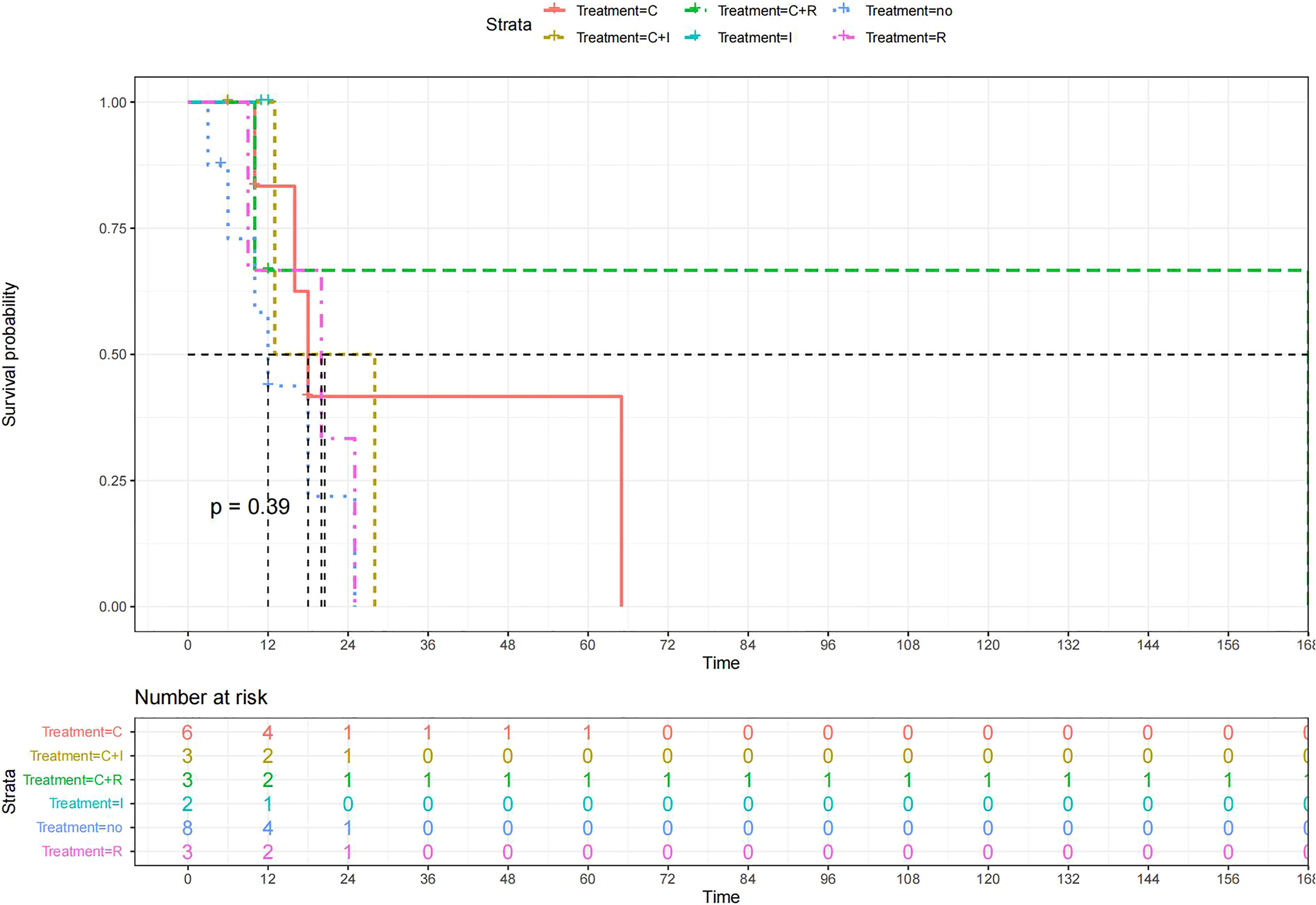
Figure 12 Kaplan-Meier survival curves for OS in patients of TAH-based surgery plus various treatments vs. no other treatments. The median OS was significantly longer for patients who added chemotherapy+radiotherapy than for those who added the remaining treatment modalities C:chemotherapy R:radiotherapy I:immunotherapy(interferon-γ or interleukin-2) PD : PD-1/PD-L1 Others:oncolytic virus Rigvir®,argon laser,Argon heliumknife+Sunitinib no:no other treatment.
Results of multivariate Cox hazard model analysis revealed that age, stage III&IV, TAH and lymph metastasis increased the risk of death, whereas RH and lymphadenectomy was associated with reduced risk of death (Figure 13). In both the univariate and multifactorial cox regression risk models, only age and lymphatic resection showed consistency and could therefore be used as independent prognostic factors (Table 2). For patients who have undergone RH-based surgery, lymphadenectomy reduces the risk of death, while lymphatic metastases and complementary other treatments increase the risk of death (Figure 14). For patients who have undergone TAH-based surgery, lymphadenectomy seems to have little effect, while complementary treatment reduces the risk of death (Figure 15).
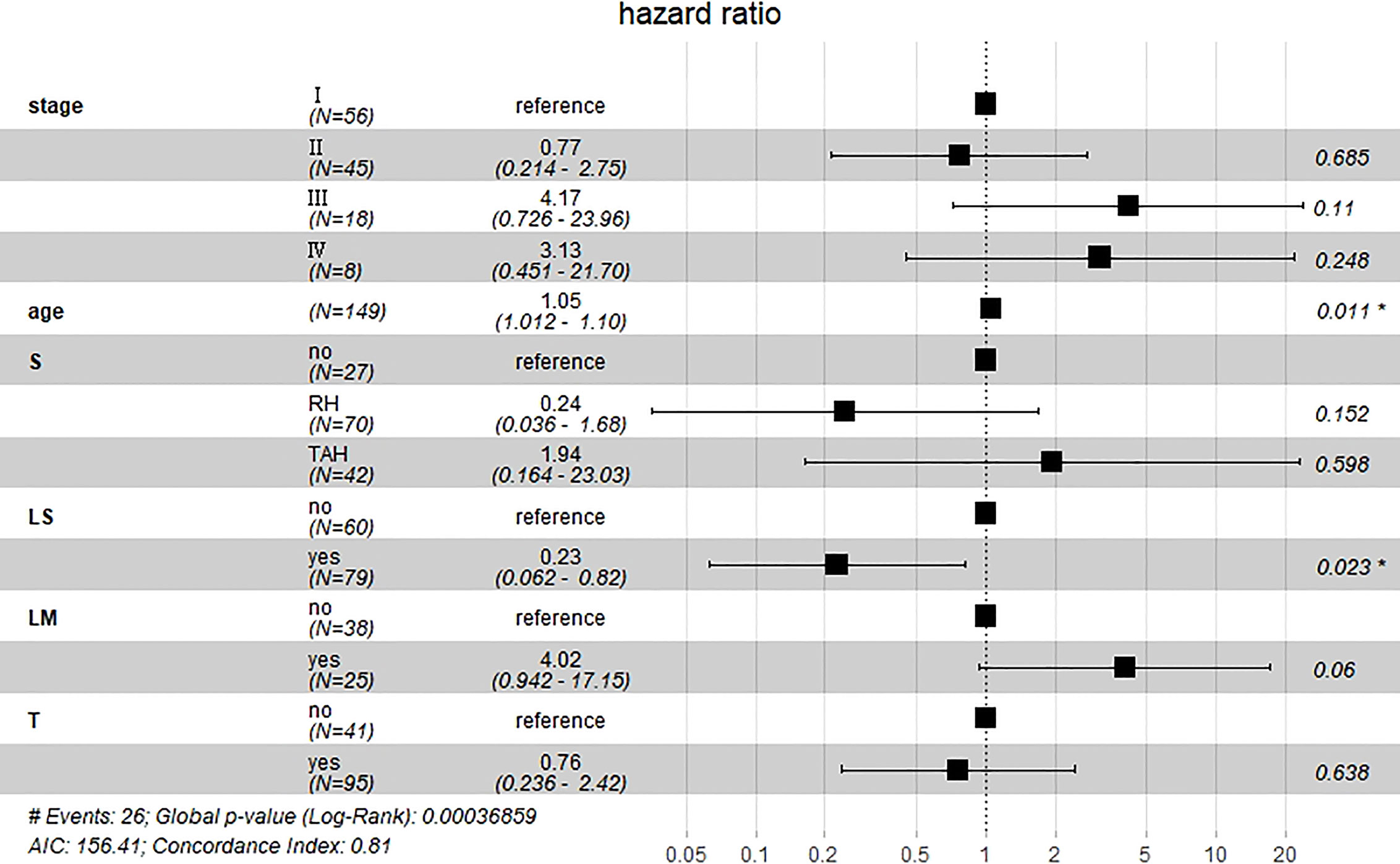
Figure 13 Multivariate Cox hazard model analysis for all patients. Age, stage III&IV, TAH, and lymph metastasis increased the risk of death, whereas RH and lymphadenectomy was associated with reduced risk of death. *means P<0.05.
