
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 29 July 2022
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.899761
This article is part of the Research Topic Clinicopathological Factors and Staging in Gastrointestinal Cancers View all 74 articles
 Zhen Yang
Zhen Yang Guangjun Shi*
Guangjun Shi*Background: Intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN) are two main histological subtypes of pancreatic cystic neoplasms with rapidly increasing incidence recently. The natural histories, treatment patterns, and survival outcomes of invasive IPMN and invasive MCN have not been well explored.
Methods: Patients with a diagnosis of invasive IPMN and invasive MCN in the SEER database from 2000 through 2018 were retrospectively identified. Multivariable Cox regression analysis was conducted to determine the independent risk factors associated with overall survival (OS). Subgroup analyses of survival outcomes for invasive IPMN and invasive MCN were conducted. The OS for invasive IPMN was compared between patients who underwent surgery alone and those who received surgery plus chemotherapy by propensity score matching (PSM).
Results: A total of 2,505 patients were included, of whom 2,300 were diagnosed with invasive IPMN and 205 were diagnosed with invasive MCN. Half of the invasive IPMN (48.4%) and three-quarters of the invasive MCN (76.1%) patients were female. Of all patients, both the OS and cancer-specific survival were significantly better in the invasive MCN cohort compared to the invasive IPMN cohort. In subgroup analyses, while invasive MCN experienced better OS compared to invasive IPMN in the subgroups of patients with local–regional disease, the survival advantages disappeared in patients at a distant stage. In addition, surgery plus chemotherapy in invasive IPMN patients was associated with significantly better survival compared to surgery alone after PSM.
Conclusion: We examined the demographic and clinical characteristics between invasive IPMN and invasive MCN patients using a large-population-based analysis. Although the OS is significantly better for invasive MCN versus invasive IPMN, the difference disappeared in patients with distant disease. A combination of surgery and chemotherapy in selected invasive IPMN patients could confer survival benefits compared to surgery alone.
Pancreatic cystic neoplasms (PCNs) are regarded as challenging entities as they can exhibit a disease spectrum from benign to malignant lesions (1). The incidence of incidentally discovered PCNs has been gradually rising worldwide over the last few decades due to the increased use of cross-sectional imaging (2, 3). Intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN) are two main histological subtypes which have been recognized as precursors of pancreatic cancer (4). IPMN is a mucin-producing clinical entity arising from the pancreatic duct system, while MCN is a distinctive subtype with the presence of ovarian-type stroma and almost exclusively occurs in middle-aged females (5, 6). The risk of progression to malignancy varies between IPMN and MCN (7). However, the natural history and malignant potentials were not fully understood yet. Given the divergent malignant potentials and increasing frequency of these two predominant lesions, it is crucial to differentiate between these various types and formulate clinical guidelines for decision-making. Regarding the variable clinical course and limited high-quality research on invasive IPMN and invasive MCN, there also remain ambiguities in distinction between these two diseases. Moreover, the management of pancreatic cysts remains controversial (8, 9). The European guidelines suggest conservative treatment for IPMN and MCN with less than 4 cm in size and in the absence of worrisome features (10), while the international and American guidelines recommend aggressive surgical resection for all MCNs and IPMN >3 cm (11, 12). Additionally, larger population-based analyses investigating the clinicopathologic features and survival outcomes are scarce.
Thus, the purpose of this current study was to determine and compare the demographic and clinical characteristics of invasive IPMN versus invasive MCN as well as evaluate the treatment strategies using a large population in the United States.
Data regarding patients with invasive IPMN or invasive MCN between 2000 and 2018 were retrieved from the SEER database which represents about 30% of the population in the United States (13). The inclusion criteria were as follows: (1) patients with a histological confirmation of invasive IPMN or invasive MCN, (2) patients with invasive IPMN or invasive MCN labeled as the first and only primary tumor, and (3) patients with a follow-up duration of more than 1 month. Patients with missing information on survival status or treatment details were excluded. The parameters collected in our study included age at diagnosis (<70/≥70 years), gender (female/male), race (white/black/other), marital status (married/other), tumor size, tumor location (head/body and tail), tumor differentiation (well differentiated/poorly differentiated/unknown), lymph node status (positive/negative/unknown), tumor stage (localized/regional/distant), treatment details (surgery/chemotherapy/radiation), and survival data (time and status). The primary outcomes were overall survival (OS) and cancer-specific survival (CSS).
Continuous variables were presented as mean ± standard deviation and compared using independent-samples t-test, while categorical variables were described as number (percentage) and compared using chi-square analysis. OS and CSS were calculated by the Kaplan–Meier method, and comparisons were conducted with log-rank test. Univariate and multivariate COX analyses were performed to identify independent risk factors associated with OS or CSS. Additionally, in order to compare the efficacy between surgery and surgery plus chemotherapy in patients with invasive IPMN, propensity score matching (PSM) analysis was utilized to balance the baseline characteristics and increase between group comparability (14, 15). A p-value <0.05 was considered statistically significant. All analyses were performed by SPSS, version 26.0 and R version 4.0.3.
