
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 23 June 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.896764
 Qi Li1†
Qi Li1† Jian Zhang1†
Jian Zhang1† Chen Chen1
Chen Chen1 Tianqiang Song2
Tianqiang Song2 Yinghe Qiu3
Yinghe Qiu3 Xianhai Mao4
Xianhai Mao4 Hong Wu5
Hong Wu5 Yu He6
Yu He6 Zhangjun Cheng7
Zhangjun Cheng7 Wenlong Zhai8
Wenlong Zhai8 Jingdong Li9
Jingdong Li9 Dong Zhang1
Dong Zhang1 Zhimin Geng1*
Zhimin Geng1* Zhaohui Tang10*
Zhaohui Tang10*Background: The influence of different postoperative recurrence times on the efficacy of adjuvant chemotherapy (ACT) for intrahepatic cholangiocarcinoma (ICC) remains unclear. This study aimed to investigate the independent risk factors and establish a nomogram prediction model of early recurrence (recurrence within 1 year) to screen patients with ICC for ACT.
Methods: Data from 310 ICC patients who underwent radical resection between 2010 and 2018 at eight Chinese tertiary hospitals were used to analyze the risk factors and establish a nomogram model to predict early recurrence. External validation was conducted on 134 patients at the other two Chinese tertiary hospitals. Overall survival (OS) and relapse-free survival (RFS) were estimated by the Kaplan–Meier method. Multivariate analysis was conducted to identify independent risk factors for prognosis. A logistic regression model was used to screen independent risk variables for early recurrence. A nomogram model was established based on the above independent risk variables to predict early recurrence.
Results: ACT was a prognostic factor and an independent affecting factor for OS and RFS of patients with ICC after radical resection (p < 0.01). The median OS of ICC patients with non-ACT and ACT was 14.0 and 15.0 months, and the median RFS was 6.0 and 8.0 months for the early recurrence group, respectively (p > 0.05). While the median OS of ICC patients with non-ACT and ACT was 41.0 and 84.0 months, the median RFS was 20.0 and 45.0 months for the late recurrence group, respectively (p < 0.01). CA19-9, tumor size, major vascular invasion, microvascular invasion, and N stage were the independent risk factors of early recurrence for ICC patients after radical resection. The C-index of the nomogram was 0.777 (95% CI: 0.713~0.841) and 0.716 (95%CI: 0.604~0.828) in the training and testing sets, respectively.
Conclusion: The nomogram model established based on the independent risk variables of early recurrence for curatively resected ICC patients has a good prediction ability and can be used to screen patients who benefited from ACT.
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer and accounts for about 10% to 15% (1, 2). Over the past two decades, the incidence of ICC has been increasing throughout the world, also accompanied by an increase in mortality (3, 4). At present, surgical resection is considered the only curative treatment for ICC patients, but only a small fraction (15%) of patients are eligible for surgery (5). The occurrence of postoperative recurrence and metastasis leads to poor survival even after curative hepatectomy, with a 3-year relapse-free survival (RFS) rate below 30% and a 5-year overall survival (OS) rate ranging from 20% to 40% (6–8). Therefore, identifying patients who are at risk for early recurrence is important to construct individualized surveillance strategies for ICC patients after radical resection. Recently, more and more scholars are concerned about the risk factors of early recurrence, while the definition of early recurrence is different because definitive guidelines do not exist (9–11).
Currently, the effect of adjuvant chemotherapy (ACT) on the prognosis of ICC patients is still controversial (12–14), although studies have proved that ACT can improve the prognosis (15, 16). Because many factors may affect the efficacy of ACT, it is very important to screen potential patients who could benefit from ACT. Few published studies focused on the recurrence time of ICC patients accompanied by ACT or not, which to some extent could help choose patient groups that are suitable for ACT. This study aimed to investigate the independent risk factors and establish a nomogram model to predict early recurrence to screen ICC patients for ACT.
All patients undergoing curative resection for histologically confirmed ICC between 2010 and 2018 at ten tertiary hospitals in China (Tianjin Medical University Cancer Institute and Hospital; Hunan Provincial People’s Hospital; The First Hospital Affiliated to Army Medical University; The First Affiliated Hospital of Xi’an Jiaotong University; Zhongda Hospital of Southeast University; The First Affiliated Hospital of Zhengzhou University; Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine; Affiliated Hospital of North Sichuan Medical College; Oriental Hepatobiliary Hospital Affiliated to Naval Medical University; West China Hospital of Sichuan University) were considered for inclusion. The study was approved by the ethics committee of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine (No. XHEC-JDYXY-2018-002), Shanghai, China, as well as by the ethics committees of the other centers. Written informed consent was obtained from all included patients and their families before study enrollment.
According to previous studies (10, 17, 18), a postoperative recurrence within 1 year was defined as early recurrence, while a recurrence of >1 year was a late recurrence. The inclusion criteria were as follows (1): patients underwent radical resection and the margin status of the initial resection was microscopically negative (R0) (2); patients had a detailed postoperative recurrence record (3); patients received ACT with complete and systematic regimens; and (4) patients without a history of other malignancies. Exclusion criteria were as follows (1): hilar cholangiocarcinoma invading the liver (2); mixed cholangiocarcinoma-hepatocellular carcinoma (3); incomplete clinical data; and (4) patients died within 30 days after surgery.
In this study, patients with ACT were strictly performed as follows. The regimens included gemcitabine (1,000 mg/m2 on days 1 and 8) + capecitabine (1,250 mg/m2 twice daily on days 1–14) of a 3-week cycle; gemcitabine (1,000 mg/m2 on days 1 and 8) + cisplatin (30 mg/m2 on days 1 and 8) of a 3-week cycle; gemcitabine (1,000 mg/m2 on days 1 and 8) + oxaliplatin (100 mg/m2 on day 1) of a 3-week cycle; gemcitabine (1,000 mg/m2 on days 1 and 8) + tegafur (40~60 mg twice daily on days 1–14) of a 3-week cycle.
