- 1Department of Pharmacy, National Cancer Center Hospital, Tokyo, Japan
- 2Division of Pharmaceutical Care Sciences, Keio University Graduate School of Pharmaceutical Sciences, Tokyo, Japan
- 3Division of Pharmaceutical Care Sciences, Center for Social Pharmacy and Pharmaceutical Care Sciences, Keio University Faculty of Pharmacy, Tokyo, Japan
- 4Department of Pharmacy, National Cancer Center Hospital East, Chiba, Japan
- 5Department of Pharmacy, Center Hospital of the National Center for Global Health and Medicine, Tokyo, Japan
- 6Department of Medical Oncology, National Cancer Center Hospital, Tokyo, Japan
Background: Olaparib-induced anemia is a frequently occurring complication in patients with advanced ovarian cancer, fallopian tube cancer, or primary peritoneal cancer and is associated with a marked deterioration in patients’ health-related quality of life. This study aimed to clarify patient-specific risk factors for severe anemia in patients with advanced ovarian or breast cancer receiving olaparib monotherapy in a real-world setting.
Methods: This multicenter, retrospective, observational study enrolled consecutively presenting patients with advanced ovarian or breast cancer who received olaparib monotherapy as maintenance or palliative treatment between April 2018 and December 2020 at three participating medical institutions in Japan. The primary endpoint was patient-associated risk factors underlying the onset of grade ≥3 anemia from olaparib treatment initiation to 90 days after treatment. Receiver operating characteristic curves were constructed and univariable and multivariable logistic regression analyses were performed to evaluate the association between patient-associated risk factors and grade ≥3 anemia.
Results: Of 113 patients evaluated in this study, 32.7% (n = 37) had grade ≥3 anemia. Multivariable logistic regression analysis revealed that low baseline red blood cell (RBC) count (<3.3 × 106 cells/μL), low baseline hematocrit level (<35%), low baseline hemoglobin level (<11.6 g/dL), and breast cancer susceptibility (BRCA1/2) mutation were significantly associated with the onset of grade ≥3 anemia (adjusted odds ratio [OR], 3.39; 95% confidence interval [CI], 1.28–9.62; P = 0.017, adjusted OR, 3.63; 95% CI, 1.28–11.64; P = 0.021, adjusted OR, 3.89; 95% CI, 1.39–12.21; P = 0.014, and adjusted OR, 4.09; 95% CI, 1.55–11.67; P = 0.006, respectively).
Conclusions: Our findings suggest that low baseline RBC count, low baseline hematocrit level, and low baseline hemoglobin level might be the patient-associated risk factors for severe anemia induced by olaparib monotherapy. Additionally, BRCA1/2 mutation was suggested to be a patient-related risk factor for anemia regardless of severity. Therefore, applying these patient-associated risk factors would help classify and screen patients at risk of severe anemia.
Introduction
According to a leading statistical report regarding global cancer incidence and prevalence, breast cancer in women was the most commonly diagnosed incident cancer (11.7%) and was ranked as the fourth leading cause of cancer-associated mortality (6.9%) among women in 2020. Ovarian cancer was reported to be the 19th most commonly diagnosed incident cancer (1.6%) and the 13th leading cause of cancer-associated mortality (2.1%) (1). Recently, three pivotal phase III clinical trials have revealed that drugs such as olaparib, polyadenosine, and poly (adenosine diphosphate–ribose) polymerase (PARP) inhibitors show a different mechanism of action as compared to the traditional cytotoxic chemotherapy. These findings have greatly changed the clinical outcomes of patients with advanced ovarian or breast cancer (2–4). Currently, olaparib monotherapy is considered the new treatment standard for patients with advanced ovarian or breast cancer (5, 6).
Nonetheless, in clinical practice, olaparib-induced severe anemia is a frequently occurring complication that is associated with a marked deterioration in patients’ health-related quality of life. A phase III clinical trial was conducted on patients newly diagnosed with ovarian cancer who also had breast cancer susceptibility (BRCA1/2) mutation. The trial also included patients with primary peritoneal cancer and fallopian tube cancer. The overall incidence rate of anemia after using olaparib as maintenance therapy was 39%, whereas that of grade ≥3 anemia was 22% (2). Similarly, a phase III clinical trial conducted on patients with platinum-sensitive relapsed ovarian cancer with BRCA1/2 mutation, primary peritoneal cancer, and fallopian tube cancer reported that the overall incidence of anemia after the use of olaparib as maintenance therapy was 43%, whereas that of grade ≥3 anemia was 19% (3). In a phase II clinical trial involving patients with recurrent platinum-sensitive ovarian cancer, irrespective of the BRCA1/2 mutation, primary peritoneal cancer, and fallopian tube cancer, the overall incidence of anemia was 17%, whereas that of grade ≥3 anemia was 5% when olaparib was used as maintenance therapy (7). Additionally, in a phase III clinical trial on breast cancer patients, the incidence rates of anemia (any grade) and grade ≥3 anemia were 40% and 16%, respectively (4). Taken together, the incidence of olaparib-induced severe anemia was found to be consistent among patients with ovarian and breast cancer. Moreover, a meta-analysis reported that the relative risk of anemia was significantly higher in patients treated with olaparib than in those who received a placebo (8).
Preclinical research has shown that PARP-2-deficient mice exhibited a significant decrease in hemoglobin levels as compared to the wild-type mice (9). This suggests that the detected decrease in hemoglobin levels may be caused by a decrease in the number of erythrocytes and that this mechanism involves the differentiation of immature erythrocytes due to deoxyribonucleic acid damage and apoptosis as mediated by the TP53 gene.