During the study period, a total of 2,505 patients diagnosed with invasive IPMN (N = 2,300) or invasive MCN (N = 205) between 2000 and 2018 in the United States with complete data were identified and analyzed in our study according to the inclusion and exclusion criteria. In the invasive IPMN cohort, most of the cases were localized at the pancreatic head (62.7%), with single tumor (94.0%), and only 42.6% received surgery. While in the invasive MCN cohort, the vast majority of patients were female (76.1%), younger than 70 years old (67.3%), white (76.1%), with single tumor (90.7%), and more than 85% had a surgical resection performed. In addition, invasive MCNs were more commonly found in the pancreatic body and tail (68.3%). As for the tumor characteristics, the tumor diameters were 4.26 ± 3.52 and 6.87 ± 8.08 cm in the invasive IPMN and invasive MCN groups, respectively. The most common tumor stage at presentation was distant in patients with invasive IPMN while localized in patients with invasive MCN. Unknown regional node status in these two cohorts accounted for 54.8 and 23.4%, respectively. The more detailed demographic and treatment data are summarized in Table 1. As presented in Table 1, the variables including gender, age at diagnosis, tumor size, tumor location and number, tumor stage, regional node status, distant metastasis, and treatment modalities were significantly different between invasive IPMN and MCN cohorts.
Of all patients in our study, both the OS and CSS were significantly better in the invasive MCN cohort compared to the invasive IPMN cohort (Figure 1). Among the patients with invasive IPMN, the 1-, 3-, and 5-year OS was 46.3, 22.2, and 16.6%, respectively. With respect to patients with invasive MCN, the 1-, 3-, and 5-year OS was 69.9, 49.7, and 45.3%, respectively. The median OS was 11 months for invasive IPMN and 36 months for invasive MCN. As for CSS, the survival probability at 1, 3, and 5 years was 48.3, 24.2, and 19.3% in the IPMN cohort, respectively, and 73.6, 55.2, and 52.4% in the MCN cohort, respectively. The median CSS was more favorable in the MCN group than in the IPMN group (Table 2).
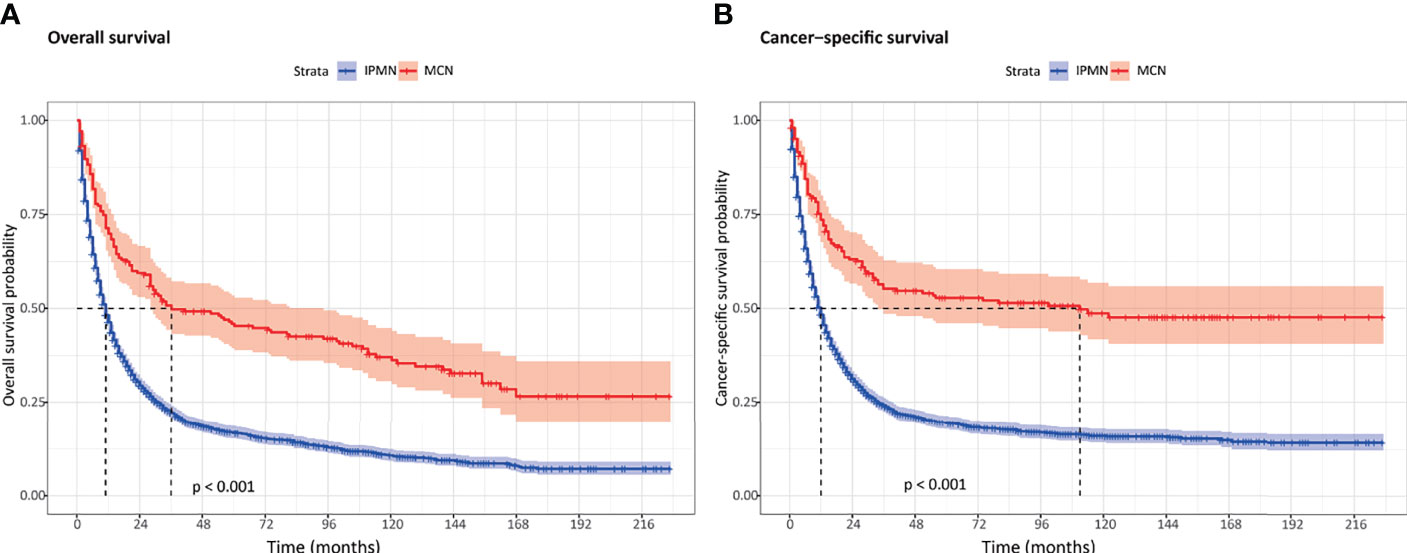
Figure 1 The overall survival and cancer-specific survival between invasive intraductal papillary mucinous neoplasm and invasive mucinous cystic neoplasm. (A) Overall survival. (B) Cancer-specific survival.
