- 1Oncology and Immunology Section, Department of Surgery, Oncology and Gastroenterology, University of Padova, Padova, Italy
- 2Multivisceral Transplant Unit, Department of Surgery, Oncology and Gastroenterology, Padova University Hospital, Padova, Italy
- 3Immunology and Diagnostic Molecular Oncology Unit, Veneto Institute of Oncology IOV-IRCCS, Padova, Italy
- 4Cancer Epidemiology Unit, Centro di Riferimento Oncologico di Aviano (CRO) IRCCS, Aviano, Italy
- 5Clinical Research Unit, Veneto Institute of Oncology IOV-IRCCS, Padova, Italy
- 6Clinical Epidemiology Unit, Istituto Nazionale per le Malattie Infettive (INMI) “L. Spallanzani” IRCCS, Rome, Italy
- 7Hepatobiliary Surgery and Liver Transplantation Unit, Department of Surgery, Oncology, and Gastroenterology, Padova University Hospital, Padova, Italy
- 8Sir Peter MacCallum Department of Oncology, The University of Melbourne, Melbourne, VIC, Australia
- 9Department of Microbiology and Immunology, The University of Melbourne, Melbourne, VIC, Australia
Liver transplanted (LT) patients for hepatocellular carcinoma (LT-HCC) or for other causes (LT-no-HCC) may develop post-transplantation malignancies. Although immune activation and senescence are frequently implicated in cancer development, no data is available on their possible role as biomarkers predictive of tumor onset in this setting. A total of 116 patients were investigated: the 45 LT-HCC patients were older than the 71 LT-non-HCC (p=0.011), but comparable for sex, HCV, HBV infection and immunosuppressive treatment. At baseline, the numbers of activated and senescent-like circulating cells were significantly higher in LT-HCC patients than in LT-no-HCC ones. After a median follow-up of 26.8 months, 6 post-transplant malignancies (PTM) occurred: 4 in LT-HCC (8.9%) and 2 in LT-no-HCC (2.8%) patients. Overall, subjects with high percentages of activated and exhausted T and B cells at baseline were at higher risk of PTM. Notably, within the LT-HCC group, a higher percentage of senescence-like T cells was also associated with cancer development. Moreover, patients with PTM had higher telomere erosion and higher levels of circulating PAMPs (16S rDNA) and DAMPs (mtDNA) when compared with matched patients without PTM. Overall, these findings suggest that immune activation and exhaustion may be useful to predict the risk of PTM occurrence, regardless of the cause of transplantation. In LT-HCC, T-cell senescence represents an additional risk factor for tumor onset.
Introduction
Liver transplantation is the treatment of choice for patients with end-stage liver disease, acute liver failure or hepatocellular carcinoma (HCC). In Italy, a total of 12,519 liver transplants were performed between 2010 and 2020 (1). The lifelong use of immunosuppressive anti-rejection treatment substantially increases the risk of post-transplant malignancies (PTM). Although advances in immunosuppressive therapy have contributed to improve patient and graft survival, cancers represent a major adverse outcome in liver transplanted (LT) patients, including a negative impact on morbidity and mortality. Furthermore, PTM are a leading cause of death in LT recipients (2–5).
HCC is the most common type of liver cancer, accounting for 75%–85% of cases (6, 7), and is characterized by rapid progression, frequent metastasis, late diagnosis, high post-operative recurrence and poor prognosis (6). It has been reported that liver transplanted patients for HCC (LT-HCC) tended to be at higher risk of developing de novo malignancies (DNMs) compared to liver transplanted patients for other causes (LT-no-HCC) (7). Recently, an earlier onset of solid DNMs in LT-HCC compared to LT-no-HCC has been documented (8).
Chronic inflammation is a well-known factor promoting cancerogenesis, including hepatocarcinogenesis, a multistep process induced by exposure to agents triggering insults in the liver (e.g., chronic viral hepatitis infection, alcohol use) followed by chronic local inflammation (9–11). After transplantation, events such as the ischaemia-reperfusion injury and/or immunosuppressive treatments also induce an inflammatory status through the release of pathogen-associated molecular patterns (PAMPs), and damage-associated molecular patterns (DAMPs) into circulation. The subsequent induction of pro-inflammatory cytokines leads to the activation of immune system, which in turn contributes to maintain the inflammatory environment, a pivotal step for tumorigenesis (12–14).
Senescence may also play an important role in the carcinogenesis process; indeed, senescent cells secrete pro-inflammatory cytokines and growth factors, known as the senescence-associated secretory phenotype (SASP), which have been implicated in both aging and cancer development (15, 16). In a previous study, we found that immune senescence biomarkers were significantly higher in elderly colon cancer patients than in age-matched controls; in particular, cancer patients exhibited higher percentages of senescent CD8+ T cells. Consistently, lower thymic output and shorter telomeres were associated with a higher risk of developing cancer in the same cohort (17). Furthermore, cancer patients with high percentage of senescent and activated CD8+ T cells had a worse disease outcome (18).
Considering the possible clinical relevance of the availability of suitable biomarkers for the prediction of tumor onset in LT patients, in the present study we have characterized immune activation, exhaustion, and senescence markers in the blood of a prospective series of LT patients with the final goal to assess their possible usefulness in the definition of the risk of PTM development.
Materials and Methods
Study Population and Sampling
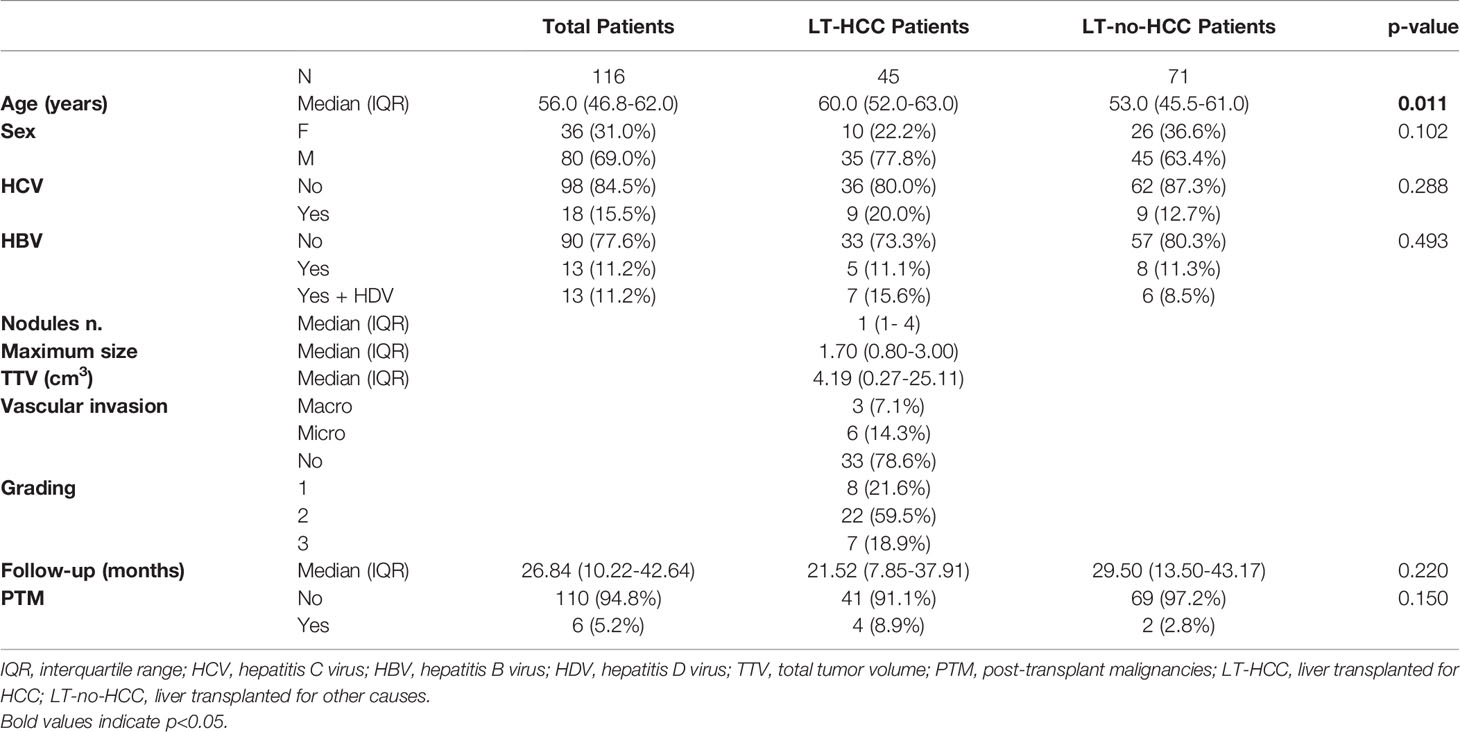
