- 1Department of Radiation Oncology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin Key Laboratory of Cancer Prevention and Therapy, Tianjin's Clinical Research Center for Cancer, Tianjin, China
- 2Department of Radiation Oncology, Affiliated Hospital of Hebei University, Baoding, China
- 3Department of Oncology, Shandong Provincial Third Hospital, Shandong University, Jinan, China
Background and Purpose: On the basis of the promising clinical study results, thoracic radiotherapy (TRT)1 has become an integral part of treatment of synchronous oligometastatic non–small cell lung cancer (SOM-NSCLC). However, some of them experienced rapid disease progression after TRT and showed no significant survival benefit. How to screen out such patients is a more concerned problem at present. In this study, we developed a risk-prediction model by screening hematological and clinical data of patients with SOM-NSCLC and identified patients who would not benefit from TRT.
Materials and Methods: We investigated patients with SOM-NSCLC between 2011 and 2019. A formula named Risk-Total was constructed using factors screened by LASSO-Cox regression analysis. Stabilized inverse probability treatment weight analysis was used to match the clinical characteristics between TRT and non-TRT groups. The primary endpoint was overall survival (OS).
Results: We finally included 283 patients divided into two groups: 188 cases for the training cohort and 95 for the validation cohort. Ten prognostic factors included in the Risk-Total formula were age, N stage, T stage, adrenal metastasis, liver metastasis, sensitive mutation status, local treatment status to metastatic sites, systemic inflammatory index, CEA, and Cyfra211. Patients were divided into low- and high-risk groups based on risk scores, and TRT was found to have improved the OS of low-risk patients (46.4 vs. 31.7 months, P = 0.083; 34.1 vs. 25.9 months, P = 0.078) but not that of high-risk patients (14.9 vs. 11.7 months, P = 0.663; 19.4 vs. 18.6 months, P = 0.811) in the training and validation sets, respectively.
Conclusion: We developed a prediction model to help identify patients with SOM-NSCLC who would not benefit from TRT, and TRT could not improve the survival of high-risk patients.
Introduction
Non–small cell lung cancer (NSCLC)2 is a common malignant tumor that accounts for 70%–80% of all lung cancer cases worldwide. NSCLC is associated with high morbidity and mortality rates (1). More than half of patients with NSCLC have stage IV disease at the time of diagnosis, and up to one-third of these patients have synchronous oligometastatic (SOM) disease (2, 3).
SOM disease has been described as a distinct disease entity characterized by reduced metastatic potential with a limited number of metastatic sites (4), which renders it amenable to local treatment (LT). There is no consensus on what specific criteria define SOM-NSCLC. Of note, inclusion criteria for previously cited studies were very different. Recently, the European Organization for Research and Treatment of cancer (EORTC) and the European Society of Radiotherapy & Oncology-American Society for Therapeutic Radiology and Oncology (ESTRO-ASTRO) conferences had attempted to standardize the definition of oligometastatic disease (2, 5). The documents showed that the definition of oligometastatic disease should base on safety of radical treatment rather than the number of metastases, and it would be better the number of metastatic lesions ≤ 5 and the number of metastatic sites ≤ 3, with or without primary sites, and mediastinal metastatic lymph nodes were included. Several clinical trials and multiple retrospectives series have reported favorable outcomes of thoracic radiotherapy (TRT) in highly selected patients with SOM-NSCLC (6–14). However, some of them experienced rapid disease progression after TRT and showed no significant survival benefit. And, to date, no effective predictive model has been developed to help identify patients with SOM-NSCLC who would not benefit from TRT. In this study, we sought to establish a risk prediction model to predict the mortality risk of these patients using baseline hematologic and clinical data and to identify patients who would not benefit from TRT.
Materials and methods
Patient Selection
We retrospectively reviewed the medical records of consecutive patients who received a diagnosis of advanced NSCLC at our hospital between January 2011 and December 2019. Clinical staging of the disease at the time of presentation was again determined with reference to the eighth edition of tumor node metastasis classification (15). The inclusion criteria for this study were as follows: (1) confirmed diagnosis of NSCLC based on pathological or cytological specimens, or both; (2) patients were allowed to have up to five lesions of metastatic disease (do not include primary site and enlarged lymph nodes in the mediastinum and supraclavicular) with no more than three sites (2, 5); and (3) availability of gene mutation status information. To determine metastasis status, patients needed to undergo comprehensive imaging tests, including head contrast-enhanced MRI, neck ultrasound, chest–abdomen contrast-enhanced CT plus ECT, or PET-CT. If there was ambiguous metastatic lesion in the liver, then contrast-enhanced abdominal MRI was also necessary. Meanwhile, patients were excluded if they had second primary tumor, pleural or pericardial effusion, meningeal or peritoneal metastases, a metastatic site with ambiguous diagnosis, or incomplete medical records.
Definition of Special Concept
In this study, positively sensitive mutations (SM+) included the following: EGFR (epidermal growth factor receptor) exon 19 deletion, EGFR exon 21 Leu858Arg mutation, ROS proto-oncogene 1, receptor tyrosine kinase (ROS1) fusion mutation, and ALK (anaplastic lymphoma kinase) mutation. EGFR uncommon mutations, such as exon 18 mutations, exon 20 insertion mutations, and so on, and other non-targeted therapeutic mutations or without any mutation, were defined as sensitive mutation negative (SM−).
Hematological Markers
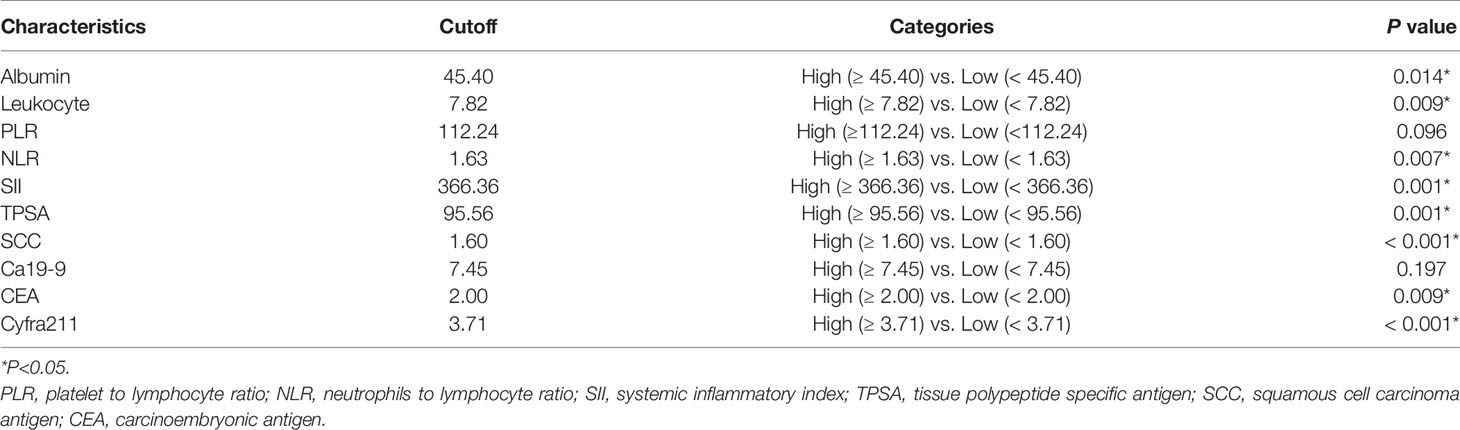
Laboratory examinations including routine blood tests, hepatic and renal function tests, and tumor markers of patients were collected before initial treatment. The calculation formulas of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic inflammatory index (SII) were as follows: NLR = neutrophil number (109/L)/lymphocyte count (109/L); PLR = number of platelets (109/L)/number of lymphocytes (109/L); SII = number of platelets (109/L) × number of neutrophils (109/L)/number of lymphocytes (109/L). The optimal cutoff levels for albumin, leukocyte, PLR, NLR, SII, tissue polypeptide–specific antigen (TPSA), squamous cell carcinoma antigen (SCC), Ca19-9, carcinoembryonic antigen (CEA), and Cyfra211 were obtained according to overall survival (OS).
