- 1Department of Radiology, The Second Affiliated Hospital of Shandong First Medical University, Tai’an, China
- 2Department of Radiology, The Fourth People’s Hospital of Taian, Tai’an, China
Background: To evaluate and compare the potential performance of various diffusion parameters obtained from mono-exponential model (MEM)-, bi-exponential model (BEM)-, and stretched exponential model (SEM)-based diffusion-weighted imaging (DWI) in grading of esophageal squamous carcinoma (ESC).
Methods: Eighty-two patients with pathologically confirmed ESC without treatment underwent multi-b-value DWI scan with 13 b values (0~12,00 s/mm2). The apparent diffusion coefficient (ADC) deriving from the MEM; the pure molecular diffusion (ADCslow), pseudo-diffusion coefficient (ADCfast), perfusion, and fraction (f) deriving from the BEM; and the distributed diffusion coefficient (DDC) and water molecular diffusion heterogeneity index (α) deriving from the SEM were calculated and compared between poorly differentiated and well/moderately differentiated ESC, respectively. The prediction parameters and diagnostic efficiency were compared by drawing receiver operating characteristic (ROC) curves.
Results: The ADC, ADCslow, ADCfast, and DDC in poorly ESC were significantly lower than those in well/moderately differentiated ones. By using only one parameter, ADCslow, DDC had the moderate diagnostic efficiency and the areas under the curve (AUC) were 0.758 and 0.813 in differentiating ESC. The DDC had the maximum AUC with sensitivity (88.00%) and specificity (68.42%). Combining ADC with ADCfast, ADCslow, and DDC and combining ADCslow with ADCfast can provide a higher diagnostic accuracy with AUC ranging from 0.756, 0.771, 0.816, and 0.793, respectively.
Conclusion: Various parameters derived from different DWI models including MEM, BEM, and SEM were potentially helpful in grading ESC. DDC obtained from SEM was the most promising diffusion parameter for predicting the grade of ESC.
Introduction
Esophageal squamous carcinoma (ESC) is one of the leading reasons of cancer-related mortality (1). Preoperative staging and pathological grading represent important prognostic indicators and determine different treatments. However, preoperative staging of ESC by conventional radiograph can only reflect the morphological changes by visual observation (2). Endoscopy-guided biopsy as a gold standard procedure is widely employed to diagnose ESC as early as possible, but it cannot reflect the grade of whole tumor and is sometimes limited by sampling errors of different observers (3). Magnetic resonance imaging (MRI) without ionizing radiation can provide excellent morphological and functional information for ESC because of its multimodal imaging sequences (4).
Diffusion-weighted imaging (DWI) is a non-invasive functional image sequence in the field of MRI, which uses the movement of water molecules in tissues. The diffusion of water can be quantitatively described by the apparent diffusion coefficient (ADC) (5). The mono-exponential model (MEM), bi-exponential model (BEM), and stretched exponential model (SEM) are all based on standard DWI with varying underlying models and differential governing parameters. Applying multiple models based on DWI protocol, perfusion information could be obtained without the need of intravenous contrast media, which is especially helpful for patients who cannot receive intravenous gadolinium-based contrast media due to severe allergies or compromised renal function. However, as we all know, the ADC featured by a simple mono-exponential decay is obtained from diffusion images with a postulation that the water molecular diffusion is a random motion, which would misestimate the influence of the microcirculation of blood in capillaries and could not reflect the true water diffusion (6). In fact, there are two main aspects that affect the measured diffusion signals in living tissues: one is the motion of water molecules, and the other is the perfusion of blood microcirculation with low b values (less than 200 s/mm2), which may lead to an inaccurate estimation of the diffusion. In 1986, Le Bihan et al. (7) by using multi-b-value DWI with a bi-exponential curve fitting firstly described a new imaging technique named intravoxel incoherent motion (IVIM), which has been used to quantitatively assess the microscopic translational motion on MRI. Also, later in 2003, Bennett et al. (8) initially introduced the stretched exponential model, which can assess the diffusion and heterogeneity of living tissues and has been used in several clinical studies (9).
To our knowledge, BEM-based DWI had been used to evaluate the tumor stage and pathological grade of ESC as well as predict treatment response (10). However, there was a lack of research of the SEM. Therefore, the purpose of this study was to investigate the ability and potential additional values of SEM-based DWI in differentiating the pathological grade of ESC.
Materials and methods
Patient Population
This study was approved by the ethics committee of our hospital, and informed consent was obtained from each patient. Eighty-six patients with ESC in our hospital from January 2018 to December 2019 were collected in the present study. The inclusion criteria were as follows: (1) MRI plain scans including multi-b-value DWI performed in patients with suspicious ESC by barium study of the gastrointestinal tract or CT examinations, and the tumors were all considered resectable. (2) Before the MRI scans, the patients were not treated with radiation therapy, chemotherapy, or biotherapy. The MRI images showed no artifact, which could affect the diagnosis. (3) All patients underwent surgical resections and confirmed with ESC by pathology ultimately. The exclusion criteria were as follows: (1) The quality of the MR images was poor. (2) The tumor was too small (diameter less than 1 cm) to draw the region of interest (ROI). Finally, three patients with poor multi-b-value DWI scans and one patient with large necrosis of tumor (more than 1/4 quadrant of tumor area) were excluded.
Magnetic Resonance Imaging
The MRI examinations were performed on a 3T scanner (Discovery 750, GE Healthcare, Chicago, IL, USA) with an eight-channel phase array coil. All the patients were in supine position. The scanning range was centered on ESC and covered the whole tumor. All patients were given breath training before examinations. Routine MRI was acquired with a fast spin echo (FSE) sequence with respiratory gating. Axial T2-weighted images were obtained with TR/TE of 9,230/85 ms (effective), and the slice thickness was 8.0 mm with spacing of 0.5 mm; field of view (FOV) 40 × 40 cm2; acquisition matrix, 288 × 256; NEX, 2; and acquisition time, 3 min 14 s. Sagittal fat-saturation T2-weighted images were obtained with TR/TE of 10,909/85 ms (effective), and the slice thickness was 6.0 mm with spacing of 0.5 mm; FOV, 40 × 40 cm2; acquisition matrix, 288 × 256; NEX, 2; and acquisition time was 3 min 49 s. The axial and sagittal T2-weighted fat-suppressed images were performed for the localization of ESC so as to plan the multi-b-value DWI scans of tumor. The multi-b-value DWI was performed with the following parameters: repetition time/echo time (TR/TE), 4,500/85 ms; FOV, 24 × 24 cm2; acquisition matrix, 128 × 128; slice thickness, 6.0 mm; and spacing, 0.5 mm. Thirteen b values from 0 to 1,200 s/mm2 (0, 10, 20, 50, 80, 100, 150, 200, 400, 600, 800, 1,000, 1,200) were used in three diffusion directions, and the number of excitations (NEXs) for each b was 2, 2, 2, 2, 2, 2, 2, 2, 2, 4, 6, 8, and 10. The acquisition time was 10 min 3 s.
