
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 06 June 2022
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.898119
This article is part of the Research Topic Neoadjuvant Treatment for Resectable and Borderline Resectable Pancreatic Cancer View all 5 articles
 Changchang Lu1,2†
Changchang Lu1,2† Yahui Zhu1†
Yahui Zhu1† Hao Cheng3†
Hao Cheng3† Weiwei Kong1
Weiwei Kong1 Linxi Zhu3
Linxi Zhu3 Lei Wang4
Lei Wang4 Min Tang5
Min Tang5 Jun Chen6
Jun Chen6 Qi Li6
Qi Li6 Jian He7
Jian He7 Aimei Li7
Aimei Li7 Xin Qiu2
Xin Qiu2 Dongsheng Chen8
Dongsheng Chen8 Fanyan Meng1
Fanyan Meng1 Xiaoping Qian1
Xiaoping Qian1 Baorui Liu1*
Baorui Liu1* Yudong Qiu3*
Yudong Qiu3* Juan Du1*
Juan Du1*Immune monotherapy does not appear to work in patients with pancreatic cancer so far. We are conducting a clinical trial that combines programmed cell death protein-1 (PD-1) inhibitor with chemotherapy and concurrent radiotherapy as induction therapy for patients with locally advanced pancreatic cancer (LAPC) and borderline resectable pancreatic cancer (BRPC). Here, we report a case with a pathologic complete response (pCR) and no postoperative complications after the induction therapy. The patient received four cycles of induction therapy and achieved a partial response (PR) with a significant decline of tumor marker carbohydrate antigen 19-9 (CA19-9). Also, peripheral blood samples were collected during the treatment to investigate serial circulating tumor DNA (ctDNA) dynamic changes in predicting the tumor response and outcomes in patients. Our result suggested that PD-1 blockade plus chemotherapy and concurrent radiotherapy is a promising mode as induction therapy for patients with potentially resectable pancreatic cancer. In this case, serial ctDNA alterations accurately provide a comprehensive outlook of the tumor status and monitor the response to the therapy, as validated by standard imaging.
Pancreatic cancer is one of the worst poor-prognosis gastrointestinal tumors with the lowest 5-year relative survival rate (10%) (1). According to the cancer incidence and deaths in the United States, pancreatic cancer is projected to become the second leading cause of cancer-related death by 2030 (2). So far, surgery remains the first option for patients with resectable pancreatic cancer, while only less than 20% of the patients may be possible to have surgery at definite diagnosis (3). Chemoradiation is still recommended as the best choice for locally advanced and metastatic disease (4). The National Comprehensive Cancer Network (NCCN) guidelines have endorsed neoadjuvant therapy for patients with resectable or borderline resectable pancreatic cancer (BRPC).
Recently, immunotherapy especially the immune checkpoint inhibitors has been a research hot spot (5). There is research suggesting that first-line pembrolizumab monotherapy significantly improved the overall survival (OS) and progression-free survival in patients with metastatic non-small-cell lung cancer (NSCLC) (6). In addition, a previous study has found that pancreatic cancer treated with chemotherapy plus immunotherapy has a higher survival rate in comparison to chemotherapy alone based on the exploration of the signaling pathways involved in Pancreatic Ductal Adenocarcinoma(PDAC) development (7). Moreover, other types of immunotherapies including tumor vaccine (8) and chimeric antigen receptor T-cell (CAR-T) therapy (9) have also gradually emerged in antitumor therapy. However, all kinds of immunotherapies failed to make a breakthrough in the treatment of pancreatic cancer because of its unique immunosuppressive tumor microenvironment (TME) (10). There are some findings that indicate that radiation may induce antitumor immunity (11), promote the recruitment of effective T cells, and enhance the susceptibility of tumor cells to T cell-mediated antitumor effects (12). The combination of radiation and immune checkpoint blockades has increased T-cell priming and augmented activation in a poorly immunogenic mouse model of pancreatic cancer (13).
