- Department of Gynecology and Obstetrics, Yantai Yuhuangding Hospital Affiliated to Qingdao University, Yantai, China
Background: According to current research, the objective response rate and overall survival of pembrolizumab in the treatment of several types of solid tumors have been significantly improved. Some high-quality clinical trials have studied the effect of applying pembrolizumab in treating cervical cancer. Multiple clinical trials have been conducted, and some of them have shown good results as expected. Therefore, we performed this meta-analysis on existing studies to reveal the efficacy and safety of pembrolizumab in treating cervical cancer.
Methods: PubMed, Embase, Cochrane Library and Web of Science were searched for literatures published until October 31, 2021. Outcomes included complete response (CR), partial response (PR), stable disease (SD), disease progression (PD), objective response rate (ORR), disease control rate (DCR), overall survival (OS), progression-free survival (PFS), the best time to response (TTR), death rate, adverse events (AE).
Results: A total of 7 studies with 727 patients were included. The results were as follows: CR (0.027, 95%CI: 0.008-0.053), PR (0.104, 95% CI: 0.074-0.145), SD (0.190, 95% CI: 0.149-0.240), PD (0.541, 95% CI: 0.421-0.661). ORR was 0.155 (95% CI: 0.098-0.236) and DCR was 0.331 (95% CI: 0.277-0.385). OS was 10.23 months (95% CI: 8.96-11.50) and PFS was 4.27 months (95% CI: 1.57-6.96). TTR was 2.10 months (95%CI: 1.69-2.51). The 1-year death rate was 0.388 (95% CI: 0.230-0.574). Main adverse events included abnormal liver function, hypothyroidism, neutropenia, anemia, decreased appetite, fatigue, fever, etc. The total incidence of the adverse events of grade 3 and above was 0.212 (95% CI: 0.065-0.509).
Conclusions: Pembrolizumab provides significant benefits in response rate and survival for cervical cancer patients. The results from recent high-quality clinical trials are expected to validate these findings.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42021291723.
1 Introduction
Cervical cancer is the most common malignancy in female reproductive system. It is the fourth most common cancer in women worldwide and the fourth leading cause of cancer-related death (1). With the popularization of HPV vaccine and applying systemic screening of disease in recent years, the incidence of cervical cancer has decreased significantly in developed countries (2–4), but the incidence and mortality rate in developing countries still remained high (5, 6). According to the statistics by World Health Organization, in 2020, an estimated 604,127 women will be diagnosed with cervical cancer globally and 341,831 women will die from the disease, with approximately 90% of the cases occurring in low- and middle-income countries (7). The treatment of cervical cancer is mainly determined by the disease stage at diagnosis, and the commonly used methods include surgical resection, radiotherapy and chemotherapy (8). Among the cervical cancer patients at early stage without evidence of lymph node metastasis, approximately 11-22% of them relapse after receiving primary standard treatment. In patients with lymph node metastasis and/or disease progression at local lesion, the recurrence rate is as high as 28-64% (9, 10). Compared with the cervical cancer patients at an early stage, patients with recurrent/metastatic cervical cancer have a poor prognosis, which is an important cause of death. In addition, the treatment approaches are quite limited. The effects of traditional chemotherapy are unsatisfactory, and there are various complications related to surgery and radiotherapy. Thus, choosing proper therapeutic strategies for patients with recurrent/metastatic cervical cancer remains challenging and is a tough issue in the treatment of gynecological tumors (11, 12). Therefore, improving the efficacy of cervical cancer treatment has been a major medical challenge worldwide.
In 1992, PD-1 was first discovered in mice (13) as a member of the CD28 superfamily, and PD-L1 (also known as CD274 or B7-H1) was its ligand (14). PD-L1 is expressed by tumor cells and can bind with PD-1 to inhibit the activation of T cell and cytokine production. PD-1/PD-L1 antibody blocks the pathway by binding to PD-1/PD-L1, which contributes to the growth and proliferation of T cells, reduces the apoptosis of T cell, activates the attack and killing abilities of T cell, and restores the sensitivity of the immune response to enhance antitumor activity (15, 16). At present, there are a large number of clinical trials evaluating the efficacy and safety of PD-1/PD-L1 immune checkpoint inhibitors (ICIs) in patients with different types of tumors (17). It has been found that PD-L1 expression was increased in HPV-induced cervical cancer, suggesting that PD-1 may be an effective therapeutic target for cervical cancer patients (18). Among them, pembrolizumab is a highly selective human monoclonal antibody that can block the interaction between PD-1 and PD-L1, thereby promoting the process of killing tumor cells by immune system (19). According to statistics, since pembrolizumab was first approved to be used in the treatment of advanced melanoma in September 2014, at least 500 clinical studies have been conducted on 20 solid tumors and hematological malignancies (20). In June 2018, FDA approved pembrolizumab for recurrent/metastatic cervical cancer patients who had tumors expressing PD-L1 after chemotherapy or had progressed disease. To date, approximately seven clinical trials or case series have reported the final or mid-term results of studies on the efficacy of pembrolizumab in patients with advanced cervical cancer (21–27), and three other trials are in progress (NCT04221945, NCT05007106, EUCTR2020 -000172-38-FR). There is still a lack of evidence supported by evidence-based medicine for using pembrolizumab in patients with advanced cervical cancer. Therefore, we performed this systematic review and single-arm meta-analysis to assess the efficacy and safety of pembrolizumab in patients with advanced cervical cancer.
2 Materials and methods
This meta-analysis strictly abided by the PRISMA statement and was registered on PROSPERO (registration number CRD42021291723).
2.1 Search strategy
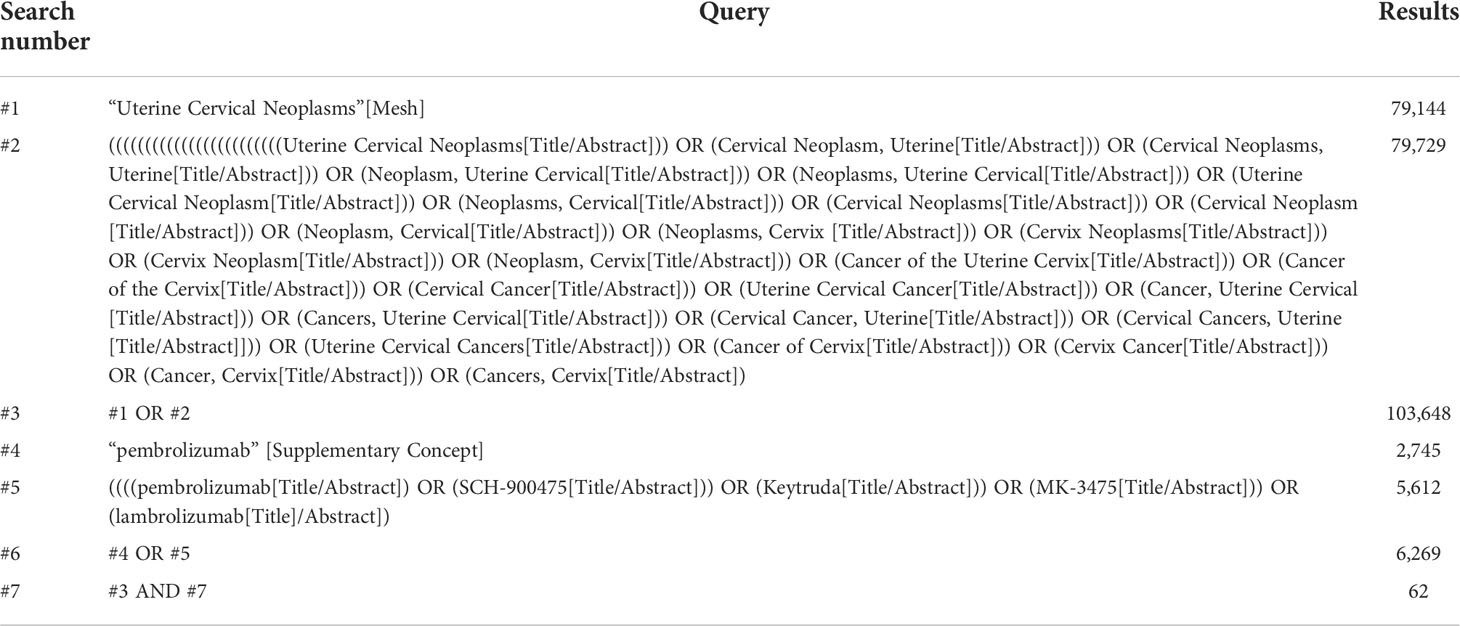
We searched PubMed, Embase, Cochrane Library and Web of Science databases for eligible studies published before 31 October 2021. Subject terms and free terms were used in the search. The subject terms used in PubMed were Uterine Cervical Neoplasms [Mesh], and pembrolizumab [Mesh]. The detailed search strategy is shown in Table 1.