The indications for ACT were ICC patients with T2~4 stage, N1 stage, combined with major vascular invasion, microvascular invasion, perineural invasion, etc., which was associated with high postoperative recurrence risk.
Follow-up was performed in outpatient or telephone. Liver function, tumor biomarkers, ultrasound, contrast-enhanced CT, or MRI examinations were reviewed every 2 to 3 months within 1 year after surgery, and then once every 3–6 months for more than 1 year after surgery. Postoperative recurrence was defined as the discovery of new lesions by two or more imaging examinations. All included patients were followed up through December 2020.
All statistical analyses were performed using SPSS version 25 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as the mean ± standard deviation. Categorical variables were examined using the χ2-test. The Kaplan–Meier method and Log-rank test were conducted for univariate analysis, and the Cox proportional hazard regression model was conducted for multivariate analysis. A logistic regression model was further used to screen independent risk variables for early recurrence. Survival analysis curves were conducted by GraphPad Prism (version 8.0, San Diego, California, USA). p < 0.05 was considered statistically significant.
A total of 444 ICC patients were finally included in the study; 310 patients from 8 medical centers were included as the training set, and 134 patients from the Oriental Hepatobiliary Hospital Affiliated to Naval Medical University and West China Hospital of Sichuan University were included as the testing set. R software version 3.6.1 (http://www.r-project.org/) was used to produce a nomogram prediction model based on the independent risk variables for early recurrence of ICC patients after surgery. The performance of the nomogram was evaluated based on the concordance index (C-index), calibration plot, and decision curve analysis (DCA). DCA was performed by calculating the benefit of a series of threshold probabilities and was conducted to evaluate the clinical practicability of the nomogram (19).
A total of 444 patients undergoing radical resection for histologically confirmed ICC between 2010 and 2018 were considered for inclusion. The 1-, 3-, and 5-year OS rates of patients were 80.9%, 40.4%, and 19.4%, and the 1-, 3-, and 5-year RFS rates of patients were 55.5%, 17.4%, and 13.3%, respectively. Median survival time was 26.0 and 14.8 months for OS and RFS in the training dataset, respectively.
To further explore the survival difference between early recurrence and late recurrence groups, the results showed that early recurrence (HR: 6.585, 95% CI:4.454~9.736) was a risk factor for OS of ICC patients after radical resection compared to the late recurrence group (p < 0.001). Furthermore, the median OS was 15.0 and 56.5 months (Figure 1A, p < 0.001), and the median RFS were 7.0 and 15.0 months for early recurrence and late recurrence groups of ICC patients, respectively (Figure 1B, p < 0.001). Therefore, the results showed that early recurrence was an adverse factor for the prognosis of ICC after radical resection.
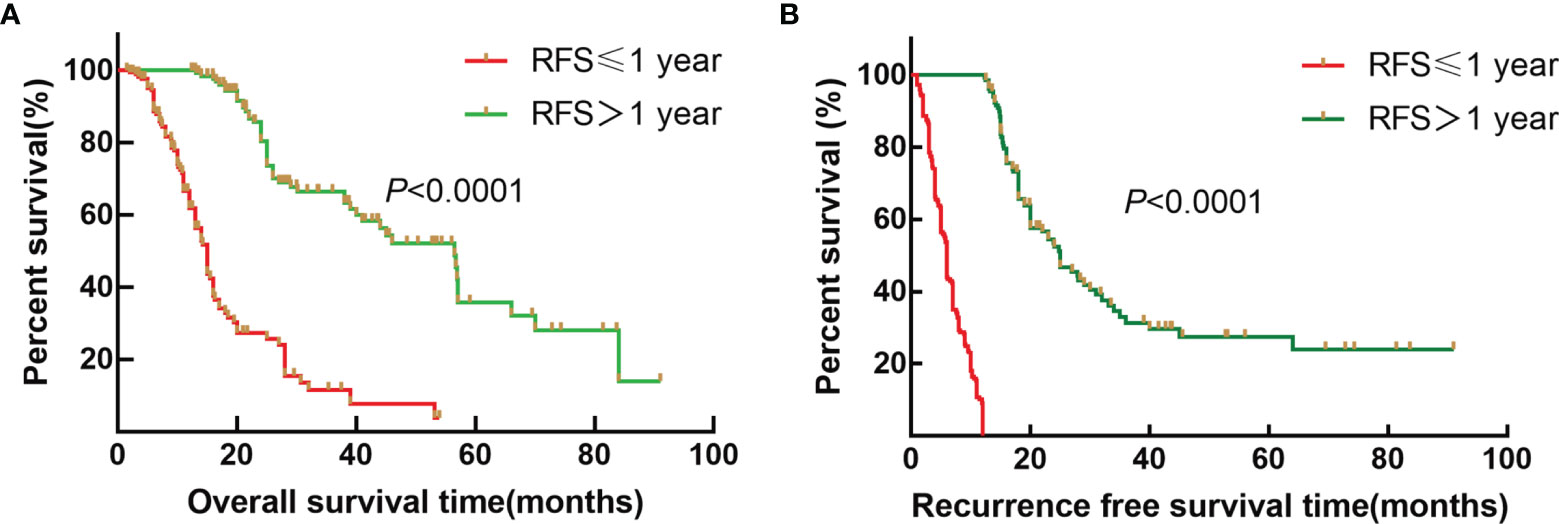
Figure 1 Survival curves of patients with ICC after radical resection between the early recurrence group and late recurrence group. (A) Kaplan–Meier OS curve for ICC patients between the early recurrence group and late recurrence group. (B) Kaplan–Meier RFS curve for ICC patients between the early recurrence group and late recurrence group.