Advances in neoadjuvant therapy and induction therapy in various tumors open a new avenue to improve the resection rate of pancreatic cancer and prolong survival. A growing body of study shows that patients with BRPC or locally advanced pancreatic cancer (LAPC) tend to benefit from neoadjuvant or induction therapy, which increases the rate of margin-negative resections and decreases recurrence rates (14–16). Based on preliminary research and the curative effects in clinical patients treated with different therapeutic schedules, we conducted a clinical trial on the combination of PD-1 blockade and concurrent radiotherapy for patients with potentially resectable pancreatic cancer, mainly referring to BRPC and LAPC.
Circulating tumor DNA (ctDNA) has been proven to have worth in multiple perspectives in pancreatic cancer (17). For resected pancreatic cancers, postoperative ctDNA is applied in predicting prognosis (18). As for localized and advanced pancreatic cancers, the value of ctDNA is monitoring relapse or progression (19). On account of the noninvasive advantages, ctDNA could provide longitudinal and dynamic surveillance of the tumor-specific genetic characteristics. In this case, serial ctDNA samples were collected to identify genetic alterations in baseline and explore the predictive role of ctDNA in pancreatic cancer patients treated with immunological induction therapy.
Here, we report a case with a clear beneficial outcome from this treatment that exhibited a pathologic complete response (pCR) and a significant decrease in tumor index. Serial ctDNA alterations accurately provide a comprehensive outlook of the tumor status and monitor the response to the therapy.
A 62-year-old man was hospitalized for surgery for intermittent epigastric pain in September 2019. Tumor markers carbohydrate antigen 19-9 (CA19-9) and carbohydrate antigen 125 (CA125) were 3,958 U/ml and 50.3 U/ml, respectively. Contrast-enhanced computed tomography (CT) showed a mass at the head of the pancreas, this mass was suspected to have invaded the superior mesenteric vein, but no distant metastasis was detected. Positron emission tomography computed tomography (PET-CT) further exhibited a mass and hypermetabolism of glucose in the uncinate process of the pancreas (Size = 4.1 cm * 3.6 cm; SUV = 18.2) and has an unclear boundary with the duodenum. The result of an endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNA) was concordant with adenocarcinoma, and the patient was diagnosed with potentially resectable pancreatic cancer. After discussion, the Multiple Disciplinary Team (MDT) recommended him to participate in our clinical trial of induction therapy.
The patient firstly received 4 cycles of gemcitabine 1,000 mg/m2 and nab-paclitaxel 125 mg/m2 on day 1 and day 8, along with tislelizumab (a PD-1 monoclonal antibody) 200 mg on day 1 every 3 weeks, concurrently with tomotherapy (TOMO) with a total radiation dose of 30 Gy/10f at the planning target volume (PTV) and 50 Gy/10f at the planning gross tumor volume (PGTV) during the third cycle. Timeline of events and the radiation target volume and precise radiotherapy planning are detailed in Figure 1. The patient had repeat CT about every two cycles. After 4 cycles of the treatment, PET-CT revealed that the pancreatic tumor size and glucose metabolism had decreased remarkably, and glucose metabolism in peripheral lymph nodes returned to normal (Figure 2A) . As shown in Figure 2B, tumor marker CA19-9 decreased to 51.4 U/ml. The curative effect was evaluated as partial response (PR). Based on these signs of improvement, the MDT agreed to give him surgical exploration. Surprisingly, not only did the patient receive pylorus-preserving pancreaticoduodenostomy (PPPD) successfully but also the postoperative pathology showed a pCR (Figure 3). Neither residual tumor cells nor metastases in 16 regional lymph nodes were detected, and the surgical margin was negative. The final TNM stage was ypT0N0M0. Better yet, the patient had no serious postoperative complications and recovered smoothly. We planned to give him at least 2 cycles of adjuvant therapy at postoperative 1 month.
