
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 31 October 2022
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.898966
Colorectal diseases are threatening human health, especially inflammatory bowel disease (IBD) and colorectal cancer (CRC). IBD is a group of chronic, recurrent and incurable disease, which may affect the entire gastrointestinal tract, increasing the risk of CRC. Eukaryotic gene expression is a complicated process, which is mainly regulated at the level of gene transcription and mRNA translation. Protein translation in tissue is associated with a sequence of steps, including initiation, elongation, termination and recycling. Abnormal regulation of gene expression is the key to the pathogenesis of CRC. In the early stages of cancer, it is vital to identify new diagnostic and therapeutic targets and biomarkers. This review presented current knowledge on aberrant expression of eIFs, eEFs and eRFs in colorectal diseases. The current findings of protein synthesis on colorectal pathogenesis showed that eIFs, eEFs and eRFs may be potential targets for CRC treatment.
Colorectal cancer (CRC) is the third highest morbidity rate worldwide. It is also the second most common cause of cancer-related death (1). The incidence and fatality rate of CRC had increased in recent years, especially in developing countries (2). Even though many CRC patients are diagnosed early and undergo therapeutic surgery, chemotherapy and radiation therapy, the metastases and relapses still occurred in many patients (3, 4).
Inflammatory bowel disease (IBD) is an idiopathic, chronic inflammatory disorder of uncertain etiology with an underlying genetic predisposition. It includes Crohn’s disease (CD) and ulcerative colitis (UC) (5, 6). Although some intrinsic factors, such as host genetics, dysregulated immune responses, and microbial dysbiosis have been identified, the pathogenesis of IBD remained unclear (7–9). Nevertheless, there are unequivocal evidences of an association between IBD and CRC. A chronic inflammatory process is one of the responsible causes for the development of CRC (10).
Protein translation comprises several steps: initiation, elongation, termination and ribosomal recycling (11). Eukaryotic initiation factors (eIFs) played an essential part in the initiation of translation. The eIF signaling cascade is mainly influenced by the PI3K/AKT/mTOR pathway, regulating cell growth and proliferation (12–14). Eukaryotic elongation factors (eEFs) are active during protein chain elongation. They had been reported to be aligned by aminoacyl-tRNAs via their specific codons in mRNA (decoding), peptide bond synthesis, and movement of the mRNA associated with ribosome translocation. eEF1α, eEF1βγ and eEF2 facilitate these processes on the ribosome. Finally, the termination process is a release of the completed polypeptide chain, which requires eukaryotic release factors (eRFs).
Deregulation of protein synthesis results in abnormal gene expression, possibly bringing about uncontrolled cell growth, cancer development and progression (15). Deregulation of translation is related to colorectal pathogenesis of IBD and CRC. Previous studies had shown that dysregulation of eIFs, eEFs and eRFs is associated with cancer progression and malignant transformation (16, 17). Here, we reviewed the current research findings about eIFs, eEFs and eRFs, to demonstrate that they may be potential targets for IBD and CRC treatment.
eIF1 is a universally conserved translation factor with 113 amino acid (AA) and an important intermediary for initiating codon recognition in negative regulation. eIF1a is a 144AA long protein encoded on the X chromosome. eIF1a together with eIF2, 3, 4A, 4B and 4F triggers preinitiation complex formation. eIF1 and eIF1a have synergistically effects. Translation initiation induced by eIF1 had been shown to occur independently of p53. eIF1 was found to be increased a series of disease risks, such as aneurysmal bone cyst (18), Parkinson’s disease (19), hepatocellular carcinoma (HCC) (20), breast cancer (21) and ductal adenocarcinoma (22).
Enhanced expression levels of eIF1 indicates poor prognosis of CRC patients (14). eIF1 gene knockdown led to a significantly reduced proliferation rate and clonogenicity. eIF1 protein was overexpressed in low-grade and high-grade colon cancer (CC), and eIF4B protein was elevated in low-grade CC. The mRNA and protein expression level of eIF1 was significantly increased in rectum carcinoma (RC) tissues compared to normal colorectal mucosa tissues and low-grade CC. Although IBD is also closely associated with an increased risk of CRC, the relationship between eIF1 and IBD remained elusive.
eIF2 is a ternary complex involved in the formation of the eIf2-Met-tRNAi-GTP complex. At the initial stage of translation, the eIf2-GTP is hydrolyzed to eIF2-GDP. Then, the eIF2 gene, called eIF2AKs, is phosphorylated by four stress-responsive kinases. These include double-stranded heme-regulated kinase (HRI, also known as EIF2AK1), RNA-induced protein kinase (PKR, EIF2AK2), PKR like endoplasmic reticulum kinase (PERK, EIF2AK3) and control non-repressed two kinases (GCN2, EIF2AK4) at Ser51.
These four kinases phosphorylate eIF2 upon different events. eIF2B levels are lower than eIF2 in cells, so partial phosphorylation is sufficient to attenuate the initiation of protein synthesis. The mRNAs of a series of stress responses are resistant or stimulated by decreased the eIF2-GTP/Met-tRNAi ternary complex levels. The response is often been called the integrated stress response (ISR), and aberrant ISR has been linked to many human diseases (23, 24). eIF2a was been found differentially expressed in gastrointestinal cancer and lymphoma subtypes (25, 26).
PRK was found to play a critical role in inflammasome activation, and interact with multiple inflammasome components, including the pyrin domain-containing 3 (NLRP3) of NLR family. The NLRP3 inflammasome is involved in the pathogenesis of IBD (27). Previous studies had shown that overexpression of EIF2AK2 could increase the activity of NLRP3 polymorphism during the development of IBD (28, 29). GCN2 is one of the vital coordinating factors of ISR. Researches reported that GCN2 has a protective effect on DSS-induced colitis in mice by inducing autophagy (30). Phosphorylated eIF2α had been reported to promote translation of the activating transcription factor 4 (ATF4) (31). PERK was known as one of the sensors of the ISR. Regulation of the PERK-eIF2α-ATF4 signaling pathway by inhibiting the dephosphorylation of eIF2α improves the clinical and histological effects of DSS-induced colitis in mice. PERK-eIF2α-ATF4 signaling pathway is a potential therapeutic target for IBD therapy (Figure 1) (28, 32). PERK activation had been shown to play an essential part in chemically induced apoptosis and contributes to G2/M arrest (33). The small-molecule PERK inhibitor may be used to activate the proapoptotic processin CRC cells (34). In addition, the study had shown that SPARC combined with GRP78 makes CRC cells sensitive to PERK/eIF2α and IRE1α/XBP-1 UPR signals by interfering with ER stress, resulting in the death of CRC cells (35). Smad7 knockout was related to inactivation of small eIF2 cells, decreased CDC25A expression, and partial reduction of proliferative cells in human CRC explants, as well as reduction numbers of intestinal tumors in Apc(min/+) mice (36).
