
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Oncol. , 27 September 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.902955
This article is part of the Research Topic Epidemiology, Screening and Diagnosis of Lung Cancer View all 24 articles
 Aiping Ma1†
Aiping Ma1† Guangdong Wang1†
Guangdong Wang1† Yan Du1†
Yan Du1† Weixi Guo2†
Weixi Guo2† Jiaxi Guo1
Jiaxi Guo1 Yi Hu3
Yi Hu3 Dongyu Bai4
Dongyu Bai4 Huiping Huang5
Huiping Huang5 Lianjin Zhuang6
Lianjin Zhuang6 Jinhan Chen7*
Jinhan Chen7* Qun Liu1*
Qun Liu1*Background: Chronic obstructive pulmonary disease (COPD) coexisting with lung cancer is associated with severe mortality and a worse prognosis. Inflammation plays an important role in common pathogenic pathways and disease progression. However, a few studies have identified the clinical value of the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in COPD with lung cancer, which are systemic inflammatory response markers in the blood. This study aimed to determine the association of the NLR or PLR with clinical characteristics and whether NLR or PLR can be diagnostic markers for COPD with lung cancer.
Methods: Between 2015 and 2021, we conducted a retrospective analysis of 236 COPD patients with lung cancer and 500 patients without lung cancer (control group). Clinical information, blood routine examination, and spirometry results were collected and analyzed. The receiver operating characteristic (ROC) curve was used to identify the best cutoff point of NLR or PLR. Multivariate logistic regression analysis was performed to evaluate the association of NLR or PLR with the diagnosis and prognosis of COPD with lung cancer.
Results: Compared to patients in the COPD-only group, patients in the lung cancer group had a higher percentage of current smoking and emphysema, and it was found that NLR or PLR was significantly higher in the lung cancer group. Multivariate analysis showed that age, smoking status, FEV1%pred, emphysema, NLR, and PLR were independent risk factors for lung cancer development in COPD. Furthermore, the high level of NLR or PLR was associated with age over 70 years old, current smoking status, and ineligible surgery treatment. The level of PLR or NLR markedly increased with hypercoagulation status, the severity of airflow limitation, and advanced progression of lung cancer. Additionally, the ROC analysis also revealed that elevated NLR or PLR was an independent predictor of COPD in lung cancer patients, TNM stages IIIB–IV at first diagnosis in lung cancer, and ineligible surgery in lung cancer patients.
Conclusion: Increased NLR or PLR values might be an important and easily measurable inflammation biomarker to predict the diagnosis and severity of lung cancer with COPD.
Chronic obstructive pulmonary disease (COPD) and lung cancer comprise the major causes of lung disease-related deaths worldwide (1). It was estimated that the total number of newly diagnosed cases of lung cancer in China in 2015 was about 787,000 (2). Furthermore, COPD is one of the leading causes of morbidity and mortality worldwide (3). In 2016, COPD was the fifth leading cause of death in China (4). Overall, lung cancer and COPD present a major public health issue and an enormous burden on society in China (5–7). Several studies describe that there is an association between COPD and the development of lung cancer. An increase in the incidence and mortality of lung cancer was observed in patients with COPD (8). Machida and colleagues investigated the incidence of lung cancer in the stable COPD population which was 1.85% per year during a median follow-up period of 4.58 years (9). Moreover, the prevalence of COPD in lung cancer patients was 32.6%, and with the increased severity of COPD, the prognosis of lung cancer gradually worsened (10). Current research has indicated that lung cancer accounts for 12% to 14% of COPD-related deaths (11, 12). Additionally, never-smokers of non-small cell lung cancer (NSCLC) patients with COPD had shorter overall survival (OS) times, compared to non-COPD never-smoker NSCLC patients (13). It has been also found that even in patients with early-stage COPD, the prevalence of postoperative pulmonary complications (PPCs) was higher than in patients with NSCLC with normal spirometry (14). Therefore, patients with lung cancer and coexisting COPD may have a much worse prognosis than those with COPD only.
The common pathogenic pathways contributing to both diseases share underlying etiologies, such as tobacco, gene expressions, susceptibility to DNA damage, environmental factors, and inflammation (1, 15, 16). However, more details of the relationship between COPD and lung cancer remain uncertain. Some studies have demonstrated that chronic inflammation could be one of the underlying mechanisms, which has been associated with carcinogenesis in lung cancer, airway remodeling, and severe comorbidities in COPD (17–20). Although some studies have demonstrated that high levels of several inflammation markers were related to progression-free survival (PFS) and OS in lung cancer patients with and without COPD, there is still a lack of effective markers to evaluate COPD with lung cancer (21).
The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are the available markers of systemic inflammation and routine clinical laboratory tests (22–24). Recent studies have indicated that NLR and PLR were higher in acute exacerbation of COPD and used for predicting hospitalization (3, 25). Furthermore, they were related to short-term mortality in patients hospitalized with acute exacerbation of COPD (AECOPD) (26, 27). NLR and PLR values were also found to be higher in advanced lung cancer (28). It was reported that NLR has been associated with worse survival and recurrence (29). The combination of NLR and PLR has increased the diagnostic value and distinguished lung cancer patients from healthy subjects (30). However, few studies have reported whether NLR and PLR could better evaluate COPD with lung cancer. In the study, we aimed to identify the baseline clinical characteristics of COPD coexisting with lung cancer and investigate whether NLR or PLR can be diagnostic markers for COPD with lung cancer.
We retrospectively reviewed the medical records of 1,264 patients diagnosed with COPD between January 2015 and August 2021 at the inpatient clinic of our respiratory department at The First Affiliated Hospital of Xiamen University, Fujian, People’s Republic of China. Patients who have incomplete medical records, with contraindications, or are unable to perform spirometry correctly and those who were diagnosed with asthma and interstitial lung disease were excluded. Finally, a total of 736 patients were eligible for this study. All participants underwent spirometry to measure pulmonary function, and their complete medical records were retrieved for analysis. Spirometry was done according to the American Thoracic Society recommendations with central quality assurance of spirometry tracings (19). Among the 1,264 patients, a total of 236 COPD patients with lung cancer were diagnosed through histological and/or cytological specimens.
Basic clinical information on age, sex, smoking status, body mass index (BMI), lung cancer diagnosis, histologic type, routine blood, coagulation, and cancer stage was taken from the medical records for all patients. As a retrospective study, all data were anonymous. We declared that the patients’ data were confidential and did not compromise the patients’ interests. Data collection was completed by two independent authors. The ethics approval of the study was obtained from the Clinical Research Ethics Committee of the First Affiliated Hospital of Xiamen University (2021064).