2.2 Inclusion and exclusion criteria
This study was a meta-analysis based on published data, and the inclusion criteria were as follows (1): the literature which research subject was on human cervical cancer patients (2); single-arm study or RCT in which the intervention method was pembrolizumab treatment (3); English literature. Exclusion criteria (1): in-vitro experiments, reviews, abstracts, letters, pathological studies, etc. (2) literature in other languages (3); original literature unavailable. If authors published several studies using the same data, the most recent or comprehensive ones were included.
2.3 Literature screening and data extraction
Two investigators independently screened the literature according to the inclusion and exclusion criteria to determine the final included studies, and then independently extracted information. Before information extraction, a standard spreadsheet for information extraction was created and the extracted data included basic information (article title, first author, publication year, national clinical trial (NCT) registration number, country, study type, intervention method, sample size, age, disease stage and type) and outcome indicators [complete response (CR), partial response (PR), stable disease (SD), disease progression (PD), objective response rate (ORR), disease control rate (DCR), overall survival (OS), progression-free survival (PFS), the best time to response (TTR), death rate, adverse events (AE)].
Two researchers independently screened the literature, extracted information and then cross-checked their work. If there was any dissent, a third researcher was consulted to make a determination.
2.4 Quality Evaluation
The quality of the included studies was assessed using the Methodological Evaluation Metrics for Non-Randomized Controlled Trials (MINORS) (28). MINORS includes 12 evaluation indicators and each one can be scored 0-2. The first 8 items are for studies without a control group and the maximum score is 16. The last 4 items and the first 8 items are for studies with a control group and the maximum score is 24. 0 means that the data is not reported. 1 means that the data is reported but without sufficient information. 2 means that the data is reported with sufficient information.
2.5 Statistical analysis
R4.04 software (R development Core Team, Vienna, http://www.R-project.org) with metafor, matrix and meta packages was used to conduct single-arm meta-analysis. I2 was used to measure the heterogeneity, and the Q test was used to test the significance of heterogeneity. When I2 ≥ 50%, heterogeneity was considered to be significant, and the random-effects model was used to pool the effect size. When I2 <50%, the fixed-effects model was used to pool effect size.
3 Results
3.1 Selection of literature
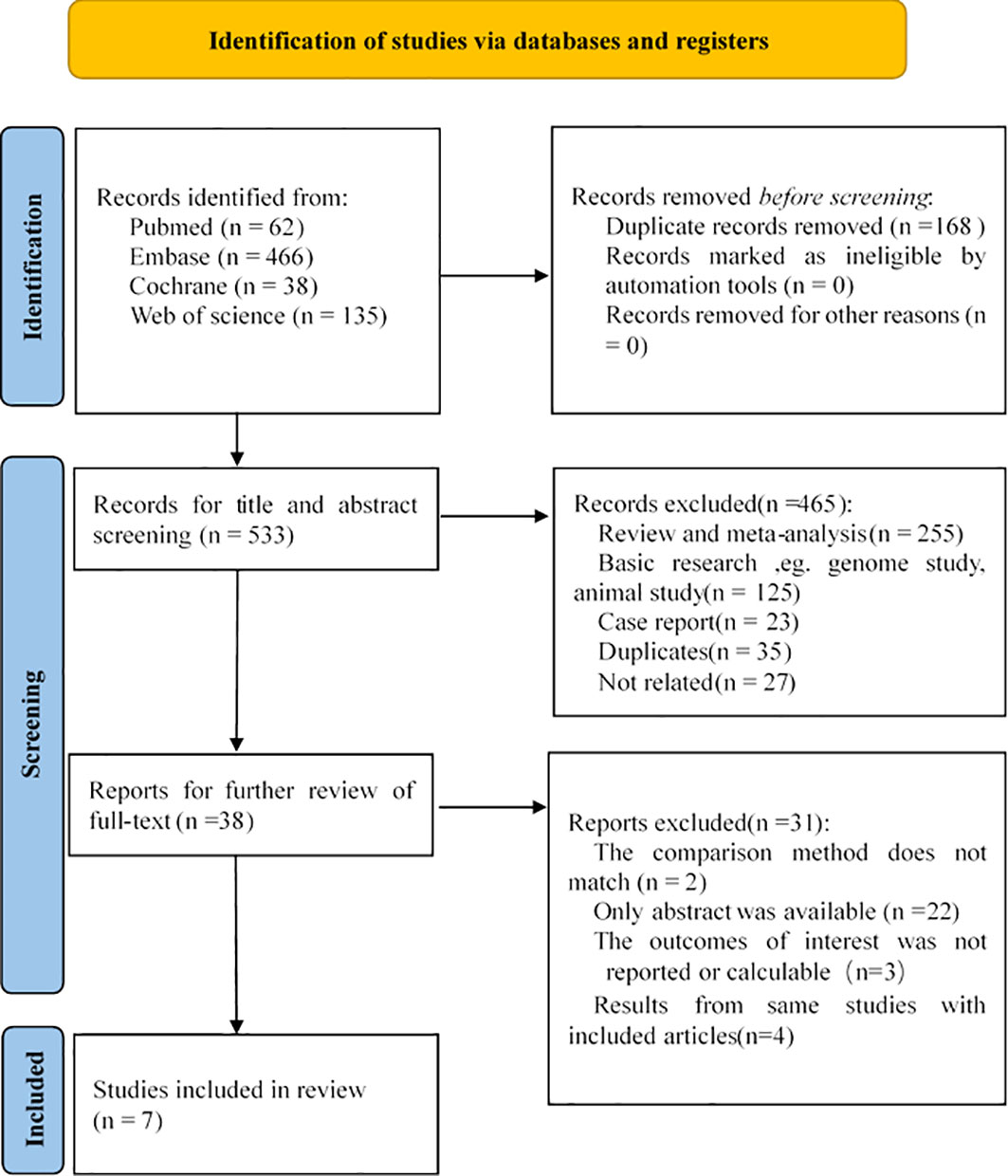
After a thorough search, a total of 701 related studies were found, of which 62 studies were from PubMed, 466 studies from Embase, 38 studies from Cochrane, and 135 studies from WOS. By reading the titles, abstracts and full texts, meta-analyses, reviews, letters, conference abstracts, animal experiments, and case reports were excluded, and 7 studies were finally included in our research (21–27). The selection process is shown in Figure 1. The 7 selectively included articles were assessed and checked by two investigators. According to the MINORS scale, the included articles had a high level of methodological quality.