A total of 116 LT patients have been prospectively enrolled between October 2016 and February 2021 at the Multivisceral Transplant Unit, Padova University-Hospital. Forty-five patients were LT-HCC and 71 LT-no-HCC (i.e. decompensated cirrhosis, cholestatic liver disease, acute liver failure). Patients’ clinical information were recorded and included personal details (age at transplant, sex), transplant details (date of liver transplantation, underlying disease, Model for End-Stage Liver Disease (MELD) score and alpha-fetoprotein levels at liver transplantation) and post-transplantation immunosuppressive schedule, considering modifications during follow-up. For LT-HCC patients, tumor characteristics were assessed through explant pathology evaluation for the following characteristics: number of HCC nodules, maximum nodular diameter, tumor differentiation and presence of macro/micro-vascular invasion. Vital tumor volume was quantified according to the following equation: “Tumor volume mm3 = 4/3 x 3.14 x (radius of the tumor nodule in mm3)”. Total tumor volume (TTV) was calculated as the sum of the tumor volume of each nodule. LT-HCC patients were followed for HCC recurrence through CT-scan or MRI every 3 months during the first year, and every 6 months thereafter, according to the European Association for the Study of the Liver/European Organization for Research and Treatment of Cancer (EASL-EORTC) clinical practice guidelines for the management of hepatocellular cancer (19). As they were candidates for LT, patients were not treated with systemic anticancer drugs and underwent locoregional bridge treatments.
After a median follow-up of 26.8 months following liver transplantation, 6 patients developed a PTM (LT-PTM): 5 were DNMs and 1 was an HCC recurrence. DNMs were defined as neoplasms developing after transplantation in patients negative at pre-transplant screening for the HCC, or related pre-malignant lesions/conditions. DNMs were coded according to the International Classification of Diseases and Related Health Problems, 10th revision (ICD-10). Diagnosis of DNMs was established by histology on biopsies or surgical specimens of the tumor. Date of biopsy or surgical procedure was designated as the date of cancer diagnosis. All patients underwent a full pre-transplant screening aimed at excluding any type of cancer other than HCC, or related premalignant lesions/conditions, which was negative in all patients. Moreover, to avoid any accidental missing pre-LT diagnosis, lesions that appeared within 6 months after transplantation were excluded from the definition of DNM. In our cohort, the 5 DNMs were: infiltrating ductal carcinoma of the breast, ovarian cancer, larynx carcinoma, basal cell carcinoma of the external auditory canal, and prostate cancer. Among the 110 patients without PTM (LT-no-PTM), 26 subjects were selected as a matched (m)LT-no-PTM control group according to the following inclusion criteria: age, sex difference, immunosuppressive combined strategies, and a median time interval from baseline to follow-up. This study was approved by the Ethical Committee of Padova University Hospital (Prot. 4231/AO/17).
Sample Collection
Blood samples were collected in EDTA-containing tubes at baseline (at liver transplantation) and at follow-up (6, 12 months and every year after transplantation and/or at cancer onset). From samples at baseline and at closest time to the tumor onset in LT-PTM patients, or with a median time interval similar for mLT-no-PTM patients, peripheral blood mononuclear cells (PBMC) were isolated by centrifugation on a Ficoll-Paque (Pharmacia, Uppsala, Sweden) gradient. PBMC were cryopreserved in liquid nitrogen, and plasma samples at - 80°C, until use.
Flow Cytometry
Immunophenotyping was performed on cryopreserved PBMC. Cells were thawed, washed, stained for 20 min in the dark with the Live/Dead Fixable Near-IR Dead Cell Stain Kit (Life Technologies, Carlsbad, California, USA) and the following labelled monoclonal antibodies (mAbs): anti-CD3 [fluorescein isothiocyanate (FITC)], anti-CD4 [peridinin chlorophyll protein (PerCP)], anti-CD38 [phycoerythrin (PE)], anti-HLA-DR [allophycocyanin (APC)], anti-CD279 (programmed cell death 1, PD-1) [PE-Cy7], anti-CD57 [PE], anti-CD21 [BV421], anti-CD27 [PE-Cy7], anti-IgD [PE], anti-CD274 (programmed cell death ligand 1, PD-L1) [BV421] (Becton-Dickinson, San Diego, California, USA); anti-CD8 [VioGreen], anti-CD28 [APC], anti-CD19 [VioBright515], anti-CD10 [APC] (Miltenyi Biotec, Auburn, California USA). Cells were then washed and resuspended in phosphate-buffered saline supplemented with 1% paraformaldehyde. All samples were analyzed using LSRII Flow cytometer (Becton-Dickinson). A total of 50000 events were collected in the lymphocyte gate using morphological parameters (forward and side-scatter). Data were processed with FACSDiva Software (Becton-Dickinson) and analyzed using Kaluza Analyzing Software v.1.2 (Beckman Coulter) (Figure 1 supplementary).
Determination of Telomere Length
DNA was extracted from PBMC, using QIAmp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Relative telomere length (RTL) was determined by monochrome quantitative multiplex real-time PCR, as previously described (20, 21).
Quantification of PAMPs and DAMPs
DNA extraction from 200 µl of plasma was performed using QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany), and eluted in 50 µl of AE Buffer. A quantitative method based on real-time PCR assay was performed to quantify plasma levels of 16S rDNA with primer pair (forward 5’-AGTTTGATCCTGGCTCAG-3’ and reverse 5’-GWATTACCGCGGCKGCTG-3’) and probe (5’-FAM-GCTGCCTCCCGTAGGAGT-BHQ-3’), as previously described (22, 23). Results were expressed as 16S rDNA copies/μl plasma. Plasma levels of mitochondrial DNA (mtDNA) were quantified by real-time PCR assay with primer pair (forward 5’-AGGACAAGAGAAATAAGGCC-3’ and reverse 5’-TAAGAAGAGGAATTGAACCTCTGACTGTAA-3’) and probe (5’-FAM-TTCACAAAGCGCCTTCCCCCGTAAATGA-BHQ-3’), as previously described (23, 24). Results were expressed as mtDNA copies/μl plasma.