Histologic lung cancer was categorized as small-cell lung cancer (SCLC) and non-SCLC (NSCLC) according to the 2015 WHO classification of lung tumors (31). NSCLC was then further categorized as adenocarcinoma, squamous cell carcinoma (SCC), or other, which consisted primarily of large-cell carcinoma and undifferentiated carcinoma of the lungs. Cancer was staged in accordance with the Union for International Cancer Control, Tumour, Node, Metastasis 7 (TNM7) classification and staging I–IV. All lung cancer patients with COPD were diagnosed through CT-guided lung tissue biopsy, thoracic surgery, or bronchoscopic biopsy.
Peripheral venous blood was collected from all patients into Vacutainer tubes in the morning on the first day of admission and processed immediately. The differential blood counts (white blood cell, neutrophil, lymphocyte) and platelet count were measured using the hematology analyzer Sysmex XN-9000 (XN, Sysmex, Kobe, Japan).
Spirometry tests were performed by qualified technicians on a computerized spirometer MasterScreen, (Jaeger, Freistaat Bayern, Germany), following the criteria for the standardization of pulmonary function tests recommended by the American Thoracic Society/European Respiratory Society (ATS/ERS) Task Force (32). According to The Global Initiative for Chronic Obstructive Lung Disease (GOLD) (http://goldcopd.org) guidelines, forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) were recorded in medical records in liters and percentage predicted values, as well as FEV1/FVC%. The post-bronchodilator fixed criteria FEV1/FVC <0.7 was applied to define COPD. The GOLD criteria were also used to assign a grade of clinical severity to COPD based on FEV1%pred and FEV1/FVC: patients with an FEV1/FVC <0.7 were classified as having COPD in all grades; GOLD 1 was defined as having an FEV1%pred >80; GOLD 2 as 50 ≤ FEV1%pred <80; GOLD 3 as 30% ≤ FEV1%pred <50%; and GOLD 4 as FEV1%pred <30% (33).
All patient data were presented in terms of frequencies and mean ± standard deviations as appropriate. Normally distributed continuous variables were evaluated using the unpaired Student’s t-test. Continuous variables with a skewed distribution were analyzed using the Mann–Whitney U test. Categorical variables were assessed using the chi-square test. Correlations were done using Pearson’s correlation, and “r” is the correlation coefficient. It ranged from −1 to +1. Receiver operating characteristic (ROC) curve analysis was used to evaluate the sensitivity and specificity of NLR and PLR for predicting the diagnosis of COPD with lung cancer. We performed a univariate analysis with age, BMI, smoking status, FEV1%pred, NLR, PLR, and emphysema. The variables with P <0.05 in the univariate analysis were entered into a multivariate logistic regression analysis. P-value <0.05 was assumed for statistical significance in all the tests. Data were analyzed with SPSS 26.0 (SPSS Inc., Chicago, IL, USA).
A total of 736 patients were eligible for this study: 236 patients were diagnosed as having COPD with lung cancer, and 500 patients were categorized as COPD (Figure 1). Patients’ baseline characteristics included median age, gender, BMI, smoking history, pulmonary function, and inflammatory markers (Table 1). As shown in the table, there were no significant differences between BMI and gender. The median onset age was earlier in the COPD with lung cancer group than in the COPD group (66.44 ± 8.37 vs. 69.87 ± 9.37, P < 0.001). The proportion of current smoking was significantly higher in the COPD with lung cancer group [178 (75.4%)] than that in the COPD-only group [254 (50.8%)]. Moreover, the proportion of ever smoker was significantly lower in the COPD with lung cancer group [58 (24.6%)] compared with that in the COPD-only group [246 (49.2%)] (P < 0.001). In addition, there were significant differences in the degree of airflow obstruction in lung function. The values for FEV1/FVC and FEV1% predicted were all significantly higher in the COPD with lung cancer group than in the COPD group (0.59 ± 0.10 vs. 0.52 ± 0.11, P < 0.001; 66.46 ± 20.54 vs. 47.39 ± 21.01, P < 0.001, respectively).
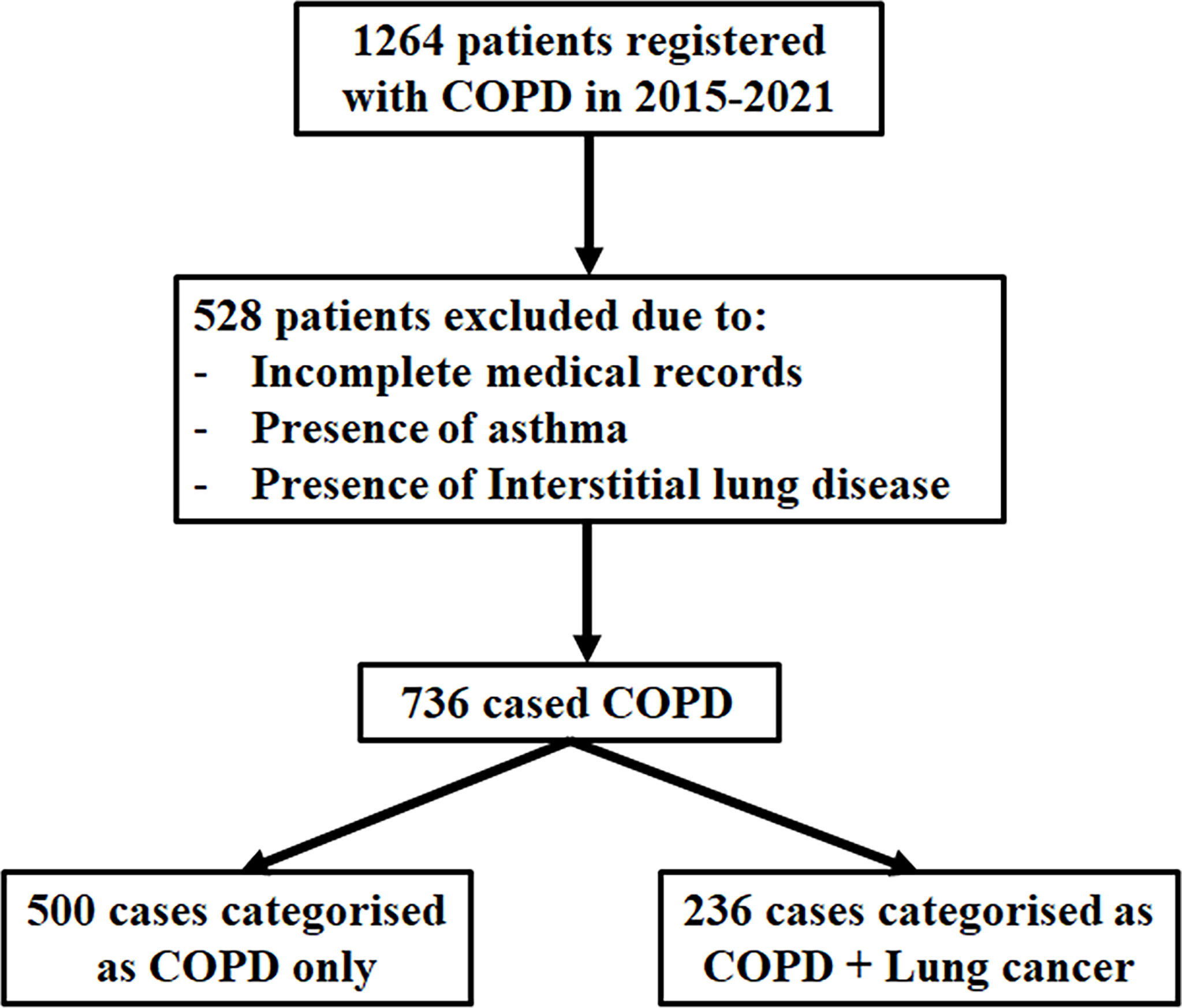
Figure 1 Flowchart of the population included in the study. Between 2015 and 2021, a total of 1,264 patients were retrospectively reviewed; 528 were excluded from the analysis due to missing medical records, asthma, and interstitial lung disease. The number of patients with COPD (FEV1/FVC < 70%) was 736, which were eligible for this study. Of the 736 patients included, 236 cases had COPD with lung cancer, and 500 controls had COPD only.