To determine whether the ACT regimens affected the prognosis of patients, we first analyzed the prognosis differences among the four regimens for patients treated with ACT. The results showed that there was no difference in OS and RFS among different chemotherapy regimens (p > 0.05). Univariate analysis then showed that ACT (HR: 0.523, 95% CI:0.364~0.753; HR: 0.653, 95% CI:0.488~0.875) was the prognostic factor for OS and RFS of patients with ICC after radical resection (p < 0.01). Multivariate analysis showed that ACT (HR: 0.403, 95% CI: 0.269~0.603; HR: 0.672, 95% CI: 0.502~0.900) was an independent prognostic factor for OS and RFS (p < 0.01) (Table 1).
To further stratify analysis in the training set, the results also showed that the median OS of ICC patients with non-ACT and ACT was 14.0 and 15.0 months; the median RFS was 6.0 and 8. months for the early recurrence group, respectively (Figures 2A, B, p > 0.05); the median OS of ICC patients with non-ACT and ACT was 41.0 and 84.0 months, and the median RFS was 20.0 and 45.0 months for the late recurrence group, respectively (Figures 2C, D, p < 0.01).
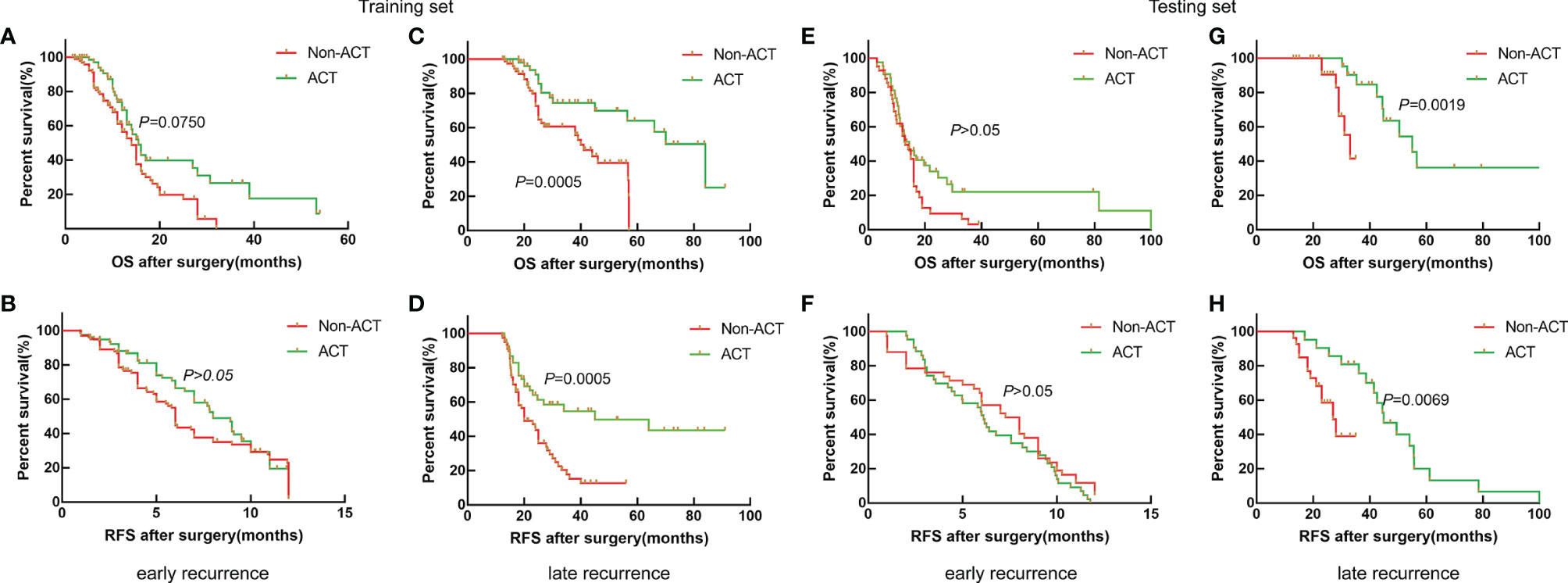
Figure 2 Survival curves of patients with ICC after radical resection between non-ACT and ACT for the early recurrence group and late recurrence group. (A, B) Kaplan–Meier OS curve and RFS curve for the early recurrence group in the training set. (C, D) Kaplan–Meier OS curve and RFS curve for the late recurrence group in the training set. (E, F) Kaplan–Meier OS curve and RFS curve for the early recurrence group in the testing set. (G, H) Kaplan–Meier OS curve and RFS curve for the late recurrence group in the testing set.
Similarly, the results also showed that ACT could be beneficial to the late recurrence group for OS and RFS in the testing set (Figures 2G, H, p < 0.01), while the OS and RFS of ICC patients with early recurrence were not significantly improved after receiving ACT (Figure 2E, F, p > 0.05). Thus, the results showed that ACT can improve the prognosis for ICC patients with late recurrence significantly.
CA19-9, tumor size, major vascular invasion, microvascular invasion, and N stage were the independent risk factors for early recurrence of ICC patients after radical resection. A nomogram to predict early recurrence was established based on the above independent risk factors. Detailed results of the logistic regression are shown on the right-hand side of Table 2. The nomogram is shown in Figure 3, and an online calculator for the nomogram model was established, which is available at https://doczj.shinyapps.io/icc_early.
