
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 22 June 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.905809
This article is part of the Research Topic Advances in Prognosis and Treatment of Endometrial Cancers View all 12 articles
Background: The role of androgen receptor (AR) in evaluating the prognosis of patients with endometrial cancer (EC) remains controversial. Here, we performed a meta-analysis to assess whether AR expression improves EC survival outcomes.
Methods: We searched related articles published before August 2021 in PubMed, EMBASE, and Web of Science. The association between AR expression and patient prognosis was estimated with hazard ratios (HRs) and odds ratios (ORs) with their corresponding 95% confidence intervals (95% CIs). The review is registered on PROSPERO, registration number: CRD42021268591.
Results: Ten studies including 1,485 patients were enrolled in the meta-analysis. The results showed that AR expression in EC tissues was associated with a better survival in crude analyses (HR = 1.63, 95% CI = 1.32–2.02, P < 0.001). However, no significant relation was found after the adjustment of the confounding factors (HR = 1.68, 95% CI = 0.75–3.75, P = 0.205). In subgroup analyses, grade 1–2 disease, stage I–II disease, negative lymph node status, and lack of the lymphovascular invasion were more common in AR-positive groups (OR = 0.47, 0.48, 0.37, and 0.57; 95% CI = 0.45–0.62, 0.35–0.65, 0.24–0.56, and 0.37–0.89). Furthermore, AR expression was more common in endometrioid cancers (OR = 2.39, 95% CI = 1.79–3.20).
Conclusions: AR expression is significantly associated favorable characteristics including low-grade disease, early-stage disease, negative lymph node status, and lack of the lymphovascular invasion and a specific histology—endometrioid cancer. However, AR is not an independent prognostic factor.
Endometrial cancer (EC) is the most common gynecologic malignancy and continues to increase by about 1% per year (1). During 2021, almost 66,570 new cases of uterine corpus cancer and 12,940 deaths are projected to occur due to this cancer in the United States (2).
An excess-estrogen environment is linked with EC development, especially type I cancer (3). As the main source of estrogen especially in postmenopausal women, the importance of androgens in EC has been recognized for the last decades. In addition, androgen receptor (AR) also has been evaluated for its prognostic power in EC. In some studies, AR expression has been reported to be associated with better survival in patients with EC (4–8), whereas the better prognosis was not noted in other studies (9, 10). For explaining better prognosis in patients with EC, some investigators thought that the heterogeneity of histology resulted in the different patient survival of EC. However, the identical findings were not identified (5, 8–10).
With the aim of disentangling these controversial issues, we present a systematic review and meta-analysis to evaluate the association between the AR expression and the prognosis of patients with EC.
This research was conducted according to Preferred Reporting for Systematic Reviews and Meta-Analyses (PRISMA) principles.
We performed a comprehensive search in PubMed, EMBASE, and Web of Science. The search terms included “endometrial cancer” or “endometrial carcinoma” or “endometrial neoplasms” in combination with “androgen receptors”. Titles and abstracts were checked to identify potential eligible articles by two researchers, who then reviewed full texts. In addition, the references of included articles were checked manually for more related studies.
The inclusion criteria were as follows: (1) studies published in English; (2) studies on EC that confirmed by histopathological examination; (3) studies assessing AR expression with positive or negative labels; and (4) studies comparing the relationship between AR and clinic-pathological characteristics or prognosis. However, we excluded studies as follows: (1) studies based on animals or in vitro experiments; (2) review articles, meta-analyses, letters, or case reports; and (3) non-English literature.
For included articles, two investigators independently extracted the related data using a fixed form. The form included the name of the first author, the year of publication, age, the expression level of AR, clinic-pathological characteristics, hazard ratios (HRs), and 95% confidence intervals (CIs) for survival analysis. If the HRs and 95% CIs could not be acquired directly, then they were estimated from Kaplan–Meier curves using the method described by Parmar et al. (11). Two studies (6, 7) were excluded because of the significant difference between the estimated and actual HR. Any disagreements were resolved by discussion and consultation with the third author.
