
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 22 June 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.911303
This article is part of the Research TopicConcomitant Pathogenic Mutations in Oncogene-Driven Subgroups: When Next Generation Biology Meets Targeted Therapy in NSCLCView all 5 articles
 Jingjing Qu1,2†
Jingjing Qu1,2† Qian Shen1,2†
Qian Shen1,2† Yuping Li3
Yuping Li3 Farhin Shaheed Kalyani1
Farhin Shaheed Kalyani1 Li Liu4*
Li Liu4* Jianya Zhou1,2*
Jianya Zhou1,2* Jianying Zhou1,2
Jianying Zhou1,2Background: Limited treatment outcome data is available for advanced non-small cell lung cancer (NSCLC) patients with BRAF V600E mutations. In this multicenter study, we describe therapeutic options and survival outcomes for patients with mutated BRAF V600E.
Method: This was a retrospective study in which BRAF V600E-mutated advanced NSCLC patients were retrospectively recruited between January 2015 and December 2021 and had their clinical characteristics, co-mutations, and treatment efficacy assessed.
Results: Fifty-three patients with BRAF V600E-mutant advanced NSCLC were included in the study, of which 64.2% were non-smokers, and the BRAF V600E mutation was more prevalent in men (52.8%). In addition, 96.2% of the patients had adenocarcinoma, and most (96.2%) received first-line therapy (23.5% anti-BRAF), with a progression-free survival (PFS) and overall survival (OS) of 10.0 [95% confidence interval (CI): 1.5–36.0 months] and 24.0 months [95% CI: 3.0–53.0 months], respectively. Twenty-three patients (43.4%) received second-line treatment (39.1% anti-BRAF), and PFS and OS were 5.0 [95% CI: 1.0–21.0 months] and 13.0 months [95% CI: 1.5–26.0 months], respectively. BRAF and MEK-targeted therapy (dabrafenib plus trametinib) produced longer PFS compared with that of chemotherapy with or without bevacizumab as a first-line (NA vs. 4.0 months, P = 0.025) or second-line therapy (6.0 vs. 4.6 months, P = 0.017). NSCLC patients harboring driver oncogene mutations such as BRAF V600E, EGFR, or ALK should be treated using targeted therapies. Concurrent TP53 mutations were the most common, affecting 11.3% (n = 6) of the patients, followed by EGFR 19 Del (n = 5). Patients with concurrent mutations had shorter PFS (9.0 vs. 10.0 months, P = 0.875) and OS (14.0 vs. 15.0 months, P = 0.555) than those without these mutations.
Conclusion: These results suggest that combined BRAF- and MEK-targeted therapy is effective in BRAF V600E-mutated advanced NSCLC patients. Dabrafenib and trametinib re-challenge is also an option for patients with BRAF V600E-mutated NSCLC.
Recent non-small cell lung cancer (NSCLC) therapy research has concentrated on developing drugs targeting driver gene mutations, particularly for adenocarcinoma (1, 2). All patients with advanced adenocarcinoma undergo routine genetic testing for clinically targetable genomic alterations (3). Clinical targeted therapy is imperative for NSCLC patients with epidermal growth factor receptor (EGFR) mutation (4, 5). EGFR-targeting tyrosine kinase inhibitors (TKIs), such as gefitinib, erlotinib, and osimertinib, extend median progression-free survival (PFS) and overall survival (OS) in NSCLC patients with EGFR mutations (6, 7). Additionally, the anaplastic lymphoma kinase (ALK) inhibitor crizotinib has transformed treatment for NSCLC patients with ALK rearrangement (8–10). Currently, through the clinical application of comprehensive genome sequencing, many potential targetable genomic alterations, such as v-Raf murine sarcoma viral oncogene homolog B1 (BRAF), mesenchymal-epithelial transition (MET) exon 14 skipping mutations, HER2 (human epidermal growth factor receptor 2), and rearranged during transfection (RET) gene rearrangements, have been verified.
BRAF-encoded protein-RAF kinase is one of the main regulators of the MAPK/ERK pathway and activates downstream MEK through phosphorylation (11). The MAPK/ERK signaling pathway regulates cell growth, proliferation, differentiation, migration, and apoptosis (12–14). BRAF mutations occur in approximately 2–4% of NSCLC patients with adenocarcinoma (15, 16). In the BRAF oncogene, over 50% of mutations are associated with glutamate-valine substitution of the 600-position codon (V600E, Val600Glu) in the 15th exon of the kinase domain. This mutation results in a 500-fold increase in BRAF kinase activity compared with that of the wild-type (17). The BRAF V600E mutation is common in older patients (>60 years), patients with adenocarcinoma (18–20). Recently, the United States Food and Drug Administration approved BRAF (dabrafenib) and MEK inhibitor (trametinib) treatment for NSCLC patients with the BRAF V600E mutation. In a clinical trial of advanced NSCLC with BRAF V600E mutations, the combination of BRAF and MEK inhibitors (dabrafenib and trametinib) had a 64% overall response rate (ORR) in formerly untreated patients (21). In Europe, a real-world study assessed the efficacy of combined dabrafenib and trametinib in treating BRAF V600E mutated advanced NSCLC. Across the entire cohort, median PFS and OS were 17.5 months (95% confidence interval [CI] 7.1–23.0 months) and 25.5 months (95% CI 16.6–not reached), respectively (22). Another study reported that the OS in BRAF-targeted therapy was longer (56.5 months) than that of conventionally treated patients (27.2 months), further emphasizing the importance of targeting treatments for BRAF V600E mutated NSCLC (23).
In Chinese advanced NSCLC patients with the BRAF V600E mutation, the clinical efficacy of chemotherapy, immunotherapy, targeted therapy, and other conventional therapies has not been well explored. This is probably because the BRAF V600E mutation in NSCLC is rare, and there are no approved treatments targeting the mutation prior to the use of BRAF and MEK inhibitors. To address this issue, we conducted a retrospective study to assess the clinical characteristics and outcomes of advanced NSCLC patients with the BRAF V600E mutation.
Clinical data from January 2015 to December 2021 were collected. The primary objective was to describe the clinical characteristics and determine the efficacy of dabrafenib and trametinib in advanced NSCLC patients with the BRAF V600E mutation. Fifty-three patients were recruited from multiple centers, including (I) the First Affiliated Hospital, College of Medicine, Zhejiang University; (II) Hunan Cancer Hospital, Affiliated Tumor Hospital of Xiangya Medical School of Central South University; and (III) Wenzhou Medical University. Patients at these centers who met the following inclusion criteria were enrolled in this study: (I) patients with a histological or cytological diagnosis of NSCLC; (II) patients with the BRAF V600E mutation detected by multiplex polymerase chain reaction (PCR) or next-generation sequencing (NGS); and (III) patients diagnosed with advanced NSCLC, including those with stage IV metastatic disease and with inoperable locally advanced stage IV disease. Using the response evaluation criteria in solid tumors (RECIST) version 1.1 (24), patients received an Eastern Cooperative Oncology Group (ECOG) performance status score to measure disease severity. After obtaining informed consent from the study patients, patient medical records were analyzed, and patient characteristics, including age, sex, smoking history, ECOG score, histology, staging, co-occurring mutations, and treatment history, were recorded. Disease staging was determined using the American Joint Council on Cancer (AJCC) Staging System, Version 8. This clinical study was approved by the Institutional Review Boards of the First Affiliated Hospital, College of Medicine, Zhejiang University, Hunan Cancer Hospital, and Wenzhou Medical University.
NSCLC patients with the BRAF V600E mutation received dabrafenib (150 mg BID) plus trametinib (2 mg QD) as first-line or follow-up therapy. Other therapies included platinum-based chemotherapy, immune checkpoint inhibitors (ICIs), EGFR-TKI, and ALK-TKI targeted therapy. The main objective of this clinical trial was to assess PFS and OS from initiation of treatment in patients with the BRAF V600E mutation. Using the Solid Tumor Version 1.1 (RECIST 1.1) criteria, PFS was defined as radiological or clinical progression (deterioration of clinical status, prophylactic systemic therapy) or death, while OS was defined from the start of therapy to death. Assessment was performed at each participating center without centralized imaging.