Thoracic Radiotherapy
In this study, 150 patients received TRT, and TRT could be carried out before, concomitant or after the systemic treatment. The specific radiotherapy target was determined by patient’s attending physician. Generally, gross tumor volume (GTV) included primary lesions with or without mediastinal metastatic lymph nodes, and planning GTV (PGTV) extends 5 mm across the GTV margin. Radiation therapy technology could apply conventional fractionated radiotherapy, hypo-fractionated radiotherapy, and stereotactic body radiotherapy, and the radiation doses were 1.8–2.1 Gy/50–66 Gy, 3 Gy/36–45 Gy, and 9–17 Gy/50–60 Gy, respectively.
First-line Systemic Treatment Strategy
All patients with EGFR non-SMs, untargeted therapy mutations or without mutation, underwent first-line chemotherapy after confirmation of the initial NSCLC diagnosis. The treatment included platinum-based doublet chemotherapy such as pemetrexed, paclitaxel, docetaxel, or gemcitabine combined with cisplatin, carboplatin, or nedaplatin. Each chemotherapy session was separated by an interval of 3 to 4 weeks.
Patients with EGFR-SMs (exon 19 deletion, exon 21 Leu858Arg mutations) were administered first-line treatment with EGFR tyrosine kinase inhibitors (TKIs), such as gefitinib, erlotinib, and icotinib, or with chemotherapy mentioned above and then TKIs after disease progression. Patients with ALK and ROS1 mutation were administered first-line treatment with crizotinib or with chemotherapy as aforesaid and then TKIs after disease progression.
Data Analysis and Statistical Considerations
The primary endpoint was OS defined as the time from the date of diagnosis until death or the most recent follow-up. The follow-up schedule began from the time of treatment to the final follow-up on December 17, 2021. The data on the date of death or at the final follow-up visit were acquired from hospital records or through direct correspondence with the family of the patient. R 4.1.1 and SPSS 24.0 software were used for statistical analyses. The Chi-squared test (or the Fisher’s exact test as applicable) was used to compare the clinical characteristics between groups. OS was estimated using the Kaplan–Meier method, and between-group differences in OS were assessed using the log-rank test. The optimal cutoff values of hematological markers were determined using the package “survminer” based on OS. Using the “glmnet” and “survival” packages and a backward–forward stepwise method, LASSO-Cox regression analysis was performed to select the optimal prognostic factors. The “predict” function of package “survival” was used to calculate the risk score of each patient. Time-dependent receiver operator characteristic (ROC) analyses were conducted using the “timeROC” package. Package “IPWsurvival” was used for stabilized inverse probability treatment weight (IPTW) analyses.
