- 1Gastroenterology and Urology Department II, Hunan Cancer Hospital and the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
- 2Department of Lamphoma and Abdominal Radiotherapy, Hunan Cancer Hospital and the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
- 3Department of Hepatobiliary and Intestinal Surgery, Hunan Cancer Hospital and the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
- 4Department of Gastroduodenal and Pancreatic Surgery, Hunan Cancer Hospital and the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
Objective: To investigate the association between body mass index (BMI) and overall survival (OS) of patients with stage II/III gastric cancer (GC) after radical gastrectomy, and evaluate the potential influence of perioperative adjuvant chemotherapy (PAC).
Methods: Medical records of 2,510 consecutive stage II/III GC patients who underwent curative resection between November 2010 and December 2020 were retrospectively reviewed. The optimal cutoff value of BMI for OS was determined by X-tile. The independent predictive factors for completeness of PAC were identified using univariate and multivariate logistic regression analyses. Cox regression analyses assessed the association among BMI, completeness of PAC, and OS.
Results: Of the 2,510 patients, 813 cases with BMI < 20.3 kg/m2 were classified as belonging in the low BMI group. Further analyses confirmed that low BMI was an independent predictor for incomplete PAC (< 6 cycles, n = 920) and poorer OS (hazard ratio: 1.317, 95% confidence interval: 1.162-1.494, P < 0.001), but neo-adjuvant chemotherapy (NAC) was a protective factor. An additive effect was found in those with both low BMI and incomplete PAC, as they had even worse OS. However, in patients with low BMI, completion of PAC (≥ 6 cycles) significantly improved OS, which became comparable to that in the high BMI group (P = 0.143).
Conclusions: Low preoperative BMI independently affects completion of PAC and prognosis of patients with stage II/III GC, but completing PAC can compensate for the adverse influence of low BMI on OS. Thus, strategies designed to ensure the completion of PAC, such as NAC and nutritional support, should be further investigated.
Introduction
Gastric cancer (GC) ranks as the fifth for incidence and fourth for cancer-related deaths globally, with almost 50% occurring in China, and surgery being the only possible curative management so far (1, 2). Unfortunately, only a few patients in China and Western countries are diagnosed at an early stage. Even after undergoing D2 gastrectomy, relapse can be seen within 5 years in about half of those with stage II or III GC (3). To improve overall survival (OS), perioperative adjuvant chemotherapy (PAC), including both pre- and/or postoperatively, has been considered as standard care (4, 5). However, many patients cannot complete all of the allocated courses of chemotherapy for various reasons, such as severe morbidity due to surgery, poor nutritional status or chemotherapy-induced adverse events. In fact, nearly a half of all patients could not complete the planned perioperative management even in recent prospective phase 3 studies (6–8). In one of our previous studies, which included 1,288 stage II/III GC patients (9), only 31.5% completed ≥ 6 cycles of PAC. Further analyses confirmed that completion of at least 6 cycles of PAC was significantly associated with prolonged cancer-specific survival. This was echoed by Noh et al. (3) who reported that those with high relative dose intensity (≥ 6 courses of regimens) had significantly better outcomes in a second post-hoc analysis of the well-known CLASSIC study.
There is a growing body of evidence showing that nutritional status not only relates to postoperative morbidity but also oncological outcomes of different types of cancer (9–12). Body mass index (BMI) is a simple and commonly used indicator for assessing nutritional status in the clinic, and several studies have found that low BMI is a significant predictor of poor prognosis in GC patients (13–15). However, some researchers argued that BMI was not related to oncological outcomes (16, 17). While possible explanations for the discordant results include the inconsistency in patient inclusion and BMI classification criteria, the relationship between BMI and prognosis of GC needs further clarification.
Considering that malnutrition also significantly influences chemotherapy-induced adverse events, as well as the completion of PAC (9, 11, 18, 19), we hypothesized that low BMI would be a useful predictor of poor compliance with PAC. Therefore, in this large sample-size study from a high-volume center, we retrospectively assessed the influence of low BMI on the completion of PAC in patients with stage II or III GC. We also explored whether completing PAC could compensate for the adverse impact of low BMI on survival.
Patients and Methods
Patients
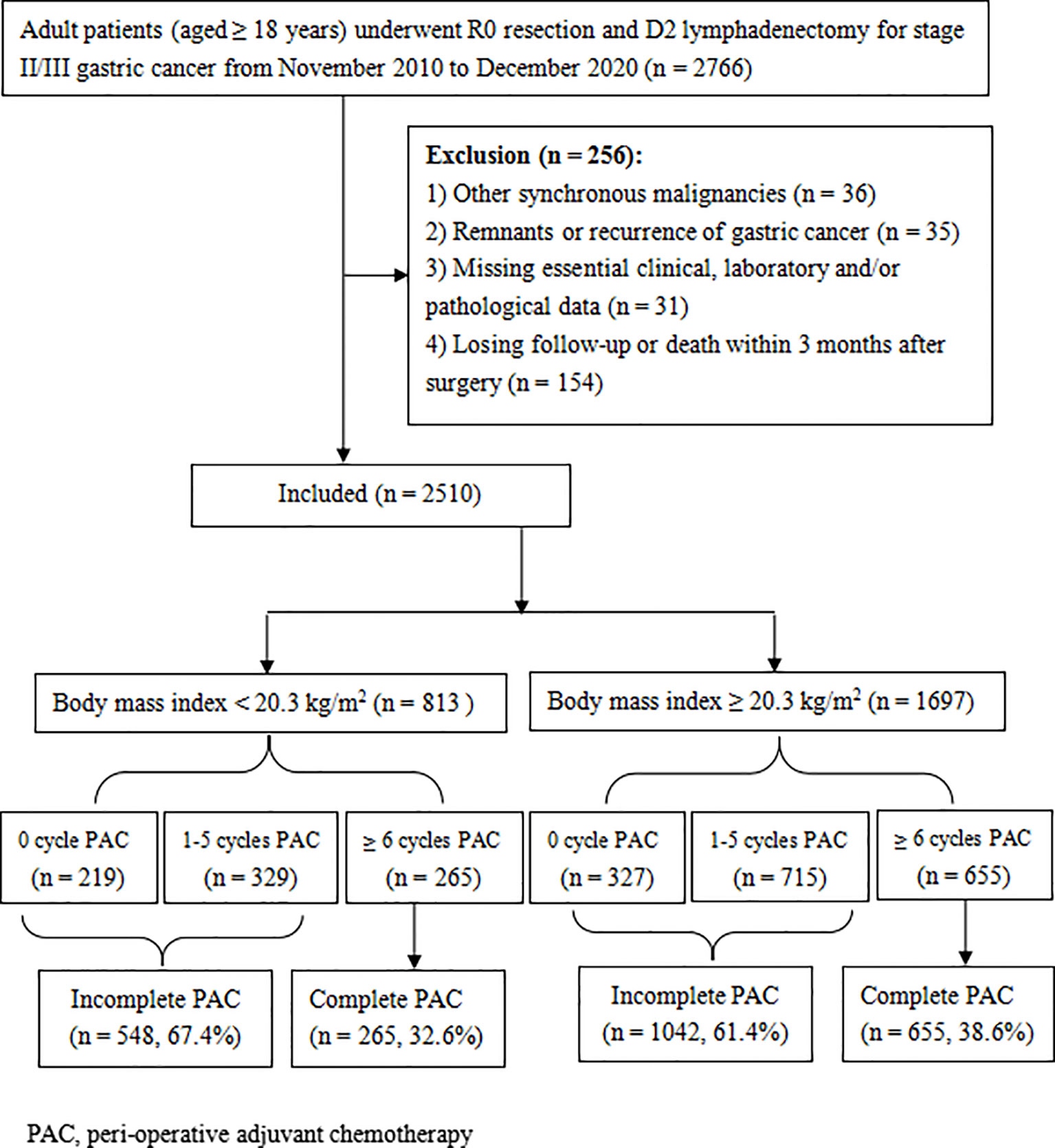
The medical records of all adult patients (≥ 18 years old) who received radical gastrectomy and D2 lymphadenectomy for stage II/III gastric adenocarcinoma in Hunan Cancer Hospital between November 2010 and December 2020 were retrospectively reviewed. The flowchart and exclusion criteria for this study are described in Figure 1. This study was conducted in accordance with the guidelines laid down in the Declaration of Helsinki and approved by the ethics committee of the Hunan Cancer Hospital (No. 16 in 2022). Written informed consent for gastrectomy, and the use of their clinical data, has been obtained from all patients prior to surgery.