As shown in Figure 2, the survival outcomes in all prespecified subgroups were estimated and compared according to age at diagnosis, sex, race, tumor location, tumor grade, and tumor stage, respectively. Significant differences were observed for OS in the subgroup analysis except for male, of tumor located at the pancreatic head, and distant disease (Figure 2). The 1-, 3-, and 5-year OS and CSS probabilities at different stages are summarized in Table 3.
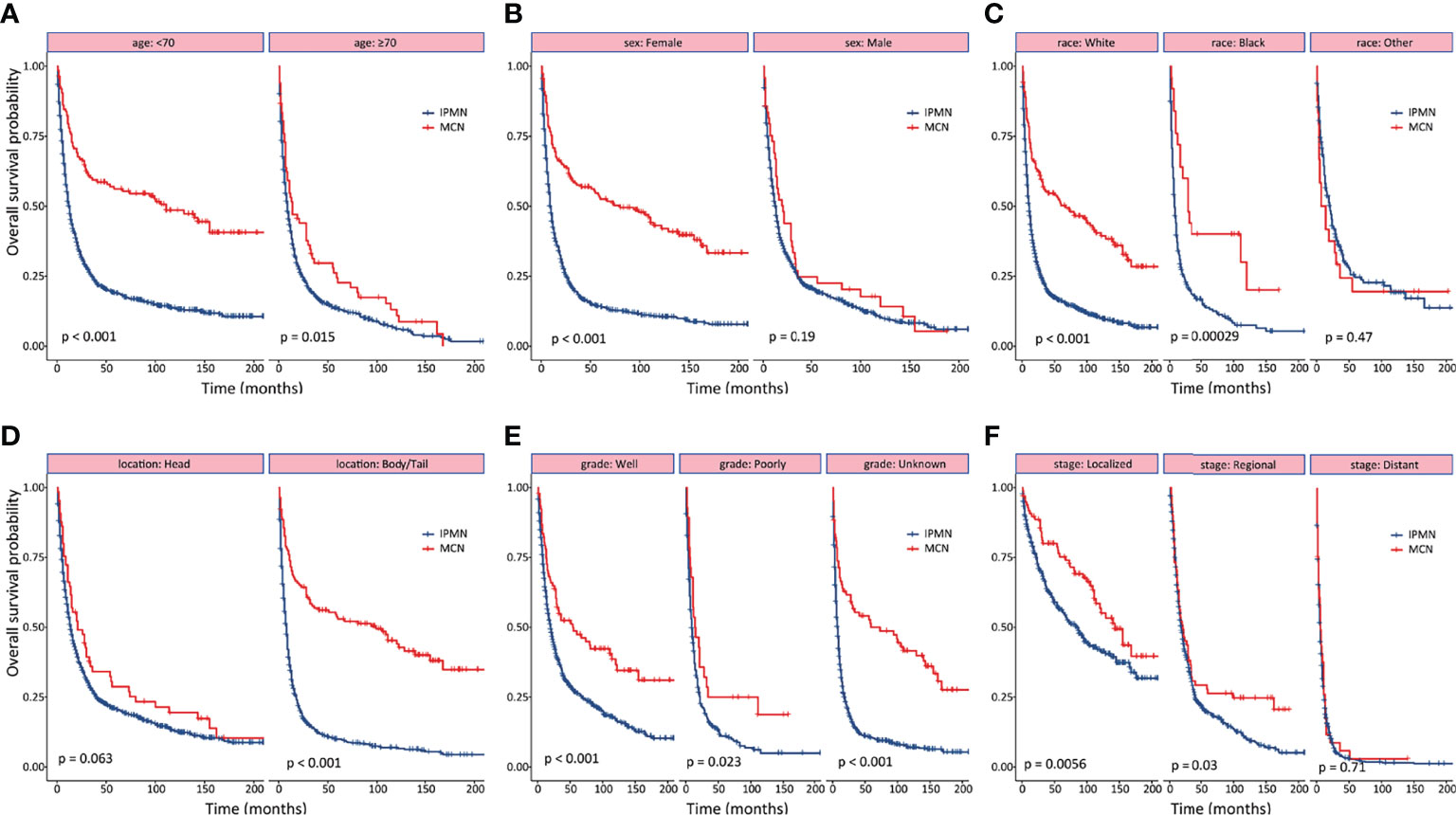
Figure 2 Subgroup analyses of overall survival between patients with invasive intraductal papillary mucinous neoplasm and invasive mucinous cystic neoplasm. (A) age. (B) gender. (C) race. (D) tumor location. (E) tumor differentiation. (F) tumor stage.
The treatment patterns of the different stages are summarized in Table 4. Surgery remained the main treatment option for patients with localized disease in the invasive IPMN cohort, whereas chemotherapy was more commonly performed in the distant disease. IPMN patients at a localized stage who only underwent surgery were found to associate with a similar overall survival compared to surgery plus other treatment, whereas surgery plus chemotherapy provided survival benefits in patients with regional disease (Figures 3A, B). However, patients with distant disease were associated with poor prognosis, and there were no effective treatments as yet (Figure 3C). In terms of the patients with invasive MCN, we found that surgery yielded comparable survival results compared to surgery plus other treatments (Figure 3D).