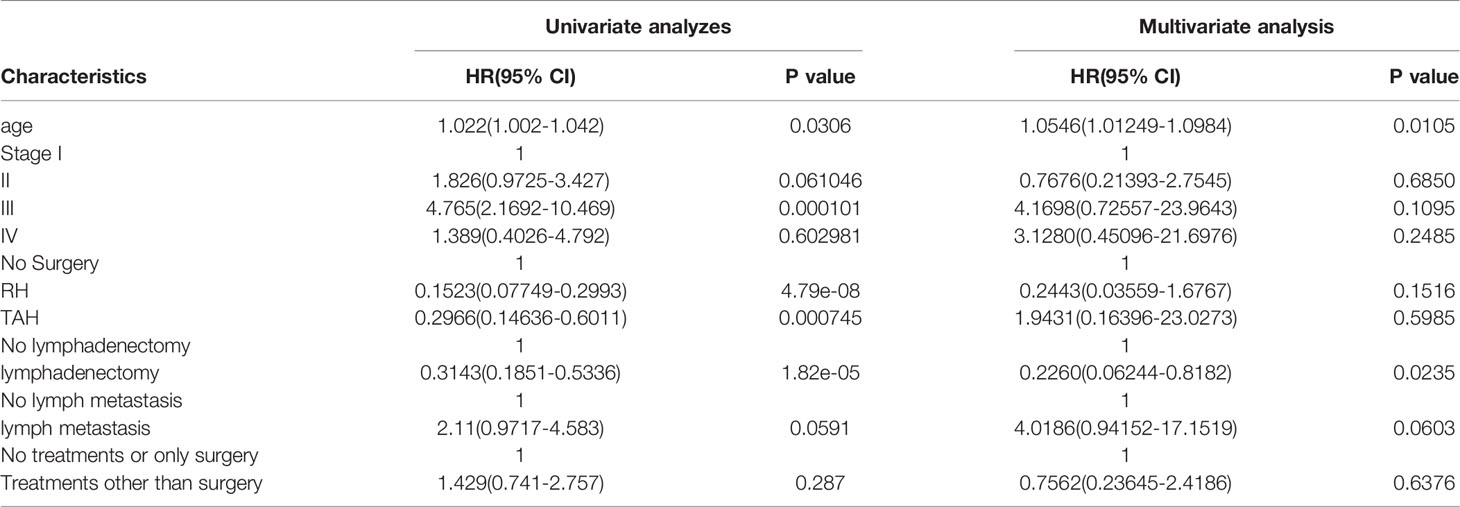
Table 2 Clinical factors effect on overall survival by univariate and multivariate cox proportional hazard regression analysis.
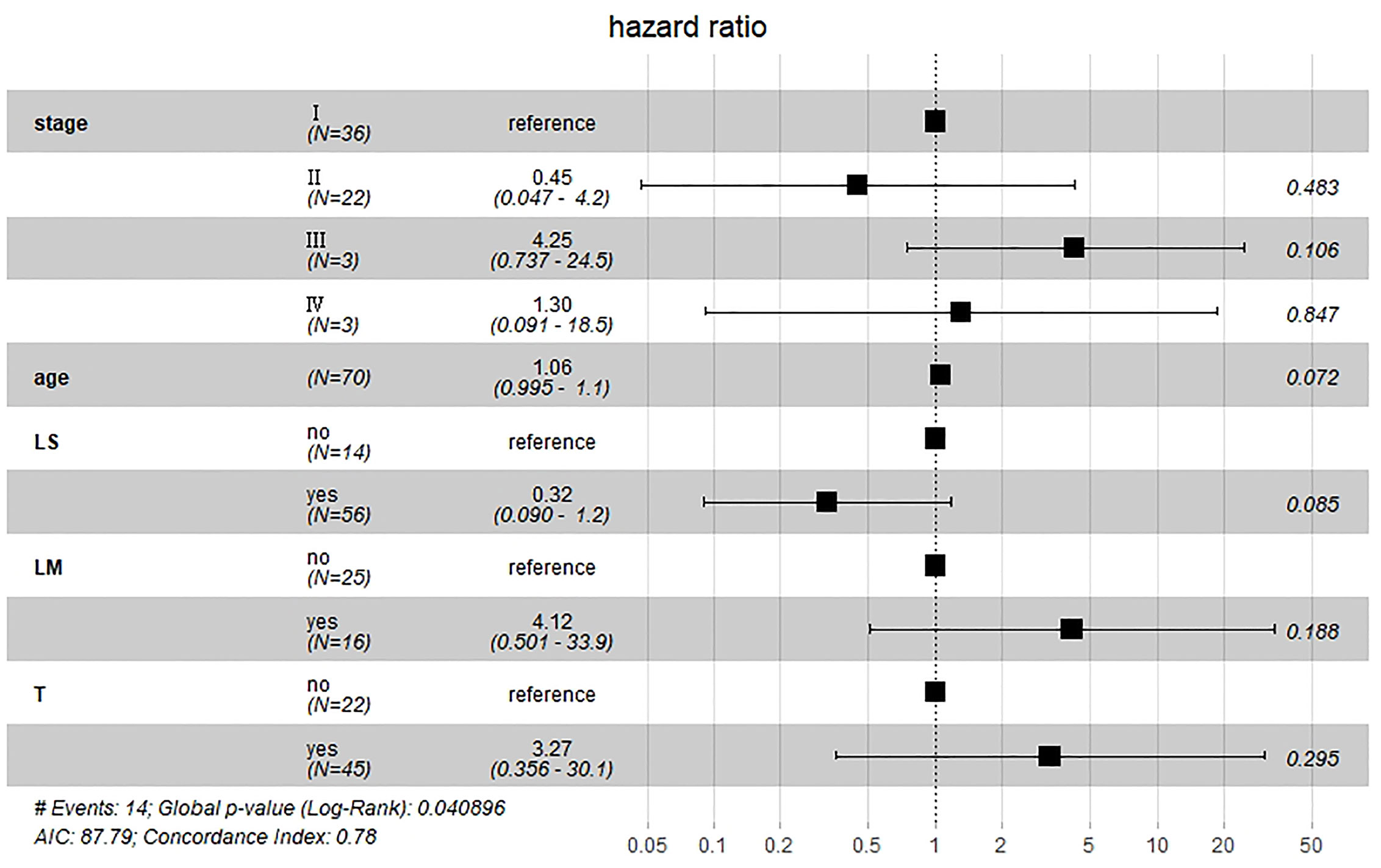
Figure 14 Multivariate Cox hazard model analysis for RH-based patients. Lymphadenectomy reduces the risk of death, while lymphatic metastases and complementary other treatments increase the risk of death.