The guidelines from the Newcastle-Ottawa Scale (NOS) criteria were used to evaluated the quality of studies (12). The NOS criteria included three domains: (1) selection: 0–4; (2) comparability: 0–2; and (3) exposure or outcomes: 0–3. Good quality was considered when the NOS scores ≥6.
Dichotomous data eligible in each research were shown as a odds ratio (OR) with its 95% CI.
Moreover, the pooled HRs and 95% CIs were calculated to evaluate the associations between AR and prognosis of patients with EC. Heterogeneity between studies was assessed using I2 (13). If I2 >50%, substantial heterogeneity was considered and the random effects model was implemented. When I2 ≤50%, the fixed effect model was used in this meta-analysis.
Publication and selection bias was investigated by funnel plots and the Egger and Begg test. All analyses were performed in STATA software, and P < 0.05 was considered statistically significant.
A total of 660 studies were identified. After removal of 298 duplicates, 362 records were checked based on title and/or abstract and 17 studies remained. The full texts of remaining articles were further assessed for more details, and seven articles were excluded for the lack of data on prognosis or clinicopathological characteristics. Finally, 10 studies including 1,485 patients were enrolled in the meta-analysis (Figure 1). The main characteristics of included studies are shown in Table 1. Briefly, all of the articles investigated the association between AR and various clinicopathologic factors (4–10, 14–16), among which five of them further performed survival analysis (4–8).
Given the effect of the confounding factors, a stratified analysis was conducted on the subsets of survival analysis. The two available studies on univariate survival analysis suggest that AR overexpression predicted a favorable survival (HR = 1.63, 95% CI = 1.32–2.02, P < 0.001; Figure 2A) (5, 8). However, in two studies using multivariate survival analysis (4, 8), no significant relation was observed after adjustment for potential confounding factors (HR = 1.68, 95% CI = 0.75–3.74, P = 0.205; Figure 2B).
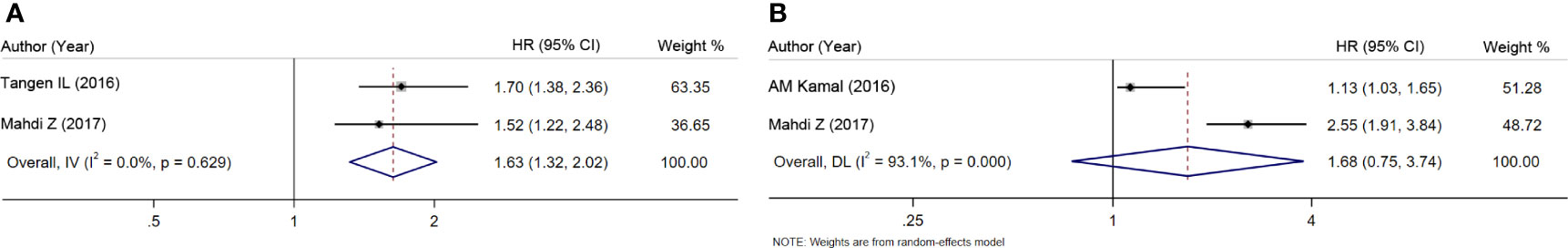
Figure 2 Meta-analysis of the association between AR and patient survival. (A) Univariate survival analysis. (B) Multivariate survival analysis.
Finally, we evaluated clinicopathologic characteristics between AR-positive and AR-negative groups. In crude analyses, low grade (OR = 0.466, 95% CI = 0.352–0.618, P < 0.001; Figure 3B), negative lymph nodes (OR = 0.367, 95% CI = 0.239–0.564, P < 0.001; Figure 3C), FIGO stage I–II disease (OR = 0.480, 95% CI = 0.353–0653, P < 0.001; Figure 3F), and negative lymphovascular invasion (OR = 0.572, 95% CI = 0.368–0.890, P = 0.013; Figure 3G) were more common in AR-positive group. However, the associations between AR expression and age, myometrial invasion and cervical invasion were not statistically significant (Figures 3A, D, E; P=0.941, P=0.063, and P=0.317, respectively).
