- 1Oncology, Guangdong Medical University, Zhanjiang, China
- 2Department of Head and Neck/Thoracic Medical Oncology, The First People’s Hospital of Foshan, Foshan, China
- 3Medical Department Shanghai OrigiMed Co., Ltd, Shanghai, China
Non-small cell lung cancer (NSCLC) patients harboring MET exon 14 skipping or high MET amplification display a high rate of response to MET inhibitors. However, MET fusions in NSCLC have rarely been revealed. In this report, a 63-year-old woman with lung adenocarcinoma (LADC), harboring EGFR exon 18 G719D and exon 21 L861Q mutations, received first-generation, EGFR-tyrosine kinase inhibitor (TKI) icotinib therapy. Next generation sequencing (NGS) results only displayed an EGFR T790M point mutation following icotinib resistance. Thus, the patient was treated with osimertinib and achieved a stable disease (SD). However, disease progressed after 15 months and a novel MET fusion (CUX1 exon14-MET exon15) in addition to EGFR G719D/L861Q mutations were simultaneously detected in a tissue biopsy sample. After more than nine months, the patient subsequently achieved a PR with the combination of icotinib and crizotinib. To our knowledge, this is the first case of LADC patient displaying the presence of EGFR double uncommon mutations and an acquired novel CUX1-MET fusion that has benefited from icotinib plus crizotinib treatment. Following nine months of PR with icotinib plus crizotinib, the patient, until the time of publication, is exhibiting stable disease. The results suggest that the CUX1-MET fusion may be sensitive to crizotinib, although previous reports indicated that some MET fusion cases did not respond to crizotinib. Given this disparity, distinguishing MET fusion partners when crizotinib is used in LADC treatment is also very important.
Introduction
Mutations within the epidermal growth factor receptor (EGFR) are an important driver of lung cancers. Targeting EGFR with tyrosine kinase inhibitors (TKIs) is the main precision medicine treatment option and the standard treatment for lung adenocarcinoma (LADC) patients harboring EGFR activating mutations (1, 2). EGFR-TKIs have markedly improved the prognoses of patients with non-small cell lung cancer (NSCLC) harboring EGFR-activating mutations. Mutations such as EGFR 19del and EGFR L858R are known as “classical mutations” that often confer a better response to EGFR-TKIs (3, 4). Other mutations within EGFR exons 18-21 such as EGFR L861Q and G719D mutations, which are non-classical mutations with a low incidence, a poor response, and an uncertain resistance when treated with an EGFR-TKI, have also been reported (5). In recent years, several EGFR-TKIs that are used as a first-line treatment for NSCLC with EGFR mutations, such as the first-generation TKIs erlotinib, gefitinib and icotinib, second-generation TKIs afatinib and dacotinib, and third-generation TKI osimertinib, have been approved by the United States (US) Food and Drug Administration (FDA). Osimertinib benefits NSCLC patients with EGFR T790M mutation who fail in first-generation EGFR-TKIs treatment (6, 7). However, osimertinib resistances have been reported and MET amplification is one mechanism for acquired resistance to third generation EGFR-TKIs (8). A previous study indicated that the combinatorial treatment of first/third-generation EGFR-TKIs and crizotinib was efficaciously treated two patients with newly acquired MET amplification following osimertinib resistance (9). However, MET fusion is relatively rare in NSCLC. In this case study, we report a unique LADC case with two uncommon EGFR mutations, who had a partial response to icotinib plus crizotinib, with an acquired novel MET fusion (CUX1 exon14-MET exon15) following osimertinib treatment. Tolerance for the addition of crizotinib to the EGFR-TKI treatment was quite good and proceeded without the emergence of unexpected toxicities.