The Kaplan–Meier curve and log-rank statistics were used to analyze the survival of each group. All statistical and graphing analyses were performed using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). A statistically significant difference was defined as a P-value < 0.05.
A total of 53 patients with BRAF V600E mutation advanced NSCLC met the inclusion criteria for this study (Table 1). The median age was 58 years (range: 40–75 years). The cohort included a higher proportion of men (28/53, 52.8%) than women (25/53, 47.2%), and 64.2% (34/53) of the patients were non-smokers. Most patients (51/53, 96.2%) had stage IV NSCLC, with only two patients (3.8%) diagnosed as stage IIIc. Most patients had an ECOG performance status of 0–1 (46/53, 86.8%). The location of metastases at the time of diagnosis differed, with bone (25/53, 47.2%) being the most common location, followed by pleura (16/53, 30.2%), lung (10/53, 18.9%), liver (7/53, 13.2%), and brain (6/53, 11.3%). There are several clinical methods for detecting the BRAF V600E mutation. In our study, PCR was the most common method (31/53, 58.5%), followed by NGS (22/53, 41.5%). The median follow-up time for each patient from diagnosis to the last follow-up was 16.8 months.
Of the 53 patients with advanced NSCLC who were scheduled to undergo first-line treatment, two refused treatment. At 31.4% (16/51), platinum-based dual chemotherapy with or without bevacizumab was the most common treatment regimen. Meanwhile, 23.5% (12/51) of the patients received the BRAF-MEK-directed therapy, dabrafenib-trametinib, as first-line therapy, while 23.5% (12/51) received platinum-based doublet chemotherapy combined with anti-programmed death-1 (PD-1) ICIs. For patients with EGFR/ALK combined with a BRAF V600E mutation, 15.7% (8/51) received EGFR-TKIs, 3.9% (2/51) received EGFR-TKIs plus bevacizumab, and 2.0% (1/51) received the EML4-ALK inhibitor crizotinib. The median duration of first-line treatment was 9.2 months (range: 1.5–36 months) (Figure 1A).
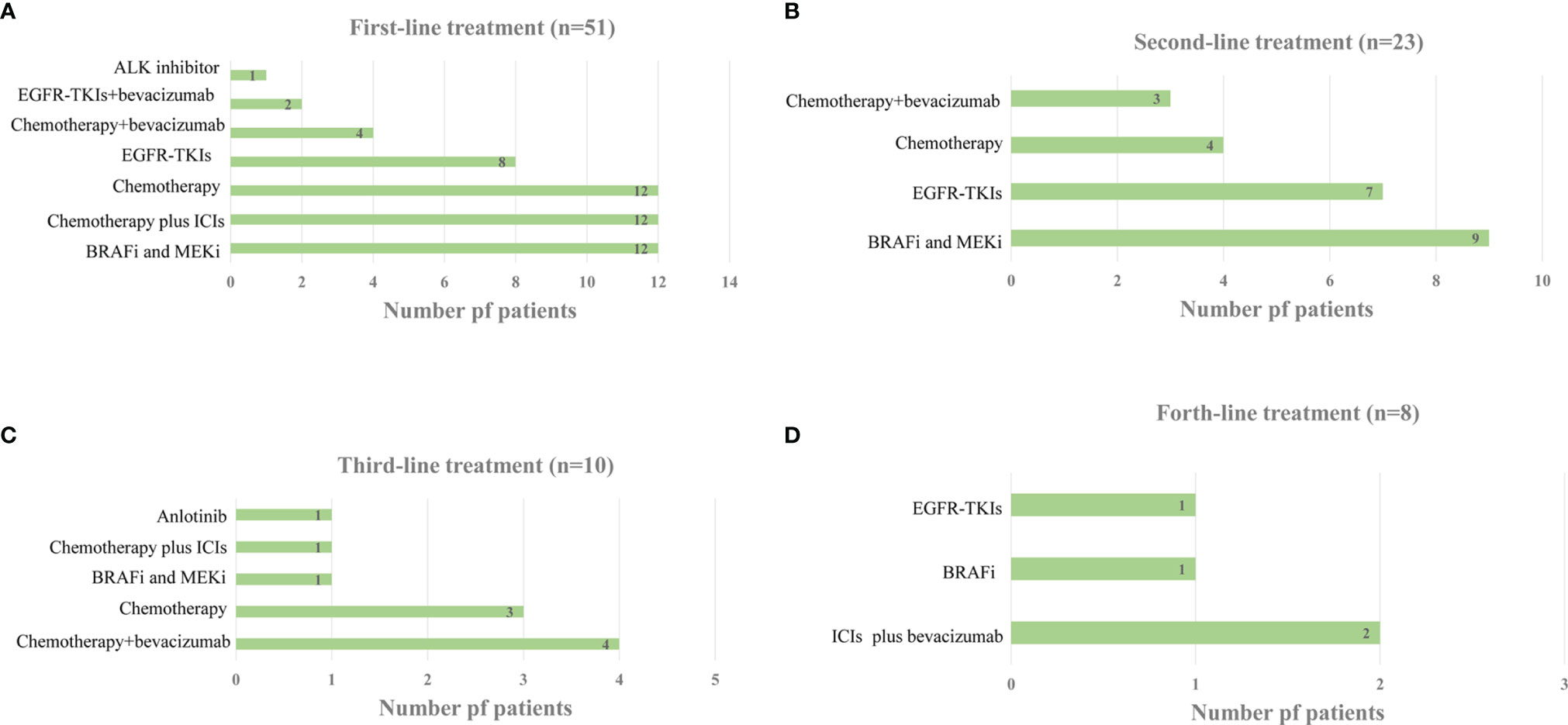
Figure 1 (A) Distribution of Systemic Treatment Regimens in the first-line Setting. (B) Distribution of Systemic Treatment Regimens in the second-line Setting. (C) Distribution of Systemic Treatment Regimens in the third-line Setting. (D) Distribution of Systemic Treatment Regimens in the forth-line Setting.
A total of 15 patients continued first-line therapy, whereas 11 gave up treatment after disease progression. Another two patients had to stop treatment because of severe renal insufficiency and infection after first-line therapy. Therefore, 23 patients received second-line therapy. BRAF and MEK inhibitors were administered to 39.1% (9/23) of second-line therapy patients, followed by EGFR-TKIs (7/23, 30.4%) and chemotherapy with or without bevacizumab (7/23, 30.4%) (Figure 1B). The median duration of second-line therapy was 6.2 months (range: 1.0–21.0 months). Additional data on subsequent therapy lines are presented in Figures 1C, D. Among the 51 patients who obtained at least one line of systemic therapy, 43.1% (21/51) received targeted treatment with BRAF/MEK inhibitors. Of these 21 patients, one received BRAF/MEK inhibitors as both first- and third-line therapies, with none receiving BRAF/MEK inhibitor therapy beyond fifth-line therapy.
In the 51 patients treated with systemic therapy, the median PFS from first-line therapy initiation was 10.0 months (95% CI, 1.5–36.0 months). The median first-line PFS was longer in the BRAF and MEK inhibitor cohorts compared with that in patients who received chemotherapy with or without bevacizumab (NA vs. 4.0 months, P = 0.025, hazard ratio [HR]: 0.33, 95% CI: 0.13–0.83). For patients who received EGFR-TKIs with or without bevacizumab/ALK inhibitor treatment, the median PFS was 16.0 months, with no significant difference compared with that of the BRAF and MEK inhibitor cohorts (16.0 months vs. NA, P = 0.710, HR: 1.20, 95% CI: 0.36–4.10). Regarding BRAF and MEK inhibitors, median PFS from first-line treatment did not significantly differ relative to that of patients who received chemotherapy plus ICIs (NA vs. 8.5 months, P = 0.211, HR: 0.49, 95% CI: 0.16–1.50) (Figures 2A, B and Table 2).