Perioperative Management and Follow-Up
Surgeons with sufficient experience performed or supervised all surgeries, according to the Japanese gastric cancer treatment guidelines (5) and staged by the 8th edition of TNM classifications (20). D2 gastrectomy and postoperative adjuvant chemotherapy (AC) was applied to the overwhelming majority of stage II/II GC patients in our institution, as described in our previous studies (9, 21), except for a few patients with cT3-4/N+ diseases, who received 2 to 4 cycles of neo-adjuvant chemotherapy (NAC) before surgery, using platinum and fluorouracil based regimens such as FLOT and XELOX (3, 22). Fluorouracil and platinum-based AC was generally initiated about 3 to 4 weeks following resection and lasted for half a year (3, 22, 23).
Postoperative adverse events were diagnosed within 30 days following gastrectomy and staged by the Clavien-Dindo classification system (21, 24). Patients were followed-up at 1 month after gastrectomy, and once every quarter in the first 2 years, then every half year for the 3rd to 5th year, and once a year thereafter, through December 2021. At each follow-up, patients underwent physical and laboratory measurements, ultrasonography, or a CT scan, and endoscopy was recommended every 2 years.
Evaluation
Clinicopathological data, including patients’ height and weight, were obtained within 7 days prior to surgery. BMI was calculated as body weight divided by square of height (kg/m2), and the cut-off BMI value for OS was selected by X-tile, as described in our previous study (25). For other variables such as age, albumin, and hemoglobin levels, generally accepted or standard clinical thresholds were used. Complete PAC was defined as receiving ≥ 6 cycles of adjuvant chemotherapy perioperatively, because patients receiving less than 6 cycles of PAC had significantly poorer prognosis, according to published literature (3, 9). The evaluated primary outcome was OS, which was defined as the time from gastrectomy until death or the last follow-up, whichever occurred first.
Statistical Analysis
Continuous variables were compared using Student’s t-test or Mann-Whitney U test, and are presented as mean and standard deviation (SD). Categorical data were compared by χ2 or Fisher exact test, and are described as numbers (%). Univariate and multivariate regression analyses were utilized to explore factors related to completeness of PAC. The optimal cutoff BMI value for OS was set by X-tile when reaching the maximum χ2 log-rank value. The differences of OS in subgroups were compared by Kaplan-Meier curves and log-rank test. Multivariate regression analyses using a forward conditional method were carried out for factors with a P-value < 0.05 after univariate analysis. Data was analyzed by SPSS 24.0 software (IBM Corporation, NY, USA) and a two-sided P value < 0.05 was considered as statistically significant.
Results
Clinicopathological Characteristics
A total of 2,510 consecutive patients were enrolled in this study, and their basic characteristics are presented in Table 1. The majority of patients were male (65.6%), with stage III disease (73.0%), who underwent subtotal gastrectomy (71.2%) by open procedure (73.3%). The mean age was 56.1 years (range 19 - 86), with a mean BMI of 21.88 kg/m2 (range 13.84 - 37.18), and the mean postoperative duration of hospital stay was 11.7 days (range 3 - 87). Two hundred and sixty-nine patients (10.7%) developed some morbidity within 30 days following resection, defined as Clavien-Dindo grade II or greater.
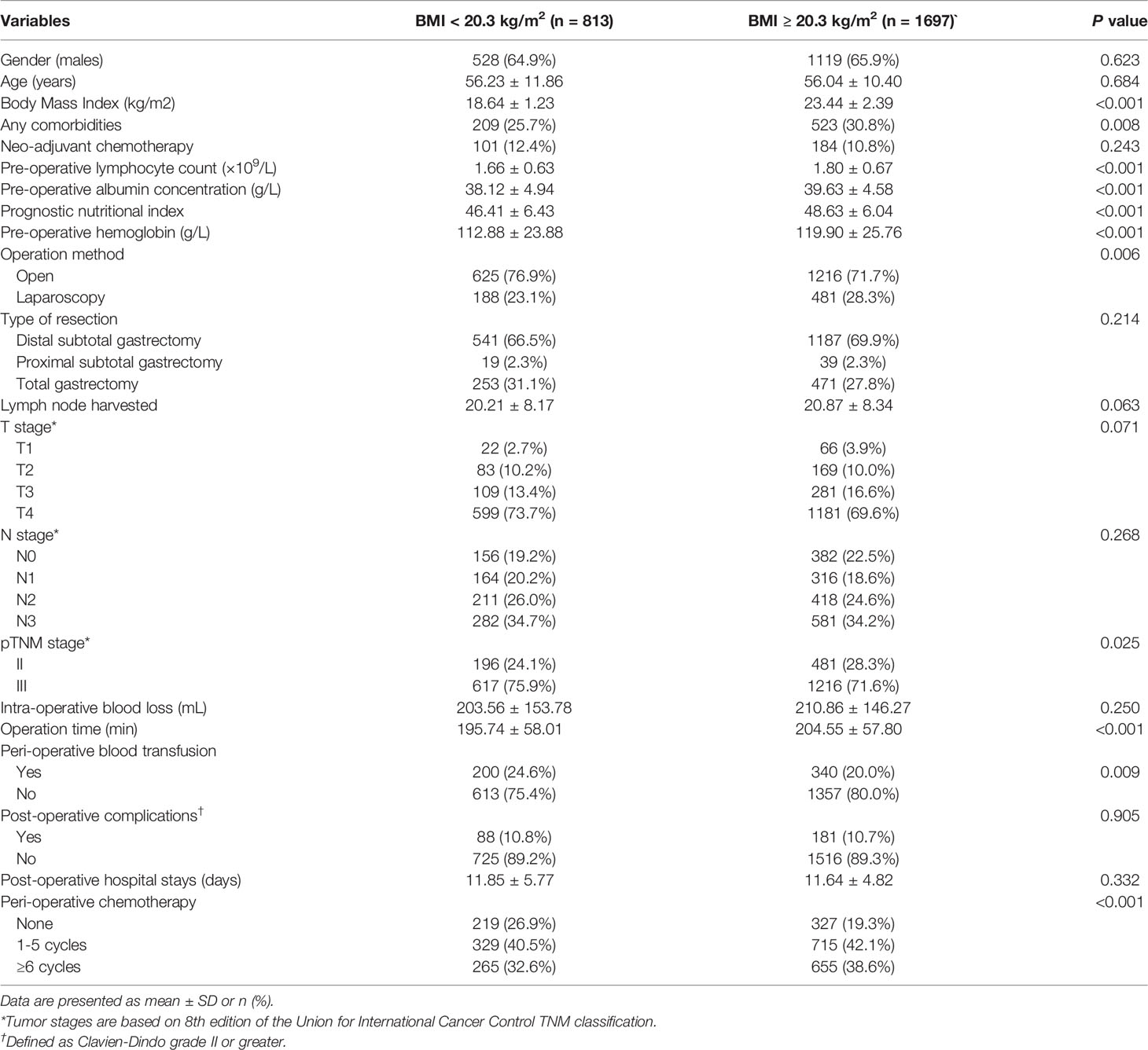
Table 1 Clinicopathological characteristics of the entire cohort, classified by body mass index (BMI) (n =2510).