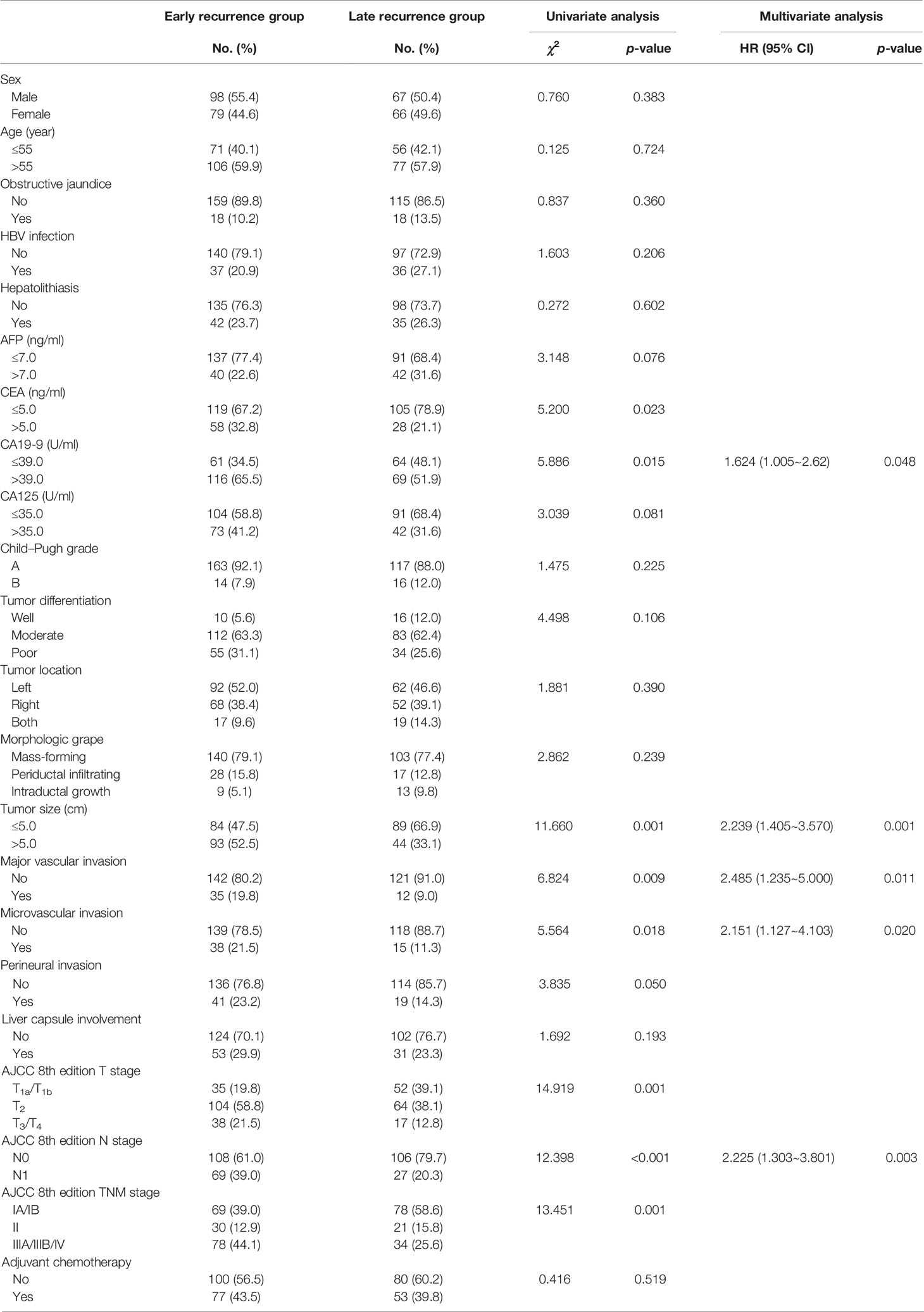
Table 2 Comparison of clinicopathologic characteristics of early recurrence and late recurrence for ICC after radical resection.
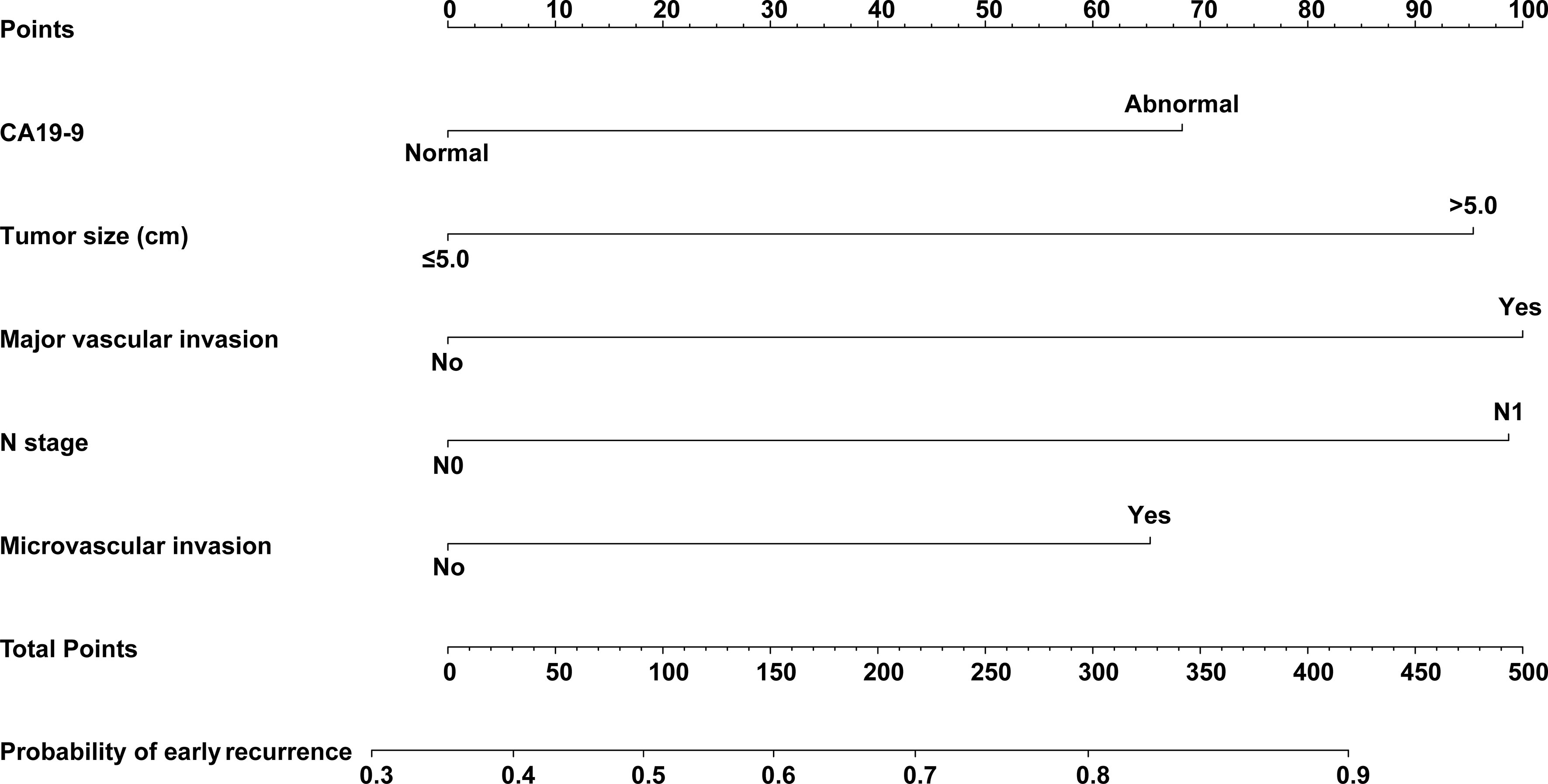
Figure 3 Nomogram prediction model for predicting early recurrence of patients with ICC after radical resection.
The C-index of the nomogram model was 0.777 (95% CI: 0.713~0.841) and 0.716 (95% CI: 0.604~0.828) in the training and testing sets, respectively. The calibration plots are shown in Figures 4A, B, which showed the prediction results were more consistent