- 1The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Institutes of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou, China
- 2Zhejiang Provincial Research Center for Upper Gastrointestinal Tract Cancer, Zhejiang Cancer Hospital, Hangzhou, China
- 3Zhejiang Key Laboratory of Prevention, Diagnosis and Therapy of Upper Gastrointestinal Cancer, Zhejiang Cancer Hospital, Hangzhou, China
- 4First Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, China
- 5Department of Integrated Chinese and Western Medicine, Institute of Basic Medicine and Cancer (IBMC), The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Chinese Academy of Sciences, Hangzhou, China
- 6Key Laboratory of Traditional Chinese Medicine Oncology, Zhejiang Cancer Hospital, Hangzhou, China
- 7Key Laboratory of Head and Neck Cancer Translational Research of Zhejiang Province, Hangzhou, China
The protein encoded by CD3D is part of the T-cell receptor/CD3 complex (TCR/CD3 complex) and is involved in T-cell development and signal transduction. Previous studies have shown that CD3D is associated with prognosis and treatment response in breast, colorectal, and liver cancer. However, the expression and clinical significance of CD3D in gastric cancer are not clear. In this study, we collected 488 gastric cancer tissues and 430 paired adjacent tissues to perform tissue microarrays (TMAs). Then, immunohistochemical staining of CD3D, CD3, CD4, CD8 and PD-L1 was conducted to investigate the expression of CD3D in gastric cancer and the correlation between the expression of CD3D and tumor infiltrating lymphocytes (TILs) and PD-L1. The results showed that CD3D was highly expressed in gastric cancer tissues compared with paracancerous tissues (P<0.000). Univariate and multivariate analyses showed that CD3D was an independent good prognostic factor for gastric cancer (P=0.004, HR=0.677, 95%CI: 0.510-0.898 for univariate analyses; P=0.046, HR=0.687, 95%CI: 0.474-0.994 for multivariate analyses). In addition, CD3D was negatively correlated with the tumor location, Borrmann type and distant metastasis (P=0.012 for tumor location; P=0.007 for Borrmann type; P=0.027 for distant metastasis). In addition, the expression of CD3D was highly positively correlated with the expression of CD3, CD4, CD8, and PD-L1, and the combination of CD3D with CD3, CD4, CD8 and PD-L1 predicted the best prognosis (P=0.043). In summary, CD3D may play an important regulatory role in the tumor immune microenvironment of gastric cancer and may serve as a potential indicator of prognosis and immunotherapy response.
Introduction
Gastric cancer is one of the most common malignant tumors in the world. According to the latest data, there are more than 1 million new cases of gastric cancer in the world, and approximately 770,000 people have died of gastric cancer. Mortality and morbidity rank 4th and 5th, respectively, and seriously threaten human life and health (1). At present, the diagnosis of gastric cancer mainly relies on endoscopy and tissue biopsy. However, due to its invasiveness and high cost, more than 60% of patients with gastric cancer are in the middle or advanced stage at the time of diagnosis, and the prognosis is poor (2). At present, surgery is the main treatment for early gastric cancer, and comprehensive treatments such as surgery, radiotherapy and chemotherapy, targeted therapy, and immunotherapy are the main strategies to combat advanced gastric cancer. However, due to the lack of therapeutic targets and the high tumor heterogeneity of gastric cancer, the population of patients who benefit from targeted therapy and immunotherapy is limited. Therefore, it is urgent to improve the level of early diagnosis and identify new therapeutic targets.
The protein product of the CD3D gene is part of the T-cell receptor/CD3 complex (TCR/CD3 complex) and is involved in T-cell development and signal transduction (3). The integrity of the TCR/CD3 complex is crucial for the effector and regulatory functions of peripheral T lymphocytes (4). An increasing number of studies have shown that CD3D is closely related to the occurrence, development, prognosis, immune microenvironment and treatment response of tumors. Chen et al. have found that CD3D was significantly negatively correlated with PD1 and could predict immunochemotherapy response in diffuse large B-cell lymphoma (5). Kang et al. have demonstrated that CD3D expression reduced the prognostic risk of bladder cancer (6). Saiz-Ladera et al. have confirmed that CD2, CD3D, CD3E and CXCR6 combined gene expression was associated with an improvement in the outcomes of head and neck squamous cell carcinoma patients and an increase in infiltrating immune effector cells (7). However, the expression and clinical significance of CD3D in gastric cancer are still unclear and are worthy of further study.
In this study, we collected 488 gastric cancer tissues and 430 paired adjacent tissues to explore the difference in CD3D expression between tumor tissues and paracancerous tissues, as well as the correlation between the expression of CD3D and clinical information, prognosis, tumor-infiltrating lymph nodes (CD3+ T cells, CD4+ T cells and CD8+ T cells) and PD-L1. The aim of this study was to clarify the value of CD3D in the diagnosis and prognosis of gastric cancer and its role in the tumor immune microenvironment.
Materials and Methods
Clinical Specimens
We retrospectively collected 488 patients with gastric cancer treated at The Affiliated Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital) from January 2013 to December 2017. The inclusion criteria were as follows: patients with gastric cancer confirmed by pathological diagnosis; and no prior antitumor treatment, such as chemoradiotherapy, targeted therapy and immunotherapy. The exclusion criteria were as follows: patients with gastric cancer and other types of malignant tumors at the same time; patients with metastasis from other tumor species to the stomach; and patients who received prior antitumor treatment. Overall survival (OS) was defined as the duration from the initial surgery to the date of death or the last follow-up.
All clinical information of the participants was collected, including age, gender, height, weight, family history, smoking status, alcohol consumption, TNM staging, blood tumor markers, etc. Pathological staging was based on the American Joint Committee on Cancer 8th edition staging (8). The study was approved by the Research Ethics Committee of the Zhejiang Cancer Hospital (IRB-2021-431).
Tissue Microarray (TMA) Construction and Immunohistochemistry Analysis
We collected 488 formalin-fixed, paraffin-embedded gastric cancer tissue specimens and 430 corresponding adjacent noncancerous tissue specimens. Two pathologists independently selected the most representative tumors and paired adjacent tissues, and TMAs were performed as described previously (9). For immunohistochemistry assays, after treatment with 3% H2O2/methyl alcohol solution for 10 min at room temperature, 5% normal goat serum buffer was used to block the tissue at 37 °C for 30 min. Slides were then incubated with primary antibodies at 4°C overnight. After washing, the slides were incubated with biotin-labeled goat anti-rabbit IgG and HRP-conjugated streptavidin at 37°C for 1 h. Immunoreaction was visualized by diaminobenzidine (DAB) (Cat# ZLI-9065, ZSGB-BIO Corp., Shanghai, China). After DAB staining, all tissues were counterstained with hematoxylin (Cat# ZLI-9609 ZSGB-BIO Corp., Shanghai, China), dehydrated and then blocked. The antibodies against CD3D (Cat# 109531), CD3 (Cat# 16669), CD4 (Cat# 133616), and CD8 (Cat# 17147) were purchased from Abcam (Cambridge, UK). PD-L1 (Cat# SK006) was purchased from Dako Denmark A/S (Copenhagen, Denmark).
The immunohistochemical staining results were interpreted by two experienced pathologists. The expression of PD-L1 was evaluated by the combined positive score (CPS) score (CPS=PD-L1-positive cells (tumor cells, lymphocytes, macrophages)/total tumor cells). The CPS evaluation criteria were as follows: tumor cells with membrane staining at any intensity directly related to tumor cells, and membrane/cytoplasmic staining of lymphocytes/macrophages. These stained cells accounted for a percentage of the total tumor cells. The stained cells should exclude all necrotic cells, mesenchymal cells, carcinoma in situ and other immune cells (including but not limited to neutrophils, eosinophils and plasma cells). The expression of CD3D, CD3, CD4, and CD8 was divided into a high-expression group and a low-expression group according to the median number of positive cells (10, 11). The median numbers of CD3D-, CD3-, CD4-, and CD8-positive cells were 120, 190, 30 and 110, respectively.