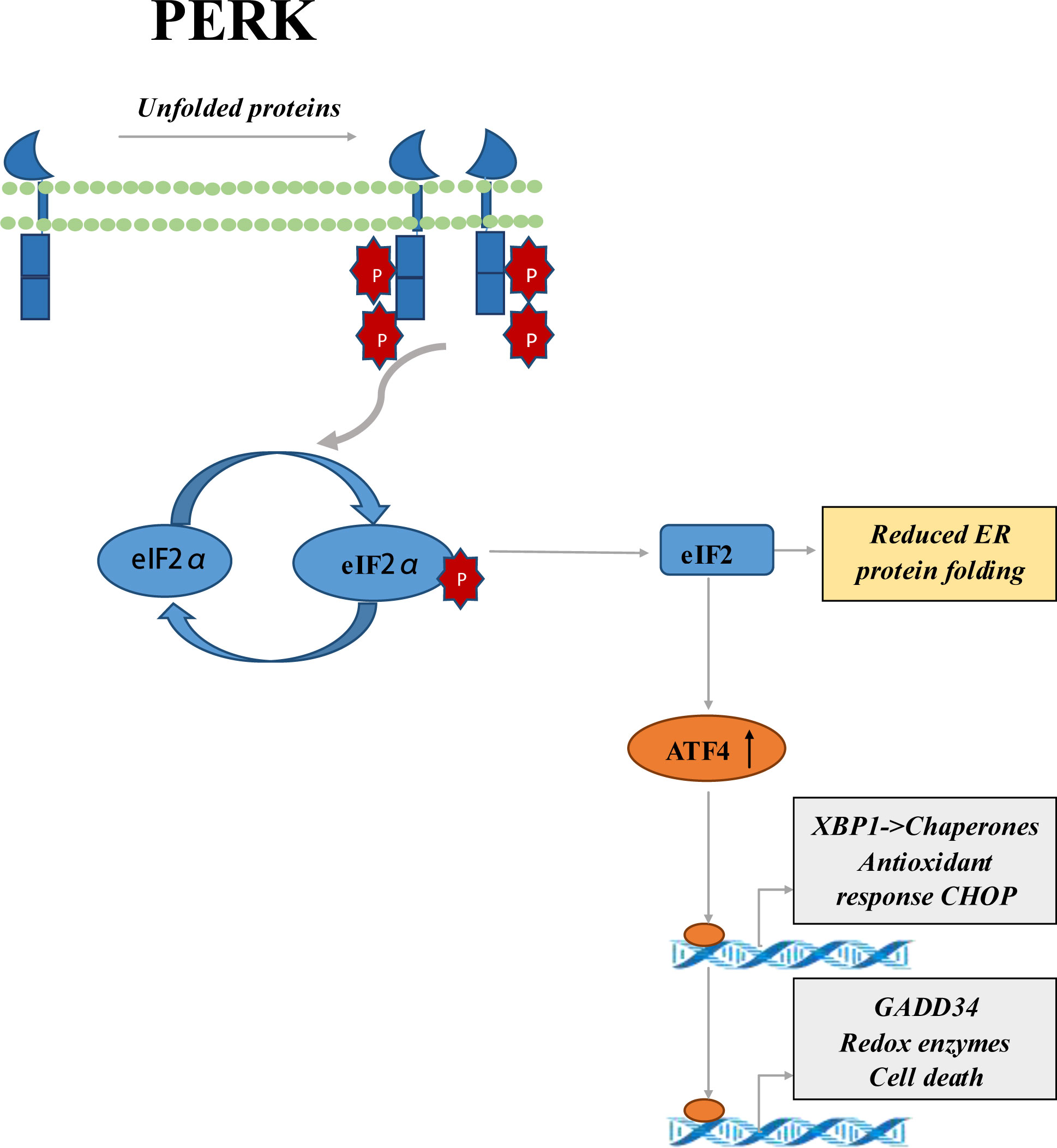
Figure 1 Schematic representation of PERK-eIF2α-ATF4 signaling pathway. It’s sensitive to ER stress, so PERK is activated. PERK activation leads to phosphorylation of the eIF2α ring, which inhibits protein translation. Phosphorylated eIF2α enhances the translation of ATF4, a transcriptional activator of genes associated with metabolism and nutrition, cellular redox status, and apoptosis regulation. ATF4 mediates the production of transcriptional factor CHOP, and the upregulation of CHOP can aggravate the development of colitis.
eIF3 is the largest and most complex translation initiation factor in mammalian cells, with a molecular weight of about 550-800kDa. The eIF3 complex consists of 13 subunits, called EIF3A-M, and members of eIF3 family undertake various tasks during translation initiation.
The overexpression of eIF3a had been reported in several cancers, such as breast (37), lung (38), esophagus (39) and cervical cancer (40). eIF3a may play an important part in colon epithelial cell differentiation (41). The expression of eIF3a was also increased in CRC (42). eIF3a binds to phosphorylated eIF4b, facilitating the translation of IRES dependent proteins such as myc. The adenomatous polyposis coli (APC) gene mutations are tightly related to CRC (43). eIF3b was overexpressed in pancreatic Cancer (44), gastric cancer (45), as well as chronic myeloid leukemia (46). Overexpressed eIF3c was found in cholangiocarcinoma, lung adenocarcinoma and prostate cancer (47, 48). eIF3d is responsible for protein synthesis, which had been reported to play a carcinogenic part in CRC. eIF3d knockout significantly induced more HCT116 cells to accumulate in the sub-G1 phase, suggesting apoptotic cells increased after eIF3d knockout (49). eIF3e, also termed INT6, interacts with the interferon-induced protein P56. It reported that overexpression of eIF3e promoted CRC cell proliferation and decreased the overall survival of CRC patients (50). Decreased expression of eIF3e was also reported in breast (51) and lung cancer (52). eIF3f may be a vital regulator of cell migration, invasion, bioenergetics and metastasis. It is downregulated in cancers exemplified in melanoma, lung cancer and pancreatic cancer (53). A study showed that eIF3g is a targeted regulator of CRC chemotherapeutic resistance (54). A variant of eIF3h (rs16892766) was discovered to be associated with higher CRC risk (55). eIF3i is a proto-oncogene that causes CRC by directly upregulating the synthesis of COX-2 protein, activating the β-catenin/TCF4 signaling pathway (56). eIF3m is considered an indicator of poor prognosis in patients with CRC (57, 58).
eIF4 is a protein complex that promotes mRNA recruitment to a preassembled 43S preinitiation complex. eIF4 complex is composed of eIF4b and eIF4F complex, which is composed of eIF4a, eIF4e and eIF4g (59, 60). The presence of eIF4F complexe is critical for cap binding and subsequent RNA helicase activity that leads to protein translation. eIF4a is independent of the eIF4F complex, which stimulates eIF4a activity and promotes mRNA recruitment to the ribosome. eIF4b and eIF4e are regulated by the PI3K/AKT/mTOR signaling pathway. eIF4b activates S6K kinase, which is responsible for the phosphorylation of eIF4b at Ser-422120. The ability of eIF4E recognizing caps is regulated by binding to eIF4E binding proteins (4E-BP). Phosphorylation mediated by mTORC1 inhibited the activity of 4E-BP. When the binding of 4E-BP1 to eIF4e was weakened, subsequently the release of eIF4e was weakened (61–63). The result suggested that increased expression of eIF4E may be a vital factor for development of breast cancer (64). A case-matched and sex-matched transcriptome screening identified that eIF4E and eIF5 act as potential prognostic markers for male breast cancer (65). Studies had shown that targeting MUC1-C by GO-203 can inhibit the AKT-S6K-eIF4A pathway and block the proliferation and survival of CRC cells (66). Overexpression of eIF4e was reported to associated with poor prognosis in CRC patients (67, 68).
eIF5 is the GTPase activating protein (GAP) of eIF2 and plays a vital role in the initiation of translation, which may inhibit the guanine nucleotide exchange factor eIF2B (69). eIF5A, containing the unusual amino acid hypusine, which had been shown to stimulate ribosomal peptidyl activity and promote prolonged translation (70, 71). Overexpression of eIF5A may cause increased expression of p53 targets as well as p53-dependent apoptosis, so it was described as positive regulator of p53 (72).