Results
Patient Characteristics
This study had been approved by the Ethics Committee of Tianjin Medical University Cancer Hospital (ab2022138). A total of 2,194 patients were diagnosed with advanced NSCLC at our hospital during the study reference period. Of these, 1,624, 23, 54, 76, and 134 patients were excluded due to extensive metastatic lesions, second primary tumors, pleural effusion, lack of gene sequencing results, and incomplete medical records, respectively.
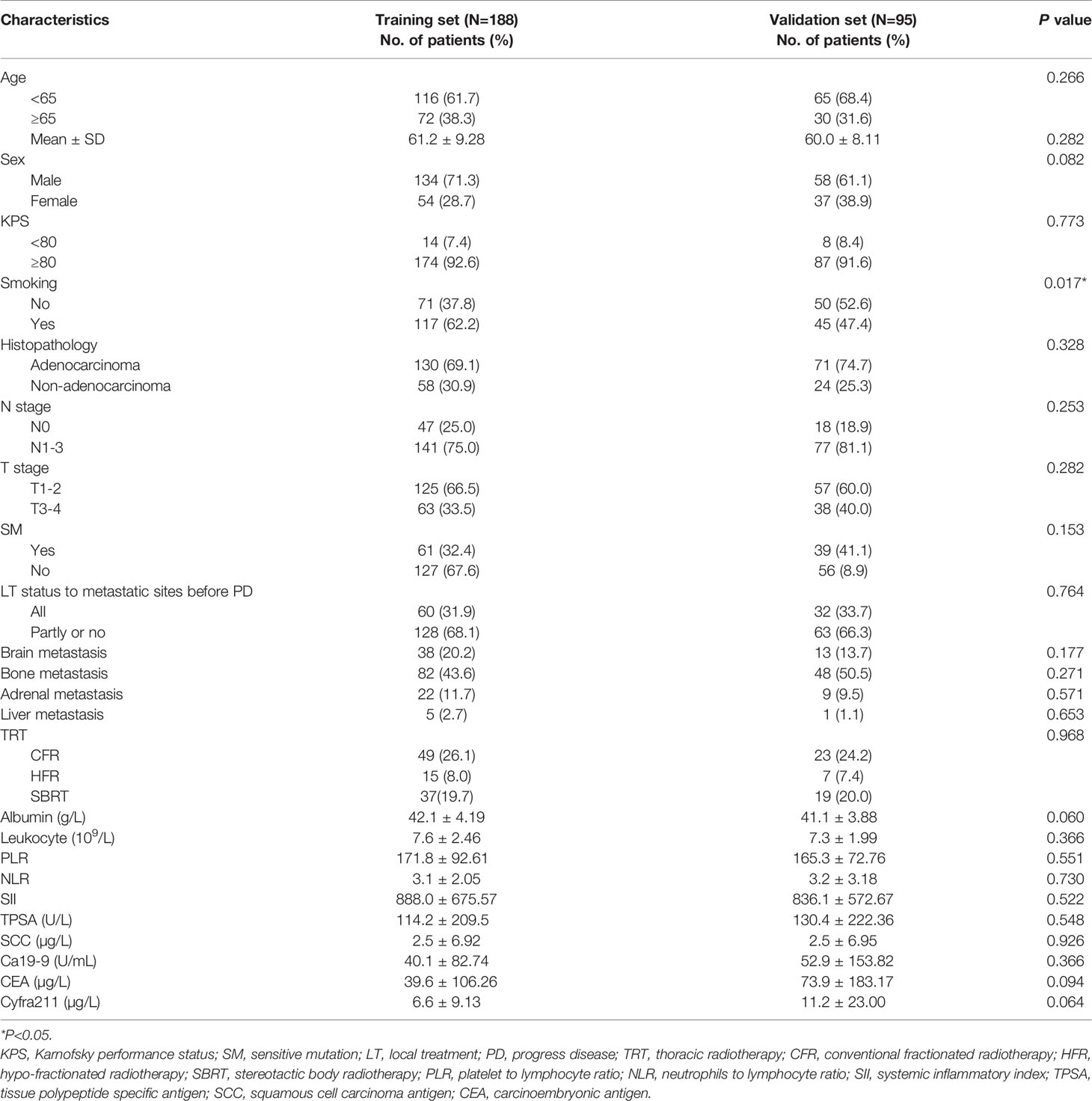
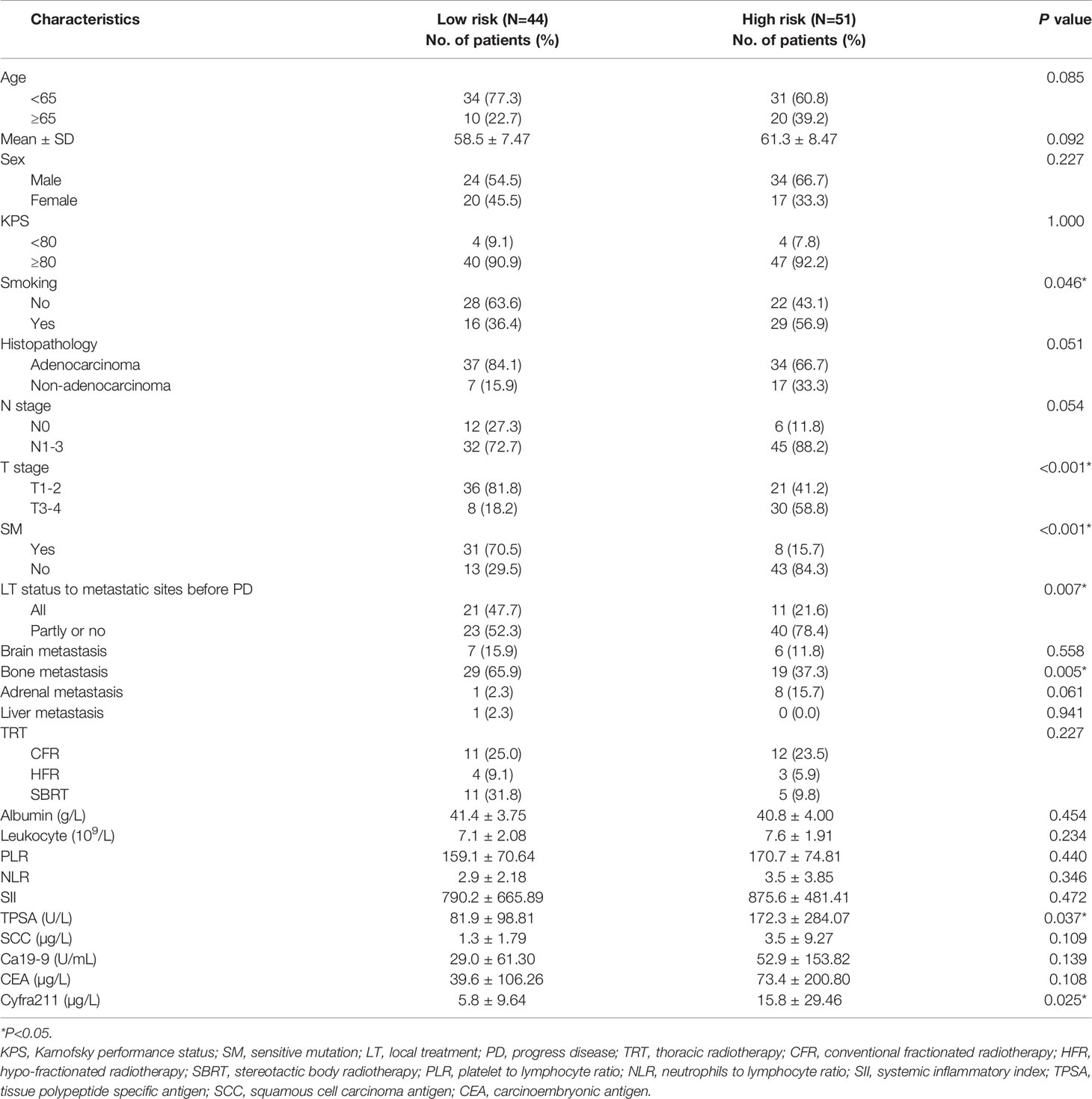
Finally, 283 patients with SOM-NSCLC fulfilled the inclusion criteria for this study. The median OS was 23.4 months, and the 1-, 3-, and 5-year OS rates were 73.3%, 30.1%, and 11.5%, respectively. The entire cohort was randomly divided into two groups by a ratio of 2:1, 188 cases in the training set and 95 cases in the validation set, respectively. The median OS were 22.7 and 24.4 months, respectively; and 1-, 3-, and 5-year OS rates were 72.1%, 31.4%, and 12.7% and 75.6%, 27.0%, and 9.1%, respectively; and there was no difference in survival between sets (P = 0.655). The patient characteristics were summarized in Table 1.
Construction of Risk-Total Formula in the Training Set
In the training set, hematological markers, including albumin, leukocyte, PLR, NLR, SII, TPSA, SCC, Ca199, CEA, and Cyfra211, were divided into low and high groups according to the respective optimal cutoff levels (Table 2).
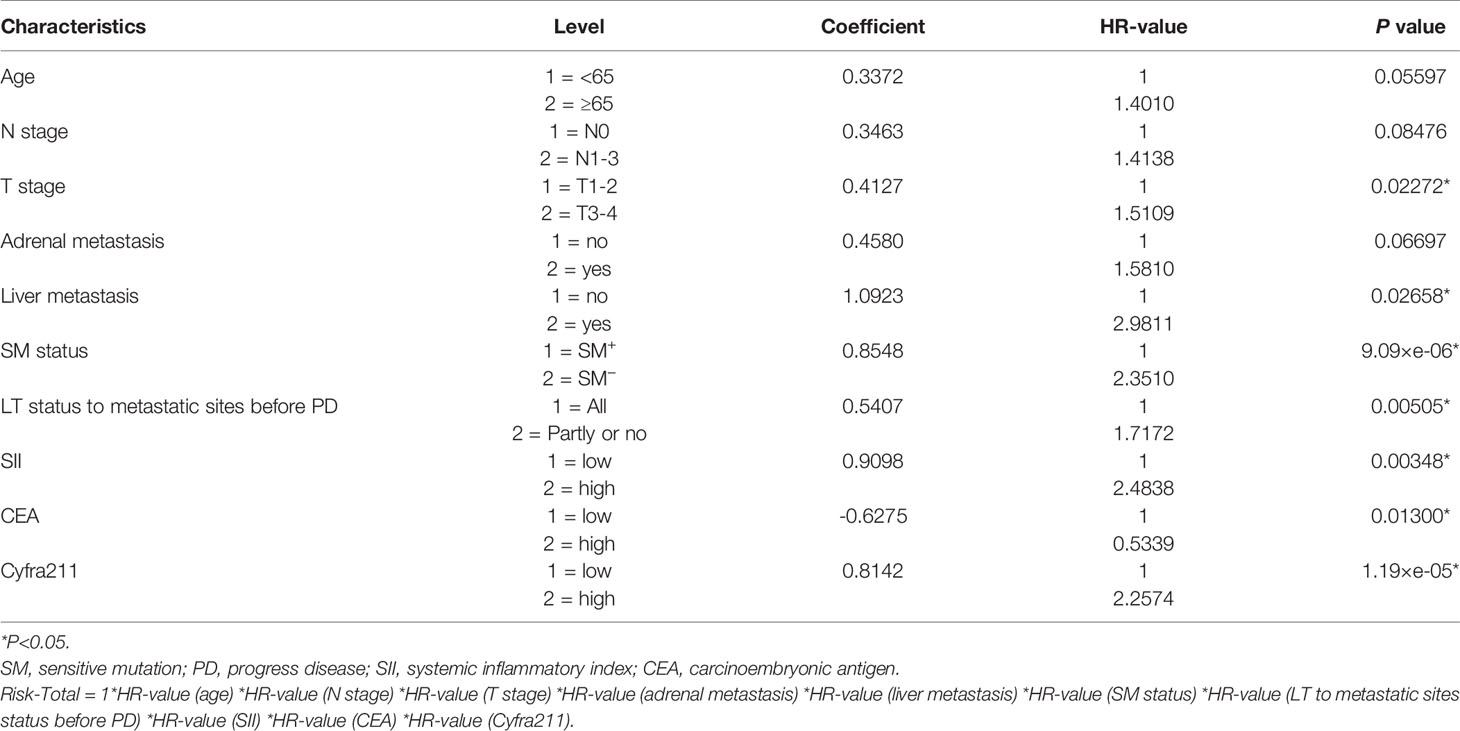
To assess the mortality risk of each patient in the training set, we established a prognostic scoring system named Risk-Total using LASSO-Cox regression model (Figure A.1). Hematological markers mentioned above and other clinical variables, such as age, sex, Karnofsky performance status (KPS), smoking, histopathology, T stage, N stage, brain metastasis, bone metastasis, adrenal metastasis, liver metastasis, SM status, and LT status to metastatic site status before progression disease (PD), were included in the analysis. In this model, low albumin, high leukocyte, high PLR, high NLR, high SII, high TPSA, high SCC, high Ca199, high CEA, high Cyfra211, age ≥ 65, male, KPS < 80, smoking, N1–3, T3–4, non-adenocarcinoma, presence of brain metastasis, bone metastasis, adrenal metastasis, liver metastasis, SM−, and metastatic sites receiving partial or no LT before PD were assigned in level 2, and the corresponding alternatives were assigned in level 1.