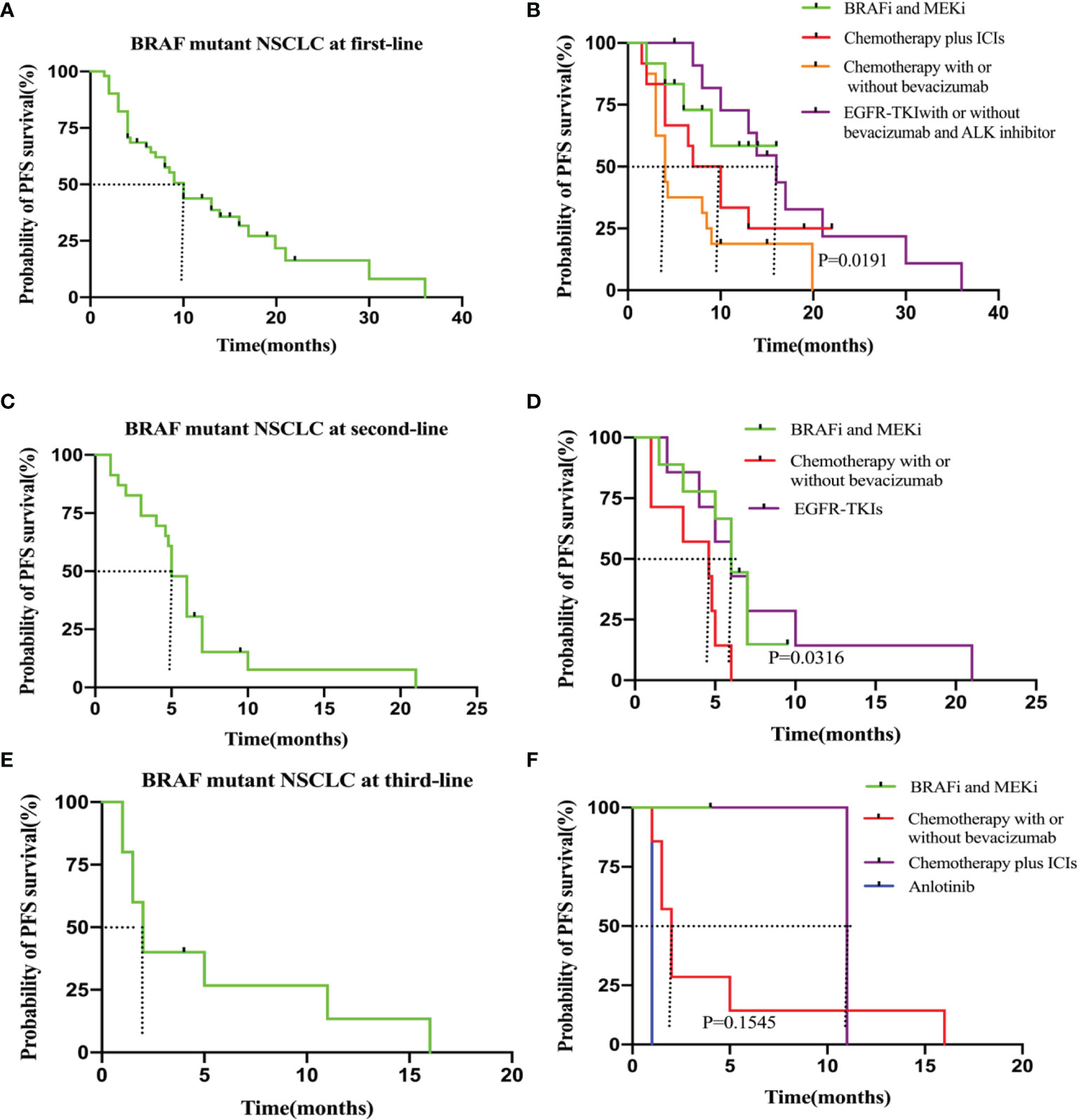
Figure 2 (A) PFS in the all patients at first-line. (B) PFS in the subgroup of patients who received BRAF and MEK inhibitor (n = 12), chemotherapy plus ICIs (n = 12), chemotherapy with or without bevacizumab(n=16) and EGFR-TKIs with or without bevacizumab/ALK inhibitor (n = 11). (C) PFS in the all patients at second-line. (D) PFS in the subgroup of patients who received BRAF and MEK inhitor (n = 9), chemotherapy with or without bevacizumab (n=7) and EGFR-TKIs (n=7). (E) PFS in the all patients at third-line. (F) PFS in the subgroup of patients who received chemotherapy with or without bevacizumab (n = 7), BRAF and MEK inhitor (n = 1), chemotherapy plus ICIs (n=2) and anlotinib (n=1).
In the 23 patients who accepted second-line treatment, the median PFS was 5.0 months (95% CI: 1.0–21.0). The PFS was significantly longer for the nine patients who received BRAF and MEK inhibitor therapy than that for the seven patients who received chemotherapy with or without bevacizumab (6.0 vs. 4.6 months, P = 0.017, HR: 0.34; 95% CI: 0.10–1.10). The PFS was the same for BRAF/MEK inhibitor and EGFR-TKI therapies (6.0 vs. 6.0 months, P = 0.834, HR: 1.1, 95% CI: 0.33–4.00). Therefore, for patients without EGFR-sensitive mutations, we recommend BRAF and MEK inhibitor therapy as second-line therapy (Figures 2C, D and Table 2).
In the 10 patients who received third-line therapy, median PFS was shorter (2.0 months, 95% CI: 1.0–16.0) than for those who received first and second-line therapies. For the one patient who underwent BRAF and MEK inhibitor targeted therapy, median third-line PFS was not reached relative to the seven patients who received chemotherapy with or without bevacizumab (NA vs. 2.0 months, P = 0.276, HR: 0.23; 95% CI: 0.02–3.20). The Kaplan–Meier survival curves and outcomes for all PFS estimates are provided in Figures 2E, F and Table 2.
Ten patients tested positive for programmed death-ligand 1 (PD-L1) during the course of the disease, among whom, five received ICIs. Considering the various antibodies for PD-L1 and the sample size being too small in study patients, the relationships among PD-L1, BRAF mutation, and treatment efficacy were not analyzed. However, three patients had PD-L1 expression ≥50%, and their efficacy evaluation following first-line ICI therapy indicated a partial response (PR), with PFS of 13.0, 8.0, and 18.5 months. These results indicate that for patients with the BRAF V600E mutation, PD-L1 expression should be determined, and once the PD-L1 expression is ≥50%, ICIs may be an optimal choice for these patients.
Among patients receiving first-line therapy, the median OS from the start of first-line therapy was 24.0 months (95% CI: 3.0–47.0). Median first-line OS was not reached for patients who received BRAF and MEK inhibitor targeted therapy, whereas it was 14.0 months for those who received chemotherapy plus ICIs (P = 0.408, HR: 0.56, 95% CI: 0.18–2.00). Median first-line OS did not significantly differ between the BRAF and MEK inhibitor cohort and the chemotherapy with or without bevacizumab cohort (NA vs. 14.0 months, P = 0.528, HR: 0.68, 95% CI: 0.21-2.20). For the patients treated with BRAF/MEK inhibitors and those treated with EGFR or ALK mutation targeted therapy, the median OS significantly differed (NA vs. 33.0 months, P = 0.039, HR: 3.0, 95% CI: 0.46–20.0) (Figures 3A, B and Table 2). The median OS for second- and third-line therapy was 13.0 (95% CI: 1.5–26.0) and 12.0 months (95% CI: 2.0–16.7), respectively. Patients receiving targeted treatment with BRAF and MEK inhibitors had a longer OS upon second-line therapy than that for patients receiving conventional therapy (except for EGFR-TKI therapy) (Figures 3C, D and Table 2). Figures 3E, F and Table 2 show Kaplan–Meier survival outcomes for OS of third-line therapy patients.