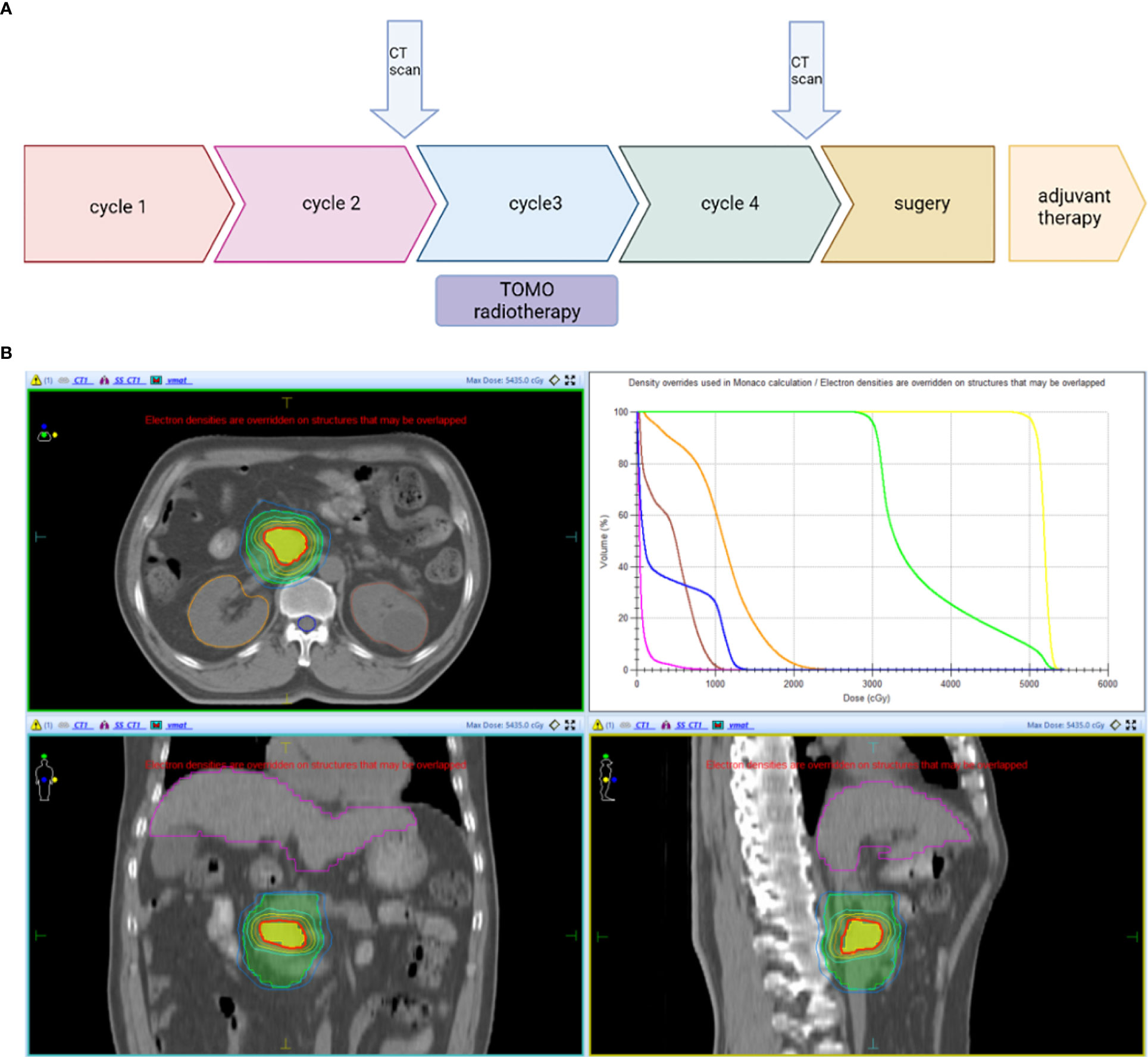
Figure 1 (A) Timeline of the treatment: Every 3 weeks one cycle: Tislelizumab (200 mg, on day 1), gemcitabine (1,000 mg/m2, on days 1 and 8), and nab-paclitaxel (125 mg/m2, on days 1 and 8). (B) Radiotherapy: PTV: 3 Gy * 10f; PGTV: 5 Gy * 10f. CT scan, Computed Tomography scan; PTV, planning target volume; PGTV, planning gross tumor volume.