Statistical Analysis
SPSS 23.0 software was used for statistical analysis of all data, and the measurement data are expressed as the median ± standard error. GraphPad Prism 8.3.0 was used for mapping. The relationship between CD3D expression and clinicopathological features, tumor-infiltrating lymphocytes and PD-L1 expression was analyzed by the chi-square test or Fisher’s exact test. Kaplan–Meier analysis was used to draw survival curves, and univariate and multivariate Cox regression analyses were used to determine the prognostic factors of gastric cancer. P<0.05 indicates a significant difference.
Results
Characteristics of the Participants
A total of 488 gastric cancer patients were recruited, ranging in age from 28 to 91, with an average age of 64.05 ± 0.48, including 360 males (73.77%) and 128 females (26.23%), which is consistent with the epidemiological situation of gastric cancer, indicating that the included cases were generally representative. In terms of TNM staging, 22 patients had stage I, 70 patients had stage II, 357 patients had stage III, and 32 patients had stage IV disease. In terms of the pathological types, gastric adenocarcinoma was the main type, accounting for 454 cases (93.03%), and only 34 (6.97%) were of other types. More detailed information is shown in Table 1 and Table 1S in Supplementary Material.
CD3D Is Highly Expressed in Gastric Cancer Tissues and Predicts a Good Prognosis
As shown in Figure 1A, CD3D was mainly expressed on lymphocytes. The average number of CD3D-positive cells in the tumor tissues was 195.45 ± 10.96, while that in the paracancerous tissues was 107.23 ± 6.05, indicating a significant difference (Figure 1B and Table 2, P<0.000). Kaplan–Meier survival analysis indicated that the 5-year overall survival rate of patients with high CD3D expression in tumor tissues was 57.4%, while that of patients with low CD3D expression was 46.1%, showing a significant difference (Figure 1C, P=0.006). We also analyzed the effect of CD3D expression in paracancerous tissues on prognosis, and the results showed that the expression of CD3D in paracancerous tissues was not associated with prognosis (Figure 1D, P=0.154).
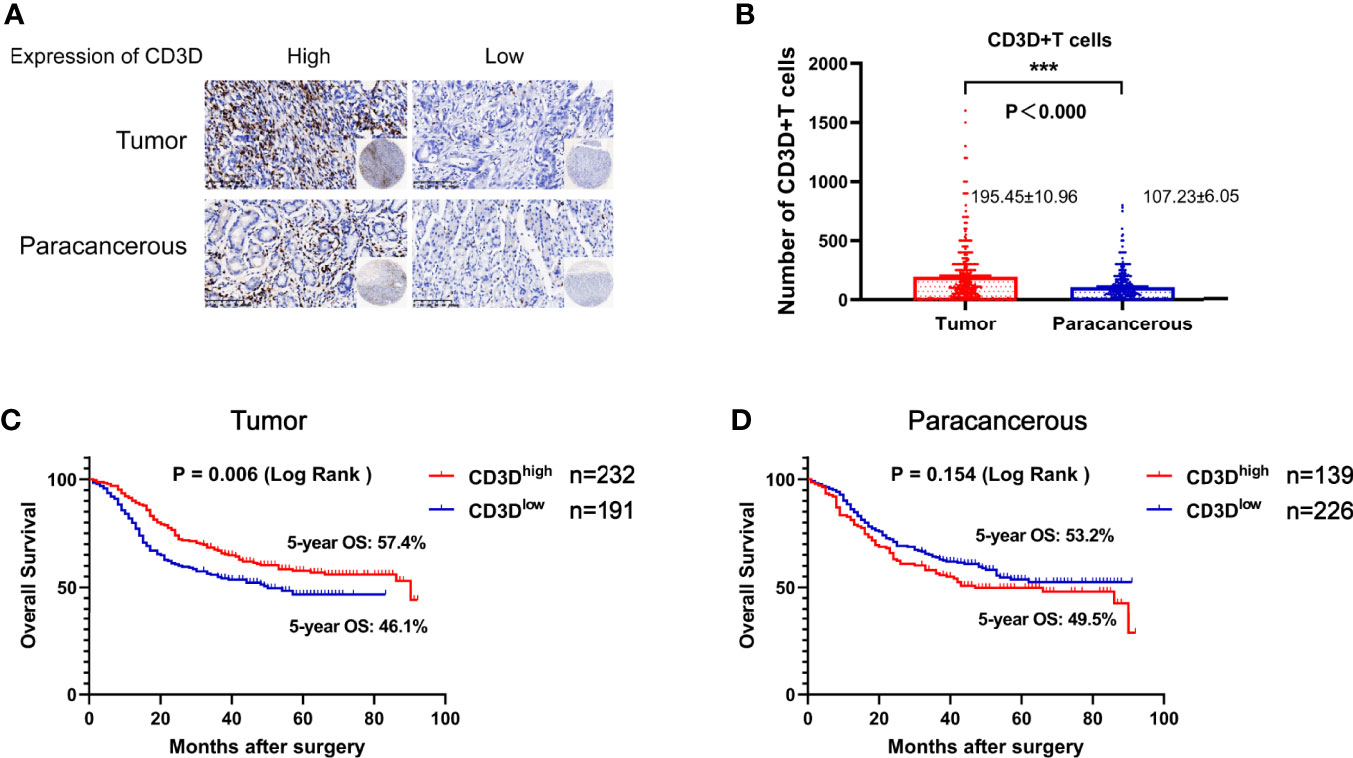
Figure 1 CD3D is overexpressed in gastric cancer tissues compared with adjacent tissues, and a high expression of CD3D is associated with a good prognosis in gastric cancer patients. (A) Representative images of CD3D staining in TMAs as determined by immunohistochemical analysis. (B) Differential expression of CD3D in gastric cancer tissue and paracancerous tissue. (C) The overall survival (OS) curves of gastric cancer patients with different CD3D expression levels in tumor tissues as determined by Kaplan–Meier analysis (log-rank test). (D) The OS curves of gastric cancer patients with different CD3D expression levels in paracancerous tissues as determined by Kaplan–Meier analysis (log-rank test). ***p < 0.001.
Expression of CD3D Is Correlated With the Tumor Location, Borrmann Type and Distant Metastasis
To further observe the significance of CD3D expression in gastric cancer, we analyzed the correlation between its expression and all clinical information, such as age, gender, smoking status, and alcohol consumption. We found that The expression of CD3D was correlated with tumor location, and is significantly higher in proximal gastric cancer than in distal gastric cancer (Table 3, P=0.012). Besides, the CD3D positivity rate was 65.63% among patients with Borrmann type I+II but only 49.55% among those with type III+IV, showing a significant difference (Table 3, P=0.004). In addition, the CD3D positivity rate was 31.25% in gastric cancer patients with distant metastases, while it reached as high as 51.45% in patients without distant metastases, indicating a significant difference (Table 3, P=0.027). To summarize, the expression of CD3D was found to be significantly reduced in patients with distant metastasis or in patients with Borrmann type III+IV. In addition, the expression of CD3D was not associated with clinical characteristics such as age, gender, height, weight, family history, smoking status, alcohol consumption, tumor size, or serum tumor markers (Table 3).