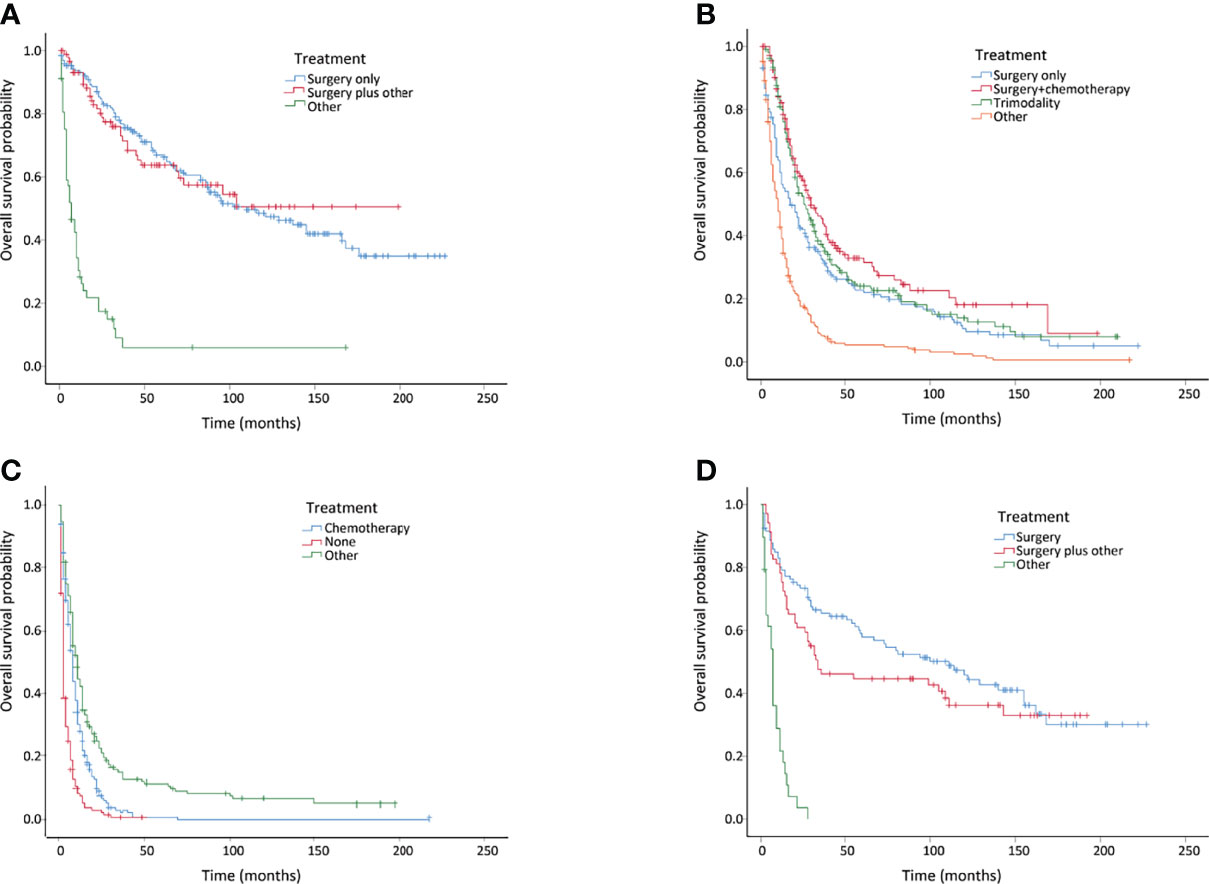
Figure 3 Kaplan–Meier curves of overall survival in invasive intraductal papillary mucinous neoplasm (IPMN) and invasive mucinous cystic neoplasm (MCN) patients with different treatments. (A) Invasive IPMNs with localized disease. (B) Invasive IPMNs with regional disease. (C) Invasive IPMNs with distant disease. (D) Entire invasive MCNs.
On multivariate analysis of patients with invasive IPMN (n = 2,300), age at diagnosis, race, marital status, tumor location, tumor grade, tumor stage, regional nodes status, liver involvement, surgery, and radiation were significant prognostic factors for OS. With respect to OS in patients with invasive MCN, age at diagnosis, tumor grade, tumor stage, and surgery were independent risk factors (Table 5).
In order to examine the survival benefits of surgery plus chemotherapy in patients with invasive IPMN, we compared the therapeutic efficacy between surgery and surgery plus chemotherapy using the PSM method. Before PSM, age at diagnosis, tumor grade, and tumor stage were significantly different between the two cohorts. Surgery plus chemotherapy was more frequently performed in patients with older age (≥70), poorly differentiated tumor, and advanced stages. The overall survival was similar in the unmatched cohorts (Figure 4A). After PSM, 234 patients were matched in each group, and the baseline characteristics were well balanced between these two groups. As can be seen in Table 6, the vast majority of patients was at the regional and distant stages. Using matched data, surgery plus chemotherapy was associated with survival advantages than surgery alone for selected invasive IPMN patients (P = 0.0072) (Figure 4B).