Fanconi anemia (FA) is an inherited condition associated with developmental abnormalities, bone marrow failure, and increased risk of cancer, with a prevalence of 1–5 per million births. FA is reported to be caused by a disruption in the Fanconi anemia/breast cancer (FA/BRCA) pathway. Moreover, 22 genes involved in the FA/BRCA pathway have been identified, including BRCA1 and BRCA2 (10–13). This implies that when BRCA1/2 mutations occur, bone marrow failure, resulting in anemia, is likely to occur.
On the contrary, it has been reported that bone marrow suppression caused by traditional cytotoxic chemotherapy leads to anemia, tumor invasion, and bone marrow destruction, which results in anorexia and malnutrition. These have been reported as direct contributory factors in the development of anemia (14). Moreover, hemoglobin, hematocrit, and body mass index (BMI) have been reported as patient-specific risk factors for chemotherapy-induced severe anemia (15). Importantly, the association between patient-associated risk factors and severe anemia in patients receiving olaparib monotherapy has not yet been established. Considering all the facts, we hypothesized that these patient-associated risk factors could predict severe anemia induced by olaparib monotherapy in clinical practice.
Therefore, this study aimed to clarify the patient-associated risk factors for severe anemia in patients with advanced ovarian or breast cancer treated with olaparib monotherapy in a real-world setting.
Materials and methods
Study design and patients
This multicenter, retrospective, observational study was conducted at three participating medical institutions: the National Cancer Center Hospital (Tokyo, Japan), the National Cancer Center Hospital East (Chiba, Japan), and the Center Hospital of the National Center for Global Health and Medicine (Tokyo, Japan). Patient data were extracted from the electronic medical records. Data integration and subsequent analyses were performed at the Keio University Graduate School of Pharmaceutical Sciences (Tokyo, Japan). The methodology adopted in this study followed the STROBE guidelines (16). Some methods used in the present study were previously reported by our co-authors (17–19).
The inclusion criteria for the patients were as follows (1): consecutively presenting and aged ≥20 years, with a diagnosis of advanced ovarian or breast cancer and (2) receipt of olaparib monotherapy (300 mg taken orally twice daily) as maintenance or palliative treatment between April 2018 and December 2020.
The exclusion criteria were as follows (1): incomplete data from the patients’ medical records or lack of baseline laboratory data (2); a lower olaparib dosage at therapy initiation (100–250 mg taken orally twice daily) (3); an olaparib treatment period of <90 days; and (4) restarting the treatment after the discontinuation of olaparib monotherapy due to any reason (except resumption after drug withdrawal).
The study protocol was approved by the Ethics Committees of the National Cancer Center (approval number: 2021-052) and the Center Hospital of the National Center for Global Health and Medicine (approval number: NCGM-G-004274-00). The study was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research involving Human Subjects by the Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labour, and Welfare of Japan. The need for written or oral informed consent was waived by the ethics review committees due to the retrospective nature of the study. Accordingly, we used the official website of the hospital to provide an opt-out option (via a web form) rather than directly acquiring written or verbal informed consent from each patient.
Data collection
Patients’ data were de-identified and anonymously analyzed. We extracted the necessary baseline clinical and demographic data (defined as the most recent blood count within 4 weeks prior to treatment initiation, as well as other clinical data prior to treatment initiation). The following data were collected: age, sex, type of cancer, BMI, Eastern Cooperative Oncology Group performance status (ECOG PS), medical history of chemotherapy, presence or absence of BRCA1/2 mutation, and routinely available peripheral blood data, including red blood cell (RBC) count and hemoglobin and hematocrit levels. The follow-up period ended on March 31, 2021.
Endpoints
The primary endpoint was patient-associated risk factors underlying the onset of grade ≥3 anemia. The secondary endpoint was the onset of grade ≥1 anemia. These endpoints were graded in accordance with the Common Terminology Criteria for Adverse Events (v5.0) (20). Furthermore, based on the common criteria range delineated by the Japan Clinical Oncology Group (21), grades 3 and 1 anemia were defined as hemoglobin levels of <8.0 g/dL (for both sexes) and <11.6 g/dL (for females), respectively. In each patient, anemia was evaluated as the least hemoglobin value measured from treatment initiation to 90 days after the completion of olaparib treatment. If no changes were observed in the anemia grade before and after olaparib administration, it was not considered as the onset of anemia.
Statistical analyses
Patient characteristics were summarized using descriptive statistics, including frequencies and proportions. Receiver operating characteristic curve analyses and Youden’s index were used to determine the optimal cut-off values for continuous variables to predict the onset of anemia (22). Youden’s index was calculated as the maximum value using the following formula: sensitivity - (1 – specificity). We also calculated the positive and negative predictive values (PPV and NPV, respectively).
Univariable and multivariable logistic regression analyses were performed to evaluate the association between patient-associated risk factors and the onset of anemia. Potential explanatory variables reported by several previous studies, specifically baseline RBC count, baseline hematocrit level, baseline hemoglobin level, age, and baseline BMI values, were included as covariates in the univariable and multivariable models (14, 15). Furthermore, we included BRCA1/2 mutations since they are part of the genes responsible for FA (10–13). We chose to evaluate only four covariates as a multivariable analysis requires at least 10 events per variable to characterize the outcomes reliably (23, 24).
The results are presented as odds ratios (ORs) and 95% confidence intervals (CIs). All statistical analyses were performed using JMP software version 16.2.0 (SAS Institute Inc., Cary, NC, USA). All P-values were two-sided, and a P-value of <0.05 was considered statistically significant.