In a recent study of Golob-Schwarzl and colleagues, overexpression of eIF5 was observed in CC and RC, and associated with survival rate (14). The overexpression of eIF5A had also been reported to be associated with poor prognosis in CRC (73). eIF5a was showed to induce apoptosis in CRC cells (HTC116 and HT29) and was associated with response of nucleus to tumor necrosis factor (TNF) signaling (74). The study had shown that upregulation of eIF5A2 could enhance epithelial mesenchymal transition (EMT) in CRC cells (HCT116 and HT29), and downregulation of eIF5A2 enhanced the chemosensitivity to doxorubicin in eIF5A2-positive cells (75). Deletion of eIF5B led an increase in ATF4 transcriptional translation through another mechanism. eIF5B silencing increased the expression of an ATF4-luciferase translational reporter by a mechanism requiring the repressive uORF2 (76). The ATF4 level was found reduced in the inflammatory intestinal mucosa of patients with IBD, so ATF4 plays a crucial role in maintaining intestinal homeostasis (77).
eIF6 is an anti-association factor in translation initiation, by binding to 60S subunits. It prevents premature connection of 40S and the interaction of 60S and 40S subunit, thus preventing the initiation of translation (78). eIF6 was phosphorylated by the complex of RACK1-PKCβII and thus cascaded by Ras. Another studies found that gene transcription of encoding eIF6 was regulated by the receptor Notch-1, which is a key downstream medium of oncogenic Ras (79–82). eIF6 was overexpressed in gallbladder cancer, head and neck cancer, non-small cell lung cancer and ovarian serous adenocarcinoma (12, 83–85), particularly in metastatic CRC (86). eIF6 plays a role in downstream protein synthesis of PI3K/AKT/mTOR (12). Overexpression of eIF6 increased cancer cell motility and invasion in CRC, in turn silencing of eIF6 significantly reduced the proliferation rate and the clonogenicity in HCT-116 CRC cell lines (12). A recent study by LJ found that eIF6 activated a variety of AKT-related cancer signaling pathways, such as p-AKT\MMP1\cyclinD1\Bcl2. Therefore, eIF6 could regulate cell proliferation, invasion, cell cycle and apoptosis under the background of CRC (87).
eEF1 is a complex factor composed of multiple subunits, responsible for binding to aminoacyl-tRNAs and transferring it to the A-site of ribosomes (88). Ras-driven cancers utilize methyltransfer-like 13 demethylations of eEF1A Lysine55 to increase translation output and promote tumorigenesis in vivo (89, 90). eEF1A1 and eEF1A2 can control cell motility, growth and death (91). Overexpression of eEF1A1 and eEF1A2 was related to a few different cancer types, such as plasmacytomas (92), HCC (93), clear cell renal cell carcinoma (94), breast cancer (95), gastric cancer (96), prostate cancer (97), ovarian cancer (98) and CRC (99). At the genomic level, a significant higher frequency of EEF1A2 copy number variation was found in patients with metastatic than non-metastatic CRC (99). Pellegrino found that EEF1A2 mediated the expression of PI3K/AKT/mTOR axis stabled oncogene MDM4 in HCC (100). eEF1G was found overexpressed in CRC (101).
eEF2 is answerable for the ribosomal translocation at the elongation stage of a polypeptide chain. Another important extension regulator is eEF2K. eEF2K is a Ca2+/calmodulin (CaM)-dependent kinase and a negative regulator of protein synthesis. eEF2 is overexpressed in lung cancer, esophageal squamous cell carcinoma, head and neck squamous cell carcinoma, pancreatic cancer, breast cancer, prostate cancer, non-Hodgkin’s lymphoma, melanoma, GBM and other human cancers (102, 103). It had been reported that in gastric cancer and CRC, overexpression of eEF2 could promote G2/M progression and cell growth in vitro and in vivo (104). Vasamsetti found that Muscarinic acetylcholine receptor (mAChRs) promoted the synthesis of the global protein of SNU-407 CC cells. mAChR-mediated dephosphorylation of eEF2 is regulated by the MEK1/2-ERK1/2 and the PKC pathway (105). mTORC1 gained power from eEF2K to promote translation elongation through S6K (106). mTORC1 had been shown to be an important downstream effector of Wnt signaling in the intestinal tract. The intestinal cell proliferation associated with Wnt signaling requires the mTORC1-S6K-eEF2K-eEF2 axis. eEF2K plays an important role in controlling the initiation of intestinal cancer and adenoma cell proliferation (107). eEF2K was downregulated in CRC, which was independently associated with poor overall survival in CRC patients (108). eEF2K acts as a tumor-suppressor in CRC. By contrast, it is established as an oncogene in other cancer entities like HCC, lung cancer, or triple-negative breast cancer (109).
The termination process of protein synthesis involves the hydrolysis of the final peptide-tRNA bond and the release of the nascent polypeptide. The reaction is mediated by eukaryotic releasing factor 1 (eRF1) and eRF3 proteins. In eukaryotes, when the eRF1-eRF3-GTP ternary complex binds to the termination codon of the ribosome A site, translation terminates (110). eRF1 is responsible for terminating protein biosynthesis by recognizing stop codon, binding ribosome and stimulating peptidyl-tRNA bond (111). eRF3 is a small GTPase that enhances the activity of eRF1. eRF3 is involved in key cellular processes, such as cell cycle regulation, cytoskeleton and apoptosis (112, 113). The N-terminal region of eRF3 contains polyglycine amplification encoded by a stable (GGC) channel in the eRF3/GSPT1 exon 1 gene. Overexpression of GSPT1 mRNA had been reported to be connected with gastric and breast cancer (114, 115). Malta-Vacas found that the GGC12 was present in 2.2% of CRC patients, but not in the CD cases (116).
Ribosome recycling usually occurs after a regular termination triggered by a termination codon. Recycling enables ribosomes and mRNAs to participate in more than one translation (117). ATPase ABCE1 had been shown to be a major ribosome recycling factor, while ABCE1-mediated post-TCs recycling is dependent on the presence of eRF1 at site A (118). eIF3 plays an important part in the post-TCs recycling of eukaryotic cells, promoting ribosome division into 40S subunits of 60S subunits and tRNA-and mRNA-bound after termination. eIF1 also mediates the release of tRNA at the P site, while eIF3j ensures subsequent mRNA dissociation (119).
The occurrence and development of IBD are affected by many factors, such as host genetic susceptibility, intestinal flora, environmental factors and host immune system. Chronic inflammation may significantly increase the risk of cancer. Elevation levels of inflammation-related factors may also interfere with the control of cell proliferation.
eIFs, eEFs and eRFs play major roles in protein synthesis steps. Investigating the details of the eIF2 complex reveals that different eIF2 subunits play important roles in IBD. PRK plays an important part in inflammatorome activation and interacts with a variety of inflammatorome components, including NLRP3. The PERK-eIF2α-ATF4 signaling pathway is a potential therapeutic target for IBD. eIF2a was found overexpressed in gastrointestinal cancer. On the other hand, ATF4 plays a vital role in maintaining intestinal homeostasis, and the loss of eIF5B leads to increased translation of ATF4 transcripts through some mechanisms. There may be an association between eIF5B and IBD. eIFs may represent a new set of players associated with IBD and CRC, opening the door to an new area of GI tract research.
Changes in the expression of eIFs, eEFs and eRFs had been reported in a wide range of tumors, which played different roles in cell proliferation and tumorigenesis. Some of them may act as tumor suppressors, while others may contribute to the occurrence and progression of tumors. The effects of eIFs, eEFs and eRFs on CRC were shown in Table 1. These studies suggested that eIFs, eEFs and eRFs play a key role in CRC development and may be potential targets for CRC therapy.
mTOR, RAS signaling pathways and cell cycle regulation are critical for CRC development. RAS/MAPK and PI3K/AKT/mTOR pathways play key roles in promoting cell proliferation from membrane receptors to the nucleus. 4E-BP1 is one of the downstream molecules that receive signals from several intracellular pathways, including PI3K/AKT/mTOR and RAS/MAPK. 4E-BP1 is phosphorylated by AKT and MAPK. Phosphorylation induced by mTOR at 4E-BP triggers the release of eIF4e, enabling it to cooperate with the eIF4F complex and activate translation initiation. Vascular endothelial growth factor (VEGF) is a translation target for the upregulation of eIF4e, which is targeted directly or via Ras activation. VEGF is highly correlated with the neovascularization often observed in malignant tumors. Phosphorylation of eIF4b increases translation efficiency and binding affinity with eIF3a, which in turn promotes IRES dependent translation of proteins such as myc. Myc activates the transcription of eIF4e through a feedback mechanism, thereby increasing myc expression. The APC gene mutations had been observed in up to 80% of sporadic CRCs, and it plays a role in transcription regulation. Due to APC absent, c-myc expression was upregulated. In addition, mTORC1 had been shown to be an important downstream effector of Wnt signaling in the intestinal tract, while intestinal cells proliferation associated with Wnt signaling requires the mTORC1-S6K-eEF2K-eEF axis (Figure 2).
