
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 30 June 2022
Sec. Cancer Genetics
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.898954
This article is part of the Research Topic Case Reports in Cancer Genetics : 2022 View all 16 articles
Background: Expanding the druggable novel anaplastic lymphoma kinase (ALK) fusions list is crucial to the precise treatment of patients with cancer with positive ALK fusions. The intergenic-ALK fusions accounted for a substantial proportion of ALK fusions. However, they were typically considered of limited clinical significance due to the obscure functional partners. In this case report, a patient carrying intergenic-ALK fusion presents an excellent outcome after taking the new second-generation tyrosine kinase inhibitor (TKI) candidate, WX-0593.
Case Presentation: A 47-year-old Chinese female patient diagnosed with IVB lung adenocarcinoma was admitted to the hospital with large dimension lesions in the left lobe of the lung. After 1 week of first line chemotherapy, no response was found. A novel ALK rearrangement generated by a fusion of the intergenic region between SLC8A1 and PKDCC to the intron 19 of ALK was presented after next-generation sequencing and was further confirmed by Sanger’s sequencing. High expression of ALK was revealed by immunohistochemistry. The patient was directed to engage in phase III clinical trial (NCT04632758) and received an orally active second-generation ALK inhibitor WX-0593. Over the course of 17 months, the partial response was obtained without significant side effects.
Conclusion: In summary, a patient with non–small cell lung cancer harboring a novel intergenic-ALK fusion, whose intergenic breakpoint was located between SLC8A1 and PKDCC, benefited from a potent ALK TKI candidate WX-0593. This finding extended the scope of targetable ALK fusions. More importantly, it highlighted the advantages of next-generation sequencing in identifying rare but functional ALK fusions, which eventually benefit patients.
Anaplastic lymphoma kinase (ALK) gene fusions drive genetic alterations and critical molecular targets in around 3%~5% of non–small cell lung cancer (NSCLC) (1). For the treatment of patients with ALK rearrangement-positive NSCLC, the first-generation ALK tyrosine kinase inhibitor (TKI) crizotinib (2), second-generation [ceritinib (3), alectinib (4), and brigatinib (5)], and third-generation [lorlatinib (6)] ALK TKIs have been approved as an effective treatment. Because of their rarity, newly confirmed ALK fusions, in addition to the conventional EML4-ALK fusion, represent significant difficulties in targeted ALK TKI therapy in the clinic. Clinical outcomes vary according to fusion partners and specific TKIs (7). Therefore, an accurate diagnosis of functional ALK fusions is crucial for successful NSCLC treatment. In contrast to the typical form of ALK fusions, the rare intergenic-ALK fusions, whose breakpoint localized in the intergenic regions, are theoretically to be unfunctional due to the missing chimeric full coding transcripts. Here, for the first time, we reported a novel intergenic-ALK fusion, whose intergenic region was between SLC8A1 and PKDCC and fused with the exon 20 of ALK in a patient with NSCLC. The patient achieved a long-term therapeutic benefit after receiving the potent second-generation candidate ALK TKI WX-0593 treatment.
This case report was approved by the Ethics Committee of Daping Hospital [2022(03)]. A 47-year-old Chinese female patient was admitted to the hospital with paroxysmal abdominal pain in June 2020. Computed tomography (CT) scans revealed a space-occupying lesion in the left lung lobe, as well as multiple masses in the liver and skeleton. IVB lung adenocarcinoma (cT2N1M1c) was diagnosed on the basis of the pathological results from tissue biopsy and CT. Lung tumor biopsies were submitted to Genetron Health Inc. (Beijing, China) for Next Generation Sequencing (NGS) with an 825 cancer-related gene DNA panel (Onco Panscan™) for comprehensive molecular profiling. During the DNA-NGS analysis, the patient received 1 week of chemotherapy with pemetrexed disodium (0.8 g) and nedaplatin (100 mg) and did not show any response (Figure 1).