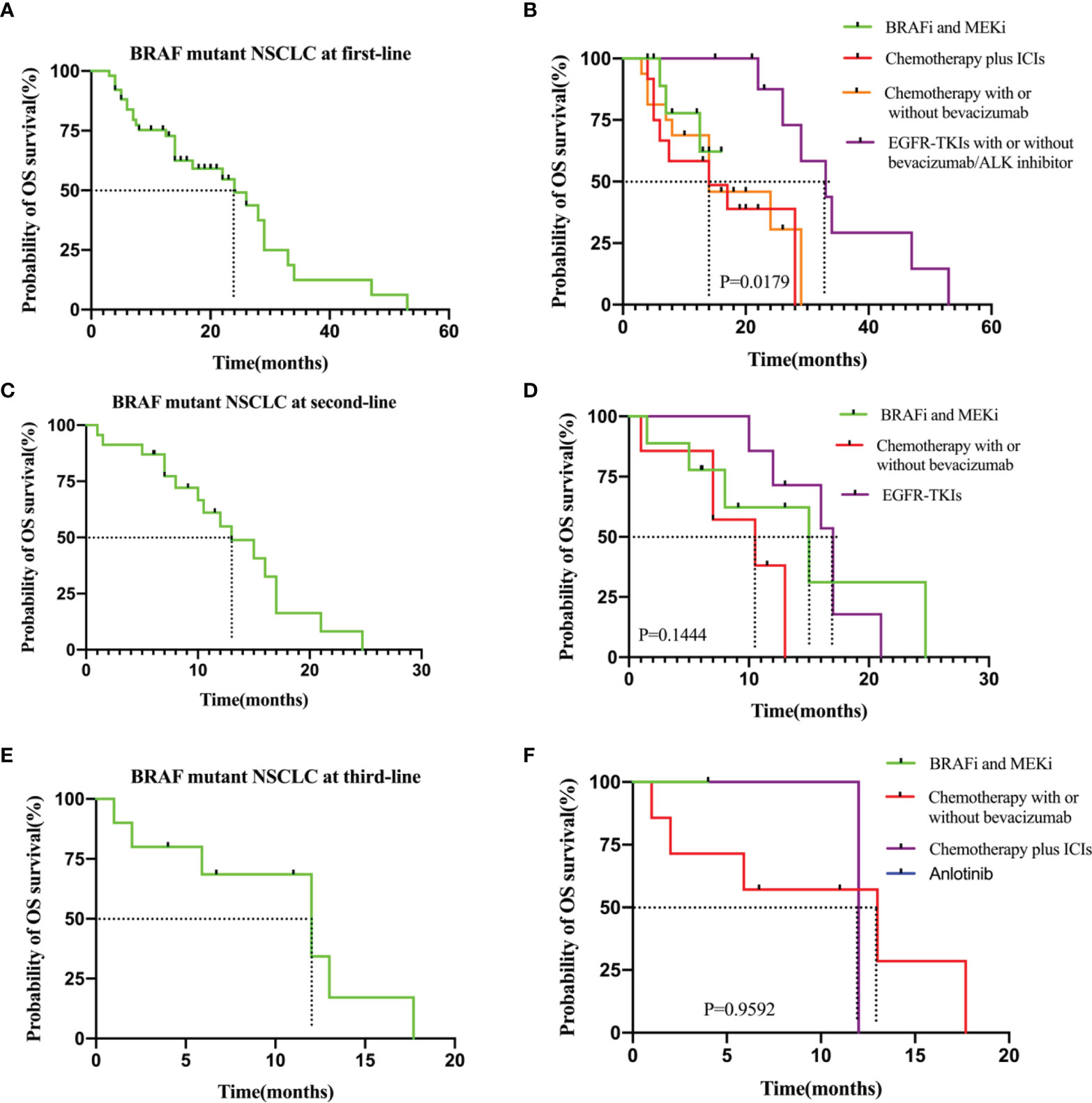
Figure 3 (A) OS in the all patients at first-line. (B) OS in the subgroup of patients who received BRAF and MEK inhitor (n = 12), chemotherapy plus ICIs (n = 12), chemotherapy with or without bevacizumab (n=16) and EGFR-TKIs with or without bevacizumab/ALK inhibitor (n = 11). (C) OS in the all patients at second-line. (D) OS in the subgroup of patients who received BRAF and MEK inhitor (n = 9), chemotherapy with or without bevacizumab (n=7) and EGFR-TKIs (n=7). (E) OS in the all patients at third-line.(F) OS in the subgroup of patients who received chemotherapy with or without bevacizumab (n = 7), BRAF and MEK inhitor (n = 1), chemotherapy plus ICIs (n=2) and anlotinib (n=1).
PCR and NGS tests were performed in patients with advanced NSCLC to elucidate their baseline genetic mutation status. Results showed that 30.2% (16/53) of the BRAF mutation patients had other concurrent mutations, while the rest did not. Concurrent TP53 mutations were the most common, affecting 11.3% (6/53) of the patients. In addition, other co-mutations, including EGFR-19del (p.E746_A750del, n = 5), EGFR-L858R (leucine to arginine at position 858, n = 3), SETD2 mutation (n = 2), KRAS mutation (p.G12D, n = 1; p.G12C, n = 1), EGFR-T790M (substitutes methionine for threonine at amino acid position 790, n = 1), EML4-ALK rearrangement (n = 1), c-MET amplification (n = 1), MSH2 mutation (n = 1), AXIN2 mutation (n = 1), and ARIDIA mutation (n = 1) were also detected. The concurrent mutations observed are summarized in Figure 4. Furthermore, we determined the presence or absence of concurrent mutations associated with survival outcomes that excluded sensitive mutations in the 51 patients, including those that were EGFR- and ALK-positive (n = 10). Patients with concurrent mutations (n = 6) had shorter, albeit statistically non-significant, PFS (9.0 vs. 10.0 months, P = 0.875, Figure 4B) and OS (14.0 vs. 15.0 months, P = 0.555; Figure 4C) than those without (n = 35).