Next, to verify the predictive value of inflammatory markers in COPD with lung cancer, peripheral blood analyses of total white blood cell, neutrophil counts, lymphocyte, NLR, and PLR were measured. The results indicated that COPD with lung cancer patients had significantly higher levels of white blood cell and neutrophil counts (P < 0.001). Moreover, a statistical significance was found in NLR or PLR between COPD and COPD coexisting with lung cancer. The levels of NLR and PLR were significantly increased in the COPD with lung cancer group compared with those in the COPD group (3.51 ± 0.72 vs. 2.45 ± 0.81, 172.72 ± 24.96 vs. 142.64 ± 37.56, P < 0.001). Finally, the results showed a significant increase in the proportion of emphysema in the COPD with lung cancer group than in the COPD group.
Of the total population, 236 cases were diagnosed with lung cancer. Histologic type analyses showed that adenocarcinoma was the most frequent histological subtype in the COPD with lung cancer group (51.3%), and 30.9% were diagnosed with squamous cell carcinoma, 3.4% with large cell lung cancer, and 13.1% with SCC (Table 2). Among the 236 patients, 132 (55.9%) had lung cancer located in the right lobe of the lungs. Moreover, the TNM stage of lung cancer in COPD patients tended to be advanced, with stages III–VI accounting for 63.6% (Supplementary Table 1).
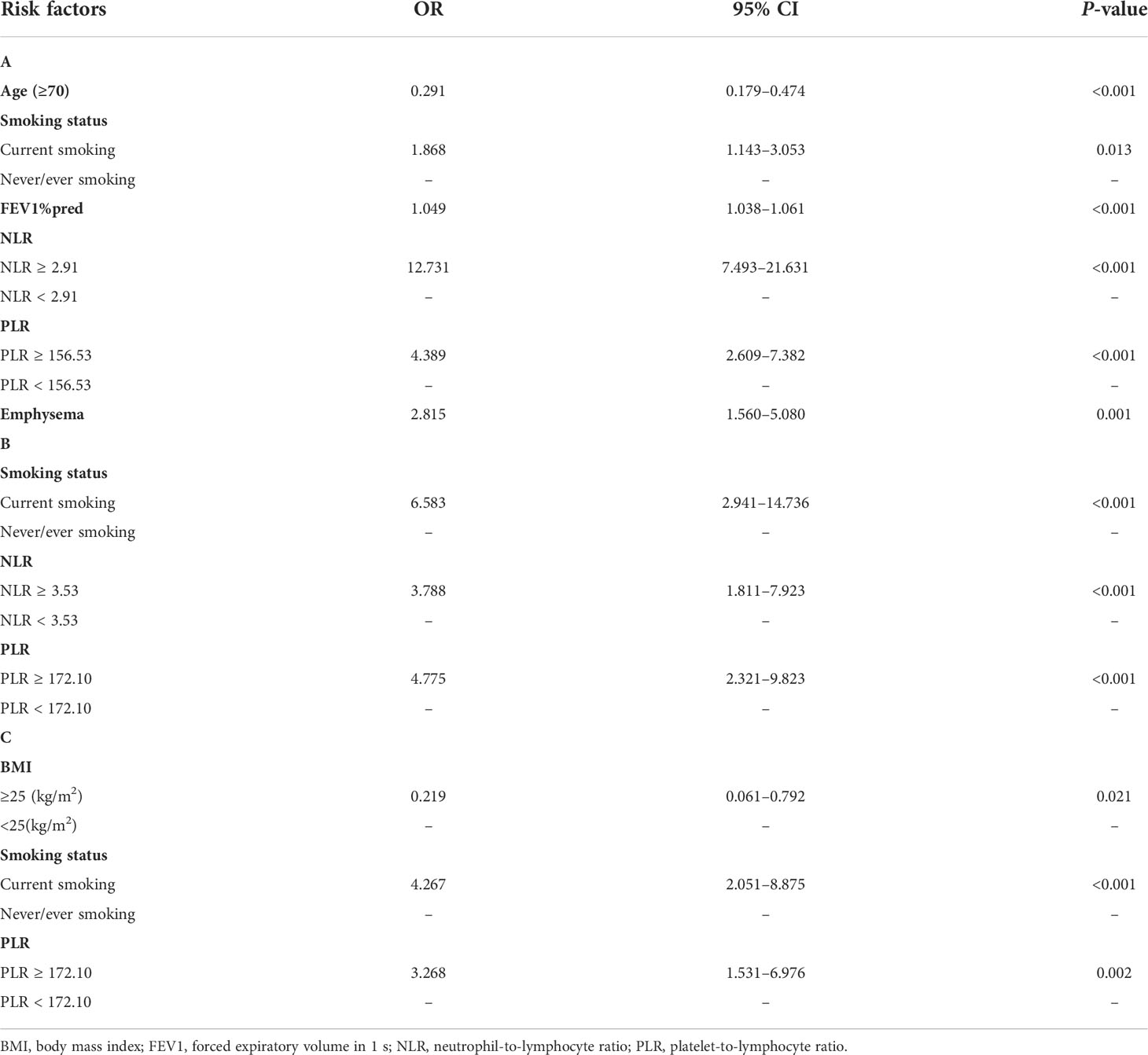
Table 2 Multivariate analysis of independent predictors of (A) lung cancer diagnosis in COPD patients, (B) TNM stages IIIB–IV at first diagnosis in the COPD with lung cancer patients, and (C) ineligible surgery at first diagnosis in the COPD with lung cancer patients.