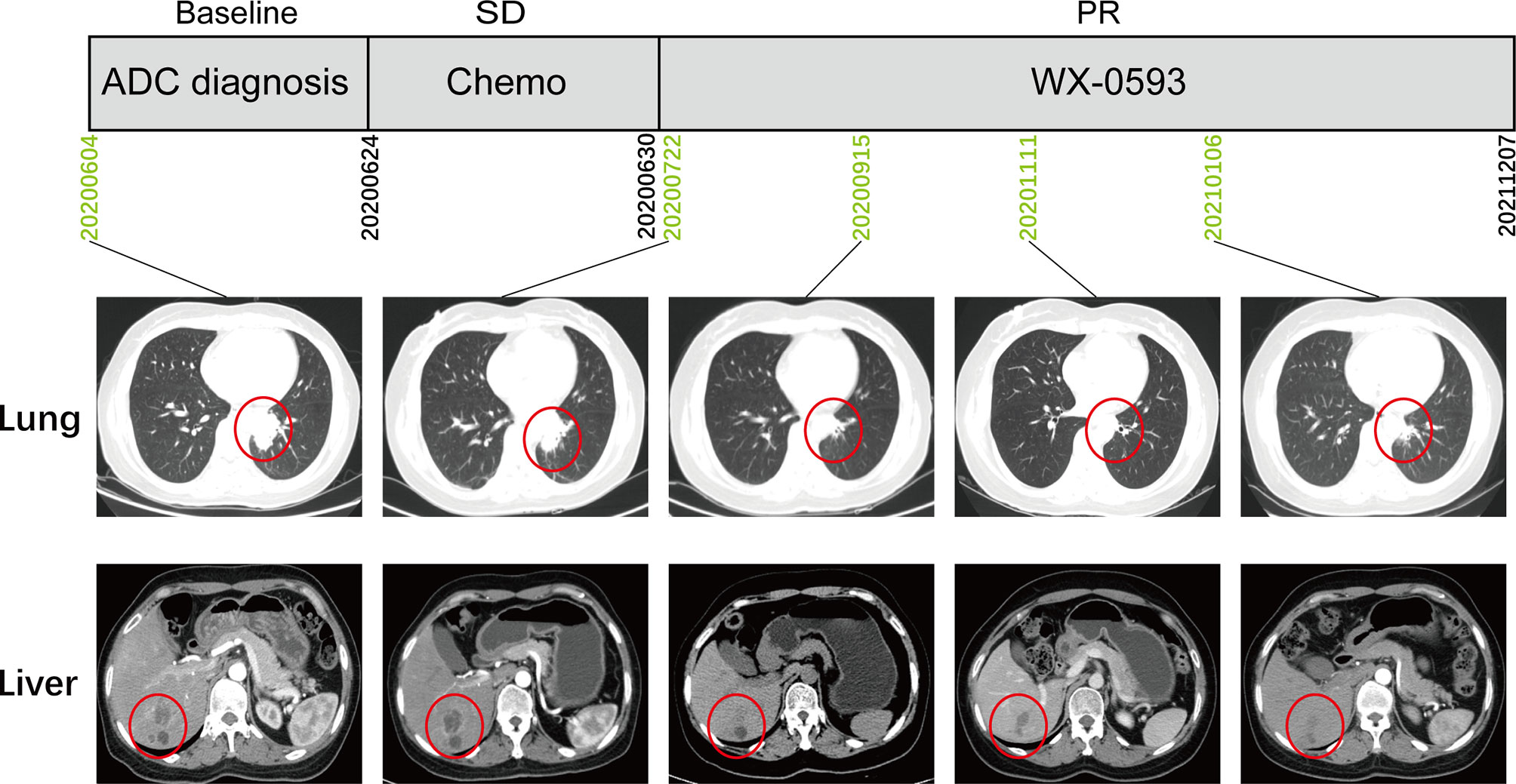
Figure 1 The treatment scheme and representative CT scan images during treatment. ADC, adenocarcinoma; SD, stable disease; PR, partial response.