Data Analysis
The DWI original imaging was processed using the Advantage Workstation (ADW 4.6 version, GE, US) and post-processed by Functool Workstation to obtain ADC maps. All MRI examinations were independently processed by two radiologists with 15 and 10 years of experience in reading MR imaging. They evaluated the multi-b-value DWI data and were blinded to histopathological results. The ROIs were placed to cover as much of the solid part of the tumor as possible on three consecutive maximal slices in the axial plane. All parameters were measured twice of each three representative slices, and their average values were calculated for future statistical analysis to reduce the effect of different ROI delineation and measurement by different observers.
Histopathological Examination
All resected specimen were examined by pathological examinations. The interval between MRI scan and the last surgery was less than 7 days, and these patients did not receive any treatment during the interval. According to the seventh edition of the American Joint Committee on Cancer Stage (AJCC, 7th) (11), the pathological differentiation of ESC was categorized into poorly differentiated, moderately differentiated, and well-differentiated carcinoma.
Statistical Analysis
The IBM SPSS Statistics 23 software (Armonk, NY) and MedCalc 15.8 (Mariakerke, Belgium) were used for statistical analysis. Quantitative parameters were expressed as the means ± standard deviation. Data were tested for normality analysis using the Kolmogorov–Smirnov test and then with the Levene test for variance homogeneity analysis. Independent t test or Mann–Whitney U test was used to compare the difference of each parameter between the poorly differentiated group and well/moderately differentiated group. The interobserver agreement and variability were evaluated by ICC and Bland–Altman analysis. Values of the first set of measurement were regarded as the parameters for the tumors when the ICC was more than 0.75 (12). When the ICC was less than 0.75, an average of different measurements of two readers was used as the final result for the subsequent analysis. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic performance of each DWI parameter in distinguishing the poorly differentiated group from the well/moderately differentiated group, and the sensitivity and specificity of these parameters were calculated. The area under the curve (AUC) of the ROC curve for the significant parameter was calculated and compared by MedCalc. P values of less than 0.05 were considered as statistically significant.
Results
Patients and Histology Results
Eighty-two cases of patients with ESC were enrolled in this study, including 49 men and 33 women (age range 42–77 years, median age 54 years). There were 12 cases of well-differentiated ESC, 31 cases of moderately differentiated ESC, 14 cases of mix well/moderately differentiated ESC, and 25 cases of poorly differentiated ESC by histopathological examinations, as well as 7 cases located in the upper esophagus, 35 cases in the middle esophagus, 40 cases in the lower esophagus.
Interobserver Agreement of Measurements Derived From Different DWI Models
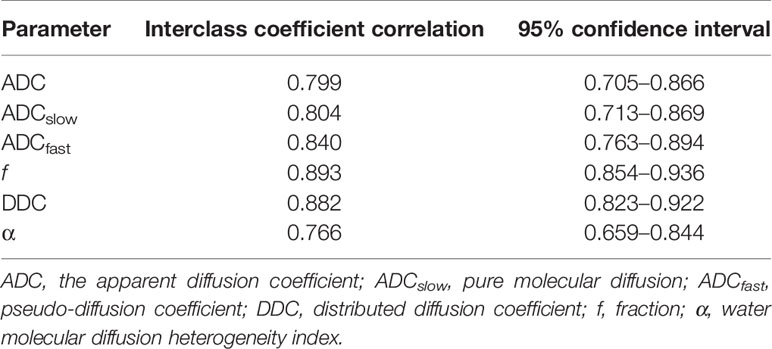
The ICC values (Table 1) were 0.799 for ADC, 0.804 for ADCslow, 0.840 for ADCfast, 0.893 for f, 0.882 for DDC, and 0.766 for α. The Bland–Altman plots representing the interobserver reproducibility between the two readers are shown in Figure 1. The center solid line represents the mean of differences.