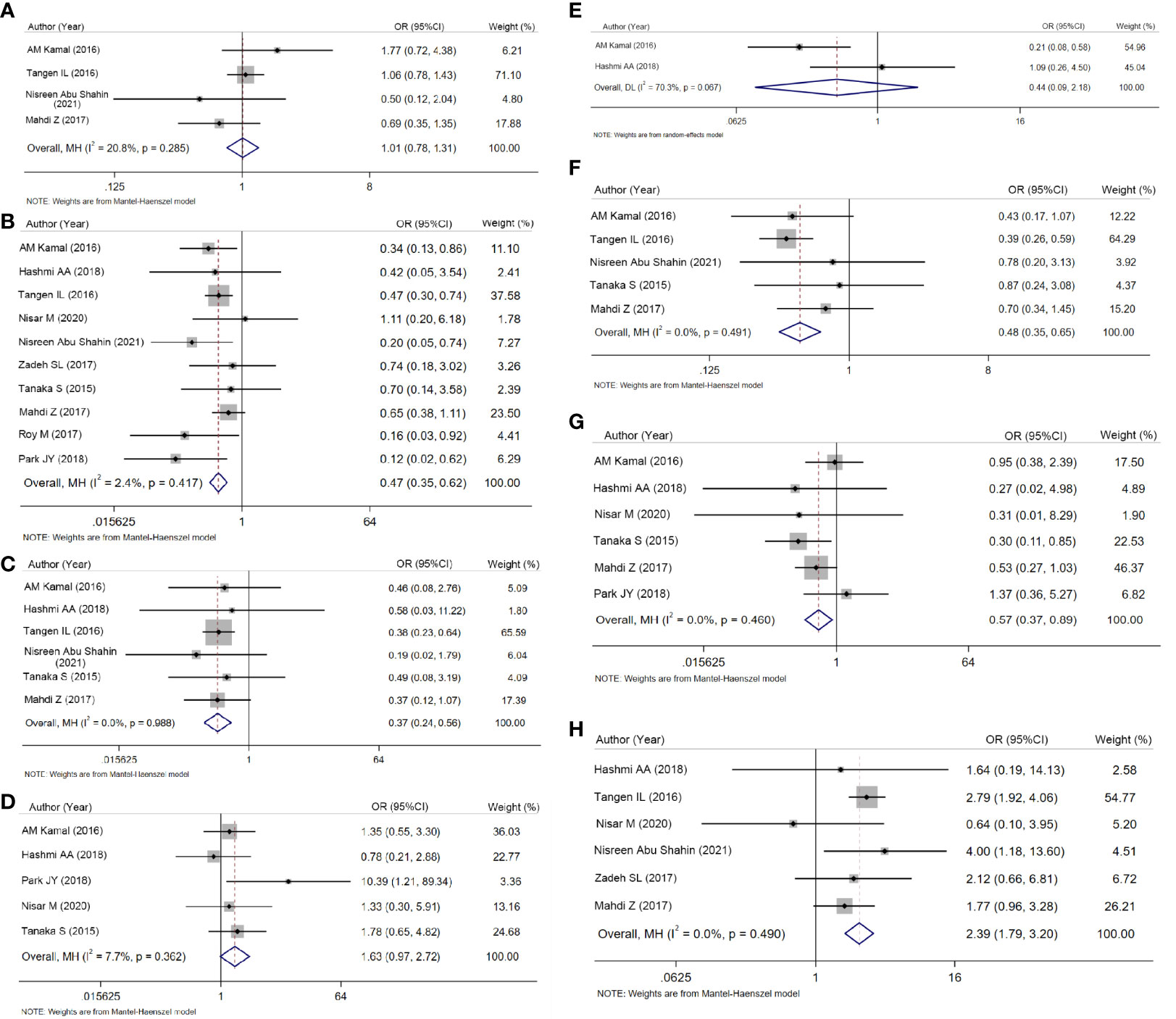
Figure 3 Forest plots for ORs and 95% CIs to compare clinicopathologic characteristics. (A) Age. (B) Grade. (C) Lymph node status. (D) Myometrial invasion. (E) Cervical invasion. (F) Stage (I + II vs. III + IV). (G) Lymphovascular invasion. (H) Histological type (I vs. II).
In terms of histology, crude analysis showed type I cancers were more frequent in AR-positive group (OR = 2.393, 95% CI = 1.789–3.202, P < 0.001; Figure 3H).