The DNA sequencing identified a novel ALK fusion with a mutation frequency of 5.3% (Figure 2A). Integrative Genomics Viewer revealed a novel intergenic-ALK rearrangement generated by a fusion of the intergenic region between SLC8A1 and PKDCC to the intron 19 of ALK, and Sanger’s sequencing result supported the accuracy of DNA-NGS detection. Furthermore, the gene fusion was confirmed by fluorescence in situ hybridization (FISH) with ALK break-apart probe (Healthcare, NMPA: 20183400004) (Figure 2B). A high level of ALK protein expression was further validated with immunohistochemistry (Ventana ALK (D5F3®) XP®, #3633, Cell Signaling Technology) (Figure 2C).
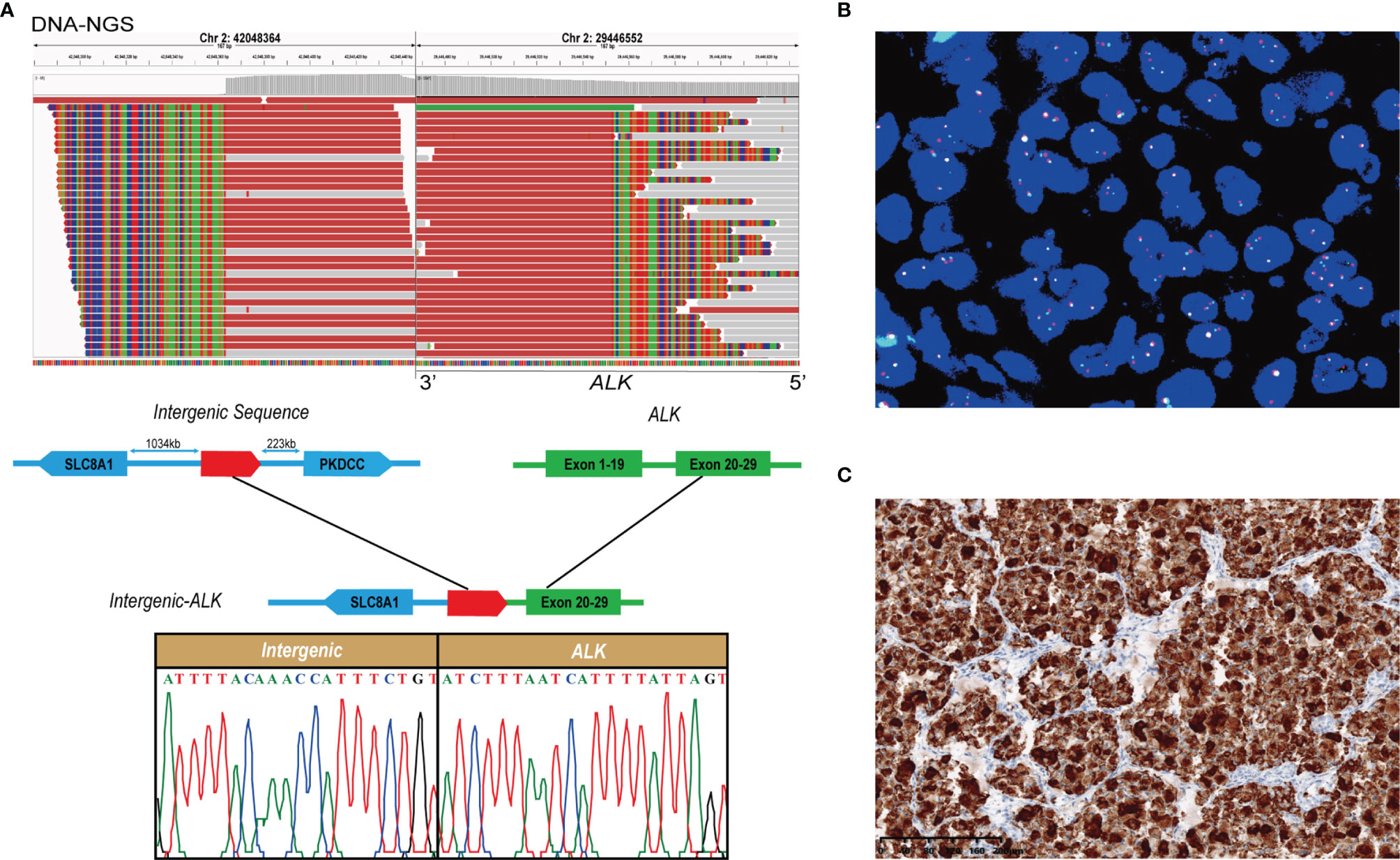
Figure 2 Identification of a novel intergenic ALK fusion. (A) Sequence analysis of the intergenic-ALK fusion. Upper panel showed reads of fusion on Integrative Genomic Viewer. Lower panel showed a schematic presentation of breakpoints and the Sanger’s sequencing result. (B) FISH analysis showed fused yellow signals (negative signal), single green signals (positive signal), and single red signals (positive signal) in the patient’s specimen. (C) Immunohistochemical staining with anti-ALK antibody (D5F3) revealed a high level of ALK protein.