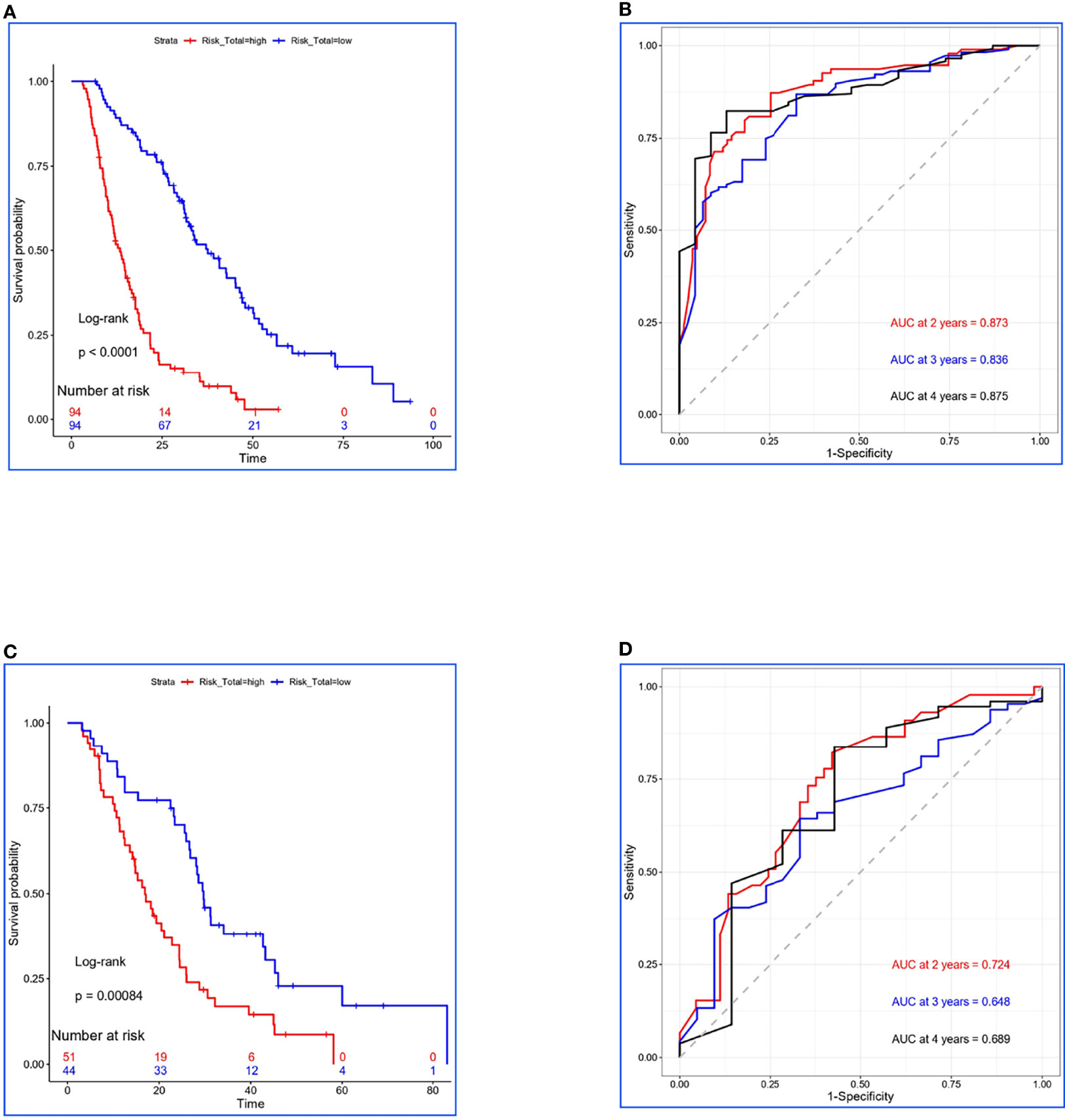
Figure 1 Construction and validation for Risk-Total. (A, C) Kaplan–Meier survival analyses of Risk-Total in the training set and the validation set. (B, D) Risk-Total performance in time-dependent receiver operating characteristic (ROC) curves in the training set and the validation set.
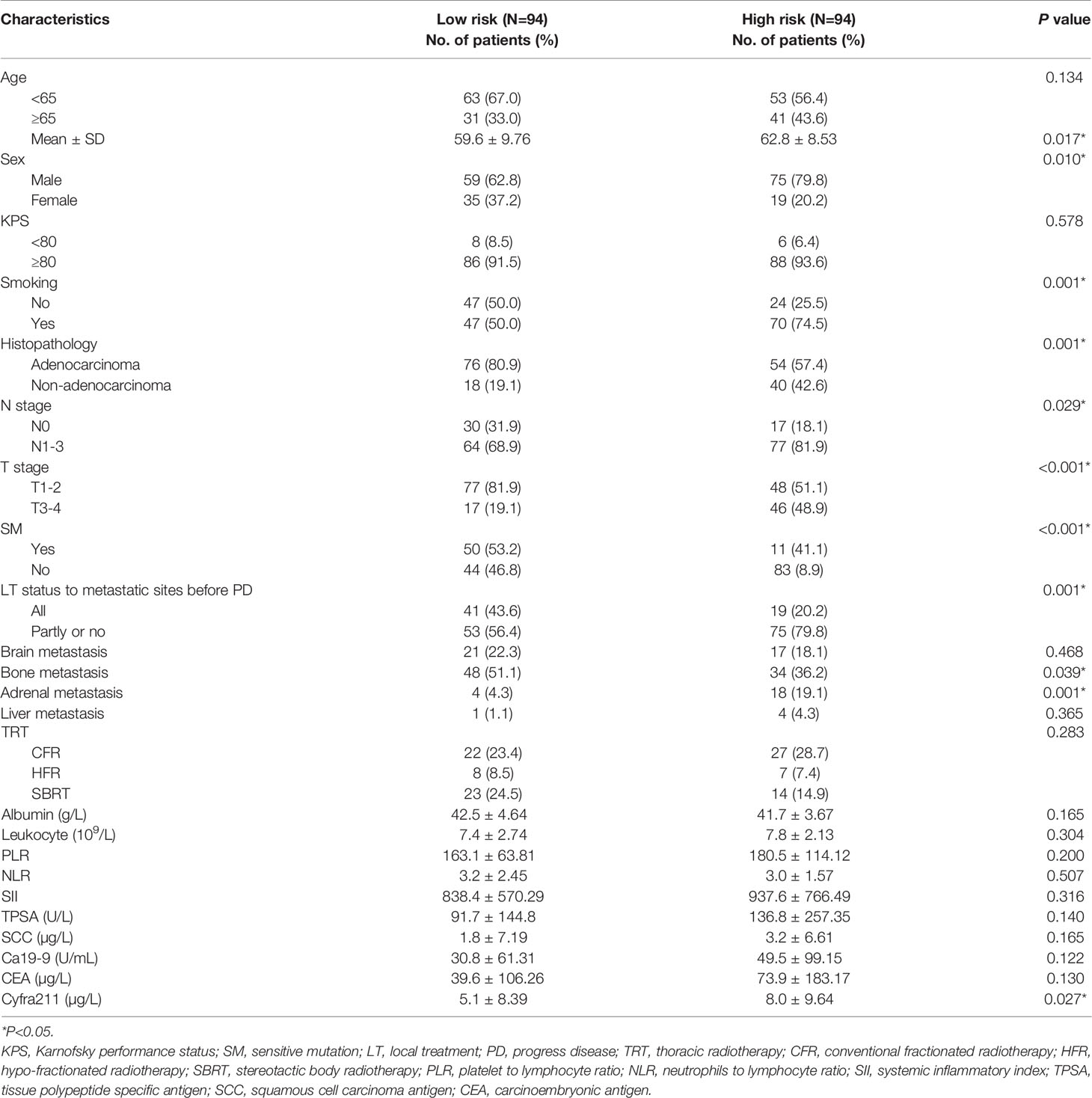
Finally, 10 variables were included in the optimal model (AIC = 1,251.94, P < 2.2 × 10−16) as follows: Risk-Total = 1 × HR-value (age) × HR-value (N stage) × HR-value (T stage) × HR-value (adrenal metastasis) × HR-value (liver metastasis) × HR-value (SM status) × HR-value (LT status to metastatic sites before PD) × HR-value (SII) × HR-value (CEA) × HR-value (Cyfra211) (Table 3). According to the median Risk-Total value (10.0658), patients were divided into low-risk and high-risk groups, and the median survival time (MST) were 37.6 and 13.4 months, respectively (P < 0.001; Figure 1A). Meanwhile, the prognostic accuracy of Risk-Total was evaluated by time-dependent ROC analyses, with 2-, 3-, and 4-year AUC values of 0.873, 0.836, and 0.875, respectively, which confirmed the excellent prognostic power of it (Figure 1B). The patient characteristics between low- and high-risk groups were displayed in Table 4.