with the actual results. In addition, DCAs are shown in Figures 4C, D, which showed that the predictive ability of the nomogram model was better than TNM staging in the training set and testing set.
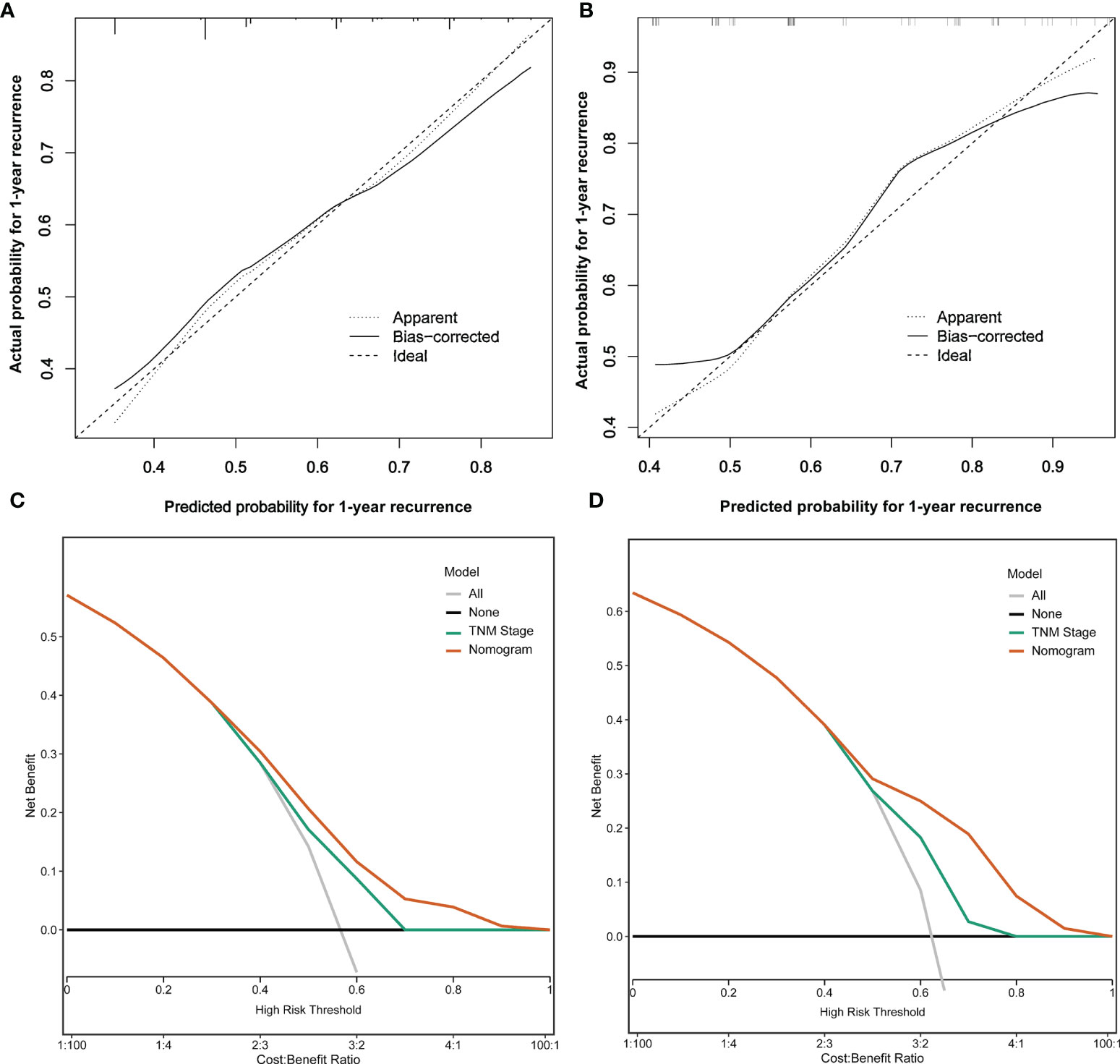
Figure 4 Analysis of calibration plots and decision curves for the nomogram prediction model. (A) Calibration plot for the nomogram in the training set. (B) Calibration plot for the nomogram in the testing set. (C) Decision curve analysis for the nomogram in the training set. (D) Decision curve analysis for the RFS in the testing set.