3.2 Basic characteristics of the included studies
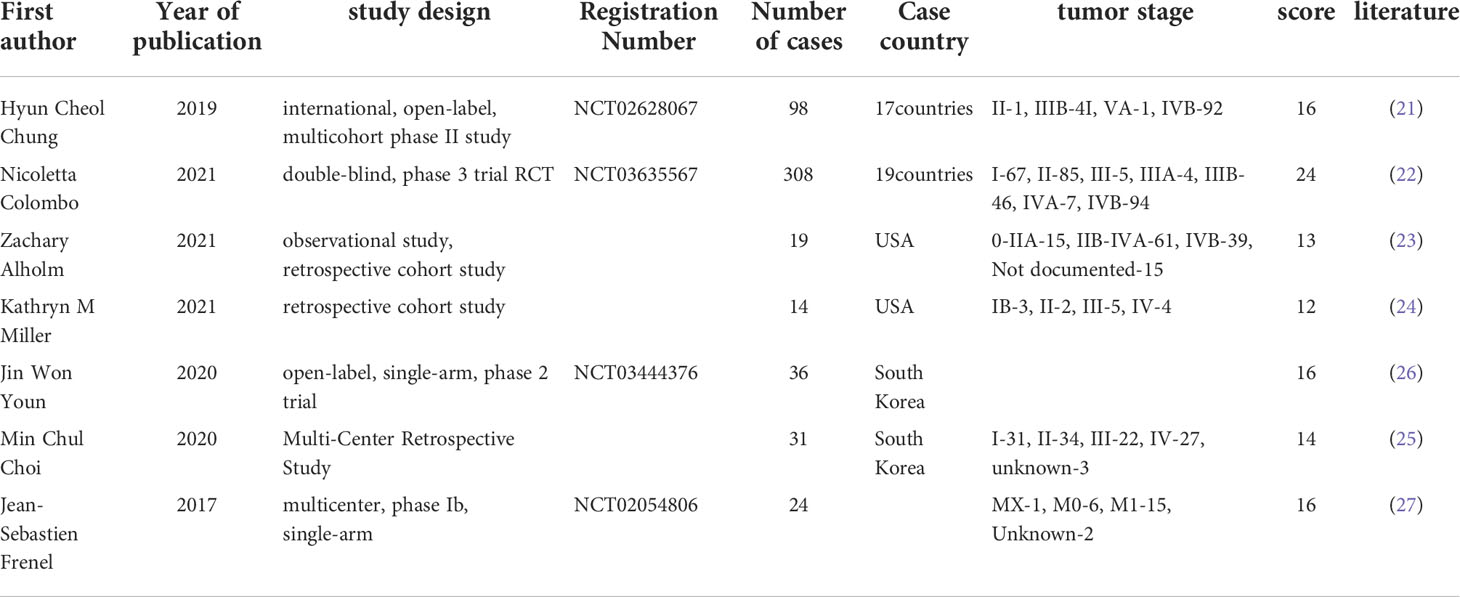
Seven studies with a total of 727 patients were included, of which 1 study was phase III RCT, 4 phase II RCTs, 1 phase I RCT and 1 observational study. Seven studies were published within the past 5 years (2017–2021), which indicated that this study was innovative and the immune checkpoint inhibitors were developed rapidly in cervical cancer treatment. Six of the studies used monotherapy of pembrolizumab and one used combination therapy. The main characteristics, treatment strategies and quality evaluation of the included articles are shown in the Table 2.
3.3 Results of meta-analysis
3.3.1 Response rate (%) of CR, PR, SD and PD
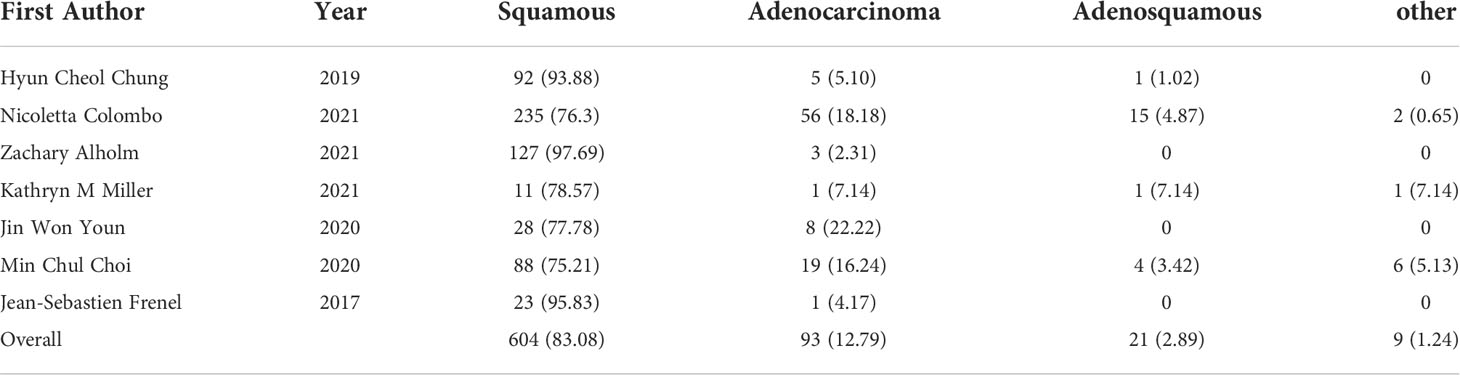
Five studies reported the response rate (%) of CR, PR, SD and PD. For cervical cancer patients treated with pembrolizumab, 11 out of 289 patients achieved CR (0.027, 95%CI: 0.008-0.053) (Figure 2A), 30 out of 289 patients achieved PR (0.104, 95%CI: 0.074-0.145) (Figure 2B), 55 out of 289 patients achieved SD (0.190, 95%CI: 0.149-0.240) (Figure 2C), and 158 out of 289 patients achieved PD (0.541, 95%CI: 0.421-0.661) (Figure 2D).