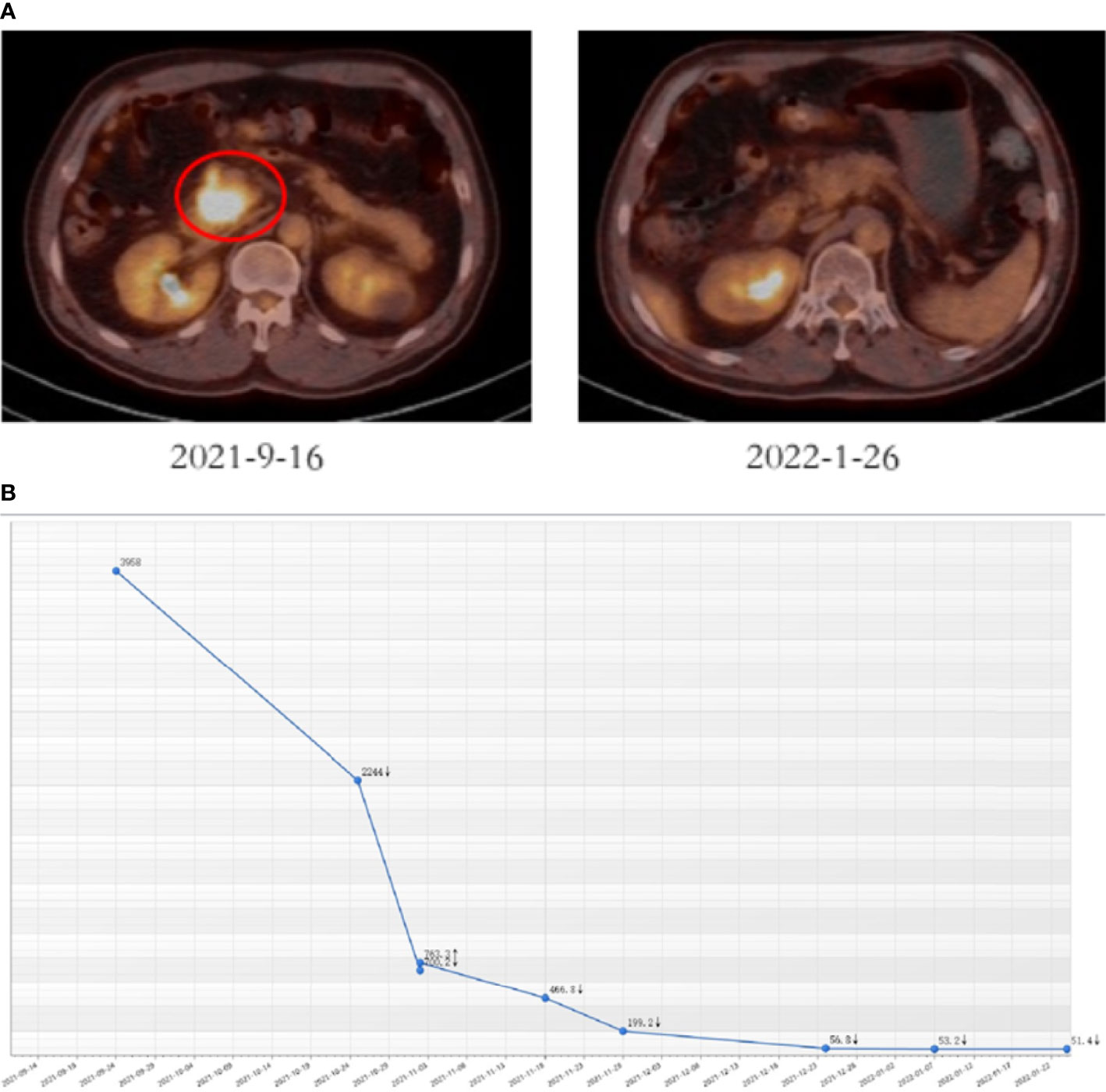
Figure 2 Tumor responses after induction therapy. (A) PET-CT was performed before (size: 4.1 cm * 3.6 cm, SUV = 18.2) and after 4 cycles of the treatment (size: 2.3 cm * 1.5 cm, SUV = 4.8). (B) CA19-9 levels of this patient during the treatment.