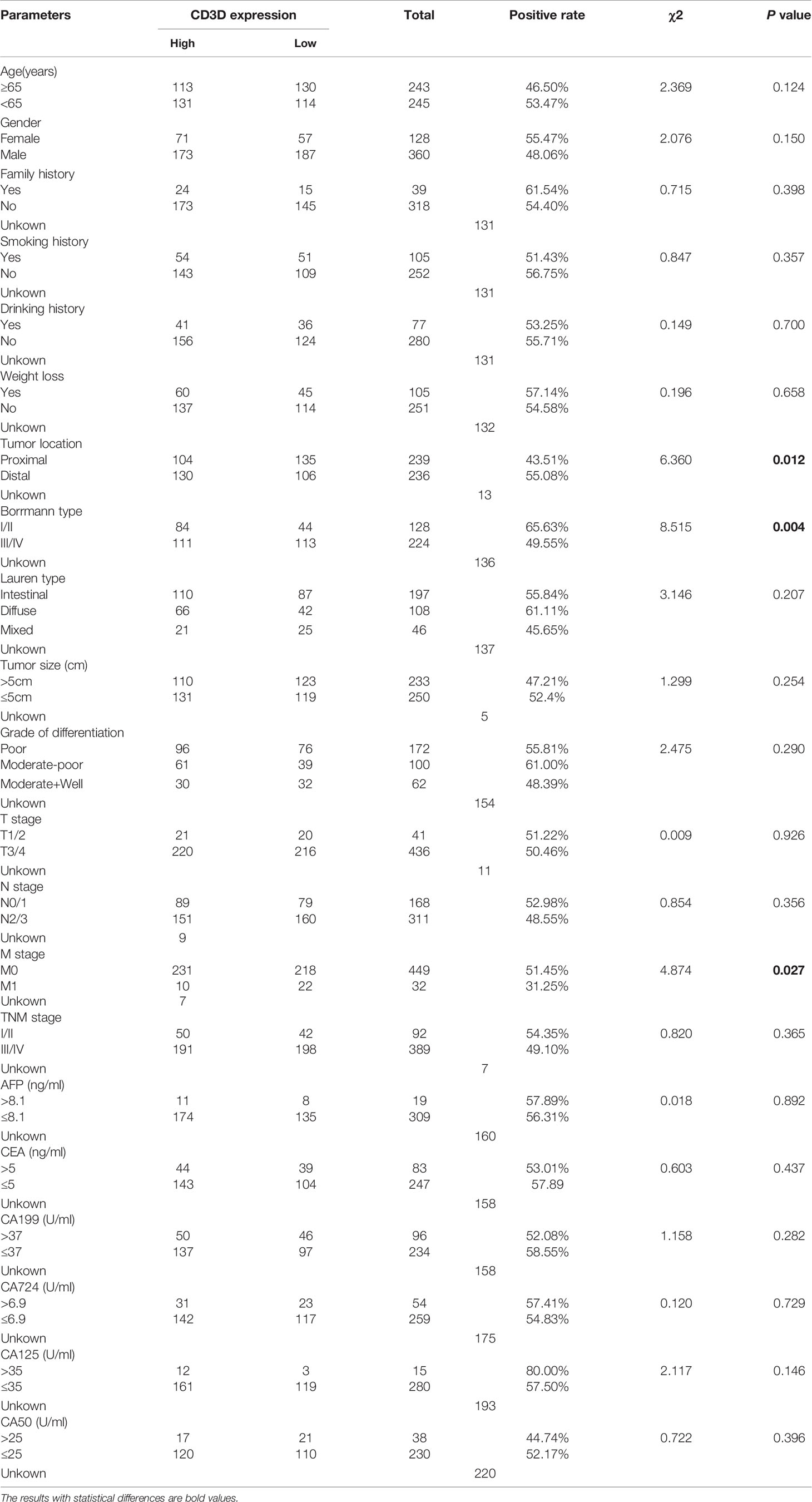
Table 3 The correlation between CD3D expression and clinicopathological characteristics in gastric cancer.
CD3D Regulates the Immune Microenvironment of Gastric Cancer
Some studies have shown that CD3D is involved in regulating the tumor immune microenvironment in cervical cancer, liver cancer, breast cancer and other tumors (12–14). Therefore, we further detected the expression of CD3, CD4, CD8 and PD-L1 in the tumor tissues of the included gastric cancer patients and evaluated their relationship with CD3D to explore the regulatory role of CD3D in the immune microenvironment of gastric cancer. We divided the patients into a high-expression group and a low-expression group according to the median number of cells positive for CD3, CD4 and CD8 (Figures 2A, C, E). For PD-L1 interpretation, a CPS score greater than or equal to 10 was considered positive, and a CPS score less than 10 was considered negative (15–17) (Figure 2G). Kaplan–Meier survival analysis showed that the expression of CD4 and CD8 was correlated with the survival of gastric cancer patients, while the expression of CD3 and PD-L1 was not (Figures 2B, D, F, H). The 5-year overall survival rates of the CD4 and CD8 high-expression groups were 60.2% and 60.3%, respectively, while those of the CD4 and CD8 low-expression groups were 48.2% and 48.0%, respectively (Figure 2D, P=0.024 and 2F, P=0.034).
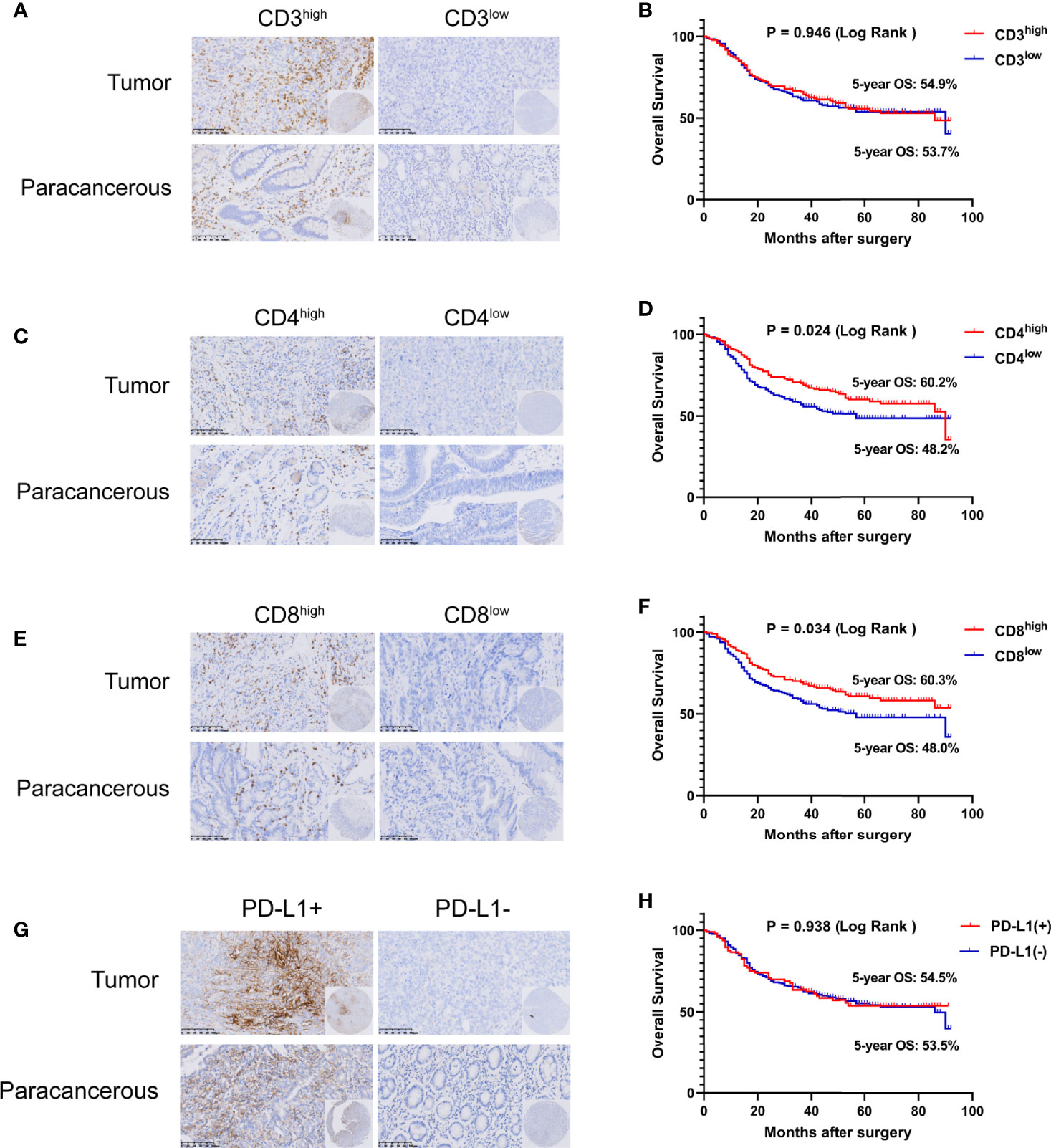
Figure 2 Tumor infiltrating lymphocytes (TILs) and PD-L1 are closely related to the prognosis of gastric cancer. Representative images of CD3 (A), CD4 (C), CD8 (E), and PD-L1 (G) staining in TMAs as determined by immunohistochemical analysis. The overall survival (OS) curves of gastric cancer patients with different CD3 (B), CD4 (D), CD8 (F), and PD-L1 (H) expression levels in tumor tissues as determined by Kaplan–Meier analysis (log-rank test).