The cutoff value of BMI for OS was selected as 20.3 kg/m2 by X-tile (Supplementary Figure 1). A total of 813 patients (32.4%) had a BMI of less than 20.3 kg/m2. As shown in Table 1, lower BMI was associated with poorer nutritional and immunological status (such as having lower albumin concentration and lymphocyte count), lower hemoglobin level, more advanced tumor stage, and being less likely to receive PAC.
Predictors for Incomplete PAC
Of a total of 2,510 patients, 1,964 (78.2%) received PAC, but only 920 cases completed at least 6 cycles (36.7%, complete PAC group). In contrast, the remaining 1,590 cases (63.3%) received none or 1 to 5 cycles of PAC, and were considered as incomplete PAC. Not surprisingly, oncological outcomes were significantly better in patients receiving complete PAC compared with those patients who received none or 1 to 5 cycles of PAC (the mOS were 108, 54, and 36 months, respectively, P < 0.001), regardless of having stage II or III disease (Supplementary Figure 2).
The clinicopathological variables were retrospectively analyzed to evaluate their potential influence on compliance with PAC. Univariate analyses revealed that age, BMI, albumin levels, NAC, operation procedure, operative time, perioperative blood transfusion, and tumor stage potentially affect the completeness of PAC (all P < 0.05). Further multivariate analyses confirmed that PAC completeness was negatively impacted only by older age (≥ 65 years), lower albumin level (< 35 g/L), and lower BMI (< 20.3 kg/m2), while NAC was identified as a protective factor (Table 2). In fact, 38.6% (655/1697) of patients with BMI ≥ 20.3 kg/m2 received at least 6 cycles of PAC, which was significantly better than that in the low BMI group (32.6%, 265/813, P = 0.003) (Figure 1).
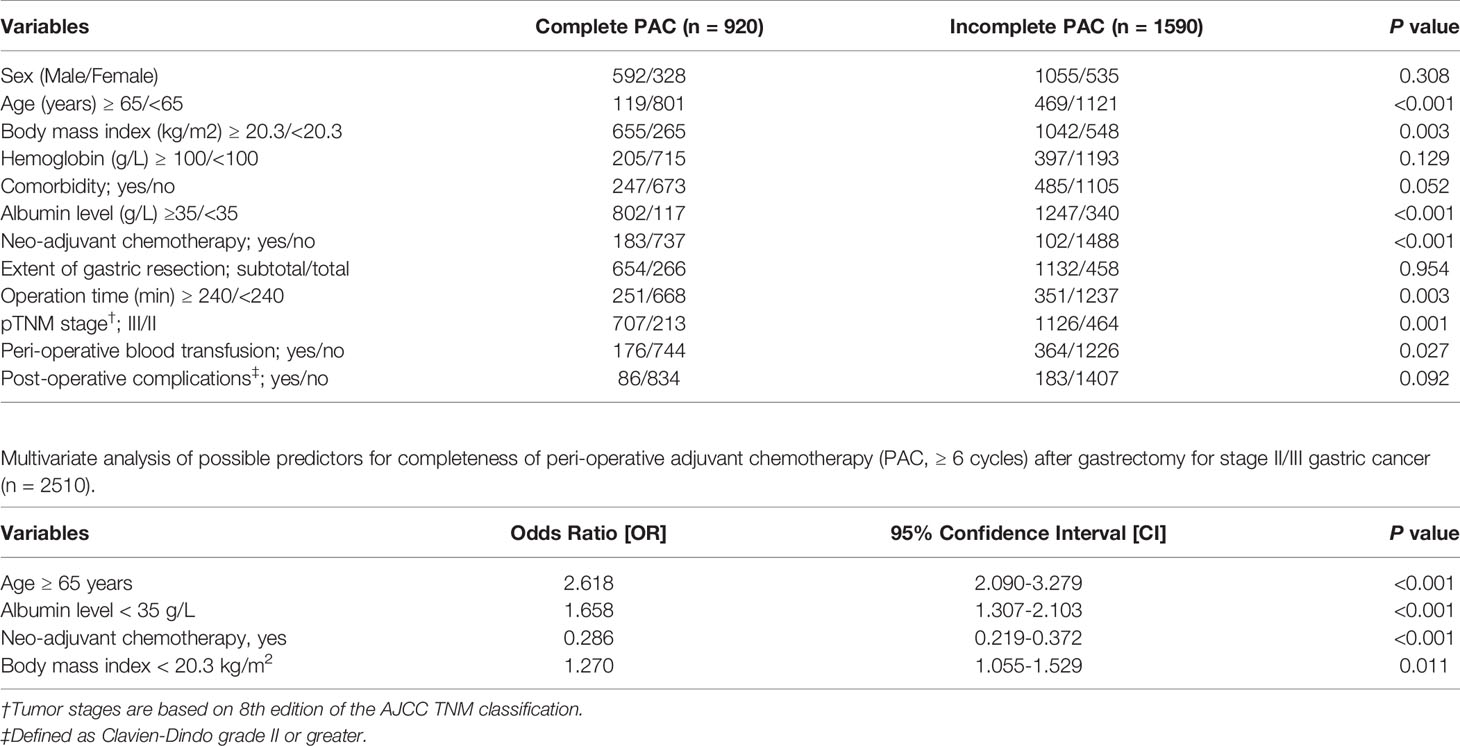
Table 2 Association between clinicopathological characteristics and completeness of peri-operative adjuvant chemotherapy (PAC, ≥ 6 cycles) after gastrectomy for stage II/III gastric cancer (n = 2510).