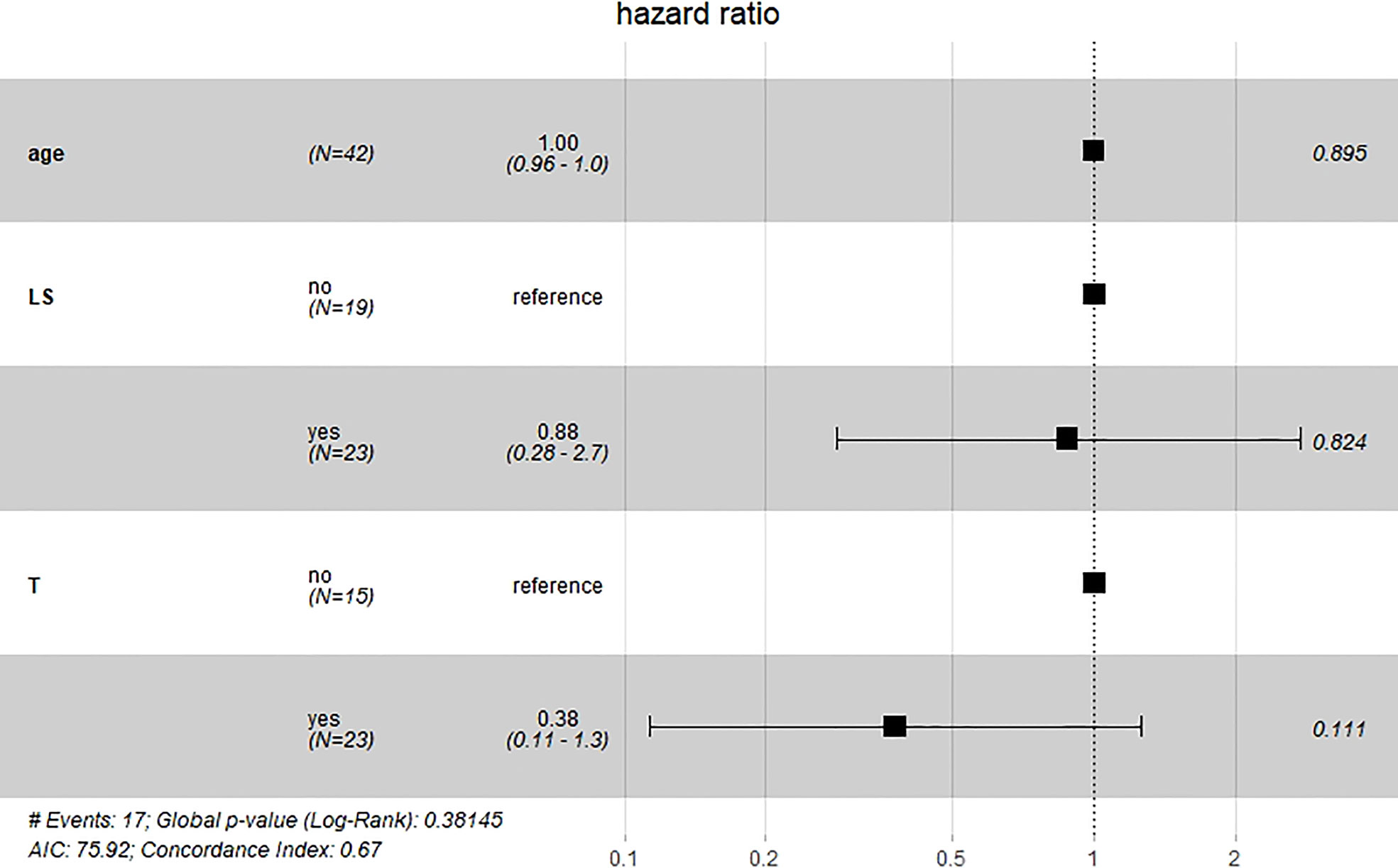
Figure 15 Multivariate Cox hazard model analysis for TAH-based patients. Lymphadenectomy seems to have little effect, while complementary treatment reduces the risk of death.
Discussion
Primary malignant melanoma of the cervix is an extremely rare disease. According to Norris and Taylor (129), cervical melanoma can be diagnosed based on four criteria, namely: presence of melanin in the normal cervical epithelium; absence of melanoma elsewhere in the body; junction changes in the cervix; and metastasis following a pattern of cervical cancer. The disease is characterized by poor patient prognosis, especially if it is not detected in time or treated correctly (95). Previous studies have shown that the 5-year OS for patients with this cancer is approximately 10% and many patients die within three years of diagnosis (87.5%) (13). However, the present study revealed contrasting results. as evidenced by a 5-year OS of 27% and death rate of 65% within 3 years. We attribute this to the continuous improvement in surgery and other treatment modalities. The main prognostic factor for cervical melanoma is the FIGO stage at the time of diagnosis (13). This is consistent with the results the present study, which revealed that median survival time gradually decreased with increasing FIGO stage.
Although no standard treatment modality has been developed for this condition, RH with pelvic lymph node dissection, partial vaginectomy remain the first-choice therapy for patients suitable for the procedure (130, 131). Results of the present study indicated that RH-based surgery did improve patient survival times, which were significantly better than those of patients who had TAH-based surgery and those who did not have surgery. However, a total hysterectomy was performed in some cases. Adjuvant pelvic radiotherapy may be considered for patients with positive surgical margins, parametrial involvement or histologically positive nodes. On the other hand, patients who are not suitable for radical surgery may be subjected to definitive external pelvic radiotherapy with/without brachytherapy, primarily for palliative purposes (131). Although our results were consistent with this conclusion, we believe that adjuvant other treatments are counterproductive in patients who have undergone RH-based surgery. This conclusion is contrary to common sense. It may be related to the following reasons:1.The difference in LM numbers between the two groups was significant, while the median survival time was significantly lower for patients with lymph node metastases than for those without; 2.the sample size of patients supplemented with other treatments was not large enough; 3. each case came from a different study unit, so there was some variation in the quality of the procedure even for radical surgery. If the extent of surgery is inadequate, for example TAH, adjuvant other treatments may improve their median survival time.
Immunotherapy, particularly immune checkpoint inhibitors, has shown great promise in cancer treatment (132), whereas immunotherapy based on immune checkpoint blockade is efficacious in treating melanoma (133). Although some studies have suggested that anti-PD-1 is associated with better OS compared to anti-CTLA4 in advanced/recurrent female genital tract melanoma (134), our results demonstrated that PD agents were not superior to the other adjuvant treatment modalities in patients with PMMC. This may be due to the small sample size of patients enrolled in this study.