Results
Patient characteristics
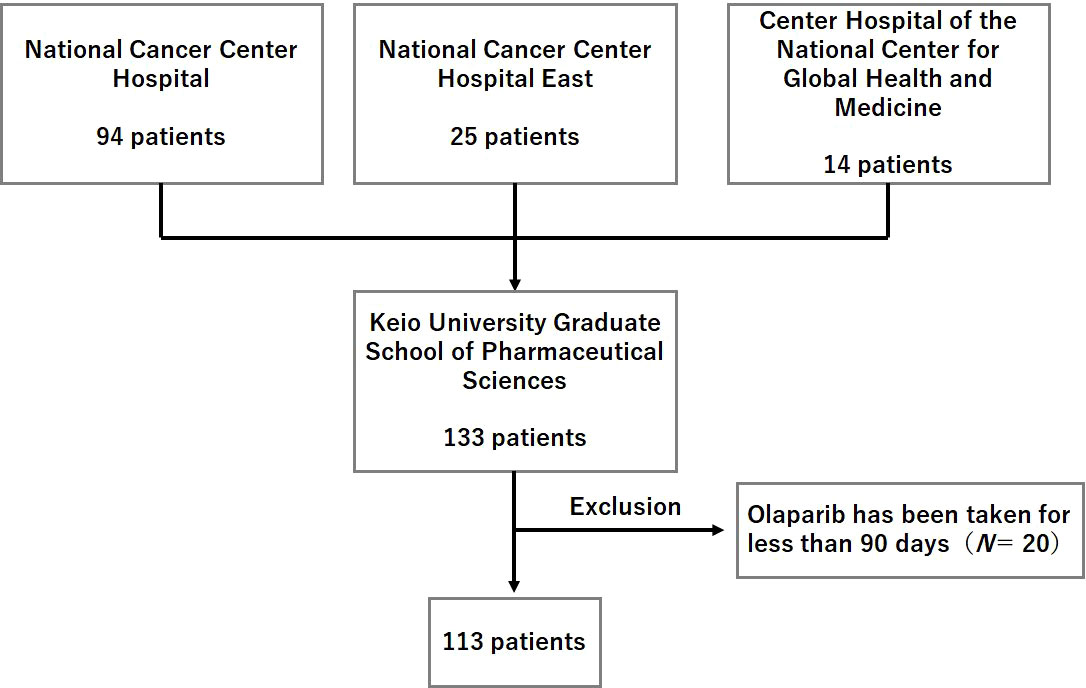
The patient enrollment flowchart is shown in Figure 1. Of the 133 initially identified patients, 20 were excluded from the study based on the aforementioned exclusion criteria. Thus, a total of 113 patients formed the study population.
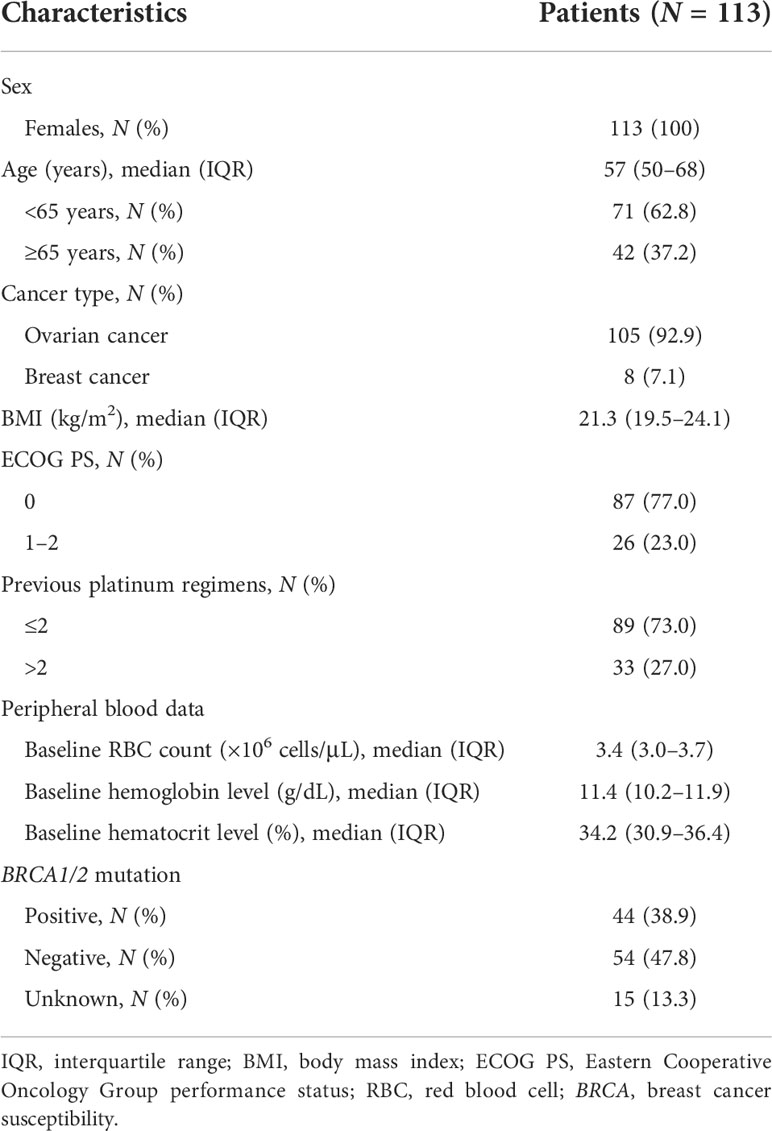
Patient demographic data are summarized in Table 1. All patients were females, and the median age of the enrolled patients was 57 years (interquartile range [IQR], 50–68 years). Olaparib monotherapy was administered to 105 (92.9%) patients with ovarian cancer and eight (7.1%) patients with breast cancer. Moreover, 87 patients (77.0%) were in good condition, with an ECOG PS of 0. The median baseline RBC count was 3.4 × 106 cells/μL (IQR, 3.0–3.7 × 106 cells/μL). The median baseline hematocrit level was 34.2% (IQR, 30.9–36.4%), whereas the median baseline hemoglobin level was 11.4 g/dL (IQR, 10.2–11.9 g/dL). Of all the patients, 44 (38.9%) were positive for BRCA1/2 mutation, 54 (47.8%) were negative, and 15 (13.3%) had an unknown mutation status.