Case presentation
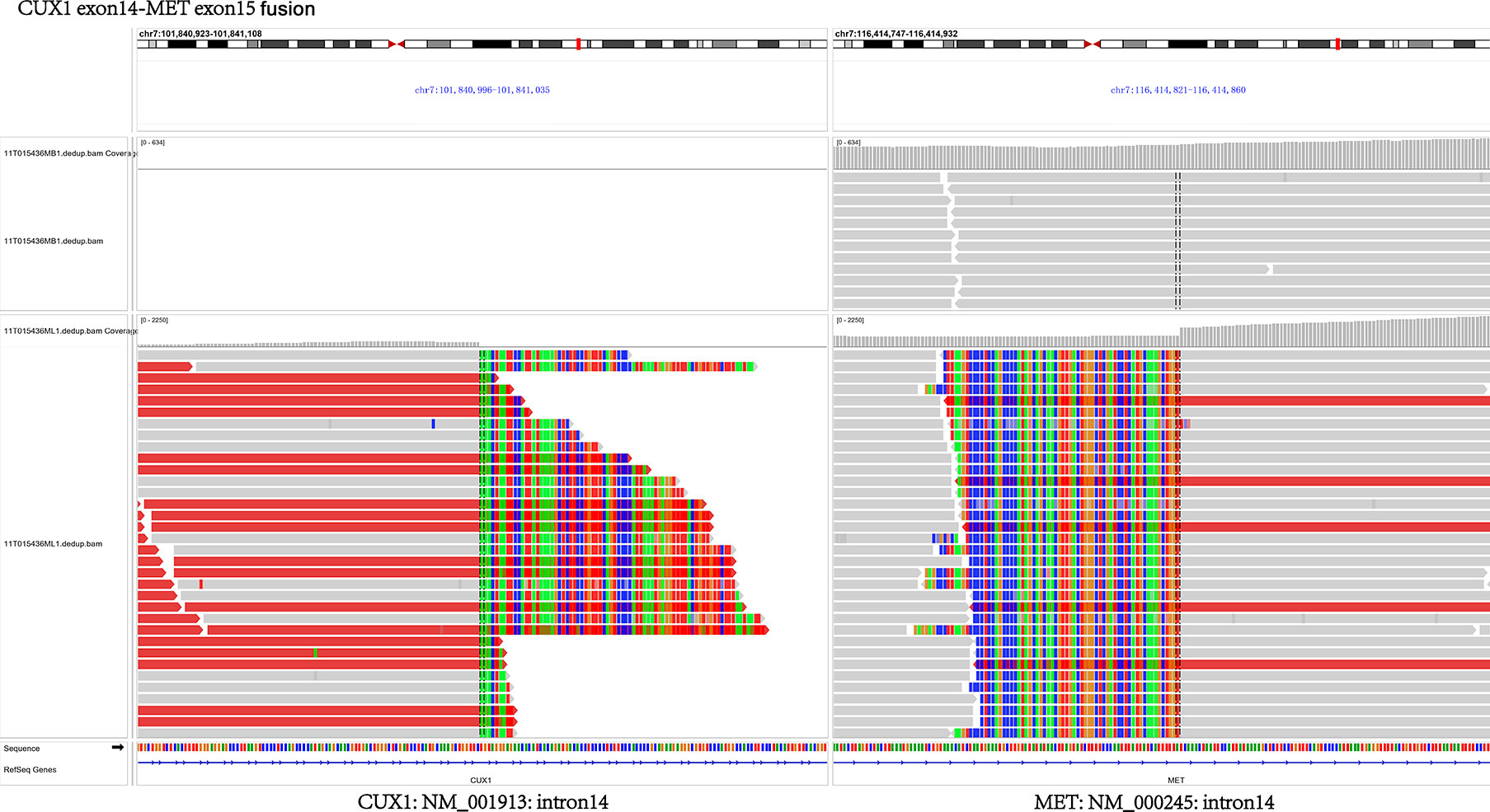
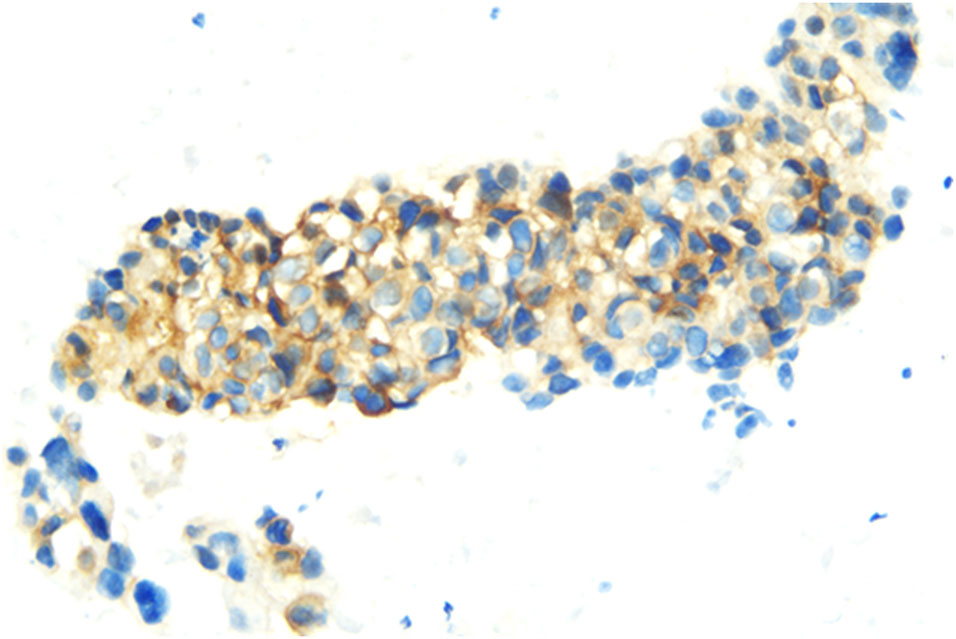
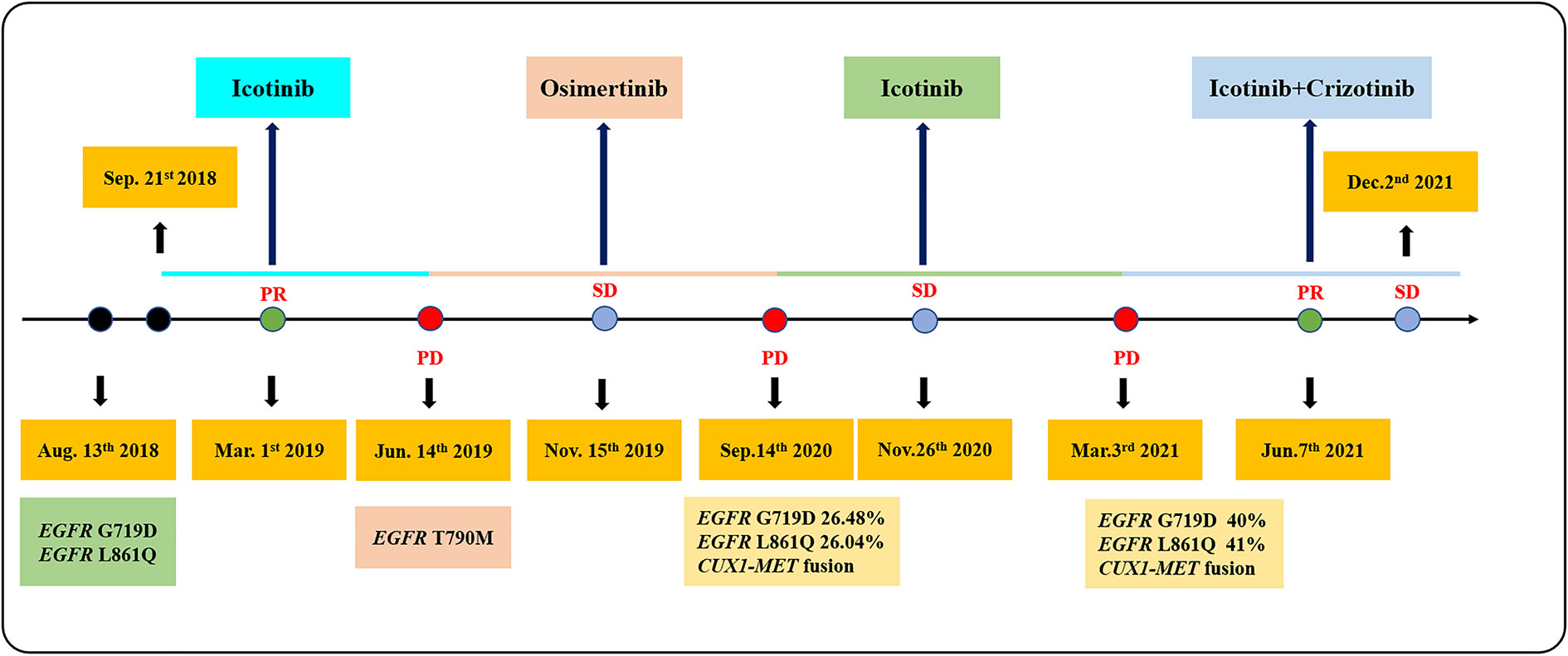
In July 2018, a 63-year-old woman, who had never smoked, presented with a cough and sputum, and was diagnosed with LADC (Stage cT4N2M0 IIIB). Biopsy tissue was sent for next generation sequencing (NGS) testing, and two EGFR uncommon mutations (exon 21 L861Q and exon 18 G719D) were identified. The patient received the first-generation EGFR-TKI icotinib, at a dose of 125 mg, a TID standard daily dose on September 21, 2018, and had a good response upon first evaluation on March 1, 2019. A computed tomographic (CT) scan revealed that the paramediastinal mass, located within the right upper lung, was significantly smaller than previously determined, and the patient achieved PR according to RECIST version 1.1. The patient was re-examined on June 14, 2019 and discouraging information was determined. The CT scan performed at this time indicated an enlarged pulmonary mass and the patient experienced progressive disease. NGS testing was performed again and an EGFR T790M point mutation was identified. The patient was then treated with the third-generation EGFR-TKI osimertinib. A chest CT on November 15, 2019 revealed disease stability. On September 14, 2020, the CT scan showed an enlargement of lung lesions and the patient, once again, experienced disease progression. An NGS test