In summary, this is the first pooled analysis including 149 cases of primary cervical melanoma. We found that patients who underwent RH-based surgery, those with non-lymph nodes metastatic and those who underwent lymphadenectomy had significantly higher survival rates. Based on the results of the analysis, the addition of other treatment options for patients who undergoing RH-based surgery is subject to further study, but for those who had TAH-based surgery, the addition of other treatments to prolong median survival may be considered. Notably, age and lymphadenectomy were associated with increased and reduced risk of death in these patients, respectively. Although there was no statistically significant difference, stage III&IV, TAH and lymph metastasis increased the risk of death, whereas RH was associated with reduced risk of death. For patients who have undergone RH-based surgery, lymphadenectomy reduces the risk of death, while lymphatic metastases and complementary other treatments increase the risk of death. For patients who have undergone TAH-based surgery, lymphadenectomy seems to have little effect, while complementary treatment reduces the risk of death. Future collaborative epidemiological studies are needed to further validate these findings. Therefore, via summarizing previous reports, the recommended treatment procedure for PMMC are radical hysterectomy and lymphadenectomy. The addition of other treatment options for patients who undergoing RH-based surgery need further study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contribution
HC wrote the manuscript. AM and AF participated in the search strategy development. AM assisted in acquisition, analysis, or interpretation of data for the current work. HC prepared figures and tables. HW contributed to study concept and design. HC and MH double-checked the data and corrected the error in the tables. All authors contributed to critical manuscript revision.
Funding
This study was financially supported by Annual Science and Technology Steering Plan Project of Zhuzhou and Sichuan Science and Technology 259 Program (2021YFS0126).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.913964/full#supplementary-material
References
1. Hu W, Zhu L, Pei W, Pan S, Guo Z, Wu A, et al. Overexpression of Ras-Related C3 Botulinum Toxin Substrate 2 Radiosensitizes Melanoma Cells In Vitro and In Vivo. Oxid Med Cell Longevity (2019) 2019:5254798. doi: 10.1155/2019/5254798
2. Schneider C, Gebhardt L, Arndt S, Karrer S, Zimmermann JL, Fischer M, et al. Acidification is an Essential Process of Cold Atmospheric Plasma and Promotes the Anti-Cancer Effect on Malignant Melanoma Cells. Cancers (2019) 11(5):671. doi: 10.3390/cancers11050671
3. Kumar D, Gorain M, Kundu G, Kundu GC. Therapeutic Implications of Cellular and Molecular Biology of Cancer Stem Cells in Melanoma. Mol Cancer (2017) 16(1):7. doi: 10.1186/s12943-016-0578-3
4. Hellmund P, Schmitt J, Roessler M, Meier F, Schoffer O. Targeted and Checkpoint Inhibitor Therapy of Metastatic Malignant Melanoma in Germany, 2000-2016. Cancers (2020) 12(9):2354. doi: 10.3390/cancers12092354
5. Kim MS, Choi CH, Kim TJ, Lee JW, Lee J, Bae DS, et al. Primary Malignant Melanoma of the Uterine Cervix Treated With Pembrolizumab After Radical Surgery: A Case Report and Literature Review. Ob. gynecol. Sci (2018) 61(4):524–8. doi: 10.5468/ogs.2018.61.4.524
6. Wicklein D, Otto B, Suling A, Elies E, Lüers G, Lange T, et al. CEACAM1 Promotes Melanoma Metastasis and is Involved in the Regulation of the EMT Associated Gene Network in Melanoma Cells. Sci Rep (2018) 8(1):11893. doi: 10.1038/s41598-018-30338-4
7. Mashima E, Inoue A, Sakuragi Y, Yamaguchi T, Sasaki N, Hara Y, et al. Nivolumab in the Treatment of Malignant Melanoma: Review of the Literature. OncoTar. Ther (2015) 8:2045–51. doi: 10.2147/OTT.S62102
8. Ma M, Ghosh S, Tavernari D, Katarkar A, Clocchiatti A, Mazzeo L, et al. Sustained Androgen Receptor Signaling is a Determinant of Melanoma Cell Growth Potential and Tumorigenesis. J Exp Med (2021) 218(2):e20201137. doi: 10.1084/jem.20201137
9. Pang Y, Yuan H, Ren A, Zhang S, Liu P. Primary Malignant Melanoma of the Female Genital Tract Synchronously Involving the Vulva and Uterine Cervix: A Case Report. Medicine (2019) 98(30):e16366. doi: 10.1097/MD.0000000000016366
10. DeMatos P, Tyler D, Seigler HF. Mucosal Melanoma of the Female Genitalia: A Clinicopathologic Study of Forty-Three Cases at Duke University Medical Center. Surgery (1998) 124(1):38–48. doi: 10.1016/S0039-6060(98)70073-X
11. Mistry B, Patel RV, Keum YS, Kim DH. Synthesis of N-Mannich Bases of Berberine Linking Piperazine Moieties Revealing Anticancer and Antioxidant Effects. Saudi J Biol Sci (2017) 24(1):36–44. doi: 10.1016/j.sjbs.2015.09.005
12. Liu T, Jin L, Chen M, Zheng Z, Lu W, Fan W, et al. Ku80 Promotes Melanoma Growth and Regulates Antitumor Effect of Melatonin by Targeting HIF1-α Dependent PDK-1 Signaling Pathway. Redox Biol (2019) 25:101197. doi: 10.1016/j.redox.2019.101197
13. Min KJ, Kim YS, Hong JH, Lee JK, Yang DS. Primary Malignant Melanoma of Uterine Cervix: A Suggestion of New Scheme of Treatment Combination. Chin J Cancer Res (2014) 26(3):351–4. doi: 10.3978/j.issn.1000-9604.2014.06.03
14. Pusceddu S, Bajetta E, Carcangiu ML, Formisano B, Ducceschi M, Buzzoni R. A Literature Overview of Primary Cervical Malignant Melanoma: An Exceedingly Rare Cancer. Crit Rev Oncol Hematol (2012) 81(2):185–95. doi: 10.1016/j.critrevonc.2011.03.008
15. NCCN. The NCCN Cervical Cancer Clinical Practice Guidelines in Oncology (Version 1.2022) [EB/OL]. Fort Washington: NCCN (2022). Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
16. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer (2000). doi: 10.1007/978-1-4757-3294-8
19. Taylor CE, Tuttle HK. Melanocarcinoma of the Cervix Uteri or Vaginal Vault. Arch Pathol (1944) 38:60–1.
21. ALLEN WM, SHERMAN AI, ARNESON AN. Carcinoma of the Cervix: Results Obtained From the Irradiation of the Parametrium With Radioactive Colloidal Gold. Am J Obstet Gynecol. (1954) 68(6):1433–46. doi: 10.1016/0002-9378(54)90295-1
22. Wimhofer H, Stoll P. Bericht Über Ein Malignes Melanom Der Portio Vaginalis Uteri [Report on a Malignant Melanoma of the Portio Vaginalis Uteri]. Zentralbl Gynakol (1954) 76(40-41):1840–5.
23. Merkas Z. Malignant Melanoma of the Portio Vaginalis Uteri. Zrpski Archiv Celok Lek (1959) 87:1046–9.
24. Stegner HE. [On Melanin-Forming Pigment Cells and Pigment Tumors of the Portio Vaginalis Uteri]. Zentralbl Gynakol. (1959) 81:1686–92.
25. Fluhman CF. Invasive Carcinoma in the Cervix Uteri and its Diseases. Philadelphia. PA: Sanders; (1961), 349–51.
26. Abell MR. Primary Melanoblastoma of the Uterine Cervix. Am J Clin Pathol (1961) 36:248–55. doi: 10.1093/ajcp/36.3.248
27. Cadek K. Primäres Malignes Melanoblastom Der Portio Vaginalis Uteri [Primary Malignant Melanoblastoma of the Portio Vaginalis Uteri]. Zentralbl Gynakol. (1966) 88(28):915–9.