Endpoints
The incidence of grade ≥3 anemia was 32.7% (n = 37), whereas the incidence of grade ≥1 anemia was 61.1% (n = 69). The optimal cut-off values for age, baseline RBC count, baseline hematocrit level, and baseline hemoglobin level in predicting the onset of grade ≥3 anemia were initially determined to be 66 years, 3.33 × 106 cells/μL, 33.2%, and 10.8 g/dL, respectively; with corresponding Youden’s index values of 0.126, 0.266, 0.253, and 0.226, respectively. The sensitivity, 1 – specificity, PPV, and NPV for the determined optimal age cut-off value were 78.4%, 65.8%, 29.0%, and 26.0%, respectively. The sensitivity, 1 – specificity, PPV, and NPV for the determined optimal baseline RBC count cut-off value were 62.2%, 35.5%, 23.0%, and 49.0%, respectively. The sensitivity, 1 – specificity, PPV, and NPV for the determined optimal baseline hematocrit level cut-off value were 62.2%, 36.8%, 23.0%, and 48.0%, respectively. Finally, the sensitivity, 1 – specificity, PPV, and NPV for the determined optimal baseline hemoglobin level cut-off value were 56.8%, 34.2%, 21.0%, and 50.0%, respectively. The areas under the curve for age, baseline RBC count, baseline hematocrit level, and baseline hemoglobin level were 0.540, 0.607, 0.634, and 0.622, respectively. Therefore, we decided that an age of 65 years, baseline RBC count, hematocrit level, and