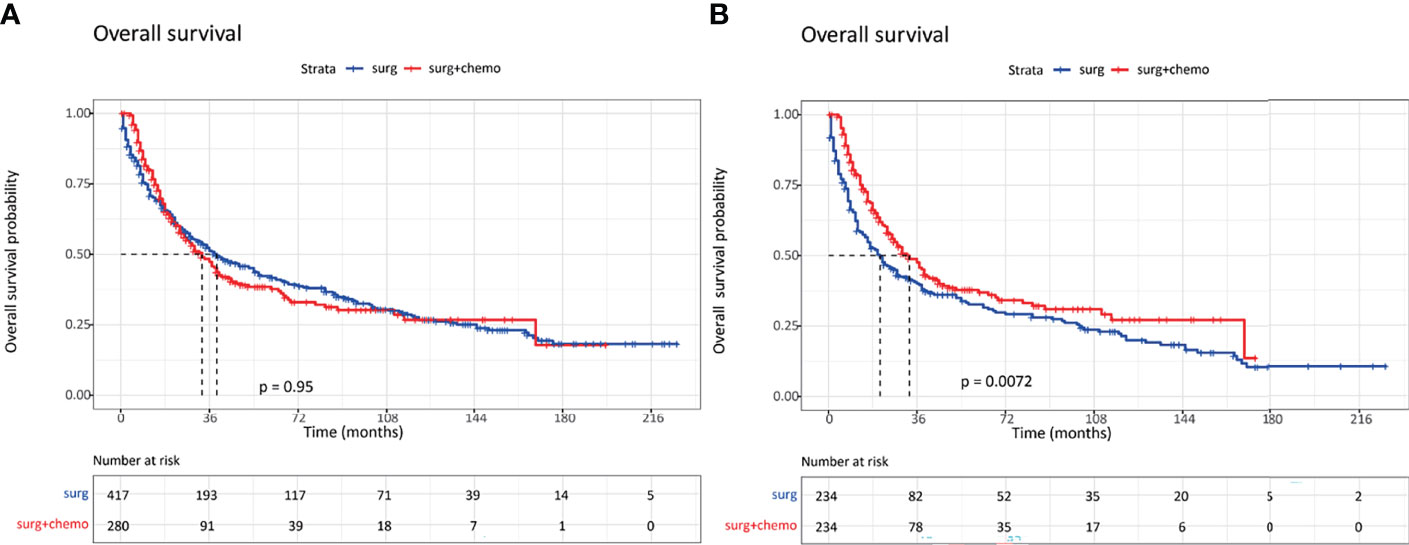
Figure 4 Kaplan–Meier curves of overall survival in invasive intraductal papillary mucinous neoplasm patients with surgery alone and surgery plus chemotherapy. (A) Survival curves in unmatched patients. (B) Survival curves in matched patients.
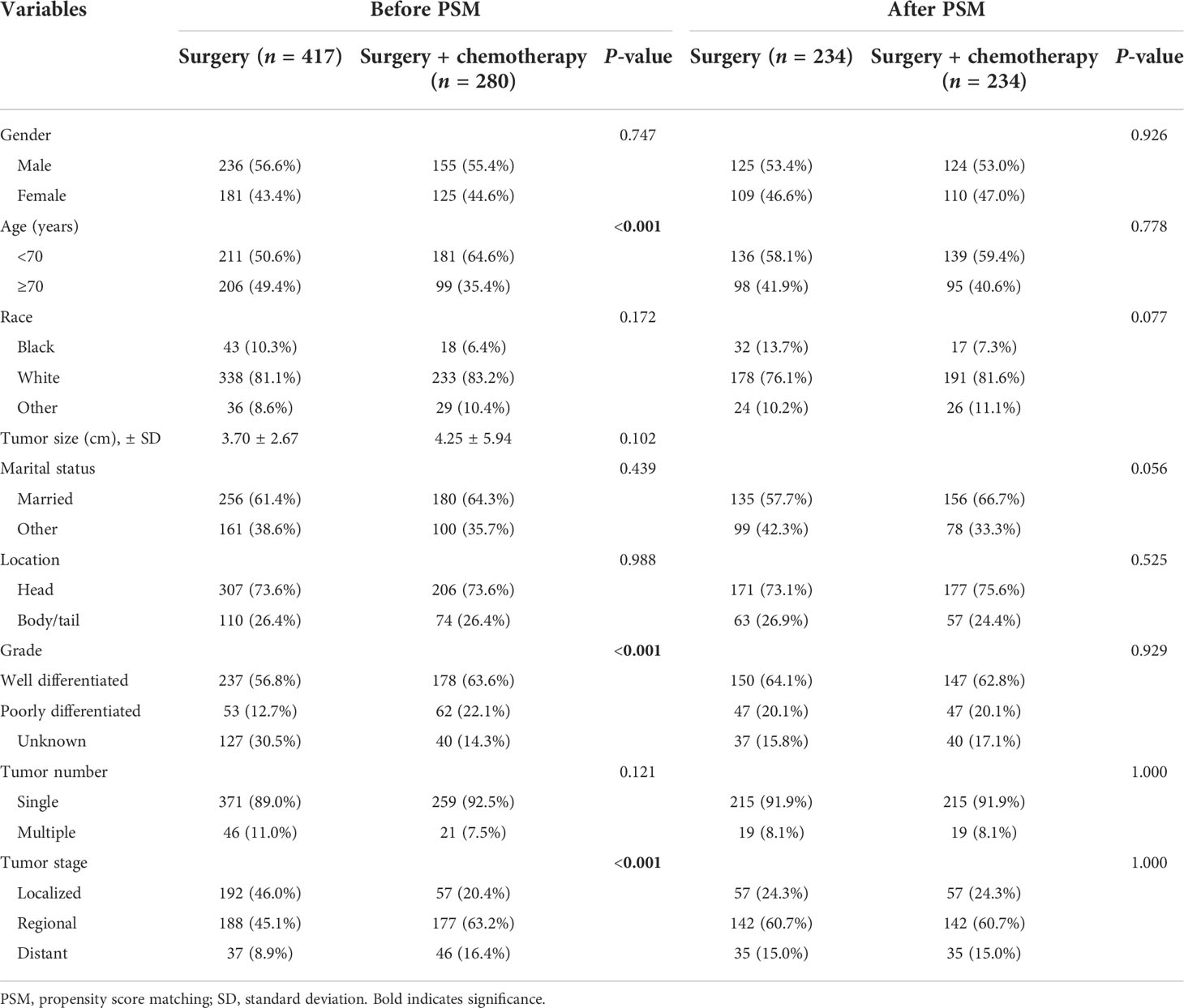
Table 6 Demographic and clinical characteristics of IPMN patients undergoing surgery or surgery plus chemotherapy before and after PSM.
IPMN and MCN are the most common pancreatic cystic neoplasms. Differences in clinical characteristics and prognosis have not been well investigated in a large-cohort study. The current study demonstrated that the demographic and clinical characteristics were significantly different between invasive IPMN and invasive MCN using the SEER database. Compared with invasive IPMN patients, invasive MCN patients were more likely to be younger, female, with a larger tumor size, and with tumors of the pancreatic body or tail, and patients with invasive IPMN presented at more advanced tumor stages and were associated with worse long-term survival outcomes compared to those with invasive MCN. With respect to the management of invasive IPMN, surgery remained the cornerstone of treatment for localized stage patients, while surgery plus chemotherapy may provide additional survival benefits in selected patients after adjusting for baseline characteristics, especially in patients with advanced stages.
Among the clinical variables, female sex predominance was observed in the invasive MCN cohort, which was in line with previous studies (16, 17), while the incidence rates of invasive IPMN were similar in both sexes. However, the exact cause of gender distribution was not well learned. Further studies are required to fully understand the gender differences of these two diseases.
It is noteworthy that the tumor size in patients with invasive MCN was significantly larger compared to that in patients with invasive IPMN. However, the survival outcomes were even worse in invasive IPMN patients, and the tumor size was not significantly associated with overall survival in invasive MCN patients. The underlying reason for the association between tumor size and prognosis remained unclear. Previous studies suggested that larger MCNs tend to be symptomatic and more indolent. Compared to invasive IPMN, invasive MCN was more commonly associated with a lower aggressive behavior (18).