28. Ferretti G, Catastini M. Rara Osservazione Di Melanoma Della Portio Uterine Con Ripetizione Vaginale [Unusual Case of Melanoma of the Uterine Cervix With Vaginal Recurrence]. Arch Vecchi Anat Patol. (1967) 49(3):791–807.
29. Jansen HH, Bräunig G, Stückle H. Morphologische Besonderheiten Eines Malignen Melanoblastoms Des Collum Uteri [Morphologic Peculiarities of a Malignant Melanoblastoma of the Uterine Cervix]. Frankf Z Pathol (1967) 77(2):150–9.
30. Cabanne F, Michiels R, Gauliard M, Jahier J, Dusserre P, Guerrin J, et al. Les Naevocarcinomes Primitifs Du Col Utérin [Primary Nevocarcinoma of the Cervix Uteri]. Rev Fr Gynecol Obstet. (1970) 65(11):649–58.
31. Weghaupt K. Primäres Melanokarzinom Der Portio Vaginalis Uteri [Primary Melanocarcinoma of the Portio Vaginalis Uteri]. Zentralbl Gynakol. (1970) 92(15):469–73.
32. Jones HW 3rd, Droegemueller W, Makowski EL. A Primary Melanocarcinoma of the Cervix. Am J Obstet Gynecol. (1971) 111(7):959–63. doi: 10.1016/0002-9378(71)90953-7
33. Puri S, Yoonessi M, Romney SL. Malignant Melanoma of the Cervix Uteri. Obstet Gynecol. (1976) 47(4):459–62.
34. Kanajet D. Primarni Melanomalignom Cervusa Uterusa [Primary Malignant Melanoma of the Uterine Cervix]. Jugosl Ginekol Opstet (1979) 19(1-2):67–72.
35. Vrousos C, Bolla M, Schaerer R, Mallard G, Francillard JL, Widgren S. Mélanome Malin Du Col Utérin [Malignant Melanoma of the Cervix Uteri]. Nouv Presse Med (1979) 8(15):1264–5.
36. Ohashi H, Nagano M. [A Primary Malignant Melanoma of the Cervix Uteri (Author's Transl)]. Nihon Gan Chiryo Gakkai Shi. (1979) 14(2):138–42.
37. Hall DJ, Schneider V, Goplerud DR. Primary Malignant Melanoma of the Uterine Cervix. Obstet Gynecol. (1980) 56(4):525–9.
38. Ramsey HE, Smith HB. Primary Melanoma of the Cervix: Report of a Case. J Natl Med Assoc (1981) 73(12):1149–51.
39. Genton CY, Kunz J, Schreiner WE. Primary Malignant Melanoma of the Vagina and Cervix Uteri. Report of a Case With Ultrastructural Study. Virchows Arch A Pathol Anat Histol. (1981) 393(2):245–50. doi: 10.1007/BF00431080
40. Mudge TJ, Johnson J, MacFarlane A. Primary Malignant Melanoma of the Cervix. Case Report. Br J Obstet Gynaecol. (1981) 88(12):1257–9. doi: 10.1111/j.1471-0528.1981.tb01208.x
41. Krishnamoorthy A, Desai M, Simanowitz M. Primary Malignant Melanoma of the Cervix. Case Report. Br J Obstet Gynaecol. (1986) 93(1):84–6. doi: 10.1111/j.1471-0528.1986.tb07820.x
42. Yu HC, Ketabchi M. Detection of Malignant Melanoma of the Uterine Cervix From Papanicolaou Smears. A Case Report. Acta Cytol. (1987) 31(1):73–6.
43. Holmquist ND, Torres J. Malignant Melanoma of the Cervix. Rep case. Acta Cytol. (1988) 32(2):252–6.
44. Owens OJ, Pollard K, Khoury GG, Dyson JE, Jarvis GJ, Joslin CA. Primary Malignant Melanoma of the Uterine Cervix. Clin Radiol (1988) 39(3):336–8. doi: 10.1016/s0009-9260(88)80565-8
45. Chua S, Viegas OA, Wee A, Ratnam SS. Malignant Melanoma of the Cervix. Gynecol Obstet Invest. (1989) 27(2):107–9. doi: 10.1159/000293632
46. Mordel N, Mor-Yosef S, Ben-Baruch N, Anteby SO. Malignant Melanoma of the Uterine Cervix: Case Report and Review of the Literature. Gynecol Oncol (1989) 32(3):375–80. doi: 10.1016/0090-8258(89)90645-8
47. Vleugels MP, Brölmann HA, van Beek M. Primary Melanoma of the Cervix Uteri, an Avis Rara? A Review of the Literature. Acta Obstet Gynecol Scand (1990) 69(3):259–64. doi: 10.3109/00016349009028690
48. Podczaski E, Abt A, Kaminski P, Larson J, Sorosky J, DeGeest K, et al. A Patient With Multiple, Malignant Melanomas of the Lower Genital Tract. Gynecol Oncol (1990) 37(3):422–6. doi: 10.1016/0090-8258(90)90380-4
49. Santoso JT, Kucera PR, Ray J. Primary Malignant Melanoma of the Uterine Cervix: Two Case Reports and a Century's Review. Obstet Gynecol Surv. (1990) 45(11):733–40. doi: 10.1097/00006254-199011000-00003
50. Pinedo F, Ingelmo JM, Miranda P, Garzón A, López JI. Primary Malignant Melanoma of the Uterine Cervix: Case Report and Review of the Literature. Gynecol Obstet Invest. (1991) 31(2):121–4. doi: 10.1159/000293117
51. Khoo US, Collins RJ, Ngan HY. Malignant Melanoma of the Female Genital Tract. A Report of Nine Cases in the Chinese of Hong Kong. Pathology. (1991) 23(4):312–7. doi: 10.3109/00313029109063595
52. Joura E, Gitsch G, Kainz C, Berger E, Breitenecker G. Kasuistik Eines Primär Bestrahlten Melanoms Der Cervix Uteri [Case Report of Primary Irradiated Melanoma of the Cervix Uteri]. Gynakol Geburtshilfliche Rundsch. (1992) 32 Suppl 1:134. doi: 10.1159/000271977
53. Kristiansen SB, Anderson R, Cohen DM. Primary Malignant Melanoma of the Cervix and Review of the Literature. Gynecol Oncol (1992) 47(3):398–403. doi: 10.1016/0090-8258(92)90148-c
54. Chang SC, Chen CJ, Tseng HH. Primary Malignant Melanoma of the Vagina and Cervix Uteri: Case Report and Literature Review. Zhonghua Yi Xue Za Zhi (Taipei). (1992) 50(4):341–6.
55. Räber G, Mempel V, Jackisch C, Patt V, Schneider HP. Das Primär Maligne Melanom Der Cervix Uteri–Fallbericht Und Literaturüberblick [Primary Malignant Melanoma of the Uterine Cervix–Case Report and Review of the Literature]. Zentralbl Gynakol. (1993) 115(11):503–7.
56. Fleming H, Mein P. Primary Melanoma of the Cervix. A Case Report. Acta Cytol. (1994) 38(1):65–9.
57. Feichter G, Curschellas E, Gobat S, Rickli J. Malignant Melanoma of the Uterine Cervix; Case Report Including Cytology, Histology and Immunocytochemistry. Cytopathology. (1995) 6(3):196–200.