Guidelines for the management of recurrent ICC remain controversial and poorly defined, and a few studies have analyzed the risk factors of early recurrence with different criteria. Tsilimigras et al. (9) analyzed the risk factors of very early recurrence (≤6 months) and developed an easy-to-use online calculator to help clinicians predict the chance of very early recurrence, which provided treatment and surveillance strategies for ICC patients after surgery. Zhang et al. (11) revealed that the patterns of early recurrence (≤2 years) and late recurrence were different, and early recurrence of extrahepatic recurrence was more common, whereas late recurrence was often only intrahepatic recurrence. Importantly, patients’ recurrence within 1 year after surgery may represent more aggressive tumor biology, and a cutoff of 1 year after surgery has been used to distinguish the early recurrence and late recurrence in most studies (10, 17, 20, 21). Wang et al. (20) showed that specific risk factors, including CA 19-9, microvascular invasion, and multiple tumors, may relate to the early recurrence of ICC after curative resection. Xing et al. (22) revealed that CA 19-9, tumor number at recurrence, and treatment for recurrence could be used to assess survival for post-operative recurrence, and time to recurrence, especially within a year after resection, had a significant impact on postrecurrence survival. In this study, CA19-9, tumor size, major vascular invasion, microvascular invasion, and N stage were identified as the independent risk factors for early recurrence, in which CA19-9 and N stage were the independent risk factors for OS and RFS of ICC patients after radical resection. Many studies (11, 17, 20, 23–25) have proved that the above five variables were the independent risk factors for early recurrence and prognosis, which also provided a basis for establishing an effective predictive model.
Many studies (15, 16, 26) revealed that ACT was beneficial to ICC patients after radical resection, but which patients were suitable for ACT also required further study. In this study, ACT was also a protective prognostic factor for ICC patients in the late recurrence group. Unfortunately, patients in the early recurrence group did not benefit from ACT. Hence, ACT seemed to have less benefit for curatively resected ICC patients when analyzed as a complete group. However, an obvious survival benefit was shown when all patients were divided into early and late recurrence groups (27, 28).
Moreover, early recurrence assessment could provide references for repeat hepatic resection to produce long-term survival outcomes in previous studies (10, 17, 29). Therefore, an accurate prediction of early recurrence was of great value for appropriate treatment strategies for ICC patients after surgery, particularly because this study identified that ACT would not benefit patients at a high risk of early recurrence. In addition, the exploration of an effective treatment to improve prognosis is of great importance for early recurrence patients.
To our knowledge, this study is the first to establish a nomogram prediction model for early recurrence including the above independent risk variables. The C-index of the nomogram model was 0.777 and 0.716 in the training and testing sets, respectively. The calibration plots showed that the prediction results were more consistent with the actual results, and DCAs showed that the predictive ability of the nomogram model was better than TNM staging in the training and testing sets. Different from our nomogram model, many scholars (21, 30, 31) developed radiomics nomograms by using the radiomics signature and other clinicopathological characteristics to predict the early recurrence of ICC after surgery, but the inclusion of radiomics signature also brought certain difficulties to clinical applications. Jeong et al. (32) established a nomogram model to allow precise estimation of the risk of 1-, 3-, and 5-year RFS for ICC after resection by the combined Cox and logistic ranking system based on 10 and 11 covariates; however, it could not evaluate and predict early recurrence and was complex in the application despite its good predictive ability. Yu et al. (33) established a nomogram for 1-, 3-, and 5-year RFS based on tumor size, tumor number, direct invasion, and triosephosphate isomerase (TPI1), while they did not provide treatment decisions for postoperative early and late recurrence, although they showed the prognostic model was accurate in predicting recurrence for ICC patients. Therefore, our nomogram model has better clinical practicability and applicability for early recurrence of ICC patients by using the online calculator.
However, several limitations must be acknowledged in this study. It is difficult to avoid selection bias in the retrospective design and the different definitions of early recurrence. In addition, the reasons why ICC patients with early recurrence cannot benefit from ACT have not been further analyzed. Accordingly, we recommend that more patients with ICC after radical resection from other medical centers could be collected in future studies to validate our results, and the molecular biomarkers should be added into the study to improve the predictive ability of the nomogram model, which can provide decision support for ACT of ICC patients more effectively.
In summary, this study retrospectively analyzed 444 patients with ICC after radical resection and developed a nomogram prediction model based on the risk factors of early recurrence, including CA19-9, tumor size, major vascular invasion, microvascular invasion, and N stage with a good predictive ability, which can be used to screen patients with ICC who benefit from ACT effectively. We expect that the nomogram model can help to screen appropriate ICC patients who could benefit from ACT and achieve widespread clinical application in the future.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the ethics committee of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine (No. XHEC-JDYXY-2018-002), Shanghai, China. The patients/participants provided their written informed consent to participate in this study.