Validation of Risk-Total Formula in the Validation Set
In the validation set, patients’ hematological markers were grouped on the basis of cutoff value, as shown in Table 2, and the risk score were calculated on the basis of Risk-Total formula, as shown in Table 3. Then, according to the median value (10.0658) mentioned above, patients were divided into low-risk and high-risk groups, and the MST were 29.7 and 16.9 months, respectively (P = 0.00084; Figure 1C). Similarly, the prognostic accuracy of Risk-Total was also evaluated by time-dependent ROC analyses, with 2-, 3-, and 4-year AUC values of 0.724, 0.648, and 0.689, respectively (Figure 1D). These results confirmed the super prognostic power of Risk-Total in another heterogeneous population. The patient characteristics between low- and high-risk groups are shown in Table 5.
Prognostic Value of TRT for Low- and High- risk Patients
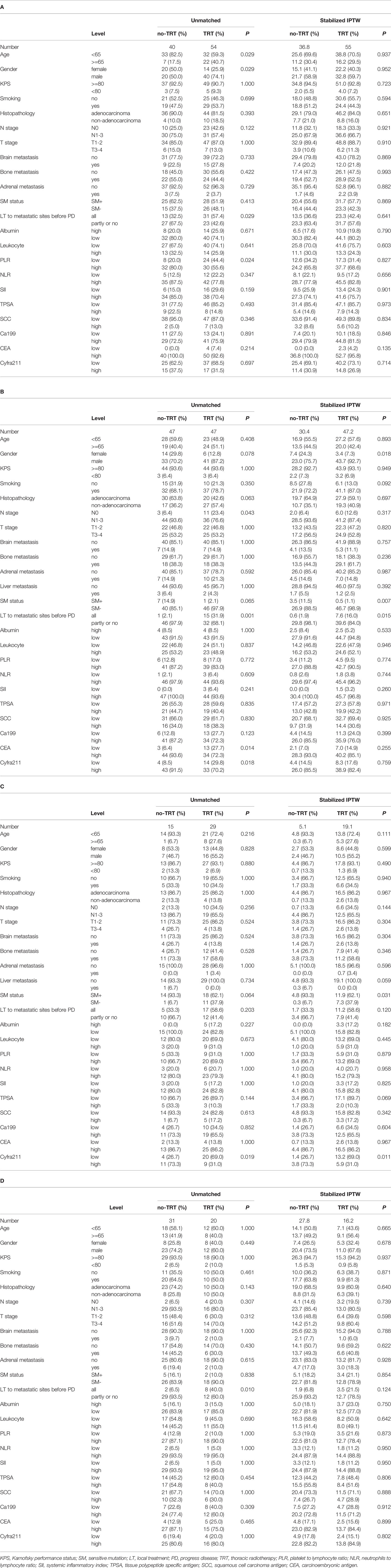
In the training set, 54 of 94 patients with low-risk received TRT, and survival analysis showed improvement in OS (42.8 vs. 32.4 months, P = 0.070; Figure 2A). However, the inter-group clinical characteristics were very unbalanced, especially with respect to age, gender, LT status to metastatic sites, and PLR (Table 6A). Therefore, we applied the stabilized IPTW analysis to calculate the weights of clinical variables and match them. After matching, TRT was still found to improve the OS (46.4 vs. 31.7 months, P = 0.083; Figure 2B). Whereas, 47 of 94 patients with high-risk received TRT, but the OS was not prolonged (15.5 vs. 11.4 months, P = 0.300; Figure 2C). When the clinical variables were calculated weights and matched (Table 6B), the survival time was not improved all the same (14.9 vs. 11.7 months, P = 0.663; Figure 2D).
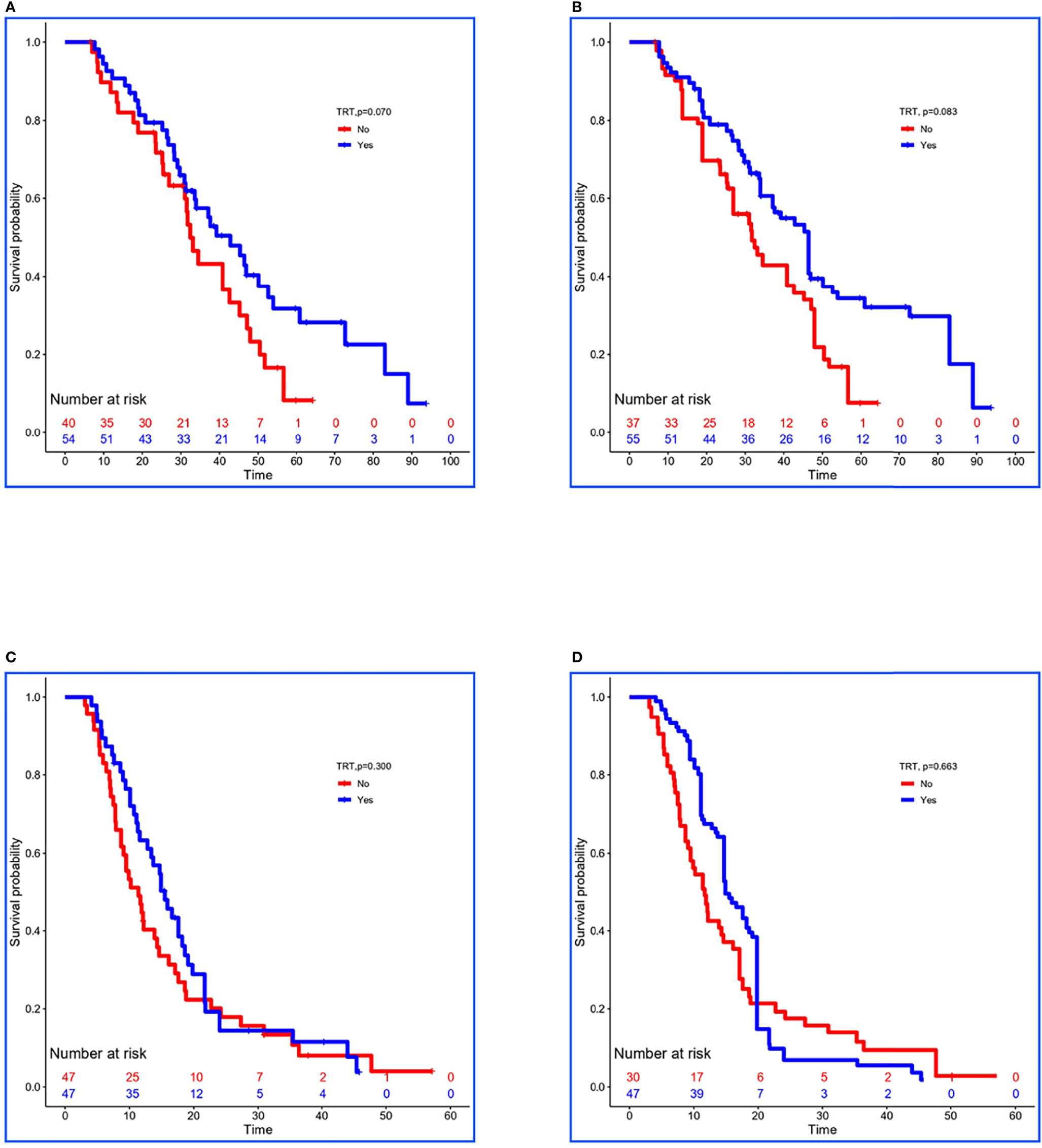
Figure 2 Kaplan–Meier survival analyses for patients between groups. (A, B) Survival curves for low-risk patients between non-TRT and TRT groups when clinical characteristics were unmatched and matched using stabilized IPTW analysis in the training set. (C, D) Survival curves for high-risk patients between non-TRT and TRT groups when clinical characteristics were unmatched and matched using stabilized IPTW analysis in the training set.