The patient was referred to enroll in phase III clinical trial (NCT04632758) and was randomly administered an orally active second-generation ALK inhibitor WX-0593. An initial dose of 60 mg was administered for 1 week, followed by a maintenance dose of 180 mg. According to the Response Evaluation Criteria for Solid Tumors 1.1 guidelines, the CT scan indicated a partial response (PR) lasting longer than 22 months, with no noticeable side effects (Figure 1).
With the development of NGS technology, more than 90 ALK fusion partners have been discovered to date (8). It is generally believed that the functional protein of the partner gene at the N-terminus of ALK could result in continuous ALK gene activation driven by the promoter of the partner gene. Theoretically, the rare type of intergenic-ALK fusion, whose partner lacked a promoter, will fail to respond to TKIs. Sporadic cases showed that some intergenic-ALK fusions were sensitive to the ALK TKIs (8). However, it has not been fully identified due to a lack of knowledge concerning the precise mechanism (9). Therefore, it is vital to expand the list of targetable intergenic fusions.
To date, three generations of ALK TKIs, including but not limited to crizotinib, alectinib, and lorlatinib, have been developed. These inhibitors present an exceptional capacity to lengthen the lives of patients with ALK fusions. Despite the excellent results of these TKIs, newly developed local ALK TKIs were required to reduce the financial burden on the patients and national health insurance. In vitro and in vivo preclinical models revealed that WX-0593, a potent orally active second-generation ALK and ROS1 inhibitor, showed robust antitumor activity. Furthermore, it presented the noticeable safety and efficacy in patients with ALK-positive and ROS1-positive NSCLC in phase I clinical trial (NCT03389815) (10).
In this study, the patient with novel intergenic-ALK (chr2_42048364; A20) fusion showed an excellent prognosis outcome after being treated with WX-0593. The soft tissue masses in the lung and liver diminished dramatically, which had obtained a PR after 17 months of therapy. The patient continues to receive WX-0593 medication while preparing this manuscript. Positive therapeutic outcomes in our investigation demonstrated that intergenic-ALK fusion could be considered as a potential oncogenic mutation by stimulating the overexpression of ALK proteins. We infer that the intergenic region at the 5’ end functions as a strong promoter in this unique fusion variant. Nonetheless, the oncogenic and molecular processes of this fusion will need to be further investigated.
Despite the great novelty of our results, several limitations still need to be discussed. RNA-based NGS is preferred to DNA-based NGS for fusion detection based on the NCCN Guidelines Version 1.2022 for NSCLC. However, because of the limited number of retained specimens, the RNA-NGS was not performed. It could not be precisely annotated whether the newly discovered fusion forms differed at the DNA and RNA levels. To confirm the biological function of the intergenic-ALK fusion in cancer, additional research is required.
In summary, an ALK-TKI candidate, WX-0593, was effectively treated a patient with NSCLC with novel intergenic-ALK fusion. This case broadened the breadth of ALK fusions that can be targeted and highlighted the utility of NGS in mining rare but functional ALK fusions, which eventually bring benefits to the patients.