Predictors for OS
During a median follow-up of 28 months (range 4 - 132), 1,050 of 2,510 patients (41.8%) died, with a mOS of 63 months. Death was more common in patients with BMI < 20.3 kg/m2 (48.3%, 393/813) compared with that in patients with higher BMI (38.7%, 657/1697, P < 0.001).
Univariate analyses revealed that age, BMI, American Society of Anesthesiologist (ASA) score, albumin level, operation procedure, length of operation, intra-operative blood loss, type of resection, TNM stage, perioperative blood transfusion, postoperative complications, and PAC were potentially related to OS (all P < 0.05). After multivariate Cox regression analyses, BMI < 20.3 kg/m2 was confirmed to adversely affect OS (hazard ratio (HR): 1.317, 95% confidence interval (CI): 1.162 - 1.494, P < 0.001). In contrast, complete PAC (≥ 6 cycles) was confirmed to be a protective variable (HR: 0.527, 95% CI: 0.458 - 0.607, P < 0.001) (Table 3).
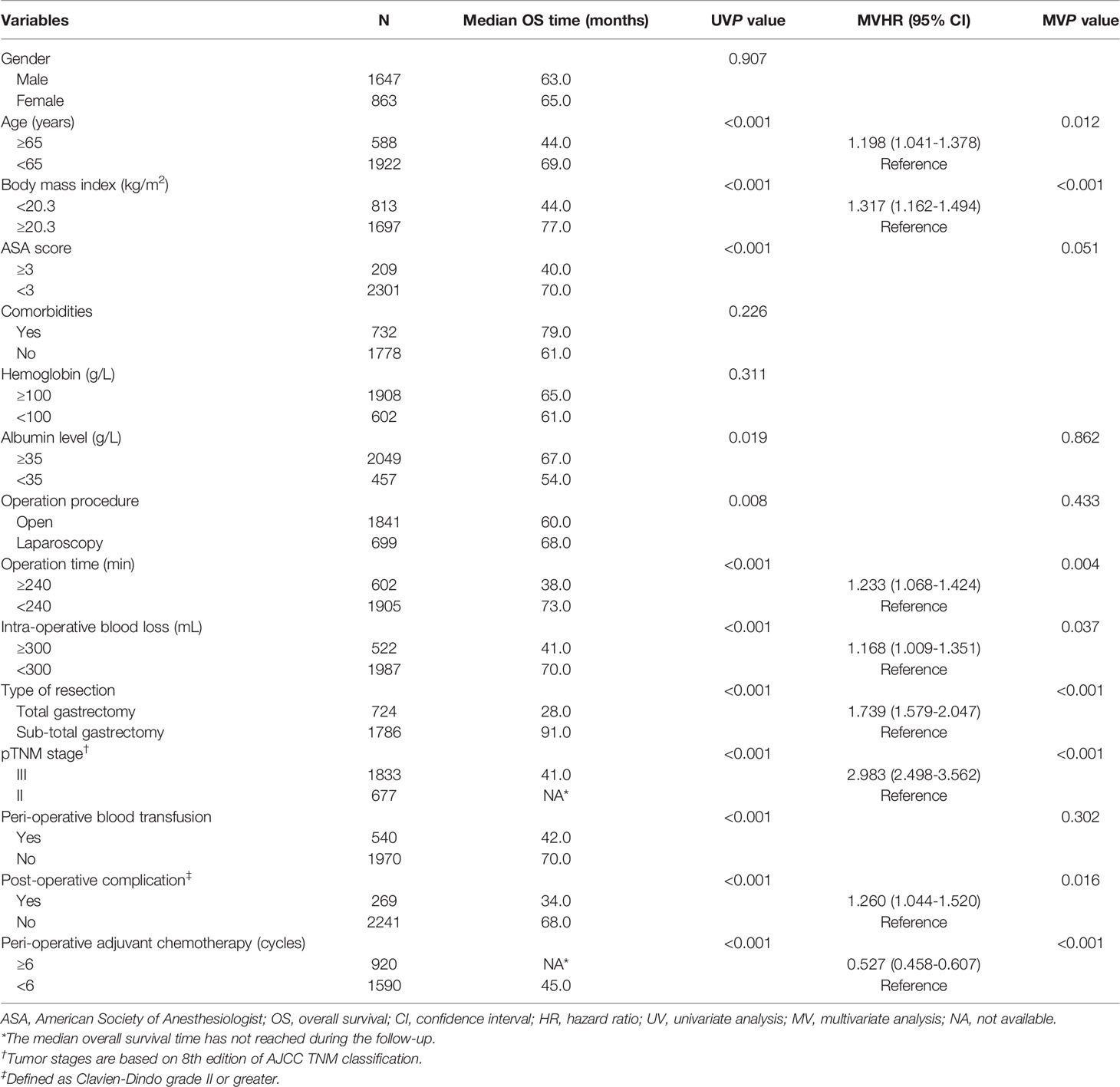
Table 3 Univariate and multivariate analyses of prognostic factors for overall survival following radical gastrectomy of stage II/III gastric cancer (n = 2510).
Relationship Among BMI, PAC, and OS
The mOS in patients with higher BMI (≥ 20.3 kg/m2) was 77 months, which was significantly better than 44 months in those with lower BMI (P < 0.001) (Figure 2A). Although the difference was still significant among patients receiving incomplete PAC (56 months vs. 34 months, P < 0.001, Figure 2B), mOS became comparable in patients who received at least 6 cycles of PAC, regardless of BMI (not available vs. 79 months, P = 0.143, Figure 2C). Results were similar when patients were classified to 3 subgroups according to the World Health Organization (WHO) classification of BMI (< 18.5, 18.5-24.9 and ≥ 25.0 kg/m2) (Figure 3).