Begg’s funnel plot was conducted to assess the publication bias of included studies and no evidence of publication bias was seen (Supplementary Figure 1).
The role of AR in EC has been widely discussed for decades. However, the prognostic usefulness of AR is still controversial. This is the first systematic review with meta-analysis to examine the effect of AR on survival outcomes in patients with EC. We found that AR expression imparts a better survival outcome. The effect on better prognosis was consistently observed in subgroup analyses according to clinicopathologic characteristics. EC is a biologically and histologically diverse group of neoplasms characterized by a dualistic model of pathogenesis. Unlike type II EC, type I endometrial tumors usually portend a less aggressive clinical course (17). Our meta-analytic results showed that AR may have favorable characteristics of type I EC including early-stage disease, low-grade disease, negative lymph node status, and lack of the lymphovascular invasion. Indeed, we found that the expression of AR significantly increased in type I cancers. These findings mean that AR plays a crucial role in type I rather than type II cancers.
Notably, numerous studies have also examined the potential role of androgens as risk factors for EC. In addition, most of them claimed to have found that elevated serum testosterone level increased EC risk (18–21). It is tempting to speculate that AR is one of negative prognostic factors in EC. However, our meta-analysis reports that AR expression is a favorable prognostic indicator. It is well known that testosterone can be metabolized by aromatase and 5α-reductase to estradiol and dihydrotestosterone (DHT), respectively (22). An excess-estrogen environment can trigger the development and progression of EC, especially for type I. It is reported that the inhibition of aromatase activity has been applied to the treatment of EC. A retrospective cohort study recently reported longer PFS (HR = 0.23; 95% CI = 0.04–1.27) and OS (HR = 0.11; 95% CI = 0.01–1.36) in patients receiving aromatase inhibitors (AIs) (23). On the other hand, Hashimoto et al. have reported that DHT could inhibit the proliferation of EC cells (24). Consistent with these findings, the results in our study indirectly show that the conversion of testosterone to DHT and further activation of AR by DHT inhibit the continuum of EC progression.
Two of the included articles performed multivariate Cox survival analysis including tumor stage, myometrial invasion, race, BMI, diabetes, and AR, ER, and PR expression (4, 8). This meta-analysis integrated these disparate results, and the data in these studies were not always consistent. This might be ascribable to the following factors. First, AR signaling may have both oncogenic and tumor suppressive roles. In mouse models of type I EC, short-term enzalutamide treatment, an inhibitor of AR signaling, reduced endometrial tumor burden and increased cancer cell apoptosis in a dose-dependent way. However, enzalutamide increased the incidence of invasive and metastatic tumor (25). Oncogenic role of AR may be more involved in EC initiation. Later stages of invasion and metastasis in EC maybe partly due to inactivation of cancer suppressive AR signaling. Second, the histological structures and the carcinogenesis are different in type I and II cancers. Type I cancers are hormone-dependent. Our meta-analytic results showed AR expression was more likely to be observed in type I cancers. This might indicate that the impact of AR may be more inclined to type I EC. Further studies should also focus on the evaluation of the role of AR in type I cancers. Third, studies in the analysis employed different antibodies and cutoff values that led to variations of the results. Fourth, the numbers of patients and outcome events were small that implied poor statistical precision.
This is the first meta-analysis to uncover the prognostic value of AR in patients in EC. However, some limitations in our study should be mentioned. First, some of the studies in the meta-analyses did not mention any preoperative and/or postoperative therapies. Radiotherapy and/or chemotherapy are usually offered for those in advanced stage (26, 27). Such variations in treatment modalities must have an impact on the prognosis and prognostic analyses. Second, the numbers of patients and outcome events were mostly small implying poor statistical precision. Third, heterogeneity was evident among the included studies with respect to the specifics of staining methods, cutoff values, and so on.
In summary, the results from this meta-analysis suggested that AR may be useful prognostic biomarkers for EC. Further well-designed, multi-center, and larger-scale trials are needed to confirm our findings.