indicated that in addition to the coexistence of the EGFR L861Q/G719D double mutations, a novel acquired CUX1 (exon 14)-MET (exon 15) fusion was identified for the first time (Figure 1). An immunohistochemistry assay indicated strong expression for MET (Figure 2). From September 14, 2020, the patient was, once again, treated with icotinib (125 mg, TID), and a chest CT on November 26, 2020 revealed disease stability. The patient again experienced disease progression on March 3, 2021 that included the presence of an enlarged lung lesion (74 mm × 78 mm), pleural thickening, and a small amount of pleural effusion (Figure 3A). NGS results, concurrent with EGFR double uncommon mutations and a CUX1-MET fusion, led us to a combined targeted strategy with icotinib (125 mg, TID) and crizotinib (250 mg, BID), inhibitors that targeted EGFR and MET, respectively, from March 3, 2021. On June 7, 2021, a CT scan revealed that the paramediastinal mass within the lung was significantly smaller (45 mm × 42 mm) than previously determined, and the patient reached PR (Figure 3B). To date, nine months have passed, and the patient is still at stable disease (SD) (Figures 3C, D). Figure 4 provides a timeline that includes relevant care data.
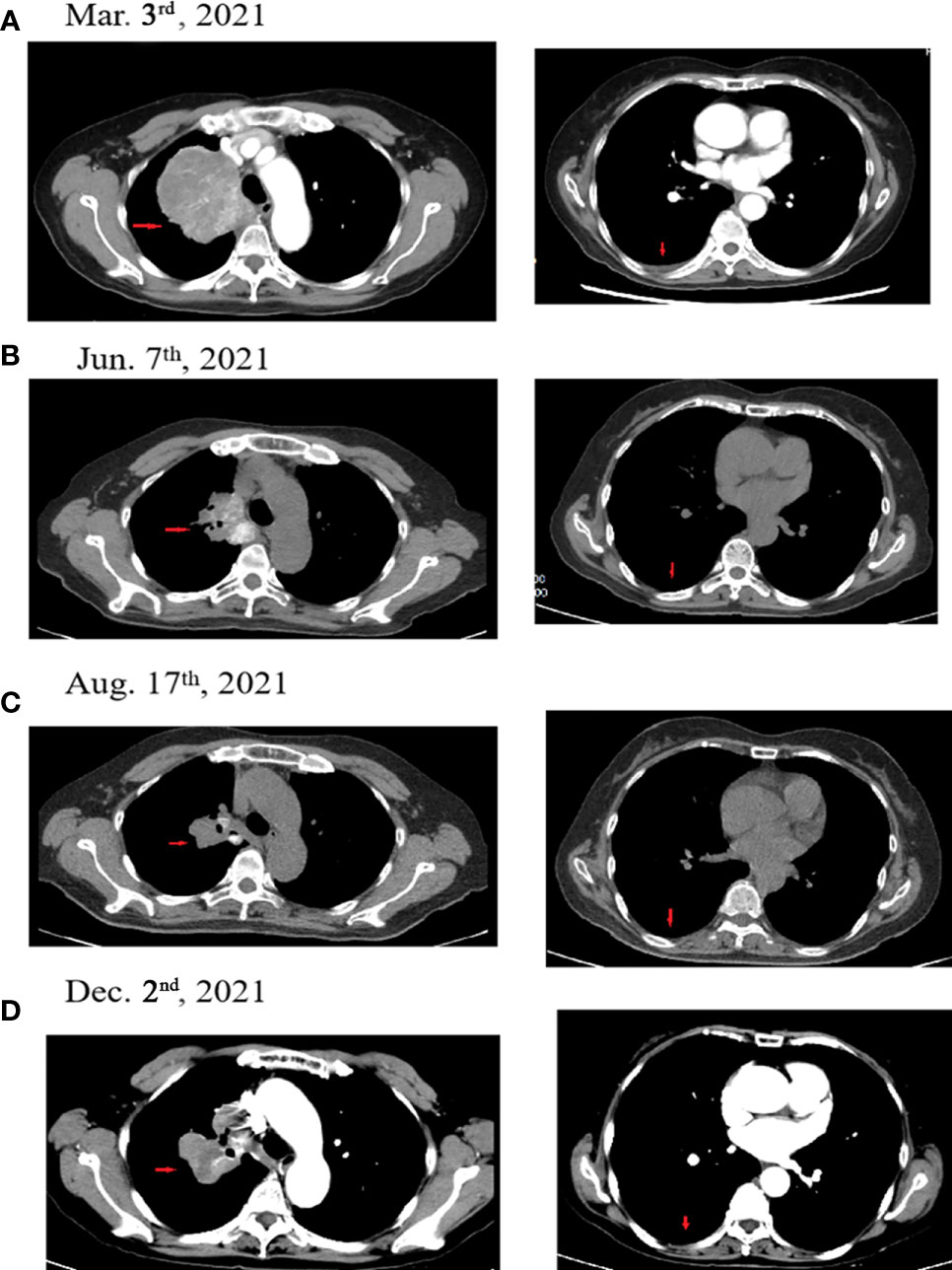