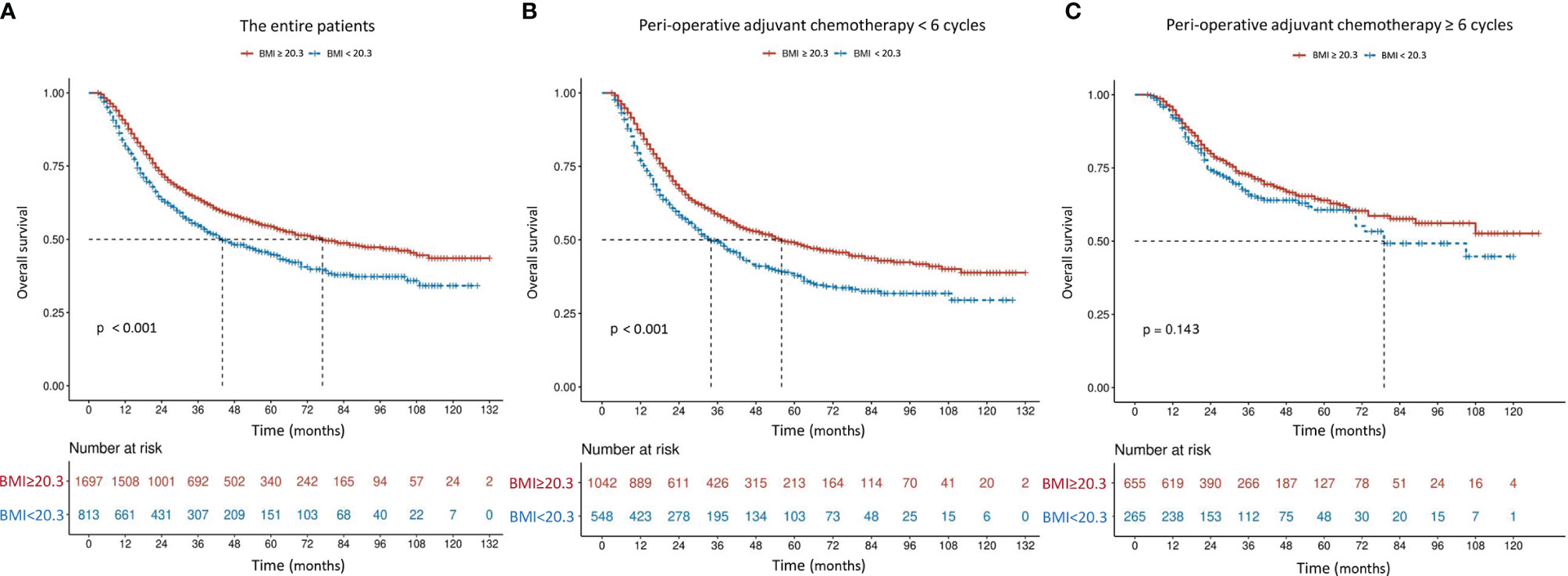
Figure 2 Overall survival curves in 2,510 patients who underwent curative resection for stage II/III gastric cancer classified by body mass index [BMI, < 20.3 or ≥ 20.3 kg/m2 (A)] and further stratified by perioperative adjuvant chemotherapy [< 6 or ≥ 6 cycles (B, C)]. The differences of overall survival in subgroups were compared by log-rank test.
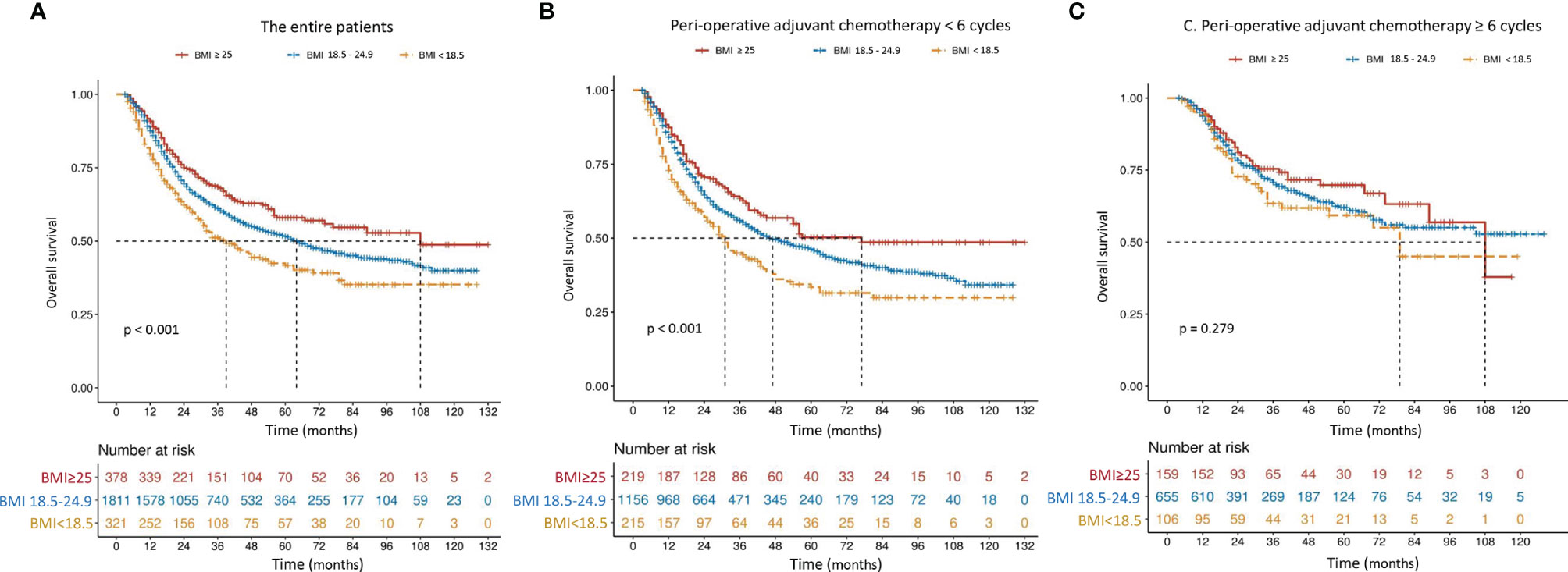
Figure 3 Overall survival curves in 2,510 patients who underwent curative resection for stage II/III gastric cancer classified by body mass index [BMI, < 18.5, 18.5-24.9 or ≥ 25.0 kg/m2 (A)] and further stratified by perioperative adjuvant chemotherapy [< 6 or ≥ 6 cycles (B, C)]. The differences of overall survival in subgroups were compared by log-rank test.
The mOS in patients with high BMI (≥ 20.3 kg/m2) who received complete PAC (≥ 6 cycles, n = 655) did not reach statistical significance when compared with that in patients with low BMI and complete PAC (79 months, n = 265, P = 0.145), but was significantly better than that in patients with high BMI and incomplete PAC (56 months, n = 1,042, P < 0.001), and in patients with low BMI and incomplete PAC (34 months, n = 548, P < 0.001) (Figure 4). In addition, a synergistic effect was identified in the incomplete PAC/low BMI group, when using the incomplete PAC/high BMI group as a reference (HR: 1.384, 95% CI: 1.199-1.599, P < 0.001).
