- 1Department of Health Sciences, University of Florence, Florence, Italy
- 2School of Human Health Sciences, University of Florence, Florence, Italy
- 3Oncology Unit, Macerata Hospital, Macerata, Italy
- 4Histopathology and Molecular Diagnostics, Careggi Teaching Hospital, Florence, Italy
- 5Department of Health Sciences, University of Catanzaro, Catanzaro, Italy
- 6Department of Urology, Faculty of Medicine, Medical University of Gdansk, Gdansk, Poland
- 7Department of Medical Oncology, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) Dino Amadori, Meldola, Italy
Urothelial carcinoma of the bladder is one of the most prevalent cancers worldwide, diagnosed as muscle invasive in 25% of cases. Although several studies have demonstrated an overall 5% absolute survival benefit at 5 years with cisplatin-based combination neoadjuvant treatment, administration of chemotherapy prior to radical cystectomy (RC) in muscle-invasive bladder cancer (MIBC) patients is still a matter of debate. This may be due to the perceived modest survival benefit, cisplatin-based chemotherapy ineligibility, or fear of delaying potentially curative surgery in non-responders. However, immunotherapy and novel targeted therapies have shown to prolong survival in advanced disease and are under investigation in the neoadjuvant and adjuvant settings to reduce systemic relapse and improve cure rates. Genomic characterization of MIBC could help select the most effective chemotherapeutic regimen for the individual patient. Large cohort studies on neoadjuvant treatments with immune checkpoint inhibitors (ICIs) and molecular therapies, alone or combined with chemotherapy, are ongoing. In this review, we trace the development of neoadjuvant therapy in MIBC and explore recent advances that may soon change clinical practice.
Introduction
Bladder cancer (BC) accounts for almost 600,000 new cases and over 200,000 deaths worldwide (1). Muscle-invasive bladder cancer (MIBC) constitutes 25% of newly diagnosed BC cases (2), and in approximately 50% of these patients treated with radical cystectomy (RC), the disease recurs within two years (3). To date, cisplatin-based neoadjuvant chemotherapy (NAC) is the standard of care for MIBC and is associated with a 5% absolute survival benefit at 5 years and a 14% relative risk reduction for death (4). Chemotherapy prior to RC has long been a matter of debate. Although administration of NAC for MIBC has increased over the years, it still does not meet actual needs (5), particularly in cT2 BC for which it is currently recommended in clinical guidelines (6, 7). Multidisciplinary management is of paramount importance in this disease setting. Indeed, with the development of new cytotoxic and targeted therapies, and specifically immune checkpoint inhibitors (ICIs), large ongoing prospective studies have been designed to test their efficacy either alone or in combination in the neoadjuvant setting. Furthermore, identification of biomarkers, such as molecular phenotype and DNA damage repair, appears to predict response to cisplatin-based NAC. In this article, we review data in support of chemotherapy, molecular therapy and immunotherapy in early-stage MIBC, and discuss the impact of molecular biology in clinical practice.
Methods
From October 2021 to February 2022, we searched PubMed database for studies containing the keywords “neoadjuvant chemotherapy”, “muscle-invasive bladder cancer”, “neoadjuvant immunotherapy”, “biomarkers of response”, and “neoadjuvant combination therapy”. Several results were analyzed for review; all studies involved MIBC patients who were candidates for surgery upfront or after neoadjuvant therapy. We also searched the clinical trial.gov database for all phase II/III “active” or “active, not recruiting” studies on neoadjuvant therapy for MIBC.
Neoadjuvant Chemotherapy in MIBC
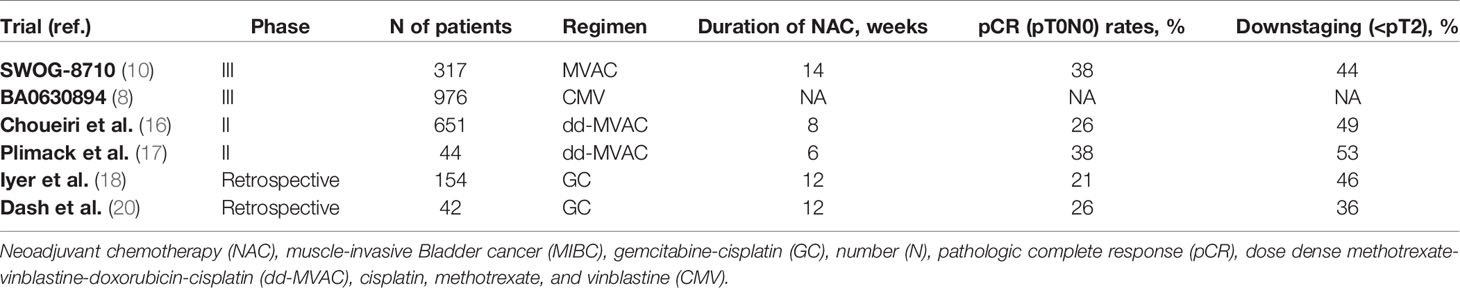
Cisplatin-based NAC is the treatment recommended by the National Comprehensive Cancer Network (NCCN) and the European Association of Urology (EAU) for patients with MIBC (cT2-4a or positive lymph nodes, N1) and fit for cisplatin (6, 7). Compared with RC alone, neoadjuvant cisplatin-based combination chemotherapy has improved overall survival (OS) and lowered the risk of recurrence. The clinical benefits of NAC in MIBC have been highlighted by several randomized phase III studies, although the ideal NAC regimen has not yet been established (8–10). Cisplatin-based NAC was first tested in the 1980s as a potential treatment strategy for MIBC. NAC based on methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) was administered to 30 MIBC patients treated with RC, achieving a 33% pathologic complete response (pCR) and 17% disease downstaging to <pT2N0 (11).
A combined analysis of two separate trials with similar patient populations showed an 8% improvement in the 5-year OS rate with NAC (56%) compared with the control group (48%), and a 20% reduction in the relative probability of death (9). Regarding local radical treatment alone versus neoadjuvant cisplatin, methotrexate and vinblastine (CMV), an international multicenter study (BA06 30894 trial) demonstrated on first analysis a non-significant 15% reduction in the risk of death with neoadjuvant CMV (8). Updated results revealed a statistically significant 16% reduction in the risk of death and a 6% increase in the 10-year survival rate with neoadjuvant CMV compared to the control group (12). Further meta-analyses assessing the clinical benefits of NAC confirmed a 5% improvement of OS in MIBC (13–15).
In the SWOG trial, MVAC-based NAC was tested against surgery alone in 317 patients with cT2-4aN0M0 BC. Median OS was 77 months in the NAC group and 46 months in the surgery alone group, while 5-year survival rate was 57% and 43%, respectively (10).
Two small, single-arm phase II trials investigated a modified MVAC regimen consisting of dose dense MVAC (dd-MVAC) with granulocyte colony-stimulating factor (G-CSF) support, evaluating NAC efficacy and safety in cT2-4N0 BC (16, 17). Of 39 patients, 49% achieved pathologic response, defined as downstaging to ≤pT1N0M0, with 10% showing grade 3 or higher treatment-related toxicities (16). Likewise, 38% pCR (pT0) rates and 52% downstaging to non-muscle-invasive disease (NMIBC) were observed by Plimack et al., with the majority of patients (82%) experiencing only grade 1–2 treatment-related toxicities (17).
The combination of gemcitabine and cisplatin (GC) is another regimen utilized in the neoadjuvant setting, showing similar OS, progression-free survival (PFS), and downstaging to pT0/pT1, but lower toxicity when compared to conventional MVAC (18–23). A randomized phase III trial assessed the efficacy of neoadjuvant treatment with GC and dd-MVAC in 537 patients (24). Overall, pCR was seen in 36% and 42% of GC and dd-MVAC patients (p=0.2), while downstaging to organ-confined disease (<ypT3pN0) was achieved in 63% and 77% (p=0.001), respectively. Grade 3 or higher hematologic toxicities were similar, 55% in the GC group and 52% in the dd-MVAC group. Contrariwise, grade 3 or higher gastrointestinal toxicities (p=0.003) and asthenia (p<0.001) were more frequent in the dd-MVAC arm. Results of NAC trials in MIBC are summarized in Table 1.
Cystectomy and Lymphadenectomy in Patients Treated With Neoadjuvant Therapy
Surgery is the standard approach for patients with MIBC or refractory NMIBC. Selection of MIBC patients as candidates for NAC requires careful consideration. It has recently emerged that impaired nutritional status due to neoadjuvant therapy is a key factor. In a study led by Cohen et al., variations in nutritional status were assessed by changes in smooth muscle index (SMI), calculated through cross-sectional imaging of psoas muscle area (25). These authors reported that SMI decline after neoadjuvant therapy was significantly associated with the risk of post-RC complications, including ileus and infections.
With the intent of determining the outcome of patients subjected to RC following NAC, Mir et al. developed and internally validated a nomogram predicting BC-specific mortality (BCSM) in MIBC patients (26). At multivariate analysis, lymph node metastasis (hazard ratio [HR] 1.90, 95% CI: 1.4-2.6), positive surgical margins (HR 2.01, 95% CI: 1.3-2.9) and pathologic stage ypT3-4 (HR 5.9, 95% CI: 3.8-9.3) were correlated with reduced BCSM, thus suggesting the potential use of this nomogram to identify patients eligible for adjuvant approaches or personalized follow-up.
Pre-surgical evaluation through [18F] Fluoro-Deoxy-Glucose Positron Emission Tomography (FDG-PET) is reserved for patients with suspected lymph node involvement at computed tomography (CT) scan. In patients receiving neoadjuvant anti-programmed cell death-1 (PD-1) immunotherapy by pembrolizumab, the sensitivity and specificity of PET/CT to predict lymph node metastasis was investigated before and after treatment (27). In this study, 4 of 7 patients (57%) with baseline FDG-uptake showed pathologic lymph node involvement versus 11 of 101 (11%) with no baseline FDG-uptake. Six of the 7 patients responded to neoadjuvant pembrolizumab, implying the necessity to further investigate and validate the use of PET/CT to determine those MIBC patients who are better candidates for neoadjuvant immunotherapy. Briganti et al. were the first to demonstrate the surgical safety of RC and pelvic lymph node dissection (PLND) in non-metastatic MIBC patients receiving neoadjuvant therapy with checkpoint inhibitors (28). They found that 77% and 34% of patients experienced any-grade and high-grade complications, respectively. The most frequent complications were fever (52%) and ileus (31%), with no perioperative mortality cases observed at 90 days.
According to the EAU guidelines, the high specificity of DWI-MRI seems to accurately predict pCR and allow better patient selection for bladder-sparing protocols (7). Pre-operative MRI in different settings may therefore provide useful information regarding treatment response.
Predictive Biomarkers of Response in Cisplatin-Based Chemotherapy
Cisplatin-based chemotherapy remains the standard treatment for advanced disease and perioperative (neoadjuvant) treatment of BC (29). Cisplatin crosslinks DNA in different ways, mainly forming adducts that prevent cell replication and induce cell death. DNA damage can manifest as single-strand breaks (SSBs), double-strand breaks (DSBs) or interstrand-crosslinks (30). Cancer cells rely on various mechanisms to repair DNA damage: excision repair, mismatch repair (MMR) or nucleotide excision repair (NER) for SSBs, while non-homologous end joining or homologous recombination (HR) can correct DSBs.