In survival analysis, we found that the OS and CSS were significantly better in patients with invasive MCN compared to those with invasive IPMN. Interestingly, among patients who were with older age (≥70), male, other race, of tumors in the pancreatic head, and with distant disease, no significant survival differences were observed between invasive IPMN and MCN cohorts. This might be attributed to the limited samples of invasive MCN in these subgroups. Further investigations to account for this discrepancy based on a large sample size are needed to conduct a more specific and detailed subgroup analysis.
On the basis of available guidelines and consensus, surgical resection for resectable and borderline-resectable invasive IPMN patients has been strongly recommended due to the encouraging survival results following surgery (19, 20). In our study, a subgroup analysis of overall survival was performed according to the tumor stage. As shown in the stage-matched survival analysis, patients who only underwent surgery have a more favorable prognosis than those who received other treatments in localized stage. As for the regional stage, surgery plus chemotherapy tended to result in significantly better overall survival. In terms of the patients with invasive MCN, surgery alone was associated with a similar overall survival compared to surgery plus chemotherapy in our study. According to the 2015 AGA and 2017 IAP guidelines (11, 12), surgery was strongly recommended for all MCNs regardless of the tumor diameter, whereas the 2018 European guideline suggested that surgical operations should only be performed in patients with a tumor size larger than 4 cm or with the presence of a nodule. Some studies argued that a conservative management for asymptomatic MCNs was feasible (21). A systematic review including 52 papers was conducted to investigate the natural history and prognosis of pancreatic MCNs, showing that the surveillance of MCNs less than 4 cm in size appeared to be acceptable and safe (22). Our study displayed that more than 85% of MCNs received surgery between 2000 and 2018, and the long-term survival outcomes were satisfactory. Given the small but measurable risk of malignant transformation, the observation strategy should be undertaken with caution. Further high-quality studies are needed to develop the optimal treatment strategies of invasive MCN.