In the validation set, 29 of 44 low-risk patients received TRT, and the OS was prolonged 8.2 months (34.1 vs. 25.9 months, P = 0.080; Figure 3A). In addition, stabilized IPTW analysis was used to match the clinical characteristics (Table 6C), and the between-group differences in OS were close to statistical as ever (34.1 vs. 25.9 months, P = 0.078; Figure 3B). Meanwhile, 51 patients were divided into high-risk group, and 20 of them received TRT with no improvement in OS (17.1 vs. 14.7 months, P = 0.400; Figure 3C). On the basis of the clinical characteristics, the TRT group had more patients with no treatment to metastatic sites, which may have influenced the result (Table 6D). Similarly, we applied stabilized IPTW analysis to match the groups. After matching, TRT was not found to have improved survival as before (19.4 vs. 18.6 months, P = 0.811; Figure 3D).
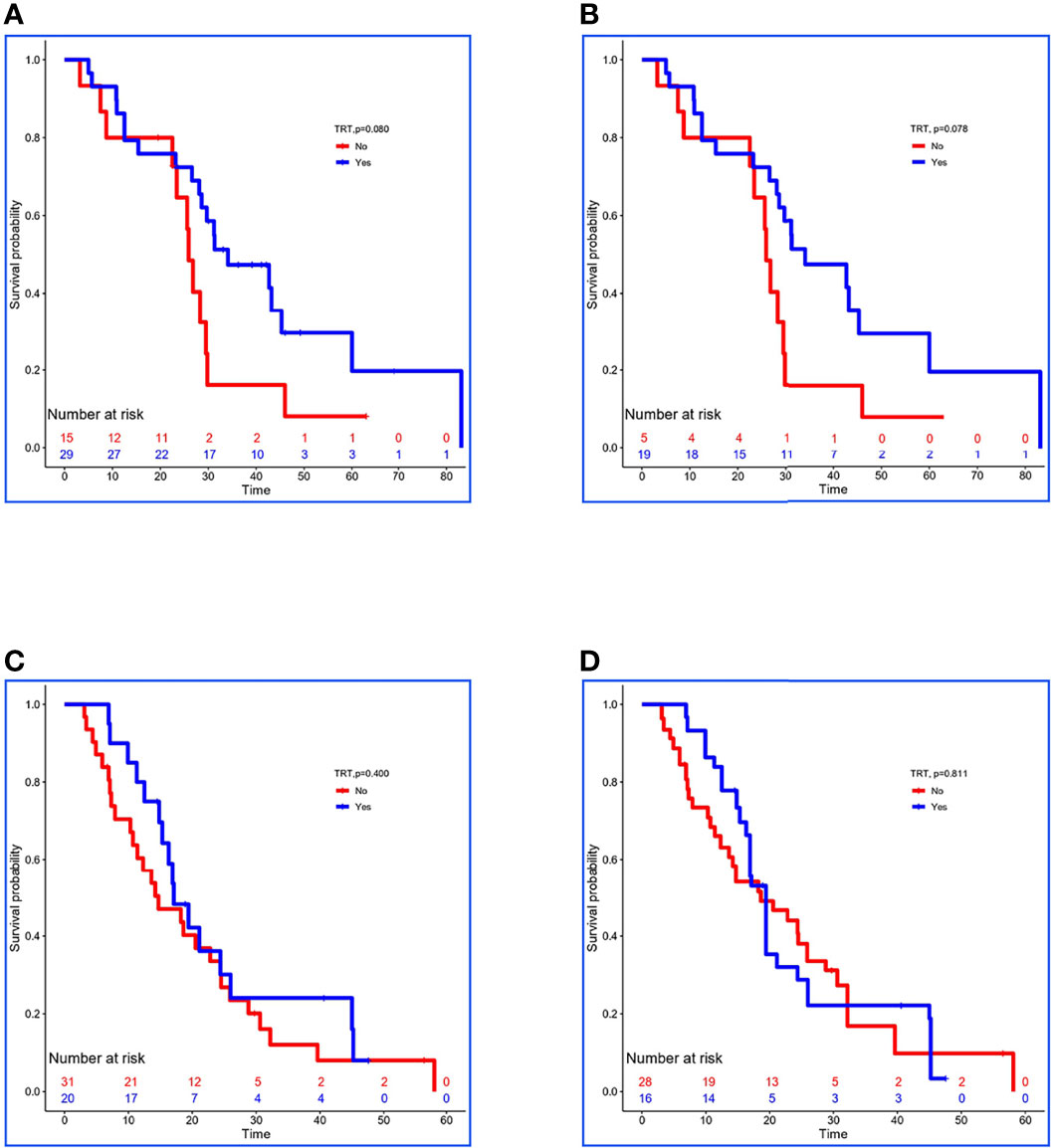
Figure 3 Kaplan–Meier survival analyses for patients between groups. (A, B) Survival curves for low-risk patients between non-TRT and TRT groups when clinical characteristics were unmatched and matched using stabilized IPTW analysis in the validation set. (C, D) Survival curves for high-risk patients between non-TRT and TRT groups when clinical characteristics were unmatched and matched using stabilized IPTW analysis in the validation set.
Discussion
In the current study, we established a risk prediction model to predict the mortality risk of patients with SOM-NSCLC and, further, to identify patients who would not benefit from TRT. Eventually, a total of 283 cases met the inclusion criteria and were divided into the training and validation sets. A Risk-Total formula constructed by 10 clinical prognostic factors was used to calculate each patient’s risk score, and patients were divided into low- and high-risk groups according to the median value (10.0658) in the training set. Then, TRT was found to just have improved the survival of low-risk patients (P = 0.083) but not that of high-risk patients (P = 0.663) in the training set. Similarly, patients in the validation set were estimated risk-score on the basis of the Risk-Total formula, and were grouped into low- and high-risk groups basing on the median value (10.0658), and TRT only prolonged the OS of low-risk patients (P = 0.078) but not that of the high-risk patients (P = 0.811).