In order to confirm whether NLR or PLR served as an independent inflammation biomarker for predicting the progression of lung cancer in COPD patients, the level of NLR or PLR was compared among these groups with different clinical features. The results showed that there were no significant differences in NLR or PLR values between gender and BMI (Supplementary Table 2). NLR or PLR levels could markedly increase with advanced age (P < 0.001). In addition, the level of NLR or PLR in current smoker was higher than in never/ever smoker (P < 0.05), and the NLR or PLR was significantly higher in GOLD stages 3–4 than 1–2 (P < 0.001). Moreover, lung cancer patients at TNM stages IIIB–IV had higher levels of NLR and PLR than those at stages I–IIIA (P < 0.001). Finally, the ineligible surgery group had higher levels of NLR and PLR than the eligible for surgery lung cancer patients (P < 0.001).
To further evaluate the correlation between NLR or PLR and other clinical parameters, Pearson’s correlation coefficients were analyzed. It was indicated that a positive correlation was observed between NLR or PLR value and age, PT, FIB, and D-dimer (P < 0.05) (Supplementary Figure 1). Conversely, NLR or PLR value was negatively correlated with FEV1%pred (r = −0.299, P < 0.001; r = −0.300, P < 0.001, respectively). We also found a negative correlation between PLR and BMI (r = −0.146, P = 0.025). In addition, PLR was positively correlated with PLT and APTT (r = 0.167, P < 0.01; r = 0.148, P = 0.023, respectively).
ROC curve analysis was also performed for the NLR and PLR values to detect the diagnosis of lung cancer in COPD patients. The best NLR cutoff value was defined as 2.91, the sensitivity was 84.3%, and the specificity was 74.4%, with the best AUC of 0.84. The optimal PLR cutoff value was 156.53 and the AUC was 0.74, with 81.8% sensitivity and 62.8% specificity (Figure 2A). Moreover, the ROC curve analysis using NLR and PLR to predict TNM stages IIIB–IV at first diagnosis in the COPD with lung cancer patients indicated an optimal cutoff NLR of 3.53, AUC of 0.74, sensitivity of 60.9%, and specificity of 75.9%. The best cutoff of PLR was 172.10 and the AUC was 0.73, with 70.3% sensitivity and 70.4% specificity (Figure 2B). Finally, the predictive accuracy of NLR and PLR in ineligible surgery at first diagnosis in the COPD with lung cancer patients was also analyzed with the ROC curve, the best cutoff of NLR was 3.49, and the AUC was 0.68, with 55.6% sensitivity and 74.3% specificity. The ROC curve for PLR had an AUC of 0.69 with a cutoff value of 172.10, a sensitivity of 63.0%, and s specificity of 73.0% (Figure 2C).
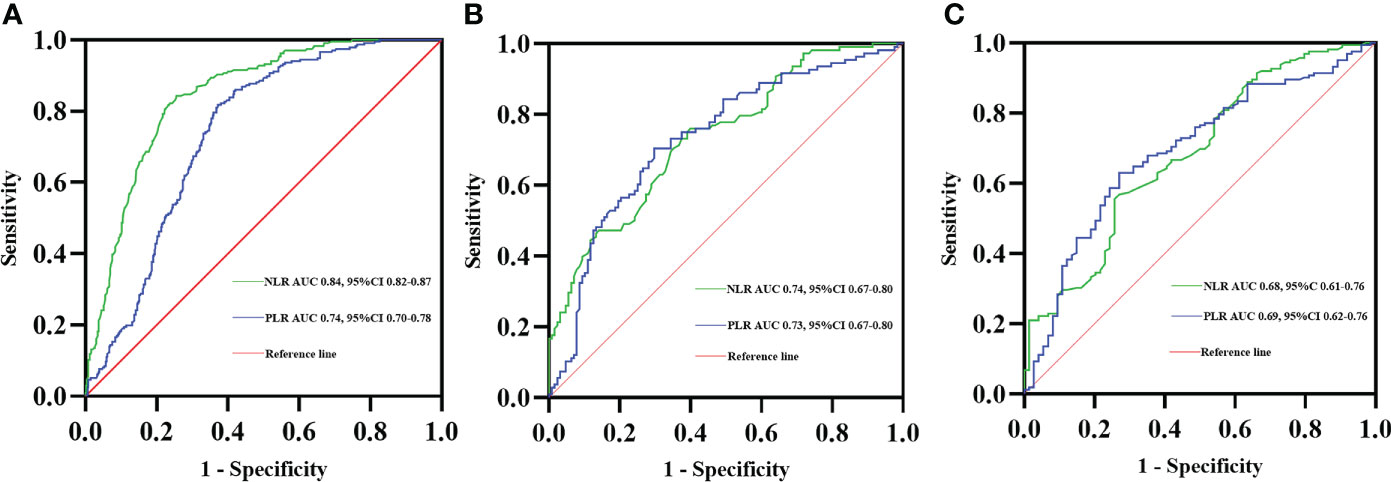
Figure 2 Receiver operating characteristic (ROC) curves of NLR and PLR predicting lung cancer diagnosis in COPD patients (A). ROC curves of NLR and PLR predicting TNM stages IIIB–IV at first diagnosis in the COPD with lung cancer patients (B). ROC curves of NLR and PLR predicting ineligible surgery at first diagnosis in the COPD with lung cancer patients (C).