As shown in Figure 3, the expression of CD3D was closely related to that of CD3, CD4, CD8 and PD-L1. We found that CD3D was positively correlated with the expression of CD3, CD4, and CD8, and the expression of CD3, CD4, and CD8 in the CD3D high-expression group was higher than that in the CD3D low-expression group, with significant differences observed (Figures 3A–C, all P<0.000). Again, Pearson correlation coefficients showed the same result (Table 4). In addition, we found that CD3D was positively correlated with the expression of PD-L1. The PD-L1 positivity rate was 34.34% in the group with high CD3D expression but only 17.5% in the group with low CD3D expression (Figure 3D, P<0.000). The Pearson correlation coefficient also found a positive correlation between CD3D and PD-L1 expression (Table 4). In conclusion, CD3D may play an important regulatory role in the tumor immune microenvironment by regulating tumor infiltrating lymphocytes (TILs) and immune checkpoints and may be a potential immunotherapy target.
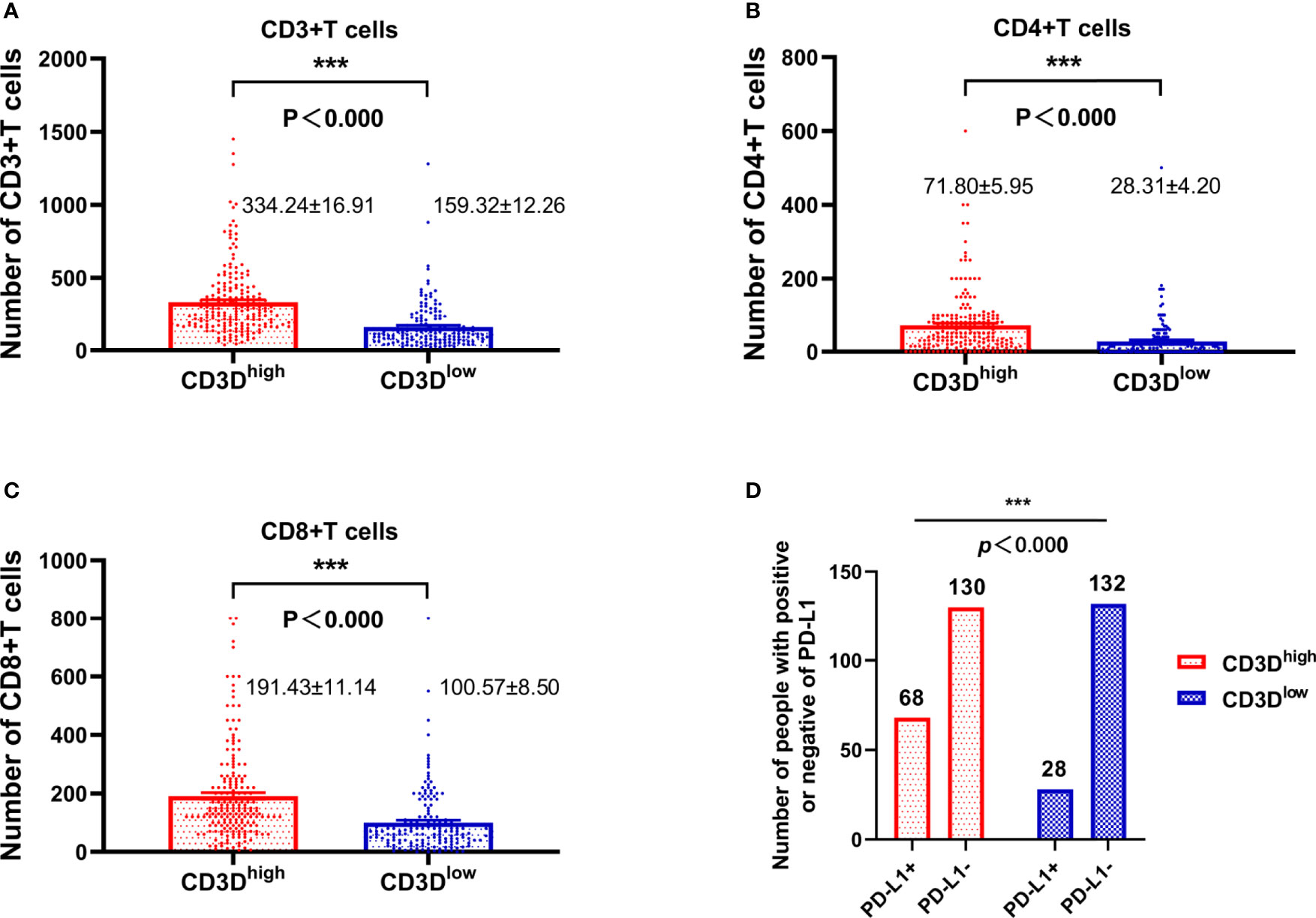
Figure 3 The expression of CD3D is highly positively correlated with the expression of CD3, CD4, CD8, and PD-L1. (A) The relationship between the expression of CD3D and CD3. (B) The relationship between the expression of CD3D and CD4. (C) The relationship between the expression of CD3D and CD8. (D) Relationship between the expression of CD3D and PD-L1. ***p < 0.001.
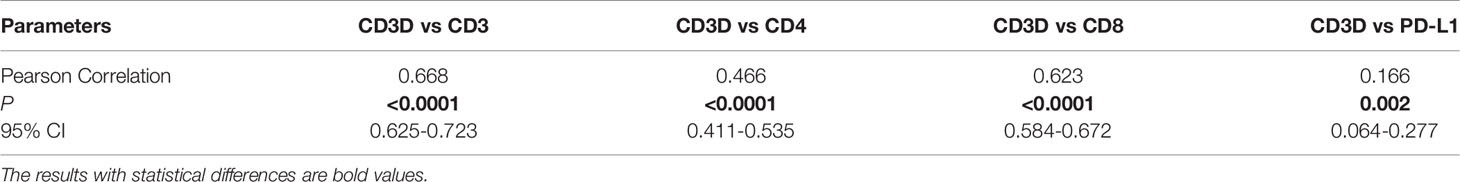
Table 4 The pearson correlation coefficients between CD3D expression and CD3, CD4, CD8 and PD-L1 expression in gastric cancer.