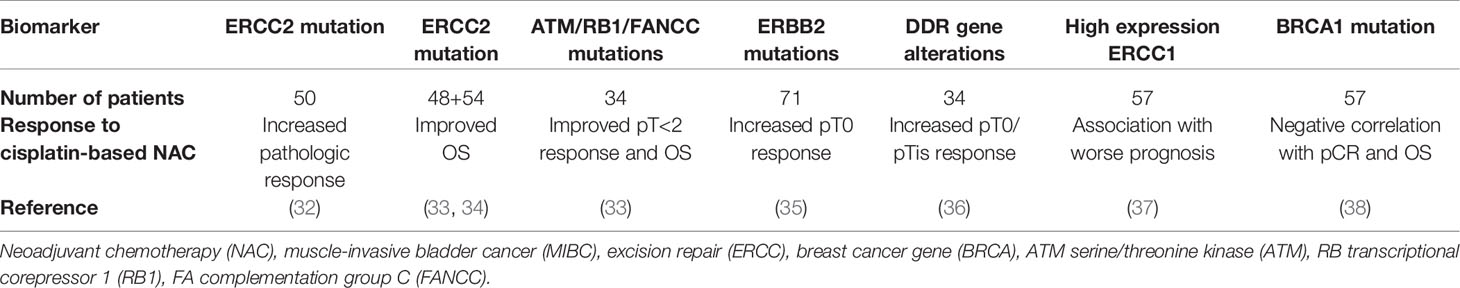
There are several reports on the genes involved in DNA damage repair (DDR) pathways, highlighting their predictive role as biomarkers of response to cisplatin (31) (Table 2). A panel of 34 DDR genes was analyzed in a study enrolling 100 advanced BC patients treated with platinum-based chemotherapy. Overall, 47 patients had at least one alteration, and median OS was significantly higher in these patients than in those without (23.7 vs. 13.0 months, p=0.006). A recent phase II trial, investigating a panel of 29 DDR genes in 49 patients administered neoadjuvant dose-dense GC, showed a greater response to chemotherapy, with a positive predictive value of 89% and a 2-year relapse-free survival of 100%, in patients with deleterious mutations (39).
Excision repair 1 and 2 (ERCC1 and ERCC2) proteins, belonging to the NER pathway, have been correlated with cisplatin-based chemotherapy response. High ERCC1 expression has been associated with gain of NER pathway function that leads to increased DNA repair capacity and platinum resistance (40, 41). In preclinical studies, ERCC2 mutations have been linked to loss of NER pathway function that confers sensitivity to cisplatin and carboplatin, but not to doxorubicin and ionizing radiation or poly (ADP-ribose) polymerase (PARP) inhibitors (42). Van Allen et al. detected ERCC2 mutations in 36% of patients who responded effectively to chemotherapy (<ypT1) but not in non-responders (>ypT2) (32). Further studies reported ERCC2 mutations in 38% (17/45) of responders and in only 6% (3/53) of non-responders (30). Recently, ERCC2 mutations were observed more frequently in primary than in secondary MIBC (12% vs. 1.2%), and patients with primary MIBC attained higher pathologic response rates following NAC (42).
Efficacy of NAC in MIBC has also been related to mutations in the ATM serine/threonine kinase (ATM), RB transcriptional corepressor 1 (RB1) and FA complementation group C (FANCC) repair genes. Plimack et al. detected genomic alterations in these genes in 13 of 15 cisplatin-responders (87%), while none of the non-responders harbored these mutations (33). A recent update of this study revealed a statistically significant improved 5-year disease-specific survival in carriers of at least one mutation compared to patients with no mutation (90% vs. 49%, p=0.0015) (43). The phase II RETAIN trial is currently evaluating bladder preservation in patients with ATM, RB1, FANCC or ERCC2 mutations who have achieved complete response with NAC (44). The presence of DDR genomic alterations could well identify those patients likely to respond to NAC and benefit from a bladder-sparing approach.
Breast cancer type 1 and 2 (BRCA1 and BRCA2) are among frequently mutated homologous recombination (HR) genes in urothelial carcinoma (45). According to Font et al., increased BRCA1 mRNA expression is negatively associated with pathologic response and survival in MIBC patients receiving NAC (38).
Current evidence indicates that alterations in DNA repair pathways can provide prognostic and predictive information in cisplatin-treated BC patients. Prospective studies including a large number of patients are needed to confirm these findings, which could pave the way for novel treatments, such as PARP inhibitors in HR-deficient cancers (46).
Neoadjuvant Immunotherapy in MIBC
Lately, immunotherapy has become an integral part of advanced and metastatic BC treatment (47–56). Between 2016 and 2017, monoclonal antibodies against the negative immunoregulatory human cell surface receptor PD-1 (nivolumab and pembrolizumab), and its ligand programmed death ligan 1 (PD-L1) (atezolizumab, avelumab and durvalumab) have been approved for metastatic urothelial cancer by the United States Food and Drug Administration (FDA). Owing to their clinical benefits in the metastatic setting, several ICIs are being investigated in neoadjuvant (Table 3) and adjuvant settings (65).
ICIs as Single Agents
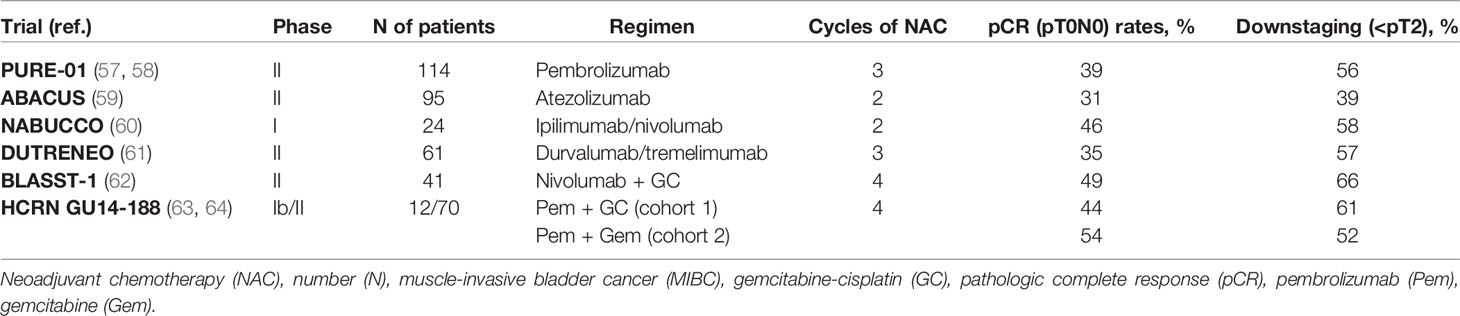
In two single-arm phase II trials, pembrolizumab and atezolizumab have been tested in the neoadjuvant setting. The PURE-01 trial assessed the activity of pembrolizumab (200 mg every 3 weeks) for three cycles as neoadjuvant treatment before RC in patients with cT2-3bN0 MIBC and predominant urothelial cancer histology (57). Of these patients, 92% were eligible for cisplatin. Neoadjuvant pembrolizumab yielded 42% pCR and 54% downstaging to NMIBC. In addition, pCR was recorded in 54.3% of patients with PD-L1 combined positive score (CPS) ≥10 and in 13.3% of patients with PD-L1 CPS <10. High-grade complications, defined according to the Clavien-Dindo classification, were observed in 34% of patients, with no perioperative mortality at 90 days (7). Pembrolizumab response was maintained after cystectomy in most patients, with 1- and 2-year event-free survival (EFS) rates of 84.5% and 71.7%, respectively (58). A statistically significant longer EFS was found in patients with complete response and high PD-L1 CPS.
The ABACUS trial investigated the efficacy and safety of two cycles of neoadjuvant atezolizumab (1200 mg every 3 weeks) prior to RC for MIBC (59). Contrary to the PURE-01 trial, all patients were ineligible for or refused cisplatin-based NAC. The rates of pCR and downstaging to NMIBC were 31% and 39%, respectively. Treatment-related grade 3-4 toxicities occurred in 12% of patients, and grade 3 or 4 surgical complications in 31% of cases.
ICIs as Combination Therapy
ICI combination has proved promising in different settings and types of cancer (66). Indeed, combined anti-PD-1 and anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) blockade prompts complementary mechanisms of therapeutic checkpoint inhibition, leading to greater antitumor activity than via a single pathway (67–69).
In the NABUCCO study, 24 patients with stage III urothelial cancer were administered 3 mg/kg ipilimumab (day 1), 1 mg/kg nivolumab plus 3 mg/kg ipilimumab (day 22), and 3 mg/kg nivolumab (day 43) in the neoadjuvant setting (60). The primary endpoint was feasibility to resect within 12 weeks from start of treatment. A total of 23 (96%) patients underwent surgery within 12 weeks, and grade 3-4 immune-related adverse events (iAEs) manifested in 55% of cases. Furthermore, 46% of patients showed pCR, and 58% had no remaining invasive disease (pCR or pTisN0/pTaN0).
Another randomized phase II trial (DUTRENEO) compared neoadjuvant durvalumab plus tremelimumab versus chemotherapy in cisplatin-eligible patients with cT2-4aN0-1 BC, classified as immunologically “hot” or “cold” according to the tumor immune score devised by NanoString Technologies (61). Patients with “hot” tumors were randomized to three cycles of durvalumab 1500 mg plus tremelimumab 75 mg every 4 weeks or standard cisplatin-based NAC, while patients in the “cold” arm received cisplatin-based NAC. In the “hot” arm, pCR was recorded in 36.4% of patients treated with NAC and in 34.8% of patients receiving durvalumab/tremelimumab. In the “cold” arm, as many as 68.8% of patients achieved pCR. Grade 3-4 toxicities occurred more frequently in the NAC group.
ICIs and Chemotherapy
Conventional chemotherapy can elicit a tumor-specific immune response by inducing immunogenic cell death of neoplastic cells or engaging immune effector mechanisms (70). The combination of chemotherapy with immunotherapy has been extensively investigated. A phase II, single-arm trial, BLASST-1, examined the efficacy and safety of neoadjuvant nivolumab with GC for MIBC (cT2-T4aN ≤ 1M0) (62). Patients received four cycles of GC with nivolumab every 21 days, followed by RC within 8 weeks. Pathologic response (≤pT1N0) was observed in 65.8% of patients, including those presenting N1 disease. Safety profile was favorable, with 20% of grade 3-4 AEs mainly due to GC.