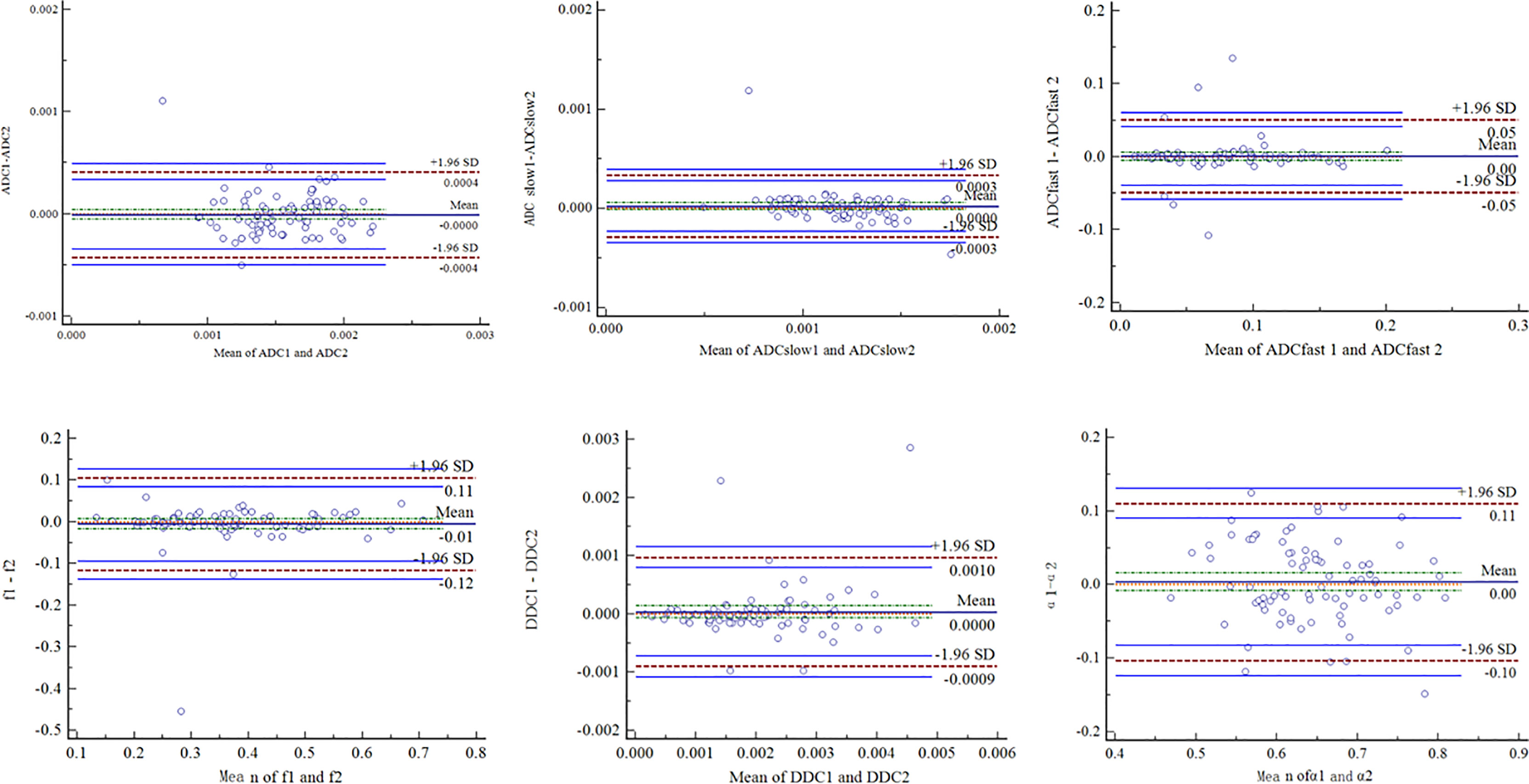
Figure 1 Bland–Altman plots showed interobserver reliability for measurement of different DWI parameters. SD = standard deviation.
Comparisons of Parameters Derived From Various Quantitative DWI Models
The ADC, ADCslow, ADCfast, f, DDC, and α of different pathologically differentiated ESCs derived from various DWI models are shown in Table 2 (Figures 2, 3). The results showed that the ADC, ADCslow, ADCfast, and DDC in the poorly differentiated group were significantly lower than those in the well-/moderately differentiated group (Table2). However, there was no significant difference in f and α values (Table 2).
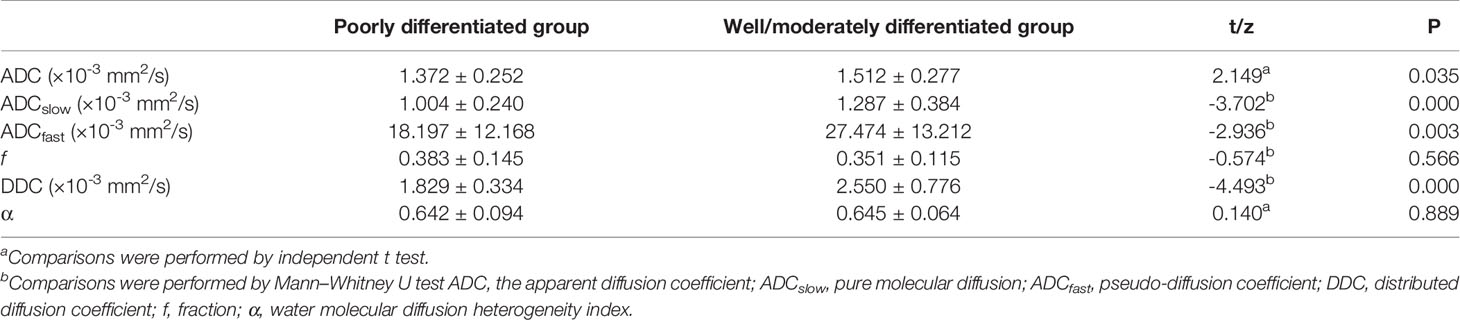
Table 2 Comparison between poorly and well-/moderately differentiated group of different parameters.
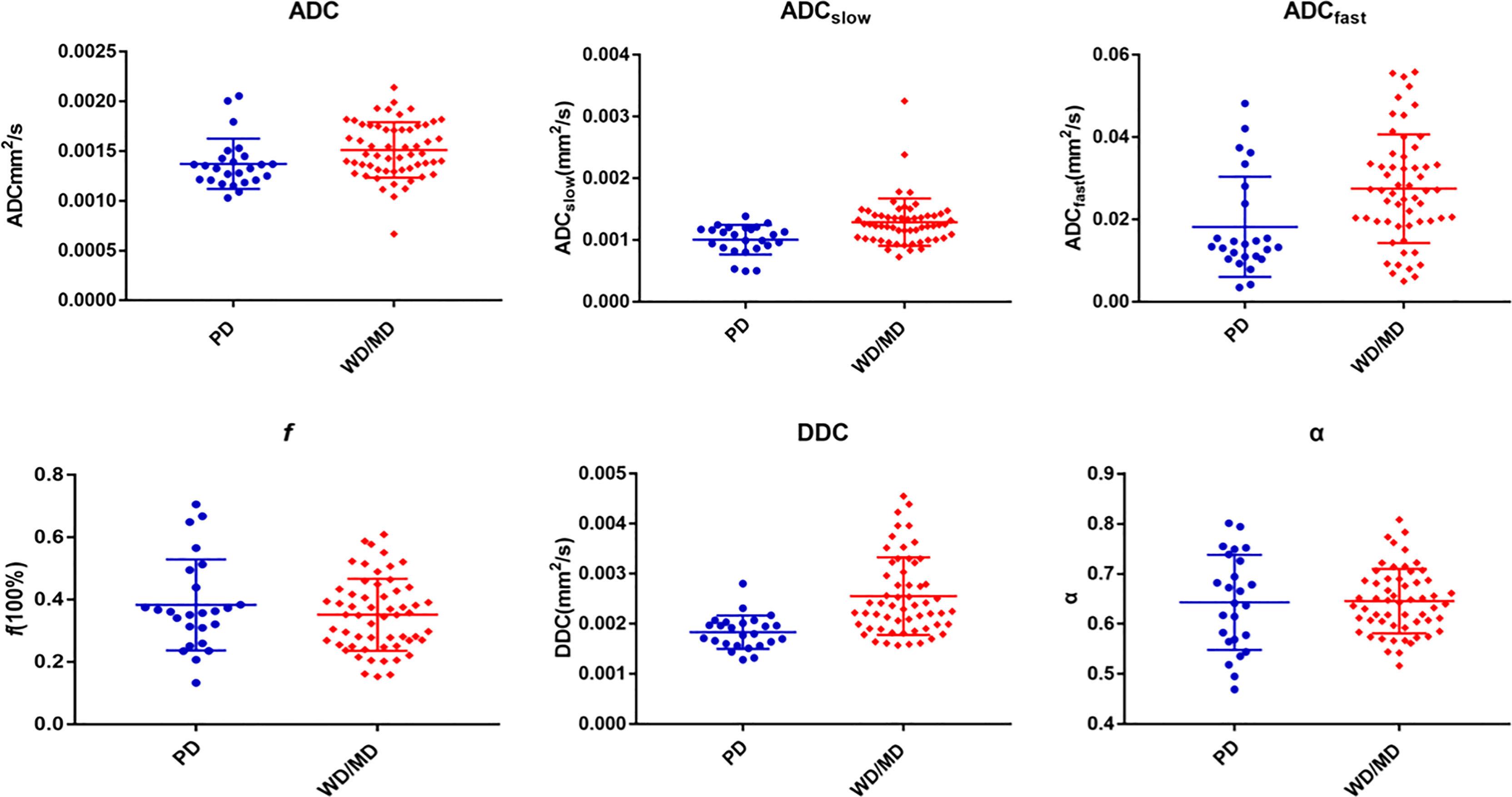
Figure 2 Various parameters derived from different DWI models for comparing PD (poorly differentiated) group with WD/MD (moderately/well-differentiated) group of ESC.
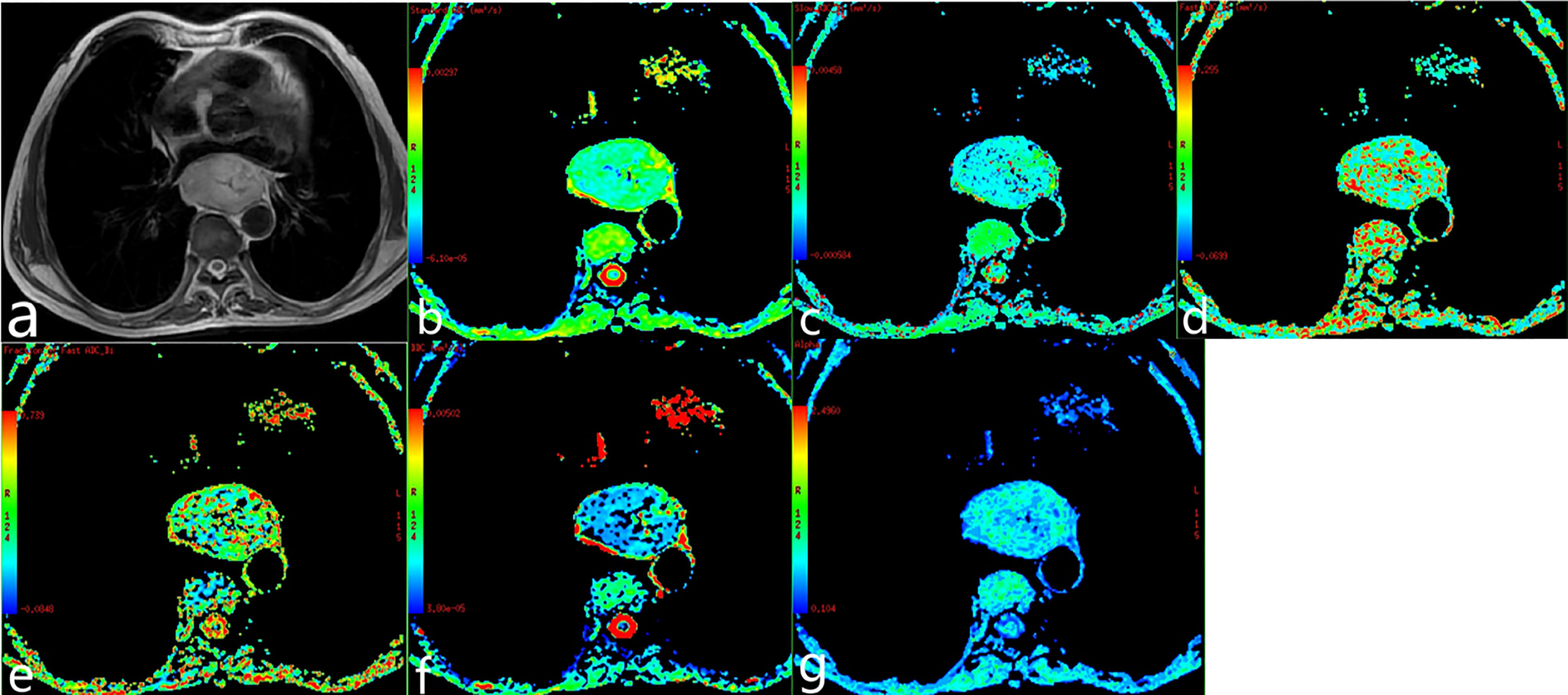
Figure 3 Poorly differentiated esophageal carcinoma of a 62-year-old man. (A) T2-weighted image. (B–G) ADC map, ADCslow map, ADCfast map, f map, DDC map, and α map.
Diagnostic Performance of Various Quantitative DWI Models
Based on the previous results of the independent t test and Mann–Whitney U test for comparisons, we performed an ROC analysis of the parameters with significant difference for distinguishing the poorly differently group from the well-/moderately differentiated group, as shown in Figure 4. The maximum Youden index, AUC, sensitivity, and specificity are illustrated in Table 3. By using only one parameter, ADCslow and DDC had moderate diagnostic efficiency and the areas under the curve were 0.758 and 0.813, respectively. The ROC curves show that DDC had the maximum AUC with sensitivity of 88.00% and specificity of 68.42%; ADC had the minimum AUC. The AUC of DDC was higher than that of ADC, ADCslow, and ADCfast but showed no statistical difference (P = 0.110, 0.331, and 0.226). ADC, ADCslow, and DDC demonstrated the highest sensitivity (88.00%), but DDC had a higher specificity (68.42%); ADCfast demonstrated the highest specificity with 80.70%. Combining ADC with ADCfast, ADCslow, DDC, and ADCslow with ADCfast can provide a higher diagnostic accuracy with AUC ranging from 0.756, 0.771, 0.816, to 0.793 respectively (Table 3).