Statistical Analysis
Continuous variables were summarized using median and interquartile range (IQR), categorical variables as frequencies and percentages. The median follow-up time was based on the reverse Kaplan-Meier estimator. Clinical characteristics at baseline were compared between LT-HCC and LT-no-HCC patients using the Kruskal-Wallis test, or Fisher exact test as appropriate. A Mann-Whitney test, adjusted for age, was used to address comparisons of each immunological parameter distribution between groups of interest. The impact of the immunological parameters on the probability of experiencing HCC or PTM was estimated in univariate logistic regression models fitted by penalized maximum likelihood to address low frequency data. Each covariate was considered as a categorical variable according to high and low levels. Optimal cut-points were selected using a criterion based on maximizing the Youden index, being the summary measure of the ROC curve, over all possible cut-points. The odds ratios (ORs) were reported with their 95% confidence interval (CI). ORs were also adjusted for age as continuous variable. Subgroup analyses were performed with explorative intent. All statistical tests were two-sided and a p value <0.05 was considered statistically significant. Statistical analyses were performed using the RStudio (RStudio: Integrated Development for R. RStudio Inc., Boston, MA, US).
Results
Patients’ Characteristics
The patients’ characteristics are summarized in Table 1. Overall, 69% of LT patients were male. Median age was 56.0 years (IQR 46.8-62.0); LT-HCC were significantly older than LT-no-HCC patients: 60.0 years (52.0-63.0) vs 53.0 years (45.5-61.0), p=0.011.
Immunological Profile in LT-HCC and LT-no-HCC Patients at Baseline
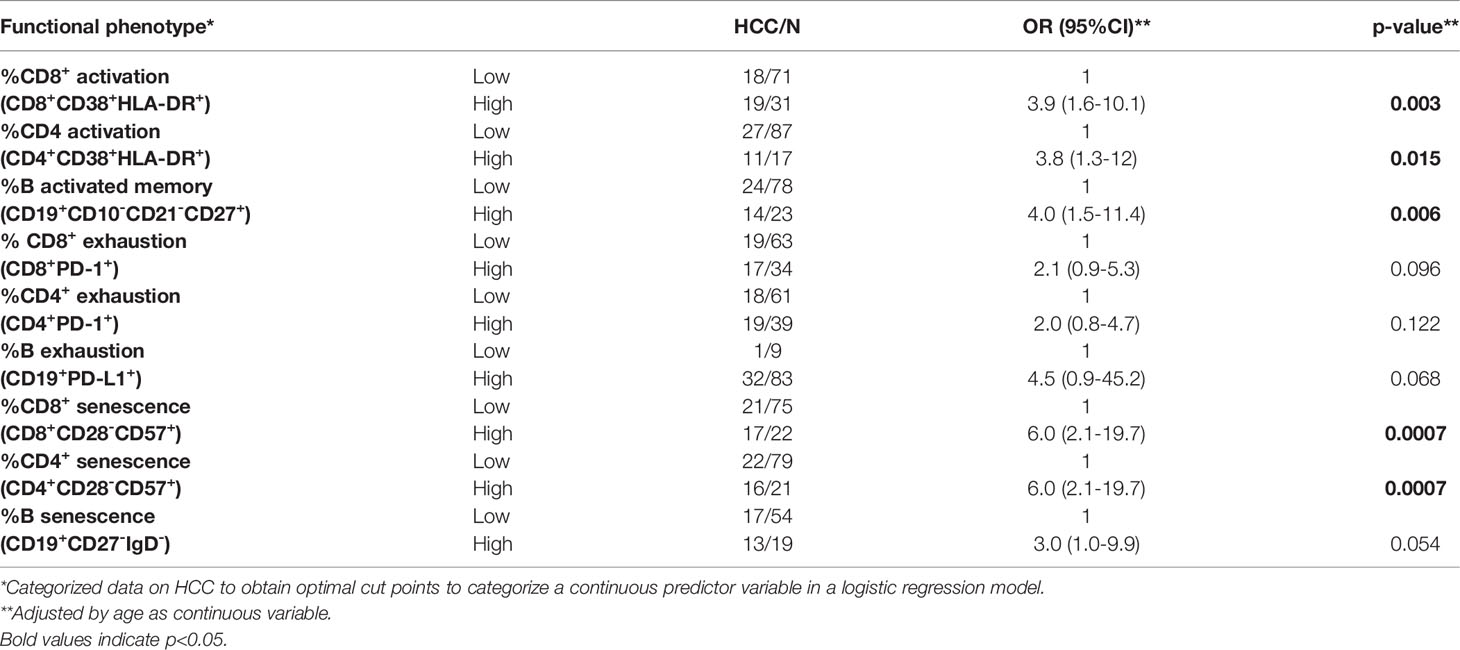
At baseline, the percentages of activated cells were higher in LT-HCC than in LT-no-HCC patients (Figure 1A); in particular, the percentages of activated CD8+ T cells and memory B cells were significantly higher in LT-HCC compared to LT-no-HCC patients (%CD8+CD38+HLA-DR+: 10.89 (5.61-18.52) vs 6.59 (4.26-9.25), p=0.003; %CD19+CD10-CD21-CD27+: 10.97 (5.59-20.68) vs 7.60 (3.00-13.72), p=0.040) (Figure 1A), and a trend to significance was also observed for activated CD4+ T cells (%CD4+CD38+HLA-DR+: 7.23 (4.11-14.12) vs 6.21 (3.49-9.23), p=0.092) (Figure 1A). These results were confirmed in a logistic regression model; subjects with high percentages of activated CD8+, CD4+ T cells and B cells were at higher risk of being LT-HCC than subjects with low percentages of cells showing features of immune activation (Table 2).
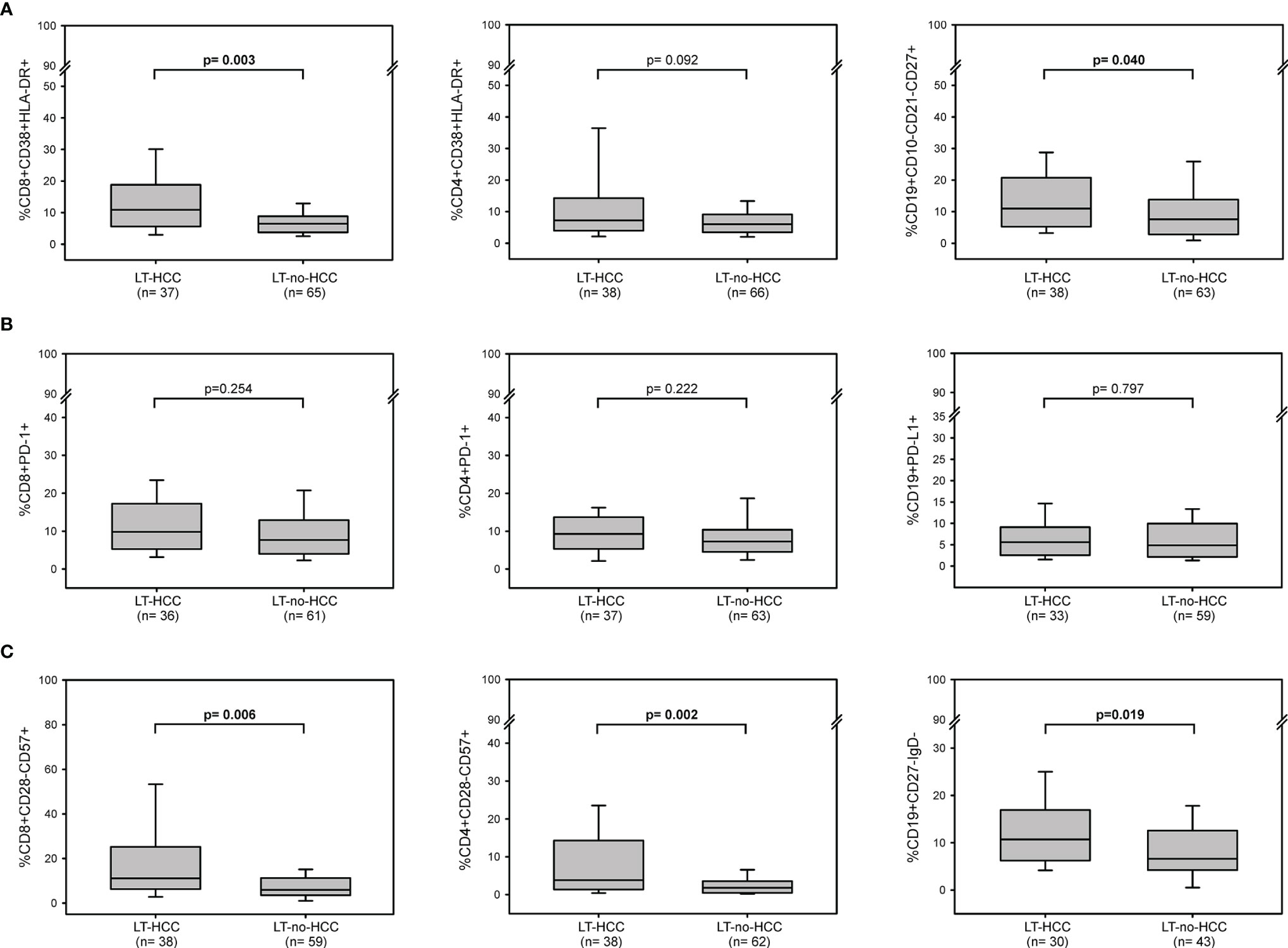
Figure 1 Baseline immune activation, exhaustion and senescence profiles in LT-HCC and LT-no-HCC patients. Percentages of (A) activated CD8+ (CD3+CD8+CD38+HLA-DR+), CD4+ (CD3+CD4+CD38+HLA-DR+) and memory B (CD19+CD10-CD21-CD27+) cells; (B) exhausted CD8+ (CD3+CD8+PD-1+), CD4+ (CD3+CD4+PD-1+) and B (CD19+PD-L1+) cells, (C) senescent-like CD8+ (CD3+CD8+CD28-CD57+), CD4+ (CD3+CD4+CD28-CD57+), and B (CD19+CD27-IgD-) cells in LT-HCC and LT-no-HCC patients. All p-values were adjusted by age.