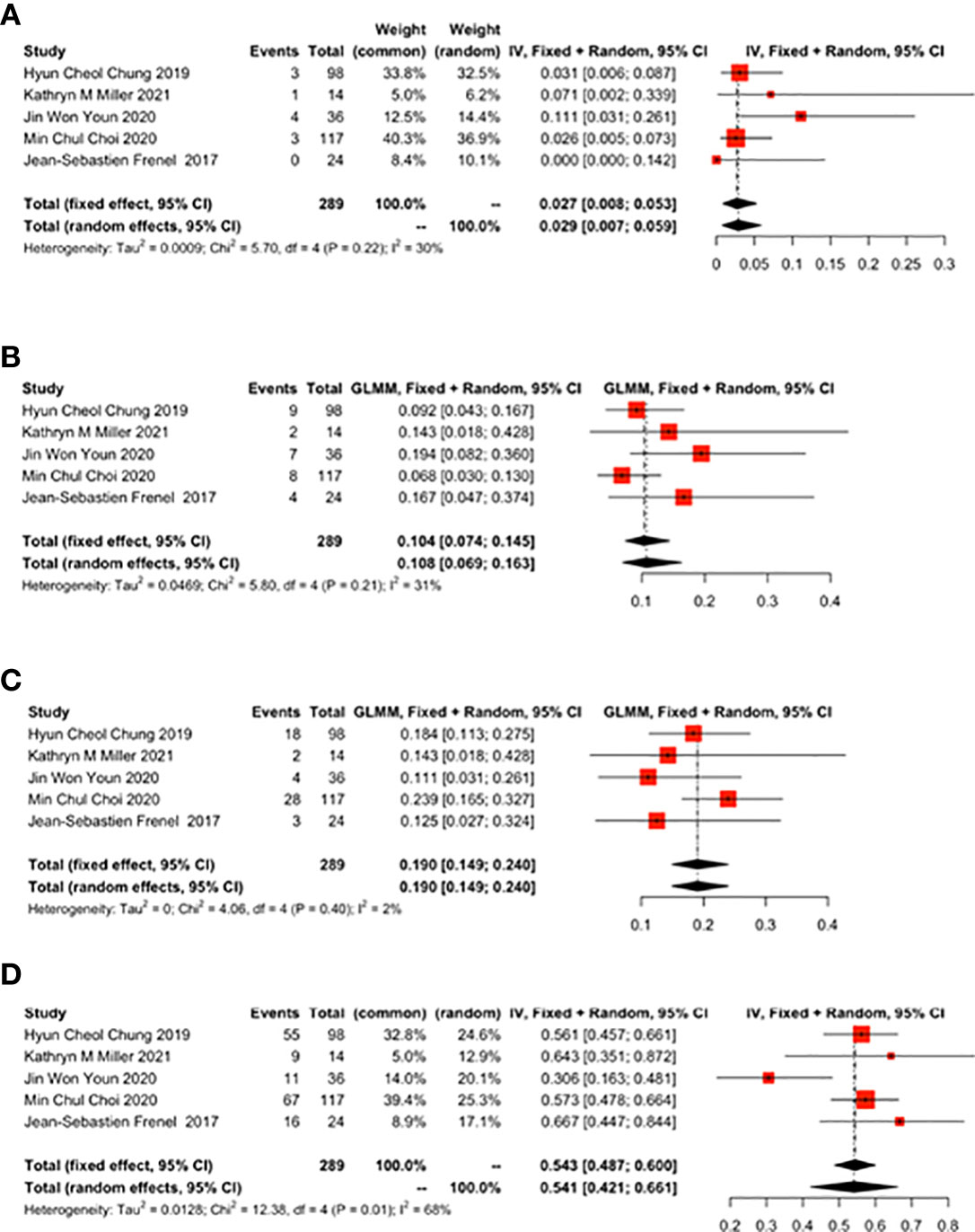
Figure 2 Complete response (CR), partial response (PR), stable disease (SD), disease progression (PD) for patients with cervical cancer receiving pembrolizumab therapy. (A) CR, (B) PR, (C) SD, (D) PD.
3.3.2 The ratio of ORR to DCR (%)
Five studies reported the ratio of ORR to DCR. ORR was 0.155 (95% CI: 0.098-0.236) (Figure 3A) and DCR was 0.331 (95% CI: 0.277-0.385) (Figure 3B).
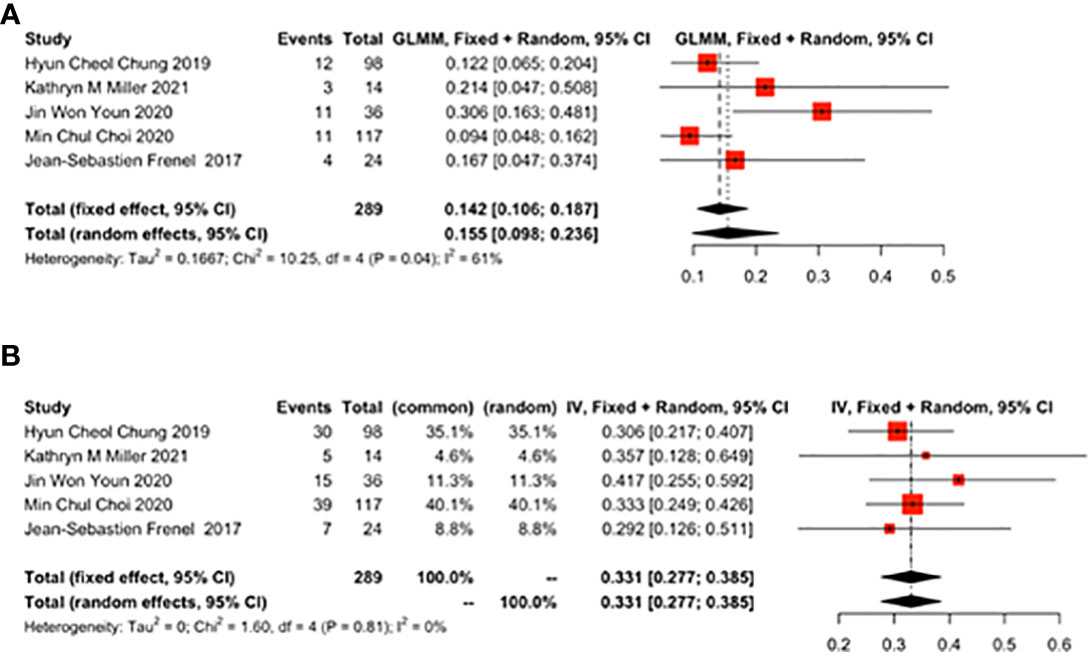
Figure 3 Overall results of objective response rate (ORR) and disease control rate (DCR) for patients with cervical cancer receiving pembrolizumab therapy. (A) ORR, (B) DCR.
3.3.3 OS and PFS
Six studies reported the effect of pembrolizumab on OS and PFS in cervical cancer patients. OS was 10.23 months (95% CI: 8.96-11.50) (Figure 4A) and PFS was 4.27 months (95% CI: 1.57-6.96) (Figure 4B). There was a significant heterogeneity in the overall results of PFS (I2 = 97%, p<0.01) (Figure 4B). However, heterogeneity was not observed in OS (I2 = 0%, p=0.85) (Figure 4A).
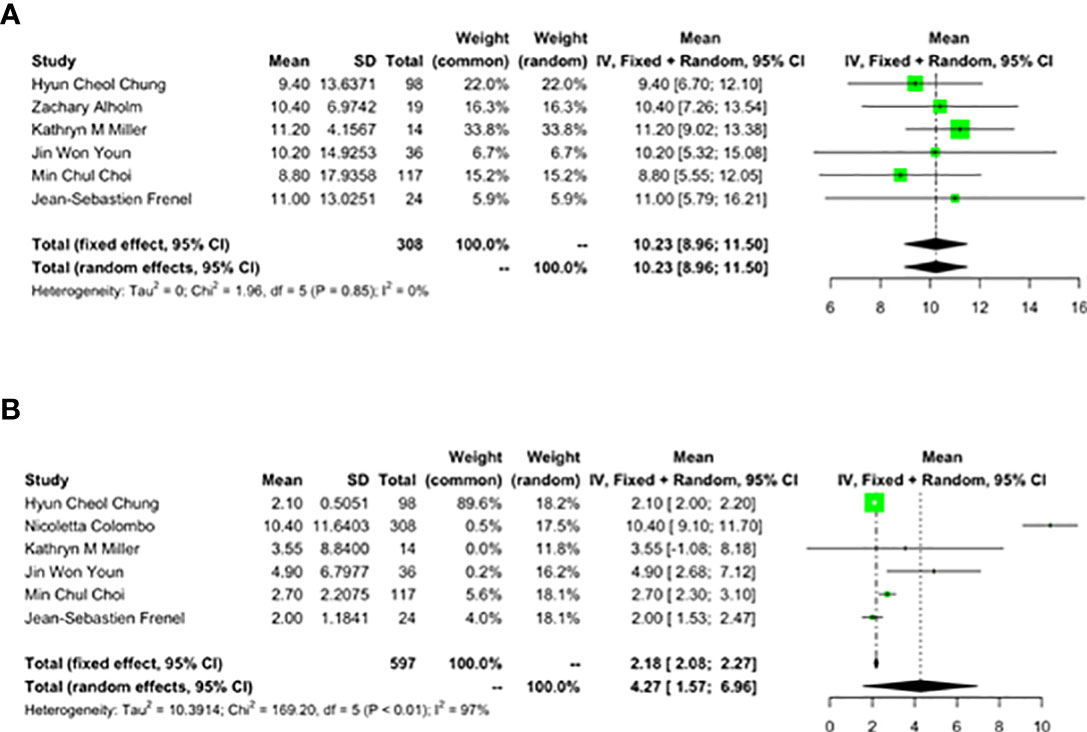
Figure 4 Overall survival (OS) and progression-free survival (PFS) for patients with cervical cancer receiving pembrolizumab therapy. (A) OS, (B) PFS.