hemoglobin level of 3.3 × 106 cells/μL, 35%, and 11.6 g/dL, respectively, were the appropriate cut-off values for further analysis. The optimal cut-off value for BMI was set at 23 kg/m2, based on the results of a previous study (15). The median treatment duration was 269 days (IQR, 181–407 days).
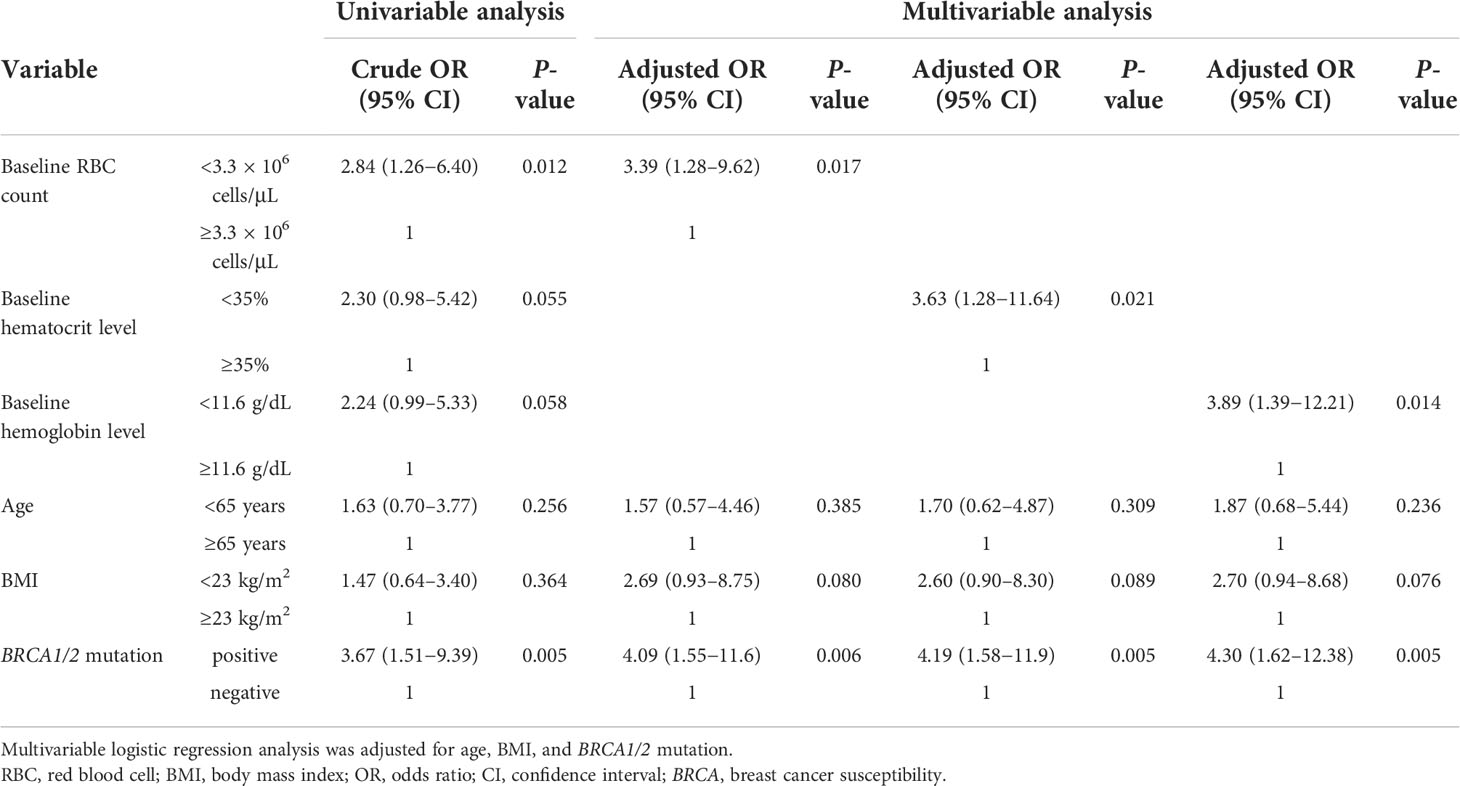
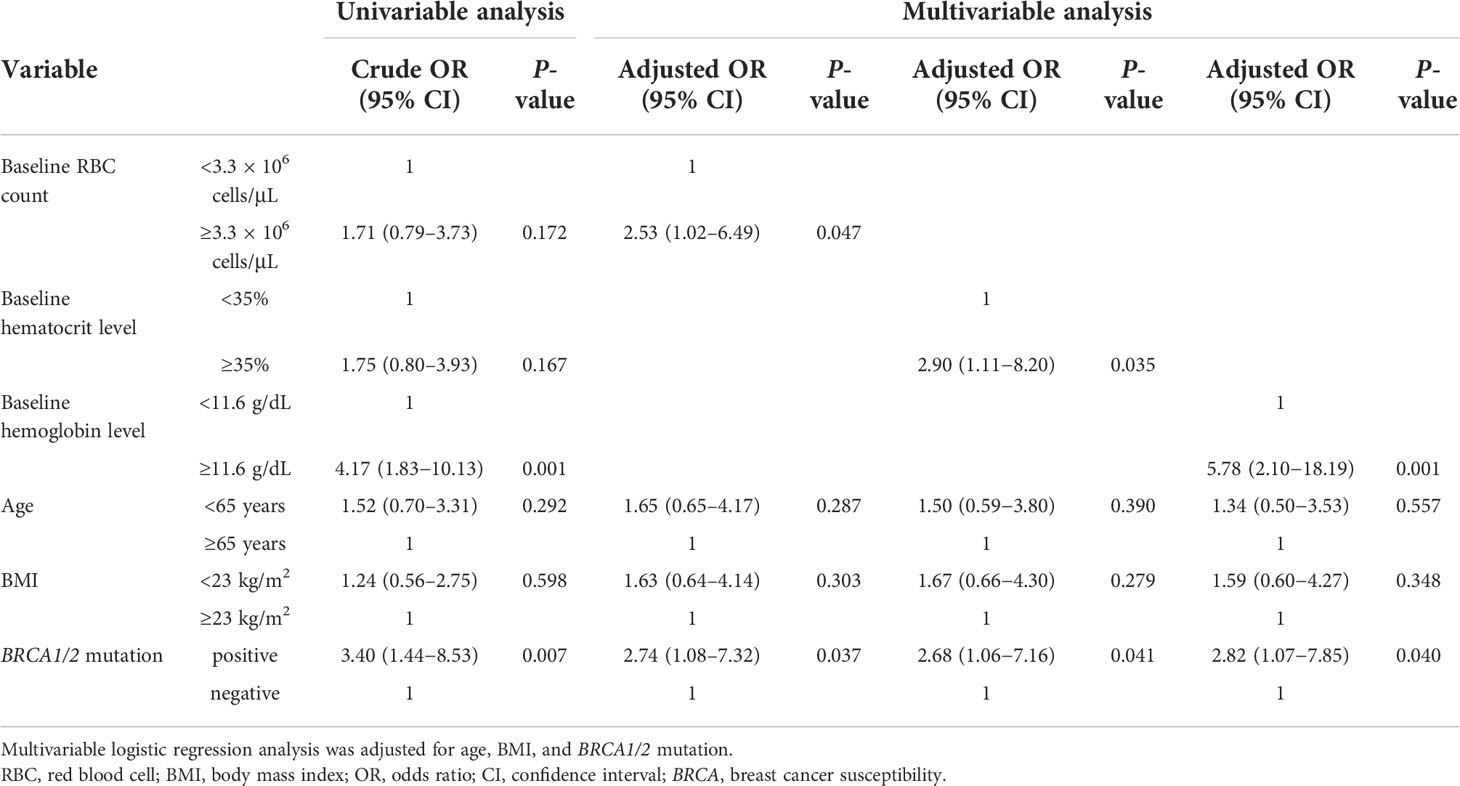
The primary endpoints are listed in Table 2. Multivariable logistic regression analysis revealed that low baseline RBC count (<3.3 × 106 cells/μL), low baseline hematocrit level (<35%), low baseline hemoglobin level (<11.6 g/dL), and BRCA1/2 mutation were significantly associated with the onset of grade ≥3 anemia (adjusted OR, 3.39; 95% CI, 1.28–9.62; P = 0.017, adjusted OR, 3.63; 95% CI, 1.28–11.64; P = 0.021, adjusted OR, 3.89; 95% CI, 1.39–12.21; P = 0.014, and adjusted OR, 4.09; 95% CI, 1.55–11.67; P = 0.006, respectively). In contrast, high baseline RBC count (≥3.3 × 106 cells/μL), high baseline hematocrit level (≥35%), high baseline hemoglobin level (≥11.6 g/dL), and BRCA1/2 mutation were significantly associated with the onset of grade ≥1 anemia (adjusted OR, 2.53; 95% CI, 1.02–6.49, P = 0.047, adjusted OR, 2.90; 95% CI, 1.11–8.20, P = 0.035, adjusted OR, 5.78; 95% CI, 2.10–18.19, P = 0.001, and adjusted OR, 2.74; 95% CI, 1.08–7.32, P = 0.037, respectively) (Table 3).