To identify the risk factors related to lung cancer in patients with COPD, univariate and stepwise multivariate logistic regression analyses were performed. In the subgroup analysis, the results showed that age (≥70) (OR: 0.291; 95% CI: 0.179–0.474; P < 0.001), current smoker (OR: 1.868; 95% CI: 1.143–3.053; P = 0.013), FEV1%pred (OR: 1.049; 95% CI: 1.038–1.061; P < 0.001), NLR ≥2.91 (OR: 12.731; 95% CI: 7.493–21.631; P < 0.001), PLR ≥156.53 (OR: 4.389; 95% CI: 2.609–7.382; P < 0.001), and emphysema (OR: 2.815; 95% CI: 1.560–5.080; P = 0.001) were independent risk factors related to the COPD with lung cancer patients (Table 2A). Then, we evaluated the risk factors associated with TNM stages IIIB–IV at first diagnosis in the COPD with lung cancer patients. On multivariate analysis, current smoker (OR: 6.583; 95% CI: 2.941–14.736; P < 0.001), NLR ≥3.53 (OR: 3.788; 95% CI: 1.811–7.923; P < 0.001), and PLR ≥172.10 (OR: 4.775; 95% CI: 2.321–9.823; P < 0.001) were independent predictors of TNM stages IIIB–IV at first diagnosis in the COPD with lung cancer patients (Table 2B). Finally, we extended our analysis to identify the factors that contributed to ineligible surgery at first diagnosis in the COPD with lung cancer patients. BMI ≥25 (kg/m2) (OR: 0.219; 95% CI: 0.061–0.792; P = 0.021), current smoker (OR: 4.267; 95% CI: 2.051–8.875; P < 0.001), and PLR ≥172.10 (OR: 3.268; 95% CI: 1.531–6.976; P = 0.002) were the statistically significant risk factors for ineligible surgery treatment in the COPD with lung cancer patients (Table 2C).
In the present study, we investigated the clinical features of COPD-associated lung cancer in a large cohort of COPD patients and identified the value of NLR or PLR that served as an independent predictive factor in COPD with lung cancer. The results indicated that age, smoking status, pulmonary function, serum inflammation biomarkers, and emphysema were associated with COPD coexisting with lung cancer. In terms of median age, the lung cancer group was younger than the COPD group. Airflow limitation assessed by FEV1%pred in COPD was more severe than in lung cancer. The possible reason is that low-dose spiral computed tomography (LDCT) screening was initiated in the year 2010 in China, based on the development of the National Lung Cancer Screening Program (NLST) (34). LDCT was considered as a prevalent screening tool for the early detection of lung cancer in high-risk asymptomatic individuals (35, 36). Another possible reason is that spirometry screening for airflow obstruction in asymptomatic smokers is still controversial (37). Many smokers never experienced lung function decline during the early stage (38). Therefore, based on CT, screening in smoking patients resulted in a higher detection rate of early-stage lung cancer, especially for mild-to-moderate COPD. It has been previously reported that smokers with COPD have a two- to fourfold higher risk of developing lung cancer compared with smokers without COPD (39, 40). It was elucidated that the prevalence of lung cancer was significantly higher in patients with COPD than in the average non-smoking population, reflecting the impact of cigarette smoking on both diseases (41). It has been well acknowledged that cigarette smoking exposure which caused lung barrier dysfunction and inflammation-associated COPD should be considered as an important risk factor for lung cancer (42). In our research, current smoking was an independent risk factor for lung cancer in the COPD group and related to TNM stages IIIB–IV and ineligible surgery treatment. Furthermore, there have been several studies showing that emphysema was an independent poor prognostic factor for tumor recurrence in completely resected NSCLC patients (43). Emphysema severity and centrilobular subtype were associated with a greater risk of lung cancer (44). It was considered that emphysema is an independent predictor of lung cancer diagnosis (45). Other researchers have shown that quantitative emphysema severity of the whole lung and stage III were independent predictors of lung cancer recurrence after adjusting for age, gender, smoking status, and FEV1 (46). Lung adenocarcinomas in the emphysema group have a more aggressive pathologic grade and a higher prevalence of solid lesions (47). Consistent with previous studies, our study showed that emphysema was also independently associated with lung cancer coexisting with COPD.
On the other hand, COPD is characterized as a chronic systemic inflammatory disease, which also has been found to be related to exacerbation (48). Several reports have shown that peripheral blood neutrophils and T lymphocytes were activated and there were increased levels of proinflammatory cytokines in the plasma (49–51). A recent study also demonstrated that increased baseline neutrophil counts were significantly associated with worse OS but not for lymphocyte counts in NSCLC (52). Wong et al. reported positive associations between white blood counts and lung cancer risk among never-smoking women (53). Furthermore, another study found that lymphocyte percentage exhibited a high correlation with the clinical characteristics and metastasis of lung cancer patients (54). In this study, we observed that circulating white blood counts and neutrophil counts were significantly higher in the lung cancer group than in the COPD-only group. It was likely that neutrophils were quickly recruited from the circulation during pulmonary inflammation (55). Thus, we have considered that persistent inflammation could mediate the progression of COPD with lung cancer. Several reports have shown that the levels of NLR or PLR significantly increased in the lung cancer and COPD groups, respectively (56, 57). However, few studies have reported the levels of NLR or PLR in lung cancer with COPD. Our data revealed that NLR and PLR levels were significantly higher in the COPD with lung cancer group than in the COPD group. Then, to further confirm the clinical relevance of inflammation biomarkers, we analyzed the correlation between NLR, PLR, and clinical data from lung cancer patients with COPD. Some studies showed that elevated PLR was associated with shorter OS and poor PFS in NSCLC (58, 59). Another study suggested that elevated PLR might be a predictive factor of poor prognosis for NSCLC patients (60). Yao et al. found that the levels of NLR and PLR were significantly higher among non-survivors compared to survivors of AECOPD, and NLR may be a simple and useful prognostic marker for hospital mortality in patients with AECOPD (61). Elevated NLR could be used as a marker similar to CRP, WBC, and ESR in the determination of increased inflammation in acutely exacerbated COPD and could be beneficial for the early detection of potential acute exacerbations in patients with COPD who have normal levels of traditional markers (62). In the present study, the levels of NLR and PLR had statistical significance among the multiple stratifications in clinical parameters, especially those with a high NLR or PLR were associated with age over 70 years old, current smoking status, and ineligible surgery treatment. The level of PLR or NLR markedly increased with the severity of airflow limitation accessed with GOLD stages 3–4 and advanced progression of lung cancer evaluated with TNM stages IIIB–IV. Additionally, NLR or PLR was positively associated with age, FIB, and D-dimer. We also found a negative association between NLR and FEV1%pred. PLR was negatively associated with BMI and FEV1%pred. The results revealed that PLR or NLR values were inversely associated with the severity of airflow limitation. Also, it was suggested that higher PLR or NLR was positively associated with hypercoagulation status. In our study, the applicable thresholds for NLR and PLR were observed using the ROC curve. Multivariate analysis revealed that NLR ≥2.91 and PLR ≥156.53 were independent risk factors for lung cancer development in COPD patients; the COPD with lung cancer patients with NLR ≥3.53 and PLR ≥172.10 are more likely to be diagnosed in TNM stages IIIB–IV at first diagnosis, while patients with PLR ≥172.10 had fewer surgical opportunities. We believe that NLR or PLR could be part of an important and easily measurable inflammation biomarker system to predict the diagnosis and severity of COPD with lung cancer.