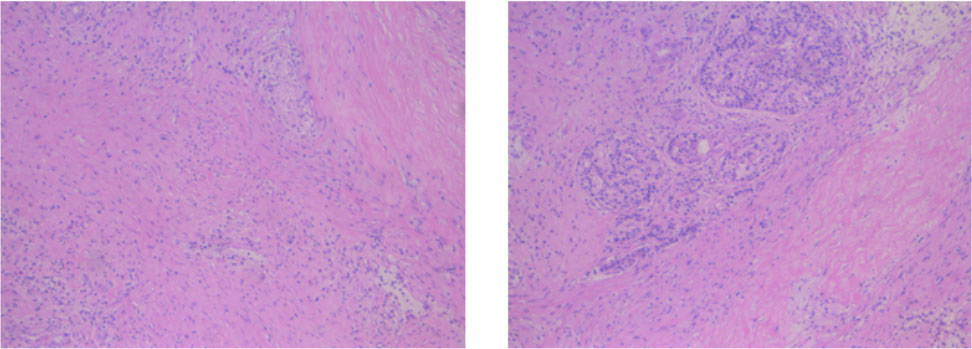
Figure 3 Postoperative pathology of the patient. No residual tumor cells, and the surgical margin was negative.
To investigate serial ctDNA dynamic changes in predicting the tumor response and outcomes in this patient, we collected peripheral blood samples in three periods. At baseline, CDKN2A p.A17Gfs*9 (26.34% abundance), TP53 p.C176* (19.72% abundance), and KRAS p.G12V (14.79% abundance) were detected, the TMB value was 1.42 muts/Mb, and the sample presented microsatellite stability status (MSS). These baseline profiles do not suggest that the patient may be a potential immunotherapy beneficiary. Interestingly, two cycles after the initiation of induction therapy, testing result clarified that no baseline gene mutations were detected, except for an extremely low abundance of newly generated DNMT3A p.R326C mutation (0.37% abundance). The dynamic changes of ctDNA during treatment with baseline strongly suggested perfect treatment efficacy. Preoperative blood was then collected and tested; in line with the finding during treatment, a low abundance of MITF p.R324H mutation (0.62% abundance) was detected, highlighting a process of continuous clinical treatment benefit. The variation diagram of the maximum mutation abundance of ctDNA dynamic changes in three defined time points was shown in Figure 4.
Increasing the R0 resection rate and prolonging OS are the main aims of preoperative treatment for initially non-resectable pancreatic cancer. This is a case of induction therapy with significant benefit. Our results suggested that induction anti-PD-1 antibody plus chemotherapy and concurrent radiotherapy may be a potential regimen for patients with LAPC.
PD-1/PD-L1 antibody is currently one of the most used immunotherapies of solid tumors and has shown great advantage in carcinomas such as NSCLC (20) and head and neck squamous cell carcinoma (HNSCC) (21). Microsatellite instability-high (MSI-H) and/or defect mismatch repair (dMMR) gene incidence in pancreatic cancer is less than 1%, which precludes the potential application of PD-1 blockade in pancreatic cancer (22, 23). Furthermore, pancreatic cancer has a denser stroma and a more complex tumor immune microenvironment compared with other solid tumors (24). For the purpose of improving the treatment effect of PD-1 inhibitors, researchers have tried a variety of methods and found that radiotherapy could modulate the tumor environment, thereby improving the antitumor response. The combination pattern has succeeded in NSCLC (25) and esophageal squamous cell carcinoma (26). At present, immune checkpoint inhibitors plus chemotherapy or radiotherapy have also gradually entered the public eye. The combination of chemotherapy and PD-1 antibody showed great advantages in metastatic pancreatic cancer (27), and there was a patient with LAPC who achieved near pCR after the neoadjuvant PD-1 inhibitor plus radiotherapy (28). During the 2021 meeting of the American Association for Cancer Research (AACR), professor Forde concluded the data of the phase III trial Checkmate-816 and suggested that patients in earlier-stage NSCLC who achieved a pCR with neoadjuvant chemotherapy may live longer than those who did not (29). Also, a meta-analysis of randomized phase II/III studies of neoadjuvant therapy for patients with HER-2-positive early breast cancer concluded that patients experienced improved recurrence-free survival (RFS) and OS if they achieved a pCR (30). Thus, it is valid to consider that combined PD-1 inhibitor with chemotherapy and concurrent radiotherapy as induction therapy may be a potential way to improve the resection rate and prolong the survival. This case is the first patient enrolled in our clinical trial who had a pCR after the induction therapy, and we will continue to follow up to record the OS. In addition, the successful surgery of the patient without postoperative complications increased our confidence to continue the clinical trial. We are looking forward to enrolling more subjects and expecting more patients to achieve a pCR to the therapy so we can analyze the role of pCR as a predictor of OS in patients treated with induction therapy.