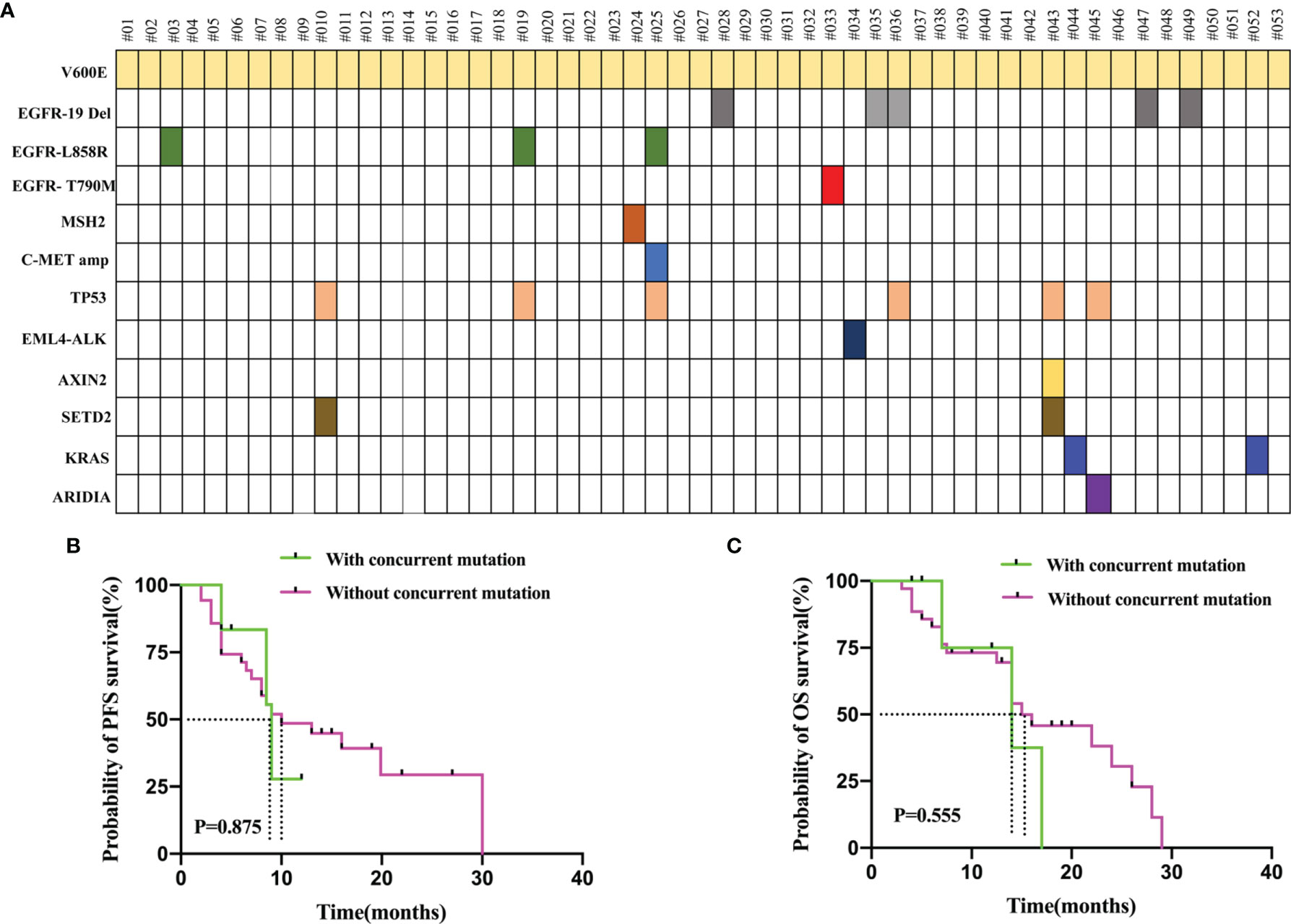
Figure 4 (A) Genomic Alterations Found in Each patient’s Tumor are Shown. 30.2% (16/53) of the BRAF mutation patients had other concurrent mutations. Concurrent TP53 mutations were the most common. (B) PFS in the patients who with concurrent mutation and without concurrent mutation. (C) OS in the patients who with concurrent mutation and without concurrent mutation. The concurrent mutation is excluded the sensitive mutations patients including EGFR and ALK positive.
A 52-year-old female patient (case #10) came to our hospital, and a computed tomography (CT) scan detected a mass in the left lung, which was suspected to be cancer with multiple lymph node metastases to the left hilar mediastinum and both clavicles. Pleural effusion was also noted. Positron emission tomography/CT showed clavicular, mediastinal and peritoneal lymph node, adrenal gland, liver, right humeral, left knee, vertebral (T8,11, and 12), and bilateral ischial metastases, as well as multiple brain metastases. Thoracentesis fluid histology demonstrated adenocarcinoma cells. She was diagnosed and confirmed as having stage IV lung adenocarcinoma based on a bronchoscopic biopsy in August 2020. Immunohistochemical biopsy results revealed TTF-1, Napsin A, and CK7 positivity in the tumor cells. Genetic testing revealed a BRAF V600E mutation.
First-line therapy with dabrafenib-trametinib resulted in a PR in September 2020. Gamma knife treatment was also prescribed for the brain metastases. Significant symptom improvement and pleural effusion reduction were observed after treatment, and the patient was discharged with outpatient follow-up. Re-examination of brain magnetic resonance imaging 1 month post-gamma knife treatment showed reductions in the size of brain metastases. Nine months later (May 2021), an apparent increase in left pleural effusion was noted. A pemetrexed-carboplatin-bevacizumab combination was administered as second-line therapy. Three months later, the pleural effusion was still not controlled, and serum creatinine levels were also elevated. Therefore, puncture and drainage of pleural effusion were performed, in which adenocarcinoma cells were found by histology. BRAF V600E, TP53, and SETD2 mutations were also identified by targeted next-generation DNA sequencing of the pleural fluid samples. A dabrafenib-trametinib re-challenge plus pemetrexed was initiated. She achieved a PR in September 2021, which continued to date with no adverse effects noted (Figure 5). These findings indicate that dabrafenib-trametinib re-challenge is an alternative therapy for patients with the BRAF V600E mutation in NSCLC.
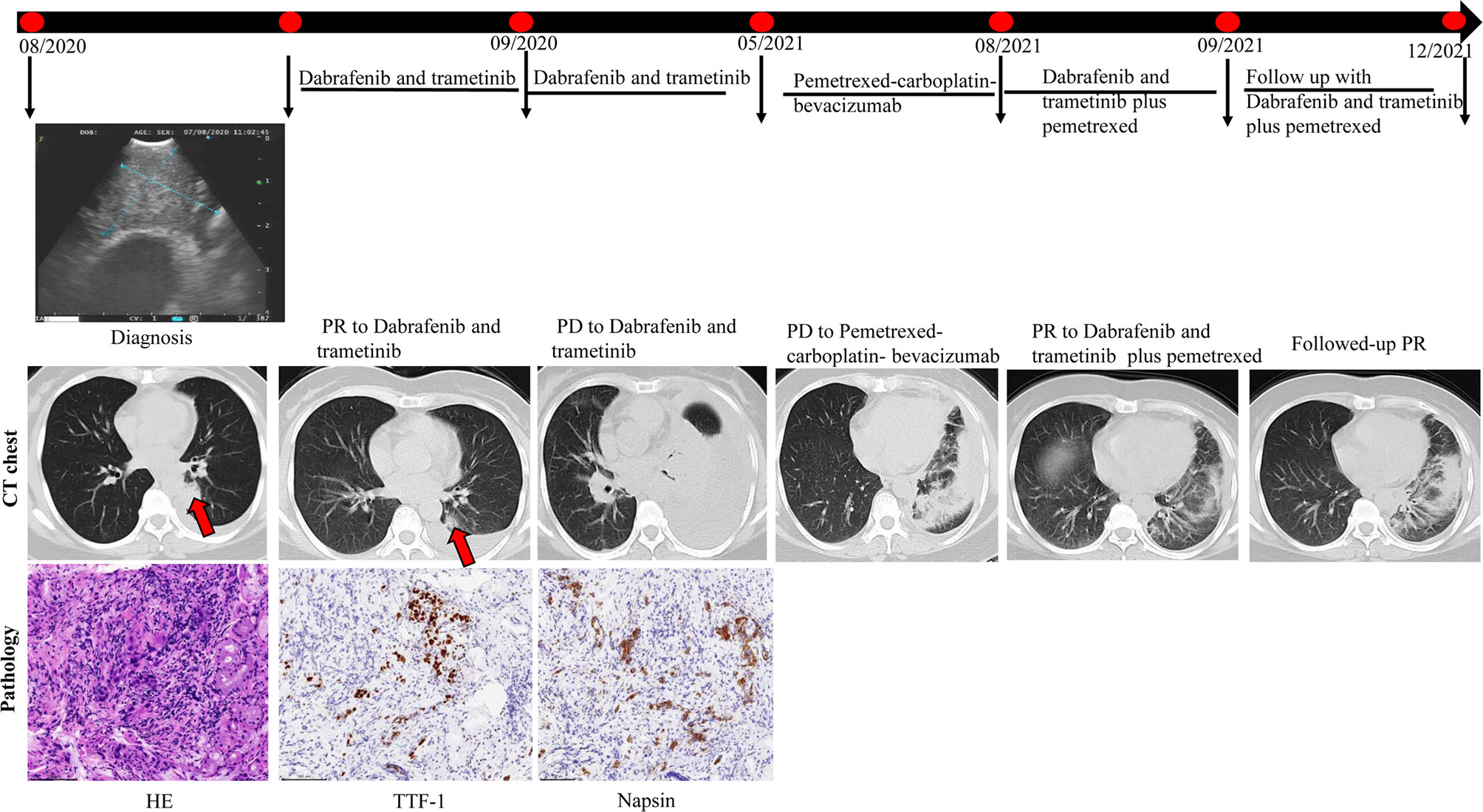
Figure 5 Computed tomography (CT) imaging reveals initial and re-challenge response to dabrafenib-trametinib combination therapy.