58. Butt A, Roberts DL, Calvert JP, Williams S. Primary Malignant Melanomas of Cervix Uteri and Vulva. Br J Dermatol (1996) 135(5):858–9. doi: 10.1111/j.1365-2133.1996.tb03908.x
59. Kedzia W, Sajdak S, Kedzia H, Spaczyński M. Pierwotny Czerniak Szyjki Macicy U 19 Letniej Kobiety–Opis Przypadku [Primary Melanoma of the Uterine Cervix in a 19 Year Old Woman–Case Report]. Ginekol Pol (1997) 68(8):386–9.
60. Miyagi Y, Yamada S, Miyagi Y, Yamamoto J, Kawanishi K, Yoshinouchi M, et al. Malignant Melanoma of the Uterine Cervix: A Case Report. J Obstet Gynaecol Res (1997) 23(6):511–9. doi: 10.1111/j.1447-0756.1997.tb00880.x
61. Teixeira JC, Salina JR, Teixeira LC, Andrade LA. Primary Melanoma of the Uterine Cervix Figo Stage III B. Sao Paulo Med J (1998) 116(4):1778–80. doi: 10.1590/s1516-31801998000400007
62. Salle E, Houvenaeghel G, Bladou F, Jacquemier J, Antoine K, Delpero JR. Mélanome Malin Du Col Utérin. A Propos D'un Cas, Avec Colpohystérectomie Totale Et Reconstruction Vaginale Par Lambeau De Rectus Abdominis [Malignant Melanoma of the Uterine Cervix. Apropos of a Case, With Total Colpo-Hysterectomy and Vaginal Reconstruction Using a Rectus Abdominis Flap]. Ann Chir. (1998) 52(1):93–6.
63. Ishikura H, Kojo T, Ichimura H, Yoshiki T. Desmoplastic Malignant Melanoma of the Uterine Cervix: A Rare Primary Malignancy in the Uterus Mimicking a Sarcoma. Histopathology. (1998) 33(1):93–4. doi: 10.1046/j.1365-2559.1998.0415h.x
64. Cantuaria G, Angioli R, Fernandez-Abril A, Penalver M. Primary Malignant Melanoma of the Uterine Cervix: Case Report and Review of the Literature. Prim Care Update Ob Gyns. (1998) 5(4):159–60. doi: 10.1016/s1068-607x(98)00052-3
65. Schlosshauer PW, Heller DS, Koulos JP. Malignant Melanoma of the Uterine Cervix Diagnosed on a Cervical Cytologic Smear. Acta Cytol. (1998) 42(4):1043–5.
66. Takehara M, Ito E, Saito T, Nishioka Y, Kudo R. Primary Malignant Melanoma of the Uterine Cervix: A Case Report. J Obstet Gynaecol Res (1999) 25(2):129–32. doi: 10.1111/j.1447-0756.1999.tb01134.x
67. Wasef WR, Roberts JK, Dixon GR. Primary Malignant Melanoma of the Cervix Uteri. J Obstet Gynaecol. (1999) 19(6):673–4. doi: 10.1080/01443619964094
68. Cantuaria G, Angioli R, Nahmias J, Estape R, Penalver M. Primary Malignant Melanoma of the Uterine Cervix: Case Report and Review of the Literature. Gynecol Oncol (1999) 75(1):170–4. doi: 10.1006/gyno.1999.5491
69. Clark KC, Butz WR, Hapke MR. Primary Malignant Melanoma of the Uterine Cervix: Case Report With World Literature Review. Int J Gynecol Pathol (1999) 18(3):265–73. doi: 10.1097/00004347-199907000-00013
70. Benson RJ, Tan LT. Radiation-Induced Malignant Melanoma of the Cervix. Clin Oncol (R Coll Radiol). (2000) 12(4):234–7. doi: 10.1053/clon.2000.9162
71. Furuya M, Shimizu M, Nishihara H, Ito T, Sakuragi N, Ishikura H, et al. Clear Cell Variant of Malignant Melanoma of the Uterine Cervix: A Case Report and Review of the Literature. Gynecol Oncol (2001) 80(3):409–12. doi: 10.1006/gyno.2000.6091
72. Zamiati S, Sahraoui S, Jabri L, Louahlia S, Sqalli S, Kahlain A. Mélanome Malin Primitif Du Col Utérin: À Propos D'un Cas Avec Revue De La Littérature [Primary Malignant Melanoma of the Cervix Uteri: Apropos of 1 Case With Review of the Literature]. Gynecol Obstet Fertil. (2001) 29(5):381–5. doi: 10.1016/s1297-9589(01)00148-5
73. Deshpande AH, Munshi MM. Primary Malignant Melanoma of the Uterine Cervix: Report of a Case Diagnosed by Cervical Scrape Cytology and Review of the Literature. Diagn Cytopathol. (2001) 25(2):108–11. doi: 10.1002/dc.2014
74. Amenssag L, Idrissi F, Erchidi I, Melhouf A, Mrabet F, Brhami R, et al. Mélanome Malin Primitif Du Col Utérin [Primary Malignant Melanoma of the Cervix]. Presse Med (2002) 31(21 Pt 1):976–8.
75. Boldt C, Lehmann R, Osmers R, Bürrig KF. Primäres Malignes Melanom Der Cervix Uteri. Zwei Fallberichte Und Literaturübersicht [Primary Malignant Melanoma of the Uterine Cervix. Report of Two Cases and Review of the Literature]. Pathologe. (2003) 24(3):226–35. doi: 10.1007/s00292-002-0596-3
76. Makovitzky J, Schmitz C, Vogt-Weber B, Nizze H. Primary Malignant Melanoma of the Cervix Uteri: A Case Report of a Rare Tumor. Anticancer Res (2003) 23(2A):1063–7.
77. Gupta S, Sodhani P, Jain S. Primary Malignant Melanoma of Uterine Cervix: A Rare Entity Diagnosed on Fine Needle Aspiration Cytology–Report of a Case. Cytopathology. (2003) 14(3):153–6. doi: 10.1046/j.1365-2303.2003.00014.x
78. Kudrimoti J, Bindu R, Hayatnagarkar N, Bhople K. Primary Malignant Melanoma of Cervix: A Case Report. Indian J Pathol Microbiol (2004) 47(2):257–8.
79. Gupta R, Singh S, Mandal AK. Primary Malignant Melanoma of Cervix - a Case Report. Indian J Cancer. (2005) 42(4):201–4.
80. Siozos C, Bhat A, Lonsdale R, Nieto JJ, Crocker SG. Malignant Melanoma of the Uterine Cervix. J Obstet Gynaecol. (2005) 25(8):826–7. doi: 10.1080/01443610500338305
81. Ma SQ, Bai CM, Zhong S, Yu XH, Lang JH. Clinical Analysis of Primary Malignant Melanoma of the Cervix. Chin Med Sci J (2005) 20(4):257–60.