CD3Dhigh Combined With CD4high, CD8 high and PD-L1- Predicts the Best Prognosis of Gastric Cancer
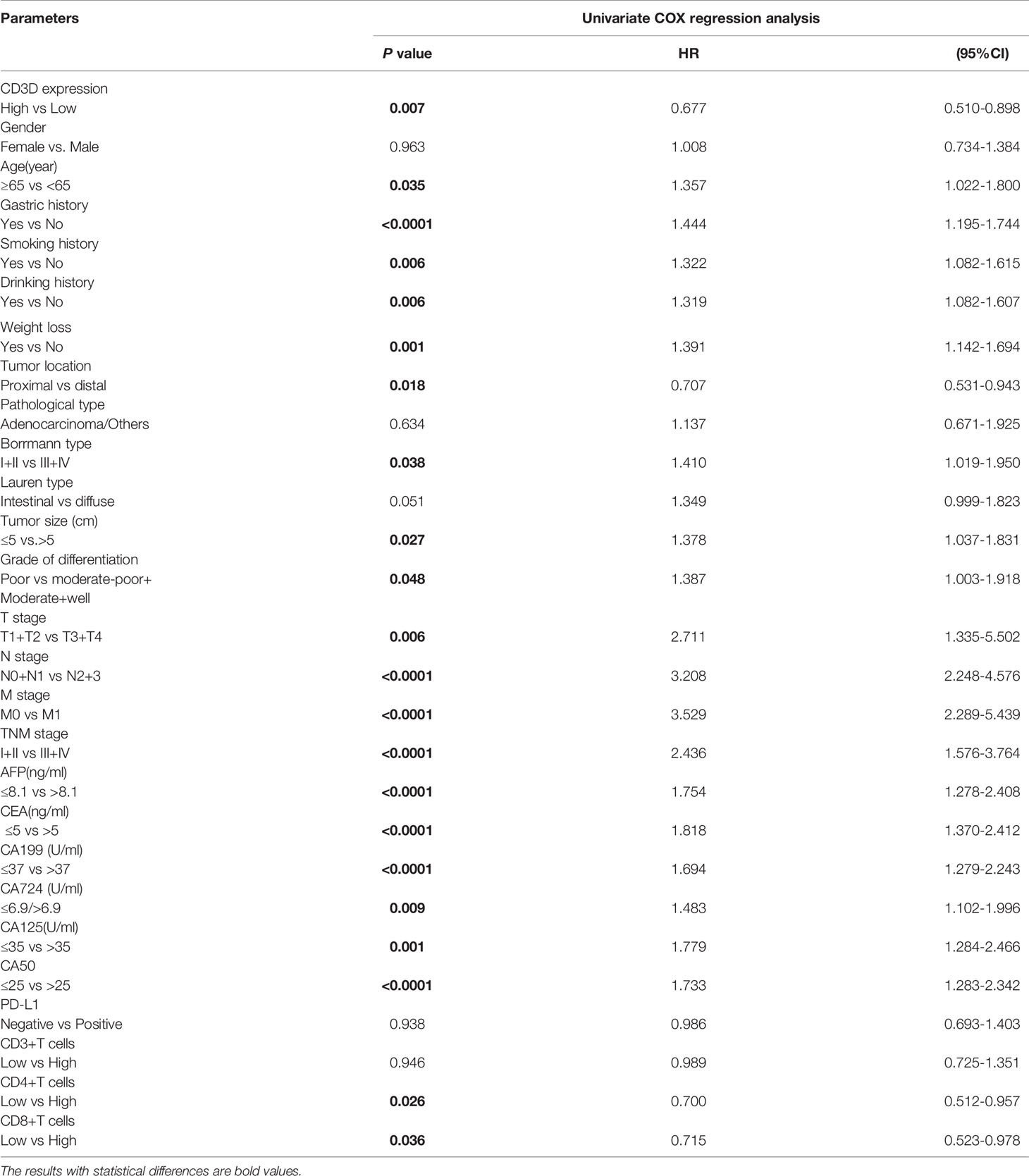
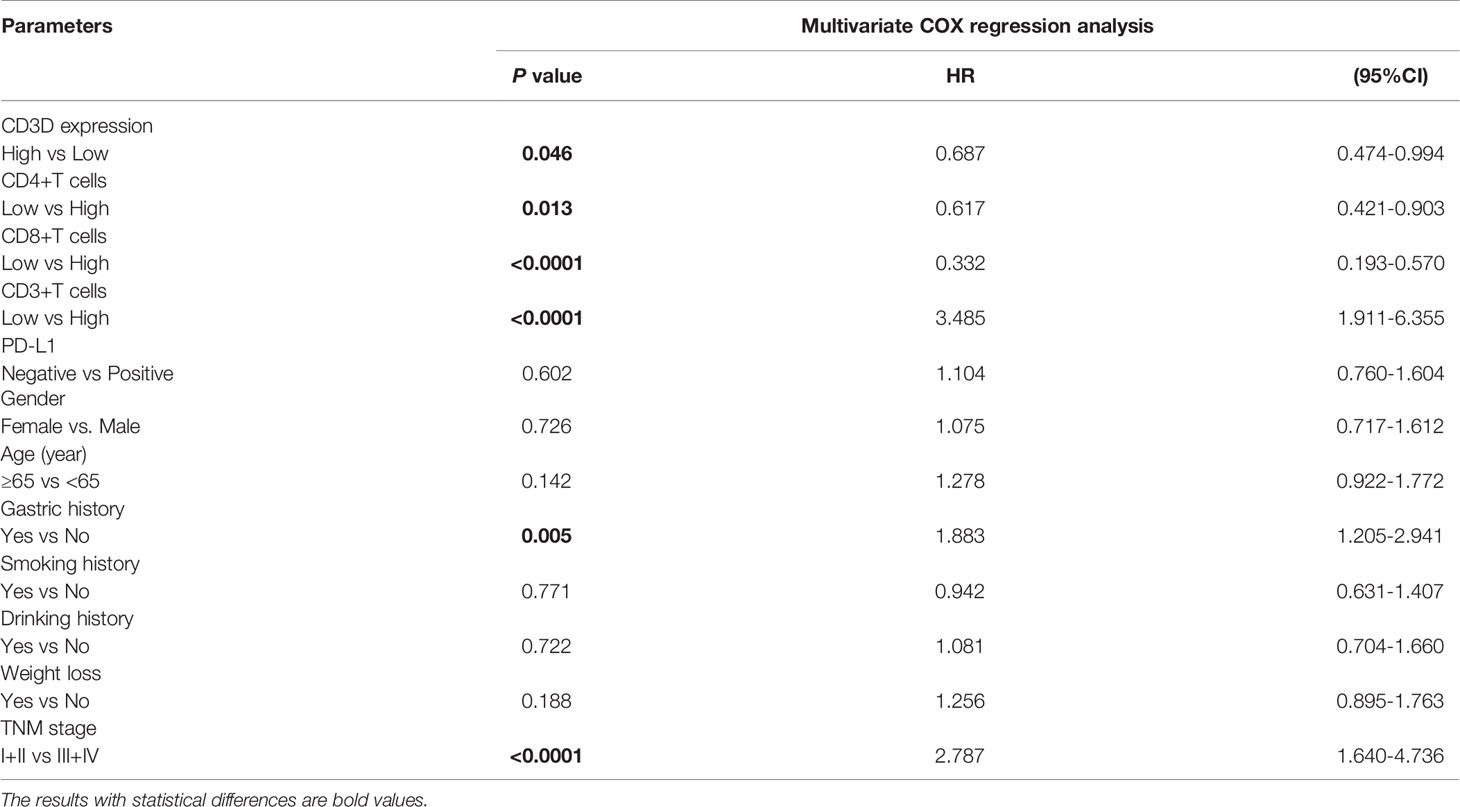
Risk factors were determined by univariate and multivariate logistic regression analyses. Univariate logistic regression analysis found that CD3D, CD8, CD4, age, family history, tumor location, tumor size, grade of differentiation and TNM stage were all prognostic factors for gastric cancer (Table 5). In addition, we further carried out multivariate analysis with these important factors, and the results showed that CD3D, CD4, and CD8 were independent prognostic factors for gastric cancer in both the univariate and multivariate analyses (Table 6).