In patients with operable MIBC (cT2-4aN0-1), the open-label single-arm phase II trial, SAKK 06/17, tested neoadjuvant durvalumab plus GC (4 cycles every 21 days) followed by durvalumab monotherapy (10 cycles every 28 days) after surgery. Pathologic response was observed in 60% of patients, with 18 (34%) achieving pCR. Treatment demonstrated acceptable safety, and data regarding the primary endpoint, i.e. event-free survival (EFS) at 2 years, are awaited (71). Another multicenter, single-arm phase II trial enrolled eligible patients with MIBC (cT2-4aN0M0) to receive a dose of atezolizumab, followed 2 weeks later by GC plus atezolizumab every 21 days for 4 cycles, and after a further 3 weeks by a dose of atezolizumab prior to RC. The primary endpoint, downstaging to < pT2N0, was met in 27 (69%) patients including 16 (41%) pT0N0, all of whom experienced improved relapse-free survival. Grade 3 iAEs occurred in 5 (11%) patients with 2 (5%) requiring systemic steroids (72).
Efficacy and tolerability of neoadjuvant pembrolizumab and GC were assessed in a phase I/II trial, HCRN GU14-188, where patients with MIBC (cT2-4aN0M0) were subdivided into two cohorts: cisplatin-eligible (cohort 1) and cisplatin-ineligible (cohort 2) (63, 64). In cohort 1, pathologic response (≤pT1N0) and pCR were seen in 61.1% and 44.4% of patients, respectively. Median time from last dose to RC was 5.3 weeks; the 36-month relapse-free survival and OS were 63% and 82%, respectively. One death from mesenteric ischemia was recorded.
Phase III trials of neoadjuvant immunotherapy, comprising nivolumab, pembrolizumab and toripalimab, in combination with cisplatin-based chemotherapy are ongoing, and results are eagerly awaited (NCT03732677, NCT03661320, NCT03924856, NCT04861584). Several trials are also evaluating immunotherapy with non-cisplatin-based chemotherapy, including nab-paclitaxel and gemcitabine as neoadjuvant treatment. Among these, tislelizumab (BGB-A317), a humanized monoclonal antibody against PD-1, is being tested with nab-paclitaxel in MIBC (NCT04730219) (Table 4).
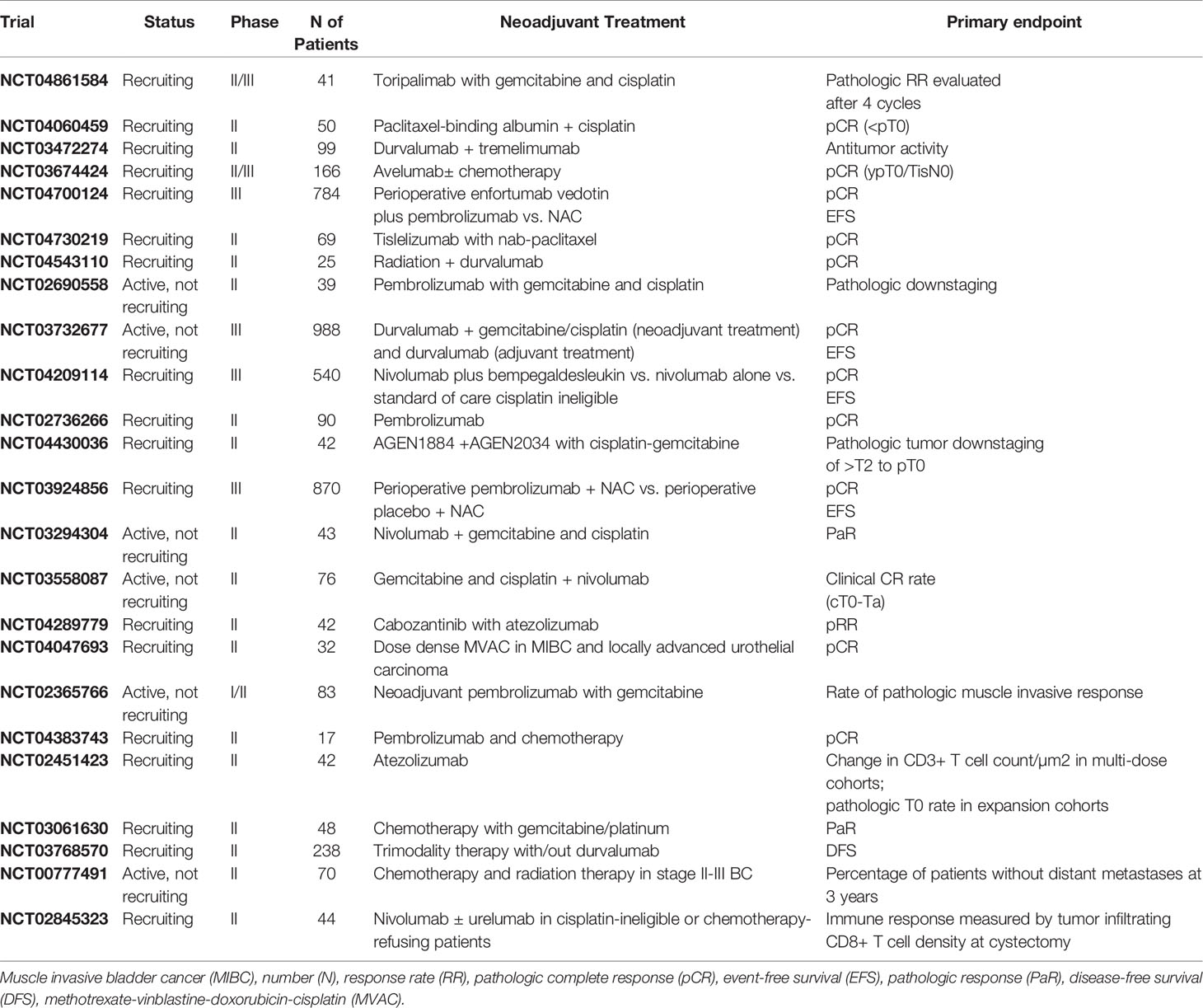
Table 4 Recruiting or active, not recruiting phase II and III clinical trials with neoadjuvant therapy for MIBC.
ICIs and Antibody-Drug Conjugates
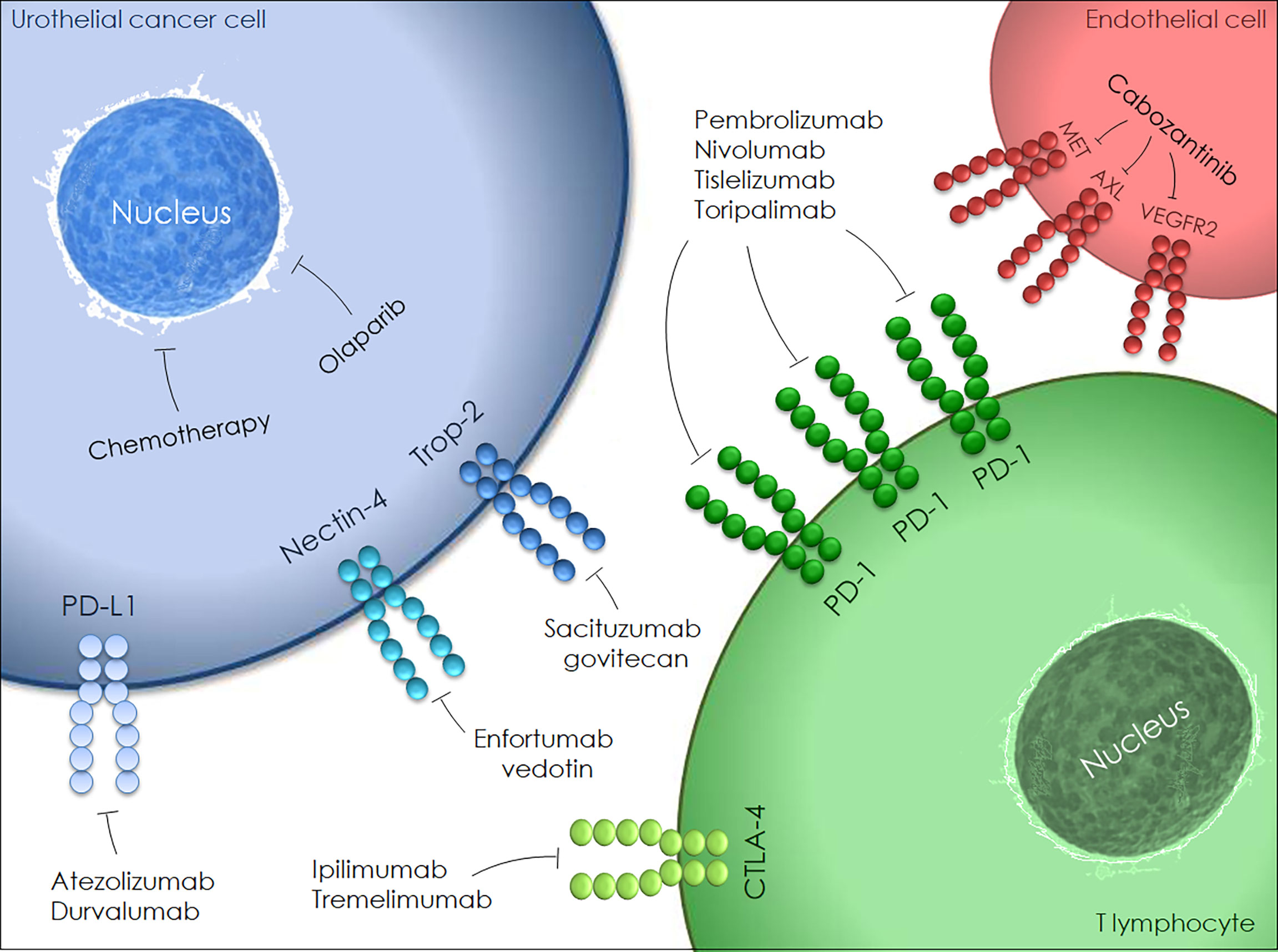
Antibody-drug conjugates (ADCs), i.e. enfortumab vedotin and sacituzumab govitecan, are complex engineered therapeutics consisting of monoclonal antibodies directed toward tumor-associated antigens, to which highly potent cytotoxic agents are attached by chemical linkers (73). Enfortumab vedotin, a fully human monoclonal antibody conjugated to a clinically validated microtubule-disrupting agent, has shown encouraging results. Accordingly, the FDA has granted its accelerated approval in patients with locally advanced or metastatic urothelial carcinoma, formerly treated with PD-1/PD-L1 inhibitors and platinum-containing chemotherapy (74). At the 2022 American Society of Clinical Oncology (ASCO) meeting, Petrylak et al. presented the EV-103 phase Ib/II study evaluating antitumor activity of neoadjuvant treatment with enfortumab vedotin monotherapy in cisplatin-ineligible MIBC patients (75). Two randomized phase III trials are currently comparing perioperative enfortumab vedotin plus pembrolizumab with chemotherapy in cisplatin-eligible patients (NCT04700124) and with cystectomy alone in cisplatin-ineligible patients (NCT03924895). Sacituzumab govitecan is a humanized anti-trophoblast surface antigen 2 (Trop-2) antibody conjugated with SN-38, the active metabolite of irinotecan (76). The FDA has recently approved sacituzumab govitecan for patients with locally advanced or metastatic BC, previously administered platinum-based chemotherapy and PD-1 or PD-L1 inhibitors. At the 2021 GU ASCO Annual Meeting, Necchi et al. presented the design for the SURE trial assessing the efficacy of neoadjuvant sacituzumab govitecan, either as a single-agent (SURE-01) or combined with pembrolizumab (SURE-02), prior to RC in MIBC patients unfit for or refusing cisplatin-based chemotherapy (77).