However, this study also had several limitations. Because the pulmonary function tests cannot be properly performed on a number of elderly people in this study, this may lead to the possibility of selection bias in the COPD population. Another limitation is the lack of data on follow-up survival information. We did not evaluate the outcomes of the surgical treatment and did not perform a prognostic analysis due to the high rate of missing follow-up data. Longitudinal studies are needed to evaluate the long-term clinical prognosis of COPD with lung cancer. In addition, the study has a retrospective design, in which a number of patients with severe COPD were unable to undergo lung tissue pathologic diagnosis. Thus, it may have caused bias in the selection of the study group. Moreover, this study could not provide information about the use of inhaled corticosteroids, which is a potential influencing factor for the prevention of lung cancer (63, 64).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethical Committee of the Clinical Research Ethics Committee of the First Affiliated Hospital of Xiamen University. The patients/participants provided their written informed consent to participate in this study.
AM and WG drafted the manuscript. GW and YD collected the associated clinical data. HH and LZ contributed to the data statistical analyses. DB and YH prepared the figures and tables. JC and QL wrote the manuscript and conceptualized the framework for this research. All authors read and approved the final manuscript.
This work was supported the Xiamen Science and Technology Bureau (3502Z20194004) and Xiamen Science and Health Joint Project of Fujian Natural Science Foundation (2020J011229).
The authors would like to thank all the patients who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.902955/full#supplementary-material
1. Parris BA, O'Farrell HE, Fong KM, Yang IA. Chronic obstructive pulmonary disease (Copd) and lung cancer: Common pathways for pathogenesis. J Thorac Dis (2019) 11(Suppl 17):S2155–S72. doi: 10.21037/jtd.2019.10.54
2. Gao S, Li N, Wang S, Zhang F, Wei W, Li N, et al. Lung cancer in people's republic of China. J Thorac Oncol (2020) 15(10):1567–76. doi: 10.1016/j.jtho.2020.04.028
3. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Gold Exec Summary. Am J Respir Crit Care Med (2017) 195(5):557–82. doi: 10.1164/rccm.201701-0218PP
4. Collaborators GBDCoD. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: A systematic analysis for the global burden of disease study 2016. Lancet (2017) 390(10100):1151–210. doi: 10.1016/S0140-6736(17)32152-9
5. Zhu B, Wang Y, Ming J, Chen W, Zhang L. Disease burden of copd in China: A systematic review. Int J Chron Obstruct Pulmon Dis (2018) 13:1353–64. doi: 10.2147/COPD.S161555
6. Yang L, Xue T, Wang N, Yuan Y, Liu S, Li H, et al. Burden of lung cancer attributable to ambient fine particles and potential benefits from air quality improvements in Beijing, China: A population-based study. Sci Total Environ (2020) 738:140313. doi: 10.1016/j.scitotenv.2020.140313
7. Wu F, Wang L, Zhou C. Lung cancer in China: Current and prospect. Curr Opin Oncol (2021) 33(1):40–6. doi: 10.1097/CCO.0000000000000703
8. Wasswa-Kintu S, Gan WQ, Man SF, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: A systematic review and meta-analysis. Thorax (2005) 60(7):570–5. doi: 10.1136/thx.2004.037135
9. Machida H, Inoue S, Shibata Y, Kimura T, Ota T, Ishibashi Y, et al. The incidence and risk analysis of lung cancer development in patients with chronic obstructive pulmonary disease: Possible effectiveness of annual ct-screening. Int J Chron Obstruct Pulmon Dis (2021) 16:739–49. doi: 10.2147/COPD.S287492
10. Wang W, Dou S, Dong W, Xie M, Cui L, Zheng C, et al. Impact of copd on prognosis of lung cancer: From a perspective on disease heterogeneity. Int J Chron Obstruct Pulmon Dis (2018) 13:3767–76. doi: 10.2147/COPD.S168048
11. Jenkins CR, Jones PW, Calverley PM, Celli B, Anderson JA, Ferguson GT, et al. Efficacy of Salmeterol/Fluticasone propionate by gold stage of chronic obstructive pulmonary disease: Analysis from the randomised, placebo-controlled torch study. Respir Res (2009) 10:59. doi: 10.1186/1465-9921-10-59
12. Bale G, Martinez-Camblor P, Burge PS, Soriano JB. Long-term mortality follow-up of the Isolde participants: Causes of death during 13 years after trial completion. Respir Med (2008) 102(10):1468–72. doi: 10.1016/j.rmed.2008.04.001
13. Lim JU, Yeo CD, Rhee CK, Kang HS, Park CK, Kim JS, et al. Comparison of clinical characteristics and overall survival between spirometrically diagnosed chronic obstructive pulmonary disease (Copd) and non-copd never-smoking stage I-iv non-small cell lung cancer patients. Int J Chron Obstruct Pulmon Dis (2019) 14:929–38. doi: 10.2147/COPD.S190244
14. Kim ES, Kim YT, Kang CH, Park IK, Bae W, Choi SM, et al. Prevalence of and risk factors for postoperative pulmonary complications after lung cancer surgery in patients with early-stage copd. Int J Chron Obstruct Pulmon Dis (2016) 11:1317–26. doi: 10.2147/COPD.S105206
15. Mouronte-Roibas C, Ruano-Ravina A, Fernandez-Villar A. Lung cancer and chronic obstructive pulmonary disease: Understanding the complexity of carcinogenesis. Transl Lung Cancer Res (2018) 7(Suppl 3):S214–S7. doi: 10.21037/tlcr.2018.08.11
16. Eapen MS, Hansbro PM, Larsson-Callerfelt AK, Jolly MK, Myers S, Sharma P, et al. Chronic obstructive pulmonary disease and lung cancer: Underlying pathophysiology and new therapeutic modalities. Drugs (2018) 78(16):1717–40. doi: 10.1007/s40265-018-1001-8
17. Tan Z, Xue H, Sun Y, Zhang C, Song Y, Qi Y. The role of tumor inflammatory microenvironment in lung cancer. Front Pharmacol (2021) 12:688625. doi: 10.3389/fphar.2021.688625
18. Nakano-Narusawa Y, Yokohira M, Yamakawa K, Ye J, Tanimoto M, Wu L, et al. Relationship between lung carcinogenesis and chronic inflammation in rodents. Cancers (Basel) (2021) 13(12): 2910. doi: 10.3390/cancers13122910
19. Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol (2016) 138(1):16–27. doi: 10.1016/j.jaci.2016.05.011
20. Wang Y, Xu J, Meng Y, Adcock IM, Yao X. Role of inflammatory cells in airway remodeling in copd. Int J Chron Obstruct Pulmon Dis (2018) 13:3341–8. doi: 10.2147/COPD.S176122
21. Berg J, Halvorsen AR, Bengtson MB, Tasken KA, Maelandsmo GM, Yndestad A, et al. Levels and prognostic impact of circulating markers of inflammation, endothelial activation and extracellular matrix remodelling in patients with lung cancer and chronic obstructive pulmonary disease. BMC Cancer (2018) 18(1):739. doi: 10.1186/s12885-018-4659-0
22. Kurtipek E, Bekci TT, Kesli R, Sami SS, Terzi Y. The role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in exacerbation of chronic obstructive pulmonary disease. J Pak Med Assoc (2015) 65(12):1283–7.