Since CD3D was positively correlated with the expression of CD3, CD4, CD8 and PD-L1, the effect of CD3D in combination with these factors on the prognosis of gastric cancer was analyzed. As shown in Figures 4A–D, the 5-year overall survival rate of CD3Dhigh + CD3high patients was 59.5%, that of CD3Dhigh + CD4high patients was 65.4%, that of CD3Dhigh + CD8high patients was 63.1%, and that of CD3Dhigh + PD-L1- was 60.7%, which was higher than all other groups. As shown in Figures 4E–H, the 5-year overall survival rate of CD3Dhigh + CD4high + CD8high patients was 68.0%, that of CD3Dhigh + CD4high + CD8high + CD3high was 66.1%, that of CD3Dhigh + CD4high + CD8high + PD-L1- was 69.8%, and that of CD3Dhigh + CD4high + CD8high + CD3 high + PD-L1- was 67.7%. Thus, the combination of CD3Dhigh with CD4high, CD8 high and PD-L1- predicts the best prognosis of gastric cancer.
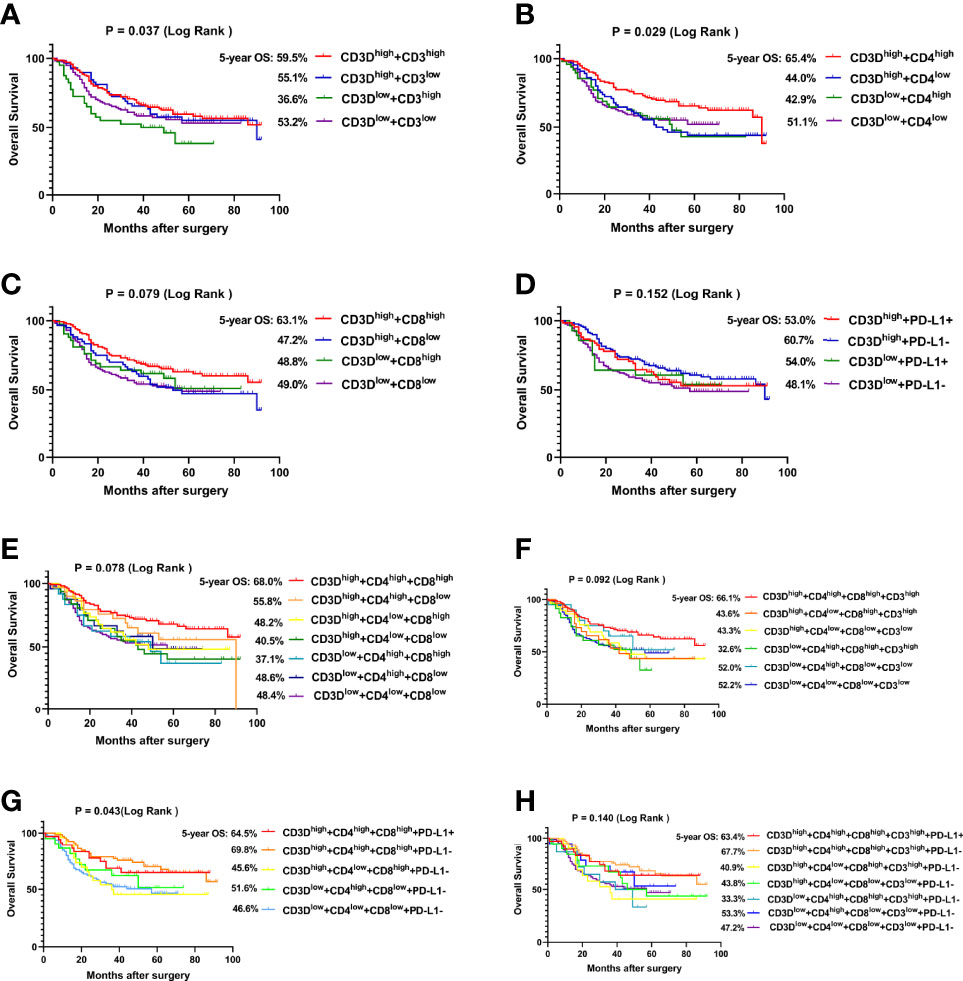
Figure 4 CD3Dhigh combined with CD4high, CD8high and PD-L1- predicts the best prognosis of gastric cancer. (A) The overall survival (OS) curves of gastric cancer patients with different CD3D combined with CD3 expression levels in tumor tissues. (B) The OS curves of gastric cancer patients with different CD3D and CD4 expression levels in tumor tissues. (C) The OS curves of gastric cancer patients with different CD3D and CD8 expression levels in tumor tissues. (D) The OS curves of gastric cancer patients with different CD3D combined with PD-L1 expression levels in tumor tissues. (E) The OS curves of gastric cancer patients with different CD3D, CD4 and CD8 expression levels in tumor tissues. (F) The OS curves of gastric cancer patients with different CD3D, CD4, CD8 and CD3 expression levels in tumor tissues. (G) The OS curves of gastric cancer patients with different CD3D, CD4, CD8 and PD-L1 expression levels in tumor tissues. (H) The OS curves of gastric cancer patients with different CD3D, CD4, CD8, CD3 and PD-L1 expression levels in tumor tissues. E-H: Because of the large number of groups, we only show some groups with large number of cases.
Discussion
The exploration of gene expression profiles, the tumor mutational burden, PD-L1 and other biomarkers have yielded great progress in predicting patient prognosis and immunotherapy efficacy, but there are still some problems that make it difficult to meet clinical needs (18, 19). In addition to clarifying the role of TILs, a comprehensive understanding of the tumor immune microenvironment can help to guide individualized immunotherapy, making it a hot spot in cancer immunotherapy research (20). Multiple studies have shown that TILs are often associated with better treatment response and prognosis (21–23). However, due to the heterogeneity of gastric cancer and the complexity of the immune microenvironment, PD-L1-based immune checkpoint inhibitors have limited benefits in the treatment of gastric cancer (24). Moreover, TILs have also been found to play a limited role in the prognosis and efficacy prediction of gastric cancer. Therefore, it is urgent to find new targets to aid in diagnosis and in predicting prognosis and immunotherapy response to improve the diagnosis and treatment of gastric cancer patients.
CD3D is part of the T-cell receptor/CD3 complex (TCR/CD3 complex), and defects in this gene cause severe combined immunodeficiency (25). CD3D has been found to be associated with prognosis in various tumors. Shi et al. have demonstrated that the CD3D/CD4 ratio was a stable independent prognostic factor in muscle-invasive bladder cancer (26). In addition, other studies have shown that high CD3D expression is strongly associated with poor survival in breast carcinoma (27). In contrast, in patients with colorectal cancer, high CD3D expression was found to predict better clinical outcomes (28). In other words, CD3D may play completely opposite roles in different tumors. In our study, we found that CD3D was highly expressed in gastric cancer tissues compared with paracancerous tissues. Univariate and multivariate analyses showed that CD3D was an independent prognostic factor for gastric cancer, and patients with high expression of CD3D had a better prognosis. In addition, CD3D was negatively correlated with the Borrmann type and distant metastasis.
TILs refer to lymphocytes that leave the blood stream to enter the tumor, constituting an important part of the tumor microenvironment (29). They have been found to have an important role in predicting tumor prognosis and have even been employed as a means of cell therapy (30). The PD-1/PD-L1 signaling pathway has been shown to be important for tumor immunosuppression, inhibiting the activation of T lymphocytes and enhancing the immune tolerance of tumor cells, exhibiting an important regulatory role in the tumor immune microenvironment. Many studies have shown that the expression of TILs (CD3, CD4, CD8) is associated with a favorable prognosis in gastric cancer (31–33). However, the relationship between PD-L1 and gastric cancer prognosis is controversial. Some studies have shown that positive PD-L1 expression is significantly related to poor overall survival (34–36), but some studies have failed to confirm this finding (37, 38). In our study, we found that CD4 and CD8 were good prognostic factors for gastric cancer in both univariate and multivariate analyses. CD3 was not found to be associated with prognosis in the univariate analysis but was identified as a good prognostic factor for gastric cancer in the multivariate analysis. In contrast, PD-L1 was not associated with gastric cancer prognosis in either the univariate or multivariate analysis.