At baseline, no difference in the number of circulating immune cells showing markers of exhaustion was found between the two groups (%CD8+PD-1+: 9.82 (5.30-17.00) vs 7.54 (4.00-12.92), p=0.254; %CD4+PD-1+: 9.28 (5.66-13.50) vs 7.27 (4.54-10.34), p=0.222; %CD19+PD-L1+: 4.96 (3.12-9.75) vs 5.16 (2.18-9.00), p=0.797) (Figure 1B). However, in the logistic regression model, high percentage of exhausted T and B cells tended to be associated with a higher risk of being LT-HCC (Table 2).
LT-HCC patients had significantly higher percentage of senescent-like T and B cells compared to LT-no-HCC (%CD8+CD28-CD57+: 11.06 (6.24-25.16) vs 5.92 (3.54-10.97), p=0.006; %CD4+CD28-CD57+: 3.80 (1.36-14.03) vs 1.80 (0.48-3.41), p=0.002; %CD19+CD27-IgD-: 12.20 (6.28-17.67) vs 6.59 (4.24-12.50), p=0.019) (Figure 1C). These results were confirmed by the logistic model; patients with high percentages of senescent-like CD8+, CD4+ T and B cells were at higher risk of being LT-HCC (Table 2).
Immune Activation and Immune Senescence Profiles at Baseline as Prognostic Markers of PTM
After a median period of follow-up of 26.8 months (10.2-42.6) following LT, 6 patients developed a PTM: 4 patients (8.9%) (3 DNMs and 1 an HCC recurrence) were in the LT-HCC group, and 2 (2.8%, both DNMs) were in the LT-no-HCC group (Table 1).
To evaluate the role of immune activation, exhaustion and senescence as possible markers predictive of the risk of tumor development in this setting, we analyzed these immunological parameters at baseline in samples obtained from the 6 LT-PTM patients in comparison with those of 110 LT-no-PTM patients. At baseline, the two groups did not differ by age, sex, HCV and HBV infection (Table 1a Supplementary).
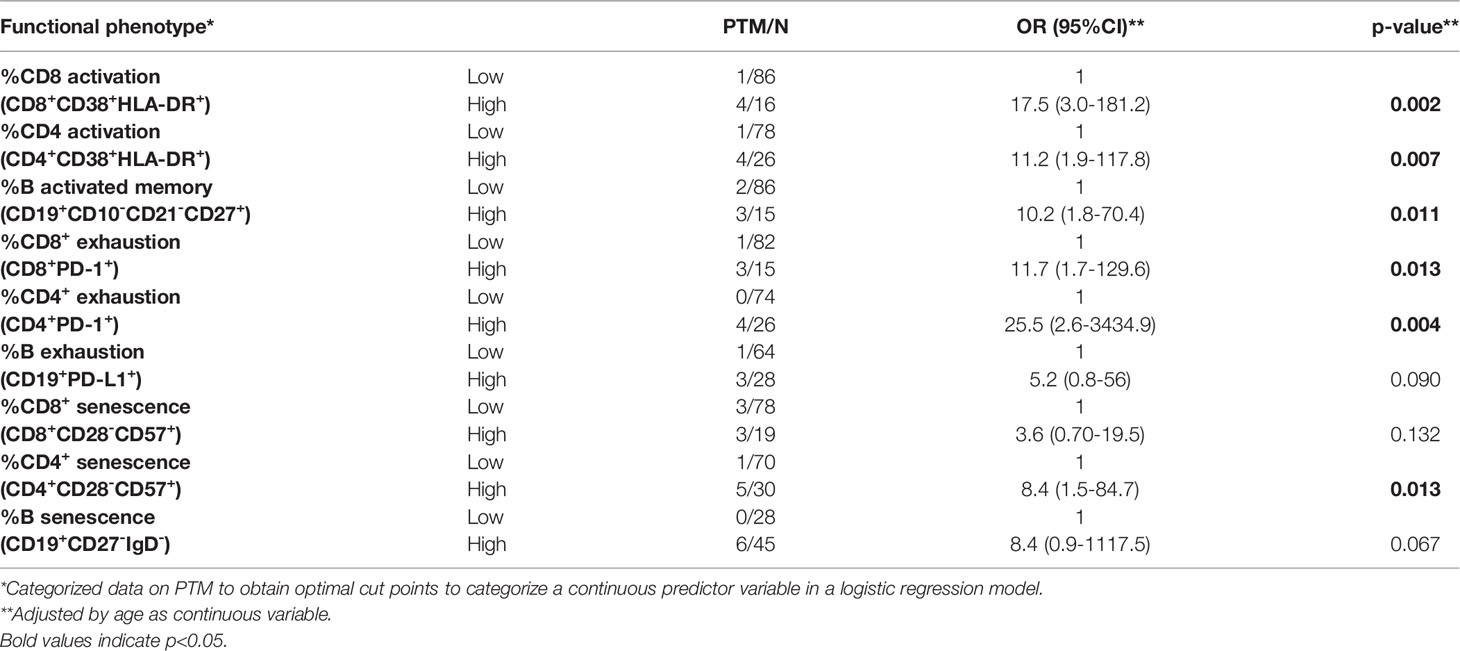
The percentages of activated CD8+, CD4+ T cells and memory B cells were significantly higher in LT-PTM than in LT-no-PTM patients (%CD8+CD38+HLA-DR+: 19.00 (18.76-19.76) vs 7.14 (4.48-11.98), p=0.002; %CD4+CD38+HLA-DR+: 14.28 (10.53-16.84) vs 6.26 (3.58-9.77), p=0.0001; %CD19+CD10-CD21-CD27+: 25.57 (10.09-28.57) vs 8.55 (4.20-15.40), p=0.024) (Figure 2A). These results were confirmed in the logistic regression model (Table 3).