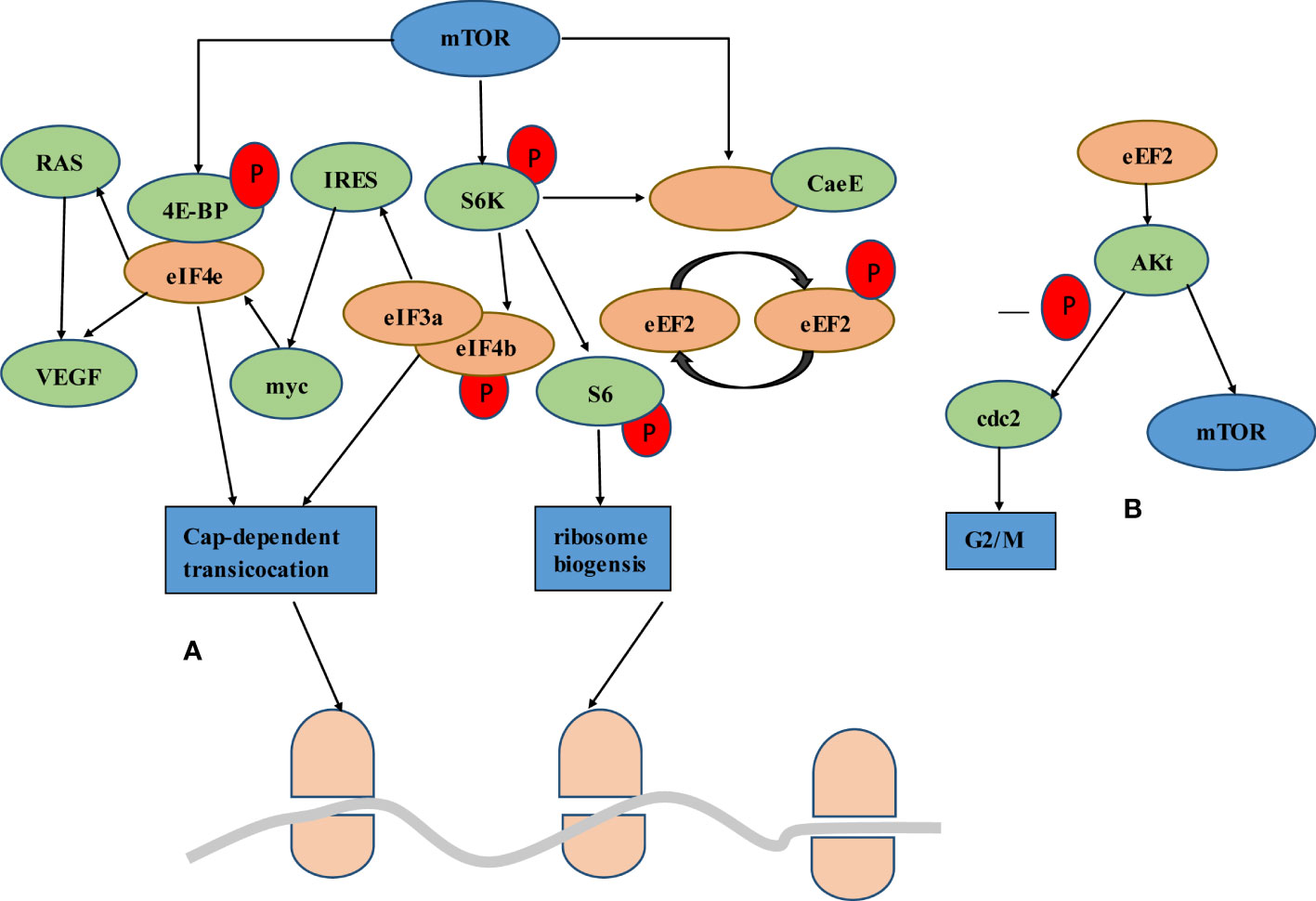
Figure 2 Interplay of mTOR signaling through protein translation. (A) The mTOR signal either goes through the 4eBP bound to eIF4e or through the S6K. mTOR phosphorylation of 4E-BP triggers the release of eIF4e and activates translation initiation. Upregulation of eIF4e triggers direct or Ras-induced VEGF translation. Activation of S6K leads to phosphorylation of eIF4b, which increases the binding affinity to eIF3a and thus promotes the translation of IRES dependent proteins such as myc. Myc in turn promotes the expression of eIF4e at the transcriptional level. S6K can affect the change of elongation. eEF2K is a negative regulator of eEF2, giving mTORC1 the ability to promote translation elongation through S6K. (B) Akt is a downstream molecule of eEF2, which regulates its activity. On the one hand, Akt activates the mTOR signaling pathway by inactivating the upstream regulator of mTOR, TSC2. On the other hand, Akt mediated eEF2 promotes G2/M progression through CDC2 activation.
Recent research had found that dysregulation of protein translation may be one of the causes of cancer. Dysregulation translation results in abnormal gene expression, which was also found involved in cell proliferation or apoptosis, leading to abnormal cell growth and malignant transformation. Some abnormalities of the mRNA and protein levels of eIFs, eEFs and eRFs in colorectal diseases had been published. For example, eIF2a is overexpressed in IBD, most isoforms of eIFs and eEFs are overexpressed in CRC. In contrast, eEF-2K downregulation in CRC was associated with reduced overall survival.
Recent findings of protein translation based drugs, such as thymoquinone rapamycin, rapalogs and imatinib, may be available in the treatment of cancer. To sum up, these studies suggested that protein translation plays an important part in IBD and CRC development, and may be a potential therapeutic target for them.
HCG made major contributions to the data analysis and manuscript writing. ZQ and ZXQ collected the data and participated in the manuscript revision. HR and DY collected the data. JH and YZH had the main primary idea and participated in the manuscript writing and revision. All authors contributed to the article and approved the submitted version.