Little is known about the clinical features and treatment efficacy for patients with BRAF V600E mutated NSCLC, as the BRAF V600E mutation rarely occurs in NSCLC (25). Our cohort comprised patients with BRAF V600E-mutant advanced NSCLC, including 64.2% non-smokers with a slight male dominance (52.8%). Marchetti et al. proposed that patients with the BRAF-V600E mutation were significantly predominated by women or those who had never smoked (26). Ding et al. suggested that BRAF mutation in Chinese patients was more likely to occur in non-smokers (27). In terms of pathological features, BRAF-mutated NSCLC mostly comprises adenocarcinoma, and other histological types, including squamous cell carcinoma, have also been detected (28, 29). In our study, 96.2% (51/53) of the patients had the adenocarcinoma subtype. This clinical characteristic data is comparable with that of other studies. The 262 BRAF-mutated patients recorded by Barlesi et al. were characterized by an average age of 65.9 years, and 87% of the patients had adenocarcinoma (30).
In our cohort, 30.2% (16/53) of patients with the BRAF V600E mutation had other concurrent mutations. Concurrent TP53 mutations were the most common, affecting 11.3% (6/53) of the patients. The other concurrent mutations detected were EGFR-19del, EGFR-L858R, KRAS mutation, SETD2 mutation, EGFR-T790M, EML4-ALK rearrangement, and c-MET amplification. Co-occurrence rates in BRAF-mutated NSCLC are reported to be 14–16% (27, 31). KRAS Q61R mutation was examined in three patients who progressed before combined treatment with dabrafenib and trametinib (32). In other studies, KRAS mutation has been found in melanoma patients who progressed following combined BRAF and MEK inhibition therapy (33, 34). Another report revealed that KRAS mutation acts as a mechanism to resist BRAF and MEK inhibitors in NSCLC (35). In our study, a patient (case 44) with concurrent KRAS mutation still received treatment with dabrafenib-trametinib. Therefore, to determine whether KRAS co-mutation affects the response to BRAF-targeted therapy, further investigation is required to clarify the relationship between KRAS and BRAF. In addition, it has been reported that tumors containing TP53 co-mutations are related to worse clinical outcomes. Unfortunately, due to the limited number of clinical samples with BRAF V600E and TP53 co-mutations, the clinical implications of treatment for these patients were not studied.
Auliac previously reported that among the 46 advanced NSCLC patients with the BRAF V600E mutation registered in France between 2012 and 2014, those with the BRAF V600E mutation had 8.7 months of PFS with first-line therapy and 4.1 months with second-line therapy (36). In our study, patients with the BRAF V600E mutation had a PFS of 10.0 months (95% CI: 1.5–36.0) and OS of 24.0 months (95% CI: 3.0–53.0) with first-line therapy. The second-line therapy patients had a PFS of 5.0 months (95% CI: 1.0–21.0) and OS of 13.0 months (95% C: 1.5–26.0), consistent with Auliac (36). It is well known that NGS technologies have played an essential role in understanding the altered genetic pathways involved in human cancer. NGS can quantitate the proportion of reads for a given mutation, also known as mutant allele frequency (MAF), which represents the percentage of tumor cells that harbor a specific mutation in neoplastic tissue. One study investigated the value of BRAF V600E MAF variability within primary melanomas (MMp) and its potential prognostic implications. Results indicated that High-BRAFV600E MMp were all located on the trunk, had lower Breslow and mitotic indices, and were predominantly first nodal metastases. The high-BRAFV600E MMp patients had better prognostic features and first nodal metastasis (37). In the present study, we did not investigate whether the MAF of BRAF V600E potentially impacts the clinical outcome of NSCLC patients. In the future, we intend to pay attention to determine MAF in clinical practice for use as a prognostic indicator.
A retrospective multicenter study that included 40 advanced NSCLC patients with the BRAF V600E mutation treated with dabrafenib and trametinib was conducted. Among the nine patients receiving first-line therapy, median PFS and OS were 16.8 (95% CI: 6.1–23.2) and 21.8 months (95% CI: 1.0–not achieved), respectively. Median PFS and OS were 16.8 months (95% CI: 6.1–23.2) and 25.5 months (95% CI: 16.6 months–not met), respectively, in 31 patients receiving second-line therapy or above (22). In our study, 23.5% (12/51) and 39.1% (9/23) of the patients received BRAF/MEK-targeted therapy as first- and second-line therapies, respectively. The PFS and OS in patients who received dabrafenib-trametinib first-line treatment were not reached. The PFS and OS for second-line therapy patients who underwent dabrafenib-trametinib therapy were 6.0 and 15.0 months. The clinical role of dabrafenib and trametinib in advanced NSCLC patients with the BRAF V600E mutation was explored in an unselected real-world study. Among patients who did not receive prior treatment, PFS and OS were 10.8 (7.0–14.5) and 17.3 (12.3–40.2) months, respectively, whereas for formerly treated patients, these were 10.2 months (95% CI: 6.9–16.7) and 18.2 months (95% CI: 14.3–28.6), respectively (21, 38, 39). Our results were also consistent with the findings of other real-world studies. For instance, in a study where 31 patients received BRAF inhibitors, the median PFS for those on anti-BRAF treatment was 5.0 months, with an OS of 10.8 months (40).
In our study, the other first-line therapies included chemotherapy with/without bevacizumab, chemotherapy plus ICIs, and EGFR-TKIs with or without bevacizumab/ALK inhibitor. Other treatments used as second-line therapy were chemotherapy with or without bevacizumab and EGFR-TKIs. The PFS and OS in the first-line therapy patients who received chemotherapy with or without bevacizumab were 4.0 and 14.0 months, those for chemotherapy plus ICIs were 8.5 and 14.0 months, and for EGFR-TKIs with or without bevacizumab/ALK inhibitor 16.0 and 33.0 months, respectively. For second-line therapy, the PFS and OS for chemotherapy with or without bevacizumab were 4.6 and 11.0 months and those for EGFR-TKIs were 6.0 and 17.0 months, respectively. Zhuang conducted a study showing that first-line anti-BRAF-targeting treatment was superior to chemotherapy in 46 patients with advanced BRAF-V600E mutation (9.8 vs. 5.4 months, P = 0.149) (41). Similarly, in the present study, the PFS for first- and second-line BRAF/MEK-targeted therapy was also longer than that for chemotherapy with or without bevacizumab (first-line: NA vs. 4.0 months, P = 0.025; second-line: 6.0 vs. 4.6 months, P = 0.017), indicating that BRAF/MEK-targeted therapy is a viable choice for advanced NSCLC patients with the BRAF-V600E mutation. The PFS for first-, third-, and fourth-line therapies in the ICI-treated cohort was 8.5, 11.0, and 2.3 months, respectively. We intended to compare the PFS and OS between BRAF/MEK-targeted and ICI treatments; however, the PFS and OS in BRAF/MEK-targeted therapy were not reached. In fourth-line therapy, the PFS was longer for BRAF/MEK-targeted therapy than that for ICI treatment (6.0 vs. 3.4 months, P = 0.317). Another study by Gautschi reported that in the nine patients treated with ICIs, the median PFS was 3.0 months (40). Therefore, the effect of ICIs and therapeutic options for the BRAF V600E mutated population requires further exploration.