ICIs and Emerging Agents
A single-arm, phase II trial (NEODURVARIB) explored the impact of neoadjuvant durvalumab plus olaparib, a poly ADP-ribose polymerase inhibitor, in cT2-4N0 urothelial carcinoma (78). Patients received durvalumab 1500 mg every 4 weeks for a 2-month maximum (up to 2 doses/cycle) plus olaparib 300 mg for up to 56 days (2 cycles of 28 days each cycle). The pCR rate was 44.5% and grade 3-4 AEs occurred in 8.3% of patients, with one death related to postoperative complications. In the ongoing ABATE trial, the efficacy and safety of cabozantinib (a multikinase inhibitor of c-MET, AXL and VEGFR2) plus atezolizumab is being tested as neoadjuvant therapy for cT2-T4N0M0 BC patients who are either ineligible for or decline cisplatin (79).
Predictive Biomarkers of Immunotherapy Response
With the emergence of immunotherapy, attempts have been made to identify biomarkers to predict clinical response. To date, potential biomarkers such as PD-L1 expression, CD8+ T-cell infiltration, DDR gene alterations, tumor mutational burden (TMB) and immune and stromal gene expression signatures have been correlated with immunotherapy response (80, 81). Nevertheless, none of these markers has shown consistent findings to warrant incorporation into BC routine management.
Controversial results on the role of PD-L1 as a predictive biomarker have been reported (82). PD-L1 positivity, detected in 20-30% of bladder tumors, appears to correlate with more advanced disease and poor prognosis (83). However, PD-L1 expression may depend on biopsy site and previous treatments, but primarily on the assays to test PD-L1 status (i.e. Dako 22C3, Ventana SP142, and Dako 28.8) based on the different ICI used. It should therefore be interpreted in the context of a broader biomarker panel, including neutrophil/lymphocyte ratio, albumin levels, high C-reactive protein and interleukin-6 (IL-6) levels. These can be easily integrated into clinical practice, unlike other more complex biomarkers such as gene expression signatures (84).
Investigation into the DDR pathways could be valuable for establishing the potential utility of immunotherapy. In a study analyzing a panel of 34 DDR genes, patients with advanced BC and deleterious mutations in these genes significantly benefitted from immunotherapy compared to those without DDR alterations (85). Moreover, ICIs have recently been reported to be highly effective in tumors with defects in the MMR/microsatellite instability pathway (86).
In the early phases of BC, the integrity of the immune system seems to induce a greater T-cell expansion than in advanced stages, characterized by increased impairment of T-cell function and cancer-associated inflammation. As a consequence, immunotherapy efficacy with checkpoint inhibitors has been explored in early-stage disease (87).
Recently, Mariathasan et al. demonstrated that inflamed and desert immune phenotypes were associated with response and resistance to atezolizumab, respectively (88). In this study, CD8 levels were higher in responding tumors, while elevated levels of fibroblast activation protein (FAP) were linked to immunotherapy resistance. In the PURE-01 study, PD-L1 expression, TMB, DDR and RB1 gene alterations were significantly related to pCR (57). Conversely, in the ABACUS trial, pCR correlated with granzyme B (GZMB) expression, a surrogate marker of activated CD8+ T cells (59).
Since several biomarkers, such as TMB and DDR alterations, are associated with the efficacy of both chemotherapy and immunotherapy, it may be difficult to select cisplatin-eligible patients and decide upon integration and sequencing of different therapeutic options in the multimodal management of MIBC (89, 90). Notably, cytotoxicity induced by NAC can elicit an immune effect by activating CD8+ T cells and decreasing Tregs (91). This would impede T-cell response when NAC and immunotherapy are administered concurrently, and partly explain the limited benefit of NAC plus immunotherapy compared with NAC alone in advanced urothelial cancer (92). NAC followed by immunotherapy could therefore be a more effective approach.
Potential Neoadjuvant Agents in MIBC
Emerging neoadjuvant agents are illustrated in Figure 1. The genetic alteration of the fibroblast growth factor receptor (FGFR) pathway has been widely investigated in BC with subsequent approval of FGFR inhibitors in advanced and metastatic settings. Infigratinib (FGFR1-3-selective tyrosine kinase inhibitor) monotherapy is currently being tested as neoadjuvant treatment for locally advanced urothelial cancer (NCT0422804).
Bempegaldesleukin (BEMPEG/NKTR-214) is a PEGylated interleukin-2 (IL-2) designed to activate and proliferate CD8+ T cells and natural killer (NK) cells. An in-progress randomized study is comparing BEMPEG plus nivolumab with nivolumab alone for neoadjuvant and adjuvant treatment of cisplatin-ineligible resectable MIBC patients (NCT04209114).
Urelumab is a fully human IgG4 monoclonal antibody that targets the CD137 extracellular domain stimulating cytotoxic T cell responses against tumor cells. A trial assessing the efficacy of nivolumab monotherapy or combined with urelumab in cisplatin-ineligible or chemotherapy-refusing MIBC patients is ongoing (NCT02845323). Further trials are assessing novel agents, namely CD73 inhibitor (NCT03773666), replication-competent oncolytic adenovirus (NCT04610671) and synthetic benzamide-derivative histone deacetylase inhibitor (NCT03978624).
Radiotherapy in MIBC
Neoadjuvant radiation should not be used in patients with MIBC prior to RC. Although preoperative radiotherapy, as a single modality, can eradicate disease in a small proportion of patients undergoing cystectomy, it seems to improve local control rather than survival when compared with RC alone (93). However, radiation can synergize with immunotherapy to improve clinical outcomes by causing immunogenic cell death and increasing expression of immune markers (94). Following this hypothesis, several trials, such as RADIANT (durvalumab + radiotherapy) (NCT04543110) and RACE IT (nivolumab + radiotherapy) (NCT03529890) prior to cystectomy in MIBC, are still active. The efficacy of chemotherapy and radiation therapy in stage II and III bladder carcinoma patients is also being tested (NCT00777491).
Bladder Cancer Molecular Subtypes and Therapeutic Implications
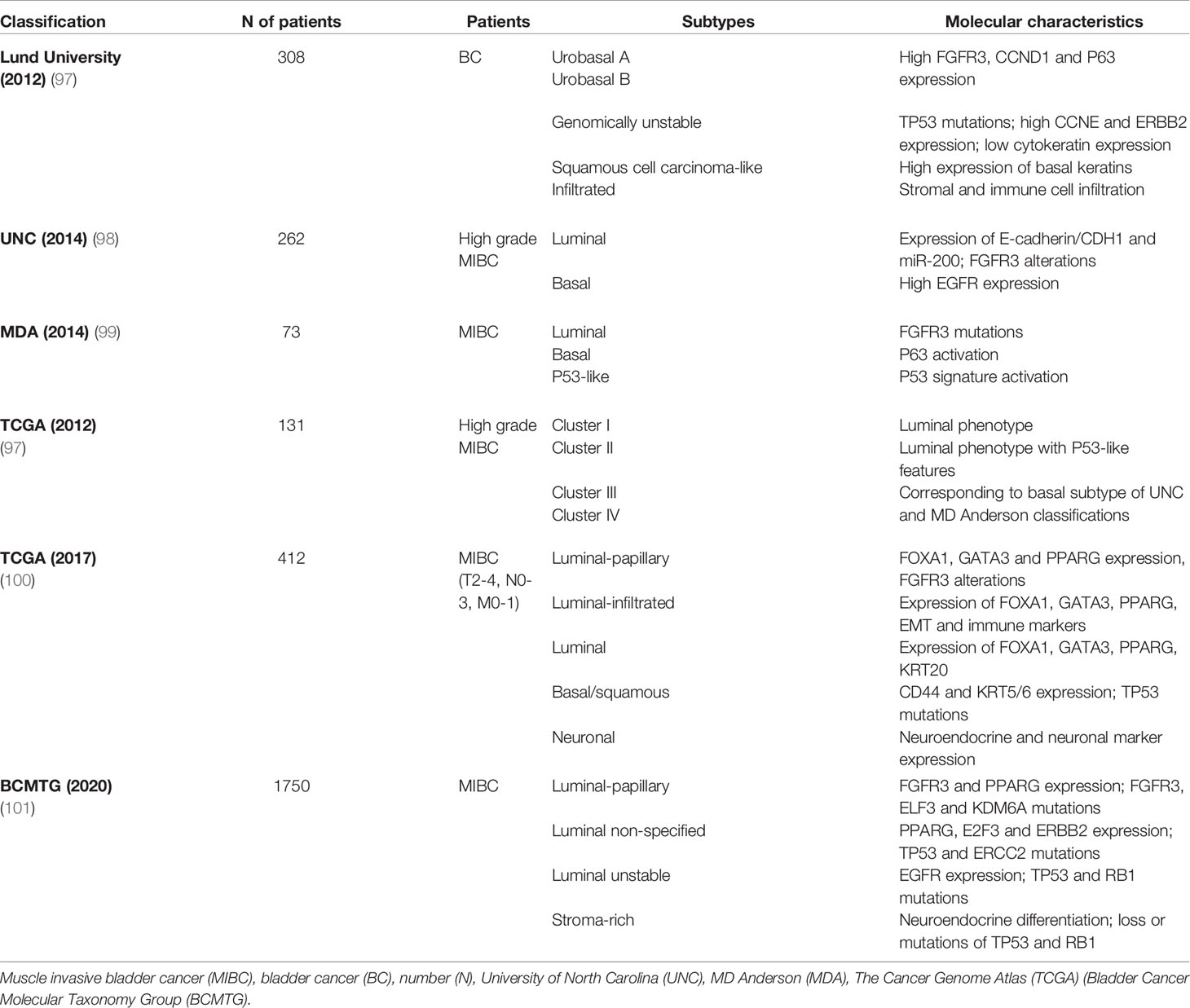
Potential markers and gene expression models have been correlated to chemotherapy response in BC (32, 95, 96), but none have been approved for clinical practice as yet. However, new insights into BC molecular pathology could lead to a shift toward individualized treatment and consequently better patient outcomes (Table 5 and Figure 2).