XW conceived and designed the study, interpreted the data, and drafted the manuscript. XY and YZ designed and revised the manuscript. XZ and JZ selected the articles. XZ and XH retrieved the data. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This study was funded by a grant (no. QB2021002) from the Youth Research grant of Nantong Commission of Health.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.905809/full#supplementary-material
1. Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International Patterns and Trends in Endometrial Cancer Incidence, 1978-2013. J Natl Cancer Institute (2018) 110(4):354–61. doi: 10.1093/jnci/djx214
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
3. Kaaks R, Lukanova A, Kurzer MS. Obesity, Endogenous Hormones, and Endometrial Cancer Risk: A Synthetic Review. Cancer epidemiology Biomarkers Prev Publ Am Assoc Cancer Research cosponsored by Am Soc Prev Oncol (2002) 11(12):1531–43.
4. Kamal AM, Bulmer JN, DeCruze SB, Stringfellow HF, Martin-Hirsch P, Hapangama DK. Androgen Receptors Are Acquired by Healthy Postmenopausal Endometrial Epithelium and Their Subsequent Loss in Endometrial Cancer Is Associated With Poor Survival. Br J cancer (2016) 114(6):688–96. doi: 10.1038/bjc.2016.16
5. Tangen IL, Onyango TB, Kopperud R, Berg A, Halle MK, Øyan AM, et al. Androgen Receptor as Potential Therapeutic Target in Metastatic Endometrial Cancer. Oncotarget (2016) 7(31):49289–98. doi: 10.18632/oncotarget.10334
6. Park JY, Baek MH, Park Y, Kim YT, Nam JH. Investigation of Hormone Receptor Expression and Its Prognostic Value in Endometrial Stromal Sarcoma. Virchows Archiv an Int J pathol (2018) 473(1):61–9. doi: 10.1007/s00428-018-2358-5
7. Tanaka S, Miki Y, Hashimoto C, Takagi K, Doe Z, Li B, et al. The Role of 5α-Reductase Type 1 Associated With Intratumoral Dihydrotestosterone Concentrations in Human Endometrial Carcinoma. Mol Cell endocrinol (2015) 401:56–64. doi: 10.1016/j.mce.2014.11.022
8. Mahdi Z, Abdulfatah E, Pardeshi V, Hassan O, Schultz D, Morris R, et al. The Impact of Androgen Receptor Expression on Endometrial Carcinoma Recurrence and Survival. Int J gynecol Pathol Off J Int Soc Gynecol Pathologists (2017) 36(5):405–11. doi: 10.1097/PGP.0000000000000355
9. Hashmi AA, Hussain ZF, Qadri A, Irfan M, Ramzan S, Faridi N, et al. Androgen Receptor Expression in Endometrial Carcinoma and Its Correlation With Clinicopathologic Features. BMC Res notes (2018) 11(1):289. doi: 10.1186/s13104-018-3403-9
10. Nisar M, Mushtaq S, Hassan U, Akhtar N, Azma M. Androgen Receptor Expression In Endometrial Carcinoma And Its Correlation With Estrogen Receptor And Progesterone Receptor And Clinicopathological Findings. J Ayub Med College Abbottabad JAMC (2020) 32(2):160–4.
11. Parmar MK, Torri V, Stewart L. Extracting Summary Statistics to Perform Meta-Analyses of the Published Literature for Survival Endpoints. Stat Med (1998) 17(24):2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8
12. Stang A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
13. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. BMJ (Clinical Res ed) (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
14. Abu Shahin N, Aladily T, Abu Alhaj N, Al-Khader A, Alqaqa S, Aljaberi R, et al. Differential Expression of Androgen Receptor in Type I and Type II Endometrial Carcinomas: A Clinicopathological Analysis and Correlation With Outcome. Oman Med J (2021) 36(2):e245. doi: 10.5001/omj.2021.53
15. Zadeh SL, Duska LR, Mills AM. Androgen Receptor Expression in Endometrial Carcinoma. Int J gynecol Pathol Off J Int Soc Gynecol Pathologists (2018) 37(2):167–73. doi: 10.1097/PGP.0000000000000401
16. Roy M, Kumar S, Bhatla N, Ray MD, Kumar L, Jain D, et al. Androgen Receptor Expression in Endometrial Stromal Sarcoma: Correlation With Clinicopathologic Features. Int J gynecol Pathol Off J Int Soc Gynecol Pathologists (2017) 36(5):420–7. doi: 10.1097/PGP.0000000000000353