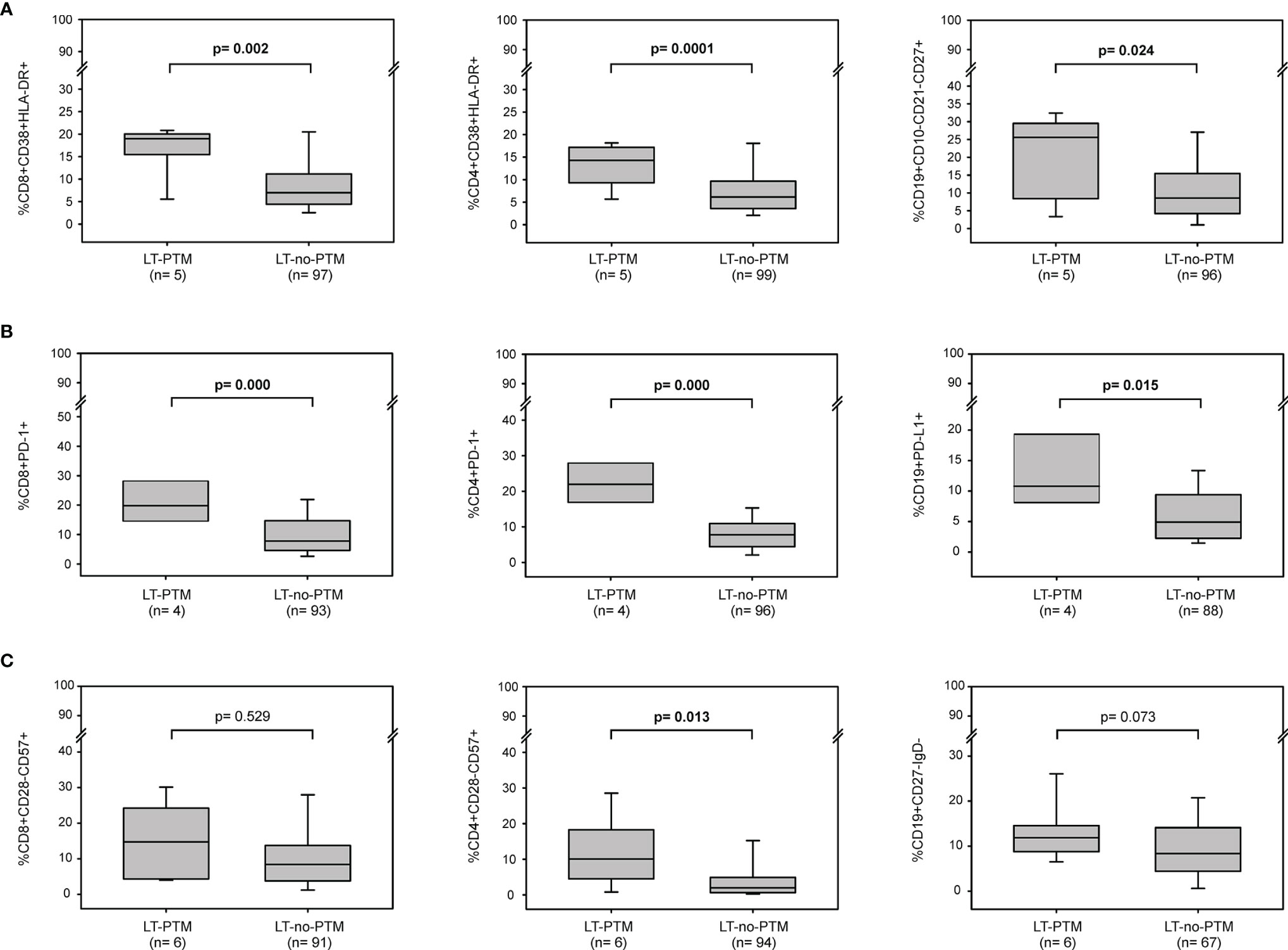
Figure 2 Baseline immune activation, exhaustion and senescence profiles in LT-PTM and LT-no-PTM patients. Percentages of (A) activated CD8+ (CD3+CD8+CD38+HLA-DR+), CD4+ (CD3+CD4+CD38+HLA-DR+) and memory B (CD19+CD10-CD21-CD27+) cells; (B) exhausted CD8+ (CD3+CD8+PD-1+), CD4+ (CD3+CD4+PD-1+) and B (CD19+PD-L1+) cells, (C) senescent-like CD8+ (CD3+CD8+CD28-CD57+), CD4+ (CD3+CD4+CD28-CD57+), and B (CD19+CD27-IgD-) cells in LT-PTM and LT-no-PTM patients. All p-values were adjusted by age.
The percentage of circulating immune cells expressing markers of exhaustion was significantly higher in LT-PTM than in LT-no-PTM patients (%CD8+PD-1+: 19.81 (16.97-28.90) vs 7.78 (4.62-14.67), p=0.000; %CD4+PD-1+: 22.71 (17.96-27.89) vs 7.75 (4.47-10.85), p=0.000; %CD19+PD-L1+: 10.15 (7.82-19.75) vs 5.01 (2.23-9.09), p=0.015) (Figure 2B). These results were confirmed in the logistic regression model, which showed that high percentages of exhausted T and B cells were associated with a higher risk of being in the PTM group (Table 3).
The percentage of senescent-like CD4+ T cells and B cells tended to be higher in the LT-PTM than in LT-no-PTM patients (Figure 2C). This trend to significance was confirmed in the logistic regression model; high percentages of senescent-like CD4+ T cells and B cells were associated with a higher risk of being in the PTM group (Table 3).
To estimate the impact of HCC as a cause of transplantation on the development of a PTM, the LT-PTM patients were stratified according to their LT-HCC and LT-no-HCC status and compared to overall LT-no-PTM patients. Several observations, taken with caution given the low number of PTM, were possible. At baseline, percentages of activated and exhausted CD8+, CD4+ T cells and B cells were higher in LT-PTM patients compared to LT-no-PTM patients in both groups (Figure 2 supplementary). Notably, within the LT-HCC group the percentages of senescent-like CD8+ and CD4+ T cells were significantly higher in LT-PTM patients than in LT-no-PTM patients (Figure 2 supplementary).
Immune Activation and Immune Senescence Profiles at Follow-Up in LT-PTM and mLT-no-PTM Patients
To further investigate overtime the association between immune activation and immune senescent profiles and onset of PTM, the immunological parameters were evaluated and compared between LT-PTM patients and a matched group of 26 mLT-no-PTM (see Materials and Methods and Table 1b Supplementary) at follow-up (i.e. closest to the tumor onset in LT-PTM patients and with the same median time interval for mLT-no-PTM patients) (Table 2a Supplementary).
At follow-up, the percentages of activated and exhausted CD8+, CD4+ T cells and B cells were significantly higher in LT-PTM patients compared to mLT-no-PTM (Table 2a Supplementary). Regarding senescence, levels of senescent-like B cells were significantly higher in LT-PTM compared to mLT-no-PTM (%CD19+CD27-IgD-: 19.39 (18.56-20.95) vs 10.08 (5.33-12.83), p=0.002), while no difference was observed in senescent-like CD8+ and CD4+ T cells (Table 2a Supplementary). These results were confirmed by logistic regression model (Table 2b Supplementary).