3.3.4 TTR and Death
As reported by 4 studies, TTR was 2.10 months (95% CI: 1.69-2.51) (Figure 5A). As reported by 7 studies, the 1-year mortality rate was 0.388 (95% CI: 0.230-0.574) (Figure 5B) in cervical cancer patients treated with pembrolizumab.
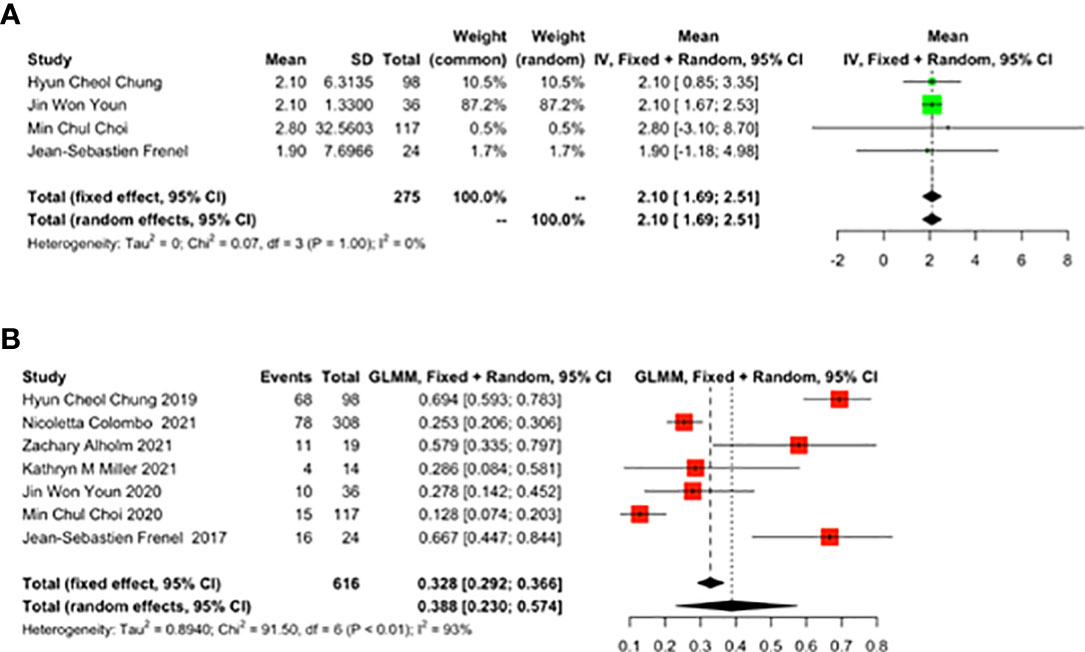
Figure 5 The best time to response (TTR) and 1-year mortality rate for patients with cervical cancer receiving pembrolizumab therapy. (A) TTR, (B) Death.
3.3.5 Adverse events AE
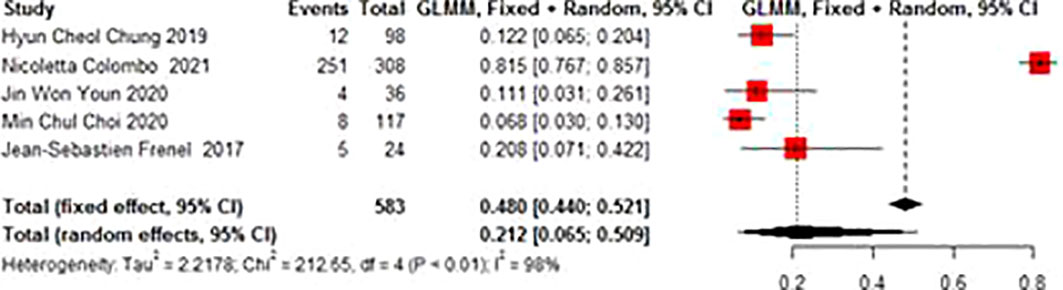
Adverse events mainly included abnormal liver function, hypothyroidism, neutropenia, anemia, decreased appetite, fatigue, fever, etc. The total incidence of the adverse events of grade 3 and above was 0.212 (95% CI 0.065-0.509). There was no significant heterogeneity in AE (I2 = 98%, p<0.01) (Figure 6).
4 Discussion
The overall quality of the 7 studies included in this meta-analysis was high, indicating that a relatively objective result could be obtained by our study. A total of 727 cervical cancer patients received pembrolizumab in this meta-analysis. Among 727 patients, Squamous (604 cases, 83.08%), Adenocarcinoma (93 cases, 12.79%), Adenosquamous (21 cases, 2.89%) and other types (9 cases, 1.24%) were the main pathological types. Among them, Squamous accounts for more than 70% of all studies. The specific distribution is shown in the following Table 2 cont. As demonstrated by this single-arm meta-analysis, the complete response rate of this treatment was 2.7%, the partial response rate was 10.4%, the rate of stable disease was 19%, the disease progression rate was 54.1%, the objective response rate was 15.5%, the disease control rate was 33.1%, the median overall survival was about 10.23 months, the median progression-free survival was 4.27 months, the best response time was 2.1 months, and one-year death rate was 38.8%. Major adverse events included tolerable hepatic dysfunction, hypothyroidism, neutropenia, decreased appetite and fatigue, and the total incidence of adverse events of grade 3 and above was 21.2%.