In this case, we implemented the targeted Next-generation sequencing technology (NGS) using a 539-gene panel to investigate the serial changes of ctDNA status during the treatment. Genomic features at baseline are believed to hold great potential to predict tumor response to immunological induction therapy. However, the Tumor Mutation (TMB) value and MSS status do not suggest that the patient may be a potential immunotherapy beneficiary. Interestingly, ctDNA testing during treatment clarified that no baseline gene mutations were detected, which strongly suggested a perfect treatment efficacy. This is consistent with the changes that reflected in standard imaging and CA19-9 alterations, indicating that serial ctDNA alterations accurately provide a comprehensive outlook of the tumor status and monitor the response to the therapy.
Indeed, serial ctDNA has been proven to predict and monitor the effect of neoadjuvant chemoradiotherapy in patients with rectal cancers; ctDNA becomes a real-time monitoring indicator that can accurately reflect the tumor burden. The median variant allelic frequency (VAF) of baseline ctDNA is a strong independent predictor of survival outcome (31). In addition, ctDNA can also serve as a potential biomarker of the response to immunotherapy in advanced gastric cancers, and ctDNA presented a potential role in predicting Immune-Related Adverse Events (irAEs) (32).
In conclusion, we provide a reference for the use of induction PD-1 blockade plus chemoradiotherapy in patients with potentially resectable pancreatic cancer. This is a typical case that proves the prognostic value of ctDNA in pancreatic cancer. However, there are some limitations, and more randomized clinical trials are needed to verify the safety and efficacy of the induction therapy mode in LAPC and BRPC. Furthermore, evidence of the ctDNA change in a single case is not very valuable; these differences should be further validated in a larger cohort.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Drum Tower Hospital Affiliated to Nanjing University Medical School. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CL, YZ, and HC have contributed equally to this work. BL, YQ, and JD designed the clinical trial. CL, YZ, and HC composed the article. MT, JH, and AL provided the imaging report, and JC and QL provided the pathology report. WK, LW and XgQ revised the article. LZ, XnQ, FM and DC did the work of the acquisition, analysis, and interpretation of the data. All authors have agreed to the final version of the article.
National Natural Science Foundation of China (82072926); National Key Research and Development Program of China (2020YFA0713804); Special Fund of Health Science and Technology Development of Nanjing (YKK20080)
Author DC was employed by The State Key Laboratory of Translational Medicine and Innovative Drug Development, Medical Department, Jiangsu Simcere Diagnostics Co., Ltd., Nanjing, China.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
2. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res (2014) 74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155
3. Klaiber U, Hackert T. Conversion Surgery for Pancreatic Cancer-The Impact of Neoadjuvant Treatment. Front Oncol (2019) 9:1501. doi: 10.3389/fonc.2019.01501
4. Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2021) 19:439–57. doi: 10.6004/jnccn.2021.0017