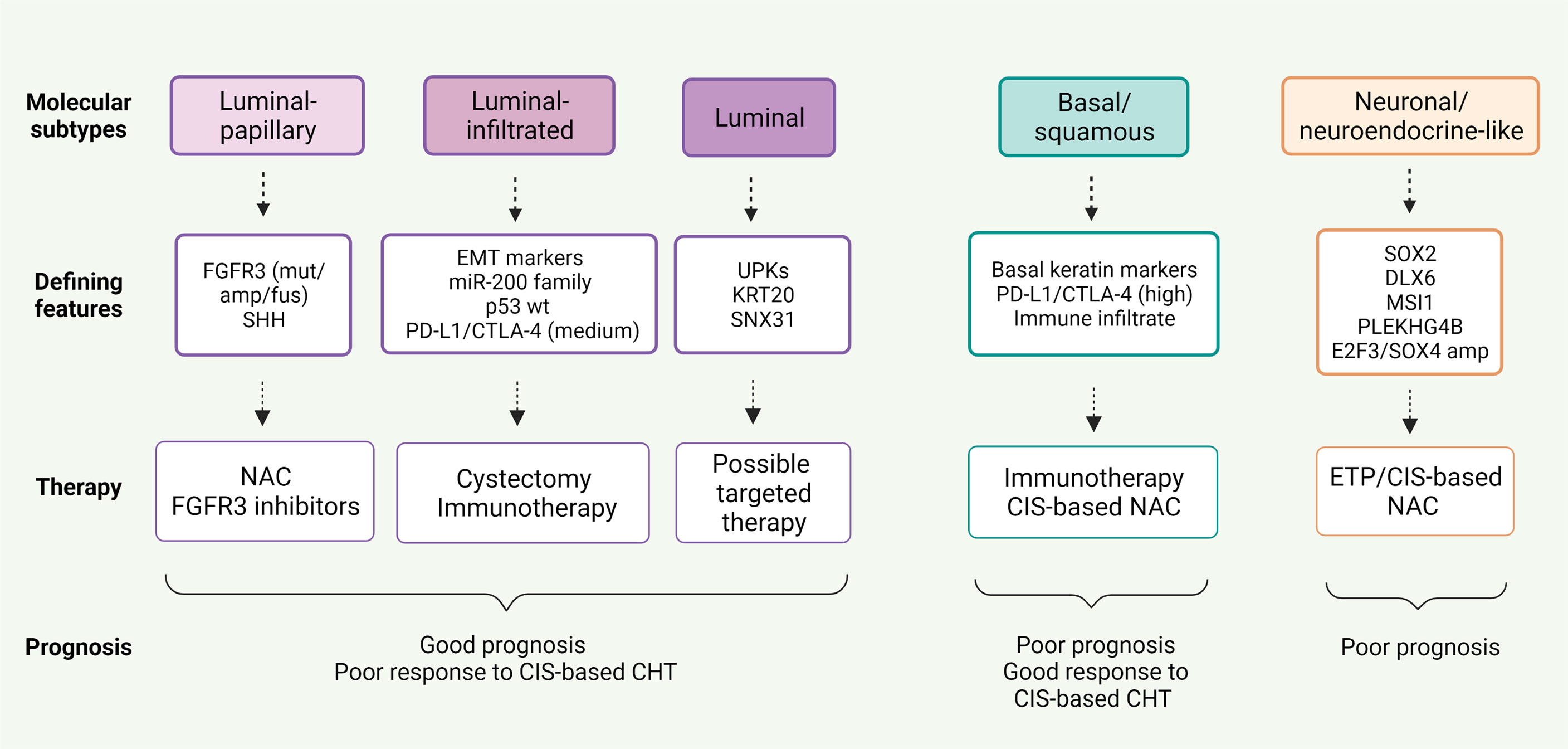
Figure 2 Correlation between five mRNA-based expression subtypes according to The Cancer Genome Atlas (TCGA) analysis and response to neoadjuvant therapy in MIBC.
Basal/squamous tumors, defined by the Cancer Genome Atlas (TCGA), University of North Carolina (UNC) and MD Anderson classifications (98, 99, 102), are associated with more advanced stages and worse prognosis, whereas luminal tumors appear to be less aggressive (37, 100). Patients with basal tumors seem to profit more from NAC than those with luminal tumors who derive little or no benefit. Irrespective of treatment strategy, luminal-papillary tumors bear the best prognosis, unlike luminal-infiltrated tumors that have an unfavorable prognosis regardless of NAC (103, 104).
Furthermore, these molecular classifications can balance standard histologic classifications burdened by intra- and intertumoral heterogeneity of primary MIBC, with relevant clinical implications. Compared with transcriptome analysis, immunohistochemistry (IHC) is a simpler and more accessible method to classify urothelial carcinoma into molecular subtypes, comprising basal (KRT5/6, KRT14, and p63) and luminal (GATA3, FOXA1, uroplakins and HER2) phenotypes.
To stratify BC patients, Makboul et al. utilized a simple IHC panel of five biomarkers, i.e. FGFR3, KRT5, cyclin B1, HER2 and p53 (105). The molecular classes identified were correlated with clinico-pathologic variables and patient survival: basal/squamous tumors showed the lowest OS (38.5%), while urobasal A (UroA) tumors, expressing luminal markers, had the best prognosis with OS of 75% and no metastatic events. In addition, Choi et al. found that basal tumors had a significantly higher response rate to cisplatin-based chemotherapy, and all chemoresistant tumors exhibited a p53-like phenotype (99).
A recent study by Font et al. stratified MIBC patients receiving NAC into three subgroups, i.e. basal/squamous (KRT5/6 and KRT14 high; FOXA1 and GATA3 low), luminal (FOXA1 and GATA3 high; KRT5/6 and KRT14 low) and mixed (FOXA1 and GATA3 high; KRT5/6 high and KRT14 low), using IHC combined with hierarchical clustering analysis. Overall, pathologic response to NAC was significantly higher in patients with basal/squamous tumors (p=0.017) (106).
Through the use of transcriptome-wide gene expression and IHC, Seiler et al. categorized the residual tumor at cystectomy after NAC, and outlined the greatest potential benefit from second-line treatments, such as checkpoint inhibition, in tumors with high immune infiltration, elevated expression of immune-associated genes (i.e. CTLA4, MPEG1 and CD27) and low expression of basal or luminal markers (107).
Likewise, correlation between tumor subtypes and efficacy of immunotherapy has recently been explored. The revised TCGA classification suggested that patients with luminal-infiltrated tumors benefit most from immunotherapy (100). In the IMvigor 210 study, treatment with atezolizumab was most beneficial in advanced BC classified as TCGA cluster II (108), whereas basal tumors were more likely to respond to nivolumab in the CheckMate 275 study (49). Despite the high immune infiltration, response to immunotherapy was poor in claudin-low tumors, defined by biologic characteristics of the claudin-low subtype of breast cancer, probably due to more effective T cell suppression in cluster IV than cluster II tumors (109). IMvigor 210 trial also showed that survival advantage of atezolizumab was greater in TCGA neuronal-subtype tumors, without this being related to other predictors of immunotherapy response, such as TMB and tumor neo-antigen load (110).
Molecular classification of BC according to gene expression profiles can play a crucial role in determining the most suitable treatment. Immunotherapy and chemotherapy appear to be advantageous in complementary patient populations. Patients with luminal tumors show better prognosis but poor response to cisplatin-based chemotherapy. Cystectomy is the best option in these patients, however, immunotherapy may be beneficial in luminal-infiltrated tumors. On the contrary, chemotherapy proves to be the treatment of choice in basal tumors.
Conclusions
Despite guideline recommendations, NAC prior to cystectomy is still seldom adopted. Newly developed therapies, such as immunotherapy, targeted therapy and combination strategies, are being tested in clinical trials. The use of biomarkers to predict response to cisplatin-based NAC or ICIs is largely investigational, but molecular signatures are showing promise in reshaping selection for tailored treatment and disease monitoring.
Author Contributions
Study concepts: GR, GN. Study design: GR, MC. Data acquisition: RS, MS, IG. Quality control of data and algorithms: UG. Manuscript preparation: MC, GR, GN. Manuscript editing: MS, WP, AA. Manuscript review: UG, AA. All authors contributed to the article and approved the submitted version.
Conflict of Interest
UG received honoraria for advisory boards or invited speaker fees from Pfizer, BMS, MSD, PharmaMar, Astellas, Bayer, Ipsen, Novartis, Roche, Clovis, AstraZeneca, institutional research grants from AstraZeneca, Sanofi and Roche.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Patel VG, Oh WK, Galsky MD. Treatment of Muscle-Invasive and Advanced Bladder Cancer in 2020. CA Cancer J Clin (2020) 70:404–23. doi: 10.3322/caac.21631
3. Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical Cystectomy in the Treatment of Invasive Bladder Cancer: Long-Term Results in 1,054 Patients. J Clin Oncol (2001) 19:666–75. doi: 10.1200/JCO.2001.19.3.666
4. Leow JJ, Fay AP, Mullane SA, Bellmunt J. Perioperative Therapy for Muscle Invasive Bladder Cancer. Hematol Oncol Clin North Am (2015) 29:301–18. doi: 10.1016/j.hoc.2014.11.002
5. Reardon ZD, Patel SG, Zaid HB, Stimson CJ, Resnick MJ, Keegan KA, et al. Trends in the Use of Perioperative Chemotherapy for Localized and Locally Advanced Muscle-Invasive Bladder Cancer: A Sign of Changing Tides. Eur Urol (2015) 67:165–70. doi: 10.1016/j.eururo.2014.01.009
6. Flaig TW, Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2020) 18:329–54. doi: 10.6004/jnccn.2020.0011
7. Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol (2021) 79:82–104. doi: 10.1016/j.eururo.2020.03.055
8. International Collaboration of Trialists, Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group), European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group, Australian Bladder Cancer Study Group; National Cancer Institute of Canada Clinical Trials Group, Finnbladder, Norwegian Bladder Cancer Study Group, et al. International Phase III Trial Assessing Neoadjuvant Cisplatin, Methotrexate, and Vinblastine Chemotherapy for Muscle-Invasive Bladder Cancer: Long-Term Results of the BA06 30894 Trial. J Clin Oncol (2011) 29:2171–7. doi: 10.1200/JCO.2010.32.3139
9. Sherif A, Holmberg L, Rintala E, Mestad O, Nilsson J, Nilsson S, et al. Neoadjuvant Cisplatinum Based Combination Chemotherapy in Patients With Invasive Bladder Cancer: A Combined Analysis of Two Nordic Studies. Eur Urol (2004) 45:297–303. doi: 10.1016/j.eururo.2003.09.019
10. Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant Chemotherapy Plus Cystectomy Compared With Cystectomy Alone for Locally Advanced Bladder Cancer. N Engl J Med (2003) 349:859–66. doi: 10.1056/NEJMoa022148
11. Scher HI, Yagoda A, Herr HW, Sternberg CN, Bosl G, Morse MJ, et al. Neoadjuvant M-VAC (Methotrexate, Vinblastine, Doxorubicin and Cisplatin) Effect on the Primary Bladder Lesion. J Urol (1988) 139:470–4. doi: 10.1016/s0022-5347(17)42495-5
12. Neoadjuvant Cisplatin, Methotrexate, and Vinblastine Chemotherapy for Muscle-Invasive Bladder Cancer: A Randomised Controlled Trial. International Collaboration of Trialists. Lancet (1999) 354:533–40.