This work was financially supported by the project of department of science and Technology, Sichuan province (No.22ZDYF3780).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol (2019) 16(12):713–32. doi: 10.1038/s41575-019-0189-8
2. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut (2017) 66(4):683–91. doi: 10.1136/gutjnl-2015-310912
3. Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American cancer society. CA Cancer J Clin (2018) 68(4):250–81. doi: 10.3322/caac.21457
4. Caputo F, Santini C, Bardasi C, Cerma K, Casadei-Gardini A, Spallanzani A, et al. BRAF-mutated colorectal cancer: Clinical and molecular insights. Int J Mol Sci (2019) 20(21):5369. doi: 10.3390/ijms20215369
5. Frenkel S, Bernstein CN, Sargent M, Kuang Q, Jiang W, Wei J, et al. Genome-wide analysis identifies rare copy number variations associated with inflammatory bowel disease. PloS One (2019) 14(6):e0217846. doi: 10.1371/journal.pone.0217846
6. Eftychi C, Schwarzer R, Vlantis K, Wachsmuth L, Basic M, Wagle P, et al. Temporally distinct functions of the cytokines IL-12 and IL-23 drive chronic colon inflammation in response to intestinal barrier impairment. Immunity (2019) 51(2):367–80 e4. doi: 10.1016/j.immuni.2019.06.008
7. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol (2015) 12(12):720–7. doi: 10.1038/nrgastro.2015.150
8. Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol (2010) 28:573–21. doi: 10.1146/annurev-immunol-030409-101225
9. Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al. British Society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut (2019) 68(Suppl 3):s1–s106. doi: 10.1136/gutjnl-2019-318484
10. Hnatyszyn A, Hryhorowicz S, Kaczmarek-Rys M, Lis E, Slomski R, Scott RJ, et al. Colorectal carcinoma in the course of inflammatory bowel diseases. Hered Cancer Clin Pract (2019), 17:18. doi: 10.1186/s13053-019-0118-4
11. Silvera D, Arju R, Darvishian F, Levine PH, Zolfaghari L, Goldberg J, et al. Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat Cell Biol (2009) 11(7):903–8. doi: 10.1038/ncb1900
12. Golob-Schwarzl N, Wodlej C, Kleinegger F, Gogg-Kamerer M, Birkl-Toeglhofer AM, Petzold J, et al. Eukaryotic translation initiation factor 6 overexpression plays a major role in the translational control of gallbladder cancer. J Cancer Res Clin Oncol (2019) 145(11):2699–711. doi: 10.1007/s00432-019-03030-x
13. Spilka R, Ernst C, Mehta AK, Haybaeck J. Eukaryotic translation initiation factors in cancer development and progression. Cancer Lett (2013) 340(1):9–21. doi: 10.1016/j.canlet.2013.06.019
14. Golob-Schwarzl N, Schweiger C, Koller C, Krassnig S, Gogg-Kamerer M, Gantenbein N, et al. Separation of low and high grade colon and rectum carcinoma by eukaryotic translation initiation factors 1, 5 and 6. Oncotarget (2017) 8(60):101224–43. doi: 10.18632/oncotarget.20642
15. Bhat KP, Umit Kaniskan H, Jin J, Gozani O. Epigenetics and beyond: targeting writers of protein lysine methylation to treat disease. Nat Rev Drug Discovery (2021) 20(4):265–86. doi: 10.1038/s41573-020-00108-x
16. Li S, Li Y, Bai Y. What is the impact of eukaryotic elongation factor 2 kinase on cancer: A systematic review. Eur J Pharmacol (2019) 857:172470. doi: 10.1016/j.ejphar.2019.172470
17. Biterge-Sut B. Alterations in eukaryotic elongation factor complex proteins (EEF1s) in cancer and their implications in epigenetic regulation. Life Sci (2019) 238:116977. doi: 10.1016/j.lfs.2019.116977
18. Sekoranja D, Zupan A, Mavcic B, Martincic D, Salapura V, Snoj Z, et al. Novel ASAP1-USP6, FAT1-USP6, SAR1A-USP6, and TNC-USP6 fusions in primary aneurysmal bone cyst. Genes Chromosomes Cancer (2020) 59(6):357–65. doi: 10.1002/gcc.22836
19. Garcia-Esparcia P, Hernandez-Ortega K, Koneti A, Gil L, Delgado-Morales R, Castano E, et al. Altered machinery of protein synthesis is region- and stage-dependent and is associated with alpha-synuclein oligomers in parkinson's disease. Acta Neuropathol Commun (2015) 3:76. doi: 10.1186/s40478-015-0257-4
20. Zhou JW, Li Y, Yue LX, Luo CL, Chen Y, Zhang JY. Autoantibody response to Sui1 and its tissue-specific expression in hepatocellular carcinoma. Tumour Biol (2016) 37(2):2547–53. doi: 10.1007/s13277-015-4074-y
21. Coignard J, Lush M, Beesley J, O'Mara TA, Dennis J, Tyrer JP, et al. A case-only study to identify genetic modifiers of breast cancer risk for BRCA1/BRCA2 mutation carriers. Nat Commun (2021) 12(1):1078. doi: 10.1038/s41467-020-20496-3
22. Golob-Schwarzl N, Puchas P, Gogg-Kamerer M, Weichert W, Goppert B, Haybaeck J. New pancreatic cancer biomarkers eIF1, eIF2D, eIF3C and eIF6 play a major role in translational control in ductal adenocarcinoma. Anticancer Res (2020) 40(6):3109–18. doi: 10.21873/anticanres.14292
23. Wek RC. Role of eIF2alpha kinases in translational control and adaptation to cellular stress. Cold Spring Harb Perspect Biol (2018) 10(7):a032870. doi: 10.1101/cshperspect.a032870
24. Adomavicius T, Guaita M, Zhou Y, Jennings MD, Latif Z, Roseman AM, et al. The structural basis of translational control by eIF2 phosphorylation. Nat Commun (2019) 10(1):2136. doi: 10.1038/s41467-019-10167-3
25. Lam N, Sandberg ML, Sugden B. High physiological levels of LMP1 result in phosphorylation of eIF2 alpha in Epstein-Barr virus-infected cells. J Virol (2004) 78(4):1657–64. doi: 10.1128/jvi.78.4.1657-1664.2004
26. Lobo MV, Martin ME, Perez MI, Alonso FJ, Redondo C, Alvarez MI, et al. Levels, phosphorylation status and cellular localization of translational factor eIF2 in gastrointestinal carcinomas. Histochem J (2000) 32(3):139–50. doi: 10.1023/a:1004091122351
27. Song Y, Zhao Y, Ma Y, Wang Z, Rong L, Wang B, et al. Biological functions of NLRP3 inflammasome: A therapeutic target in inflammatory bowel disease. Cytokine Growth Factor Rev (2021) 60:61–75. doi: 10.1016/j.cytogfr.2021.03.003
28. Larabi A, Barnich N, Nguyen HTT. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy (2020) 16(1):38–51. doi: 10.1080/15548627.2019.1635384
29. Varghese GP, Uporova L, Halfvarson J, Sirsjo A, Fransen K. Polymorphism in the NLRP3 inflammasome-associated EIF2AK2 gene and inflammatory bowel disease. Mol Med Rep (2015) 11(6):4579–84. doi: 10.3892/mmr.2015.3236
30. Ravindran R, Loebbermann J, Nakaya HI, Khan N, Ma H, Gama L, et al. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature (2016) 531(7595):523–7. doi: 10.1038/nature17186
31. Yang S, Hu L, Wang C, Wei F. PERK-eIF2alpha-ATF4 signaling contributes to osteogenic differentiation of periodontal ligament stem cells. J Mol Histol (2020) 51(2):125–35. doi: 10.1007/s10735-020-09863-y
32. Okazaki T, Nishio A, Takeo M, Sakaguchi Y, Fukui T, Uchida K, et al. Inhibition of the dephosphorylation of eukaryotic initiation factor 2alpha ameliorates murine experimental colitis. Digestion (2014) 90(3):167–78. doi: 10.1159/000366414
33. Wu MS, Chien CC, Jargalsaikhan G, Ilsan NA, Chen YC. Activation of PERK contributes to apoptosis and G2/M arrest by microtubule disruptors in human colorectal carcinoma cells (double dagger). Cancers (Basel) (2019) 12(1):97. doi: 10.3390/cancers12010097
34. Rozpedek W, Pytel D, Wawrzynkiewicz A, Siwecka N, Dziki A, Dziki L, et al. Use of small-molecule inhibitory compound of PERK-dependent signaling pathway as a promising target-based therapy for colorectal cancer. Curr Cancer Drug Targets (2020) 20(3):223–38. doi: 10.2174/1568009620666200106114826
35. Chern YJ, Wong JCT, Cheng GSW, Yu A, Yin Y, Schaeffer DF, et al. The interaction between SPARC and GRP78 interferes with ER stress signaling and potentiates apoptosis via PERK/eIF2alpha and IRE1alpha/XBP-1 in colorectal cancer. Cell Death Dis (2019) 10(7):504. doi: 10.1038/s41419-019-1687-x
36. Stolfi C, De Simone V, Colantoni A, Franze E, Ribichini E, Fantini MC, et al. A functional role for Smad7 in sustaining colon cancer cell growth and survival. Cell Death Dis (2014) 5:e1073. doi: 10.1038/cddis.2014.49
37. Chen J, Liu JY, Dong ZZ, Zou T, Wang Z, Shen Y, et al. The effect of eIF3a on anthracycline-based chemotherapy resistance by regulating DSB DNA repair. Biochem Pharmacol (2021) 190:114616. doi: 10.1016/j.bcp.2021.114616
38. Chen YX, Wang CJ, Xiao DS, He BM, Li M, Yi XP, et al. eIF3a R803K mutation mediates chemotherapy resistance by inducing cellular senescence in small cell lung cancer. Pharmacol Res (2021) 174:105934. doi: 10.1016/j.phrs.2021.105934
39. Chen G, Burger MM. p150 overexpression in gastric carcinoma: the association with p53, apoptosis and cell proliferation. Int J Cancer (2004) 112(3):393–8. doi: 10.1002/ijc.20443
40. Xu JZ, Wen F, Wang XR. The eIF3a Arg803Lys genetic polymorphism is associated with susceptibility to and chemoradiotherapy efficacy in cervical carcinoma. Kaohsiung J Med Sci (2017) 33(4):187–94. doi: 10.1016/j.kjms.2017.01.008
41. Liu Z, Dong Z, Yang Z, Chen Q, Pan Y, Yang Y, et al. Role of eIF3a (eIF3 p170) in intestinal cell differentiation and its association with early development. Differentiation (2007) 75(7):652–61. doi: 10.1111/j.1432-0436.2007.00165.x
42. Haybaeck J, O'Connor T, Spilka R, Spizzo G, Ensinger C, Mikuz G, et al. Overexpression of p150, a part of the large subunit of the eukaryotic translation initiation factor 3, in colon cancer. Anticancer Res (2010) 30(4):1047–55.