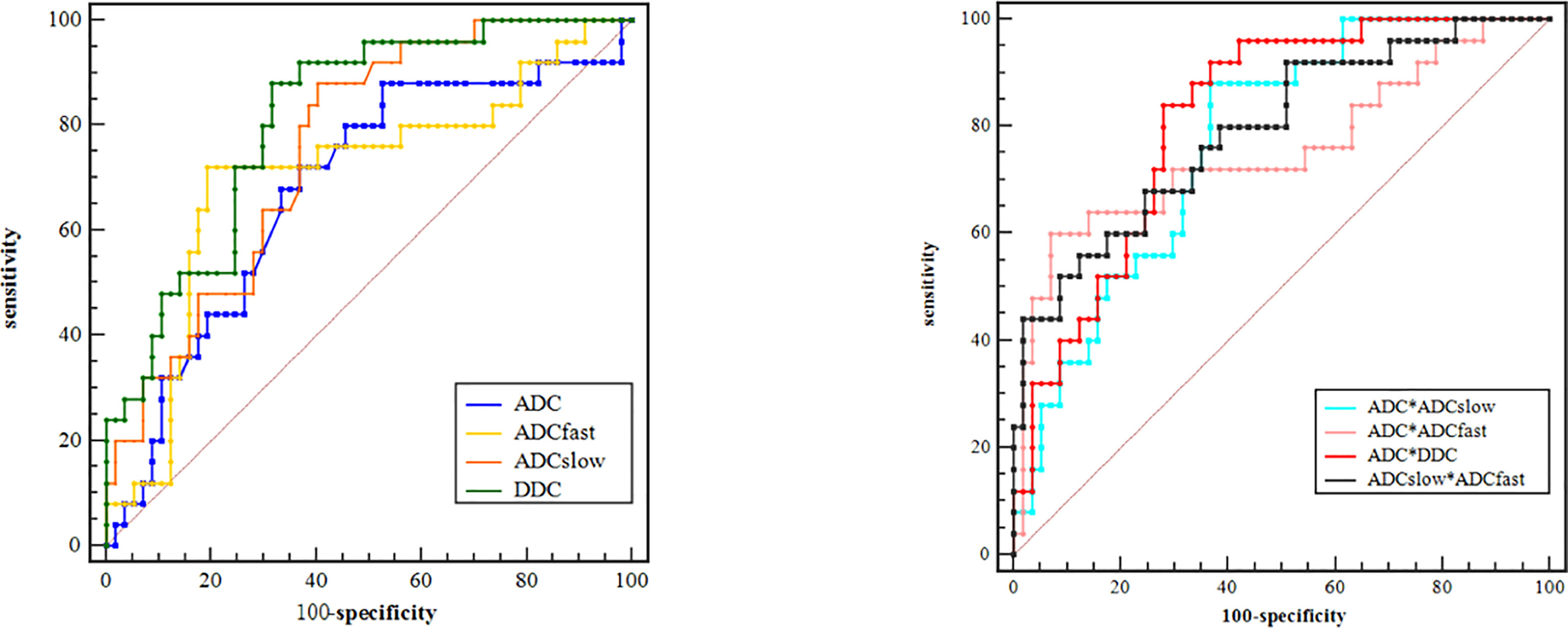
Figure 4 ROC curves of different parameters for identifying PD (poorly differentiated) group with WD/MD (moderately/well-differentiated) group of ESC.
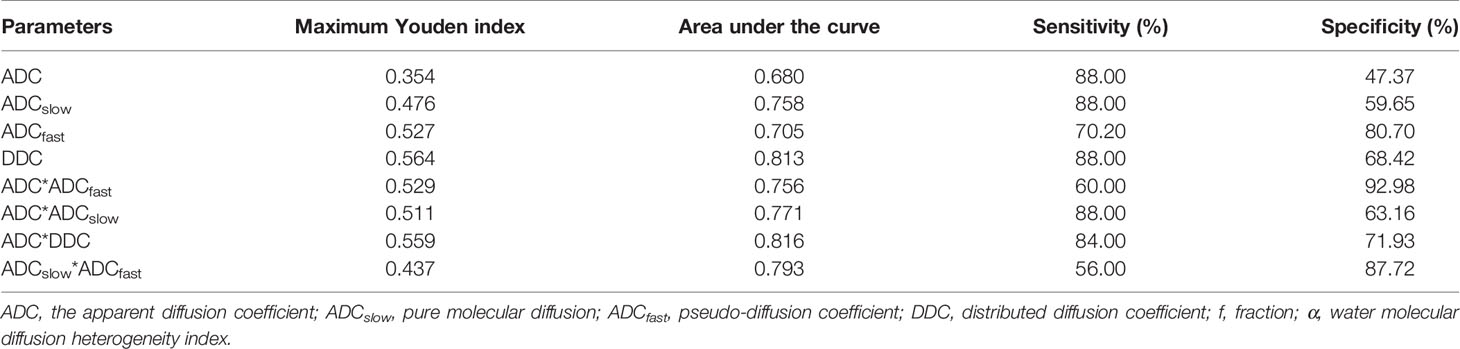
Table 3 ROC-related parameters in differentiating the poorly and well-/moderately differentiated group of ESC.
Discussion
The MRI scans were increasingly used in the staging of gastrointestinal tumors because of their good soft tissue resolution. Applying multiple models based on DWI protocol, perfusion information could be obtained without the need for intravenous contrast media. The main studies of ESC by DWI scans currently were to improve tumor’s early detection and staging accuracy and predict treatment efficacy (13–16). Therefore, the purpose of this study was to investigate the ability and potential additional values of SEM and compare with MEM, BME in differentiating the pathological grade of ESC.