Circulating Levels of PAMPs and DAMPs
At baseline, levels of 16S rDNA were significantly higher in LT-PTM compared to mLT-no-PTM (73.69 (61.38-120.94) vs 37.79 (17.99-54.06) copies/µl, p=0.008); since no difference occurred in the two groups during follow-up, this level still remained higher at follow-up (64.95 (29.51-106.15) vs 26.99 (11.27-58.74) copies/µl, p= 0.074) (Figure 3A). Similarly, at baseline, mtDNA levels tended to be higher in LT-PTM compared to mLT-no-PTM (3369 (2169-9570) vs 1822 (985-3984) copies/µl, p=0.096), but they become significantly higher at follow-up (3075 (2187-6244) vs 1668 (447-2424) copies/µl, p= 0.040) (Figure 3B).
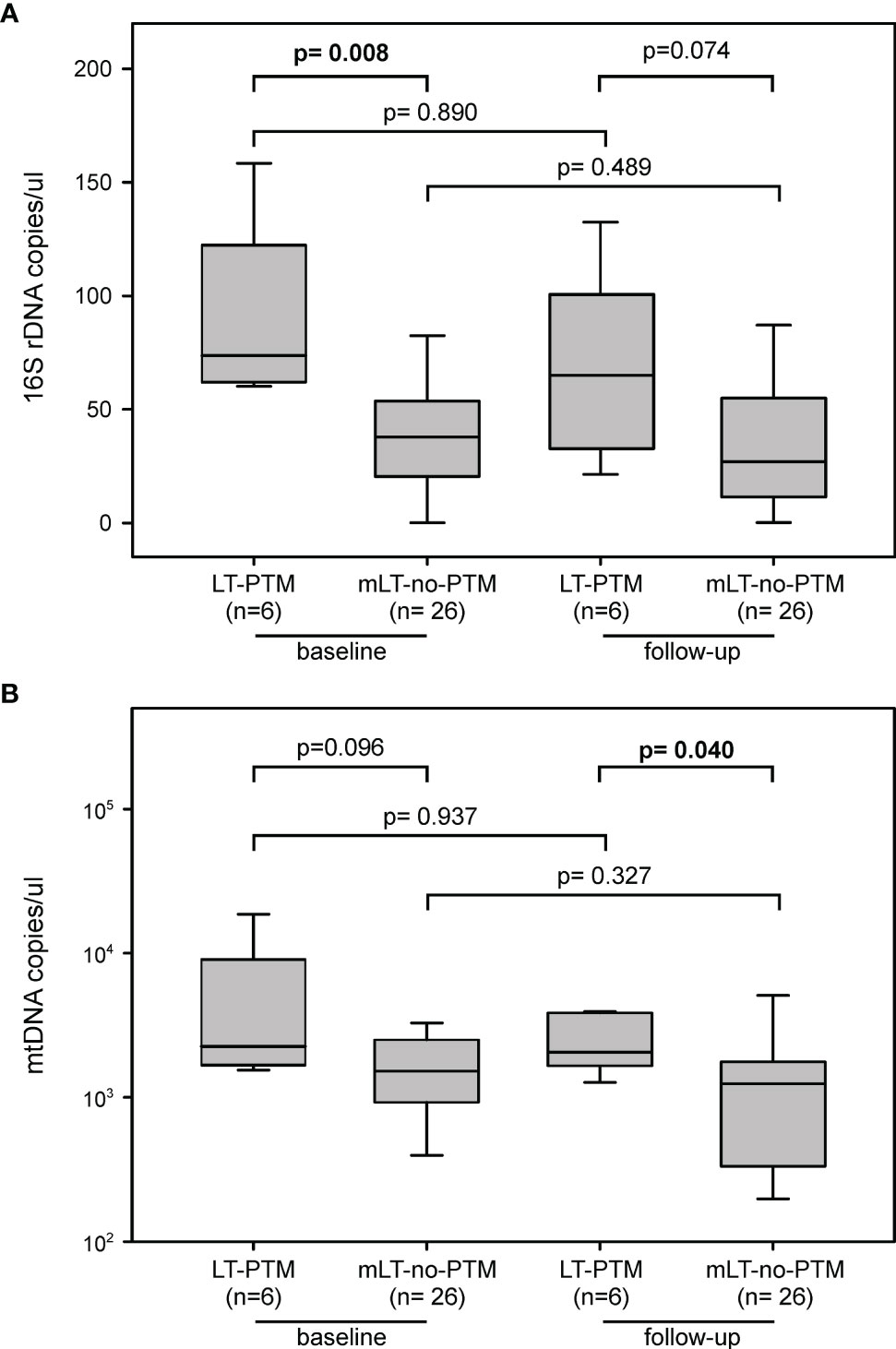
Figure 3 Circulating markers of microbial translocation between LT-PTM and mLT-no-PTM at baseline and follow-up. Circulating levels of (A) PAMPs (16S rDNA) and (B) DAMPs (mtDNA) in LT-PTM versus mLT-no-PTM patients at baseline and follow-up.
Telomere Length
At baseline, relative telomere lengths (RTL) on PBMC were longer, although not statistically significant, in LT-PTM than in mLT-no-PTM patients (RTL: 1.46 (1.03-1.63) vs 1.08 (0.80-1.63), p=0.391). Notably, at follow-up, the levels did not differ between the two groups (RTL: 0.79 (0.73-0.80) vs 0.77 (0.75-0.83), p=0.873), thus indicating that LT-PTM patients tended to have a higher telomere erosion during the same follow-up interval (ΔFU-baseline: -0.66 (-0.75; -0.29) vs -0.09 (-0.48; -0.04), p=0.091).
Discussion
Liver transplantation represents the best treatment choice for patients with acute or chronic liver failure and for a fraction of those with HCC (6); however, after successful liver transplantation, de novo malignancies (DNMs) and HCC recurrence are the most common causes of long-term morbidity and death (7, 8, 25). The need of identifying biological markers predictive of the risk to develop post-transplant malignancies is therefore clinically important. To the best of our knowledge, this is the first study assessing the possible role of immune activation, exhaustion, and senescence profiles as biomarkers potentially useful to identify liver transplanted patients at higher risk to develop post-transplant tumors.
The gut-liver axis is widely implicated in the pathogenesis of liver diseases and most of the gut bacterial communities have the capacity to colonize multiple tumor milieu (26, 27). Detrimental inflammatory responses after solid organ transplantation are initiated when immune cells bind, through the Toll-like receptor, PAMPs and DAMPs released during transplant-associated processes (i.e. ischemia/reperfusion injury, surgical trauma, and recipient conditioning) and/or the constant exposure to triggering insults in the liver (as chronic viral hepatitis infection or alcohol use) (14). Translocation of PAMPs and DAMPs into circulation may lead to the abnormal activation of the immune system, which is considered a critical step for post-transplant tumorigenesis (9, 28). However, very few data are available on the role of aberrant immune activation in the complex process of cancerogenesis occurring in liver transplanted patients. A previous study suggested no difference in the expression of activated CD8+CD38+ T cells between patients who developed malignancies and non-cancer patients (29), but in a Danish cohort of solid organ transplant recipients, older age and elevated levels of C-reactive protein, a circulating marker of inflammation, correlated with a high risk for cancer development (30). In this study, levels of 16S rDNA and mtDNA, markers of PAMPs and DAMPs respectively, were higher at baseline in LT-PTM than in LT-no-PTM patients and remained higher at tumor onset. Consistently, immune activation identified by high numbers of CD38+HLA-DR+ T cells and CD10-CD21-CD27+ B cells, was associated with a higher risk of developing a post-transplant malignancy, regardless of the cause of liver transplantation. Overall, these data suggest that the persistent immune activated status, likely induced by circulating levels of microbial translocation markers after transplantation, itself represents an important risk factor for tumor onset.