43. Liu S, Tackmann NR, Yang J, Zhang Y. Disruption of the RP-MDM2-p53 pathway accelerates APC loss-induced colorectal tumorigenesis. Oncogene (2017) 36(10):1374–83. doi: 10.1038/onc.2016.301
44. Ren H, Mai G, Liu Y, Xiang R, Yang C, Su W. Eukaryotic translation initiation factor 3 subunit b is a promoter in the development and progression of pancreatic cancer. Front Oncol (2021) 11:644156. doi: 10.3389/fonc.2021.644156
45. Wang L, Wen X, Luan F, Fu T, Gao C, Du H, et al. EIF3B is associated with poor outcomes in gastric cancer patients and promotes cancer progression via the PI3K/AKT/mTOR signaling pathway. Cancer Manag Res (2019) 11:7877–91. doi: 10.2147/CMAR.S207834
46. Huang L, He K, Wang J, Yan J, Jiang Y, Wei Z, et al. Inhibition of eukaryotic initiation factor 3B suppresses proliferation and promotes apoptosis of chronic myeloid leukemia cells. Adv Clin Exp Med (2019) 28(12):1639–45. doi: 10.17219/acem/110323
47. Xu YP, Dong ZN, Zhou YQ, Zhao YJ, Zhao Y, Wang F, et al. Role of eIF3C overexpression in predicting prognosis of intrahepatic cholangiocarcinoma. Dig Dis Sci (2022) 67(2):559–68. doi: 10.1007/s10620-021-06878-7
48. Liu H, Qin Y, Zhou N, Ma D, Wang Y. ZNF280A promotes lung adenocarcinoma development by regulating the expression of EIF3C. Cell Death Dis (2021) 12(1):39. doi: 10.1038/s41419-020-03309-9
49. Yin Y, Long J, Sun Y, Li H, Jiang E, Zeng C, et al. The function and clinical significance of eIF3 in cancer. Gene (2018) 673:130–3. doi: 10.1016/j.gene.2018.06.034
50. Traicoff JL, Chung JY, Braunschweig T, Mazo I, Shu Y, Ramesh A, et al. Expression of EIF3-p48/INT6, TID1 and patched in cancer, a profiling of multiple tumor types and correlation of expression. J BioMed Sci (2007) 14(3):395–405. doi: 10.1007/s11373-007-9149-3
51. Morris C, Durand S, Jalinot P. Decreased expression of the translation factor eIF3e induces senescence in breast cancer cells via suppression of PARP1 and activation of mTORC1. Oncotarget (2021) 12(7):649–64. doi: 10.18632/oncotarget.27923
52. Buttitta F, Martella C, Barassi F, Felicioni L, Salvatore S, Rosini S, et al. Int6 expression can predict survival in early-stage non-small cell lung cancer patients. Clin Cancer Res (2005) 11(9):3198–204. doi: 10.1158/1078-0432.CCR-04-2308
53. Esteves P, Dard L, Brillac A, Hubert C, Sarlak S, Rousseau B, et al. Nuclear control of lung cancer cells migration, invasion and bioenergetics by eukaryotic translation initiation factor 3F. Oncogene (2020) 39(3):617–36. doi: 10.1038/s41388-019-1009-x
54. Yang C, Liu X, Li C, Li S, Du W, Yang D. Eukaryotic translation initiation factor 3 subunit G (EIF3G) resensitized HCT116/5-fu to 5-fluorouracil (5-fu) via inhibition of MRP and MDR1. Onco Targets Ther (2018) 11:5315–24. doi: 10.2147/OTT.S170854
55. Noci S, Dugo M, Bertola F, Melotti F, Vannelli A, Dragani TA, et al. A subset of genetic susceptibility variants for colorectal cancer also has prognostic value. Pharmacogenomics J (2016) 16(2):173–9. doi: 10.1038/tpj.2015.35
56. Qi J, Dong Z, Liu J, Zhang JT. EIF3i promotes colon oncogenesis by regulating COX-2 protein synthesis and beta-catenin activation. Oncogene (2014) 33(32):4156–63. doi: 10.1038/onc.2013.397
57. Goh SH, Hong SH, Hong SH, Lee BC, Ju MH, Jeong JS, et al. eIF3m expression influences the regulation of tumorigenesis-related genes in human colon cancer. Oncogene (2011) 30(4):398–409. doi: 10.1038/onc.2010.422
58. Wang QH, Zhang M, Zhou MH, Gao XJ, Chen F, Yan X, et al. High expression of eukaryotic initiation factor 3M predicts poor prognosis in colon adenocarcinoma patients. Oncol Lett (2020) 19(1):876–84. doi: 10.3892/ol.2019.11164
59. Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem (1999) 68:913–63. doi: 10.1146/annurev.biochem.68.1.913
60. Mishra RK, Datey A, Hussain T. mRNA recruiting eIF4 factors involved in protein synthesis and its regulation. Biochemistry (2020) 59(1):34–46. doi: 10.1021/acs.biochem.9b00788
61. Ruoff R, Katsara O, Kolupaeva V. Cell type-specific control of protein synthesis and proliferation by FGF-dependent signaling to the translation repressor 4E-BP. Proc Natl Acad Sci USA (2016) 113(27):7545–50. doi: 10.1073/pnas.1605451113
62. Biffo S, Manfrini N, Ricciardi S. Crosstalks between translation and metabolism in cancer. Curr Opin Genet Dev (2018) 48:75–81. doi: 10.1016/j.gde.2017.10.011
63. Chen Y, Wang J, Fan H, Xie J, Xu L, Zhou B. Phosphorylated 4E-BP1 is associated with tumor progression and adverse prognosis in colorectal cancer. Neoplasma (2017) 64(5):787–94. doi: 10.4149/neo_2017_518
64. Gong C, Tsoi H, Mok KC, Cheung J, Man EPS, Fujino K, et al. Phosphorylation independent eIF4E translational reprogramming of selective mRNAs determines tamoxifen resistance in breast cancer. Oncogene (2020) 39(15):3206–17. doi: 10.1038/s41388-020-1210-y
65. Humphries MP, Sundara Rajan S, Droop A, Suleman CAB, Carbone C, Nilsson C, et al. A case-matched gender comparison transcriptomic screen identifies eIF4E and eIF5 as potential prognostic markers in Male breast cancer. Clin Cancer Res (2017) 23(10):2575–83. doi: 10.1158/1078-0432.CCR-16-1952