Previous studies have shown that CD3D can modulate the tumor immune microenvironment and affect the prognosis and immune response of breast cancer and cervical squamous cell carcinoma (39–41). In gastric cancer patients, we found that the expression of CD3D was highly positively correlated with the expression of CD3, CD4, CD8, and PD-L1. In addition, the combination of CD3D with CD3, CD4, CD8 and PD-L1 predicted the best prognosis, with the 5-year survival rate of the CD3Dhigh + CD4high + CD8high + PD-L1- group being the highest, reaching 69.8%. Thus, CD3D may play an important role in the regulation of the immune microenvironment in gastric cancer.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The study was approved by the research ethics committee of Cancer Hospital of Zhejiang Cancer Hospital (IRB-2021-431). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
QY, BZ, and JQ conceived the study and acquired the funding. LY and JX carried out clinical research, collected clinical samples, analyzed clinical data, and wrote articles. YS, ZJ, ZB, YW, PY, and YX participated in clinical sample collection. All authors have read and approved the final manuscript.
Funding
This study was supported by the National Key R&D Program of China (2021YFA0910100), Natural Science Foundation of Zhejiang Province (HDMY22H160008), Program of Zhejiang Provincial TCM Sci-tech Plan (2018ZY006), Medical Science and Technology Project of Zhejiang Province (2022KY114, WKJ-ZJ-2104), Zhejiang Provincial Research Center for Upper Gastrointestinal Tract Cancer (JBZX-202006), Science and Technology Projects of Zhejiang Province (2019C03049), and National Natural Science Foundation of China (82074245, 81973634).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciate the great technical support from the Zhejiang Provincial Research Center for Upper Gastrointestinal Tract Cancer and Zhejiang Key Lab of Prevention, Diagnosis and Therapy of Upper Gastrointestinal Cancer.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.913670/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric Cancer. Lancet (London England) (2020) 396(10251):635–48. doi: 10.1016/s0140-6736(20)31288-5
3. Riemondy KA, Ransom M, Alderman C, Gillen AE, Fu R, Finlay-Schultz J, et al. Recovery and Analysis of Transcriptome Subsets From Pooled Single-Cell RNA-Seq Libraries. Nucleic Acids Res (2019) 47(4):e20. doi: 10.1093/nar/gky1204
4. Rowe JH, Delmonte OM, Keles S, Stadinski BD, Dobbs AK, Henderson LA, et al. Patients With CD3G Mutations Reveal a Role for Human CD3γ in T(reg) Diversity and Suppressive Function. Blood (2018) 131(21):2335–44. doi: 10.1182/blood-2018-02-835561
5. Chen W, Liang W, He Y, Liu C, Chen H, Lv P, et al. Immune Microenvironment-Related Gene Mapping Predicts Immunochemotherapy Response and Prognosis in Diffuse Large B-Cell Lymphoma. Med Oncol (Northwood London England) (2022) 39(4):44. doi: 10.1007/s12032-021-01642-3
6. Kang Z, Li W, Yu YH, Che M, Yang ML, Len JJ, et al. Identification of Immune-Related Genes Associated With Bladder Cancer Based on Immunological Characteristics and Their Correlation With the Prognosis. Front Genet (2021) 12:763590. doi: 10.3389/fgene.2021.763590
7. Saiz-Ladera C, Baliu-Piqué M, Cimas FJ, Manzano A, García-Barberán V, Camarero SC, et al. Transcriptomic Correlates of Immunologic Activation in Head and Neck and Cervical Cancer. Front Oncol (2021) 11:714550. doi: 10.3389/fonc.2021.714550
8. Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, et al. Melanoma Staging: Evidence-Based Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA: Cancer J Clin (2017) 67(6):472–92. doi: 10.3322/caac.21409
9. Yuan L, Mo SW, Xu ZY, Lv H, Xu JL, Guo KB, et al. P-MEK Expression Predicts Prognosis of Patients With Adenocarcinoma of Esophagogastric Junction (AEG) and Plays a Role in Anti-AEG Efficacy of Huaier. Pharmacol Res (2021) 165:105411. doi: 10.1016/j.phrs.2020.105411
10. Kuwahara T, Hazama S, Suzuki N, Yoshida S, Tomochika S, Nakagami Y, et al. Intratumoural-Infiltrating CD4 + and Foxp3 + T Cells as Strong Positive Predictive Markers for the Prognosis of Resectable Colorectal Cancer. Br J cancer (2019) 121(8):659–65. doi: 10.1038/s41416-019-0559-6
11. Leary A, Genestie C, Blanc-Durand F, Gouy S, Dunant A, Maulard A, et al. Neoadjuvant Chemotherapy Alters the Balance of Effector to Suppressor Immune Cells in Advanced Ovarian Cancer. Cancer immunology immunotherapy: CII (2021) 70(2):519–31. doi: 10.1007/s00262-020-02670-0
12. Xu F, Shen J, Xu S. Multi-Omics Data Analyses Construct a Six Immune-Related Genes Prognostic Model for Cervical Cancer in Tumor Microenvironment. Front Genet (2021) 12:663617. doi: 10.3389/fgene.2021.663617
13. Wang Z, Chen S, Wang G, Li S, Qin X. CDCA3 Is a Novel Prognostic Biomarker Associated With Immune Infiltration in Hepatocellular Carcinoma. BioMed Res Int (2021) 2021:6622437. doi: 10.1155/2021/6622437
14. Peng L, Hayatullah G, Zhou H, Chang S, Liu L, Qiu H, et al. Tumor Microenvironment Characterization in Cervical Cancer Identifies Prognostic Relevant Gene Signatures. PloS One (2021) 16(4):e0249374. doi: 10.1371/journal.pone.0249374
15. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab Plus Chemotherapy Versus Chemotherapy Alone for First-Line Treatment of Advanced Oesophageal Cancer (KEYNOTE-590): A Randomised, Placebo-Controlled, Phase 3 Study. Lancet (London England) (2021) 398(10302):759–71. doi: 10.1016/s0140-6736(21)01234-4
16. Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-Line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol (2020) 6(10):1571–80. doi: 10.1001/jamaoncol.2020.3370
17. Carter JM, Polley MC, Leon-Ferre RA, Sinnwell J, Thompson KJ, Wang X, et al. Characteristics and Spatially Defined Immune (Micro)Landscapes of Early-Stage PD-L1-Positive Triple-Negative Breast Cancer. Clin Cancer Res (2021) 27(20):5628–37. doi: 10.1158/1078-0432.Ccr-21-0343
18. Zhang Y, Wang S, Zhang J, Liu C, Li X, Guo W, et al. Elucidating Minimal Residual Disease of Paediatric B-Cell Acute Lymphoblastic Leukaemia by Single-Cell Analysis. Nat Cell Biol (2022) 24(2):242–52. doi: 10.1038/s41556-021-00814-7