13. Advanced Bladder Cancer (ABC) Meta-Analysis Collaboration. Neoadjuvant Chemotherapy in Invasive Bladder Cancer: Update of a Systematic Review and Meta-Analysis of Individual Patient Data Advanced Bladder Cancer (ABC) Meta-Analysis Collaboration. Eur Urol (2005) 48:202–5. doi: 10.1016/j.eururo.2005.04.006
14. Winquist E, Kirchner TS, Segal R, Chin J, Lukka H. Genitourinary Cancer Disease Site Group, Cancer Care Ontario Program in Evidence-Based Care Practice Guidelines Initiative. Neoadjuvant Chemotherapy for Transitional Cell Carcinoma of the Bladder: A Systematic Review and Meta-Analysis. J Urol (2004) 171:561–9. doi: 10.1097/01.ju.0000090967.08622.33
15. Advanced Bladder Cancer Meta-Analysis Collaboration. Neoadjuvant Chemotherapy in Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. Lancet (2003) 361:1927–34. doi: 10.1016/s0140-6736(03)13580-5
16. Choueiri TK, Jacobus S, Bellmunt J, Qu A, Appleman LJ, Tretter C, et al. Neoadjuvant Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin With Pegfilgrastim Support in Muscle-Invasive Urothelial Cancer: Pathologic, Radiologic, and Biomarker Correlates. J Clin Oncol (2014) 32:1889–94. doi: 10.1200/JCO.2013.52.4785
17. Plimack ER, Hoffman-Censits JH, Viterbo R, Trabulsi EJ, Ross EA, Greenberg RE, et al. Accelerated Methotrexate, Vinblastine, Doxorubicin, and Cisplatin is Safe, Effective, and Efficient Neoadjuvant Treatment for Muscle-Invasive Bladder Cancer: Results of a Multicenter Phase II Study With Molecular Correlates of Response and Toxicity. J Clin Oncol (2014) 32:1895–901. doi: 10.1200/JCO.2013.53.2465
18. Iyer G, Tully CM, Zabor EC, Bochner BH, Dalbagni G, Herr HW, et al. Neoadjuvant Gemcitabine-Cisplatin Plus Radical Cystectomy-Pelvic Lymph Node Dissection for Muscle-Invasive Bladder Cancer: A 12-Year Experience. Clin Genitourin Cancer (2020) 18:387–94. doi: 10.1016/j.clgc.2020.02.014
19. Yuh BE, Ruel N, Wilson TG, Vogelzang N, Pal SK. Pooled Analysis of Clinical Outcomes With Neoadjuvant Cisplatin and Gemcitabine Chemotherapy for Muscle Invasive Bladder Cancer. J Urol (2013) 189:1682–6. doi: 10.1016/j.juro.2012.10.120
20. Dash A, Pettus JA4, Herr HW, Bochner BH, Dalbagni G, Donat SM, et al. A Role for Neoadjuvant Gemcitabine Plus Cisplatin in Muscle-Invasive Urothelial Carcinoma of the Bladder: A Retrospective Experience. Cancer (2008) 113:2471–7. doi: 10.1002/cncr.23848
21. Lee FC, Harris W, Cheng HH, Shenoi J, Zhao S, Wang J, et al. Pathologic Response Rates of Gemcitabine/Cisplatin Versus Methotrexate/Vinblastine/Adriamycin/Cisplatin Neoadjuvant Chemotherapy for Muscle Invasive Urothelial Bladder Cancer. Adv Urol (2013) 2013:317190. doi: 10.1155/2013/317190
22. Galsky MD, Pal SK, Chowdhury S, Harshman LC, Crabb SJ, Wong YN, et al. Comparative Effectiveness of Gemcitabine Plus Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin as Neoadjuvant Therapy for Muscle-Invasive Bladder Cancer. Cancer (2015) 121:2586–93. doi: 10.1002/cncr.29387
23. von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-Term Survival Results of a Randomized Trial Comparing Gemcitabine Plus Cisplatin, With Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin in Patients With Bladder Cancer. J Clin Oncol (2005) 23:4602–8. doi: 10.1200/JCO.2005.07.757
24. Pfister C, Gravis G, Fléchon A, Soulié M, Guy L, Laguerre B, et al. Randomized Phase III Trial of Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin, or Gemcitabine and Cisplatin as Perioperative Chemotherapy for Patients With Muscle-Invasive Bladder Cancer. Analysis of the GETUG/AFU V05 VESPER Trial Secondary Endpoints: Chemotherapy Toxicity and Pathological Responses. Eur Urol (2021) 79:214–21. doi: 10.1016/j.eururo.2020.08.024
25. Cohen S, Gal J, Freifeld Y, Khoury S, Dekel Y, Hofman A, et al. Nutritional Status Impairment Due to Neoadjuvant Chemotherapy Predicts Post-Radical Cystectomy Complications. Nutrients (2021) 13:4471. doi: 10.3390/nu13124471
26. Mir MC, Marchioni M, Zargar H, Zargar-Shoshtari K, Fairey AS, Mertens LS, et al. Nomogram Predicting Bladder Cancer-Specific Mortality After Neoadjuvant Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer: Results of an International Consortium. Eur Urol Focus (2021) 7:1347–54. doi: 10.1016/j.euf.2020.07.002
27. Marandino L, Capozza A, Bandini M, Raggi D, Farè E, Pederzoli F, et al. [18f]Fluoro-Deoxy-Glucose Positron Emission Tomography to Evaluate Lymph Node Involvement in Patients With Muscle-Invasive Bladder Cancer Receiving Neoadjuvant Pembrolizumab. Urol Oncol (2021) 39:235.e15–235.e21. doi: 10.1016/j.urolonc.2020.09.035
28. Briganti A, Gandaglia G, Scuderi S, Gallina A, Colombo R, Fossati N, et al. Surgical Safety of Radical Cystectomy and Pelvic Lymph Node Dissection Following Neoadjuvant Pembrolizumab in Patients With Bladder Cancer: Prospective Assessment of Perioperative Outcomes From the PURE-01 Trial. Eur Urol (2020) 77:576–80. doi: 10.1016/j.eururo.2019.12.019
29. Font A, Luque R, Villa JC, Domenech M, Vázquez S, Gallardo E, et al. The Challenge of Managing Bladder Cancer and Upper Tract Urothelial Carcinoma: A Review With Treatment Recommendations From the Spanish Oncology Genitourinary Group (SOGUG). Target Oncol (2019) 14:15–32. doi: 10.1007/s11523-019-00619-7
30. Siddik ZH. Cisplatin: Mode of Cytotoxic Action and Molecular Basis of Resistance. Oncogene (2003) 22:7265–79. doi: 10.1038/sj.onc.1206933
31. Curtin NJ. DNA Repair Dysregulation From Cancer Driver to Therapeutic Target. Nat Rev Cancer (2012) 12:801–17. doi: 10.1038/nrc3399
32. Van Allen EM, Mouw KW, Kim P, Iyer G, Wagle N, Al-Ahmadie H, et al. Somatic ERCC2 Mutations Correlate With Cisplatin Sensitivity in Muscle-Invasive Urothelial Carcinoma. Cancer Discov (2014) 4:1140–53. doi: 10.1158/2159-8290.CD-14-0623
33. Plimack ER, Dunbrack RL, Brennan TA, Andrake MD, Zhou Y, Serebriiskii IG, et al. Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-Based Chemotherapy in Muscle-Invasive Bladder Cancer. Eur Urol (2015) 68:959–67. doi: 10.1016/j.eururo.2015.07.009
34. Liu D, Plimack ER, Hoffman-Censits J, Garraway LA, Bellmunt J, Van Allen E, et al. Clinical Validation of Chemotherapy Response Biomarker ERCC2 in Muscle-Invasive Urothelial Bladder Carcinoma. JAMA Oncol (2016) 2:1094–6. doi: 10.1001/jamaoncol.2016.1056
35. Groenendijk FH, de Jong J, Fransen van de Putte EE, Michaut M, Schlicker A, Peters D, et al. ERBB2 Mutations Characterize a Subgroup of Muscle-Invasive Bladder Cancers With Excellent Response to Neoadjuvant Chemotherapy. Eur Urol (2016) 69:384–8. doi: 10.1016/j.eururo.2015.01.014
36. Iyer G, Balar AV, Milowsky MI, Huang WC, Woods M, Donat SM, et al. Correlation of DNA Damage Response (DDR) Gene Alterations With Response to Neoadjuvant (Neo) Dose-Dense Gemcitabine and Cisplatin (ddGC) in Urothelial Carcinoma (Uc). J Clin Oncol (2016) 34:5011–1. doi: 10.1200/JCO.2016.34.15_suppl.5011
37. Seiler R, Ashab HAD, Erho N, van Rhijn BWG, Winters B, Douglas J, et al. Impact of Molecular Subtypes in Muscle-Invasive Bladder Cancer on Predicting Response and Survival After Neoadjuvant Chemotherapy. Eur Urol (2017) 72:544–54. doi: 10.1016/j.eururo.2017.03.030
38. Font A, Taron M, Gago JL, Costa C, Sánchez JJ, Carrato C, et al. BRCA1 mRNA Expression and Outcome to Neoadjuvant Cisplatin-Based Chemotherapy in Bladder Cancer. Ann Oncol (2011) 22:139–44. doi: 10.1093/annonc/mdq333
39. Iyer G, Balar AV, Milowsky MI, Bochner BH, Dalbagni G, Donat SM, et al. Multicenter Prospective Phase II Trial of Neoadjuvant Dose-Dense Gemcitabine Plus Cisplatin in Patients With Muscle-Invasive Bladder Cancer. J Clin Oncol (2018) 36:1949–56. doi: 10.1200/JCO.2017.75.0158
40. Bellmunt J, Paz-Ares L, Cuello M, Cecere FL, Albiol S, Guillem V, et al. Gene Expression of ERCC1 as a Novel Prognostic Marker in Advanced Bladder Cancer Patients Receiving Cisplatin-Based Chemotherapy. Ann Oncol (2007) 18:522–8. doi: 10.1093/annonc/mdl435
41. Britten RA, Liu D, Tessier A, Hutchison MJ, Murray D. ERCC1 Expression as a Molecular Marker of Cisplatin Resistance in Human Cervical Tumor Cells. Int J Cancer (2000) 89:453–7.