In our present study, the results demonstrated that ADC values were significantly lower in poorly differentiated tumors than in well-/moderately differentiated ones, showing that ADC decreased with a decrease in pathological differentiation, which was consistent with the result of Zhu et al. (10), who also demonstrated that both ADC and ADCslow could assess the tumor cell grade, and the numeric values of the poorly differentiated esophageal carcinoma were lower than the moderately differentiated and well-differentiated groups. Histologically, the decreased value of ADC in poorly differentiated ESC may be related to the following reasons: the fast proliferation of malignant tumor cell, the increase in cell density, the shrinking of the extracellular space, and the increase in the nucleo-cytoplasmic ratio. Huang et al. (17) also found that ADC decreased with the increase in the T stage of ESC, and Mizumachi et al. (18) found that lower ADC values were significantly associated with a higher clinical T stage. Sakurada et al. (13) reported that combining T2WI and DWI obtained detection rates in the T staging of ESC, and they were 33% for T1, 58% for T2, 96% for T3, and 100% for T4, respectively. Kiyohiko Shuto et al. (15) reported that the clinical impact of DWI showed higher sensitivity than PET in predicting postoperative survival for patients with ESC. However, the ADC value was quantified by measuring the mean diffusivity along three orthogonal directions, which was mainly influenced by not only cellularity but also microcirculation. Cellularity and microcirculation would influence ADC measurement in a diametrically opposite direction (19). As we all know, the IVIM can assess the microscopic motion, diffusion, and heterogeneity of living tissues, considering the heterogeneity of the intravoxel diffusion rate and distributed diffusion effect in each voxel in multiple pools of water molecules.
There were few studies focusing on the evaluation of esophageal carcinoma by the IVIM-DWI model. Lei et al. (20) studied the application value of IVIM-DWI in the diagnosis of early esophageal cancer and showed that the f value could differentiate between esophageal carcinoma and normal esophagus. Huang et al. (17) found that the IVIM-DWI-derived parameters of D (pure diffusion coefficient) and f negatively correlated with the stage of ESC and the D value could distinguish the T1-staged tumor from the normal esophageal wall in detail, which might be probably related to the smooth muscle proliferation and extracellular stroma expansion that blocked the free water diffusion in the progress of ESC. Zheng et al. (21) showed that ADC, D, and f increased significantly during concurrent chemoradiotherapy (CRT) and proved that the IVIM-DWI parameters combined with ADC were useful in evaluating treatment and prognosis. Zhu et al. (10) used IVIM and conventional DWI parameters to evaluate the pathologically differentiated grade of esophageal carcinoma and showed that ADCslow and ADC had a significantly higher diagnostic performance than ADCfast and f.
As shown in our study, ADCslow representing the pure diffusion was lower than that of ADC and the AUC of ADCslow was higher than that of ADC in distinguishing poorly from well-/moderately differentiated ESC; this was because BEM-based DWI can separate the diffusion and perfusion component from the overall DWI measurement. ADCslow obtained from this model can supply a precise differential diagnosis and reduce the bias by avoiding microcirculation contributions (22). The ADCslow deriving from the BEM model can eliminate the interference of perfusion and maintain the true diffusion, suggesting that ADCslow had a higher diagnostic performance than ADC. It was noted that despite the better diagnostic performance of ADCslow, a low value of specificity (59.65%) was present in differentiating poorly from well-/moderately differentiated lesions. This may be related to the pathological heterogeneity of tumor and the overlapping grade of differentiated ESC from the point of the pathology. At the same time, the stability of the MRI parameters may be affected by the surrounding structures such as bone and air.
ADCfast represents a perfusion-related coefficient and reflects microcirculation (22). Our data demonstrated that ADCfast was statistically significant in differentiating the poorly and well-/moderately differentiated lesion. However, the f value showed no statistical difference with only a gradually increasing trend from the well-/moderately to poorly differentiated lesion, which was consistent with the research of Zhu et al. (10). However, there were different conclusions for ADCfast in distinguishing the behavior of the tumors in other systems (23, 24). ADCfast was considered proportional to the average blood velocity and capillary segment length (7). The result may be related to the different anatomical blood supply of esophageal segments and the inconsistent capillary length of different differentiated ESCs. Furthermore, because of the low signal-to-noise ratio (SNR) and the limited small b values of measurement, the value of ADCfast may not be reliable and need to be further studied.
SEM is an alternate method that may quantify both tissue heterogeneity and diffusion simultaneously, which was more reliable and reproducible than the MEM- and BEM-based DWI in prior studies (25, 26). The results of our study showed that the DDC values of poorly lesions were significantly lower than those of well-/moderately differentiated ones, and the ROC curves showed that DDC had the maximum AUC, which demonstrated excellent diagnostic performance in differentiating poorly from well-/moderately differentiated lesions. The DDC was considered to be the weighted sum of the continuous distribution of ADCs, and it can provide a more accurate and reliable depiction of tissue diffusion (26, 27). Our results can be explained by the proliferating tumor cells and the increasing nucleus-to-cytoplasm ratio of poorly lesions, which can lead to more intravoxel diffusion heterogeneity. Nevertheless, in our study, the α value was slightly lower in poorly differentiated lesions but showed no statistical difference compared with well/moderately ones. The α value described the deviation of water diffusion from a single exponential decay and was supposed to be related to intravoxel water diffusion heterogeneity, which indicated a numerically low α index (α near 0) representing a high degree of diffusion heterogeneity exhibited as multi-exponential decay, while a numerically high α index (α near 1) represented low intravoxel diffusion heterogeneity approaching mono-exponential decay. Similarly, in the previous studies, Lin et al. (28) did not find a difference between high-grade and low-grade meningiomas, and Wang et al. (29) demonstrated that α was not significantly different in the various stages and grades of bladder cancers. However, another former research indicated that the α value can be used to differentiate high-grade and low-grade gliomas with AUC of 0.892 (30). The differences in results suggested that α varied among different types of tumors, which needs further larger cohort studies.
Additionally, the ROC curves were used to distinguish poorly differentiated lesions from well-/moderately differentiated ones. By using only one parameter, DDC had the maximum AUC with sensitivity of 88.00% and specificity of 68.42%, suggesting that it was a reliable diagnostic marker compared with other parameters. Combining ADC with ADCfast, ADCslow, and DDC and combining ADCslow with ADCfast can provide a higher diagnostic accuracy with AUC ranging from 0.756, 0.771, 0.816 to 0.793, respectively. Therefore, the combination of multiple parameters of different DWI models may have a more powerful diagnostic value.