66. Ahmad R, Alam M, Hasegawa M, Uchida Y, Al-Obaid O, Kharbanda S, et al. Targeting MUC1-c inhibits the AKT-S6K1-elF4A pathway regulating TIGAR translation in colorectal cancer. Mol Cancer (2017) 16(1):33. doi: 10.1186/s12943-017-0608-9
67. Ge S, Zhang Q, Chen Y, Tian Y, Yang R, Chen X, et al. Ribavirin inhibits colorectal cancer growth by downregulating PRMT5 expression and H3R8me2s and H4R3me2s accumulation. Toxicol Appl Pharmacol (2021) 415:115450. doi: 10.1016/j.taap.2021.115450
68. Chen YT, Tsai HP, Wu CC, Wang JY, Chai CY. Eukaryotic translation initiation factor 4E (eIF-4E) expressions are associated with poor prognosis in colorectal adenocarcinoma. Pathol Res Pract (2017) 213(5):490–5. doi: 10.1016/j.prp.2017.02.004
69. Lin KY, Nag N, Pestova TV, Marintchev A. Human eIF5 and eIF1A compete for binding to eIF5B. Biochemistry (2018) 57(40):5910–20. doi: 10.1021/acs.biochem.8b00839
70. Shin BS, Katoh T, Gutierrez E, Kim JR, Suga H, Dever TE. Amino acid substrates impose polyamine, eIF5A, or hypusine requirement for peptide synthesis. Nucleic Acids Res (2017) 45(14):8392–402. doi: 10.1093/nar/gkx532
71. Gutierrez E, Shin BS, Woolstenhulme CJ, Kim JR, Saini P, Buskirk AR, et al. eIF5A promotes translation of polyproline motifs. Mol Cell (2013) 51(1):35–45. doi: 10.1016/j.molcel.2013.04.021
72. Martella M, Catalanotto C, Talora C, La Teana A, Londei P, Benelli D. Inhibition of eukaryotic translation initiation factor 5A (eIF5A) hypusination suppress p53 translation and alters the association of eIF5A to the ribosomes. Int J Mol Sci (2020) 21(13):4583. doi: 10.3390/ijms21134583
73. Coni S, Serrao SM, Yurtsever ZN, Di Magno L, Bordone R, Bertani C, et al. Blockade of EIF5A hypusination limits colorectal cancer growth by inhibiting MYC elongation. Cell Death Dis (2020) 11(12):1045. doi: 10.1038/s41419-020-03174-6
74. Taylor CA, Sun Z, Cliche DO, Ming H, Eshaque B, Jin S, et al. Eukaryotic translation initiation factor 5A induces apoptosis in colon cancer cells and associates with the nucleus in response to tumour necrosis factor alpha signalling. Exp Cell Res (2007) 313(3):437–49. doi: 10.1016/j.yexcr.2006.09.030
75. Bao Y, Lu Y, Wang X, Feng W, Sun X, Guo H, et al. Eukaryotic translation initiation factor 5A2 (eIF5A2) regulates chemoresistance in colorectal cancer through epithelial mesenchymal transition. Cancer Cell Int (2015) 15:109. doi: 10.1186/s12935-015-0250-9
76. Ross JA, Bressler KR, Thakor N. Eukaryotic initiation factor 5B (eIF5B) cooperates with eIF1A and eIF5 to facilitate uORF2-mediated repression of ATF4 translation. Int J Mol Sci (2018) 19(12):4032. doi: 10.3390/ijms19124032
77. Hu X, Deng J, Yu T, Chen S, Ge Y, Zhou Z, et al. ATF4 deficiency promotes intestinal inflammation in mice by reducing uptake of glutamine and expression of antimicrobial peptides. Gastroenterology (2019) 156(4):1098–111. doi: 10.1053/j.gastro.2018.11.033
78. Miluzio A, Beugnet A, Volta V, Biffo S. Eukaryotic initiation factor 6 mediates a continuum between 60S ribosome biogenesis and translation. EMBO Rep (2009) 10(5):459–65. doi: 10.1038/embor.2009.70
79. Benelli D, Cialfi S, Pinzaglia M, Talora C, Londei P. The translation factor eIF6 is a notch-dependent regulator of cell migration and invasion. PloS One (2012) 7(2):e32047. doi: 10.1371/journal.pone.0032047
80. Ceci M, Gaviraghi C, Gorrini C, Sala LA, Offenhauser N, Marchisio PC, et al. Release of eIF6 (p27BBP) from the 60S subunit allows 80S ribosome assembly. Nature (2003) 426(6966):579–84. doi: 10.1038/nature02160
81. Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, et al. Activation of notch-1 signaling maintains the neoplastic phenotype in human ras-transformed cells. Nat Med (2002) 8(9):979–86. doi: 10.1038/nm754
82. Komor MA, de Wit M, van den Berg J, Martens de Kemp SR, Delis-van Diemen PM, Bolijn AS, et al. Molecular characterization of colorectal adenomas reveals POFUT1 as a candidate driver of tumor progression. Int J Cancer (2020) 146(7):1979–92. doi: 10.1002/ijc.32627
83. Rosso P, Cortesina G, Sanvito F, Donadini A, Di Benedetto B, Biffo S, et al. Overexpression of p27BBP in head and neck carcinomas and their lymph node metastases. Head Neck (2004) 26(5):408–17. doi: 10.1002/hed.10401
84. Gantenbein N, Bernhart E, Anders I, Golob-Schwarzl N, Krassnig S, Wodlej C, et al. Influence of eukaryotic translation initiation factor 6 on non-small cell lung cancer development and progression. Eur J Cancer (2018) 101:165–80. doi: 10.1016/j.ejca.2018.07.001
85. Flavin RJ, Smyth PC, Finn SP, Laios A, O'Toole SA, Barrett C, et al. Altered eIF6 and dicer expression is associated with clinicopathological features in ovarian serous carcinoma patients. Mod Pathol (2008) 21(6):676–84. doi: 10.1038/modpathol.2008.33
86. Sanvito F, Vivoli F, Gambini S, Santambrogio G, Catena M, Viale E, et al. Expression of a highly conserved protein, p27BBP, during the progression of human colorectal cancer. Cancer Res (2000) 60(3):510–6.