In our large population-based analysis of 2,300 patients with invasive IPMN, multivariate COX regression analysis revealed that surgery was associated with improved overall survival, while chemotherapy was not the independent risk factor for overall survival in the entire study cohort. Several studies have substantiated the prognostic significance of surgical treatment. Unlike pancreatic ductal carcinoma, the impact of chemotherapy in patients with invasive IPMN is still a matter of debate. Within a study including 102 invasive IPMN patients treated between 1990 and 2016, Marchegiani et al. demonstrated that adjuvant chemotherapy could improve survival only in patients with positive nodal status and tubular differentiation (23). In another retrospective study on 103 patients collected from 1993 to 2018, Rodrigues et al. examined the impact of adjuvant chemotherapy and found that it could not provide survival benefits in node-negative patients but even compromise the prognosis (24). However, a systematic review of 11 studies and 3,393 invasive IPMNs found that node-positive patients could benefit from adjuvant chemotherapy (25). In order to determine the potential role of chemotherapy in the invasive IPMN cohort, we assessed the outcomes between patients who underwent surgery alone and those who received surgery plus chemotherapy after adjusting for confounding by the PSM method. Using matching data, we found that surgery plus chemotherapy could confer survival benefits in invasive IPMN patients with older age and advanced stages. Our findings confirmed that the combination of surgery and chemotherapy would translate into survival benefits for these selected patients.
There were some limitations in our study. Firstly, the current study was limited by the retrospective design. Secondly, some important factors related to long-term survival were lacking, such as patients’ performance status, surgical type and approach, underlying diseases, and comorbidities. Thirdly, disease recurrence and information on treatment schemes were not recorded in the SEER database. Finally, our analysis was based on populations in the United States, which might limit the generalization to other regions.
In conclusion, our study comprehensively investigated the clinicopathologic features, treatment patterns, and survival outcomes of patients with the two common subtypes of pancreatic cystic neoplasms, invasive IPMN and invasive MCN. While invasive MCN experienced better overall survival compared to invasive IPMN in the subgroups of patients with local–regional disease, the survival advantages disappeared in patients with distant disease. Furthermore, we found that the combination of surgery and chemotherapy was associated with survival benefits in selected invasive IPMN patients, especially those with older age and advanced tumor stages.
Publicly available datasets were analyzed in this study.These data can be found here: https://seer.cancer.gov/.
The studies involving human participants were reviewed and approved by the Qingdao Municipal Hospital. The current study is based on the SEER database, and the requirements for informed consent were waived off due to the retrospective design.
Conception and data collection: GS. Drafting and statistical analysis: ZY. All authors contributed to the article and approved the submitted version.
This study was supported by the Hepatobiliary and Pancreatic Cancer from Hubei Chen Xiaoping Foundationfor Scientific and Technological Development.(no. CXPJJH11900001-2019206)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. van Huijgevoort NCM, Del Chiaro M, Wolfgang CL, van Hooft JE, Besselink MG. Diagnosis and management of pancreatic cystic neoplasms: current evidence and guidelines. Nat Rev Gastroenterol Hepatol (2019) 16(11):676–89. doi: 10.1038/s41575-019-0195-x
2. Chandwani R, Allen PJ. Cystic neoplasms of the pancreas. Annu Rev Med (2016) 67:45–57. doi: 10.1146/annurev-med-051914-022011
3. Burk KS, Knipp D, Sahani DV. Cystic pancreatic tumors. Magn Reson Imaging Clin N Am (2018) 26(3):405–20. doi: 10.1016/j.mric.2018.03.006
4. McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol (2018) 24(43):4846–61. doi: 10.3748/wjg.v24.i43.4846
5. Keane MG, Afghani E. A review of the diagnosis and management of premalignant pancreatic cystic lesions. J Clin Med (2021) 10(6). doi: 10.3390/jcm10061284
6. Ketwaroo GA, Mortele KJ, Sawhney MS. Pancreatic cystic neoplasms: An update. Gastroenterol Clin North Am (2016) 45(1):67–81. doi: 10.1016/j.gtc.2015.10.006