5. Rijnders M, de Wit R, Boormans JL, Lolkema MPJ, van der Veldt AAM. Systematic Review of Immune Checkpoint Inhibition in Urological Cancers. Eur Urol (2017) 72:411–23. doi: 10.1016/j.eururo.2017.06.012
6. Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab Versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic Non-Small-Cell Lung Cancer (KEYNOTE-042): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet (2019) 393:1819–30. doi: 10.1016/s0140-6736(18)32409-7
7. Javadrashid D, Baghbanzadeh A, Derakhshani A, Leone P, Silvestris N, Racanelli V, et al. Pancreatic Cancer Signaling Pathways, Genetic Alterations, and Tumor Microenvironment: The Barriers Affecting the Method of Treatment. Biomedicines (2021) 9:373–95. doi: 10.3390/biomedicines9040373
8. Palmer DH, Valle JW, Ma YT, Faluyi O, Neoptolemos JP, Jensen Gjertsen T, et al. TG01/GM-CSF and Adjuvant Gemcitabine in Patients With Resected RAS-Mutant Adenocarcinoma of the Pancreas (CT TG01-01): A Single-Arm, Phase 1/2 Trial. Br J Cancer (2020) 122:971–7. doi: 10.1038/s41416-020-0752-7
9. Zhang ZZ, Wang T, Wang XF, Zhang YQ, Song SX, Ma CQ. Improving the Ability of CAR-T Cells to Hit Solid Tumors: Challenges and Strategies. Pharmacol Res (2022) 175:106036. doi: 10.1016/j.phrs.2021.106036
10. Fan JQ, Wang MF, Chen HL, Shang D, Das JK, Song J. Current Advances and Outlooks in Immunotherapy for Pancreatic Ductal Adenocarcinoma. Mol Cancer (2020) 19:32. doi: 10.1186/s12943-020-01151-3
11. Zhai D, An D, Wan C, Yang K. Radiotherapy: Brightness and Darkness in the Era of Immunotherapy. Transl Oncol (2022) 19:101366. doi: 10.1016/j.tranon.2022.101366
12. Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation Modulates the Peptide Repertoire, Enhances MHC Class I Expression, and Induces Successful Antitumor Immunotherapy. J Exp Med (2006) 203:1259–71. doi: 10.1084/jem.20052494
13. Stump CT, Roehle K, Manjarrez Orduno N, Dougan SK. Radiation Combines With Immune Checkpoint Blockade to Enhance T Cell Priming in a Murine Model of Poorly Immunogenic Pancreatic Cancer. Open Biol (2021) 11:210245. doi: 10.1098/rsob.210245
14. Deng A, Wang C, Cohen SJ, Winter JM, Posey J, Yeo C, et al. Multi-Agent Neoadjuvant Chemotherapy Improves Survival in Early-Stage Pancreatic Cancer: A National Cancer Database Analysis. Eur J Cancer (2021) 147:17–28. doi: 10.1016/j.ejca.2021.01.004
15. Katz MH, Shi Q, Ahmad SA, Herman JM, Marsh Rde W, Collisson E, et al. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg (2016) 151:e161137. doi: 10.1001/jamasurg.2016.1137
16. Gugenheim J, Crovetto A, Petrucciani N. Neoadjuvant Therapy for Pancreatic Cancer. Updates Surg (2022) 74:35–42. doi: 10.1007/s13304-021-01186-1
17. Cohen JD, Javed AA, Thoburn C, Wong F, Tie J, Gibbs P, et al. Combined Circulating Tumor DNA and Protein Biomarker-Based Liquid Biopsy for the Earlier Detection of Pancreatic Cancers. Proc Natl Acad Sci USA (2017) 114:10202–7. doi: 10.1073/pnas.1704961114
18. Groot VP, Mosier S, Javed AA, Teinor JA, Gemenetzis G, Ding D, et al. Circulating Tumor DNA as a Clinical Test in Resected Pancreatic Cancer. Clin Cancer Res (2019) 25:4973–84. doi: 10.1158/1078-0432.CCR-19-0197
19. Lee B, Lipton L, Cohen J, Tie J, Javed AA, Li L, et al. Circulating Tumor DNA as a Potential Marker of Adjuvant Chemotherapy Benefit Following Surgery for Localized Pancreatic Cancer. Ann Oncol (2019) 30:1472–8. doi: 10.1093/annonc/mdz200
20. Petrelli F, Ferrara R, Signorelli D, Ghidini A, Proto C, Roudi R, et al. Immune Checkpoint Inhibitors and Chemotherapy in First-Line NSCLC: A Meta-Analysis. Immunotherapy (2021) 13:621–31. doi: 10.2217/imt-2020-0224