Figure 3 Computed tomographic (CT) images for the patient during the treatment course. (A) The patient experienced progressive disease during icotinib therapy (March 3, 2021). (B) The patient achieved PR following icotinib plus crizotinib therapy (June 7, 2021). (C) The patient achieved PR following icotinib plus crizotinib therapy (August 17, 2021). (D) The patient achieved SD following icotinib plus crizotinib therapy (December 2, 2021). Red arrows indicate the tumor.
Discussion
EGFR-TKIs are recommended for patients with NSCLC having EGFR mutations (10). Data regarding treatment effectiveness for EGFR-TKIs in NSCLC patients with non-resistant, uncommon EGFR mutations are limited. A previous report suggested that the combination of two uncommon mutations is associated with poorer prognosis (median progression free survival (PFS): 6.5 months), as compared to combinations of two common mutations (median PFS: 10.1 months) or a common and uncommon mutation (median PFS: 10.5 months) (11). Substitution mutations G719X, L861Q, and S768I are of particular interest as uncommon mutations. According to a recent molecular epidemiological study in Asia, these three mutations constitute approximately 6% of all EGFR mutations (12). The G719X and L861Q mutations were found to be included in several commercial EGFR mutation detection kits. These uncommon mutations are occasionally found in daily clinical practice, although the clinical effectiveness of EGFR-TKIs in treating patients with these uncommon mutations remains unclear. The response rate to an EGFR-TKI treatment has been determined to be significantly higher in tumors with compound mutations (G719X + L861Q and G719X + S768I) as compared to tumors with single mutations (uncommon) (68.4% vs. 37.8%, p = 0.011) (12). Jin et al. (13) reported that icotinib is an effective treatment option for patients with the LADC harboring compound EGFR L858R and an A871G mutation (13). Another study reported a case of LADC patient with the same EGFR L861Q and R776H compound mutations, who received gefitinib and achieved stable disease (SD) with a PFS of 2.2 months (14). Here, we report a LADC patient with uncommon EGFR G719D/L861Q mutations who was treated with icotinib therapy. After five months, the patient achieved PR. However, the patient experienced progressive disease after another three months. To date, the most common acquired resistance mechanism to first generation EGFR-TKIs is the T790M mutation, which accounts for approximately 60% of resistance cases (15). With the exception of the T790M mutation (16), optimal treatment for the various mechanisms associated with acquired resistance is not yet clearly defined. Several studies have comprehensively explored the mechanisms of acquired resistance to EFGR-TKIs using re-biopsy tissue specimens. In such studies, the most common acquired resistance mechanisms have been attributed to target gene modification, alternative pathway activation, and histological or phenotypic transformation (16).