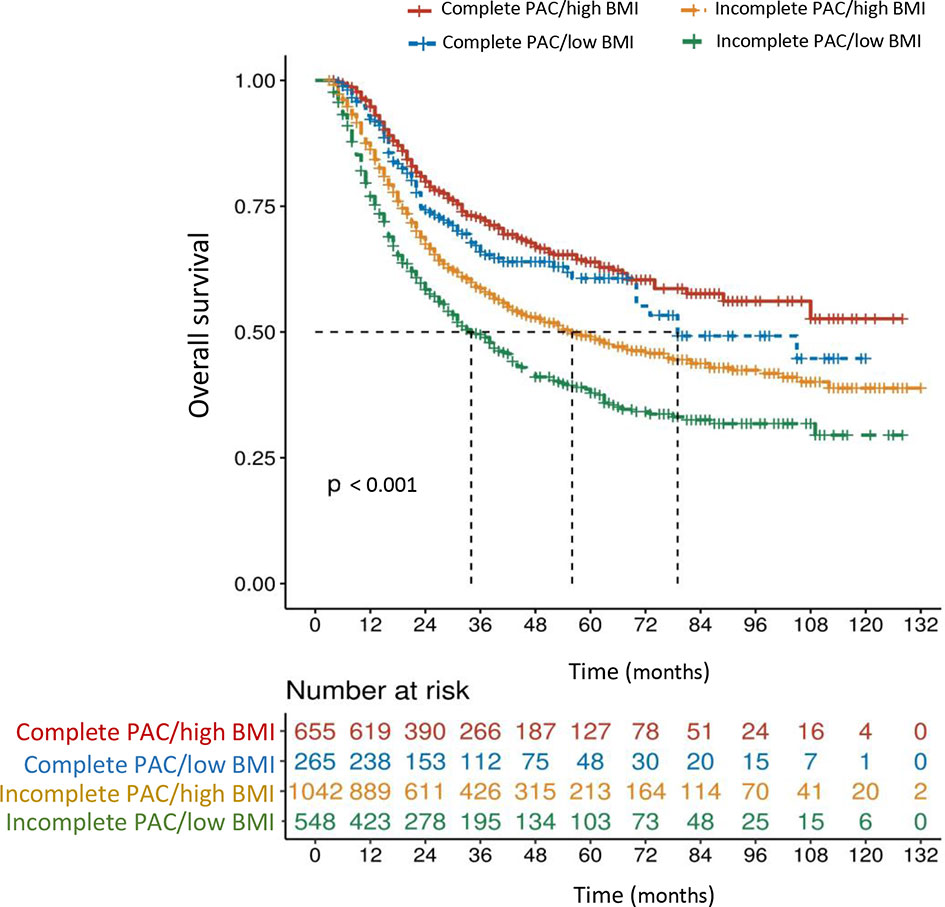
Figure 4 Overall survival curves in 2,510 patients who underwent curative resection for stage II/III gastric cancer classified by the body mass index (BMI < 20.3 or ≥ 20.3 kg/m2) and perioperative adjuvant chemotherapy (PAC) (low BMI defined as < 20.3 kg/m2, high BMI defined as ≥ 20.3 kg/m2, incomplete PAC defined as < 6 cycles, complete PAC defined as ≥ 6 cycles). The differences of overall survival in subgroups were compared by log-rank test.
Discussion
In the present large cohort study including 2,510 consecutive patients from a high-volume center, we revealed that approximately one-third of the patients (32.4%) with stage II/III GC had poor nutritional status (defined as BMI < 20.3 kg/m2 in this study), which was consistent with previous studies (13, 14). For the first time, low pre-operative BMI was confirmed as a simple but independent risk factor for poor compliance with PAC. Additionally, low BMI was identified to be associated with poor oncological outcomes, confirming the results of previous studies (13–15). However, further analyses found that if patients completed at least 6 cycles of PAC, then survival time became independent of BMI, regardless of whether they were classified by 20.3 kg/m2 or the WHO classification. Our findings suggest that completion of PAC might be a confounding factor between the relationship of low BMI and poor oncological outcomes of stage II/III GC. To compensate for the negative influence of low BMI on prognosis, strategies aimed to ensure the completion of PAC should be further investigated.
Malnutrition is commonly seen in patients with locally advanced GC because of decreased appetite and occasional pyloric obstruction. There is a growing body of evidence showing that nutritional status is significantly associated with prognosis in various malignancies, including GC (9–15). Potential mechanisms include suppression of the immune system, which plays an inevitable role in eliminating cancer cells and preventing metastasis (9, 12, 24). BMI can be easily calculated and is a widely used indicator to assess patients’ nutritional status in the clinic. Although some studies have explored the influence of BMI on the prognosis of GC, their conclusions are still controversial (13–17, 26–28). In a retrospective study containing 1,210 stage I to III GC patients treated with D2 gastrectomy, prognosis was significantly worse in patients with low BMI (< 18.5 kg/m2) than in patients with normal (18.5 - 24.9 kg/m2) or high (≥ 25.0 kg/m2) BMI, after propensity score matching for tumor depth, lymph node metastasis, and tumor stage (26). In contrast, another study analyzing 947 stage I to III GC patients, concluded that both OS and cancer-specific survival were similar among the 3 groups (BMI < 25, 25-30, and > 30 kg/m2) (27). Meanwhile, Kim and colleagues (28) reported that preoperative low BMI (< 18.5 kg/m2) significantly impacted recurrence and survival in those with stage I/II GC but lost its significance in those with stage III/IV disease. Possible explanations for these discordant findings were inconsistent patient inclusion and BMI classification criteria and relatively small sample sizes. Moreover, stage I GC patients rarely develop severe malnutrition, usually require no chemotherapy, and have significantly better outcomes, thus it seems difficult to define the influence of nutritional status on prognosis for these patients. Additionally, stage IV GC patients with metastatic disease, who usually cannot undergo curative gastrectomy and experience extremely dismal prognoses, were included in some previous studies (28).
Park et al. (14) retrospectively analyzed 1,868 stage II/III GC patients receiving gastrectomy, and concluded that pre-operative underweight (BMI < 18.5 kg/m2) was a significant predictor of recurrence, along with age and TNM stage (P < 0.001). In contrast to our findings, underweight patients seemed more likely to receive adjuvant chemotherapy, and the prognosis for these patients was still significantly poorer, regardless of chemotherapy. However, the exact number of cycles of chemotherapy has not been described and thus, the potential impact of BMI on completeness of PAC and the influence of dose intensity on prognosis could not be evaluated. To the best of our knowledge, the potential influence of completeness of PAC on the relationship between BMI and prognosis of GC has never been evaluated to date. Therefore, we conducted this study to investigate the relationship among BMI, PAC, and prognosis of patients with stage II or III GC, by utilizing the data of 2,510 patients.
Although PAC has been considered as the most effective strategy to improve oncological outcomes of those with stage II or III GC, in addition to radical gastrectomy, only 36.6% of patients completed at least 6 cycles of PAC. Further analyses found that older age and poorer nutritional status (low BMI and albumin concentration) adversely affected the compliance with PAC, which was consistent with previous studies (9, 18, 29). Seo et al. (19) reported that older age significantly increased chemotherapy-induced grade 3/4 non-hematological toxicity, but low BMI and hypoalbuminemia were independently associated with grade 3/4 hematological adverse events, which may explain our findings, at least in part. A randomized clinical trial evaluated the effects of post-discharge oral nutritional supplements (ONS) on nutritional outcomes and chemotherapy tolerance in patients at nutritional risk following resection for GC. The 171 patients receiving ONS had significantly higher BMI and less chemotherapy modifications following 3 months of intervention, compared with those who received dietary advice alone (n = 166) (30). These findings suggest that postoperative nutritional supplementation is not optional but a prerequisite, especially in those with malnutrition.
The mOS of patients with low BMI increased from 34 to 79 months when they received ≥ 6 cycles of PAC, which was comparable to those with high BMI (P = 0.143, Figure 3C). It seems that complete PAC can compensate for the adverse impact of low BMI on survival. Our findings strongly support the importance of complete and adequate PAC, especially in those with low BMI. Our analyses confirmed that NAC was a protective strategy for complete PAC. This was echoed by Li et al. (31), who conducted a retrospective study of 206 patients and concluded that NAC was a significant protective factor to ensure that patients complete all intended multimodal therapy, in order to negate the adverse influence of postoperative complications on oncological outcomes. Thus, in order to complete PAC and thereby mitigate the negative influence of low BMI on oncological outcomes, it may be preferable to perform chemotherapy preoperatively, instead of postoperatively. However, further prospective studies should be conducted to clarify this hypothesis.