42. Pietzak EJ, Zabor EC, Bagrodia A, Armenia J, Hu W, Zehir A, et al. Genomic Differences Between "Primary" and "Secondary" Muscle-Invasive Bladder Cancer as a Basis for Disparate Outcomes to Cisplatin-Based Neoadjuvant Chemotherapy. Eur Urol (2019) 75:231–9. doi: 10.1016/j.eururo.2018.09.002
43. Miron B, Hoffman-Censits JH, Anari F, O'Neill J, Geynisman DM, Zibelman MR, et al. Defects in DNA Repair Genes Confer Improved Long-Term Survival After Cisplatin-Based Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer. Eur Urol Oncol (2020) 3:544–7. doi: 10.1016/j.euo.2020.02.003
44. Geynisman DM, Abbosh P, Ross EA, Zibelman MR, Ghatalia P, Anari F, et al. A Phase II Trial of Risk Enabled Therapy After Initiating Neoadjuvant Chemotherapy for Bladder Cancer (RETAIN BLADDER): Interim Analysis. J Clin Oncol (2021) 39:397–7. doi: 10.1200/JCO.2021
45. Heeke AL, Pishvaian MJ, Lynce F, Xiu J, Brody JR, Chen WJ, et al. Prevalence of Homologous Recombination-Related Gene Mutations Across Multiple Cancer Types. JCO Precis Oncol (2018) 2:PO.17.00286. doi: 10.1200/PO.17.00286
46. Romeo M, Pardo JC, Martínez-Cardús A, Martínez-Balibrea E, Quiroga V, Martínez-Román S, et al. Translational Research Opportunities Regarding Homologous Recombination in Ovarian Cancer. Int J Mol Sci (2018) 19:3249. doi: 10.3390/ijms19103249
47. Fradet Y, Bellmunt J, Vaughn DJ, Lee JL, Fong L, Vogelzang NJ, et al. Randomized Phase III KEYNOTE-045 Trial of Pembrolizumab Versus Paclitaxel, Docetaxel, or Vinflunine in Recurrent Advanced Urothelial Cancer: Results of >2 Years of Follow-Up. Ann Oncol (2019) 30:970–6. doi: 10.1093/annonc/mdz127
48. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med (2017) 376:1015–26. doi: 10.1056/NEJMoa1613683
49. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in Metastatic Urothelial Carcinoma After Platinum Therapy (CheckMate 275): A Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol (2017) 18:312–22. doi: 10.1016/S1470-2045(17)30065-7
50. Grivas P, Plimack ER, Balar AV, Castellano D, O'Donnell PH, Bellmunt J, et al. Pembrolizumab as First-Line Therapy in Cisplatin-Ineligible Advanced Urothelial Cancer (KEYNOTE-052): Outcomes in Older Patients by Age and Performance Status. Eur Urol Oncol (2020) 3:351–9. doi: 10.1016/j.euo.2020.02.009
51. Patel MR, Ellerton J, Infante JR, Agrawal M, Gordon M, Aljumaily R, et al. Avelumab in Metastatic Urothelial Carcinoma After Platinum Failure (JAVELIN Solid Tumor): Pooled Results From Two Expansion Cohorts of an Open-Label, Phase 1 Trial. Lancet Oncol (2018) 19:51–64. doi: 10.1016/S1470-2045(17)30900-2
52. Powles T, Durán I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab Versus Chemotherapy in Patients With Platinum-Treated Locally Advanced or Metastatic Urothelial Carcinoma (IMvigor211): A Multicentre, Open-Label, Phase 3 Randomised Controlled Trial. Lancet (2018) 391:748–57. doi: 10.1016/S0140-6736(17)33297-X
53. Vuky J, Balar AV, Castellano D, O'Donnell PH, Grivas P, Bellmunt J, et al. Long-Term Outcomes in KEYNOTE-052: Phase II Study Investigating First-Line Pembrolizumab in Cisplatin-Ineligible Patients With Locally Advanced or Metastatic Urothelial Cancer. J Clin Oncol (2020) 38:2658–66. doi: 10.1200/JCO.19.01213
54. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as First-Line Treatment in Cisplatin-Ineligible Patients With Locally Advanced and Metastatic Urothelial Carcinoma: A Single-Arm, Multicentre, Phase 2 Trial. Lancet (2017) 389:67–76. doi: 10.1016/S0140-6736(16)32455-2
55. Powles T, O'Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-Label Study. JAMA Oncol (2017) 3:e172411. doi: 10.1001/jamaoncol.2017.2411
56. Galsky MD, Saci A, Szabo PM, Han GC, Grossfeld G, Collette S, et al. Nivolumab in Patients With Advanced Platinum-Resistant Urothelial Carcinoma: Efficacy, Safety, and Biomarker Analyses With Extended Follow-Up From CheckMate 275. Clin Cancer Res (2020) 26:5120–8. doi: 10.1158/1078-0432.CCR-19-4162
57. Necchi A, Anichini A, Raggi D, Briganti A, Massa S, Lucianò R, et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J Clin Oncol (2018) 36:3353–60. doi: 10.1200/JCO.18.01148
58. Bandini M, Gibb EA, Gallina A, Raggi D, Marandino L, Bianchi M, et al. Does the Administration of Preoperative Pembrolizumab Lead to Sustained Remission Post-Cystectomy? First Survival Outcomes From the PURE-01 Study. Ann Oncol (2020) 31:1755–63. doi: 10.1016/j.annonc.2020.09.011
59. Powles T, Kockx M, Rodriguez-Vida A, Duran I, Crabb SJ, van der Heijden MS, et al. Clinical Efficacy and Biomarker Analysis of Neoadjuvant Atezolizumab in Operable Urothelial Carcinoma in the ABACUS Trial. Nat Med (2019) 25:1706–14. doi: 10.1038/s41591-019-0628-7
60. van Dijk N, Gil-Jimenez A, Silina K, Hendricksen K, Smit LA, de Feijter JM, et al. Preoperative Ipilimumab Plus Nivolumab in Locoregionally Advanced Urothelial Cancer: The NABUCCO Trial. Nat Med (2020) 26:1839–44. doi: 10.1038/s41591-020-1085-z
61. Grande E, Guerrero F, Puente J, Galante I, Duran I, Dominguez M, et al. DUTRENEO Trial: A Randomized Phase II Trial of DUrvalumab and TREmelimumab Versus Chemotherapy as a NEOadjuvant Approach to Muscle-Invasive Urothelial Bladder Cancer (MIBC) Patients (Pts) Prospectively Selected by an Interferon (INF)-Gamma Immune Signature. J Clin Oncol (2020) 38:5012. doi: 10.1200/JCO.2020.38.15_suppl.5012
62. Gupta S, Sonpavde G, Weight CJ, McGregor BA, Gupta S, Maughan BL, et al. Results From BLASST-1 (Bladder Cancer Signal Seeking Trial) of Nivolumab, Gemcitabine, and Cisplatin in Muscle Invasive Bladder Cancer (MIBC) Undergoing Cystectomy. J Clin Oncol (2020) 38:439. doi: 10.1200/JCO.2020.38.6_suppl.439
63. Fu P, Goolamier G, Eitman C, Ponsky LE, Hoimes CJ. Phase II Neoadjuvant (N-) Gemcitabine (G) and Pembrolizumab (P) for Locally Advanced Urothelial Cancer (laUC): Interim Results From the Cisplatin (C)-Ineligible Cohort of GU14-188. J Clin Oncol (2020) 38:5019. doi: 10.1200/JCO.2020.38.15_suppl.5019
64. Hoimes CJ, Adra N, Fleming MT, Kaimakliotis HZ, Picus J, Smith ZL, et al. Phase Ib/II Neoadjuvant (N-) Pembrolizumab (P) and Chemotherapy for Locally Advanced Urothelial Cancer (laUC): Final Results From the Cisplatin (C)- Eligible Cohort of HCRN GU14-188. J Clin Oncol (2020) 38:5047. doi: 10.1200/jco.2020.38.15_suppl.5047
65. Roviello G, Catalano M, Santi R, Palmieri VE, Vannini G, Galli IC, et al. Immune Checkpoint Inhibitors in Urothelial Bladder Cancer: State of the Art and Future Perspectives. Cancers (Basel) (2021) 13:4411. doi: 10.3390/cancers13174411
66. Rotte A. Combination of CTLA-4 and PD-1 Blockers for Treatment of Cancer. J Exp Clin Cancer Res (2019) 38:255. doi: 10.1186/s13046-019-1259-z
67. Warner AB, Postow MA. Combination Controversies: Checkpoint Inhibition Alone or in Combination for the Treatment of Melanoma? Oncol (Williston Park) (2018) 32:228–34.
68. Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 Combination Blockade Expands Infiltrating T Cells and Reduces Regulatory T and Myeloid Cells Within B16 Melanoma Tumors. Proc Natl Acad Sci USA (2010) 107:4275–80. doi: 10.1073/pnas.0915174107
69. Selby MJ, Engelhardt JJ, Johnston RJ, Lu LS, Han M, Thudium K, et al. Preclinical Development of Ipilimumab and Nivolumab Combination Immunotherapy: Mouse Tumor Models, In Vitro Functional Studies, and Cynomolgus Macaque Toxicology. PLoS One (2016) 11:e0161779. doi: 10.1371/journal.pone.0161779
70. Hayashi H, Nakagawa K. Combination Therapy With PD-1 or PD-L1 Inhibitors for Cancer. Int J Clin Oncol (2020) 25:818–30. doi: 10.1007/s10147-019-01548-1
71. Cathomas R, Rothschild S, Hayoz S, Spahn M, Schardt J, Seiler R, et al. Safety and Efficacy of Perioperative Cisplatin/Gemcitabine (Cis/Gem) and Durvalumab (Durva) for Operable Muscle-Invasive Urothelial Carcinoma (MIUC): SAKK 06/17. J Clin Oncol (2021) 39(6_suppl). doi: 10.1200/JCO.2021.39.6_suppl.430
72. Funt SA, Lattanzi M, Whiting K, Al-Ahmadie H, Quinlan C, Teo MY, et al. Neoadjuvant Atezolizumab With Gemcitabine and Cisplatin in Patients With Muscle-Invasive Bladder Cancer: A Multicenter, Single-Arm, Phase II Trial. J Clin Oncol (2022) 40:1312–22. doi: 10.1200/JCO.21.01485
73. Birrer MJ, Moore KN, Betella I, Bates RC. Antibody-Drug Conjugate-Based Therapeutics: State of the Science. J Natl Cancer Inst (2019) 111:538–49. doi: 10.1093/jnci/djz035
74. Rosenberg JE, O'Donnell PH, Balar AV, McGregor BA, Heath EI, Yu EY, et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol (2019) 37:2592–600. doi: 10.1200/JCO.19.01140
75. Petrylak DP, Flaig TW, Mar N, Gourdin TS, Srinivas S, Rosenberg JE, et al. Study EV-103 Cohort H: Antitumor Activity of Neoadjuvant Treatment With Enfortumab Vedotin Monotherapy in Patients (Pts) With Muscle Invasive Bladder Cancer (MIBC) Who are Cisplatin-Ineligible. J Clin Oncol (2022) 40:435–5.