ZG and ZT conceived and designed the experiments. QL and JZ performed the experiments. TS, YQ, XM, HW, YH, ZC, WZ, and JL collected and offered the data. QL and JZ contributed analysis tools. QL, JZ, CC, and DZ conducted statistical analysis. QL and JZ wrote the paper. ZG and ZT reviewed the manuscript. All authors read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (No. 62076194, No. 81772521); Multicenter Clinical Research Project of Shanghai Jiaotong University, School of Medicine (DLY201807); and Clinical Training Program of Shanghai Xinhua Hospital Affiliated to Shanghai Jiaotong University, School of Medicine (17CSK06).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zhang H, Yang T, Wu M, Shen F. Intrahepatic Cholangiocarcinoma: Epidemiology, Risk Factors, Diagnosis and Surgical Management. Cancer Lett (2016) 379(2):198–205. doi: 10.1016/j.canlet.2015.09.008
2. Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control (2017) 24(3):1145164509. doi: 10.1177/1073274817729245
3. Van Dyke AL, Shiels MS, Jones GS, Pfeiffer RM, Petrick JL, Beebe-Dimmer JL, et al. Biliary Tract Cancer Incidence and Trends in the United States by Demographic Group, 1999-2013. Cancer-Am Cancer Soc (2019) 125(9):1489–98. doi: 10.1002/cncr.31942
4. Khan SA, Genus T, Morement H, Murphy A, Rous B, Tataru D. Global Trends in Mortality From Intrahepatic and Extrahepatic Cholangiocarcinoma. J Hepatol (2019) 71(6):1261–2. doi: 10.1016/j.jhep.2019.07.024
5. Buettner S, van Vugt JL, IJzermans JN, Groot KB. Intrahepatic Cholangiocarcinoma: Current Perspectives. Onco Targets Ther (2017) 10:1131–42. doi: 10.2147/OTT.S93629
6. Jutric Z, Johnston WC, Hoen HM, Newell PH, Cassera MA, Hammill CW, et al. Impact of Lymph Node Status in Patients With Intrahepatic Cholangiocarcinoma Treated by Major Hepatectomy: A Review of the National Cancer Database. HPB (Oxford) (2016) 18(1):79–87. doi: 10.1016/j.hpb.2015.07.006
7. Spolverato G, Kim Y, Alexandrescu S, Marques HP, Lamelas J, Aldrighetti L, et al. Management and Outcomes of Patients With Recurrent Intrahepatic Cholangiocarcinoma Following Previous Curative-Intent Surgical Resection. Ann Surg Oncol (2016) 23(1):235–43. doi: 10.1245/s10434-015-4642-9
8. Spolverato G, Yakoob MY, Kim Y, Alexandrescu S, Marques HP, Lamelas J, et al. The Impact of Surgical Margin Status on Long-Term Outcome After Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol (2015) 22(12):4020–8. doi: 10.1245/s10434-015-4472-9
9. Tsilimigras DI, Sahara K, Wu L, Moris D, Bagante F, Guglielmi A, et al. Very Early Recurrence After Liver Resection for Intrahepatic Cholangiocarcinoma: Considering Alternative Treatment Approaches. JAMA Surg (2020) 155(9):823–31. doi: 10.1001/jamasurg.2020.1973
10. Kojima T, Umeda Y, Fuji T, Niguma T, Sato D, Endo Y, et al. Efficacy of Surgical Management for Recurrent Intrahepatic Cholangiocarcinoma: A Multi-Institutional Study by the Okayama Study Group of Hbp Surgery. PLoS One (2020) 15(9):e238392. doi: 10.1371/journal.pone.0238392
11. Zhang XF, Beal EW, Bagante F, Chakedis J, Weiss M, Popescu I, et al. Early Versus Late Recurrence of Intrahepatic Cholangiocarcinoma After Resection With Curative Intent. Br J Surg (2018) 105(7):848–56. doi: 10.1002/bjs.10676
12. Messina C, Merz V, Frisinghelli M, Trentin C, Grego E, Veccia A, et al. Adjuvant Chemotherapy in Resected Bile Duct Cancer: A Systematic Review and Meta-Analysis of Randomized Trials. Crit Rev Oncol Hematol (2019) 143:124–9. doi: 10.1016/j.critrevonc.2019.09.002
13. Luvira V, Satitkarnmanee E, Pugkhem A, Kietpeerakool C, Lumbiganon P, Pattanittum P. Postoperative Adjuvant Chemotherapy for Resectable Cholangiocarcinoma. Cochrane Database Syst Rev (2021) 9(9):D12814. doi: 10.1002/14651858.CD012814.pub2
14. Sasaki T, Takeda T, Okamoto T, Ozaka M, Sasahira N. Chemotherapy for Biliary Tract Cancer in 2021. J Clin Med (2021) 10(14):3108. doi: 10.3390/jcm10143108
15. Schweitzer N, Weber T, Kirstein MM, Fischer M, Kratzel AM, Reineke-Plaaß T, et al. The Effect of Adjuvant Chemotherapy in Patients With Intrahepatic Cholangiocarcinoma: A Matched Pair Analysis. J Cancer Res Clin Oncol (2017) 143(7):1347–55. doi: 10.1007/s00432-017-2392-8
16. Reames BN, Bagante F, Ejaz A, Spolverato G, Ruzzenente A, Weiss M, et al. Impact of Adjuvant Chemotherapy on Survival in Patients With Intrahepatic Cholangiocarcinoma: A Multi-Institutional Analysis. HPB (Oxford) (2017) 19(10):901–9. doi: 10.1016/j.hpb.2017.06.008
17. Yoh T, Hatano E, Seo S, Okuda Y, Fuji H, Ikeno Y, et al. Long-Term Survival of Recurrent Intrahepatic Cholangiocarcinoma: The Impact and Selection of Repeat Surgery. World J Surg (2018) 42(6):1848–56. doi: 10.1007/s00268-017-4387-7