The retrospective study included patients diagnosed with NSCLC and tested for EGFR, ALK, ROS1, and BRAF mutations. Results showed that EGFR-TKI treatment was superior to chemotherapy in patients with BRAF V600E mutation concurrent with EGFR mutation (median PFS 10.8 vs. 5.2 months, P = 0.023) (41). Similarly, in the current study, the PFS and OS of patients treated with EGFR-TKIs with or without bevacizumab/ALK inhibitor were 16.0 and 33.0 months for first-line therapy, respectively. For second-line therapy, EGFR-TKI therapy was found to have a longer OS than that of BRAF/MEK-targeted therapy (17.0 vs. 15.0 months, P = 0.823). Therefore, in patients with the BRAF V600E-mutation and concurrent EGFR mutation, EGFR-TKIs may be the optimal treatment choice.
Current acquired resistance mechanisms to BRAF and MEK inhibitors in NSCLC patients are difficult to elucidate from molecular diagnosis. In our study, a patient with advanced NSCLC with BRAF V600E mutation (case 10) achieved a long-term PR to BRAF and MEK-targeted treatment re-challenge. The mechanism of action of response to re-challenge in BRAF and MEK-targeted therapy remains unclear. Reschke suggests that acquired resistance to BRAF and MEK inhibitors in metastatic melanoma might be reversible under “drug-free” conditions (42). Thus, cytotoxic chemotherapy creates a “drug-free” environment and may lead to re-challenge for some patients receiving BRAF and MEK-targeted therapy with positive outcomes. In another study, a patient with NSCLC with advanced BRAF V600E mutation was resistant to BRAF-targeted therapy in first-line therapy and therefore received chemotherapy as second-line therapy. After progression to chemotherapy, BRAF-targeted therapy was re-challenged, and the patient benefited (43).
The current study has some limitations. First, our study comprised a small sample of patients with BRAF V600E-positive NSCLC from three academic hospitals in China, and the results may not apply to other cancer centers. Second, the immunotherapy results for BRAF V600E-mutated NSCLC are limited and require further research. Therefore, due to a lack of consensus, final recommendations for immunotherapy or BRAF- and MEK-targeted therapy for patients with BRAF V600E mutation could not be reached. Third, we were unable to acquire tissue samples from patients with the BRAF V600E-mutation who were resistant to BRAF- and MEK-targeted therapy to further explore the mechanism of resistance to BRAF- and MEK-targeted therapy. In addition, we lacked an independent radiology review board to re-assess the outcomes at different medical centers. Therefore, multicenter prospective studies with a larger cohort of Chinese patients with the BRAF V600E mutation are needed.
Our study uncovered differences in clinical characteristics and treatment efficacy in patients with BRAF V600E-mutated NSCLC in the Chinese population. Our data suggest that patients with NSCLC with carcinogenic alterations such as EGFR, ALK, and BRAF V600E may receive targeted treatment. PFS and OS were longer in patients receiving BRAF and MEK inhibitors in first- and second-line therapies than in those receiving chemotherapy. The value of ICI treatment in the BRAF V600E population requires further investigation. Patients with concurrent mutations had shorter PFS and OS than those without these mutations. Dabrafenib and trametinib re-challenge has potential as an alternative treatment for patients with NSCLC with advanced BRAF V600E mutation. Taken together, these findings suggest that BRAF- and MEK-targeting is a potential treatment option for patients with BRAF V600E mutated NSCLC.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
This clinical study was reviewed and approved by the Institutional Review Board of the First Affiliated Hospital, College of Medicine, Zhejiang University and Hunan Cancer Hospital and Wenzhou Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JJQ, QS, and FSK collected and analyzed data; JJQ, QS, YPL, LL, JianyiZ, and JianyaZ wrote the manuscript. LL and JianyaZ were responsible for study conception and design and acquiring financial support. All authors have read and agreed to publish the current version of the manuscript.
This work was supported by grants from the National Natural Science Foundation of China (No. 81802278), Medicine Health Technology Plan of Zhejiang Province, China (No. 2022KY150), and the Natural Science Foundation of Zhejiang Province (No. LGF22H160005).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank Prof. Nong Yang from the Lung Cancer and Gastroenterology Department, Hunan Cancer Hospital and Affiliated Tumor Hospital of Xiangya Medical School of Central South University for technical support with this manuscript.
1. Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer (2017) 17:637–58. doi: 10.1038/nrc.2017.84
2. Succony L, Rassl DM, Barker AP, McCaughan FM, Rintoul RC. Adenocarcinoma spectrum lesions of the lung: Detection, pathology and treatment strategies. Cancer Treat Rev (2021) 99:102237. doi: 10.1016/j.ctrv.2021.102237
3. Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. : Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol (2013) 8:823–59. doi: 10.1097/JTO.0b013e318290868f
4. Muthusamy B, Pennell N. Chemoimmunotherapy for EGFR-Mutant NSCLC: Still No Clear Answer. J Thorac Oncol (2022) 17:179–81. doi: 10.1016/j.jtho.2021.11.012
5. ten Berge DMHJ, Aarts MJ, Groen HJM, Aerts JGJV, Kloover JS. A population-based study describing characteristics, survival and the effect of TKI treatment on patients with EGFR mutated stage IV NSCLC in the Netherlands. Eur J Cancer (2022) 165:195–204. doi: 10.1016/j.ejca.2022.01.038
6. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. : Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol (2012) 13:239–46. doi: 10.1016/S1470-2045(11)70393-X
7. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. : Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol (2011) 12:735–42. doi: 10.1016/S1470-2045(11)70184-X
8. Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, et al. : Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol (2011) 12:1004–12. doi: 10.1016/S1470-2045(11)70232-7
9. Novello S, Mazieres J, Oh IJ, de Castro J, Migliorino MR, Helland A, et al. : Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol (2018) 29:1409–16. doi: 10.1093/annonc/mdy121
10. Fukui T, Tachihara M, Nagano T, Kobayashi K. Review of Therapeutic Strategies for Anaplastic Lymphoma Kinase-Rearranged Non-Small Cell Lung Cancer. Cancers (2022) 14:1184. doi: 10.3390/cancers14051184
11. Martinez Fiesco JA, Durrant DE, Morrison DK, Zhang P. Structural insights into the BRAF monomer-to-dimer transition mediated by RAS binding. Nat Commun (2022) 13:486. doi: 10.1038/s41467-022-28084-3
12. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. : Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med (2011) 364:2507–16. doi: 10.1056/NEJMoa1103782
13. Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. : Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med (2012) 367:1694–703. doi: 10.1056/NEJMoa1210093
14. Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, et al. : Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med (2012) 367:107–14. doi: 10.1056/NEJMoa1203421
15. Cardarella S, Ogino A, Nishino M, Butaney M, Shen J, Lydon C, et al. : Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res (2013) 19:4532–40. doi: 10.1158/1078-0432.CCR-13-0657
16. Dankner M, Rose AAN, Rajkumar S, Siegel PM, Watson IR. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene (2018) 37:3183–99. doi: 10.1038/s41388-018-0171-x
17. Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM. et al: Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell (2004) 116:855–67. doi: 10.1016/S0092-8674(04)00215-6
18. Litvak AM, Paik PK, Woo KM, Sima CS, Hellmann MD, Arcila ME, et al. : Clinical characteristics and course of 63 patients with BRAF mutant lung cancers. J Thorac Oncol (2014) 9:1669–74. doi: 10.1097/JTO.0000000000000344
19. Kinno T, Tsuta K, Shiraishi K, Mizukami T, Suzuki M, Yoshida A, et al. : Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Ann Oncol (2014) 25:138–42. doi: 10.1093/annonc/mdt495
20. Sasaki H, Shitara M, Yokota K, Okuda K, Hikosaka Y, Moriyama S, et al. : Braf and erbB2 mutations correlate with smoking status in lung cancer patients. Exp Ther Med (2012) 3:771–5. doi: 10.3892/etm.2012.500
21. Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland A, et al. : Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol (2017) 18:1307–16. doi: 10.1016/S1470-2045(17)30679-4
22. Auliac JB, Bayle S, Do P, Le Garff G, Roa M, Falchero L, et al. : Efficacy of Dabrafenib Plus Trametinib Combination in Patients with BRAF V600E-Mutant NSCLC in Real-World Setting: GFPC 01-2019. Cancers (Basel) (2020) 12:3608. doi: 10.3390/cancers12123608
23. Horn L, Bauml J, Forde PM, Davis KL, Myall NJ, Sasane M, et al. : Real-world treatment patterns and survival of patients with BRAF V600-mutated metastatic non-small cell lung cancer. Lung Cancer (2019) 128:74–90. doi: 10.1016/j.lungcan.2018.12.003
24. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. : New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1). Eur J Cancer (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
25. Planchard D, Besse B, Groen HJM, Hashemi SMS, Mazieres J, Kim TM, et al. : Phase 2 Study of Dabrafenib Plus Trametinib in Patients With BRAF V600E-Mutant Metastatic NSCLC: Updated 5-Year Survival Rates and Genomic Analysis. J Thorac Oncol (2022) 17:103–15. doi: 10.1016/j.jtho.2021.08.011
26. Marchetti A, Felicioni L, Malatesta S, Grazia Sciarrotta M, Guetti L, Chella A, et al. : Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol (2011) 29:3574–9. doi: 10.1200/JCO.2011.35.9638
27. Ding X, Zhang Z, Jiang T, Li X, Zhao C, Su B, et al. : Clinicopathologic characteristics and outcomes of Chinese patients with non-small-cell lung cancer and BRAF mutation. Cancer Med (2017) 6:555–62. doi: 10.1002/cam4.1014
28. Luk PP, Yu B, Ng CC, Mercorella B, Selinger C, Lum T, et al. BRAF mutations in non-small cell lung cancer. Transl Lung Cancer Res (2015) 4:142–8.