19. Long J, Wang D, Wang A, Chen P, Lin Y, Bian J, et al. A Mutation-Based Gene Set Predicts Survival Benefit After Immunotherapy Across Multiple Cancers and Reveals the Immune Response Landscape. Genome Med (2022) 14(1):20. doi: 10.1186/s13073-022-01024-y
20. Schmidt M, Heimes AS. Immunomodulating Therapies in Breast Cancer-From Prognosis to Clinical Practice. Cancers (2021) 13(19):4883. doi: 10.3390/cancers13194883
21. Zhang X, Zhu X, Tang K, Zhao Y, Lu Z, Feng Q. DDTNet: A Dense Dual-Task Network for Tumor-Infiltrating Lymphocyte Detection and Segmentation in Histopathological Images of Breast Cancer. Med image analysis (2022) 78:102415. doi: 10.1016/j.media.2022.102415
22. Orhan A, Khesrawi F, Tvilling Madsen M, Peuliche Vogelsang R, Dohrn N, Kanstrup Fiehn AM, et al. Tumor-Infiltrating Lymphocytes as Biomarkers of Treatment Response and Long-Term Survival in Patients With Rectal Cancer: A Systematic Review and Meta-Analysis. Cancers (2022) 14(3):636. doi: 10.3390/cancers14030636
23. Schiza A, Thurfjell V, Stenmark Tullberg A, Olofsson H, Lindberg A, Holmberg E, et al. Tumour-Infiltrating Lymphocytes Add Prognostic Information for Patients With Low-Risk DCIS: Findings From the SweDCIS Randomised Radiotherapy Trial. Eur J Cancer (Oxford England: 1990) (2022) 168:128–37. doi: 10.1016/j.ejca.2022.01.016
24. Mimura K, Kua LF, Xiao JF, Asuncion BR, Nakayama Y, Syn N, et al. Combined Inhibition of PD-1/PD-L1, Lag-3, and Tim-3 Axes Augments Antitumor Immunity in Gastric Cancer-T Cell Coculture Models. Gastric Cancer (2021) 24(3):611–23. doi: 10.1007/s10120-020-01151-8
25. de Saint Basile G, Geissmann F, Flori E, Uring-Lambert B, Soudais C, Cavazzana-Calvo M, et al. Severe Combined Immunodeficiency Caused by Deficiency in Either the Delta or the Epsilon Subunit of CD3. J Clin Invest (2004) 114(10):1512–7. doi: 10.1172/jci22588
26. Shi MJ, Meng XY, Wu QJ, Zhou XH. High CD3D/CD4 Ratio Predicts Better Survival in Muscle-Invasive Bladder Cancer. Cancer Manage Res (2019) 11:2987–95. doi: 10.2147/cmar.S191105
27. Zhu Z, Ye W, Wu X, Lin S, Xu J, Li L, et al. Comprehensive Analysis Reveals a Prognostic and Therapeutic Biomarker CD3D in the Breast Carcinoma Microenvironment. Bioscience Rep (2021) 41(1):BSR20202898. doi: 10.1042/bsr20202898
28. Yang Y, Zang Y, Zheng C, Li Z, Gu X, Zhou M, et al. CD3D is Associated With Immune Checkpoints and Predicts Favorable Clinical Outcome in Colon Cancer. Immunotherapy (2020) 12(1):25–35. doi: 10.2217/imt-2019-0145
29. Bailey CM, Liu Y, Liu M, Du X, Devenport M, Zheng P, et al. Targeting HIF-1α Abrogates PD-L1-Mediated Immune Evasion in Tumor Microenvironment But Promotes Tolerance in Normal Tissues. J Clin Invest (2022) 132(9):e150846. doi: 10.1172/jci150846
30. Loi S, Michiels S, Adams S, Loibl S, Budczies J, Denkert C, et al. The Journey of Tumor-Infiltrating Lymphocytes as a Biomarker in Breast Cancer: Clinical Utility in an Era of Checkpoint Inhibition. Ann Oncol (2021) 32(10):1236–44. doi: 10.1016/j.annonc.2021.07.007
31. Zhang N, Cao M, Duan Y, Bai H, Li X, Wang Y. Prognostic Role of Tumor-Infiltrating Lymphocytes in Gastric Cancer: A Meta-Analysis and Experimental Validation. Arch Med science: AMS (2020) 16(5):1092–103. doi: 10.5114/aoms.2019.86101
32. Ni Z, Xing D, Zhang T, Ding N, Xiang D, Zhao Z, et al. Tumor-Infiltrating B Cell is Associated With the Control of Progression of Gastric Cancer. Immunologic Res (2021) 69(1):43–52. doi: 10.1007/s12026-020-09167-z
33. Uppal A, Dehal A, Chang SC, Barrak D, Naeini Y, Jalas JR, et al. The Immune Microenvironment Impacts Survival in Western Patients With Gastric Adenocarcinoma. J gastrointestinal Surg (2020) 24(1):28–38. doi: 10.1007/s11605-019-04403-w
34. Nishi M, Shimada M, Yoshikawa K, Higashijima J, Tokunaga T, Kashihara H, et al. Impact of CKLF-Like MARVEL Transmembrane Domain Containing 6 (CMTM6) Expression in Gastric Cancer. J Med investigation: JMI (2021) 68(3.4):362–7. doi: 10.2152/jmi.68.362
35. Qiu Z, Du Y. Clinicopathological and Prognostic Significance of Programmed Death Ligant-1 Expression in Gastric Cancer: A Meta-Analysis. J gastrointestinal Oncol (2021) 12(1):112–20. doi: 10.21037/jgo-20-568
36. Huang KH, Chen MH, Fang WL, Lin CH, Chao Y, Lo SS, et al. The Clinicopathological Characteristics And Genetic Alterations of Signet-Ring Cell Carcinoma in Gastric Cancer. Cancers (2020) 12(8):2318. doi: 10.3390/cancers12082318
37. Pereira MA, de Castria TB, Ramos M, Dias AR, Cardili L, de Moraes RDR, et al. Cytotoxic T-Lymphocyte-Associated Protein 4 in Gastric Cancer: Prognosis and Association With PD-L1 Expression. J Surg Oncol (2021) 124(7):1040–50. doi: 10.1002/jso.26604
38. Fang W, Chen Y, Sheng J, Zhou T, Zhang Y, Zhan J, et al. Association Between PD-L1 Expression on Tumour-Infiltrating Lymphocytes and Overall Survival in Patients With Gastric Cancer. J Cancer (2017) 8(9):1579–85. doi: 10.7150/jca.18729
39. Győrffy B, Bottai G, Fleischer T, Munkácsy G, Budczies J, Paladini L, et al. Aberrant DNA Methylation Impacts Gene Expression and Prognosis in Breast Cancer Subtypes. Int J cancer (2016) 138(1):87–97. doi: 10.1002/ijc.29684
40. Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-Associated Lymphocytes as an Independent Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer. J Clin Oncol (2010) 28(1):105–13. doi: 10.1200/jco.2009.23.7370
Keywords: gastric cancer, cd3d, tumor infiltrating lymphocytes (TILs), prognosis, PD-L1
Citation: Yuan L, Xu J, Shi Y, Jin Z, Bao Z, Yu P, Wang Y, Xia Y, Qin J, Zhang B and Yao Q (2022) CD3D Is an Independent Prognostic Factor and Correlates With Immune Infiltration in Gastric Cancer. Front. Oncol. 12:913670. doi: 10.3389/fonc.2022.913670
Received: 06 April 2022; Accepted: 27 April 2022;
Published: 01 June 2022.
Edited by:
Benyi Li, University of Kansas Medical Center, United StatesCopyright © 2022 Yuan, Xu, Shi, Jin, Bao, Yu, Wang, Xia, Qin, Zhang and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangjiang Qin, anFpbkB1Y2FzLmFjLmNu; Bo Zhang, emhhbmdibzE3MDVAempjYy5vcmcuY24=; Qinghua Yao, eWFvcWhAempjYy5vcmcuY24=
†These authors have contributed equally to this work
 Li Yuan
Li Yuan Jingli Xu
Jingli Xu Yunfu Shi
Yunfu Shi Zhiyuan Jin1
Zhiyuan Jin1 Jiangjiang Qin
Jiangjiang Qin Bo Zhang
Bo Zhang Qinghua Yao
Qinghua Yao