Many past studies have shown that MET alteration is a mechanism of acquired resistance to EGFR-TKIs. As a proto-oncogene, the carcinogenic effect of MET has been demonstrated for multiple tumors (17). Studies have revealed that mutations within the splice site of MET that result in the skipping of exon 14 is important molecular driver for NSCLC (18). MET inhibitors have resulted in a therapeutic response in patients with LADC (19). High-level MET amplification, MET exon 14 skipping alterations, or MET overexpression are different types of MET alternations (20). Since EGFR mutations and MET fusions are markedly sensitive to EGFR-TKIs and crizotinib, respectively, clinicians should pay more attention to the existence of EGFR mutation and MET fusion (21–23). Crizotinib is a small-molecule tyrosinase inhibitor that effectively inhibits high-level MET amplification or the MET exon 14 skipping mutation. National Comprehensive Cancer Network (NCCN) guidelines have approved crizotinib as a first-line therapy or as a subsequent therapy option for patients with metastatic NSCLC harboring MET exon 14 skipping mutation. Cohort studies of osimertinib resistance mechanism, especially MET-associated, have been poorly investigated. NSCLC patient achieved PR to the combination of first-generation EGFR-TKI icotinib and crizotinib, after the previous treatment history of third-generation TKI osimertinib resistance (9). Preclinical studies have suggested that resistance to third-generation EGFR-TKIs would primarily owing to the additional mutations in EGFR itself (for example, C797S) (24, 25). MET fusions are rare in LADC. As such, standard treatment for patients with MET fusions has not been determined. In past studies, acquired MET fusions (CAV1-MET fusion, MET-UBE2H fusion, and SPECC1L-MET fusion) have been identified in three LADC patients with EGFR L858R mutation/EGFR 19 exon mutations following EGFR-TKIs; and a partial reaction to crizotinib was determined (21, 26, 27). To our knowledge, no reports currently exist for LADC patients harboring EGFR two uncommon mutations and an acquired MET fusion. The EGFR mutations reported in previous studies in NSCLC patients with acquired MET fusions were common variants. Here, we reported a unique case of LADC patient with EGFR double uncommon mutations and an acquired novel MET fusion (CUX1 exon14-MET exon15) following icotinib resistance who had a PR to icotinib plus crizotinib administration. A previous report showed LADC patient harboring MET-ATXN7L1 fusion obtained a PR to crizotinib although the disease progressed after four months (22). Another study reported an acquired MET fusion following osimertinib therapy in a LADC patient with EGFR mutation, however, at the time of the study, the patient had only received therapy for one month and the patient’s condition was significantly declining (21). Therefore, distinguishing the MET fusion partners when the MET inhibitor crizotinib is used in clinical practice is very important. Our patient was treated using a combined targeted strategy of icotinib and crizotinib, and reached PR. To date, nine months have passed, the patient remains at SD.
In conclusion, we reported a unique case of LADC patient with EGFR double uncommon mutations and an acquired novel MET fusion (CUX1 exon14-MET exon15) following treatment with osimertinib that had a PR to the administration of icotinib plus crizotinib. Here, for the first time, we suggest that MET fusion may be one of the resistance mechanisms in patients with EGFR uncommon mutations and that distinguishing MET fusion partners is important for crizotinib treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study has been approved by ethics committee of The First People’s Hospital of Foshan. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WF, LO, YT, and YD all participated in the management of this case. LG, QH, and TH were in charge of data collection. WF and LO did the modification. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank the patient and her family for their engagement in this case study.