The 5-year survival rate of cervical cancer patients at an early stage after radical resection is about 80%. After radical radiotherapy and chemotherapy, the 5-year survival rate can still achieve about 70% even if the patients have locally advanced cervical cancer (29). However, about 28% of cervical cancer patients may have local recurrence or distant metastasis. Most cases of recurrence have been seen within 3 years, with poor prognosis, and 30-50% of the patients with locally advanced cervical cancer may relapse and eventually die of it (30, 31). Currently, the treatment approaches for recurrent/metastatic cervical cancer are limited. Treatment strategies are made primarily based on patients` physical condition, sites and ranges of recurrence/metastasis, and previous treatments they have received (32). Chemo-radiotherapy presents typically a preferred option (33, 34). Pelvic recurrence is the most common type of local recurrence, and radiotherapy or platinum-based concurrent chemoradiotherapy are standard treatments for pelvic recurrence after radical hysterectomy. Vaginal vault relapses can be treated with external radiotherapy plus brachytherapy, as compared to the nodal disease in which external radiotherapy alone is the only therapeutic option. In case when recurrent disease involves the pelvic side wall and the primary treatment included chemoradiation or surgery followed by adjuvant radiotherapy, then palliative CT is a reasonable option due to limitations of exenteration in this setting (35). However, the outcome would not be satisfactory due to complications and chronic toxic and side effects induced by radiotherapy. It has been generally accepted that cisplatin is the most effective agent for relapsed/advanced cervical cancer. Platinum-based combination regimens, such as cisplatin combined with paclitaxel, are currently recommended. For patients who had history of cis-platinum administration, carboplatin combined with paclitaxel would be the most preferred alternative, with other second-line options such as bevacizumab, docetaxel, gemcitabine, and irinotecan (36). Studies have shown that the overall survival has been improved in the cervical cancer patients who received combination treatment of bevacizumab and chemotherapy compared with those who received chemotherapy alone (37). Even so, there is no established evidence that treatment in the second-line setting improves OS compared with best supportive care. In addition, treatment of advanced cervical cancers in older patients remains controversial and poorly defined. Historically, the first reported series of patients treated at large proportion with second-line systemic treatment for recurrent or metastatic cervical cancer was performed at the Royal Marsden Hospital between 2004 and 2014. In this retrospective series, 70% of women treated with systemic therapy for recurrent or metastatic cervical cancer subsequently received second-line therapy with an ORR of 13.2%, a median PFS of 3.2 months and a median overall survival of 9.3 months (38). The only option for advanced cervical cancer patients with multiple complications is palliative care, to maintain the dignity and quality of life. Nevertheless, the treatment progress of recurrent/metastatic cervical cancer is generally slow due to the high recurrence rate after radiotherapy and chemotherapy, critical toxic and adverse events, poor tolerance, and rapid deterioration of quality of life (39).
In recent years, immunotherapy research has received increasing attention due to its sensitivity, specificity and self-renewal ability of the immune system. Good results have been achieved in the treatment of various solid tumors by immunotherapy (40). Tumor immunotherapy has also become the fourth tumor treatment strategy after surgery, chemotherapy and radiotherapy. Tumors evade the immune response by suppressing the immunosuppressive signaling pathway when the body’s immune function is at a low level. Immunotherapy aims to activate the immune system and kill tumor cells via the immune function in our body (41). Since the approval of ipilimumab in the United States in 2011, immune checkpoint inhibitors (ICIs) have made breakthroughs in tumor immunotherapy. Among them, the expression level of PD-L1 in cervical cancer patients is relatively high, ranging from 34.4% to 96.0% (42), which suggests that PD-1 inhibitors can be used in the treatment of cervical cancer. Therefore, ICIs are expected to be used as a potential treatment for cervical cancer. In particular, according to the KEYNOTE-158 (21) clinical trial, pembrolizumab was proved to be effective against solid tumors, including cervical cancer, and was approved by the U.S. Food and Drug Administration (FDA). Pembrolizumab is an effective humanized immunoglobulin G4 (IgG4) monoclonal antibody (mAb) with a high specificity of binding with the PD-1 receptor. Pembrolizumab can inhibit the PD-1 pathway (including PD-L1 and PD-L2) via dual-ligand blockade. Due to its inherent properties, pembrolizumab can inhibit tumor growth with low toxicity (43). Based on this NCCN guideline, pembrolizumab is recommended as a second-line regimen for PD-L1-positive or MSI-H/dMMR recurrent/metastatic cervical cancer. However, most of the existing studies were designed as retrospective analyses, phase I or II trials. Therefore, it is necessary to summarize the existing data to promote future research.
For recurrent/metastatic cervical cancer, cisplatin-based chemotherapy is the most common treatment (44). When using cisplatin as the only agent for chemotherapy, the median overall survival (OS) is approximately 6.5 months, the median progression-free survival (PFS) is approximately 3 months, and the remission in most patients is partial and transient (45–47). Since 1999, the American Gynecologic Oncology Group (GOG) has conducted a series of clinical trials. Among them, the GOG204 trial compared four groups of platinum-based doublet chemotherapy (including cisplatin with paclitaxel, gemcitabine, topotecan or Vinorelbine), paclitaxel combined with cisplatin was finally determined as the preferred chemotherapy regimen. The median OS was about 12.9 months, the median PFS 5.9 months, and the ORR 29.1% (48). In recent years, vascular endothelial growth factor (VEGF) has been demonstrated to promote tumor development by inducing angiogenesis, among which bevacizumab is a humanized monoclonal antibody that can specifically bind to VEGF-A (49, 50). Bradley J. Monk et al. (51) conducted a phase II trial and found that the median OS of bevacizumab was 7.29 months, the median PFS 3.4 months, and the ORR 10.9%. The results of the GOG240 study showed that the median OS of combination chemotherapy of cisplatin, paclitaxel and bevacizumab was increased to 16.8 months, and the median PFS was 8.2 months (52). Therefore, cisplatin/carboplatin-paclitaxel-bevacizumab is currently recommended as the first-line treatment in the NCCN guideline (11). Nonetheless, a significant proportion of patients died within one year after receiving systemic therapy (43%), and many patients needed second-line therapy within one year due to disease progression or toxicity (31%) (53). The results of this study showed that the median OS of pembrolizumab group was 10.23 months, the median PFS was 4.27 months, the ORR was 15.5%, and the mortality rate within one year was 38.8%, which was significantly better than that of using bevacizumab alone. The effect seemed not better compared to the first-line regimen. However, all the patients in the GOG240 study did not receive paclitaxel and platinum-based chemotherapy. In the existing studies, most patients using pembrolizumab had received at least one combination chemotherapy or radiation therapy before. According to the comparison results after disease progression, the median OS of the chemotherapy using cisplatin and paclitaxel after progression was 7.1 months, and the median OS of the chemotherapy using cisplatin + paclitaxel + bevacizumab after progression was 8.4 months (53), which were both lower than the overall survival when using pembrolizumab. At the same time, pembrolizumab in combination with standard chemotherapy has also been proposed to improve the efficacy, which is currently being explored.
In terms of safety, patients using cisplatin-based chemotherapy are prone to myelosuppression, diarrhea, and tenesmus, which may lead to treatment interruption or prolongation of treatment time. Some patients even suffer from long-term chronic diarrhea and malnutrition (54), and many patients eventually discontinue the therapy due to drug resistance (55). Bevacizumab increases the risk of specific adverse events (gastrointestinal tract perforation or fistula, thromboembolism, hypertension, etc.) especially severe adverse events, compared with chemotherapy alone (56). Current development of proteomics technology (mass spectrometry and protein array analysis) has deepened the identification of potential molecular signaling events and the proteomic characteristics of cervical and ovarian cancer, which facilitates the exploration of new therapeutic agents so as to reduce drug-resistance (57). In this meta-analysis, it was found that the incidence of the adverse events of grade 3 and above in the patients using pembrolizumab was 21.2%, mainly including tolerable liver dysfunction, hypothyroidism, neutropenia, anemia, decreased appetite, fatigue, and fever. Most of the adverse events can be improved after the therapy is discontinued. Most other PD-1/PD-L1 inhibitors, like nivolumab and atezolizumab, are still at the stage of I/II phase clinical trial so that the incidence of adverse events would be unavailable. It indicated that pembrolizumab showed significantly better safety.
On the other hand, translational research has identified a large number of potential biomarkers involved in the carcinogenesis. Within these biomarkers is included the baseline neutrophil-to-lymphocyte ratio (NLR), which is a simple haematological parameter easily obtainable in daily clinical practice. NLR has been repeatedly reported as a significant prognostic factor in advanced cancer patients. Changes in the NLR are a useful predicting factor in advanced cervical cancer patients treated with anti-PD-1/PD-L1 agents (58).