76. Trerotola M, Cantanelli P, Guerra E, Tripaldi R, Aloisi AL, Bonasera V, et al. Upregulation of Trop-2 Quantitatively Stimulates Human Cancer Growth. Oncogene (2013) 32:222–33. doi: 10.1038/onc.2012.36
77. Necchi A, Raggi D, Bandini M, Gallina A, Capitanio U, Gandaglia G, et al. SURE: An Open Label, Sequential-Arm, Phase II Study of Neoadjuvant Sacituzumab Govitecan (SG), and SG Plus Pembrolizumab (Pembro) Before Radical Cystectomy, for Patients With Muscle-Invasive Bladder Cancer (MIBC) Who Cannot Receive or Refuse Cisplatin-Based Chemotherapy. J Clin Oncol (2021) 39(6_suppl). doi: 10.1200/JCO.2021.39.6_suppl.TPS506
78. Rodriguez-Moreno JF, Sevillano E, Fenor M, Collado Martín R, Esteban E, Fernandez R, et al. Impact of the Combination of Durvalumab (MEDI4736) Plus Olaparib (AZD2281) Administered Prior to Surgery in the Molecular Profile of Resectable Urothelial Bladder Cancer: NEODURVARIB Trial. J Clin Oncol (2019) 37(7_suppl). doi: 10.1200/JCO.2019.37.7_suppl.TPS503
79. Milowsky M, Davis N, Fung C, Johnson S, Langenstroer P, Jacobsohn K, et al. A Phase II Study of Cabozantinib in Combination With Atezolizumab as Neoadjuvant Treatment for Muscle-Invasive Bladder Cancer (ABATE). Ann Oncol (2020) 31:S550–0. doi: 10.1016/j.annonc.2020.08.2072
80. Hussain SA, Birtle A, Crabb S, Huddart R, Small D, Summerhayes M, et al. From Clinical Trials to Real-Life Clinical Practice: The Role of Immunotherapy With PD-1/PD-L1 Inhibitors in Advanced Urothelial Carcinoma. Eur Urol Oncol (2018) 1:486–500. doi: 10.1016/j.euo.2018.05.011
81. Roviello G, Catalano M, Nobili S, Santi R, Mini E, Nesi G. Focus on Biochemical and Clinical Predictors of Response to Immune Checkpoint Inhibitors in Metastatic Urothelial Carcinoma: Where do We Stand? Int J Mol Sci (2020) 21:7935. doi: 10.3390/ijms21217935
82. Aggen DH, Drake CG. Biomarkers for Immunotherapy in Bladder Cancer: A Moving Target. J Immunother Cancer (2017) 5:94. doi: 10.1186/s40425-017-0299-1
83. Boorjian SA, Sheinin Y, Crispen PL, Farmer SA, Lohse CM, Kuntz SM, et al. T-Cell Coregulatory Molecule Expression in Urothelial Cell Carcinoma: Clinicopathologic Correlations and Association With Survival. Clin Cancer Res (2008) 14:4800–8. doi: 10.1158/1078-0432.CCR-08-0731
84. Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-γ-Related mRNA Profile Predicts Clinical Response to PD-1 Blockade. J Clin Invest (2017) 127:2930–40. doi: 10.1172/JCI91190
85. Joshi M, Grivas P, Mortazavi A, Monk P, Clinton SK, Sue-Ann Woo M, et al. Alterations of DNA Damage Response Genes Correlate With Response and Overall Survival in Anti-PD-1/PD-L1-Treated Advanced Urothelial Cancer. Cancer Med (2020) 9:9365–72. doi: 10.1002/cam4.3552
86. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors With Mismatch-Repair Deficiency. N Engl J Med (2015) 372:2509–20. doi: 10.1056/NEJMoa1500596
87. Versluis JM, Long GV, Blank CU. Learning From Clinical Trials of Neoadjuvant Checkpoint Blockade. Nat Med (2020) 26:475–84. doi: 10.1038/s41591-020-0829-0
88. Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. Tgfβ Attenuates Tumour Response to PD-L1 Blockade by Contributing to Exclusion of T Cells. Nature (2018) 554:544–8. doi: 10.1038/nature25501
89. Teo MY, Bambury RM, Zabor EC, Jordan E, Al-Ahmadie H, Boyd ME, et al. DNA Damage Response and Repair Gene Alterations are Associated With Improved Survival in Patients With Platinum-Treated Advanced Urothelial Carcinoma. Clin Cancer Res (2017) 23:3610–8. doi: 10.1158/1078-0432.CCR-16-2520
90. Teo MY, Seier K, Ostrovnaya I, Regazzi AM, Kania BE, Moran MM, et al. Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit From PD-1/PD-L1 Blockade in Advanced Urothelial Cancers. J Clin Oncol (2018) 36:1685–94. doi: 10.1200/JCO.2017.75.7740
91. Krantz SB, Mitzman B, Lutfi W, Kuchta K, Wang CH, Howington JA, et al. Neoadjuvant Chemoradiation Shows No Survival Advantage to Chemotherapy Alone in Stage IIIA Patients. Ann Thorac Surg (2018) 105:1008–16. doi: 10.1016/j.athoracsur.2017.10.056
92. Galsky MD, Arija JÁA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab With or Without Chemotherapy in Metastatic Urothelial Cancer (IMvigor130): A Multicentre, Randomised, Placebo-Controlled Phase 3 Trial. Lancet (2020) 395:1547–57. doi: 10.1016/S0140-6736(20)30230-0
93. Zhang S, Yu YH, Zhang Y, Qu W, Li J. Radiotherapy in Muscle-Invasive Bladder Cancer: The Latest Research Progress and Clinical Application. Am J Cancer Res (2015) 5:854–68.
94. Park I, Lee JL. Systemic Treatment for Advanced Urothelial Cancer: An Update on Recent Clinical Trials and Current Treatment Options. Korean J Intern Med (2020) 35:834–53. doi: 10.3904/kjim.2020.204
95. Chang SS. Re: Genomic Differences Between "Primary" and "Secondary" Muscle-Invasive Bladder Cancer as a Basis for Disparate Outcomes to Cisplatin-Based Neoadjuvant Chemotherapy. J Urol (2019) 202:30. doi: 10.1097/01.JU.0000557728.16853.8f
96. Lefkowitz RB, Tynan JA, Liu T, Wu Y, Mazloom AR, Almasri E, et al. Clinical Validation of a Noninvasive Prenatal Test for Genomewide Detection of Fetal Copy Number Variants. Am J Obstet Gynecol (2016) 215:227.e1–.e16. doi: 10.1016/j.ajog.2016.02.030
97. Sjödahl G, Lauss M, Lövgren K, Chebil G, Gudjonsson S, Veerla S, et al. A Molecular Taxonomy for Urothelial Carcinoma. Clin Cancer Res (2012) 18:3377–86. doi: 10.1158/1078-0432.CCR-12-0077-T
98. Damrauer JS, Hoadley KA, Chism DD, Fan C, Tiganelli CJ, Wobker SE, et al. Intrinsic Subtypes of High-Grade Bladder Cancer Reflect the Hallmarks of Breast Cancer Biology. Proc Natl Acad Sci USA (2014) 111:3110–5. doi: 10.1073/pnas.1318376111
99. Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, et al. Identification of Distinct Basal and Luminal Subtypes of Muscle-Invasive Bladder Cancer With Different Sensitivities to Frontline Chemotherapy. Cancer Cell (2014) 25:152–65. doi: 10.1016/j.ccr.2014.01.009
100. Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell (2017) 171:540–56.e25. doi: 10.1016/j.cell.2017.09.007
101. Kamoun A, de Reyniès A, Allory Y, Sjödahl G, Robertson AG, Seiler R, et al. A Consensus Molecular Classification of Muscle-Invasive Bladder Cancer. Eur Urol (2020) 77:420–33. doi: 10.1016/j.eururo.2019.09.006
102. Cancer Genome Atlas Research Network. Comprehensive Molecular Characterization of Urothelial Bladder Carcinoma. Nature (2014) 507:315–22. doi: 10.1038/nature12965
103. Carlo MI, Ravichandran V, Srinavasan P, Bandlamudi C, Kemel Y, Ceyhan-Birsoy O, et al. Cancer Susceptibility Mutations in Patients With Urothelial Malignancies. J Clin Oncol (2020) 38:406–14. doi: 10.1200/JCO.19.01395
104. Lotan Y, Boorjian SA, Zhang J, Bivalacqua TJ, Porten SP, Wheeler T, et al. Molecular Subtyping of Clinically Localized Urothelial Carcinoma Reveals Lower Rates of Pathological Upstaging at Radical Cystectomy Among Luminal Tumors. Eur Urol (2019) 76:200–6. doi: 10.1016/j.eururo.2019.04.036
105. Makboul R, Hassan HM, Refaiy A, Abdelkawi IF, Shahat AA, Hameed DA, et al. A Simple Immunohistochemical Panel Could Predict and Correlate to Clinicopathologic and Molecular Subgroups of Urinary Bladder Urothelial Carcinoma. Clin Genitourin Cancer (2019) 17:e712–9. doi: 10.1016/j.clgc.2019.04.011
106. Font A, Domènech M, Benítez R, Rava M, Marqués M, Ramírez JL, et al. Immunohistochemistry-Based Taxonomical Classification of Bladder Cancer Predicts Response to Neoadjuvant Chemotherapy. Cancers (Basel) (2020) 12:1784. doi: 10.3390/cancers12071784
107. Seiler R, Gibb EA, Wang NQ, Oo HZ, Lam HM, van Kessel KE, et al. Divergent Biological Response to Neoadjuvant Chemotherapy in Muscle-Invasive Bladder Cancer. Clin Cancer Res (2019) 25:5082–93. doi: 10.1158/1078-0432.CCR-18-1106
108. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in Patients With Locally Advanced and Metastatic Urothelial Carcinoma Who Have Progressed Following Treatment With Platinum-Based Chemotherapy: A Single-Arm, Multicentre, Phase 2 Trial. Lancet (2016) 387:1909–20. doi: 10.1016/S0140-6736(16)00561-4
109. Kardos J, Chai S, Mose LE, Selitsky SR, Krishnan B, Saito R, et al. Claudin-Low Bladder Tumors are Immune Infiltrated and Actively Immune Suppressed. JCI Insight (2016) 1:e85902. doi: 10.1172/jci.insight.85902
110. Kim J, Kwiatkowski D, McConkey DJ, Meeks JJ, Freeman SS, Bellmunt J, et al. The Cancer Genome Atlas Expression Subtypes Stratify Response to Checkpoint Inhibition in Advanced Urothelial Cancer and Identify a Subset of Patients With High Survival Probability. Eur Urol (2019) 75:961–4. doi: 10.1016/j.eururo.2019.02.017
Keywords: muscle-invasive bladder cancer, neoadjuvant chemotherapy, immunotherapy, combined therapy, biomarkers, molecular subtypes
Citation: Roviello G, Catalano M, Santi R, Santoni M, Galli IC, Amorosi A, Polom W, De Giorgi U and Nesi G (2022) Neoadjuvant Treatment in Muscle-Invasive Bladder Cancer: From the Beginning to the Latest Developments. Front. Oncol. 12:912699. doi: 10.3389/fonc.2022.912699
Received: 04 April 2022; Accepted: 22 June 2022;
Published: 22 July 2022.
Edited by:
Gianluca Ingrosso, University of Perugia, ItalyReviewed by:
Linda Cerbone, San Camillo-Forlanini Hospital, ItalyLuca Afferi, Luzerner Kantonsspital, Switzerland
Copyright © 2022 Roviello, Catalano, Santi, Santoni, Galli, Amorosi, Polom, De Giorgi and Nesi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriella Nesi, Z2FicmllbGxhLm5lc2lAdW5pZmkuaXQ=
 Giandomenico Roviello
Giandomenico Roviello Martina Catalano
Martina Catalano Raffaella Santi
Raffaella Santi Matteo Santoni
Matteo Santoni Ilaria Camilla Galli4
Ilaria Camilla Galli4 Ugo De Giorgi
Ugo De Giorgi Gabriella Nesi
Gabriella Nesi