17. Buchanan EM, Weinstein LC, Hillson C. Endometrial Cancer. Am Family physician (2009) 80(10):1075–80.
18. Teng F, Ma X, Yu X, Yan Y, Zhao J, Gao J, et al. High Serum Androgen and Insulin Concentrations Increase the Tendency of Endometrial Carcinoma. J Cancer (2020) 11(19):5656–64. doi: 10.7150/jca.46391
19. Michels KA, Brinton LA, Wentzensen N, Pan K, Chen C, Anderson GL, et al. Postmenopausal Androgen Metabolism and Endometrial Cancer Risk in the Women's Health Initiative Observational Study. . JNCI Cancer Spectr (2019) 3(3):pkz029. doi: 10.1093/jncics/pkz029
20. Audet-Walsh E, Lépine J, Grégoire J, Plante M, Caron P, Têtu B, et al. Profiling of Endogenous Estrogens, Their Precursors, and Metabolites in Endometrial Cancer Patients: Association With Risk and Relationship to Clinical Characteristics. J Clin Endocrinol Metab (2011) 96(2):E330–9. doi: 10.1210/jc.2010-2050
21. Lukanova A, Lundin E, Micheli A, Arslan A, Ferrari P, Rinaldi S, et al. Circulating Levels of Sex Steroid Hormones and Risk of Endometrial Cancer in Postmenopausal Women. Int J cancer (2004) 108(3):425–32. doi: 10.1002/ijc.11529
22. McNamara KM, Moore NL, Hickey TE, Sasano H, Tilley WD. Complexities of Androgen Receptor Signalling in Breast Cancer. Endocrine-related Cancer (2014) 21(4):T161–81. doi: 10.1530/ERC-14-0243
23. Paleari L, Rutigliani M, Siri G, Provinciali N, Colombo N, Decensi A. Aromatase Inhibitors as Adjuvant Treatment for ER/PgR Positive Stage I Endometrial Carcinoma: A Retrospective Cohort Study. Int J Mol Sci (2020) 21(6):2227–33. doi: 10.3390/ijms21062227
24. Hashimoto C, Miki Y, Tanaka S, Takagi K, Fue M, Doe Z, et al. 17β-Hydroxysteroid Dehydrogenase Type 2 Expression Is Induced by Androgen Signaling in Endometrial Cancer. Int J Mol Sci (2018) 19(4):1139–52. doi: 10.3390/ijms19041139
25. Koivisto CS, Parrish M, Bonala SB, Ngoi S, Torres A, Gallagher J, et al. Evaluating the Efficacy of Enzalutamide and the Development of Resistance in a Preclinical Mouse Model of Type-I Endometrial Carcinoma. Neoplasia (New York NY) (2020) 22(10):484–96. doi: 10.1016/j.neo.2020.07.003
26. Hoskins PJ, Swenerton KD, Pike JA, Wong F, Lim P, Acquino-Parsons C, et al. Paclitaxel and Carboplatin, Alone or With Irradiation, in Advanced or Recurrent Endometrial Cancer: A Phase II Study. J Clin Oncol Off J Am Soc Clin Oncol (2001) 19(20):4048–53. doi: 10.1200/JCO.2001.19.20.4048
Keywords: androgen receptor, clinicopathological, prognosis, endometrial cancer, meta-analysis
Citation: Wu X, Zhong X, Huo X, Zhang J, Yang X and Zhang Y (2022) The Clinicopathological Significance and Prognostic Value of Androgen Receptor in Endometrial Carcinoma: A Meta-Analysis. Front. Oncol. 12:905809. doi: 10.3389/fonc.2022.905809
Received: 27 March 2022; Accepted: 09 May 2022;
Published: 22 June 2022.
Edited by:
Shannon Neville Westin, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Yibo Dai, Peking University People’s Hospital, ChinaCopyright © 2022 Wu, Zhong, Huo, Zhang, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoqing Yang, ntyxq169@126.com; Yuquan Zhang, jsnt_zhangyuquan@163.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.