The biological characteristics of oligometastatic cancer are increasingly being defined, and the role of LT has evolved substantially during the past decade. In 2018, a prospective, multicenter, single-arm, phase 2 trial reported the long-term outcomes of consolidative radiation therapy (CRT) to the primary and metastatic sites from oligometastatic NSCLC, achieving a partial response or stable disease after three to six cycles of platinum-based chemotherapy. The median PFS and OS were 11.2 and 28.4 months, respectively, which met the primary endpoint and transcended the historical record (13). The first multicenter randomized trial of local consolidative therapy (LCT) for highly selected oligometastatic NSCLC (≤3 metastatic lesions, no progression after front-line systemic therapy) demonstrated significant PFS (14.2 vs. 4.4 months) and OS (41.2 vs. 17.0 months) benefit compared with patients who received maintenance therapy or observation (8). Another single-center randomized phase 2 study of maintenance chemotherapy alone versus stereotactic ablative radiotherapy followed by maintenance chemotherapy for patients with limited metastatic NSCLC (primary plus up to five metastatic sites) with no EGFR-targetable or ALK-targetable mutations but who did achieve a partial response or stable disease after induction chemotherapy also obtained gratifying results (7). Despite differences in the population inclusion criteria in these clinical trials, there was significant prolongation of OS (range of 28.4–41.2 months). However, some patients with SOM-NSCLC experienced rapid disease progression after TRT and showed no significant survival benefit. However, to date, no effective predictive model has been developed to help identify patients who would not benefit from TRT. Hence, in the present study, we established a risk prediction model to predict the mortality risk of patients with SOM-NSCLC and, further, to identify patients who would not benefit from TRT.
Several hematological and clinical factors have been shown to suggest a bad prognosis for lung cancer including hypoalbuminemia (16–18); increase of C-reactive protein (18, 19), lactate dehydrogenase (20), PLR (17, 21–23), NLR (17, 21–24), SII (17, 21), and tumor biomarkers (20, 25); abnormal coagulation and fibrinolysis (26, 27); high T and N stage; liver metastasis; adrenal metastasis (28, 29); absence of SMs; smoking history; male; and loss of weight (30). In the present study, 10 variables were included in the Risk-Total formula, and the level of risk score was associated with reduced survival of patients, which was consistent with previous studies. According to this model, we found that TRT just improve the survival of low-risk patients but not that of high-risk.
In recent years, immunotherapy has transformed the treatment approach for patients with advanced NSCLC. The combination of immunotherapy and LCT for these potentially curable patients is an area of active investigation. Bauml et al. (31) randomized 51 patients who had oligometastatic NSCLC (≤4 metastatic sites) and had completed LT to all known sites of disease to receive pembrolizumab. The median PFS was significantly greater than historical data (P = 0.005), and 1- and 2-year OS rates were 90.9% and 77.5%, respectively. Nevertheless, in our study, immunotherapy status was not included in the analysis, which may affect the practicality of this prediction model in the era of immunotherapy.
Limitations
Some limitations of our study should be considered. Most importantly, because of the retrospective study design, the diagnosis of metastatic sites was not based on homogenous imaging techniques. Next, local and systematic treatments were also inconsistent, which may have influenced survival. Finally, this study was based on the experience of a single institution, and the number of patients was limited. Future multicenter studies are required to verify this model and to refine the treatment method for primary lesion.
Conclusion
The prognosis of SOM-NSCLC is significantly influenced by many hematological and clinical factors. A prediction model was developed in this study to help identify patients who would not benefit from TRT, and we found that TRT improved the survival of low-risk patients but not that of the high-risk patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Department of Ethics Committee, Tianjin Medical University Cancer Institute and Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
CM: Conceptualization, Methodology, Formal analysis, Investigation, Writing - Original Draft. FW: Conceptualization, Methodology, Formal analysis, Investigation. JT, JW, and XL: Investigation. KR and LX: Methodology. LZ and PW: Writing - Review and Editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Chinese National Key Research and Development Project (Grant No. 2018YFC1315601), and the National Natural Science Foundation of China (No.81903121).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NSCLC, non–small cell lung cancer; LT, local treatment; TRT, thoracic radiotherapy; OS, overall survival; PFS, progression-free survival; TNM, tumor node metastasis; SM, sensitive mutations; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, ROS proto-oncogene 1, receptor tyrosine kinase; TPSA, tissue polypeptide–specific antigen; SCC, squamous cell carcinoma antigen; CEA, carcinoembryonic antigen; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; SII, systemic inflammatory index; LASSO, least absolute shrinkage and selection operator; MST, median survival time; ROC, receiver operator characteristic; AUC, area under the curve; KPS, Karnofsky performance status; SBRT, stereotactic body radiotherapy; IPTW, inverse probability treatment weight.
Footnotes
- ^ 1Abbreviations: TRT, thoracic radiotherapy; SOM-NSCLC, synchronous oligometastatic non–small cell lung cancer; OS, overall survival; LASSO, least absolute shrinkage and selection operator.
- ^ 2Abbreviations: NSCLC, non–small cell lung cancer; LT, local treatment; TRT, thoracic radiotherapy; OS, overall survival; PFS, progression-free survival; TNM, tumor node metastasis; SM, sensitive mutations; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, ROS proto-oncogene 1, receptor tyrosine kinase; TPSA, tissue polypeptide–specific antigen; SCC, squamous cell carcinoma antigen; CEA, carcinoembryonic antigen; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; SII, systemic inflammatory index; LASSO, least absolute shrinkage and selection operator; MST, median survival time; ROC, receiver operator characteristic; AUC, area under the curve; KPS, Karnofsky performance status; SBRT, stereotactic body radiotherapy; IPTW, inverse probability treatment weight.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–249. doi: 10.3322/caac.21660
2. Levy A, Hendriks LEL, Berghmans T, Faivre-Finn C, GiajLevra M, GiajLevra N, et al. EORTC Lung Cancer Group Survey on the Definition of NSCLC Synchronous Oligometastatic Disease. Eur J Cancer (2019) 122:109–14. doi: 10.1016/j.ejca.2019.09.012
3. Parikh RB, Cronin AM, Kozono DE, Oxnard GR, Mak RH, Jackman DM, et al. Definitive Primary Therapy in Patients Presenting With Oligometastatic non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys (2014) 89(4):880–7. doi: 10.1016/j.ijrobp.2014.04.007
4. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol (1995) 13(1):8–10. doi: 10.1200/JCO.1995.13.1.8
5. Lievens Y, Guckenberger M, Gomez D, Hoyer M, Iyengar P, Kindts I, et al. Defining Oligometastatic Disease From a Radiation Oncology Perspective: An ESTRO-ASTRO Consensus Document. Radiother Oncol (2020) 148:157–66. doi: 10.1016/j.radonc.2020.04.003