Discussion
The present study showed that low baseline RBC count, low baseline hematocrit level, and low baseline hemoglobin level might be the patient-associated risk factors for severe anemia induced by olaparib monotherapy. In addition, BRCA1/2 mutation was suggested to be a patient-related risk factor for anemia regardless of severity. RBC count, hematocrit level, and hemoglobin level are closely related to the hematopoietic function. It is considered that severe anemia is more likely to occur in patients receiving olaparib since this drug is administered during the period when the hematopoietic function is already impaired. To the best of our knowledge, this is the first report on the association between patient-associated risk factors and severe anemia in patients treated with olaparib monotherapy in a real-world setting. Patients with BRCA1/2 mutations are at risk of FA, which may have affected the frequency and severity of olaparib-induced anemia.
The mechanisms underlying the correlations of low baseline RBC count, hematocrit level, and hemoglobin level with respect to the onset of severe anemia have not yet been fully clarified. When the erythrocyte parameters of PARP-2-/- mice and wild-type mice were compared in a preclinical setting, the RBC count, hematocrit level, and hemoglobin level were significantly lower in the PARP-2-/- mice than in the wild-type mice (9). The results of the present study were in concordance with the findings reported in the aforementioned studies. Razzaghdoust et al. reported that in 305 patients with solid tumors or lymphoma who received traditional cytotoxic chemotherapy, hemoglobin level, hematocrit level, and BMI values were strong predictors of severe anemia (15). Aligning with this study, the present study demonstrated that hematocrit level complemented the prediction of severe anemia. BMI was also associated with the incidence of severe anemia. The patients’ backgrounds in the present study differed from patients enrolled in prior investigations. However, we believe that the mechanisms underlying the association between patient-associated risk factors and severe anemia are common between olaparib and traditional cytotoxic chemotherapy. Although we did not investigate mean corpuscular volume in this study, which is a part of the hematopoietic parameters, it may be a risk factor for olaparib-induced anemia as well as an explanatory factor for other hematopoietic parameters. Additionally, no patients with myelodysplastic syndrome or acute myeloid leukemia were included in the study. The prevalence of FA is 1−5 per million live births (10–13). Therefore, the effect of BRCA1/2 mutations on anemia is not significant.
To the best of our knowledge, the present study is the first to indicate that low baseline RBC count, low baseline hematocrit level, and low baseline hemoglobin level could be patient-associated risk factors for severe anemia induced by olaparib monotherapy. Moreover, the present study is also the first to identify BRCA1/2 mutation as a possible patient-related risk factor for anemia regardless of severity. These parameters are easily obtainable and do not require additional cost or time. Applying these predictors would help classify and screen patients at high risk for olaparib-induced severe anemia in clinical practice.
The incidence of anemia in the present study was higher than that in previous studies. Olaparib is reported to be metabolized by CYP3A4/5. It is reported that the incidence of CYP3A5 differs between Japanese and European and United States patients, which may have affected the results of this study (25). Moreover, unlike in Europe and the United States, specific standards for dietary folic acid intake have not been established in Japan, except during pregnancy. Additionally, there may be a dissociation between the characteristics of patients in clinical trials and those in actual clinical practice, which may have also affected our results.
Previous studies have shown that prophylactic erythropoietin reduces the negative consequences of chemotherapy-induced severe anemia and improves the patients’ health-related quality of life (26–28). However, this practice is considered an off-label use in Japan. A previous study reported that erythropoietin production is increased in a compensatory manner in PARP-2-/- mice and contributes to the maintenance of erythropoiesis, thus suggesting that erythropoietin may have an effect on ameliorating the risk of severe anemia (9). Considering all these facts, oncologists and other treating clinicians can provide personalized, evidence-based clinical management for these high-risk patients.
High baseline RBC count (≥3.3 × 106 cells/μL), high baseline hematocrit level (≥35%), and high baseline hemoglobin level (≥11.6 g/dL) were significantly associated with the onset of grade ≥1 anemia. The reason for this finding may be attributed to the criteria for evaluating anemia. In the present study, patients who did not show a change in their anemia grade before and after olaparib administration were not considered to have new onset anemia. This result suggests that progression from grade 0 to grade 1 anemia is more likely to occur than a progression from grade 1 to grade ≥2 or from grade 2 to grade ≥3.
The present study has two strengths. First, this was a multicenter study involving three participating medical institutions in Japan, including the National Cancer Center. Therefore, our data may be generalizable to similar populations in the clinical setting. Second, we focused on olaparib monotherapy for patients with advanced ovarian or breast cancer since olaparib monotherapy has become the new treatment regimen for such patients.