This study still had several limitations. First, the sample sizes of poorly differentiated ESCs were relatively small and further prospective analysis of a larger number of patients will be needed to validate the present results. Second, the ROIs were placed to cover as much of the solid part of the tumor as possible on three consecutive maximal slices and did not contain the entire volume, which might lead to bias owing to tumor heterogeneity. Therefore, entire tumors should be measured in future research.
In conclusion, various parameters derived from different DWI models including MEM, BEM, and SEM were potentially helpful in grading ESC. DDC obtained from SEM was the most promising diffusion parameter for predicting the grade of ESC.
Data Availability Statement
The datasets generated during the current study are not publicly available due our research is still ongoing, but they are available from the corresponding author on reasonable request.
Ethics Statement
This study was approved by the ethics committee of the Second Affiliated Hospital of Shandong First Medical University and informed consent was obtained from each patient. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
HY: conceptualization, writing—original draft preparation. XBG: conceptualization, methodology; XZ: visualization, software; XL: data curation, formal analysis; JL: data curation, methodology; ML: formal analysis, visualization, resources; JZ: supervision, project administration; JQ: supervision, resources, writing—reviewing and editing. All authors contributed to the article and approved the submitted version.
Funding
The present study was supported by grants from the Academic promotion program of Shandong First Medical University (No. 2019QL017).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MEM, mono-exponential model; BEM, bi-exponential model; SEM, stretched exponential model; DWI, diffusion-weighted imaging; ESC, esophageal squamous carcinoma; ADC, apparent diffusion coefficient; ADCslow, pure molecular diffusion; ADCfast, pseudo-diffusion coefficient; DDC, distributed diffusion coefficient; ROC, receiver operating characteristic; IVIM, intravoxel incoherent motion; ROI, region of interest; TR/TE, repetition time/echo time; NEXs, number of excitations; AUC, area under the curve; SNR, signal-to-noise ratio.
References
1. Gupta B, Kumar N. Worldwide Incidence, Mortality and Time Trends for Cancer of the Oesophagus. Eur J Cancer Prev (2017) 26(2):107–18. doi: 10.1097/CEJ.0000000000000249
2. Rice TW, Gress DM, Patil DT, Hofstetter WL, Kelsen DP, Blackstone EH. Cancer of the Esophagus and Esophagogastric Junction—Major Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin (2017) 67(4):304–17. doi: 10.3322/caac.21399
3. Swager A, Curvers WL, Bergman JJ. Diagnosis by Endoscopy and Advanced Imaging. Best Pract Res Clin Gastroenterol (2015) 29(1):97–111. doi: 10.1016/j.bpg.2014.11.011
4. Tirumani H, Rosenthal MH, Tirumani SH, Shinagare AB, Krajewski KM, Ramaiya NH. Esophageal Carcinoma: Current Concepts in the Role of Imaging in Staging and Management. Can Assoc Radiol J (2015) 66(2):130–9. doi: 10.1016/j.carj.2014.08.006
5. White NS, McDonald CR, Farid N, Kuperman J, Karow D, Schenker-Ahmed NM, et al. Diffusion-Weighted Imaging in Cancer: Physical Foundations and Applications of Restriction Spectrum Imaging. Cancer Res (2014) 74(17):4638–52. doi: 10.1158/0008-5472.CAN-13-3534
6. Fujima N, Sakashita T, Homma A, Shimizu Y, Yoshida A, Harada T, et al. Advanced Diffusion Models in Head and Neck Squamous Cell Carcinoma Patients: Goodness of Fit, Relationships Among Diffusion Parameters and Comparison With Dynamic Contrast-Enhanced Perfusion. Magn Reson Imag (2017) 36:16–23. doi: 10.1016/j.mri.2016.10.024
7. Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M, et al. MR Imaging of Intravoxel Incoherent Motions: Application to Diffusion and Perfusion in Neurologic Disorders. Radiology (1986) 161(2):401–7. doi: 10.1148/radiology.161.2.3763909
8. Bennett KM, Schmainda KM, Bennett R, Rowe DB, Lu H, Hyde JS, et al. Characterization of Continuously Distributed Cortical Water Diffusion Rates With a Stretched-Exponential Model. Magn Reson Med (2003) 50(4):727–34. doi: 10.1002/mrm.10581
9. Seo N, Chung YE, Park YN, Kim E, Hwang J, Kim MJ, et al. Liver Fibrosis: Stretched Exponential Model Outperforms Mono-Exponential and Bi-Exponential Models of Diffusion-Weighted MRI. Eur Radiol (2018) 28(7):2812–22. doi: 10.1007/s00330-017-5292-z
10. Zhu S, Wei Y, Gao F, Li L, Liu Y, Huang Z, et al. Esophageal Carcinoma: Intravoxel Incoherent Motion Diffusion-Weighted MRI Parameters and Histopathological Correlations. J Magn Reson Imaging (2019) 49(1):253–61. doi: 10.1002/jmri.26172
11. Rice TW, Rusch VW, Ishwaran H, Blackstone EH. Worldwide Esophageal Cancer Collaboration. Cancer of the Esophagus and Esophagogastric Junction: Data-Driven Staging for the Seventh Edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer (2010) 116(16):3763–73. doi: 10.1002/cncr.25146
12. Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med (2016) 15(2):155–63. doi: 10.1016/j.jcm.2016.02.012
13. Sakurada A, Takahara T, Kwee TC, Yamashita T, Nasu S, Horie T, et al. Diagnostic Performance of Diffusion-Weighted Magnetic Resonance Imaging in Esophageal Cancer. Eur Radiol (2009) 19(6):1461–9. doi: 10.1007/s00330-008-1291-4
14. Borggreve AS, Heethuis SE, Boekhoff MR, Goense L, van Rossum PSN, Brosens LAA, et al. Optimal Timing for Prediction of Pathologic Complete Response to Neoadjuvant Chemoradiotherapy With Diffusion-Weighted MRI in Patients With Esophageal Cancer. Eur Radiol (2020) 30(4):1896–907. doi: 10.1007/s00330-019-06513-0
15. Shuto K, Kono T, Shiratori T, Akutsu Y, Uesato M, Mori M, et al. Diagnostic Performance of Diffusion-Weighted Magnetic Resonance Imaging in Assessing Lymph Node Metastasis of Esophageal Cancer Compared With PET. Esophagus (2020) 17(3):239–49. doi: 10.1007/s10388-019-00704-w
16. Cheng B, Yu J. Predictive Value of Diffusion-Weighted MR Imaging in Early Response to Chemoradiotherapy of Esophageal Cancer: A Meta-Analysis. Dis Esophagus (2019) 32(4):doy065. doi: 10.1093/dote/doy065
17. Huang YC, Chen TW, Zhang XM, Zeng NL, Li R, Tang YL, et al. Intravoxel Incoherent Motion Diffusion-Weighted Imaging of Resectable Oesophageal Squamous Cell Carcinoma: Association With Tumour Stage. Br J Radiol (2018) 91(1084):20170421. doi: 10.1259/bjr.20170421
18. Mizumachi R, Hayano K, Hirata A, Ohira G, Imanishi S, Tochigi T, et al. Development of Imaging Biomarker for Esophageal Cancer Using Intravoxel Incoherent Motion MRI. Esophagus (2021) 18(4):844–50. doi: 10.1007/s10388-021-00851-z
19. Liu C, Liang C, Liu Z, Zhang S, Huang B. Intravoxel Incoherent Motion (IVIM) in Evaluation of Breast Lesions: Comparison With Conventional DWI. Eur J Radiol (2013) 82(12):e782–9. doi: 10.1016/j.ejrad.2013.08.006
20. Lei J, Tian Y, Zhu SC, Han Q, Wei Y, Yang S, et al. Preliminary Study of IVIM-DWI and DCE-MRI in Early Diagnosis of Esophageal Cancer. Eur Rev Med Pharmacol Sci (2015) 19(18):3345–50.