87. Lin J, Yu X, Xie L, Wang P, Li T, Xiao Y, et al. eIF6 promotes colorectal cancer proliferation and invasion by regulating AKT-related signaling pathways. J BioMed Nanotechnol (2019) 15(7):1556–67. doi: 10.1166/jbn.2019.2792
88. Vislovukh A, Kratassiouk G, Porto E, Gralievska N, Beldiman C, Pinna G, et al. Proto-oncogenic isoform A2 of eukaryotic translation elongation factor eEF1 is a target of miR-663 and miR-744. Br J Cancer (2013) 108(11):2304–11. doi: 10.1038/bjc.2013.243
89. Jakobsson ME, Malecki JM, Halabelian L, Nilges BS, Pinto R, Kudithipudi S, et al. The dual methyltransferase METTL13 targets n terminus and Lys55 of eEF1A and modulates codon-specific translation rates. Nat Commun (2018) 9(1):3411. doi: 10.1038/s41467-018-05646-y
90. Liu S, Hausmann S, Carlson SM, Fuentes ME, Francis JW, Pillai R, et al. METTL13 methylation of eEF1A increases translational output to promote tumorigenesis. Cell (2019) 176(3):491–504 e21. doi: 10.1016/j.cell.2018.11.038
91. Abbas W, Kumar A, Herbein G. The eEF1A proteins: At the crossroads of oncogenesis, apoptosis, and viral infections. Front Oncol (2015) 5:75. doi: 10.3389/fonc.2015.00075
92. Li Z, Qi CF, Shin DM, Zingone A, Newbery HJ, Kovalchuk AL, et al. Eef1a2 promotes cell growth, inhibits apoptosis and activates JAK/STAT and AKT signaling in mouse plasmacytomas. PloS One (2010) 5(5):e10755. doi: 10.1371/journal.pone.0010755
93. Biterge Sut B. Data article on genes that share similar expression patterns with EEF1 complex proteins in hepatocellular carcinoma. Data Brief (2020) 29:105162. doi: 10.1016/j.dib.2020.105162
94. Bao Y, Zhao TL, Zhang ZQ, Liang XL, Wang ZX, Xiong Y, et al. High eukaryotic translation elongation factor 1 alpha 1 expression promotes proliferation and predicts poor prognosis in clear cell renal cell carcinoma. Neoplasma (2020) 67(1):78–84. doi: 10.4149/neo_2019_190224N158
95. Lin CY, Beattie A, Baradaran B, Dray E, Duijf PHG. Contradictory mRNA and protein misexpression of EEF1A1 in ductal breast carcinoma due to cell cycle regulation and cellular stress. Sci Rep (2018) 8(1):13904. doi: 10.1038/s41598-018-32272-x
96. Jia L, Yang T, Gu X, Zhao W, Tang Q, Wang X, et al. Translation elongation factor eEF1Balpha is identified as a novel prognostic marker of gastric cancer. Int J Biol Macromol (2019) 126:345–51. doi: 10.1016/j.ijbiomac.2018.12.126
97. Worst TS, Waldbillig F, Abdelhadi A, Weis CA, Gottschalt M, Steidler A, et al. The EEF1A2 gene expression as risk predictor in localized prostate cancer. BMC Urol (2017) 17(1):86. doi: 10.1186/s12894-017-0278-3
98. Anand N, Murthy S, Amann G, Wernick M, Porter LA, Cukier IH, et al. Protein elongation factor EEF1A2 is a putative oncogene in ovarian cancer. Nat Genet (2002) 31(3):301–5. doi: 10.1038/ng904
99. Zhou B, Guo R. Integrative analysis of significant RNA-binding proteins in colorectal cancer metastasis. J Cell Biochem (2018) 119(12):9730–41. doi: 10.1002/jcb.27290
100. Pellegrino R, Calvisi DF, Neumann O, Kolluru V, Wesely J, Chen X, et al. EEF1A2 inactivates p53 by way of PI3K/AKT/mTOR-dependent stabilization of MDM4 in hepatocellular carcinoma. Hepatology (2014) 59(5):1886–99. doi: 10.1002/hep.26954
101. Mathur S, Cleary KR, Inamdar N, Kim YH, Steck P, Frazier ML. Overexpression of elongation factor-1gamma protein in colorectal carcinoma. Cancer (1998) 82(5):816–21. doi: 10.1002/(sici)1097-0142(19980301)82:5<816::aid-cncr3>3.0.co;2-h
102. Yu P, Wang HY, Tian M, Li AX, Chen XS, Wang XL, et al. Eukaryotic elongation factor-2 kinase regulates the cross-talk between autophagy and pyroptosis in doxorubicin-treated human melanoma cells in vitro. Acta Pharmacol Sin (2019) 40(9):1237–44. doi: 10.1038/s41401-019-0222-z
103. Oji Y, Tatsumi N, Fukuda M, Nakatsuka S, Aoyagi S, Hirata E, et al. The translation elongation factor eEF2 is a novel tumorassociated antigen overexpressed in various types of cancers. Int J Oncol (2014) 44(5):1461–9. doi: 10.3892/ijo.2014.2318
104. Nakamura J, Aoyagi S, Nanchi I, Nakatsuka S, Hirata E, Shibata S, et al. Overexpression of eukaryotic elongation factor eEF2 in gastrointestinal cancers and its involvement in G2/M progression in the cell cycle. Int J Oncol (2009) 34(5):1181–9.
105. Vasamsetti BMK, Liu Z, Park YS, Cho NJ. Muscarinic acetylcholine receptors regulate the dephosphorylation of eukaryotic translation elongation factor 2 in SNU-407 colon cancer cells. Biochem Biophys Res Commun (2019) 516(2):424–9. doi: 10.1016/j.bbrc.2019.06.059
106. Ryazanov AG, Shestakova EA, Natapov PG. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature (1988) 334(6178):170–3. doi: 10.1038/334170a0
107. Faller WJ, Jackson TJ, Knight JR, Ridgway RA, Jamieson T, Karim SA, et al. mTORC1-mediated translational elongation limits intestinal tumour initiation and growth. Nature (2015) 517(7535):497–500. doi: 10.1038/nature13896
108. Ng TH, Sham KWY, Xie CM, Ng SSM, To KF, Tong JHM, et al. Eukaryotic elongation factor-2 kinase expression is an independent prognostic factor in colorectal cancer. BMC Cancer (2019) 19(1):649. doi: 10.1186/s12885-019-5873-0
109. Pott LL, Hagemann S, Reis H, Lorenz K, Bracht T, Herold T, et al. Eukaryotic elongation factor 2 is a prognostic marker and its kinase a potential therapeutic target in HCC. Oncotarget (2017) 8(7):11950–62. doi: 10.18632/oncotarget.14447
110. Brown A, Shao S, Murray J, Hegde RS, Ramakrishnan V. Structural basis for stop codon recognition in eukaryotes. Nature (2015) 524(7566):493–6. doi: 10.1038/nature14896
111. Janzen DM, Frolova L, Geballe AP. Inhibition of translation termination mediated by an interaction of eukaryotic release factor 1 with a nascent peptidyl-tRNA. Mol Cell Biol (2002) 22(24):8562–70. doi: 10.1128/MCB.22.24.8562-8570.2002
112. Lawson MR, Lessen LN, Wang J, Prabhakar A, Corsepius NC, Green R, et al. Mechanisms that ensure speed and fidelity in eukaryotic translation termination. Science (2021) 373(6557):876–82. doi: 10.1126/science.abi7801
113. Beissel C, Neumann B, Uhse S, Hampe I, Karki P, Krebber H. Translation termination depends on the sequential ribosomal entry of eRF1 and eRF3. Nucleic Acids Res (2019) 47(9):4798–813. doi: 10.1093/nar/gkz177
114. Malta-Vacas J, Chauvin C, Goncalves L, Nazare A, Carvalho C, Monteiro C, et al. eRF3a/GSPT1 12-GGC allele increases the susceptibility for breast cancer development. Oncol Rep (2009) 21(6):1551–8. doi: 10.3892/or_00000387
115. Malta-Vacas J, Aires C, Costa P, Conde AR, Ramos S, Martins AP, et al. Differential expression of the eukaryotic release factor 3 (eRF3/GSPT1) according to gastric cancer histological types. J Clin Pathol (2005) 58(6):621–5. doi: 10.1136/jcp.2004.021774
116. Malta-Vacas J, Ferreira P, Monteiro C, Brito M. Differential expression of GSPT1 GGCn alleles in cancer. Cancer Genet Cytogenet (2009) 195(2):132–42. doi: 10.1016/j.cancergencyto.2009.08.010
117. Franckenberg S, Becker T, Beckmann R. Structural view on recycling of archaeal and eukaryotic ribosomes after canonical termination and ribosome rescue. Curr Opin Struct Biol (2012) 22(6):786–96. doi: 10.1016/j.sbi.2012.08.002
118. Hellen CUT. Translation termination and ribosome recycling in eukaryotes. Cold Spring Harb Perspect Biol (2018) 10(10):a032656. doi: 10.1101/cshperspect.a032656
Keywords: colorectal cancer, inflammatory bowel disease, eukaryotic gene expression, protein translation, colorectal pathogenesis
Citation: Huang CG, Zhao Q, Zhou XQ, Huang R, Duan Y, Haybaeck J and Yang ZH (2022) The progress of protein synthesis factors eIFs, eEFs and eRFs in inflammatory bowel disease and colorectal cancer pathogenesis. Front. Oncol. 12:898966. doi: 10.3389/fonc.2022.898966
Received: 18 March 2022; Accepted: 14 October 2022;
Published: 31 October 2022.
Edited by:
Rocco Ricciardi, Massachusetts General Hospital, Harvard Medical School, United StatesReviewed by:
Yeun-po Chiang, Downstate Health Sciences University, United StatesCopyright © 2022 Huang, Zhao, Zhou, Huang, Duan, Haybaeck and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihui Yang, eXpoaWg3M0AxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.