7. Del Chiaro M, Verbeke C, Salvia R, Klöppel G, Werner J, McKay C, et al. European Experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis (2013) 45(9):703–11. doi: 10.1016/j.dld.2013.01.010
8. Hasan A, Visrodia K, Farrell JJ, Gonda TA. Overview and comparison of guidelines for management of pancreatic cystic neoplasms. World J Gastroenterol (2019) 25(31):4405–13. doi: 10.3748/wjg.v25.i31.4405
9. Lanke G, Lee JH. Similarities and differences in guidelines for the management of pancreatic cysts. World J Gastroenterol (2020) 26(11):1128–41. doi: 10.3748/wjg.v26.i11.1128
10. European Study Group on Cystic Tumours of the Pancreas. European Evidence-based guidelines on pancreatic cystic neoplasms. Gut (2018) 67(5):789–804. doi: 10.1136/gutjnl-2018-316027
11. Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology (2017) 17(5):738–53. doi: 10.1016/j.pan.2017.07.007
12. Vege SS, Ziring B, Jain R, Moayyedi P. American Gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology (2015) 148(4):819–822; quize812-813. doi: 10.1053/j.gastro.2015.01.015
13. Doll KM, Rademaker A, Sosa JA. Practical guide to surgical data sets: Surveillance, epidemiology, and end results (SEER) database. JAMA Surg (2018) 153(6):588–9. doi: 10.1001/jamasurg.2018.0501
14. Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg (2018) 53(6):1112–7. doi: 10.1093/ejcts/ezy167
15. Kuss O, Blettner M, Börgermann J. Propensity score: an alternative method of analyzing treatment effects. Dtsch Arztebl Int (2016) 113(35-36):597–603. doi: 10.3238/arztebl.2016.0597
16. Luo G, Fan Z, Gong Y, Jin K, Yang C, Cheng H, et al. Characteristics and outcomes of pancreatic cancer by histological subtypes. Pancreas (2019) 48(6):817–22. doi: 10.1097/mpa.0000000000001338
17. Reid MD, Choi H, Balci S, Akkas G, Adsay V.. Serous cystic neoplasms of the pancreas: clinicopathologic and molecular characteristics. Semin Diagn Pathol (2014) 31(6):475–83. doi: 10.1053/j.semdp.2014.08.009
18. Griffin JF, Page AJ, Samaha GJ, Christopher A, Bhaijee F, Pezhouh MK, et al. Patients with a resected pancreatic mucinous cystic neoplasm have a better prognosis than patients with an intraductal papillary mucinous neoplasm: A large single institution series. Pancreatology (2017) 17(3):490–6. doi: 10.1016/j.pan.2017.04.003
19. Hirono S, Yamaue H. Surgical strategy for intraductal papillary mucinous neoplasms of the pancreas. Surg Today (2020) 50(1):50–5. doi: 10.1007/s00595-019-01931-5
20. Bhardwaj N, Dennison AR, Maddern GJ, Garcea G. Management implications of resection margin histology in patients undergoing resection for IPMN: A meta-analysis. Pancreatology (2016) 16(3):309–17. doi: 10.1016/j.pan.2016.02.008
21. Talukdar R, Nageshwar Reddy D. Treatment of pancreatic cystic neoplasm: surgery or conservative? Clin Gastroenterol Hepatol (2014) 12(1):145–51. doi: 10.1016/j.cgh.2013.08.031
22. Nilsson LN, Keane MG, Shamali A, Millastre Bocos J, Marijinissen van Zanten M, Antila A, et al. Nature and management of pancreatic mucinous cystic neoplasm (MCN): A systematic review of the literature. Pancreatology (2016) 16(6):1028–36. doi: 10.1016/j.pan.2016.09.011
23. Marchegiani G, Andrianello S, Dal Borgo C, Secchettin E, Melisi D, Malleo G, et al. Adjuvant chemotherapy is associated with improved postoperative survival in specific subtypes of invasive intraductal papillary mucinous neoplasms (IPMN) of the pancreas: it is time for randomized controlled data. HPB (Oxford) (2019) 21(5):596–603. doi: 10.1016/j.hpb.2018.09.013
24. Rodrigues C, Hank T, Qadan M, Ciprani D, Mino-Kenudson M, Weekes CD, et al. Impact of adjuvant therapy in patients with invasive intraductal papillary mucinous neoplasms of the pancreas. Pancreatology (2020) 20(4):722–8. doi: 10.1016/j.pan.2020.03.009
Keywords: IPMN, MCN, clinical characteristic, survival, treatment
Citation: Yang Z and Shi G (2022) Comparison of clinicopathologic characteristics and survival outcomes between invasive IPMN and invasive MCN: A population-based analysis. Front. Oncol. 12:899761. doi: 10.3389/fonc.2022.899761
Received: 19 March 2022; Accepted: 27 June 2022;
Published: 29 July 2022.
Edited by:
Ravindra Deshpande, Wake Forest School of Medicine, United StatesReviewed by:
Hassan Alkharaan, Prince Sattam Bin Abdulaziz University, Saudi ArabiaCopyright © 2022 Yang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangjun Shi, c2dqenBAaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.