21. Masarwy R, Kampel L, Horowitz G, Gutfeld O, Muhanna N. Neoadjuvant PD-1/PD-L1 Inhibitors for Resectable Head and Neck Cancer: A Systematic Review and Meta-Analysis. JAMA Otolaryngol Head Neck Surg (2021) 147:871–8. doi: 10.1001/jamaoto.2021.2191
22. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science (2017) 357:409–13. doi: 10.1126/science.aan6733
23. Zhang F, Wang Y, Yang F, Zhang Y, Jiang M, Zhang X. The Efficacy and Safety of PD-1 Inhibitors Combined With Nab-Paclitaxel Plus Gemcitabine Versus Nab-Paclitaxel Plus Gemcitabine in the First-Line Treatment of Advanced Pancreatic Cancer: A Retrospective Monocentric Study. Cancer Manag Res (2022) 14:535–46. doi: 10.2147/CMAR.S349442
24. Sunami Y, Haussler J, Zourelidis A, Kleeff J. Cancer-Associated Fibroblasts and Tumor Cells in Pancreatic Cancer Microenvironment and Metastasis: Paracrine Regulators, Reciprocation and Exosomes. Cancers (Basel) (2022) 14:744–56. doi: 10.3390/cancers14030744
25. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med (2017) 377:1919–29. doi: 10.1056/NEJMoa1709937
26. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med (2021) 384:1191–203. doi: 10.1056/NEJMoa2032125
27. Weiss GJ, Blaydorn L, Beck J, Bornemann-Kolatzki K, Urnovitz H, Schutz E, et al. Phase Ib/II Study of Gemcitabine, Nab-Paclitaxel, and Pembrolizumab in Metastatic Pancreatic Adenocarcinoma. Invest New Drugs (2018) 36:96–102. doi: 10.1007/s10637-017-0525-1
28. McCarthy PM, Rendo MJ, Uy MD, Adams AM, O'Shea AE, Nelson DW, et al. Near Complete Pathologic Response to PD-1 Inhibitor and Radiotherapy in a Patient With Locally Advanced Pancreatic Ductal Adenocarcinoma. Onco Targets Ther (2021) 14:3537–44. doi: 10.2147/OTT.S311661
29. Forde P. Neoadjuvant Nivolumab Plus Chemotherapy Increased Pathological Complete Response Rate in CheckMate-816 Lung Cancer Trial. N Engl J Med (2022) 386:1973–85. doi: 10.1056/NEJMoa2202170
30. Guarneri V, Griguolo G, Miglietta F, Conte PF, Dieci MV, Girardi F. Survival After Neoadjuvant Therapy With Trastuzumab-Lapatinib and Chemotherapy in Patients With HER2-Positive Early Breast Cancer: A Meta-Analysis of Randomized Trials. ESMO Open (2022) 7:100433. doi: 10.1016/j.esmoop.2022.100433
31. Zhou J, Wang C, Lin G, Xiao Y, Jia W, Xiao G, et al. Serial Circulating Tumor DNA in Predicting and Monitoring the Effect of Neoadjuvant Chemoradiotherapy in Patients With Rectal Cancer: A Prospective Multicenter Study. Clin Cancer Res (2021) 27:301–10. doi: 10.1158/1078-0432.CCR-20-2299
Keywords: potentially resectable pancreatic cancer, induction therapy, PD-1 inhibitor, pathologic complete response, benefit
Citation: Lu C, Zhu Y, Cheng H, Kong W, Zhu L, Wang L, Tang M, Chen J, Li Q, He J, Li A, Qiu X, Chen D, Meng F, Qian X, Liu B, Qiu Y and Du J (2022) Case Report: Pathologic Complete Response to Induction Therapy in a Patient With Potentially Resectable Pancreatic Cancer. Front. Oncol. 12:898119. doi: 10.3389/fonc.2022.898119
Received: 17 March 2022; Accepted: 22 April 2022;
Published: 06 June 2022.
Edited by:
Tommaso Stecca, ULSS2 Marca Trevigiana, ItalyReviewed by:
Kei Saito, The University of Tokyo, JapanCopyright © 2022 Lu, Zhu, Cheng, Kong, Zhu, Wang, Tang, Chen, Li, He, Li, Qiu, Chen, Meng, Qian, Liu, Qiu and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Du, ZHVqdWFuZ2x5eUAxNjMuY29t; Yudong Qiu, eXVkb25ncWl1NTEwQDE2My5jb20=; Baorui Liu, YmFvcnVpbGl1QG5qdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.