This meta-analysis has the following advantages. Firstly, this was the first article to provide evidence-based proof for the efficacy and safety of pembrolizumab in cervical cancer. Secondly, the original studies included were of high quality. At the same time, there were some limitations. First, even though we have conducted a comprehensive and systematic search in the mainstream databases, the number of the retrieved literature was still relatively small. Second, the included studies could only be used to conduct a single-arm meta-analysis, and we did not made comparison with the mainstream treatment strategies at present. Therefore, we could not directly reflect whether the treatment of pembrolizumab had advantages.
5 Conclusion
This single-arm meta-analysis showed that pembrolizumab could ameliorate cervical cancer to some extent and bring survival benefits. Therefore, pembrolizumab can be used as a promising treatment option for advanced/recurrent cervical cancer. However, due to the limitation of the published original literature, the existing evidenve is still not enough to support us to complete RCT-based meta-analysis. Thus, we look forward to more center RCTs which are not limited to ethnicity in the future to explore the specific advantages of pembrolizumab in cervical cancer treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
LQ and JC contributed the central idea, analysed most of the data and wrote the main manuscript text. NL, AL and XW contributed to refining the ideas, collecting the data, carrying out additional analyses and revising the manuscript. All authors have read and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Fontham ET, Wolf AM, Church TR, Etzioni R, Flowers CR, Herzig A, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American cancer society. CA Cancer J Clin (2020) 70(5):321–46. doi: 10.3322/caac.21628
3. Borcoman E, Le Tourneau C. Pembrolizumab in cervical cancer: latest evidence and clinical usefulness. Ther Adv Med Oncol (2017) 9(6):431–9. doi: 10.1177/1758834017708742
4. Lees BF, Erickson BK, WK H. Cervical cancer screening: evidence behind the guidelines. Am J Obstet Gynecol (2016) 214(4):438–43. doi: 10.1016/j.ajog.2015.10.147
5. Jolly S, Uppal S, Bhatla N, Johnston C, Maturen K. Improving global outcomes in cervical cancer: the time has come for international federation of gynecology and obstetrics staging to formally incorporate advanced imaging. J Glob Oncol (2018) 4:1–6. doi: 10.1200/JGO.2016.007534
6. Lee S-J, Yang A, Wu T-C, Hung C-F. Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research. J Gynecol Oncol (2016) 27(5):e51. doi: 10.3802/jgo.2016.27.e51
7. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. A Cancer Journal for Clinicians (2022). Malden, USA: Wiley-Blackwell
8. Lee SI, Atri MJR. 2018 FIGO staging system for uterine cervical cancer: enter cross-sectional imaging. Radiology (2019) 292(1):15–24. doi: 10.1148/radiol.2019190088
9. Gupta S, Maheshwari A, Parab P, Mahantshetty U, Hawaldar R, Sastri S, et al. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: a randomized controlled trial. J Clin Oncol (2018) 36(16):1548–55. doi: 10.1200/JCO.2017.75.9985
10. Gadducci A, Tana R, Cosio S, Cionini L. Treatment options in recurrent cervical cancer. Oncol Lett (2010) 1(1):3–11. doi: 10.3892/ol_00000001
11. Abu-Rustum N, Yashar C, Bean S. NCCN clinical practice guidelines in oncology: cervical cancer. Version (2019) 1:64–84. doi: 10.6004/jnccn.2019.0001
12. De Felice F, Giudice E, Bolomini G, Distefano MG, Scambia G, Fagotti A, et al. Pembrolizumab for advanced cervical cancer: safety and efficacy. Expert Rev Anticancer Ther (2021) 21(2):221–8. doi: 10.1080/14737140.2021.1850279
13. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J (1992) 11(11):3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x
14. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol (2008) 26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331
15. Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov (2019) 18(3):175–96. doi: 10.1038/s41573-018-0006-z
16. Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA (2002) 99(19):12293–7. doi: 10.1073/pnas.192461099
17. Dermani FK, Samadi P, Rahmani G, Kohlan AK, Najafi R. PD-1/PD-L1 immune checkpoint: potential target for cancer therapy. J Cell Physiol (2019) 234(2):1313–25. doi: 10.1002/jcp.27172
18. Allouch S, Malki A, Allouch A, Gupta I, Vranic S, Al Moustafa A-E. High-risk HPV oncoproteins and PD-1/PD-L1 interplay in human cervical cancer: recent evidence and future directions. Front Oncol (2020) 914. doi: 10.3389/fonc.2020.00914
19. Verhoeven Y, Quatannens D, Trinh XB, Wouters A, Smits EL, Lardon F, et al. Targeting the PD-1 axis with pembrolizumab for recurrent or metastatic cancer of the uterine cervix: A brief update. Int J Mol Sci (2021) 22(4):1807. doi: 10.3390/ijms22041807
20. Ribas A, Wolchok JDJS. Cancer immunotherapy using checkpoint blockade. Science (2018) 359(6382):1350–5. doi: 10.1126/science.aar4060
21. Chung HC, Ros W, Delord J-P, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol (2019) 37(17):1470–8. doi: 10.1200/JCO.18.01265
22. Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med (2021) 385(20):1856–67. doi: 10.1056/NEJMoa2112435
23. Alholm Z, Monk BJ, Ting J, Pulgar S, Boyd M, Sudharshan L, et al. Patient characteristics, treatment patterns, and clinical outcomes among patients with previously treated recurrent or metastatic cervical cancer: a community oncology-based analysis. Gynecol Oncol (2021) 161(2):422–8. doi: 10.1016/j.ygyno.2021.03.002
24. Miller KM, Filippova OT, Hayes SA, Abu-Rustum NR, Aghajanian C, Broach V, et al. Pattern of disease and response to pembrolizumab in recurrent cervical cancer. Gynecol Oncol Rep (2021) 37:100831. doi: 10.1016/j.gore.2021.100831
25. Choi MC, Kim Y-M, Lee J-W, Lee YJ, Suh DH, Lee SJ, et al. Real-world experience of pembrolizumab monotherapy in patients with recurrent or persistent cervical cancer: A korean multi-center retrospective study (KGOG1041). Cancers (Basel) (2020) 12(11):3188. doi: 10.3390/cancers12113188
26. Youn JW, Hur S-Y, Woo JW, Kim Y-M, Lim MC, Park SY, et al. Pembrolizumab plus GX-188E therapeutic DNA vaccine in patients with HPV-16-positive or HPV-18-positive advanced cervical cancer: interim results of a single-arm, phase 2 trial. Lancet Oncol (2020) 21(12):1653–60. doi: 10.1016/S1470-2045(20)30486-1
27. Frenel J-S, Le Tourneau C, O’Neil B, Ott PA, Piha-Paul SA, Gomez-Roca C, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1–positive cervical cancer: results from the phase ib KEYNOTE-028 trial. J Clin Oncol (2017) 35(36):4035–41. doi: 10.1200/JCO.2017.74.5471
28. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg (2003) 73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x
29. Cohen PA, Jhingran A, Oaknin A, Denny LJTL. Cervical cancer. Lancet, Vol. 393. (2019). pp. 169–82. New York, USA: Elsevier Ltd.