21. Zheng H, Ren W, Pan X, Zhang Q, Liu B, Liu S, et al. Role of Intravoxel Incoherent Motion MRI in Early Assessment of the Response of Esophageal Squamous Cell Carcinoma to Chemoradiotherapy: A Pilot Study. J Magn Reson Imaging (2018) 48(2):349–58. doi: 10.1002/jmri.25934
22. Lin M, Yu X, Chen Y, Ouyang H, Wu B, Zheng D, et al. Contribution of Mono-Exponential, Bi-Exponential and Stretched Exponential Model-Based Diffusion-Weighted MR Imaging in the Diagnosis and Differentiation of Uterine Cervical Carcinoma. Eur Radiol (2017) 27(6):2400–10. doi: 10.1007/s00330-016-4596-8
23. De Robertis R, Cardobi N, Ortolani S, Tinazzi Martini P, Stemmer A, Grimm R, et al. Intravoxel Incoherent Motion Diffusion-Weighted MR Imaging of Solid Pancreatic Masses: Reliability and Usefulness for Characterization. Abdom Radiol (NY) (2019) 44(1):131–9. doi: 10.1007/s00261-018-1684-z
24. Tan H, Chen J, Zhao YL, Liu JH, Zhang L, Liu CS, et al. Feasibility of Intravoxel Incoherent Motion for Differentiating Benign and Malignant Thyroid Nodules. Acad Radiol (2019) 26(2):147–53. doi: 10.1016/j.acra.2018.05.011
25. Li H, Liang L, Li A, Hu Y, Hu D, Li Z, et al. Monoexponential, Biexponential, and Stretched Exponential Diffusion-Weighted Imaging Models: Quantitative Biomarkers for Differentiating Renal Clear Cell Carcinoma and Minimal Fat Angiomyolipoma. J Magn Reson Imaging (2017) 46(1):240–7. doi: 10.1002/jmri.25524
26. Kim HC, Seo N, Chung YE, Park MS, Choi JY, Kim MJ. Characterization of Focal Liver Lesions Using the Stretched Exponential Model: Comparison With Monoexponential and Biexponential Diffusion-Weighted Magnetic Resonance Imaging. Eur Radiol (2019) 29(9):5111–20. doi: 10.1007/s00330-019-06048-4
27. Wang F, Wang Y, Zhou Y, Liu C, Xie L, Zhou Z, et al. Comparison Between Types I and II Epithelial Ovarian Cancer Using Histogram Analysis of Monoexponential, Biexponential, and Stretched-Exponential Diffusion Models. J Magn Reson Imaging (2017) 46(6):1797–809. doi: 10.1002/jmri.25722
28. Lin L, Xue Y, Duan Q, Chen X, Chen H, Jiang R, et al. Grading Meningiomas Using Mono-Exponential, Bi-Exponential and Stretched Exponential Model-Based Diffusion-Weighted MR Imaging. Clin Radiol (2019) 74(8):651.e15–651.e23. doi: 10.1016/j.crad.2019.04.007
29. Wang Y, Hu D, Yu H, Shen Y, Tang H, Kamel IR, et al. Comparison of the Diagnostic Value of Monoexponential, Biexponential, and Stretched Exponential Diffusion-Weighted MRI in Differentiating Tumor Stage and Histological Grade of Bladder Cancer. Acad Radiol (2019) 26(2):239–46. doi: 10.1016/j.acra.2018.04.016
Keywords: esophageal squamous carcinoma, magnetic resonance imaging, stretched exponential model, intravoxel incoherent motion, DWI
Citation: Yang H, Ge X, Zheng X, Li X, Li J, Liu M, Zhu J and Qin J (2022) Predicting Grade of Esophageal Squamous Carcinoma: Can Stretched Exponential Model-Based DWI Perform Better Than Bi-Exponential and Mono-Exponential Model? Front. Oncol. 12:904625. doi: 10.3389/fonc.2022.904625
Received: 20 April 2022; Accepted: 20 June 2022;
Published: 14 July 2022.
Edited by:
Francisco Tustumi, University of São Paulo, BrazilReviewed by:
Isabela Sunye, Albert Einstein Institute for Teaching and Research (IIEP), BrazilVitor Arienzo, Albert Einstein Israelite Hospital, Brazil
Copyright © 2022 Yang, Ge, Zheng, Li, Li, Liu, Zhu and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Qin, c2RxaW5qaWFuQDEyNi5jb20=
†These authors have contributed equally to this work
 Hui Yang
Hui Yang Xubo Ge2†
Xubo Ge2† Jiang Li
Jiang Li Min Liu
Min Liu Jian Qin
Jian Qin