82. Wydra D, Sawicki S, Ciach K, Emerich J. Malignant Melanoma of the Uterine Cervix. Eur J Obstet Gynecol Reprod Biol (2006) 124(2):257–8. doi: 10.1016/j.ejogrb.2005.06.024
83. Mousavi AS, Fakor F, Nazari Z, Ghaemmaghami F, Hashemi FA, Jamali M. Primary Malignant Melanoma of the Uterine Cervix: Case Report and Review of the Literature. J Low Genit Tract Dis (2006) 10(4):258–63. doi: 10.1097/01.lgt.0000229564.11741.4e
84. Jin B, Goldsmith A, Budev H, Al-Abbadi M. Primary Melanoma of the Uterine Cervix After Supracervical Hysterectomy. A Case Report. Acta Cytol. (2007) 51(1):86–8. doi: 10.1159/000325690
85. Pusceddu S, Bajetta E, Buzzoni R, Carcangiu ML, Platania M, Del Vecchio M, et al. Primary Uterine Cervix Melanoma Resembling Malignant Peripheral Nerve Sheath Tumor: A Case Report. Int J Gynecol Pathol (2008) 27(4):596–600. doi: 10.1097/PGP.0b013e31817323c4
86. Baruah J, Roy KK, Kumar S, Kumar L. A Rare Case of Primary Malignant Melanoma of Cervix. Arch Gynecol Obstet. (2009) 280(3):453–6. doi: 10.1007/s00404-008-0912-0
87. Yücesoy G, Kus E, Cakiroglu Y, Muezzinoglu B, Yildiz K, Yucesoy I. Primary Malignant Melanoma of the Cervix: Report of a Case. Arch Gynecol Obstet. (2009) 279(4):573–5. doi: 10.1007/s00404-008-0761-x
88. Khurana A, Jalpota Y. Primary Malignant Melanoma of the Uterine Cervix. Indian J Pathol Microbiol (2009) 52(4):575–6. doi: 10.4103/0377-4929.56164
89. Mandato VD, Kobal B, Di Stefano A, Sinkovec J, Levicnik A, Rakar S, et al. Amelanotic Malignant Melanoma of the Uterine Cervix With Ten-Year Follow-Up. Eur J Gynaecol Oncol (2009) 30(1):106–9.
90. An J, Li B, Wu L, Lu H, Li N. Primary Malignant Amelanotic Melanoma of the Female Genital Tract: Report of Two Cases and Review of Literature. Melanoma Res (2009) 19(4):267–70. doi: 10.1097/CMR.0b013e32831993de
91. Das P, Kumar N, Ahuja A, Jain A, Ray R, Sarkar C, et al. Primary Malignant Melanoma at Unusual Sites: An Institutional Experience With Review of Literature. Melanoma Res (2010) 20(3):233–9. doi: 10.1097/CMR.0b013e328334c39a
92. Duggal R, Srinivasan R. Primary Amelanotic Melanoma of the Cervix: Case Report With Review of Literature. J Gynecol Oncol (2010) 21(3):199–202. doi: 10.3802/jgo.2010.21.3.199
93. Setia N, Goulart RA, Leiman G, Otis CN, Modem R, Pantanowtiz L. Cytomorphology of Cervicovaginal Melanoma: ThinPrep Versus Conventional Papanicolaou Tests. Cytojournal. (2010) 7:25. doi: 10.4103/1742-6413.75666
94. Calderón-Salazar L, Cantú de Leon D, Perez Montiel D, Almogabar-Villagrán E, Villavicencio V, Cetina L. Primary Malignant Melanoma of the Uterine Cervix Treated With Ultraradical Surgery: A Case Report. ISRN Obstet Gynecol. (2011) 2011:683020. doi: 10.5402/2011/683020
95. Simões M, Cunha V, Nabais H, Riscado I, Jorge AF. Primary Malignant Melanoma of the Uterine Cervix–Case Report and Review. Eur J Gynaecol Oncol (2011) 32(4):448–51.
96. Zhang J, Cao Y, Xiao L, Tang J, Tang L. A Peculiar Site: Melanoma of the Cervix. Am J Obstet Gynecol. (2011) 205(5):508. doi: 10.1016/j.ajog.2011.09.016
97. Parada D, Peña KB, Riu F. Coexisting Malignant Melanoma and Blue Nevus of the Uterine Cervix: An Unusual Combination. Case Rep Pathol (2012) 2012:986542. doi: 10.1155/2012/986542
98. Tsai YJ, Shueng PW, Chan SC, Chuang WY, Shiau YC, Hsu CH. Uterine Cervical Melanoma Presenting With Rapid Progression Detected by PET/CT. Acta Radiol Short Rep (2012) 1(4):arsr.2012.120026. doi: 10.1258/arsr.2012.120026
99. Myriokefalitaki E, Babbel B, Smith M, Ahmed AS. Primary Malignant Melanoma of Uterine Cervix FIGO IIa1: A Case Report With 40 Months Ongoing Survival and Literature Review. Gynecol Oncol Case Rep (2013) 5:52–4. doi: 10.1016/j.gynor.2013.04.004
100. Singh N, Tripathi R, Mala YM. Primary Malignant Melanoma of Uterine Cervix With Probable Origin From Benign Cervical Melanosis. BMJ Case Rep (2013) 2013:bcr2013010042. doi: 10.1136/bcr-2013-010042
101. Bhargava S, Mogra N, Goyal N. Primary Malignant Melanoma of Uterine Cervix. J Obstet Gynaecol India. (2014) 64(Suppl 1):132–3. doi: 10.1007/s13224-013-0437-8
102. Liu Z, Wang H, Zhang X, Xu Q. Primary Malignant Melanoma of the Cervix: A Case Report. Oncol Lett (2014) 8(6):2661–3. doi: 10.3892/ol.2014.2555
103. Omranipour R, Mahmoodzadeh H, Jalaeefar A, Abdirad A, Parsaei R, Ebrahimi R. Primary Malignant Melanoma of Uterine Cervix. J Obstet Gynaecol. (2014) 34(1):111. doi: 10.3109/01443615.2013.834297
104. Shrivastava S, Barmon D, Deka P, Ch Kataki A, Choudhary BK. Sarcomatoid Carcinoma of the Cervix With Foci of Malignant Melanoma. J Midlife Health (2014) 5(1):41–4. doi: 10.4103/0976-7800.127792
105. Shenjere P, Fisher C, Rajab R, Patnaik L, Hazell S, Thway K. Melanoma With Rhabdomyosarcomatous Differentiation: Two Further Cases of a Rare Pathologic Pitfall. Int J Surg Pathol (2014) 22(6):512–9. doi: 10.1177/1066896914531817
106. Cetinkaya K, Benzer E, Dervisoglu H. Primary Mucosal Malignant Melanoma of the Cervix: Case Report and Review of the Literature. Tumori. (2015) 101(5):e147–50. doi: 10.5301/tj.5000304
107. Mihmanli V, Toprakci G, Cetinkaya N, Kilickaya A, Kamali G. Primary Malignant Melanoma of the Cervix: A Case Report. Eur J Gynaecol Oncol (2015) 36(5):607–9.