30. Waggoner SEJTL. Cervical cancer. Lancet, Vol. 361. (2003). pp. 2217–25. New York, USA: Elsevier Ltd.
31. Landoni F, Colombo A, Milani R, Placa F, Zanagnolo V, Mangioni C. Randomized study between radical surgery and radiotherapy for the treatment of stage IB–IIA cervical cancer: 20-year update. J Gynecol Oncol (2017) 28(3):e34. doi: 10.3802/jgo.2017.28.e34
32. Quinn M, Benedet J, Odicino F, Maisonneuve P, Beller U, Creasman W, et al. Carcinoma of the cervix uteri. Int J Gynaecol Obstet (2006) 95:S43–S103. doi: 10.1016/S0020-7292(06)60030-1
33. Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2017) 28:iv72–83. doi: 10.1093/annonc/mdx220
34. Tewari KS, Sill MW, Long HJ III, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med (2014) 370(8):734–43. doi: 10.1056/NEJMoa1309748
35. Boussios S, Seraj E, Zarkavelis G, Petrakis D, Kollas A, Kafantari A, et al. Management of patients with recurrent/advanced cervical cancer beyond first line platinum regimens: Where do we stand? a literature review. Crit Rev Oncol Hematol (2016) 108:164–74. doi: 10.1016/j.critrevonc.2016.11.006
36. Dueñas-González A, Cetina L, Coronel J, Martínez-Baños D. Pharmacotherapy options for locally advanced and advanced cervical cancer. Drugs (2010) 70(4):403–32. doi: 10.2165/11534370-000000000-00000
37. Grau JF, Farinas-Madrid L, Oaknin A. A randomized phase III trial of platinum chemotherapy plus paclitaxel with bevacizumab and atezolizumab versus platinum chemotherapy plus paclitaxel and bevacizumab in metastatic (stage IVB), persistent, or recurrent carcinoma of the cervix: the BEATcc study (ENGOT-Cx10/GEICO 68-C/JGOG1084/GOG-3030). Int J Gynecol Cancer (2020) 30(1):139–43. doi: 10.1136/ijgc-2019-000880
38. McLachlan J, Boussios S, Okines A, Glaessgen D, Bodlar S, Kalaitzaki R, et al. The impact of systemic therapy beyond first-line treatment for advanced cervical cancer. Clin Oncol (Royal Coll Radiologists (Great Britain)) (2017) 29(3):153–60. doi: 10.1016/j.clon.2016.10.002
39. Marchetti C, De Felice F, Di Pinto A, Romito A, Musella A, Palaia I, et al. Survival nomograms after curative neoadjuvant chemotherapy and radical surgery for stage IB2-IIIB cervical cancer. Cancer Res Treat (2018) 50(3):768. doi: 10.4143/crt.2017.141
40. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol (2020) 21(10):1353–65. doi: 10.1016/S1470-2045(20)30445-9
41. Cha J-H, Chan L-C, Li C-W, Hsu JL, Hung M-C. Mechanisms controlling PD-L1 expression in cancer. Mol Cell (2019) 76(3):359–70. doi: 10.1016/j.molcel.2019.09.030
42. Liu Y, Wu L, Tong R, Yang F, Yin L, Li M, et al. PD-1/PD-L1 inhibitors in cervical cancer. Front Pharmacol (2019) 65. doi: 10.3389/fphar.2019.00065
43. Marret G, Borcoman E, Le Tourneau C. Pembrolizumab for the treatment of cervical cancer. Expert Opin Biol Ther (2019) 19(9):871–7. doi: 10.1080/14712598.2019.1646721
44. Buda A, Fossati R, Colombo N, Fei F, Floriani I, Alletti DG, et al. Randomized trial of neoadjuvant chemotherapy comparing paclitaxel, ifosfamide, and cisplatin with ifosfamide and cisplatin followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: the SNAP01 (Studio neo-adjuvante portio) Italian collaborative study. J Clin Oncol (2005) 23(18):4137–45. doi: 10.1200/JCO.2005.04.172
45. Bonomi P, Blessing JA, Stehman FB, DiSaia PJ, Walton L, Major FJ. Randomized trial of three cisplatin dose schedules in squamous-cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol (1985) 3(8):1079–85. doi: 10.1200/JCO.1985.3.8.1079
46. Thigpen T, Shingleton H, Homesley H, Lagasse L, Blessing JJC. Cis-platinum in treatment of advanced or recurrent squamous cell carcinoma of the cervix: a phase II study of the gynecologic oncology group. Cancer (1981) 48(4):899–903. doi: 10.1002/1097-0142(19810815)48:4<899::AID-CNCR2820480406>3.0.CO;2-6
47. Potter ME, Hatch KD, Potter MY, Shingleton HM, Baker VVJC. Factors affecting the response of recurrent squamous cell carcinoma of the cervix to cisplatin. Cancer (1989) 63(7):1283–6. doi: 10.1002/1097-0142(19890401)63:7<1283::AID-CNCR2820630709>3.0.CO;2-U
48. Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a gynecologic oncology group study. J Clin Oncol (2009) 27(28):4649. doi: 10.1200/JCO.2009.21.8909
49. Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, et al. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev (2020) 86:102017. doi: 10.1016/j.ctrv.2020.102017
50. Tsuda N, Watari H, Ushijima K. Chemotherapy and molecular targeting therapy for recurrent cervical cancer. Chin J Cancer Res (2016) 28(2):241. doi: 10.21147/j.issn.1000-9604.2016.02.14
51. Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol (2009) 27(7):1069. doi: 10.1200/JCO.2008.18.9043
52. Penson RT, Huang HQ, Wenzel LB, Monk BJ, Stockman S, Long HJ III, et al. Bevacizumab for advanced cervical cancer: patient-reported outcomes of a randomised, phase 3 trial (NRG oncology–gynecologic oncology group protocol 240). Lancet Oncol (2015) 16(3):301–11. doi: 10.1016/S1470-2045(15)70004-5
53. Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic oncology group 240). Lancet (2017) 390(10103):1654–63. doi: 10.1016/S0140-6736(17)31607-0
54. Cetina L, Rivera L, Hinojosa J, Poitevin A, Uribe J, López-Graniel C, et al. Routine management of locally advanced cervical cancer with concurrent radiation and cisplatin. Five-year Results (2006) 6(1):1–7. doi: 10.1186/1472-6874-6-3
55. Zhu H, Luo H, Zhang W, Shen Z, Hu X, Zhu X. Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Des Devel Ther (2016) 10:1885. doi: 10.2147/DDDT.S106412
56. Chuai Y, Rizzuto I, Zhang X, Li Y, Dai G, Otter SJ, et al. Vascular endothelial growth factor (VEGF) targeting therapy for persistent, recurrent, or metastatic cervical cancer. Cochrane Database Syst Rev (2021) 3(3):Cd013348. doi: 10.1002/14651858.CD013348.pub2
57. Ghose A, Gullapalli SVN, Chohan N, Bolina A, Moschetta M, Rassy E, et al. Applications of proteomics in ovarian cancer: Dawn of a new era. Proteomes (2022) 10(2):16. doi: 10.3390/proteomes10020016
Keywords: pembrolizumab, cervical cancer, meta-analysis, single-arm, systematic review
Citation: Qi L, Li N, Lin A, Wang X and Cong J (2022) Efficacy and safety of pembrolizumab on cervical cancer: A systematic review and single-arm meta-analysis. Front. Oncol. 12:910486. doi: 10.3389/fonc.2022.910486
Received: 01 April 2022; Accepted: 15 July 2022;
Published: 10 August 2022.
Edited by:
Leonhard Müllauer, Medical University of Vienna, AustriaReviewed by:
Pierpaolo Correale, Azienda ospedaliera ‘Bianchi-Melacrino-Morelli’, ItalyNicholas Pavlidis, University of Ioannina, Greece
Copyright © 2022 Qi, Li, Lin, Wang and Cong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianglin Cong, eW43MDJAMTYzLmNvbQ==; Lin Qi, c2hlbnNob3VxbEAxNjMuY29t
 Lin Qi
Lin Qi Ning Li
Ning Li Jianglin Cong
Jianglin Cong