Conflict of interest
Authors LG, QH, and TH were employed by Shanghai OrigiMed Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen F, Liu J, Flight RM, Naughton KJ, Lukyanchuk A, Edgin AR, et al. Cellular origins of EGFR-driven lung cancer cells determine sensitivity to therapy. Adv Sci Weinh (2021) 8(22):e2101999. doi: 10.1002/advs.202101999
2. Fang YF, Liu PC. Afatinib and osimertinib in lung adenocarcinoma harbored EGFR T751_I759delinsS mutation: A case report. Thorac Cancer (2021) 12(24):3429–32. doi: 10.1111/1759-7714.14215
3. Amann J, Kalyankrishna S, Massion PP, Ohm JE, Girard L, Shigematsu H, et al. Aberrant epidermal growth factor receptor signaling and enhanced sensitivity to EGFR inhibitors in lung cancer. Cancer Res (2005) 65(1):226–35.
4. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science (2004) 304(5676):1497–500. doi: 10.1126/science.1099314
5. Floc'h N, Lim S, Bickerton S, Ahmed A, Orme J, Urosevic J, et al. Osimertinib, an irreversible next-generation EGFR tyrosine kinase inhibitor, exerts antitumor activity in various preclinical NSCLC models harboring the uncommon EGFR mutations G719X or L861Q or S768I. Mol Cancer Ther (2020) 19(11):2298–307. doi: 10.1158/1535-7163
6. Remon J, Caramella C, Jovelet C, Lacroix L, Lawson A, Smalley S, et al. Osimertinib benefit in EGFR-mutant NSCLC patients with T790M-mutation detected by circulating tumour DNA. Ann Oncol (2017) 28(4):784–90. doi: 10.1093/annonc/mdx017
7. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med (2017) 376(7):29–640. doi: 10.1056/NEJMoa1612674
8. Ou SI, Agarwal N, Ali SM. High MET amplification level as a resistance mechanism to osimertinib AZD9291 in a patient that symptomatically responded to crizotinib treatment post-osimertinib progression. Lung Cancer (2016) 98:59–61. doi: 10.1016/j.lungcan.2016.05.015
9. Wang Y, Li L, Han R, Jiao L, Zheng J, He Y. Clinical analysis by next-generation sequencing for NSCLC patients with MET amplification resistant to osimertinib. Lung Cancer (2018) 118:105–10. doi: 10.1016/j.lungcan.2018.02.007
10. Liu Q, Wu L, Zhang S. Transformation of advanced lung adenocarcinoma to acquired T790M resistance mutation adenosquamous carcinoma following tyrosine kinase inhibitor: a case report. Tumori (2021) 107(6):NP5–NP10. doi: 10.1177/0300891620973262
11. Yu X, Zhang X, Zhang Z, Lin Y, Wen Y, Chen Y, et al. First-generation EGFR tyrosine kinase inhibitor therapy in 106 patients with compound EGFR-mutated lung cancer: a single institution's clinical practice experience. Cancer Commun Lond (2018) 38(1):51. doi: 10.1186/s40880-018-0321-0
12. Chiu CH, Yang CT, Shih JY, Huang MS, Su WC, Lai RS, et al. Epidermal growth factor receptor tyrosine kinase inhibitor treatment response in advanced lung adenocarcinomas with G719X/L861Q/S768I mutations. J Thorac Oncol (2015) 10(5):793–9. doi: 10.1097/JTO.0000000000000504
13. Jin LL, Wu ZZ, Wang YL, Chen DS, Li S, Xiao M, et al. Icotinib, an effective treatment option for patients with lung adenocarcinoma harboring compound EGFR L858R and A871G mutation. Invest New Drugs (2021) 39(5):1419–21. doi: 10.1007/s10637-021-01108-3
14. Wu JY, Wu SG, Yang CH, Gow CH, Chang YL, Yu CJ, et al. Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin Cancer Res (2008) 14(15):4877–82. doi: 10.1158/1078-0432.CCR-07-5123
15. Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res (2013) 19(8):2240–7. doi: 10.1158/1078-0432.CCR-12-2246
16. Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer (2018) 17(1):1738. doi: 10.1186/s12943-018-0777-1
17. Salgia R, Stille JR, Weaver RW, McCleod M, Hamid O, Polzer J, et al. A randomized phase II study of LY2510924 and carboplatin/etoposide versus carboplatin/etoposide in extensive-disease small cell lung cancer. Lung Cancer (2017) 10:57–13. doi: 10.1016/j.lungcan.2016.12.020
18. Macaluso M, Montanari M, Giordano A. The regulation of ER-alpha transcription by pRb2/p130 in breast cancer. Ann Oncol (2005) 16(Suppl 4):4iv20–22. doi: 10.1093/annonc/mdi903
19. Ahn JS, Ahn YC, Kim JH, Lee CG, Cho EK, Lee KC, et al. Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KCSG-LU05-04. J Clin Oncol (2015) 33(24):2660–6. doi: 10.1200/JCO.2014.60.0130
20. Su S, Lin A, Luo P, Zou J, Huang Z, Wang X, et al. Effect of mesenchymal-epithelial transition amplification on immune microenvironment and efficacy of immune checkpoint inhibitors in patients with non-small cell lung cancer. Ann Transl Med (2021) 9(18):1475. doi: 10.21037/atm-21-4543
21. Nelson AW, Schrock AB, Pavlick DC, Ali SM, Atkinson EC, Chachoua A. Novel SPECC1L-MET fusion detected in circulating tumor DNA in a patient with lung adenocarcinoma following treatment with erlotinib and osimertinib. J Thorac Oncol (2019) 14(2):e27–e29. doi: 10.1016/j.jtho.2018.10.160
22. Zhu YC, Wang WX, Xu CW, Zhang QX, Du KQ, Chen G, et al. Identification of a novel crizotinib-sensitive MET-ATXN7L1 gene fusion variant in lung adenocarcinoma by next generation sequencing. Ann Oncol (2018) 29(12):2392–3. doi: 10.1093/annonc/mdy455
23. Gow CH, Liu YN, Li HY, Hsieh MS, Chang SH, Luo SC, et al. Oncogenic function of a KIF5B-MET fusion variant in non-small cell lung cancer. Neoplasia (2018) 20(8):838–47. doi: 10.1016/j.neo.2018.06.007
24. Ercan D, Choi HG, Yun CH, Capelletti M, Xie T, Eck MJ, et al. EGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitors. Clin Cancer Res (2015) 21(17):3913–23. doi: 10.1158/1078-0432.CCR-14-2789
25. Niederst MJ, Hu H, Mulvey HE, Lockerman EL, Garcia AR, Piotrowska Z, et al. The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin Cancer Res (2015) 21(17):3924–33. doi: 10.1158/1078-0432.CCR-15-0560
26. Xu W, Liu K, Chen M, Sun JY, McCaughan GW, Lu XJ, et al. Immunotherapy for hepatocellular carcinoma: recent advances and future perspectives. Ther Adv Med Oncol (2019) 11:1758835919862692. doi: 10.1177/1758835919862692
Keywords: lung adenocarcinoma, EGFR mutations, CUX1-MET fusion, icotinib plus crizotinib, partial response
Citation: Ou L, Tang Y, Deng Y, Guo L, He Q, He T and Feng W (2022) Case Report: Durable partial response to icotinib plus crizotinib in a lung adenocarcinoma patient with double uncommon EGFR G719D/L861Q mutations and an acquired novel CUX1-MET fusion. Front. Oncol. 12:911362. doi: 10.3389/fonc.2022.911362
Received: 02 April 2022; Accepted: 05 July 2022;
Published: 26 July 2022.
Edited by:
Jinpeng Shi, Shanghai Pulmonary Hospital, ChinaReviewed by:
Guangming Tian, Beijing Cancer Hospital, ChinaYuxiang Ma, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2022 Ou, Tang, Deng, Guo, He, He and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weineng Feng, ZmVuZ3duODFAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Lanzi Ou1,2†
Lanzi Ou1,2† Lijie Guo
Lijie Guo Tingting He
Tingting He Weineng Feng
Weineng Feng