Aging is a well-known risk factor for tumor development. The exhaustion and senescence of T cells are two dysfunctional states in chronic infections and cancers (31, 32). Differently from exhausted T lymphocytes, senescent cells may retain their cytotoxic potential and ability to secrete high levels of inflammatory cytokines, contributing to the SASP phenotype that plays crucial roles in promoting cancer (33–35). Senescent T cells accumulate in the peripheral blood of patients with solid tumours and represent a potential prognostic biomarker (32, 33). Here, we found that patients who developed a PTM tended to have at baseline higher levels of immune senescent-like cells than no-PTM patients; in particular, within the cohort of patients transplanted for HCC, the numbers of circulating senescent-like CD4+ and CD8+ T cells were significantly higher, thus suggesting that immune senescence at baseline may be an additional predictive marker in patients transplanted for HCC. Our results concerning the telomere length tend to support this possibility. Telomeres are repetitive DNA sequences located at the ends of the chromosomes and intact telomeres are essential for genomic integrity and the maintenance of the cellular proliferative potential (36). Inflammation and telomere shortening are hallmarks of the early stages of chronic liver disease and play a pivotal role in the early onset of neoplasia (37, 38). In a previous study, we found that low telomere length is associated with a higher risk of tumor onset (17). In the transplantation field, shorter telomere length in peripheral blood leukocytes was associated with a significantly worse clinical response in patients receiving immune checkpoint inhibitor therapy across different malignancies and the calculated relative telomere length differences defined as “delta telomere length” was found to be an independent predictor of overall survival (39). In our study, liver transplanted patients who developed a tumor presented a longer telomere length at baseline, but tended to undergo to a higher telomere erosion between the baseline and the tumor onset compared to LT-no-PTM patients during the same follow-up period.
In conclusion, our findings suggest that circulating biomarkers of immune activation and exhaustion may usefully predict the risk of tumor development in liver transplanted patients, regardless of the cause of transplantation. At time of transplantation, LT-HCC carried an immune activated status, most likely derived from the primary tumor, which was not solved by liver transplantation and persisted long after that, leading to a higher accumulation of immune senescent cells and a progressive telomere erosion. These abnormalities could contribute to an enhanced and accelerated promotion of tumorigenesis in liver transplanted patients resulting in an earlier onset of post-transplant cancers, as compared to patients transplanted for other causes. Confirmation of these findings in a larger prospective series of liver transplanted patients may provide the rationale for the exploitation of immune activation, exhaustion and senescence profiles as clinically useful markers to be included in the follow up laboratory routine to identify patients at higher risk to develop post-transplant tumors.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical Committee of Padova University Hospital (Prot. 4231/AO/17). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MRP, SS, MT, PP, DS, PB and ADR, designed the study. MRP, ER, FC, SG, performed the investigations. SS, DS, UC, PB, provided samples and reagents. PDB performed the statistical analysis. MRP wrote the manuscript. SS, MT, SG, PDB, PP, DS, UC, RD, PB and ADR supervised the project, reviewed and editing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Ricerca Corrente IRCCS Centro di Riferimento Oncologico, Aviano: Italian Association for Research on Cancer; Grant number: AIRC IG No. 19112; and “Pathogenesis of EBV-driven post-transplant malignancies” granted by Department of Surgery, Oncology and Gastroenterology (DiSCOG), University of Padova, Italy (Grant no. BIRD181981/18, PI ADR).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to Alessandra Maggiora for English editing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.899170/full#supplementary-material
References
1. Centro Nazionale Trapianti. Ministero della Salute. Available at: http://www.trapianti.salute.gov.it.
2. Acuna SA, Fernandes KA, Daly C, Hicks LK, Sutradhar R, Kim SJ, et al. Cancer Mortality Among Recipients of Solid-Organ Transplantation in Ontario, Canada. JAMA Oncol (2016) 2(4):463–9. doi: 10.1001/jamaoncol.2015.5137
3. Acuna SA. Etiology of Increased Cancer Incidence After Solid Organ Transplantation. Transplant Rev (Orlando) (2018) 32(4):218–24. doi: 10.1016/j.trre.2018.07.001
4. Taborelli M, Piselli P, Ettorre GM, Lauro A, Galatioto L, Baccarani U, et al. Risk of Virus and Non-Virus-Related Malignancies Following Immunosuppression in a Cohort of Liver Transplant Recipients. Italy, 1985-2014. Int J Cancer (2018) 143(7):1588–94. doi: 10.1002/ijc.31552
5. Sérée O, Altieri M, Guillaume E, De Mil R, Lobbedez T, Robinson P, et al. Long-Term Risk of Solid Organ De Novo Malignancies After Liver Transplantation: A French National Study on 11,226 Patients. Liver Transpl (2018) 24(10):1425–36. doi: 10.1002/lt.25310
6. Yang JD, Larson JJ, Watt KD, Allen AM, Wiesner RH, Gores GJ, et al. Hepatocellular Carcinoma Is the Most Common Indication for Liver Transplantation and Placement on the Waitlist in the United States. Clin Gastroenterol Hepatol (2017) 15(5):767–75. doi: 10.1016/j.cgh.2016.11.034
7. Kong J, Yu G, Si W, Li G, Chai J, Liu Y, et al. Second Primary Malignancies in Patients With Hepatocellular Carcinoma: A Population-Based Analysis. Front Oncol (2021) 11:713637. doi: 10.3389/fonc.2021.713637
8. Shalaby S, Taborelli M, Zanetto A, Ferrarese A, D'Arcangelo F, Gambato M, et al. Hepatocellular Carcinoma and the Risk of De Novo Malignancies After Liver Transplantation - A Multicenter Cohort Study. Transpl Int (2021) 34(4):743–53. doi: 10.1111/tri.13831
9. Yu LX, Ling Y, Wang HY. Role of Nonresolving Inflammation in Hepatocellular Carcinoma Development and Progression. NPJ Precis Oncol (2018) 2(1):6. doi: 10.1038/s41698-018-0048-z
10. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A Global View of Hepatocellular Carcinoma: Trends, Risk, Prevention and Management. Nat Rev Gastroenterol Hepatol (2019) 16(10):589–604. doi: 10.1038/s41575-019-0186-y
11. Rico Montanari N, Anugwom CM, Boonstra A, Debes JD. The Role of Cytokines in the Different Stages of Hepatocellular Carcinoma. Cancers (Basel) (2021) 13(19):4876. doi: 10.3390/cancers13194876
12. Petrara MR, Giunco S, Serraino D, Dolcetti R, De Rossi A. Post-Transplant Lymphoproliferative Disorders: From Epidemiology to Pathogenesis-Driven Treatment. Cancer Lett (2015) 369(1):37–44. doi: 10.1016/j.canlet.2015.08.007
13. Todd JL, Palmer SM. Danger Signals in Regulating the Immune Response to Solid Organ Transplantation. J Clin Invest (2017) 127(7):2464–72. doi: 10.1172/JCI90594
14. Dwyer GK, Turnquist HR. Untangling Local Pro-Inflammatory, Reparative, and Regulatory Damage-Associated Molecular-Patterns (DAMPs) Pathways to Improve Transplant Outcomes. Front Immunol (2021) 12:611910. doi: 10.3389/fimmu.2021.611910
15. Aunan JR, Cho WC, Søreide K. The Biology of Aging and Cancer: A Brief Overview of Shared and Divergent Molecular Hallmarks. Aging Dis (2017) 8(5):628–42. doi: 10.14336/AD.2017.0103
16. Niklander SE, Lambert DW, Hunter KD. Senescent Cells in Cancer: Wanted or Unwanted Citizens. Cells (2021) 10(12):3315. doi: 10.3390/cells10123315
17. Falci C, Gianesin K, Sergi G, Giunco S, De Ronch I, Valpione S, et al. Immune Senescence and Cancer in Elderly Patients: Results From an Exploratory Study. Exp Gerontol (2013) 48(12):1436–42. doi: 10.1016/j.exger.2013.09.011
18. Giunco S, Petrara MR, Bergamo F, Del Bianco P, Zanchetta M, Carmona F, et al. Immune Senescence and Immune Activation in Elderly Colorectal Cancer Patients. Aging (Albany NY) (2019) 11(11):3864–75. doi: 10.18632/aging.102022
19. European Association for the Study of the Liver, European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. Electronic address: easloffice easloffice.eu.