Although the present study has some interesting findings, it also has several limitations. First, it was a retrospective study, thus the exact reasons for termination of chemotherapy could not be determined. For example, the type of medical insurance and potential economic burden may act as confounders. In addition, some patients might experience early recurrence (adjuvant chemotherapy was usually performed within 6 months following surgery) and thus terminated PAC or received palliative chemotherapy, which may impact the reliability of our conclusions. Second, it seemed that the median follow-up of 28 months was relatively short, and as a result, later recurrence and death of patients could not be analyzed. Third, patients generally received fluorouracil and platinum based regimens for PAC in our institution. Whereas different combinations have been used, such as S-1 alone, XELOX, SOX, ECF, FLOT, and oxaliplatin plus fluorouracil/leucovorin, given the long duration over 10 years of our study (21). The convenience, incidence, and grade of chemotherapy-caused adverse events induced by different chemotherapy regimens might also affect the compliance with PAC. Last, only 11.4% of all patients received NAC, which was significantly less than in Western countries (32), given that AC following D2 gastrectomy was recommended in Asia (5, 21). As a result, this may influence the generalizability of our findings. Notwithstanding the limitations, this is the first study to evaluate the association among BMI, PAC, and oncological outcomes of patients with stage II or III GC following curative resection, based on a large number of patients.
In conclusion, the present study confirmed that preoperative low BMI is an independent predictor for incompleteness of PAC and poorer oncological outcomes in those with stage II or III GC, but complete PAC could compensate for the negative influence of low BMI on oncological outcomes. Thus, strategies to improve compliance with PAC, such as NAC and nutritional supplements, should be further investigated.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of Hunan Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HX designed the research, analyzed the data, and critically revised the manuscript. WP collected part of the data, and grafted the manuscript. JD, C-CL and DL collected part of the data. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Hunan Cancer Hospital Climb Plan (No. 2020NSFC-A004), Hunan Provincial Natural Science Foundation of China (No. 2020JJ5339), the Science and Technology Foundation of Changsha (No. kq1907125).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors gratefully thank all of the participants in this study and Hunan Cancer Hospital for supporting this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.899677/full#supplementary-material
Supplementary Figure 1 | X-tile analysis of overall survival performed using patients’ data to determine the optimal cut-off value for body mass index (BMI). In the left panels, the X-axis represents all potential cut-off values from low to high (left to right) that define a low subset, whereas the Y-axis represents the cut-off values from high to low (top to bottom) that define a high subset. Red coloration of a cut-off value indicates an inverse correlation with time to death, and the green coloration represents direct associations. The optimal cut-off value highlighted by the black circles in the left panels is shown in the histogram of the entire cohort (middle panels). Kaplan-Meier plots are displayed in the right panels, where blue represents the low subgroup and gray represents the high subgroup. The optimal cut-off values for BMI was 20.3 kg/m2, with maximum χ2 long-rank value of 26.5864 and minimum P value < 0.0001.
Supplementary Figure 2 | Overall survival curves in 2,510 patients who underwent curative resection for stage II/III gastric cancer classified by completeness of perioperative adjuvant chemotherapy (PAC, 0, 1-5 or ≥ 6 cycles), and further stratified by tumor stage (stage II or III). The differences of overall survival in subgroups were compared by log-rank test.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in China, 2015. CA Cancer J Clin (2016) 66:115–32. doi: 10.3322/caac.21338
3. Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant Capecitabine Plus Oxaliplatin for Gastric Cancer After D2 Gastrectomy (CLASSIC): 5-Year Follow-Up of an Open-Label, Randomized Phase 3 Trial. Lancet Oncol (2014) 15(12):1389–96. doi: 10.1016/S1470-2045(14)70473-5
4. Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2016) 14(10):1286–312. doi: 10.6004/jnccn.2016.0137
5. Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2018 (5th Edition). Gastric Cancer (2021) 24(1):1–21. doi: 10.1007/s10120-020-01042-y
6. Tsuburaya A, Yoshida K, Kobayashi M, Yoshino S, Takahashi M, Takiquchi N, et al. Sequential Paclitaxel Followed by Tegafur and Uracil (UFT) or S-1 Versus UFT or S-1 Monotherapy as Adjuvant Chemotherapy for T4a/b Gastric Cancer (SAMIT): A Phase 3 Factorial Randomised Controlled Trial. Lancet Oncol (2014) 15(8):886–93. doi: 10.1016/S1470-2045(14)70025-7
7. Cats A, Jansen EPM, van Grieken NCT, Skorsha K, Lind P, Nordsmark M, et al. Chemotherapy Versus Chemoradiotherapy After Surgery and Preoperative Chemotherapy for Resectable Gastric Cancer (CRITICS): An International, Open-Label, Randomised Phase 3 Trial. Lancet Oncol (2018) 19(5):616–28. doi: 10.1016/S1470-2045(18)30132-3
8. Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative Chemotherapy With Fluorouracil Plus Leucovorin, Oxaliplatin, and Docetaxel Versus Fluorouracil or Capecitabine Plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet (2019) 393(10184):1948–57. doi: 10.1016/S0140-6736(18)32557-1
9. Xiao H, Zhou H, Zhang P, Xiao H, Liu K, Chen X, et al. Association Among the Prognostic Nutritional Index, Completion of Adjuvant Chemotherapy, and Cancer-Specific Survival After Curative Resection of Stage II/III Gastric Cancer. Eur J Clin Nutr (2020) 74(4):555–64. doi: 10.1038/s41430-019-0502-1
10. Chan AW, Chan SL, Wong GL, Wong WV, Chong CC, Lai PB, et al. Prognostic Nutritional Index (PNI) Predicts Tumor Recurrence of Very Early/Early Stage Hepatocellular Carcinoma After Surgical Resection. Ann Surg Oncol (2015) 22(13):4138–48. doi: 10.1245/s10434-015-4516-1