18. Watanabe T, Tokumoto Y, Joko K, Michitaka K, Horiike N, Tanaka Y, et al. AFP and EGFR are Related to Early and Late Recurrence of HCC Following Antiviral Therapy. BMC Cancer (2021) 21(1):699. doi: 10.1186/s12885-021-08401-7
19. Kerr KF, Brown MD, Zhu K, Janes H. Assessing the Clinical Impact of Risk Prediction Models With Decision Curves: Guidance for Correct Interpretation and Appropriate Use. J Clin Oncol (2016) 34(21):2534–40. doi: 10.1200/JCO.2015.65.5654
20. Wang C, Pang S, Si-Ma H, Yang N, Zhang H, Fu Y, et al. Specific Risk Factors Contributing to Early and Late Recurrences of Intrahepatic Cholangiocarcinoma After Curative Resection. World J Surg Oncol (2019) 17(1):2. doi: 10.1186/s12957-018-1540-1
21. Liang W, Xu L, Yang P, Zhang L, Wan D, Huang Q, et al. Novel Nomogram for Preoperative Prediction of Early Recurrence in Intrahepatic Cholangiocarcinoma. Front Oncol (2018) 8:360. doi: 10.3389/fonc.2018.00360
22. Xing KL, Lu LH, Huang X, He CB, Song YD, Guo RP, et al. A Novel Prognostic Nomogram for Patients With Recurrence of Intrahepatic Cholangiocarcinoma After Initial Surgery. Front Oncol (2020) 10:434. doi: 10.3389/fonc.2020.00434
23. Jeong S, Cheng Q, Huang L, Wang J, Sha M, Tong Y, et al. Risk Stratification System to Predict Recurrence of Intrahepatic Cholangiocarcinoma After Hepatic Resection. BMC Cancer (2017) 17(1):464. doi: 10.1186/s12885-017-3464-5
24. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic Nomogram for Intrahepatic Cholangiocarcinoma After Partial Hepatectomy. J Clin Oncol (2013) 31(9):1188–95. doi: 10.1200/JCO.2012.41.5984
25. Umeda Y, Mitsuhashi T, Kojima T, Satoh D, Sui K, Endo Y, et al. Impact of Lymph Node Dissection on Clinical Outcomes of Intrahepatic Cholangiocarcinoma: Inverse Probability of Treatment Weighting With Survival Analysis. J Hepatobiliary Pancreat Sci (2022) 29(2):217–29. doi: 10.1002/jhbp.1038
26. Wang L, Deng M, Ke Q, Lou J, Zheng S, Bi X, et al. Postoperative Adjuvant Therapy Following Radical Resection for Intrahepatic Cholangiocarcinoma: A Multicenter Retrospective Study. Cancer Med (2020) 9(8):2674–85. doi: 10.1002/cam4.2925
27. Wu ZF, Zhang HB, Yang N, Zhao WC, Fu Y, Yang GS. Postoperative Adjuvant Transcatheter Arterial Chemoembolisation Improves Survival of Intrahepatic Cholangiocarcinoma Patients With Poor Prognostic Factors: Results of a Large Monocentric Series. Eur J Surg Oncol (2012) 38(7):602–10. doi: 10.1016/j.ejso.2012.02.185
28. Li J, Wang Q, Lei Z, Wu D, Si A, Wang K, et al. Adjuvant Transarterial Chemoembolization Following Liver Resection for Intrahepatic Cholangiocarcinoma Based on Survival Risk Stratification. Oncologist (2015) 20(6):640–7. doi: 10.1634/theoncologist.2014-0470
29. Si A, Li J, Xing X, Lei Z, Xia Y, Yan Z, et al. Effectiveness of Repeat Hepatic Resection for Patients With Recurrent Intrahepatic Cholangiocarcinoma: Factors Associated With Long-Term Outcomes. Surgery (2017) 161(4):897–908. doi: 10.1016/j.surg.2016.10.024
30. Zhu Y, Mao Y, Chen J, Qiu Y, Guan Y, Wang Z, et al. Radiomics-Based Model for Predicting Early Recurrence of Intrahepatic Mass-Forming Cholangiocarcinoma After Curative Tumor Resection. Sci Rep (2021) 11(1):18347. doi: 10.1038/s41598-021-97796-1
31. Xu L, Wan Y, Luo C, Yang J, Yang P, Chen F, et al. Integrating Intratumoral and Peritumoral Features to Predict Tumor Recurrence in Intrahepatic Cholangiocarcinoma. Phys Med Biol (2021) 66(12):1–10. doi: 10.1088/1361-6560/ac01f3
32. Jeong S, Luo G, Gao Q, Chen J, Liu X, Dong L, et al. A Combined Cox and Logistic Model Provides Accurate Predictive Performance in Estimation of Time-Dependent Probabilities for Recurrence of Intrahepatic Cholangiocarcinoma After Resection. Hepatobiliary Surg Nutr (2021) 10(4):464–75. doi: 10.21037/hbsn.2020.01.07
Keywords: intrahepatic cholangiocarcinoma, recurrence, prognosis, nomogram, adjuvant chemotherapy
Citation: Li Q, Zhang J, Chen C, Song T, Qiu Y, Mao X, Wu H, He Y, Cheng Z, Zhai W, Li J, Zhang D, Geng Z and Tang Z (2022) A Nomogram Model to Predict Early Recurrence of Patients With Intrahepatic Cholangiocarcinoma for Adjuvant Chemotherapy Guidance: A Multi-Institutional Analysis. Front. Oncol. 12:896764. doi: 10.3389/fonc.2022.896764
Received: 15 March 2022; Accepted: 23 May 2022;
Published: 23 June 2022.
Edited by:
Mingyu Chen, Sir Run Run Shaw Hospital, ChinaReviewed by:
Zhao Li, Peking University People’s Hospital, ChinaCopyright © 2022 Li, Zhang, Chen, Song, Qiu, Mao, Wu, He, Cheng, Zhai, Li, Zhang, Geng and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaohui Tang, dGFuZ3poYW9odWlAeWFob28uY29t; Zhimin Geng, Z2VuZ3poaW1pbkBtYWlsLnhqdHUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.