108. Geredeli C, Boruban MC, Poyraz N, Artac M, Aribas A, Koral L. Biatrial Cardiac Metastases in a Patient With Uterine Cervix Malignant Melanoma. Case Rep Cardiol (2015) 2015:958756. doi: 10.1155/2015/958756
109. Arık D, Öge T, Kabukçuoğlu S, Yalçın ÖT, Özalp S. Amelanotic Malignant Melanoma of the Uterine Cervix Diagnosed by Cervical Smear. Diagn Cytopathol. (2016) 44(6):535–7. doi: 10.1002/dc.23471
110. Lee JH, Yun J, Seo JW, Bae GE, Lee JW, Kim SW. Primary Malignant Melanoma of Cervix and Vagina. Obstet Gynecol Sci (2016) 59(5):415–20. doi: 10.5468/ogs.2016.59.5.415
111. Gupta M. Malignant Melanoma of Cervix. BMJ Case Rep (2016) 2016:bcr2016217970. doi: 10.1136/bcr-2016-217970
112. Lim KH, Tay SK, Ng AX, Mantoo S. Primary Melanoma of the Uterine Cervix: A Case Report, With Key Points on Recognition and Pathological Diagnosis. J Low Genit Tract Dis (2017) 21:e1–4. doi: 10.1097/LGT.0000000000000264
113. Julião I, Carvalho SD, Patricio V, Raimundo A. Primary Malignant Melanoma of the Cervix: A Rare Disease. BMJ Case Rep (2017) 2017:bcr2017219361. doi: 10.1136/bcr-2017-219361
114. Noguchi T, Ota N, Mabuchi Y, Yagi S, Minami S, Okuhira H, et al. A Case of Malignant Melanoma of the Uterine Cervix With Disseminated Metastases Throughout the Vaginal Wall. Case Rep Obstet Gynecol. (2017) 2017:5656340. doi: 10.1155/2017/5656340
115. Yuan G, Wu L, Li B, An J. Primary Malignant Melanoma of the Cervix: Report of 14 Cases and Review of Literature. Oncotarget. (2017) 8(42):73162–7. doi: 10.18632/oncotarget.17183
116. Sun H, Chen Y, Chen Y, Liu D, Yan Z, Meng B, et al. Primary Malignant Melanoma of the Cervix: 14 Cases and Literature Overview. Melanoma Res (2018) 28(6):578–85. doi: 10.1097/CMR.0000000000000469
117. Mukhina TS, Dmitriev VN, Tverskoi AV, Khabibullin RR, Moiseenko EA. Pervichnaia Melanoma Sheĭki Matki [Primary Uterine Cervix Melanoma]. Arkh Patol. (2018) 80(6):50–4. doi: 10.17116/patol20188006150
118. Kim MS, Choi CH, Kim TJ, Lee JW, Lee J, Bae DS, et al. Primary Malignant Melanoma of the Uterine Cervix Treated With Pembrolizumab After Radical Surgery: A Case Report and Literature Review. Obstet Gynecol Sci (2018) 61(4):524–8. doi: 10.5468/ogs.2018.61.4.524
119. Srivastava P, Rath S, Hadi R, Husain N. Primary Amelanotic Malignant Melanoma of Cervix Masquerading as Squamous Cell Carcinoma Presenting With Extensive Metastases. BMJ Case Rep (2018) 2018:bcr2018224723. doi: 10.1136/bcr-2018-224723
120. Anko M, Nakamura M, Kobayashi Y, Tsuji K, Nakada S, Nakamura Y, et al. Primary Malignant Melanoma of the Uterine Cervix or Vagina Which Were Successfully Treated With Nivolumab. J Obstet Gynaecol Res (2020) 46(1):190–5. doi: 10.1111/jog.14136
121. Yin C, Yang A, Zhang Y, Tao L, Zou H, Ren Y, et al. Primary Cervical Malignant Melanoma: 2 Cases and a Literature Review. Int J Gynecol Pathol (2019) 38(2):196–203. doi: 10.1097/PGP.0000000000000480
122. Diakosavvas M, Fasoulakis ZN, Kouroupi M, Theodora M, Inagamova L, Tsatsaris G, et al. Primary Malignant Melanoma of the Cervix: A Case Report and a Review of the Literature. Case Rep Oncol Med (2020) 2020:7206786. doi: 10.1155/2020/7206786
123. Pumpure E, Dručka E, Kigitoviča D, Meškauskas R, Isajevs S, Nemiro I, et al. Management of a Primary Malignant Melanoma of Uterine Cervix Stage IVA Patient With Radical Surgery and Adjuvant Oncolytic Virus Rigvir® Therapy: A Case Report. Clin Case Rep (2020) 8(8):1538–43. doi: 10.1002/ccr3.2928
124. Shakeel O, Ullah F, Khalid N, Ali SI, Batool S, Amjad A, et al. Malignant Melanoma of the Female Genital Tract: Experience of an Oncology Center in Pakistan. Cureus. (2020) 12(6):e8484. doi: 10.7759/cureus.8484
125. Pandey G, Dave P, Patel S, Patel B, Arora R, Parekh C, et al. Female Genital Tract Melanoma: Analysis From a Regional Cancer Institute. Turk J Obstet Gynecol. (2020) 17(1):46–51. doi: 10.4274/tjod.galenos.2020.44789
126. Suzuki R, Endo H, Sasaki T, Nakamura T, Yamanaka H, Hosonuma S, et al. Primary Malignant Melanoma of Uterine Cervix Treated With Pembrolizumab as Adjuvant Immunotherapy. Int Cancer Conf J (2021) 10(3):254–8. doi: 10.1007/s13691-021-00477-z
127. Meo N DI, Conforti C, Nan K, Romano A, Degrassi F, Cova MA, et al. Primary Malignant Melanoma of Uterine Cervix. Ital J Dermatol Venerol (2021) 156(Suppl. 1 to No. 6):110–1. doi: 10.23736/S2784-8671.19.06512-X
128. Sone K, Kukita A, Masui Y, Yamada D, Shinozaki-Ushiku A, Kawata A, et al. Recurrent Malignant Melanoma of the Uterine Cervix Treated With Anti-PD-1 Antibodies and Anti-CTLA-4 Antibodies: A Case Report. Mol Clin Oncol (2022) 16(3):63. doi: 10.3892/mco.2022.2496
129. Norris HJ, Taylor HB. Melanomas of the Vagina. Am J Clin Pathol (1966) 46(4):420–6. doi: 10.1093/ajcp/46.4.420
130. Sugiyama VE, Chan JK, Kapp DS. Management of Melanomas of the Female Genital Tract. Curr Opin Oncol (2008) 20(5):565–9. doi: 10.1097/CCO.0b013e32830b0dda
131. Gadducci A, Carinelli S, Guerrieri ME, Aletti GD. Melanoma of the Lower Genital Tract: Prognostic Factors and Treatment Modalities. Gynecol Oncol (2018) 150(1):180–9. doi: 10.1016/j.ygyno.2018.04.562
132. Li R, He Y, Zhang H, Wang J, Liu X, Liu H, et al. Identification and Validation of Immune Molecular Subtypes in Pancreatic Ductal Adenocarcinoma: Implications for Prognosis and Immunotherapy. Front Immunol (2021) 12:690056. doi: 10.3389/fimmu.2021.690056
133. Martinez-Morilla S, Zugazagoitia J, Wong PF, Kluger HM, Rimm DL. Quantitative Analysis of CMTM6 Expression in Tumor Microenvironment in Metastatic Melanoma and Association With Outcome on Immunotherapy. Oncoimmunology. (2020) 10(1):1864909. doi: 10.1080/2162402X.2020.1864909
Keywords: primary malignant melanoma, cervix, integrated analysis, case report, case series
Citation: Min A, Fu A, Huang M, Wang H and Chen H (2022) Primary Malignant Melanoma of the Cervix: An Integrated Analysis of Case Reports and Series. Front. Oncol. 12:913964. doi: 10.3389/fonc.2022.913964
Received: 06 April 2022; Accepted: 27 May 2022;
Published: 22 June 2022.
Edited by:
Anna Myriam Perrone, Sant’Orsola-Malpighi Polyclinic, ItalyCopyright © 2022 Min, Fu, Huang, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huan Chen, NzA4NTEzODRAcXEuY29t
 Aiping Min2
Aiping Min2 Hongjing Wang
Hongjing Wang Huan Chen
Huan Chen