23. Bozan N, Alpayci M, Aslan M, Cankaya H, Kiroglu AF, Turan M, et al. Mean platelet volume, red cell distribution width, platelet-to-Lymphocyte and neutrophil-to-Lymphocyte ratios in patients with ankylosing spondylitis and their relationships with high-frequency hearing thresholds. Eur Arch Otorhinolaryngol (2016) 273(11):3663–72. doi: 10.1007/s00405-016-3980-y
24. Mercan R, Bitik B, Tufan A, Bozbulut UB, Atas N, Ozturk MA, et al. The association between Neutrophil/Lymphocyte ratio and disease activity in rheumatoid arthritis and ankylosing spondylitis. J Clin Lab Anal (2016) 30(5):597–601. doi: 10.1002/jcla.21908
25. Sahin F, Kosar AF, Aslan AF, Yigitbas B, Uslu B. Serum biomarkers in patients with stable and acute exacerbation of chronic obstructive pulmonary disease: A comparative study. J Med Biochem (2019) 38(4):503–11. doi: 10.2478/jomb-2018-0050
26. Luo Z, Zhang W, Chen L, Xu N. Prognostic value of Neutrophil:Lymphocyte and Platelet:Lymphocyte ratios for 28-day mortality of patients with aecopd. Int J Gen Med (2021) 14:2839–48. doi: 10.2147/IJGM.S312045
27. Kumar P, Law S, Sriram KB. Evaluation of platelet lymphocyte ratio and 90-day mortality in patients with acute exacerbation of chronic obstructive pulmonary disease. J Thorac Dis (2017) 9(6):1509–16. doi: 10.21037/jtd.2017.05.77
28. Goksel S, Ozcelik N, Telatar G, Ardic C. The role of hematological inflammatory biomarkers in the diagnosis of lung cancer and in predicting tnm stage. Cancer Invest (2021) 39(6-7):514–20. doi: 10.1080/07357907.2021.1938110
29. Sebastian NT, Raj R, Prasad R, Barney C, Brownstein J, Grecula J, et al. Association of pre- and posttreatment neutrophil-lymphocyte ratio with recurrence and mortality in locally advanced non-small cell lung cancer. Front Oncol (2020) 10:598873. doi: 10.3389/fonc.2020.598873
30. Zhu X, Song H, Chen Y, Han F, Wang Q, Cui Y. Neutrophil-to-Lymphocyte ratio and platelet-to-Lymphocyte ratio in blood to distinguish lung cancer patients from healthy subjects. Dis Markers (2020) 2020:8844698. doi: 10.1155/2020/8844698
31. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 world health organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol (2015) 10(9):1243–60. doi: 10.1097/JTO.0000000000000630
32. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J (2005) 26(5):948–68. doi: 10.1183/09031936.05.00035205
33. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report: Gold executive summary. Respirology (2017) 22(3):575–601. doi: 10.1111/resp.13012
34. National Lung Screening Trial Research T, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med (2011) 365(5):395–409. doi: 10.1056/NEJMoa1102873
35. Zhou Q, Fan Y, Wu N, Huang Y, Wang Y, Li L, et al. Demonstration program of population-based lung cancer screening in China: Rationale and study design. Thorac Cancer (2014) 5(3):197–203. doi: 10.1111/1759-7714.12078
36. Zhao SJ, Wu N. Early detection of lung cancer: Low-dose computed tomography screening in China. Thorac Cancer (2015) 6(4):385–9. doi: 10.1111/1759-7714.12253
37. Young RP, Hopkins RJ. A clinical practice guideline update on the diagnosis and management of stable chronic obstructive pulmonary disease. Ann Intern Med (2012) 156(1 Pt 1):68–9. doi: 10.7326/0003-4819-156-1-201201030-00021
38. Walters JA, Hansen EC, Walters EH, Wood-Baker R. Under-diagnosis of chronic obstructive pulmonary disease: A qualitative study in primary care. Respir Med (2008) 102(5):738–43. doi: 10.1016/j.rmed.2007.12.008
39. Nakayama M, Satoh H, Sekizawa K. Risk of cancers in copd patients. Chest (2003) 123(5):1775–6. doi: 10.1378/chest.123.5.1775-a
40. Papi A, Casoni G, Caramori G, Guzzinati I, Boschetto P, Ravenna F, et al. Copd increases the risk of squamous histological subtype in smokers who develop non-small cell lung carcinoma. Thorax (2004) 59(8):679–81. doi: 10.1136/thx.2003.018291
41. Kim TH, Oh DK, Oh YM, Lee SW, Do Lee S, Lee JS. Fibrinogen as a potential biomarker for clinical phenotype in patients with chronic obstructive pulmonary disease. J Thorac Dis (2018) 10(9):5260–8. doi: 10.21037/jtd.2018.08.52
42. Hou W, Hu S, Li C, Ma H, Wang Q, Meng G, et al. Cigarette smoke induced lung barrier dysfunction, emt, and tissue remodeling: A possible link between copd and lung cancer. BioMed Res Int (2019) 2019:2025636. doi: 10.1155/2019/2025636
43. Heo JW, Kang HS, Park CK, Kim SK, Kim JS, Kim JW, et al. Regional emphysema score is associated with tumor location and poor prognosis in completely resected nsclc patients. BMC Pulm Med (2020) 20(1):242. doi: 10.1186/s12890-020-01268-7
44. Gonzalez J, Henschke CI, Yankelevitz DF, Seijo LM, Reeves AP, Yip R, et al. Emphysema phenotypes and lung cancer risk. PloS One (2019) 14(7):e0219187. doi: 10.1371/journal.pone.0219187