20. Gianesin K, Noguera-Julian A, Zanchetta M, Del Bianco P, Petrara MR, Freguja R, et al. Premature Aging and Immune Senescence in HIV-Infected Children. AIDS (2016) 30(9):1363–73. doi: 10.1097/QAD.0000000000001093
21. Rampazzo E, Cecchin E, Del Bianco P, Menin C, Spolverato G, Giunco S, et al. Genetic Variants of the TERT Gene, Telomere Length, and Circulating TERT as Prognostic Markers in Rectal Cancer Patients. Cancers (Basel) (2020) 12(11):3115. doi: 10.3390/cancers12113115
22. Petrara MR, Penazzato M, Massavon W, Nabachwa S, Nannyonga M, Mazza A, et al. Epstein-Barr Virus Load in Children Infected With Human Immunodeficiency Virus Type 1 in Uganda. J Infect Dis (2014) 210(3):392–9. doi: 10.1093/infdis/jiu099
23. Petrara MR, Serraino D, Di Bella C, Neri F, Del Bianco P, Brutti M, et al. Immune Activation, Immune Senescence and Levels of Epstein Barr Virus in Kidney Transplant Patients: Impact of mTOR Inhibitors. Cancer Lett (2020) 469:323–31. doi: 10.1016/j.canlet.2019.10.045
24. Petrara MR, Cattelan AM, Sasset L, Freguja R, Carmona F, Sanavia S, et al. Impact of Monotherapy on HIV-1 Reservoir, Immune Activation, and Co-Infection With Epstein-Barr Virus. PLos One (2017) 12(9):e0185128. doi: 10.1371/journal.pone.0185128
25. Taborelli M, Piselli P, Ettorre GM, Baccarani U, Burra P, Lauro A, et al. Survival After the Diagnosis of De Novo Malignancy in Liver Transplant Recipients. Int J Cancer (2019) 144(2):232–9. doi: 10.1002/ijc.31782
26. Human Microbiome Project Consortium. A Framework for Human Microbiome Research. Nature (2012) 486(7402):215–21. doi: 10.1038/nature11209
27. Lloyd-Price J, Mahurkar A, Rahnavard G, Crabtree J, Orvis J, Hall AB, et al. Strains, Functions and Dynamics in the Expanded Human Microbiome Project. Nature (2017) 550(7674):61–6. doi: 10.1038/nature23889
28. Nakagawa H, Maeda S. Inflammation- and Stress-Related Signaling Pathways in Hepatocarcinogenesis. World J Gastroenterol (2012) 18(31):4071–81. doi: 10.3748/wjg.v18.i31.4071
29. Boleslawski E, Othman SB, Aoudjehane L, Chouzenoux S, Scatton O, Soubrane O, et al. CD28 Expression by Peripheral Blood Lymphocytes as a Potential Predictor of the Development of De Novo Malignancies in Long-Term Survivors After Liver Transplantation. Liver Transplant (2011) 17(3):299–305. doi: 10.1002/lt.22232
30. Wareham NE, Li Q, Sengeløv H, Da Cunha-Bang C, Gustafsson F, Heilmann C, et al. Risk of De Novo or Secondary Cancer After Solid Organ or Allogeneic Haematopoietic Stem Cell Transplantation. J Cancer Res Clin Oncol (2019) 145(12):3125–35. doi: 10.1007/s00432-019-03039-2
31. Zhao Y, Shao Q, Peng G. Exhaustion and Senescence: Two Crucial Dysfunctional States of T Cells in the Tumor Microenvironment. Cell Mol Immunol (2020) 17(1):27–35. doi: 10.1038/s41423-019-0344-8
32. Zhang J, He T, Xue L, Guo H. Senescent T Cells: A Potential Biomarker and Target for Cancer Therapy. eBioMedicine (2021) 68:103409. doi: 10.1016/j.ebiom.2021.103409
33. Ferrara R, Naigeon M, Auclin E, Duchemann B, Cassard L, Jouniaux JM, et al. Circulating T-Cell Immunosenescence in Patients With Advanced Non-Small Cell Lung Cancer Treated With Single-Agent PD-1/PD-L1 Inhibitors or Platinum-Based Chemotherapy. Clin Canc Res (2021) 27(2):492–503. doi: 10.1158/1078-0432.CCR-20-1420
34. Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-Induced Gut Microbial Metabolite Promotes Liver Cancer Through Senescence Secretome. Nature (2013) 499(7456):97–101. doi: 10.1038/nature12347
35. Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, et al. Gut Microbiota Promotes Obesity-Associated Liver Cancer Through PGE 2-Mediated Suppression of Antitumor Immunity. Cancer Discov (2017) 7(5):522–38. doi: 10.1158/2159-8290.CD-16-0932
36. Blasco MA. Telomere Length, Stem Cells and Aging. Nat Chem Biol (2007) 3(10):640–9. doi: 10.1038/nchembio.2007.38
37. Ramakrishna G, Rastogi A, Trehanpati N, Sen B, Khosla R, Sarin SK, et al. From Cirrhosis to Hepatocellular Carcinoma: New Molecular Insights on Inflammation and Cellular Senescence. Liver Cancer (2013) 2(3-4):367–83. doi: 10.1159/000343852
38. Liu W, Deng Y, Li Z, Chen Y, Zhu X, Tan X, et al. Cancer Evo-Dev: A Theory of Inflammation-Induced Oncogenesis. Front Immunol (2021) 12:768098. doi: 10.3389/fimmu.2021.768098
Keywords: immune activation, immune senescence, post-transplant malignancy, hepatocellular carcinoma, biological predictors
Citation: Petrara MR, Shalaby S, Ruffoni E, Taborelli M, Carmona F, Giunco S, Del Bianco P, Piselli P, Serraino D, Cillo U, Dolcetti R, Burra P and De Rossi A (2022) Immune Activation, Exhaustion and Senescence Profiles as Possible Predictors of Cancer in Liver Transplanted Patients. Front. Oncol. 12:899170. doi: 10.3389/fonc.2022.899170
Received: 18 March 2022; Accepted: 16 May 2022;
Published: 13 June 2022.
Edited by:
Adriana Albini, MultiMedica Holding SpA (IRCCS), ItalyReviewed by:
Serena De Matteis, IRCCS Azienda Ospedaliero-Universitaria di Bologna, ItalyNicolo' Pernigoni, Institute of Oncology Research (IOR), Switzerland
Copyright © 2022 Petrara, Shalaby, Ruffoni, Taborelli, Carmona, Giunco, Del Bianco, Piselli, Serraino, Cillo, Dolcetti, Burra and De Rossi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anita De Rossi, YW5pdGEuZGVyb3NzaUB1bmlwZC5pdA==
 Maria Raffaella Petrara
Maria Raffaella Petrara Sarah Shalaby
Sarah Shalaby Elena Ruffoni
Elena Ruffoni Martina Taborelli
Martina Taborelli Francesco Carmona
Francesco Carmona Silvia Giunco
Silvia Giunco Paola Del Bianco
Paola Del Bianco Pierluca Piselli
Pierluca Piselli Diego Serraino
Diego Serraino Umberto Cillo
Umberto Cillo Riccardo Dolcetti
Riccardo Dolcetti Patrizia Burra
Patrizia Burra Anita De Rossi
Anita De Rossi