6. Xu Q, Zhou F, Liu H, Jiang T, Li X, Xu Y, et al. Consolidative Local Ablative Therapy Improves the Survival of Patients With Synchronous Oligometastatic NSCLC Harboring EGFR Activating Mutation Treated With First-Line EGFR-TKIs. J Thorac Oncol (2018) 13(9):1383–92. doi: 10.1016/j.jtho.2018.05.019
7. Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol (2018) 4(1):e173501. doi: 10.1001/jamaoncol.2017.3501
8. Gomez D, Tang C, Zhang J, Blumenschein G, Hernandez M, Lee J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol (2019) 37(18):1558–65. doi: 10.1200/JCO.19.00201
9. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic Ablative Radiotherapy Versus Standard of Care Palliative Treatment in Patients With Oligometastatic Cancers (SABR-COMET): A Randomised, Phase 2, Open-Label Trial. Lancet (2019) 393(10185):2051–8. doi: 10.1016/S0140-6736(18)32487-5
10. Lopez Guerra JL, Gomez D, Zhuang Y, Hong DS, Heymach JV, Swisher SG, et al. Prognostic Impact of Radiation Therapy to the Primary Tumor in Patients With non-Small Cell Lung Cancer and Oligometastasis at Diagnosis. Int J Radiat Oncol Biol Phys (2012) 84(1):e61–7. doi: 10.1016/j.ijrobp.2012.02.054
11. Ashworth AB, Senan S, Palma DA, Riquet M, Ahn YC, Ricardi U, et al. An Individual Patient Data Metaanalysis of Outcomes and Prognostic Factors After Treatment of Oligometastatic non-Small-Cell Lung Cancer. Clin Lung Cancer (2014) 15(5):346–55. doi: 10.1016/j.cllc.2014.04.003
12. Collen C, Christian N, Schallier D, Meysman M, Duchateau M, Storme G, et al. Phase II Study of Stereotactic Body Radiotherapy to Primary Tumor and Metastatic Locations in Oligometastatic Nonsmall-Cell Lung Cancer Patients. Ann Oncol (2014) 25(10):1954–9. doi: 10.1093/annonc/mdu370
13. Petty WJ, Urbanic JJ, Ahmed T, Hughes R, Levine B, Rusthoven K, et al. Long-Term Outcomes of a Phase 2 Trial of Chemotherapy With Consolidative Radiation Therapy for Oligometastatic Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys (2018) 102(3):527–35. doi: 10.1016/j.ijrobp.2018.06.400
14. Farooqi A, Ludmir EB, Mitchell KG, Antonoff MB, Tang C, Lee P, et al. Increased Biologically Effective Dose (BED) to the Primary Tumor is Associated With Improved Survival in Patients With Oligometastatic NSCLC. Radiother Oncol (2021) 163:114–8. doi: 10.1016/j.radonc.2021.08.005
15. Chansky K, Detterbeck FC, Nicholson AG, Rusch VW, Vallieres E, Groome P, et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol (2017) 12(7):1109–21. doi: 10.1016/j.jtho.2017.04.011
16. Meng C, Wei J, Tian J, Ma J, Liu N, Yuan Z, et al. Estimating Survival and Clinical Outcome in Advanced non-Small Cell Lung Cancer With Bone-Only Metastasis Using Molecular Markers. J Bone Oncol (2021) 31:100394. doi: 10.1016/j.jbo.2021.100394
17. Qi J, Zhang J, Ge X, Wang X, Xu L, Liu N, et al. The Addition of Peripheral Blood Inflammatory Indexes to Nomogram Improves the Predictive Accuracy of Survival in Limited-Stage Small Cell Lung Cancer Patients. Front Oncol (2021) 11:713014. doi: 10.3389/fonc.2021.713014
18. Ni XF, Wu J, Ji M, Shao YJ, Xu B, Jiang JT, et al. Effect of C-Reactive Protein/Albumin Ratio on Prognosis in Advanced non-Small-Cell Lung Cancer. Asia Pac J Clin Oncol (2018) 14(6):402–9. doi: 10.1111/ajco.13055
19. Cetin K, Christiansen CF, Jacobsen JB, Norgaard M, Sorensen HT. Bone Metastasis, Skeletal-Related Events, and Mortality in Lung Cancer Patients: A Danish Population-Based Cohort Study. Lung Cancer (2014) 86(2):247–54. doi: 10.1016/j.lungcan.2014.08.022
20. Gu W, Hu M, Xu L, Ren Y, Mei J, Wang W, et al. The Ki-67 Proliferation Index-Related Nomogram to Predict the Response of First-Line Tyrosine Kinase Inhibitors or Chemotherapy in Non-Small Cell Lung Cancer Patients With Epidermal Growth Factor Receptor-Mutant Status. Front Med (Lausanne) (2021) 8:728575. doi: 10.3389/fmed.2021.728575
21. Tong YS, Tan J, Zhou XL, Song YQ, Song YJ. Systemic Immune-Inflammation Index Predicting Chemoradiation Resistance and Poor Outcome in Patients With Stage III non-Small Cell Lung Cancer. J Transl Med (2017) 15(1):221. doi: 10.1186/s12967-017-1326-1
22. Chen C, Yang H, Cai D, Xiang L, Fang W, Wang R. Preoperative Peripheral Blood Neutrophil-to-Lymphocyte Ratios (NLR) and Platelet-to-Lymphocyte Ratio (PLR) Related Nomograms Predict the Survival of Patients With Limited-Stage Small-Cell Lung Cancer. Trans Lung Cancer Res (2021) 10(2):866–77. doi: 10.21037/tlcr-20-997
23. Zhang N, Jiang J, Tang S, Sun G. Predictive Value of Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio in Non-Small Cell Lung Cancer Patients Treated With Immune Checkpoint Inhibitors: A Meta-Analysis. Int Immunopharmacol (2020) 85:106677. doi: 10.1016/j.intimp.2020.106677
24. Huang W, Wang S, Zhang H, Zhang B, Wang C. Prognostic Significance of Combined Fibrinogen Concentration and Neutrophil-to-Lymphocyte Ratio in Patients With Resectable non-Small Cell Lung Cancer. Cancer Biol Med (2018) 15(1):88–96. doi: 10.20892/j.issn.2095-3941.2017.0124
25. Dall'Olio FG, Abbati F, Facchinetti F, Massucci M, Melotti B, Squadrilli A, et al. CEA and CYFRA 21-1 as Prognostic Biomarker and as a Tool for Treatment Monitoring in Advanced NSCLC Treated With Immune Checkpoint Inhibitors. Ther Adv Med Oncol (2020) 12:1758835920952994. doi: 10.1177/1758835920952994
26. Bharadwaj AG, Holloway RW, Miller VA, Waisman DM. Plasmin and Plasminogen System in the Tumor Microenvironment: Implications for Cancer Diagnosis, Prognosis, and Therapy, Cancers (Basel)13(8):1838. (2021). doi: 10.3390/cancers13081838
27. Guo J, Gao Y, Gong Z, Dong P, Mao Y, Li F, et al. Plasma D-Dimer Level Correlates With Age, Metastasis, Recurrence, Tumor-Node-Metastasis Classification (TNM), and Treatment of Non-Small-Cell Lung Cancer (NSCLC) Patients. BioMed Res Int (2021) 2021:9623571. doi: 10.1155/2021/9623571
28. Nakazawa K, Kurishima K, Tamura T, Kagohashi K, Ishikawa H, Satoh H, et al. Specific Organ Metastases and Survival in Small Cell Lung Cancer. Oncol Lett (2012) 4(4):617–20. doi: 10.3892/ol.2012.792
29. Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, et al. Metastatic Sites and Survival in Lung Cancer. Lung Cancer (2014) 86(1):78–84. doi: 10.1016/j.lungcan.2014.07.020
30. Wang J, Zhao Y, Wang Q, Zhang L, Shi J, Wang Z, et al. Prognostic Factors of Refractory NSCLC Patients Receiving Anlotinib Hydrochloride as the Third- or Further-Line Treatment. Cancer Biol Med (2018) 15(4):443–51. doi: 10.20892/j.issn.2095-3941.2018.0158
Keywords: synchronous oligometastasis, non–small cell lung cancer, thoracic radiotherapy, risk prediction model, survival
Citation: Meng C, Wang F, Tian J, Wei J, Li X, Ren K, Xu L, Zhao L and Wang P (2022) Risk Prediction Model for Synchronous Oligometastatic Non-Small Cell Lung Cancer: Thoracic Radiotherapy May Not Prolong Survival in High-Risk patients. Front. Oncol. 12:897329. doi: 10.3389/fonc.2022.897329
Received: 16 March 2022; Accepted: 16 June 2022;
Published: 15 July 2022.
Edited by:
Xin Ye, Qianfoshan Hospital, Shandong University, ChinaReviewed by:
Maurizio Valeriani, Sapienza University of Rome, ItalyMatthew Pierre Deek, Johns Hopkins Medicine, United States
Copyright © 2022 Meng, Wang, Tian, Wei, Li, Ren, Xu, Zhao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lujun Zhao, emhhb2x1anVuQHRqbXVjaC5jb20=; Ping Wang, d2FuZ3BpbmdAdGptdWNoLmNvbQ==
†These authors have contributed equally to this work
 Chunliu Meng
Chunliu Meng Fang Wang
Fang Wang Jia Tian1
Jia Tian1 Lujun Zhao
Lujun Zhao