45. Yong PC, Sigel K, de-Torres JP, Mhango G, Kale M, Kong CY, et al. The effect of radiographic emphysema in assessing lung cancer risk. Thorax (2019) 74(9):858–64. doi: 10.1136/thoraxjnl-2018-212457
46. Lee SJ, Yoo JW, Ju S, Cho YJ, Kim JD, Kim SH, et al. Quantitative severity of pulmonary emphysema as a prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. Thorac Cancer (2019) 10(3):421–7. doi: 10.1111/1759-7714.12920
47. Lim CG, Shin KM, Lim JK, Kim HJ, Kim WH, Cho SH, et al. Emphysema is associated with the aggressiveness of copd-related adenocarcinomas. Clin Respir J (2020) 14(4):405–12. doi: 10.1111/crj.13146
48. Agustí A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, Miller BE, et al. Persistent systemic inflammation is associated with poor clinical outcomes in copd: A novel phenotype. PloS One (2012) 7(5):e37483. doi: 10.1371/journal.pone.0037483
49. Agustí AG, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J (2003) 21(2):347–60. doi: 10.1183/09031936.03.00405703
50. Barnes PJ. New concepts in chronic obstructive pulmonary disease. Annu Rev Med (2003) 54:113–29. doi: 10.1146/annurev.med.54.101601.152209
51. Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl. (2001) 34:50s–9s. doi: 10.1183/09031936.01.00229701
52. Biswas T, Gawdi R, Jindal C, Iyer S, Kang KH, Bajor D, et al. Pretreatment neutrophil-to-Lymphocyte ratio as an important prognostic marker in stage iii locally advanced non-small cell lung cancer: Confirmatory results from the proclaim phase iii clinical trial. J Thorac Dis (2021) 13(10):5617–26. doi: 10.21037/jtd-21-1018
53. Wong JYY, Bassig BA, Loftfield E, Hu W, Freedman ND, Ji BT, et al. White blood cell count and risk of incident lung cancer in the uk biobank. JNCI Cancer Spectr (2020) 4(2):pkz102. doi: 10.1093/jncics/pkz102
54. Huang H, Li L, Luo W, Yang Y, Ni Y, Song T, et al. Lymphocyte percentage as a valuable predictor of prognosis in lung cancer. J Cell Mol Med (2022) 26(7) 1918–31. doi: 10.1111/jcmm.17214
55. de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: Going forward in reverse. Nat Rev Immunol (2016) 16(6):378–91. doi: 10.1038/nri.2016.49
56. Sanchez-Salcedo P, de-Torres JP, Martinez-Urbistondo D, Gonzalez-Gutierrez J, Berto J, Campo A, et al. The neutrophil to lymphocyte and platelet to lymphocyte ratios as biomarkers for lung cancer development. Lung Cancer (Amsterdam Netherlands) (2016) 97:28–34. doi: 10.1016/j.lungcan.2016.04.010
57. Günay E, Sarınç Ulaşlı S, Akar O, Ahsen A, Günay S, Koyuncu T, et al. Neutrophil-to-Lymphocyte ratio in chronic obstructive pulmonary disease: A retrospective study. Inflammation (2014) 37(2):374–80. doi: 10.1007/s10753-013-9749-1
58. Gu X, Sun S, Gao XS, Xiong W, Qin S, Qi X, et al. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: Evidence from 3,430 patients. Sci Rep (2016) 6:23893. doi: 10.1038/srep23893
59. Zhang H, Gao L, Zhang B, Zhang L, Wang C. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: A systematic review and meta-analysis. Sci Rep (2016) 6:22618. doi: 10.1038/srep22618
60. Zhao QT, Yuan Z, Zhang H, Zhang XP, Wang HE, Wang ZK, et al. Prognostic role of platelet to lymphocyte ratio in non-small cell lung cancers: A meta-analysis including 3,720 patients. Int J Cancer (2016) 139(1):164–70. doi: 10.1002/ijc.30060
61. Yao C, Liu X, Tang Z. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with aecopd. Int J Chron. Obstruct Pulmon Dis (2017) 12:2285–90. doi: 10.2147/copd.S141760
62. Taylan M, Demir M, Kaya H, Selimoglu Sen H, Abakay O, Carkanat AI, et al. Alterations of the neutrophil-lymphocyte ratio during the period of stable and acute exacerbation of chronic obstructive pulmonary disease patients. Clin Respir J (2017) 11(3):311–7. doi: 10.1111/crj.12336
63. Liu SF, Kuo HC, Lin MC, Ho SC, Tu ML, Chen YM, et al. Inhaled corticosteroids have a protective effect against lung cancer in female patients with chronic obstructive pulmonary disease: A nationwide population-based cohort study. Oncotarget (2017) 8(18):29711–21. doi: 10.18632/oncotarget.15386
Keywords: chronic obstructive pulmonary disease, lung cancer, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, inflammation biomarkers
Citation: Ma A, Wang G, Du Y, Guo W, Guo J, Hu Y, Bai D, Huang H, Zhuang L, Chen J and Liu Q (2022) The clinical relevance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in chronic obstructive pulmonary disease with lung cancer. Front. Oncol. 12:902955. doi: 10.3389/fonc.2022.902955
Received: 23 March 2022; Accepted: 31 August 2022;
Published: 27 September 2022.
Edited by:
Mohamed Rahouma, NewYork-Presbyterian, United StatesReviewed by:
Janaki Deepak, University of Maryland, Baltimore, United StatesCopyright © 2022 Ma, Wang, Du, Guo, Guo, Hu, Bai, Huang, Zhuang, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qun Liu, NDcwNzE4MzM4QHFxLmNvbQ==; Jinhan Chen, eG1qaW5oYW5AYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.