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
This case report was approved by the Ethics Committee of Daping Hospital [2022(03)]. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JD and BW contributed to the experiment performing and manuscript writing. CW and TM participated to the data analysis. ML provided the clinical samples and relevant information. JS designed and optimized the experiment. All authors contributed to the article and approved the submitted version.
Clinical medical technology innovation ability training program (2019CXLCB002)
Authors BW, CW and TM were employed by Genetron Health (Beijing), Co. Ltd.
The remaning authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the patient and her family for their collaboration.
1. Du X, Shao Y, Qin HF, Tai YH, Gao HJ. ALK-Rearrangement in Non-Small-Cell Lung Cancer (NSCLC). Thorac Cancer (2018) 9:423–30.
2. Shaw AT, Kim D-W, Nakagawa K, Seto T, Crinó L, Ahn M-J, et al. Crizotinib Versus Chemotherapy in Advanced ALK-Positive Lung Cancer. N Engl J Med (2013) 368:2385–94.
3. Shaw AT, Kim D-W, Mehra R, Tan DS, Felip E, Chow LQ, et al. Ceritinib in ALK-Rearranged Non–Small-Cell Lung Cancer. N Engl J Med (2014) 370:1189–97.
4. Peters S, Camidge DR, Shaw AT., Gadgeel S, Ahn JS, Kim D-W, et al. Alectinib Versus Crizotinib in Untreated ALK-Positive Non–Small-Cell Lung Cancer. N Engl J Med (2017) 377:829–38.
5. Camidge DR, Kim HR, Ahn M-J, Yang JC-H, Han J-Y, Lee J-S, et al. Brigatinib Versus Crizotinib in ALK-Positive non–Small-Cell Lung Cancer. N Engl J Med (2018) 379:2027–39.
6. Solomon BJ, Besse B, Bauer TM, Felip E, Soo RA, Camidge DR, et al. Lorlatinib in Patients With ALK-Positive Non-Small-Cell Lung Cancer: Results From a Global Phase 2 Study. Lancet Oncol (2018) 19:1654–67.
7. Childress MA, Himmelberg SM, Chen H, Deng W, Davies MA, Lovly C. M.. ALK Fusion Partners Impact Response to ALK Inhibition: Differential Effects on Sensitivity, Cellular Phenotypes, and Biochemical Properties. Mol Cancer Res MCR (2018) 16:1724–36. doi: 10.1158/1541-7786.Mcr-18-0171
8. Ou S-HI, Zhu VW, Nagasaka M. Catalog of 5’fusion Partners in ALK-Positive NSCLC Circa 2020. JTO Clin Res Rep (2020) 1:100015.
9. Li W, Liu Y, Li W, Chen L, Ying J. Intergenic Breakpoints Identified by DNA Sequencing Confound Targetable Kinase Fusion Detection in NSCLC. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer (2020) 15:1223–31. doi: 10.1016/j.jtho.2020.02.023
Keywords: fusion, intergenic-ALK, inhibitor, WX-0593, SLC8A1, PKDCC
Citation: Du J, Wang B, Li M, Wang C, Ma T and Shan J (2022) A Novel Intergenic Gene Between SLC8A1 and PKDCC-ALK Fusion Responds to ALK TKI WX-0593 in Lung Adenocarcinoma: A Case Report. Front. Oncol. 12:898954. doi: 10.3389/fonc.2022.898954
Received: 18 March 2022; Accepted: 30 May 2022;
Published: 30 June 2022.
Edited by:
Anurag Mehta, Rajiv Gandhi Cancer Institute and Research Centre, IndiaReviewed by:
Ullas Batra, Rajiv Gandhi Cancer Institute and Research Centre, IndiaCopyright © 2022 Du, Wang, Li, Wang, Ma and Shan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinlu Shan, amlubHVzaGFuMjAyMkB5ZWFoLm5ldA==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.