29. Tissot C, Couraud S, Tanguy R, Bringuier PP, Girard N, Souquet PJ. Clinical characteristics and outcome of patients with lung cancer harboring BRAF mutations. Lung Cancer (2016) 91:23–8. doi: 10.1016/j.lungcan.2015.11.006
30. Barlesi F, Mazieres J, Merlio JP, Debieuvre D, Mosser J, Lena H, et al. : Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet (2016) 387:1415–26. doi: 10.1016/S0140-6736(16)00004-0
31. Villaruz LC, Socinski MA, Abberbock S, Berry LD, Johnson BE, Kwiatkowski DJ, et al. : Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium. Cancer (2015) 121:448–56. doi: 10.1002/cncr.29042
32. Facchinetti F, Lacroix L, Mezquita L, Scoazec JY, Loriot Y, Tselikas L, et al. : Molecular mechanisms of resistance to BRAF and MEK inhibitors in BRAF(V600E) non-small cell lung cancer. Eur J Cancer (2020) 132:211–23. doi: 10.1016/j.ejca.2020.03.025
33. Johnson DB, Menzies AM, Zimmer L, Eroglu Z, Ye F, Zhao S, et al. : Acquired BRAF inhibitor resistance: A multicenter meta-analysis of the spectrum and frequencies, clinical behaviour, and phenotypic associations of resistance mechanisms. Eur J Cancer (2015) 51:2792–9. doi: 10.1016/j.ejca.2015.08.022
34. Long GV, Fung C, Menzies AM, Pupo GM, Carlino MS, Hyman J, et al. : Increased MAPK reactivation in early resistance to dabrafenib/trametinib combination therapy of BRAF-mutant metastatic melanoma. Nat Commun (2014) 5:5694. doi: 10.1038/ncomms6694
35. Niemantsverdriet M, Schuuring E, Elst AT, van der Wekken AJ, van Kempen LC, van den Berg A, et al. : KRAS Mutation as a Resistance Mechanism to BRAF/MEK Inhibition in NSCLC. J Thorac Oncol (2018) 13:e249–51. doi: 10.1016/j.jtho.2018.07.103
36. Auliac JB, Bayle S, Vergnenegre A, Le Caer H, Falchero L, Gervais R, et al. : Patients with non-small-cell lung cancer harbouring a BRAF mutation: a multicentre study exploring clinical characteristics, management, and outcomes in a real-life setting: EXPLORE GFPC 02-14. Curr Oncol (2018) 25:e398–402. doi: 10.3747/co.25.3945
37. Soria X, Vilardell F, Maiques O, Barcelo C, Siso P, de la Rosa I, et al. : BRAF(V600E) Mutant Allele Frequency (MAF) Influences Melanoma Clinicopathologic Characteristics. Cancers (Basel) (2021) 13:5073. doi: 10.3390/cancers13205073
38. Planchard D, Besse B, Groen H, Hashemi SM, Mazieres J, Kim TM, et al. : Updated overall survival (OS) and genomic analysis from a single-arm phase II study of dabrafenib (D) + trametinib (T) in patients (pts) with BRAF V600E mutant (Mut) metastatic non-small cell lung cancer (NSCLC). J Clin Oncol (2020) 38:9593–3. doi: 10.1200/JCO.2020.38.15_suppl.9593
39. Planchard D, Besse B, Kim TM, Quoix EA, Souquet PJ, Mazieres J, et al. : Updated survival of patients (pts) with previously treated BRAF V600E–mutant advanced non-small cell lung cancer (NSCLC) who received dabrafenib (D) or D + trametinib (T) in the phase II BRF113928 study. J Clin Oncol (2017) 35:9075–5. doi: 10.1200/JCO.2017.35.15_suppl.9075
40. Gautschi O, Milia J, Cabarrou B, Bluthgen MV, Besse B, Smit EF, et al. : Targeted Therapy for Patients with BRAF-Mutant Lung Cancer: Results from the European EURAF Cohort. J Thorac Oncol (2015) 10:1451–7. doi: 10.1097/JTO.0000000000000625
41. Zhuang X, Zhao C, Li J, Su C, Chen X, Ren S, et al. : Clinical features and therapeutic options in non-small cell lung cancer patients with concomitant mutations of EGFR, ALK, ROS1, KRAS or BRAF. Cancer Med (2019) 8:2858–66. doi: 10.1002/cam4.2183
42. Reschke R, Simon JC, Ziemer M. Rechallenge of targeted therapy in metastatic melanoma. J Dtsch Dermatol Ges (2019) 17:483–6. doi: 10.1111/ddg.13766
Keywords: non-small cell lung cancer, BRAF-V600E, dabrafenib plus trametinib, co-mutations, treatment outcomes
Citation: Qu J, Shen Q, Li Y, Kalyani FS, Liu L, Zhou J and Zhou J (2022) Clinical Characteristics, Co-Mutations, and Treatment Outcomes in Advanced Non-Small-Cell Lung Cancer Patients With the BRAF-V600E Mutation. Front. Oncol. 12:911303. doi: 10.3389/fonc.2022.911303
Received: 02 April 2022; Accepted: 24 May 2022;
Published: 22 June 2022.
Edited by:
Lucia Anna Muscarella, Home for Relief of Suffering (IRCCS), ItalyReviewed by:
Francesco Pepe, University of Naples Federico II, ItalyCopyright © 2022 Qu, Shen, Li, Kalyani, Liu, Zhou and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianya Zhou, emhvdWp5QHpqdS5lZHUuY24=; Li Liu, bGl1bGlAaG5jYS5vcmcuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.