11. Yang Y, Gao P, Song Y, Sun J, Chen X, Zhao J, et al. The Prognostic Nutritional Index Is a Predictive Indicator of Prognosis and Postoperative Complications in Gastric Cancer: A Meta-Analysis. Eur J Surg Oncol (2016) 42(8):1176–82. doi: 10.1016/j.ejso.2016.05.029
12. Kubo Y, Tanaka K, Yamasaki M, Yamashita K, Makino T, Saito T, et al. Influences of the Charlson Comorbidity Index and Nutrition Status on Prognosis After Esophageal Cancer Surgery. Ann Surg Oncol (2021) 28(12):7173–82. doi: 10.1245/s10434-021-09779-1
13. Lee JH, Park B, Joo J, Kook MC, Kim YI, Lee JY, et al. Body Mass Index and Mortality in Patients With Gastric Cancer: A Large Cohort Study. Gastric Cancer (2018) 21(6):913–24. doi: 10.1007/s10120-018-0818-x
14. Park SH, Lee S, Song JH, Choi S, Cho M, Kwon IG, et al. Prognostic Significance of Body Mass Index and Prognostic Nutritional Index in Stage II/III Gastric Cancer. Eur J Surg Oncol (2020) 46(4 Pt A):620–5. doi: 10.1016/j.ejso.2019.10.024
15. Ma S, Liu H, Ma FH, Li Y, Jin P, Hu HT, et al. Low Body Mass Index Is an Independent Predictor of Poor Long-Term Prognosis Among Patients With Resectable Gastric Cancer. World J Gastrointest Oncol (2021) 13(3):161–73. doi: 10.4251/wjgo.v13.i3.161
16. Zhao B, Zhang J, Mei D, Luo R, Lu H, Xu H, et al. Does High Body Mass Index Negatively Affect the Surgical Outcome and Long-Term Survival of Gastric Cancer Patients Who Underwent Gastrectomy: A Systematic Review and Meta-Analysis. Eur J Surg Oncol (2018) 44(12):1971–81. doi: 10.1016/j.ejso.2018.09.007
17. Zhao W, Wang P, Sun W, Gu P, Wang X, Wu Z, et al. Effects of a High Body Mass Index on the Short-Term Outcomes and Prognosis After Radical Gastrectomy. Surg Today (2021) 51(7):1169–78. doi: 10.1007/s00595-021-02259-9
18. Aoyama T, Yoshikawa T, Shirai J, Hayashi T, Yamada T, Tsuchida K, et al. Body Weight Loss After Surgery Is an Independent Risk Factor for Continuation of S-1 Adjuvant Chemotherapy for Gastric Cancer. Ann Surg Oncol (2013) 20(6):2000–6. doi: 10.1245/s10434-012-2776-6
19. Seo SH, Kim SE, Kang YK, Ryoo BY, Ryu MH, Jeong JH, et al. Association of Nutritional Status-Related Indices and Chemotherapy-Induced Adverse Events in Gastric Cancer Patients. BMC Cancer (2016) 16(1):900. doi: 10.1186/s12885-016-2934-5
20. Lu J, Zheng CH, Cao LL, Li P, Xie JW, Wang JB, et al. The Effectiveness of the 8th American Joint Committee on Cancer TNM Classification in the Prognosis Evaluation of Gastric Cancer Patients: A Comparative Study Between the 7th and 8th Editions. Eur J Surg Oncol (2017) 43(12):2349–56. doi: 10.1016/j.ejso.2017.09.001
21. Xiao H, Xiao Y, Chen P, Quan H, Luo J, Huang G. Association Among Blood Transfusion, Postoperative Infectious Complications, and Cancer-Specific Survival in Patients With Stage II/III Gastric Cancer After Radical Gastrectomy: Emphasizing Benefit From Adjuvant Chemotherapy. Ann Surg Oncol (2021) 28(4):2394–404. doi: 10.1245/s10434-020-09102-4
22. Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, et al. Histopathological Regression After Neoadjuvant Docetaxel, Oxaliplatin, Fluorouracil, and Leucovorin Versus Epirubicin, Cisplatin, and Fluorouracil or Capecitabine in Patients With Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4-AIO): Results From the Phase 2 Part of a Multicentre, Open-Label, Randomised Phase 2/3 Trial. Lancet Oncol (2016) 17(12):1697–708. doi: 10.1016/S1470-2045(16)30531-9
23. Shitara K, Chin K, Yoshikawa T, Katai H, Terashima M, Ito S, et al. Phase II Study of Adjuvant Chemotherapy of S-1 Plus Oxaliplatin for Patients With Stage III Gastric Cancer After D2 Gastrectomy. Gastric Cancer (2017) 20(1):175–81. doi: 10.1007/s10120-015-0581-1
24. Dindo D, Demartines N, Clavien PA. Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
25. Shen Q, Liu W, Quan H, Pan S, Li S, Zhou T, et al. Prealbumin and Lymphocyte-Based Prognostic Score, a New Tool for Predicting Long-Term Survival After Curative Resection of Stage II/III Gastric Cancer. Br J Nutr (2018) 120(12):1359–69. doi: 10.1017/S0007114518002854
26. Feng F, Zheng G, Guo X, Liu Z, Xu G, Wang F, et al. Impact of Body Mass Index on Surgical Outcomes of Gastric Cancer. BMC Cancer (2018) 18(1):151. doi: 10.1186/s12885-018-4063-9
27. Lin YS, Huang KH, Lan YT, Fang WL, Chen JH, Lo SS, et al. Impact of Body Mass Index on Postoperative Outcome of Advanced Gastric Cancer After Curative Surgery. J Gastrointest Surg (2013) 17(8):1382–91. doi: 10.1007/s11605-013-2238-x
28. Kim CH, Park SM, Kim JJ. The Impact of Preoperative Low Body Mass Index on Postoperative Complications and Long-Term Survival Outcomes in Gastric Cancer Patients. J Gastric Cancer (2018) 18(3):274–86. doi: 10.5230/jgc.2018.18.e30
29. Yamashita K, Kurokawa Y, Yamamoto K, Hirota M, Kawabata R, Mikami J, et al. Compliance With Adjuvant S-1 Chemotherapy for Gastric Cancer: A Multicenter Retrospective Study. Ann Surg Oncol (2017) 24(9):2639–45. doi: 10.1245/s10434-017-5923-2
30. Meng Q, Tan S, Jiang Y, Han J, Xi Q, Zhuang Q, et al. Post-Discharge Oral Nutritional Supplements With Dietary Advice in Patients at Nutritional Risk After Surgery for Gastric Cancer: A Randomized Clinical Trial. Clin Nutr (2021) 40(1):40–6. doi: 10.1016/j.clnu.2020.04.043
31. Li SS, Udelsman BV, Parikh A, Klempner SJ, Clark JW, Roeland EJ, et al. Impact of Postoperative Complication and Completion of Multimodality Therapy on Survival in Patients Undergoing Gastrectomy for Advanced Gastric Cancer. J Am Coll Surg (2020) 230(6):912–24. doi: 10.1016/j.jamcollsurg.2019.12.038
Keywords: body mass index, malnutrition, perioperative adjuvant chemotherapy, gastric cancer, prognosis
Citation: Peng W, Dai J, Liu C-c, Liu D and Xiao H (2022) Body Mass Index and Prognosis of Patients With Stage II/III Gastric Cancer After Curative Gastrectomy: Completion of Perioperative Adjuvant Chemotherapy May Be a Confounding Factor. Front. Oncol. 12:899677. doi: 10.3389/fonc.2022.899677
Received: 19 March 2022; Accepted: 16 May 2022;
Published: 13 June 2022.
Edited by:
Jinqiu Jacky Yuan, Sun Yat-sen University, ChinaReviewed by:
Manabu Ohashi, Cancer Institute Hospital of Japanese Foundation for Cancer Research, JapanNingning Mi, First Hospital of Lanzhou University, China
Copyright © 2022 Peng, Dai, Liu, Liu and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Xiao, aHVha2V4aDIwMTBAMTYzLmNvbQ==
 Wei Peng1
Wei Peng1 Hua Xiao
Hua Xiao