Nevertheless, we acknowledge some limitations in our study. First, this was a retrospective observational study. Thus, we could not exclude the possibility of information bias. However, we conducted a multivariable analysis to reduce the effects of biases inherent in observational studies and potential confounders that may be associated with clinical variations in patient characteristics. However, by definition, we could not control the unmeasured confounders during the multivariable analysis. Second, the sample size of the current study was modest; therefore, we could not include more than four covariates in the multivariable analysis. The baseline RBC count, hematocrit level, and hemoglobin level seemed to be correlated. However, in the preclinical study, RBC, hematocrit, and hemoglobin were decreased in PARP-2-deficient mice compared to wild-type mice (9); therefore, we selected these as explanatory variables. While the mechanism of olaparib-induced anemia is not clear, we believe it is important to analyze all of these entities as explanatory variables. Third, we only enrolled patients with advanced ovarian or breast cancer. In recent years, olaparib has been approved for patients with breast cancer possessing mutated BRCA1/2 gene, male patients with metastatic castration-resistant prostate cancer, and patients with metastatic pancreatic cancer in Japan (29, 30). Fourth, we neither investigated the presence or absence of therapeutic drugs for anemia nor evaluated blood transfusion as a study covariate. Moreover, risk factors for anemia in combination with other drugs (such as bevacizumab) were not investigated, and the period between previous platinum-based chemotherapy and the initiation of olaparib monotherapy was not considered. Furthermore, the presence of grade ≥3 anemia, thrombocytopenia, or neutropenia at the time of the previous platinum-based chemotherapy was not considered. No differences in neutrophil and platelet counts were found between PARP-2-deficient mice and wild-type mice in the in vivo study (9). Therefore, we limited the explanatory factors in this study to erythrocyte parameters. Olaparib monotherapy is used as maintenance therapy and is most likely to be prescribed within 1 month of concluding chemotherapy. Fifth, since this study was a retrospective study based on clinical practice, we neither investigated the erythrocyte volume by examining the relationship of the severity of anemia with the duration of olaparib administration nor evaluated the flow cytometry of peripheral blood, which may have revealed incipient myelodysplastic syndrome. Finally, the effect of the olaparib dose on anemia could not be assessed since the dose intensity was not calculated. Overall, our findings should be confirmed using an adequate sample of patients with the above-mentioned solid malignancies who were treated with olaparib.
In conclusion, our findings suggest that low baseline RBC count, low baseline hematocrit level, and low baseline hemoglobin level might be the patient-associated risk factors for severe anemia induced by olaparib monotherapy. Additionally, BRCA1/2 mutation was suggested to be a patient-related risk factor for anemia regardless of severity. Moreover, early detection of patients at high risk for developing severe anemia can prompt cautious monitoring and optimize the treatment benefit for patients treated with olaparib monotherapy. The findings of the current study can likely be generalized to other populations, thus highlighting the need for additional research in this field.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors upon request, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committees of National Cancer Center (approval number: 2021-052) and the Center Hospital of the National Center for Global Health and Medicine (approval number: NCGM-G-004274-00). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
RT, HK, YS, HH, MY, and TN: conceptualization and design. RT, KM, and KS: data acquisition and patient management. RT and HK: data analysis and interpretation. RT and HK: writing, reviewing, and revising the manuscript. TN: study supervision. All authors contributed to the article and approved the submitted version.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgments
We are grateful to all patients and medical staff at the National Cancer Center Hospital, the National Cancer Center Hospital East, and the Center Hospital of the National Center for Global Health and Medicine who were involved in this study. We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
HK received research funding from Eli Lilly. KM received payment for presentations and expert testimony from Daiichi Sankyo and payment for presentations from Merck Serono. HT received research funding from AbbVie. TN received research funding from Astellas Pharma, Chugai, Daiichi Sankyo, Kyowa Kirin, Otsuka Pharmaceutical, Sanofi, Sato Pharmaceutical, and Shionogi.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Moreover, the above funders had no role in the design, conduct, or reporting of this investigation. Thus, the authors have no actual conflicts of interest to declare.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BMI, body mass index; BRCA, breast cancer susceptibility; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; FA, Fanconi anemia; IQR, interquartile range; NPV, negative predictive value; OR, odds ratio; PARP, poly (adenosine diphosphate–ribose) polymerase; PPV, positive predictive value; RBC, red blood cell.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med (2018) 379:2495–505. doi: 10.1056/NEJMoa1810858
3. Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol (2017) 18:1274–84. doi: 10.1016/S1470-2045(17)30469-2
4. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med (2017) 377:523–33. doi: 10.1056/NEJMoa1706450
5. National Comprehensive Cancer Network (NCCN) Breast cancer. version 2.2022. NCCN clinical practice guidelines in oncology. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (Accessed January 21, 2022).
6. National Comprehensive Cancer Network (NCCN). Ovarian Cancer/Fallopian tube Cancer/Primary peritoneal cancer. version 1.2022. NCCN clinical practice guidelines in oncology. Available at: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (Accessed January 21, 2022).
7. Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med (2012) 366:1382–92. doi: 10.1056/NEJMoa1105535
8. Ruiz-Schutz VC, Gomes LM, Mariano RC, de Almeida DVP, Pimenta JM, Dal Molin GZ, et al. Risk of fatigue and anemia in patients with advanced cancer treated with olaparib: A meta-analysis of randomized controlled trials. Crit Rev Oncol Hematol (2019) 141:163–73. doi: 10.1016/j.critrevonc.2019.06.012
9. Farrés J, Llacuna L, Martin-Caballero J, Martínez C, Lozano JJ, Ampurdanés C, et al. PARP-2 sustains erythropoiesis in mice by limiting replicative stress in erythroid progenitors. Cell Death Differ (2015) 22:1144–57. doi: 10.1038/cdd.2014.202
10. García-de-Teresa B, Rodríguez A, Frias S. Chromosome instability in fanconi anemia: From breaks to phenotypic consequences. Genes (2020) 11:1528. doi: 10.3390/genes11121528
11. Renaudin X, Rosselli F. The FANC/BRCA pathway releases replication blockades by eliminating DNA interstrand cross-links. Genes (2020) 11:585. doi: 10.3390/genes11050585
12. Cheung RS, Taniguchi T. Recent insights into the molecular basis of fanconi anemia: Genes, modifiers, and drivers. Int J Hematol (2017) 106:335–44. doi: 10.1007/s12185-017-2283-4
13. Kim Y, Spitz GS, Veturi U, Lach FP, Auerbach AD, Smogorzewska A. Regulation of multiple DNA repair pathways by the fanconi anemia protein SLX4. Blood (2013) 121:54–63. doi: 10.1182/blood-2012-07-441212
14. Abdel-Razeq H, Hashem H. Recent update in the pathogenesis and treatment of chemotherapy and cancer induced anemia. Crit Rev Oncol Hematol (2020) 145:102837. doi: 10.1016/j.critrevonc.2019.102837
15. Razzaghdoust A, Mofid B, Peyghambarlou P. Predictors of chemotherapy-induced severe anemia in cancer patients receiving chemotherapy. Support Care Cancer (2020) 28:155–61. doi: 10.1007/s00520-019-04780-7
16. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
17. Ogiwara T, Kawazoe H, Egami S, Hashimoto H, Saito Y, Sakiyama N, et al. Prognostic value of baseline medications plus neutrophil-to-lymphocyte ratio in the effectiveness of nivolumab and pembrolizumab in patients with advanced non-small-cell lung cancer: a retrospective study. Front Oncol (2021) 11:770268. doi: 10.3389/fonc.2021.770268
18. Egami S, Kawazoe H, Hashimoto H, Uozumi R, Arami T, Sakiyama N, et al. Absolute lymphocyte count predicts immune-related adverse events in patients with non-small-cell lung cancer treated with nivolumab monotherapy: a multicenter retrospective study. Front Oncol (2021) 11:618570. doi: 10.3389/fonc.2021.618570
19. Egami S, Kawazoe H, Hashimoto H, Uozumi R, Arami T, Sakiyama N, et al. Peripheral blood biomarkers predict immune-related adverse events in non-small cell lung cancer patients treated with pembrolizumab: a multicenter retrospective study. J Cancer (2021) 12:2105–12. doi: 10.7150/jca.53242
20. National Cancer Institute. Common terminology criteria for adverse events (CTCAE) version 5.0. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40 (Accessed February 7, 2022).
21. Japan Clinical Oncology Group. Common criteria range of the Japan clinical oncology group (JCOG). Available at: http://www.jcog.jp/doctor/tool/kijun.html (Accessed February 8, 2022).
22. Pepe MS. The statistical evaluation of medical tests for classification and prediction. New York, NY: Oxford University Press (2003).
23. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol (1996) 49:1373–9. doi: 10.1016/s0895-4356(96)00236-3
24. Courvoisier DS, Combescure C, Agoritsas T, Gayet-Ageron A, Perneger TV. Performance of logistic regression modeling: Beyond the number of events per variable, the role of data structure. J Clin Epidemiol (2011) 64:993–1000. doi: 10.1016/j.jclinepi.2010.11.012
25. Hiratsuka M, Takekuma Y, Endo N, Narahara K, Hamdy SI, Kishikawa Y, et al. Allele and genotype frequencies of CYP2B6 and CYP3A5 in the Japanese population. Eur J Clin Pharmacol (2002) 58:417–21. doi: 10.1007/s00228-002-0499-5
26. Chang J, Couture F, Young S, McWatters KL, Lau CY. Weekly epoetin alfa maintains hemoglobin, improves quality of life, and reduces transfusion in breast cancer patients receiving chemotherapy. J Clin Oncol (2005) 23:2597–605. doi: 10.1200/JCO.2004.12.027
27. Lyman GH, Glaspy J. Are there clinical benefits with early erythropoietic intervention for chemotherapy-induced anemia? A systematic review. Cancer (2005) 106:223–33. doi: 10.1002/cncr.21570
28. Coiffier B, Guastalla JP, Pujade-Lauraine E, Bastit P. Predicting cancer-associated anemia in patients receiving nonplatinum chemotherapy: Results of a retrospective survey. Eur J Cancer (2001) 37:1617–23. doi: 10.1016/s0959-8049(01)00169-1
29. de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med (2020) 382:2091–102. doi: 10.1056/NEJMoa1911440
Keywords: olaparib (Lynparza™), severe anemia, risk factor, red blood cell count (RBC), breast cancer susceptibility (BRCA) gene
Citation: Tashiro R, Kawazoe H, Mamishin K, Seto K, Udagawa R, Saito Y, Hashimoto H, Shimoi T, Yonemori K, Yonemura M, Terakado H, Kawasaki T, Furukawa T and Nakamura T (2022) Patient-associated risk factors for severe anemia in patients with advanced ovarian or breast cancer receiving olaparib monotherapy: A multicenter retrospective study. Front. Oncol. 12:898150. doi: 10.3389/fonc.2022.898150
Received: 17 March 2022; Accepted: 16 September 2022;
Published: 04 October 2022.
Edited by:
Elisa Giovannetti, VU Medical Center, NetherlandsReviewed by:
Kyung Jin Eoh, Yonsei University, South KoreaRichard T. Penson, Massachusetts General Hospital and Harvard Medical School, United States
Copyright © 2022 Tashiro, Kawazoe, Mamishin, Seto, Udagawa, Saito, Hashimoto, Shimoi, Yonemori, Yonemura, Terakado, Kawasaki, Furukawa and Nakamura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hitoshi Kawazoe, a2F3YXpvZS1odEBrZWlvLmpw
 Ryota Tashiro
Ryota Tashiro Hitoshi Kawazoe
Hitoshi Kawazoe Kanako Mamishin
Kanako Mamishin Keisuke Seto5
Keisuke Seto5 Tatsunori Shimoi
Tatsunori Shimoi Kan Yonemori
Kan Yonemori Tomonori Nakamura
Tomonori Nakamura